Abstract
Background:
Antiretroviral options for neonates (younger than 28 days) should be expanded. We evaluated the pharmacokinetics, safety, and acceptability of the "4-in-1" fixed-dose pediatric granule formulation of abacavir/lamivudine/lopinavir/ritonavir (30/15/40/10 mg) in neonates.
Methods:
The PETITE study is an ongoing phase I/II, open-label, single-arm, 2-stage trial conducted in South Africa. In stage 1, term neonates exposed to HIV on standard antiretroviral prophylaxis (nevirapine ± zidovudine) received single dose(s) of the 4-in-1 formulation, followed by intensive pharmacokinetic sampling and safety assessments. At each PK visit, blood was drawn after an observed dose at 1, 2, 4, 8, and 12 hours postdose. In this study, we have reported the planned interim pharmacokinetic and safety analysis after completion of the single-dose administration.
Results:
Sixteen neonates, with a median (range) birth weight of 3130 g (2790–3590 g), completed 24 pharmacokinetic visits. The 4-in-1 formulation imposed relatively high doses of abacavir [8.6 mg/kg (6.6–11.4)] and lamivudine [4.3 mg/kg (3.3–5.7)] but lower doses of lopinavir [11.5 mg/kg (8.8–15.2)]. The geometric means (GM, 90% CI) AUC0–12 of abacavir, lamivudine, and lopinavir were 29.87 (26.29–33.93), 12.61 (10.72–14.83), and 3.49 (2.13–5.72) µg.h/mL, respectively. Lopinavir GM AUC0–12 was below the predefined target (20–100 µg.h/mL), and ritonavir concentrations were only detectable in 4 of the 120 (3%) samples. No adverse events were related to study drugs. No neonate had difficulty swallowing the 4-in-1 formulation.
Conclusions:
The high doses of abacavir and lamivudine (in mg/kg) and AUCs were safe, and the formulation was well tolerated; however, lopinavir/ritonavir exposures were extremely low, preventing its use in neonates use in neonates. Alternative pediatric solid antiretroviral formulations must be studied in neonates.
Key Words: neonates, HIV, pharmacokinetics, abacavir, lamivudine, lopinavir
INTRODUCTION
The clinical benefits of early antiretroviral therapy (ART) for young children living with HIV are well established,1 but appropriate antiretroviral formulations that are safe, efficacious, and acceptable for neonates (younger than 28 days) are lacking. Initiating ART at birth or in the first few weeks of life for HIV treatment or prevention is challenging in all settings. In sub-Saharan Africa, only zidovudine (ZDV), lamivudine (3TC), and nevirapine (NVP) liquid formulations are readily available for use from birth. Lopinavir/ritonavir (LPV/r) is widely used in pediatric HIV treatment, but there are no formulations approved for use from birth. LPV/r liquid formulation was initially approved for use in neonates aged 14 days or older, but the US Food and Drug Administration revised its 2013 labeling to specify for older than 42 weeks postmenstrual age as well, following reports of cardiac toxicity, acute renal failure, and respiratory complications in preterm neonates.2 Liquid LPV/r contains 42% alcohol and 15% propylene glycol, and it is unknown whether these toxicities are due to LPV/r or excessive exposure to the alcohol and/or propylene glycol in the formulation. Solid LPV/r formulations without these excipients could be advantageous for neonates, and expanding antiretroviral options for exposed neonates (born to women with HIV) remains a high priority.3
The first solid pediatric LPV/r formulation developed was the oral “pellets" (Cipla Ltd, India.) composed of mini-meltrex tablets (1.8 mm) stored in capsules containing LPV/r at 40/10 mg.4 The oral pellets were approved for use for infants not only older than 14 days but also weighing >5.0 kg because of swallowability uncertainties in neonates. Building on this formulation, Cipla in partnership with the Drugs for Neglected Disease initiative recently developed a taste-masked fixed-dose combination (FDC) pediatric granule formulation of abacavir (ABC/3TC/LPV/r—30/15/40/10 mg), also referred to as the “4-in-1,” for the treatment of infants and young children with HIV.5 These granules (0.2–0.5 mm) are up to 9-fold smaller than the pellets and considered safe to swallow. The 4-in-1 capsules are simple to open, have no cold chain requirements, and the strawberry-flavored granules can be given with liquids, for example, breastmilk. The drug doses and ratios of ABC/3TC/LPV/r in the 4-in-1 capsule were designed for use in infants aged 4 weeks or older and weighing 3.0 kg for dosing per WHO weight bands.6 Multidosing pharmacokinetics (PK) and safety for the 3TC liquid formulation use7 and single-dose data for the ABC liquid formulation8 in neonates are available but none for solid pediatric formulations. Administering the 4-in-1 to neonates with immature drug elimination pathways may potentially lead to overexposure or underexposure of individual drug components. However, given the multiple advantages of this FDC granule formulation over individual liquid formulations, coupled with the favorable safety profiles of these drugs in children,9–11 we designed this study to evaluate the PK, safety, and acceptability of the 4-in-1 in term neonates.
METHODS
Study Design
The "PETITE" study is an ongoing phase I/II, open-label, single-arm, 2-stage clinical trial conducted at the Family Center for Research with Ubuntu at Tygerberg Hospital in Cape Town, South Africa (PACTR202007806554538). The study was designed to enroll 50 neonates exposed to HIV with birth weights between ≥2000 and ≤4000 g in 2 stages. In stage 1, the safety and single-dose PK of the 4-in-1 were assessed in 2 sequential cohorts: cohort 1A (n = 8) and cohort 1B (n = 8). Clinical and laboratory safety criteria had to be met before moving from cohort 1A to 1B. If both safety and predefined PK criteria were met following stage 1 single-dose PK, stage 2 would open to assess multidosing in neonates (cohort 2, n = 34). The study was approved by the Stellenbosch University Health Research Ethics Committee and the World Health Organization Research Ethics Review Committee. In this study, we have reported the planned interim safety and PK analysis after completion of stage 1.
