Abstract
The severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) pandemic has disrupted health care and has resulted in high mortality rates.1 Vaccination is an international priority to mitigate the risks of SARS-CoV-2. The initial trials for development of SARS-CoV-2 vaccines excluded individuals with immunocompromising conditions.2
Abbreviations used in this paper: IBD, inflammatory bowel disease; IQR, interquartile range; PREVENT-COVID, Partnership to Report Effectiveness of Vaccination in populations Excluded from iNitial Trials of Coronavirus Disease; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2
The severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) pandemic has disrupted health care and has resulted in high mortality rates.1 Vaccination is an international priority to mitigate the risks of SARS-CoV-2. The initial trials for development of SARS-CoV-2 vaccines excluded individuals with immunocompromising conditions.2
Because individuals on immunosuppression, including those with inflammatory bowel diseases (IBD), may not mount as robust an antibody titer to vaccination,3 , 4 the Food and Drug Administration has recommended an additional dose after the initial series.5
To date, little is known about the effectiveness and safety of additional vaccine doses in patients with IBD. We sought to quantify the humoral immune response to an additional vaccine in this population.
The Partnership to Report Effectiveness of Vaccination in populations Excluded from iNitial Trials of Coronavirus Disease (PREVENT-COVID) is a prospective, observational, cohort of patients with IBD who have received any SARS-CoV-2 vaccine granted emergency use authorization with initial enrollment in March of 2021. Methods for PREVENT-COVID have been described previously.6 Here, we analyzed data on participants who completed baseline and follow-up surveys, had samples obtained 8 weeks after initial vaccination series, and samples 3 to 8 weeks after an additional vaccine. We excluded those who self-reported prior COVID-19 infection and/or who had a positive nucleocapsid assay at baseline.
Side effects to the vaccine were self-reported as none, mild, moderate, severe, or very severe. We performed quantitative measurement of anti–receptor binding domain IgG antibodies specific to SARS-CoV-2 using the Lab Corp Diagnostics (LabCorp Diagnostics, Calabasas, CA).6 Results of 1.0 μg/mL or greater suggest detectable serologic response to vaccination and/or prior infection with SARS-CoV-2.
We used descriptive statistics to characterize the population and anti-spike antibody levels before and after additional vaccines and determined the rate of seroconversion among those who initially had undetectable levels. Variables included age, sex, disease subtype, vaccine type (BNT162b2 vs messenger RNA [mRNA]-1273), time since vaccination, and use of IBD medications. We report the median antibody level (interquartile range [IQR]) after initial series, after additional vaccination, and the delta (SD) for each vaccine type. We used the Wilcoxon rank sum to compare the median change in antibody level with additional SARS-CoV-2 vaccination by detectable response to the initial vaccine series. We used a linear regression model in which quartiles of antibody levels were treated as a continuous variable to determine factors associated independently with the level of antibody response. All analyses were performed using SAS version 9.4 (SAS Institute, Cary, NC). The study protocol was approved by the Institutional Review Board at the University of North Carolina.
A total of 659 participants with IBD were included (415 [63%] initially received BNT162b2 [Pfizer, New York, NY-BioNTech, Mainz, Germany], 243 [37%] initially received mRNA-1273 [National Institutes of Health–Moderna, Cambridge, MA], and 5 [1%] initially received Ad26.COV2.S [Johnson & Johnson, New Brunswick, NJ]) (Supplementary Table 1). A total of 408 (98%) initial BNT162b2 vaccine recipients received an additional dose of BNT162b2, 225 (96%) initial mRNA-1273 vaccine recipients received an additional dose of mRNA-1273, and those who initially received Ad26.COV2.S received additional doses of an mRNA vaccine.
Overall, 612 (93%) had a detectable initial response to SARS-CoV-2 vaccination. Antibody response was measured at a median of 66 days (range, 61–73 d). After the additional SARS-CoV-2 immunization (median, 48 d; range, 43–53 d), 99.5% of patients had a detectable antibody titer, including 45 of 47 (95.7%) of those with undetectable antibodies at the conclusion of the initial series. Both BNT162b2 and mRNA-1273 additional vaccines were associated with a significant increase in titer as compared with baseline (P < .001 for both). On multivariate analysis, mRNA-1273 (β coefficient, 0.38; P < .001) was associated with increased titer and anti–tumor necrosis factor combination therapy (β coefficient, -0.95; P < .001) was associated with reduced titer.
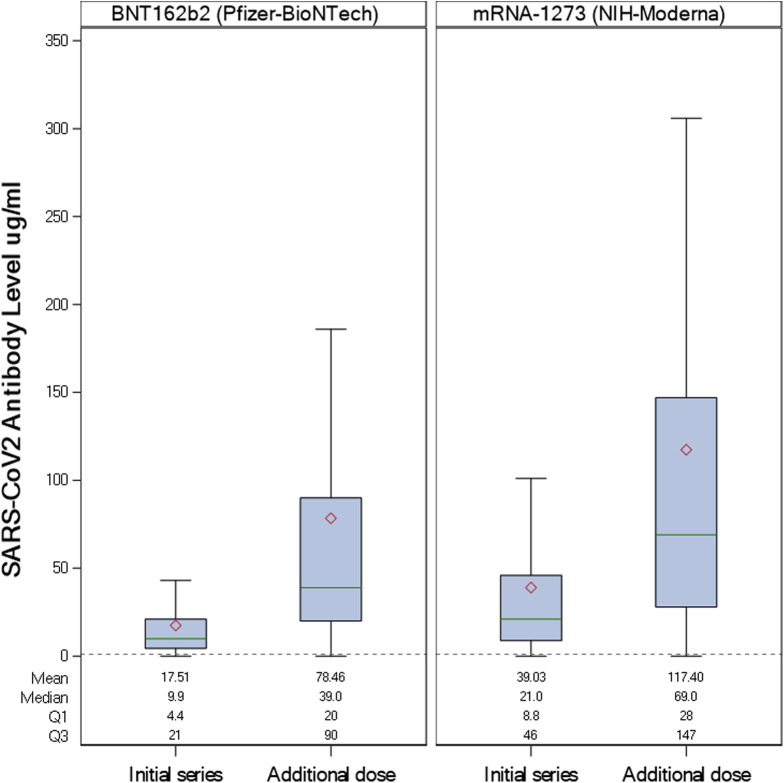
Of the 47 patients with initially undetectable antibodies, the median antibody level after the additional dose was 13 ug/mL (IQR, 5.8–24.0 ug/mL) as compared with 51 ug/mL (IQR, 26.0–115.0 ug/mL) for those with detectable antibodies after the initial series (P = .017). Change in antibody levels after the additional dose by vaccine type is shown in Figure 1 , with higher antibody titer after mRNA-1273. Additional vaccination generally was well tolerated in this population, with 44% having no side effects, 24% with mild side effects, 25% with moderate side effects, and 6% with severe side effects.
Figure 1.
Antibody change with additional vaccination in patients with inflammatory bowel disease by vaccine type (BNT162b2 vs messenger RNA [mRNA]-1273). The red diamond represents the mean antibody level, the green line represents the median, the box indicates the interquartile range, and the bottom line and top line indicate the lower extreme and upper extreme values, respectively (excluding outliers). Quantitative measurement of anti–receptor binding domain IgG antibodies specific to severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) were performed using the LabCorp Cov2Quant IgG assay. The dashed line represents the level suggestive of serologic response to vaccination (1.0 μg/L). Results of 1.0 μg/mL (lower limit of quantitation) or greater suggest vaccination and/or prior infection with SARS-CoV-2. NIH, National Institutes of Health.