Stage 1
All mothers provided written informed consent, and healthy term (gestational age: 38 weeks or older) neonates exposed to HIV were screened for enrollment. All neonates received HIV nucleic acid tests (NATs) at birth and standard antiretroviral prophylaxis (NVP with or without ZDV) per South African guidelines.12 Neonates were excluded based on the following criteria: (1) their baseline-corrected QT (QTc) intervals were >450 milliseconds (msec) on electrocardiogram (ECG); (2) aspartate transaminase (AST) or alanine aminotransferase value >1.25 the upper limit of normal (ULN); (3) creatinine value above the ULN; (4) hemoglobin level <13 g/dL; (5) hypokalemia (<3.6 mmol/L), hyperkalemia (>6.7 mmol/L), hyponatremia, (<135 mmol/L), hypernatremia (>145 mmol/L), and any other grade 3 or higher adverse event (AE) on the DAIDS toxicity table; (5) confirmed HIV infection; (6) receipt of tuberculosis treatment; or (7) if their mother was receiving LPV-containing ART. All ECGs were assessed by the study investigators and an independent pediatric cardiologist. A single 4-in-1 (ABC/3TC/LPV/r—30/15/40/10 mg) capsule was opened and mixed with expressed breastmilk or formula milk for oral administration on the PK sampling day. Acceptability and swallowability evaluations were performed at PK visits.
Cohort 1A
Screening and entry visits were performed in neonates younger than 14 days exposed to HIV with birth weights of ≥2500 to ≤4000 g. Enrolled neonates received a single observed dose of the 4-in-1 between neonates aged 14 days or older and those younger than 21 days. Thereafter, blood was drawn at 1, 2, 4, 8, and 12 hours postdose. An ECG was performed 4 hours after dosing [time to maximum concentration (Tmax) for LPV]. A clinical and laboratory safety visit (including HIV NAT) was performed 1 week after the PK visit. If 1 participant experienced a QTc interval of >500 msec, 1 participant a fatal or life-threatening–related AE, 2 participants the same related grade 3 or higher AE; or 3 participants with different related grade 3 or higher AEs (safety hold criteria), cohort 1A would be paused until the data safety and monitoring board (DSMB) assessed causality and advised on either study continuation or termination. If no major safety concerns were identified, cohort 1B would open to accrual.
Cohort 1B
Screening and entry visits of neonates were performed within the first 72 hours of life. Neonates exposed to HIV with birth weights ≥2000 to ≤4000 g were screened for enrollment. Enrolled neonates received a single dose of the 4-in-1 between ≥3 days and <14 days of age. After an observed dose, blood was drawn at 1, 2, 4, 8, and 12 hours postdose. An ECG was performed 4 hours after dosing. Ten to 14 days later, another single dose of the 4-in-1 was administered, followed by identical PK sampling and assessments. Clinical and laboratory safety visits were also performed 1 week after each PK visit. The safety hold criteria for cohort 1B were the same as those for cohort 1A.
Quantification of Plasma Drug Concentrations
Antiretroviral plasma concentrations were measured using liquid chromatography triple quadrupole mass spectrometry assays validated per US Food and Drug Administration guidelines at Bioanalytical Testing Laboratory of the Faculty of Associated Medical Sciences, Chiang Mai University. The lower limit of quantification (LLOQ) was 0.02 µg/mL for ABC/3TC and 0.05 µg/mL for LPV/RTV. This laboratory participates in the US NIH Clinical Pharmacology Quality Control program.13
Pharmacokinetic and Statistical Analysis
A noncompartmental pharmacokinetic analysis was performed to calculate the PK parameters using Phoenix WinNonLin (Certara). Maximum concentration (Cmax), Tmax, and 12-hour postdose concentration (C12) were taken directly from the observed concentration–time data. AUC0–12 was determined using the linear trapezoidal method. When possible, from the data, AUC12–infinity was estimated as the C12/λz, where λz was determined from the terminal slope. Apparent clearance (CL/F) was calculated as dose/AUC0–inf. If concentrations after the peak were <LLOQ, the first LLOQ value was set to LLOQ/2 and subsequent values to zero.
Interim Safety and Pharmacokinetic Analysis
An interim analysis was planned after completion of stage 1 to review the safety parameters and predefined PK criteria before proceeding to stage 2. Because no clear plasma exposure–response relationships exist for ABC and 3TC, the interim PK criteria focused on achieving LPV exposures comparable with those observed in adults and children on a multidose schedule. A mean LPV area under the concentration–time curve (AUC)0–12 between approximately40 and 100 µg.h/mL is safe and effective in adults and children.2,14 A mean LPV AUC0–12 between 30 and 150 µg.h/mL, that is, approximately 70%–150% of the reference range was selected as the predefined steady-state target for the multidose strategy. If the geometric mean (GM) of LPV AUC0–12 fell between 20 and 100 µg.h/mL (assuming an AUC0–12/AUC0–infinity ratio of 0.6515,16) after a single dose, it was expected that steady-state target exposures would be achieved.
RESULTS
Twenty-one term neonates exposed to HIV were screened. Three were ineligible due to grade 1 hypernatremic event, grade 1 AST (AST > 1.25 × ULN) event, and a mother on LPV-based ART. The baseline characteristics for the 18 enrolled neonates are listed in Table 1. The median (range) birth weight of neonates in stage 1 was 3143 g (2790–3590). Two neonates were withdrawn before receiving the 4-in-1, one because of new neurological signs (hypotonia, lethargy, and absence of eye contact) and one mother withdrew consent.
TABLE 1.
Baseline Characteristics of the Neonates at Enrollment
| Characteristics | Cohort 1A (N = 9) | Cohort 1B (N = 9) | Total (N = 18) | |
| Sex (female: male) | 6 (67%): 3 (33%) | 7 (78%): 2 (22%) | 13 (72%): 5 (28%) | |
| Ethnicity | Black | 9 (100%) | 8 (89%) | 17 (94%) |
| Mixed race | 0 (0%) | 1 (11%) | 1 (6%) | |
| Gestational age at birth (wk) | 39 (39–39) | 39 (39–40) | 39 (39–40) | |
| Birth weight (g) | 3090 (2840–3240) | 3195 (2955–3475) | 3143 (2890–3475) | |
| Days of life | 3 (2–4) | 2 (2–2) | 2 (2–3) | |
| Weight (g) | 3010 (2750–3140) | 3005 (2840–3355) | 3008 (2770–3355) | |
| Length (cm) | 47 (47–48) | 48 (48–50) | 48 (47–49) | |
| Primary caregiver | Mother | 9 (100%) | 9 (100%) | 18 (100%) |
| Age of primary caregiver (yr) | 30 (27–33) | 32 (28–34) | 31 (27–34) | |
| ARV prophylaxis | NVP | 4 (44%) | 3 (33%) | 7 (39%) |
| NVP + ZDV | 5 (56%) | 6 (67%) | 11 (61%) | |
| Hemoglobin (g/dL) | 17.6 (16.2–18.2) | 17.3 (17.1–19.0) | 17.5 (16.3–18.4) | |
| Sodium (mmol/L) | 143 (139–144) | 140 (138–142) | 142 (139–144) | |
| Potassium (mmol/L) | 5.2 (4.7–5.3) | 5.1 (5.0–5.4) | 5.2 (4.8–5.4) | |
| Creatinine (µmol/L) | 51 (49–63) | 68 (57–69) | 58 (51–69) | |
| ALT (U/L) | 11 (8–14) | 10 (8–11) | 11 (8–13) | |
| AST (U/L) | 54 (31–59) | 53 (50–58) | 54 (46–59) | |
| Total bilirubin (µmol/L) | 72 (30–106) | 61 (29–72) | 69 (29–79) | |
| Heart rate* (beats/min) | 143 (120–150) | 129 (120–140) | 132 (120–150) | |
| QT interval* (msec) | 280 (240–280) | 280 (248–300) | 280 (248–288) | |
| QTcF1 (msec) | 346 (331–363) | 364 (340–378) | 356 (331–365) | |
| HIV-1 NAT | Negative | 9 (100%) | 9 (100%) | 18 (100%) |
Values are n (%) or median (interquartile range).
As assessed by pediatric cardiologist.
ALT, alanine aminotransferase; ARV, antiretroviral; g, grams; HIV-1 NAT, HIV-1 nucleic acid test; QTcF, QT interval according to Fridericia formula.
Pharmacokinetics of the 4-in-1 in Neonates
Sixteen neonates completed stage 1. In cohort 1A, 8 neonates had intensive PK assessment at a median (range) of 15 (14–19) days of life, with a body weight of 3413 g (3020–4240 g). After no safety hold criteria were met, cohort 1B opened to accrual. In cohort 1B, 8 neonates had both intensive PK assessments, the first at a median of 8 (7–9) days of life, with a body weight of 3288 g (2640–3740 g). The second PK assessment was performed at 20 (18–22) days of life, with a body weight of 3715 g (3015–4525 g). At the PK visits (n = 24), the dose in mg/kg for ABC, 3TC, and LPV/RTV was 8.6 (6.6–11.4), 4.3 (3.3–5.7), and 11.5/2.9 (8.8/2.2–15.2/3.8), respectively.
All 120 PK samples were successfully collected per protocol and analyzed. ABC and 3TC plasma concentrations were above the LLOQ in all samples. LPV concentrations were greater than LLOQ in 93 of the 120 (78%) samples, but only 4 of the 120 (3.3%) samples had quantifiable RTV concentrations. The mean concentration versus time curves for ABC, 3TC, and LPV at each PK visit are shown in (Fig. 1). The GM (90% CI) for ABC AUC0–12 decreased with postnatal age: 35.90, 28.02, and 26.50 µg.h/mL at 7–9, 14–19, and 18–22 days of life, respectively. A relatively smaller decrease in 3TC AUC0–12 from 13.34 to 11.90 µg.h/mL was observed over the same period. Lopinavir and ritonavir concentrations were extremely low at each PK visit and did not allow estimation of λz and AUC0–inf. No neonates had an LPV concentration above 4.10 µg/mL at any time point. The LPV AUC0–12 was low and variable, ranging between 0 and 26.33 µg.h/mL. A summary of the PK parameters for ABC, 3TC, and LPV is summarized in Table 2. Combining all PK visits, the GM (90% CI) AUC0–12 of ABC, 3TC, and LPV was 29.87 (26.29–33.93) µg.h/mL, 12.61 (10.72–14.83) µg.h/mL, and 3.49 (2.13–5.72) µg.h/mL, respectively. The GM LPV AUC0–12 in cohort 1A/1B did not fall within the predefined target of 20–100 µg.h/mL necessary to assess multidoses of the 4-in-1 in cohort 2.