These findings show substantial immunogenicity to additional doses of SARS-CoV-2 vaccine, even among IBD patients with undetectable antibody levels after the initial series. The highest increase in antibody titer was seen with an additional dose of mRNA-1273. Combination anti–tumor necrosis factor therapy was associated with a significant reduction in antibody titer. Reassuringly, adverse event rates were low among patients receiving an additional vaccination of any type. A recently published series of cancer patients showed 93.7% mounting a detectable humoral vaccine response 2 to 9 weeks after the initial vaccine series. A third vaccine given to 30 patients with persistently low antibody titers resulted in an 88.5% seroconversion rate.7 In 17 patients with rheumatoid arthritis who did not mount an initial response to SARS-CoV-2 vaccine, 15 patients reached moderate to maximal postvaccine titers after an additional vaccine. However, in this rheumatoid arthritis population, 16 of 17 patients held their disease-modifying agents before the additional vaccine.8 In our IBD population, 95.7% of those with an initial undetectable response (n = 47) developed a detectable humoral response to an additional vaccine, comparable with results in other immunosuppressed populations. Importantly, recommendations in IBD do not include holding immunosuppressive therapies before vaccination.9
There were a number of strengths to this large prospective study of humoral vaccine response to additional SARS-CoV-2 vaccine in patients with IBD. The cohort is geographically diverse, contributing to generalizability across the US population. The large sample size allows for precise estimates of humoral vaccine response to an additional vaccine dose in patients with IBD. Study limitations included a convenience sample that may not represent the broader US population and the reliance of self-report for details regarding immunization. The relatively low rate of initial undetectable antibody titer makes subgroup analysis difficult to determine independent medication effects of seroconversion with additional vaccine. In addition, no threshold has been established for protective immunity in quantitative antibody testing.
Nevertheless, these findings provide urgently needed data regarding the effectiveness of additional mRNA vaccines in immunosuppressed individuals. These data can be used to inform vaccine decisions in patients with IBD.
Footnotes
Members of the PREVENT-COVID Study Group are listed in the Appendix
Conflicts of interest These authors disclose the following: Millie D. Long has received research/grants from Pfizer, Inc, and has consulted for AbbVie, Inc, Bristol-Myers Squibb Company, Calibr, Eli Lilly and Company, Genentech, Inc, Gilead Sciences, Inc, Janssen Pharmaceuticals, Inc, Pfizer Inc, Roche, Takeda Pharmaceuticals U.S.A., Inc, TARGET PharmaSolutions, Inc, and Theravance Biopharma; Kimberly N. Weaver has consulted for AbbVie; Kelly Y. Chun is an employee of LabCorp; and Michael D. Kappelman has consulted for AbbVie, Janssen, Pfizer, and Takeda, is a shareholder in Johnson & Johnson, and has received research support from Pfizer, Takeda, Janssen, AbbVie, Lilly, Genentech, Boehringer Ingelheim, Bristol Myers Squibb, Celtrion, and Arenapharm. The remaining authors disclose no conflicts.
Funding This research was funded by the Helmsley Charitable Trust, United States.
PREVENT-COVID Study Group The PREVENT-COVID Study Group members include the following: R. Watkins (Division of Pediatric Gastroenterology and Nutrition, University of Maryland School of Medicine, Baltimore, Maryland), J. Adler (Susan B. Meister Child Health Evaluation and Research Center, Department of Pediatrics, University of Michigan, Ann Arbor, Michigan), M.C. Dubinsky (Department of Pediatrics, Susan and Leonard Feinstein IBD Center, Icahn School of Medicine at Mount Sinai, New York, New York), A. Kastl (Division of Gastroenterology, Children’s Hospital of Philadelphia, Perelman School of Medicine, Philadelphia, Pennsylvania), A. Bousvaros (Children’s Hospital