FIGURE 1.
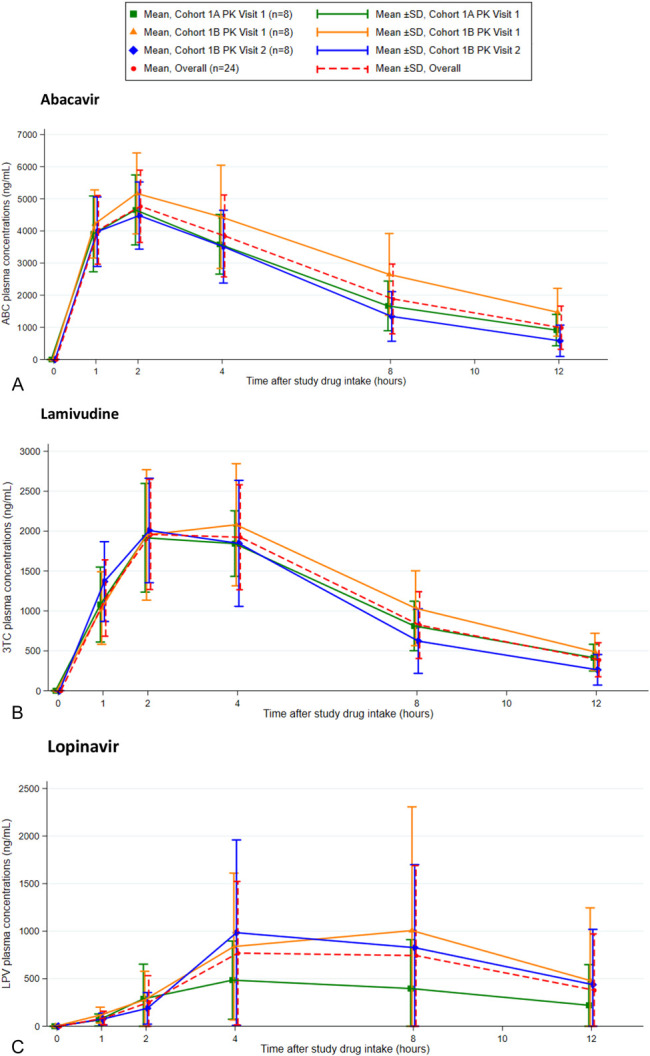
Concentration time curves for (A) abacavir, (B) lamivudine, and (C) lopinavir for each pharmacokinetic visit.
TABLE 2.
Summary of Pharmacokinetic Parameters for Abacavir, Lamivudine, and Lopinavir
| Drug | Cohort | PK Parameter | Median (Min to Max) | Geometric Mean | 90% CI |
| ABC* | 1A + 1B | AUC0–12 (µg.h/mL) | 31.12 (13.85–63.35) | 29.87 | 26.29 to 33.93 |
| AUC0–inf (µg.h/mL) | 36.12 (16.37–89.91) | 34.81 | 29.90 to 40.53 | ||
| AUC0–12/AUC0–inf ratio | 0.87 (0.70–0.98) | 0.86 | 0.83 to 0.89 | ||
| Cmax (µg/mL) | 4.93 (2.55–7.24) | 4.79 | 4.38 to 5.23 | ||
| Tmax (h) | 2.0 (0.9–4.0) | — | — | ||
| C12 (µg/mL) | 0.93 (0.12–2.95) | 0.757 | 0.57 to 1.01 | ||
| CL/F (L/h/kg) | 0.23 (0.13–0.51) | 0.25 | 0.22 to 0.29 | ||
| Dose (mg/kg) | 8.61 (6.63–11.36) | — | — | ||
| 3TC* | 1A + 1B | AUC0–12 (µg.h/mL) | 13.38 (2.80–22.14) | 12.61 | 10.72 to 14.83 |
| AUC0–inf (µg.h/mL) | 16.00 (3.26–26.47) | 14.28 | 12.06 to 16.92 | ||
| AUC0–12/AUC0–inf ratio | 0.90 (0.75–0.95) | 0.88 | 0.86 to 0.90 | ||
| Cmax (µg/mL) | 2.09 (0.57–3.18) | 2.00 | 1.75 to 2.29 | ||
| Tmax (h) | 3.1 (2.0–4.1) | — | — | ||
| C12 (µg/mL) | 0.39 (0.09–0.75) | 0.33 | 0.26 to 0.41 | ||
| CL/F (L/h/kg) | 0.27 (0.16–1.23) | 0.31 | 0.26 to 0.35 | ||
| Dose (mg/kg) | 4.30 (3.31–5.68) | — | — | ||
| LPV* | 1A + 1B | AUC0–12 (µg.h/mL) | 4.89 (0–26.33) | 3.49 | 2.13 to 5.72 |
| AUC0–inf (µg.h/mL) | NC | NC | NC | ||
| AUC0–12/AUC0–inf ratio | NC | NC | NC | ||
| Cmax (µg/mL) | 0.75 (<0.05 to 4.06) | 0.58 | 0.38 to 0.90 | ||
| Tmax (h) | 4.1 (1.1–8.1) | — | — | ||
| C12 (µg/mL) | 0.11 (<0.05 to 2.31) | 0.21 | 0.12 to 0.37 | ||
| CL/F (L/h/kg) | NC | NC | NC | ||
| Dose (mg/kg) | 11.48 (8.84–15.15) | — | — | ||
| Dose (mg/m2) | 181 (154–215) | — | — |
3TC, lamivudine; ABC, abacavir; C12, 12-hours post-dose; CL/F, apparent clearance; inf, infinity; LPV, lopinavir; NC, cannot calculate; PK, pharmacokinetic.
Reference data for comparison with PETITE study AUC0–inf: PENTA-15 children, ABC and 3TC steady state, AUC0–12SS were 5.43 µg.h/mL (95% CI: 4.45 to 6.62) at 8.0 mg/kg twice daily and 4.74 µg.h/mL (95% CI: 3.95 to 5.70) at 4.0 mg/kg twice daily, respectively.[19] In the study conducted by Foissac et al, infants aged 6 weeks, with median (range) LPV steady-state AUC0–12SS 54.5 µg.h/mL (33.9–85.8), received LPV/r 40/10 mg twice daily.[27]
Safety of the 4-in-1 in Neonates
No deaths or life-threatening events occurred. Twenty-one AEs were reported in the 16 neonates who received a least one dose of the 4-in-1; all were assessed as not related. Of the 21 AEs, 16 were classified as grade 1, 4 as grade 2, and 1 as grade 3. The grade 3 serious AE was hospitalization for respiratory syncytial virus pneumonia, with full recovery 9 days after onset of illness. Electrolyte disturbances without clinical sequela were common. Thirteen hyperkalemic episodes, primarily grade 1 and likely due to hemolysis, were reported in 11 neonates: 10 episodes were of grade 1 (5.6 to <6.0 mml/L) and 3 grade 2 (6.0 to ≤6.7 mmol/L). Three episodes of grade 1 hyponatremia, 3 grade 1 anemia, and 1 grade 2 oral candidiasis were also reported. No neonates showed abnormal creatinine, alanine aminotransferase, AST, or elevated bilirubin levels during the study.
All 42 ECG findings, 18 at screening and 24 at PK visits, were normal. Of the 24 ECGs performed 4 hours after administration of the 4-in-1, the median (range) heart rate, QT interval, and QTcF calculations were 143 (111–167) beats/minute, 260 (220–280) msec, and 344 (309–376) msec, respectively. HIV NATs showed negative results for all neonates throughout the study.
Acceptability and Tolerability
Seventeen capsule contents were administered with breast milk and 7 with formula milk. Swallowing of the 4-in-1 when mixed with milk was reported to be easy by all caregivers for all 24 administrations. No neonate refused or vomited the 4-in-1.
DISCUSSION
This is the first study to generate PK and safety data on a solid antiretroviral FDC formulation in neonates exposed to HIV. Administration of a single dose of the 4-in-1 with formula milk or breast milk was safe and well tolerated. ABC and 3TC exposures during the first 4 weeks of life were relatively high compared with those in infants and older children, but LPV and RTV exposures were very low. Unfortunately, while no safety concerns were observed, these interim PK results of the PETITE study do not support the use of the 4-in-1 FDC granule formulation in neonates for HIV prevention or treatment.
Few antiretroviral drugs are available for neonates, most being liquid formulations (eg, ABC, ZDV, 3TC, NVP, and LPV/r), and there are no appropriate FDCs. Liquids can be costly, require cold chain facility, require accurate measurement for administration, be extremely unpalatable, and may contain harmful excipients.17 The WHO advocates for individual and FDC solid pediatric antiretroviral formulations. Weight-band dosing is also recommended by the WHO, rather than mg/kg or mg/m2 dosing, for simplification and ease of implementation. the only solid formulation approved from birth but is not widely available in resource limited settings is raltegravir granules. Solid child-friendly FDC formulations have greatly simplified the administration of ART to young children and adolescents, but to date, none have been studied in neonates. The 4-in-1 was developed to address the multiple challenges related to the LPV/r liquid formulation in young children. Owing to the lack of antiretroviral options for neonates, there was consensus at the WHO Pediatric Antiretroviral Drug Optimization meeting that the 4-in-1, although designed for infants older than 4 weeks, should be studied in neonates.3
ABC is approved at a dose of 8 mg/kg twice daily in children older than 3 months.18 In the PETITE study, after a single dose of the 4-in-1 (median ABC dose of 8.6 mg/kg), ABC exposure was 36.12 μg.h/mL, consistent with another ABC single-dose study (median dose 8.1 mg/kg) using the liquid formulation in neonates where the median ABC AUC0–inf was 39.12 μg.h/mL.8 Owing to the slow maturation of the enzymes responsible for ABC metabolism in neonates, these ABC exposures are substantially higher than those reported in young children and adults at equivalent twice-daily dosages. Indeed, in the PENTA-15 study assessing the steady-state PK and safety of the ABC and 3TC liquid formulations in children aged 3 months to younger than 36 months,19 the GM ABC AUC0–12 was 5.43 μg.h/mL (median dose 8.0 mg/kg; 45% of children on LPV/r-base ART), comparable with that reported in adults.20 Thus, the ABC exposures with the 4-in-1 in neonates were 6-fold to 7-fold higher than those in children at equivalent doses.