Boston, Boston, Massachusetts), J.A. Strople (Ann and Robert H. Lurie Children’s Hospital of Chicago), R.K. Cross (Division of Gastroenterology and Hepatology, University of Maryland School of Medicine, Baltimore, Maryland), P.D.R. Higgins (Division of Gastroenterology and Hepatology, University of Michigan, Ann Arbor, Michigan), R.C. Ungaro (Susan and Leonard Feinstein IBD Center, Icahn School of Medicine at Mount Sinai, New York, New York), M. Bewtra (Division of Gastroenterology, Department of Biostatistics and Epidemiology, University of Pennsylvania, Philadelphia, Pennsylvania), E. Bellaguarda (Division of Gastroenterology and Hepatology, Northwestern University, Chicago, Illinois), F.A. Farraye (Division of Gastroenterology and Hepatology, Mayo Clinic, Jacksonville, Florida), M.E. Boccieri and A. Firestine (Division of Pediatric Gastroenterology and Hepatology, University of North Carolina, Chapel Hill, North Carolina), M. Fernando, M. Bastidas, and M. Zikry (Esoterix Specialty Laboratory, LabCorp, Calabasas, California), and X. Dai (Center for Gastrointestinal Biology and Disease, University of North Carolina at Chapel Hill, Chapel Hill, North Carolina)
Conflicts of interest The PREVENT-COVID Study Group members disclose the following: Jeremy Adler has consulted for Janssen, and has received research support from The Gary and Rachel Glick Charitable Fund, Shaevsky Family Research Fund for Crohn's Disease, the Crohn’s & Colitis Foundation, and The Leona M. and Harry B. Helmsley Charitable Trust; M.C. Dubinsky has received consultant fees from AbbVie, Arena, Bristol Myers Squibb, Celgene, Eli Lilly, Gilead, Janssen, Pfizer, Prometheus Labs, and Takeda, has received grant support from AbbVie and Prometheus Labs, and has received license fees from Takeda; Athos Bousvaros has received research support (subinvestigator on protocols) from the following companies: Janssen, AbbVie, Takeda, Buhlmann, Arena, and Eli Lilly, has consulted for Arena, Best Doctors, Eli Lilly, and Takeda, and has received royalties from Up to Date; Raymond K. Cross has participated in advisory boards and consulted for AbbVie, Bristol Myers Squibb, Eli Lilly, Janssen, LabCorp, Pfizer, Samsung Bioepis, and Takeda; P.D.R. Higgins has consulted for AbbVie, Pfizer, and Takeda, and has received grant support from the National Institutes of Health, CCF, AbbVie, Pfizer, Takeda, Genentech, Eli Lilly, Arena, and the Rainin Foundation; Ryan C. Ungaro has served as an advisory board member or consultant for AbbVie, Bristol Myers Squibb, Eli Lilly, Janssen, Pfizer, and Takeda, has received research support from AbbVie, Boehringer Ingelheim, and Pfizer; Meena Bewtra has received research funding from Janssen, GlaxoSmithKline, and Takeda, has served as a consultant for Janssen, AbbVie, BMS, and Pfizer, and has received honorarium for participation in a continuing medical education program sponsored by AbbVie; Emanuelle Bellaguarda has consulted for AbbVie, Pfizer, and Bristol Myers Squibb; F.A. Farraye is a consultant for Arena, BMS, Braintree Labs, Gilead, GI Reviewers, Innovation Pharmaceuticals, Iterative Scopes, Janssen, Pfizer, and Sebela, and is a member of the Data Safety Monitoring Board for Lilly and Theravance; and Manory Fernando, Monique Bastidas, and Michael Zikry are employees of LabCorp. The remaining PREVENT-COVID Study Group members disclose no conflicts.
Note: To access the supplementary material accompanying this article, visit the online version of Clinical Gastroenterology and Hepatology at www.cghjournal.org, and at http://doi.org/10.1016/j.cgh.2022.01.056.
Contributor Information
PREVENT-COVID Study Group:
R. Watkins, J. Adler, M.C. Dubinsky, A. Kastl, A. Bousvaros, J.A. Strople, R.K. Cross, P.D.R. Higgins, R.C. Ungaro, M. Bewtra, E. Bellaguarda, F.A. Farraye, M.E. Boccieri, A. Firestine, M. Fernando, M. Bastidas, M. Zikry, X. Dai, J. Adler, M.C. Dubinsky, A. Kastl, A. Bousvaros, R.K. Cross, P.D.R. Higgins, R.C. Ungaro, M. Bewtra, E. Bellaguarda, F.A. Farraye, M.E. Boccieri, A. Firestine, M. Fernando, M. Bastidas, M. Zikry, and X. Dai
Appendix
Supplementary Table 1.