The liquid formulation of 3TC is approved at a dose of 4 mg/kg twice daily in children older than 3 months.21 Although no dose is licensed for use in neonates, the PK and safety of 3TC liquid has been extensively studied and is recommend by the WHO for term neonates at a weight-band dose of 1.7–2.7 mg/kg twice a day. Moodley et al studied 3TC doses of 4.0 and 2.0 mg/kg twice daily in neonates with both doses well tolerated. The steady-state 3TC exposure was 16.88 μg.h/mL with 4 mg/kg twice daily,10 which is comparable with our study using a single dose of the 4-in-1 solid granule formulation. The 3TC exposures in neonates receiving 2.0 mg/kg twice daily after the first dose (within 12 hours of birth) and at steady state 7 days later were 12.08 and 5.78 μg.h/mL, respectively.7 High exposures of 3TC were also predicted in neonates when using the WHO weight-band dosing for children older than 4 weeks.22 In the PENTA-15 trial, the steady-state 3TC AUC0–12 in children was 4.74 μg.h/mL (median dose 4.0 mg/kg),19 indicating the 3TC exposures were 3-fold higher in neonates given the 4-in-1 at equivalent doses. Of note, 3TC exposures in children are lower for the liquid formulation than for the tablets (approximately40% lower relative bioavailability) despite no difference in adults,23,24 but this interaction has not been evaluated in neonates.
The LPV and RTV exposures observed in neonates after single dose(s) of the 4-in-1 formulation were extremely low. Limited PK data are available in neonates with the liquid LPV/r formulation.25,26 The ANRS 12174 trial assessed infant LPV/r versus 3TC for the prevention of mother-to-child transmission of HIV during breastfeeding (NCT00640263). Neonates weighing 2.0–3.9 kg at birth were randomized to receive 40/10 mg LPV/r liquid twice daily starting at day 7 of life. Using a population PK model, the median (range) steady-state LPV exposures were estimated at 54.5 (33.9–85.8) μg.h/mL in 69 infants at 6 weeks of age.27 Although almost 2-fold lower than those reported in adults using the LPV/r meltrex tablet formulation, these steady-state exposures were comparable with those reported in children aged 14 days or older to younger than 6 weeks receiving liquid LPV/r formulation for HIV treatment in the IMPAACT P1030 trial.14 There may be several contributing factors to the very low LPV and RTV plasma concentrations with the 4-in-1 formulation in neonates. First, the LPV dose with a single 4-in-1 capsule in neonates weighing 2000–4000 g (ie, 10–20 mg/kg) could be lower than the licensed twice-daily dose of 16 mg/kg (or 300 mg/m2) with the liquid formulation. Higher LPV doses of up to 80 mg twice daily have also been proposed to achieve target concentrations for neonates weighing 2–6 kg.25 The median LPV dose administered to the 16 neonates with the 4-in-1 was 11.5 mg/kg (or 181 mg/m2), but this lower dose was insufficient to explain the very low LPV exposures observed in the PETITE study. Second, all neonates received NVP perinatal prophylaxis (15 mg, once daily) per standard of care, and LPV exposure (at steady state) was reduced by approximately 30% when coadministrated with NVP because of induction of cytochrome P450 CYP3A.28,29 Thus, a drug–drug interaction (DDI) with the background NVP prophylaxis regimen may have contributed to the low LPV exposures observed; however, extrapolation of this DDI to neonates is difficult because CYP3A4 activity is very immature during the first weeks of life (reaching 50% of adult levels between ages of 6 and 12 months).30 It is not expected that this DDI fully explains the low LPV exposures, but it cannot be ignored, and assessment of the 4-in-1 in the absence of NVP prophylaxis could be considered. A third potential factor is related to the 4-in-1 formulation. Eudragit E-PO cationic copolymer, commonly used as an excipient in the pharmaceutical industry, was used to taste-mask both LPV and RTV.31 This polymer is insoluble at pH >5 and requires a highly acidic environment in the stomach for release.32 Neonates have a relatively high intragastric pH of >433 and are typically fed every 3 hours. In addition, their intragastric pH can increase after a meal to 6.0–6.5 and remain elevated for 1 hour after feeding.34 It is possible that the Eudragit E-PO polymer when administered with milk reduced dissolution and absorption of LPV and/or RTV in this setting.
Single dose(s) of the 4-in-1 in term neonates was safe and well tolerated, and all AEs were assessed as not related. No death or life-threatening events occurred, and the only serious AE was due to hospitalization for pneumonia. No liver or renal toxicity occurred with the single doses despite the high ABC and 3TC exposures observed. It is difficult to draw firm conclusions about the safety of the solid LPV and RTV formulation in neonates because the exposures were extremely low. Despite the previous life-threatening cardiac toxicity (arrythmia, QT interval prolongation, etc) and acute renal failure or central nervous system depression reported in preterm and term neonates receiving liquid LPV/r, we did not find any such abnormalities. Although these safety data are encouraging, care should be taken not to extrapolate these limited safety findings to settings of multidose 4-in-1 administration or other solid formulations of ABC, 3TC, and LPV/r in neonates.
The DSMB met in February 2021 and advised not to proceed to assessing multidosing of the 4-in-1 in neonates. The DSMB agreed with the PETITE team's proposal to amend the study to assess alternative solid ABC, 3TC, and LPV/r formulations in neonates. The PETITE study will now assess separate solid formulations of the ABC/3TC (120/60 mg) double-scored dispersible tablet and LPV/r (40/10 mg) granules (Viatris Ltd), which will allow more flexibility for dose frequency (eg, ABC/3 TC once daily) and dose adjustments if necessary.
In summary, we demonstrated that ABC and 3TC were well absorbed in neonates administered the 4-in-1; however, LPV/r exposures were too low to recommend its use. The low LPV exposures observed may have been due to underdosing of LPV/r, a drug–drug interaction between NVP and LPV and/or a unique property of the 4-in-1 formulation. This study highlights the importance of PK and safety studies of pediatric formulations in neonates where developmental and physiological changes differ for older infants, potentially affecting drug absorption and disposition. Integrase strand transfer inhibitor–based regimens with dolutegravir (DTG) are now preferred for HIV treatment in adults and children, but DTG is currently recommended only for infants aged 4 weeks or older and weighing >3.0 kg. While neonatal PK studies of both liquid and solid DTG formulations are planned, the use of other LPV/r solid formulations will remain relevant as an alternative to expand neonatal antiretroviral options.
ACKNOWLEDGMENTS
The authors thank all mothers and infants for participation in the study and the study team at the Family Centre for Research with Ubuntu for their assistance. The authors thank the Drugs and Neglected Disease Initiative (DNDi) and Cipla Ltd for drug support and DSMB members: Moherndran Archary, Linda Lewis, and Brookie Best.
Footnotes
Funding for this project was received from Unitaid. All authors and/ their institution received funding from Unitaid to perform this study (except M.K., E.C., and M.L.)
The authors have no conflicts of interest to disclose.
Presented in part at the International Workshop on HIV Pediatrics, July 16–17, 2021, and the International AIDS Society Conference on HIV Science, July 18–21, 2021.
Contributor Information
Helena Rabie, Email: hrabie@sun.ac.za.
Nicolas Salvadori, Email: nicolas.salvadori@phpt.org.
Samantha du Toit, Email: sdutoit2@sun.ac.za.
Kanchana Than-in-at, Email: kanchkanchana.than-in-at@phpt.org.
Marisa Groenewald, Email: groenewald@sun.ac.za.
Isabelle Andrieux-Meyer, Email: meyerisabelle20@bluewin.ch.
Mukesh Kumar, Email: Mukesh.Kumar7@Cipla.com.
Ratchada Cressey, Email: ratchada.cr@cmu.ac.th.
James Nielsen, Email: jamescordrynielsen@gmail.com.
Edmund Capparelli, Email: ecapparelli@ucsd.edu.
Marc Lallemant, Email: marclallemant@gmail.com.
Mark F. Cotton, Email: mcot@sun.ac.za.
Tim R. Cressey, Email: tim.cressey@phpt.org.
REFERENCES
- 1.Violari A, Cotton MF, Gibb DM, et al. Early antiretroviral therapy and mortality among HIV-infected infants. N Engl J Med. 2008;359:2233–2244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kaletra [package insert] (Product Ladeling). Abbott Park, IL: Abbott Laboratories; 2012. [Google Scholar]
- 3.Penazzato M, Townsend CL, Rakhmanina N, et al. Prioritising the most needed paediatric antiretroviral formulations: the PADO4 list. Lancet HIV. 2019;6:e623–e631. [DOI] [PubMed] [Google Scholar]
- 4.Musiime V, Fillekes Q, Kekitiinwa A, et al. The pharmacokinetics and acceptability of lopinavir/ritonavir minitab sprinkles, tablets, and syrups in african HIV-infected children. J Acquir Immune Defic Syndr. 2014;66:148–154. [DOI] [PubMed] [Google Scholar]
- 5.Mwanga J, Kekitiinwa A, Musiime V, et al. Safety, pharmacokinetics and acceptability of the abc/3tc/lpv/R granules (4-in-1) in children living with HIV (3–20 kg) in Uganda: LOLIPOP study. 2020. Presented at: International Workshop on HIV Pediatrics; November 16–17, 2020; Virtual. [Google Scholar]
- 6.Bouazza N, Foissac F, Fauchet F, et al. Lopinavir/ritonavir plus lamivudine and abacavir or zidovudine dose ratios for paediatric fixed-dose combinations. Antivir Ther. 2015;20:225–233. [DOI] [PubMed] [Google Scholar]
- 7.Moodley D, Pillay K, Naidoo K, et al. Pharmacokinetics of zidovudine and lamivudine in neonates following coadministration of oral doses every 12 hours. J Clin Pharmacol. 2001;41:732–741. [DOI] [PubMed] [Google Scholar]
- 8.Bekker A, Decloedt EH, Slade G, et al. Single dose abacavir pharmacokinetics and safety in neonates exposed to HIV. Clin Infect Dis. 2021;72:2032–2034. [DOI] [PubMed] [Google Scholar]
- 9.Jesson J, Dahourou DL, Renaud F, et al. Adverse events associated with abacavir use in HIV-infected children and adolescents: a systematic review and meta-analysis. Lancet HIV. 2016;3:e64–e75. [DOI] [PubMed] [Google Scholar]
- 10.Moodley J, Moodley D, Pillay K, et al. Pharmacokinetics and antiretroviral activity of lamivudine alone or when coadministered with zidovudine in human immunodeficiency virus type 1-infected pregnant women and their offspring. J Infect Dis. 1998;178:1327–1333. [DOI] [PubMed] [Google Scholar]
- 11.Chadwick EG, Capparelli EV, Yogev R, et al. Pharmacokinetics, safety and efficacy of lopinavir/ritonavir in infants less than 6 months of age: 24 week results. AIDS. 2008;22:249–255. [DOI] [PubMed] [Google Scholar]
- 12.South African HIV Clinicians Society (SAHCS). Antiretroviral Drug Dosing Chart for Children. Available at: https://sahivsoc.org/Files/PaedDosingChart_Final2019.pdf. Accessed August 24, 2021. [Google Scholar]
- 13.Difrancesco R, Rosenkranz SL, Taylor CR, et al. Clinical pharmacology quality assurance program: models for longitudinal analysis of antiretroviral proficiency testing for international laboratories. Ther Drug Monit. 2013;35:631–642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chadwick EG, Pinto J, Yogev R, et al. Early initiation of lopinavir/ritonavir in infants less than 6 weeks of age: pharmacokinetics and 24-week safety and efficacy. Pediatr Infect Dis J. 2009;28:215–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ibarra M, Fagiolino P, Vázquez M, et al. Impact of food administration on lopinavir-ritonavir bioequivalence studies. Eur J Pharm Sci. 2012;46:516–521. [DOI] [PubMed] [Google Scholar]
- 16.de Kanter CT, Colbers EP, Fillekes Q, et al. Pharmacokinetics of two generic co-formulations of lopinavir/ritonavir for HIV-infected children: a pilot study of paediatric Lopimune versus the branded product in healthy adult volunteers. J Antimicrob Chemother. 2010;65:538–542. [DOI] [PubMed] [Google Scholar]
- 17.FDA Drug Safety Communication: serious health problems seen in premature babies given Kaletra (lopinavir/ritonavir) oral solution. US FDA safety announcement. Available at: https://www.fda.gov/drugs/drug-safety-and-availability/fda-drug-safety-communication-serious-health-problems-seen-premature-babies-given-kaletra. Accessed September 1, 2021.