Characteristics of IBD Patients Receiving SARS-CoV-2 Additional Vaccine by Response to Initial Vaccine Series
| All patients (n = 659) | Detectable initial antibody (n = 612) | Undetectable initial antibody (n = 47) | P value | |||
|---|---|---|---|---|---|---|
| Age, y, means (SD) | 44.6 (14.4) | 44.6 (14.5) | 44.6 (13.8) | .999 | ||
| Female, n (%) | 475 (72) | 443 | (72) | 32 | (68) | .527 |
| Disease type, n (%) | ||||||
| Crohn’s disease | 476 (72) | 439 | (72) | 37 | (79) | |
| Ulcerative colitis/IBD-unclassified | 177 (27) | 167 | (27) | 10 | (21) | |
| Type of vaccine (initial), n (%) | <.001 | |||||
| BNT162b2 | 415 (63) | 381 | (62) | 34 | (72) | |
| mRNA-1273 | 243 (37) | 228 | (37) | 10 | (21) | |
| Ad26.COV2.S | 5 (1) | 2 | (0) | 3 | (6) | |
| Type of vaccine (additional), n (%) | .131 | |||||
| BNT162b2 | 415 (63) | 379 | (62) | 36 | (77) | |
| mRNA-1273 | 243 (37) | 232 | (38) | 11 | (23) | |
| Ad26.COV2.S | 1 (0) | 1 | (0) | 0 | (0) | |
| Medication treatment at baseline vaccination, n (% yes)a | ||||||
| No medical therapy | 15 (2) | 13 | (2) | 2 | (4) | .345 |
| Systemic steroids | 36 (5) | 31 | (5) | 5 | (11) | .105 |
| Anti-TNF monotherapy | 274 (42) | 258 | (42) | 16 | (34) | .277 |
| Anti-TNF combination therapyb | 121 (18) | 101 | (17) | 20 | (43) | <.001 |
| Thiopurine | 58 (9) | 56 | (9) | 2 | (4) | .254 |
| Methotrexate | 5 (1) | 5 | (1) | 0 | (0) | .534 |
| Mesalamine or sulfasalazine (any) | 118 (18) | 114 | (19) | 4 | (9) | .081 |
| Budesonide | 26 (4) | 22 | (4) | 4 | (9) | .095 |
| Vedolizumab | 72 (11) | 72 | (12) | 0 | (0) | .013 |
| Ustekinumab | 92 (14) | 90 | (15) | 2 | (4) | .046 |
| Tofacitinib | 11 (2) | 10 | (2) | 1 | (2) | .799 |
NOTE. Detectable initial antibody was defined as the quantitative measurement of anti–receptor binding domain IgG antibodies specific to SARS-CoV-2 using the LabCorp Cov2Quant IgG assay with results of ≥1.0 μg/mL.
IBD, inflammatory bowel disease; mRNA, messenger RNA; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; TNF, tumor necrosis factor.
Numbers do not add to total n because patients may be taking more than 1 medication class.
Including azathioprine, 6-mercaptopurine, or methotrexate.
References
- 1.Ganesh B., et al. Clin Epidemiol Global Health. 2021;10:100694. doi: 10.1016/j.cegh.2020.100694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Self W.H., et al. MMWR Morb Mortal Wkly Rep. 2021;70:1337–1343. doi: 10.15585/mmwr.mm7038e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Collier A.Y., et al. J Infect Dis. 2022;225:1124–1128. doi: 10.1093/infdis/jiab569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Buttiron Webber T., et al. Eur J Cancer. 2021;159:105–112. doi: 10.1016/j.ejca.2021.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.https://www.cdc.gov/coronavirus/2019-ncov/vaccines/recommendations/immuno.html?s_cid=10483:immunocompromised%20and%20covid%20vaccine:sem.ga:p:RG:GM:gen:PTN:FY21
- 6.Kappelman M.D., et al. Gastroenterology. 2021;161:1340–1343.e1342. doi: 10.1053/j.gastro.2021.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gounant V., et al. J Thorac Oncol. 2022;17 doi: 10.1016/j.jtho.2021.10.015. 239–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schmiedeberg K., et al. Lancet Rheumatol. 2022;4 doi: 10.1016/S2665-9913(21)00328-3. e11–e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Siegel C.A., et al. Gut. 2021;70:635–640. doi: 10.1136/gutjnl-2020-324000. [DOI] [PMC free article] [PubMed] [Google Scholar]