- 18.ZIAGEN (abacavir sulfate): [package insert], Full Prescribing Information: Revised: 11/2020. Available at: https://gskpro.com/content/dam/global/hcpportal/en_US/Prescribing_Information/Ziagen/pdf/ZIAGEN-PI-MG.PDF. Accessed August 24, 2021. [Google Scholar]
- 19.Paediatric European Network for Treatment of AIDS (PENTA). Pharmacokinetic study of once-daily versus twice-daily abacavir and lamivudine in HIV type-1-infected children aged 3-<36 months. Antivir Ther. 2010;15:297–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yuen GJ, Lou Y, Bumgarner NF, et al. Equivalent steady-state pharmacokinetics of lamivudine in plasma and lamivudine triphosphate within cells following administration of lamivudine at 300 milligrams once daily and 150 milligrams twice daily. Antimicrob Agents Chemother. 2004;48:176–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.EPIVIR (Lamivudine Tablet, Film Coated, Lamivudine Solution): [package insert]. ViiV Healthcare Company; 2017. Available at: https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/020564s37_020596s036lbl.pdf. Accessed September 1, 2021. [Google Scholar]
- 22.Tremoulet AH, Nikanjam M, Cressey TR, et al. Developmental pharmacokinetic changes of Lamivudine in infants and children. J Clin Pharmacol. 2012;52:1824–1832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chokephaibulkit K, Cressey TR, Capparelli E, et al. Pharmacokinetics and safety of a new paediatric fixed-dose combination of zidovudine/lamivudine/nevirapine in HIV-infected children. Antivir Ther. 2011;16:1287–1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kasirye P, Kendall L, Adkison KK, et al. Pharmacokinetics of antiretroviral drug varies with formulation in the target population of children with HIV-1. Clin Pharmacol Ther. 2011;91:272–280. [DOI] [PubMed] [Google Scholar]
- 25.Urien S, Firtion G, Anderson ST, et al. Lopinavir/ritonavir population pharmacokinetics in neonates and infants. Br J Clin Pharmacol. 2011;71:956–960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Holgate SL, Rabie H, Smith P, et al. Trough lopinavir concentrations in preterm HIV-infected infants. Pediatr Infect Dis J. 2012;31:602–604. [DOI] [PubMed] [Google Scholar]
- 27.Foissac F, Blume J, Treluyer JM, et al. Are prophylactic and therapeutic target concentrations different?: the case of lopinavir-ritonavir or lamivudine administered to infants for prevention of mother-to-child HIV-1 transmission during breastfeeding. Antimicrob Agents Chemother. 2017;61:e01869–e01916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Viramune (Nevirapine Tablets and Solution): [package insert]. Ridgefield, CT: Boehringer-Ingelheim Pharmaceuticals Inc; 2011. Available at: https://www.accessdata.fda.gov/drugsatfda_docs/label/2011/020636s039_020933s030lbl.pdf. Accessed September 1, 2021. [Google Scholar]
- 29.Saez-Llorens X, Violari A, Deetz CO, et al. Forty-eight-week evaluation of lopinavir/ritonavir, a new protease inhibitor, in human immunodeficiency virus-infected children. Pediatr Infect Dis J. 2003;22:216–224. [DOI] [PubMed] [Google Scholar]
- 30.de Wildt SN, Kearns GL, Leeder JS, et al. Cytochrome P450 3A: ontogeny and drug disposition. Clin Pharmacokinet. 1999;37:485–505. [DOI] [PubMed] [Google Scholar]
- 31.Patra N, Priya R, Swain S, et al. Pharmaceutical significance of Eudragit: a review. Future J Pharm Sci. 2017;3:33–45. [Google Scholar]
- 32.Qi S, Gryczke A, Belton P, et al. Characterisation of solid dispersions of paracetamol and EUDRAGIT E prepared by hot-melt extrusion using thermal, microthermal and spectroscopic analysis. Int J Pharm. 2008;354:158–167. [DOI] [PubMed] [Google Scholar]
- 33.Kearns GL, Abdel-Rahman SM, Alander SW, et al. Developmental pharmacology--drug disposition, action, and therapy in infants and children. N Engl J Med. 2003;349:1157–1167. [DOI] [PubMed] [Google Scholar]
- 34.Neal-Kluever A, Fisher J, Grylack L, et al. Physiology of the neonatal gastrointestinal system relevant to the disposition of orally administered medications. Drug Metab Dispos. 2019;47:296–313. [DOI] [PubMed] [Google Scholar]


