Abstract
Objective
The aim of this study was to evaluate the clinical impact of a 12-wk home-based digitally assisted rehabilitation program after arthroscopic rotator cuff repair against conventional home-based rehabilitation.
Design
The digital therapy group performed independent technology-assisted sessions complemented with 13 face-to-face sessions, and the conventional therapy group had conventional face-to-face physical therapy (30 sessions). Primary outcome was functional change between baseline and 12 wks, measured through the Constant-Murley score. Secondary outcomes were the change in the QuickDASH Scale and shoulder range of motion.
Results
Fifty participants enrolled; 41 completed the 12-wk program (23 digital therapy group vs. 18 conventional therapy group), and 32 (15 vs. 17) were available for the 12-mo follow-up assessment. No differences were found between groups regarding study endpoints at the end of the 12-wk program. However, follow-up results revealed the superiority of the digital therapy group for QuickDASH (P = 0.043), as well as an interaction between time and group in the Constant-Murley score (P = 0.047) in favor of the digital therapy group.
Conclusions
The results demonstrate that digital therapeutics can be used to achieve similar, if not superior, short- and long-term outcomes as conventional approaches after arthroscopic rotator cuff repair, while being far less human resource intensive than conventional care.
Level of evidence: II.
Key Words: Arthroscopic Rotator Cuff Repair, Shoulder Rehabilitation, Home-Based Rehabilitation, Digital Therapeutic
What Is Known
Rotator cuff tendinopathy is a major source of shoulder pain and disability. Surgical rotator cuff repair is many times required to tackle disability, and postsurgical rehabilitation is an essential step toward full recovery.
Evidence is growing that digitally assisted therapeutics can improve outcomes, personalize care, and decrease costs, but no such solution exists for postsurgical shoulder rehabilitation.
What Is New
This is the first study comparing a digitally assisted home-based rehabilitation program after arthroscopic rotator cuff repair with conventional care. Treatment intensity and long-term clinical outcomes were maximized, while decreasing the dependency on human resources.
Shoulder pain is the third main complaint in primary care settings.1–3 Approximately 65%–70% of these involve problems in the rotator cuff tendons (ROCTs),1,4 with incidence rising higher after the fourth decade of life.1,5–7 The ROCT dysfunction represents, therefore, a huge burden for healthcare systems, insurance companies, and employers alike.1,8,9 In the United Kingdom, nearly £310 million is spent on medical appointments in the first 6 mos of pain onset, with costs of surgical procedures estimated at £30 million per year.9 In the United States, the annual financial burden of ROCT management was estimated at US $3 billion.10
Most cases are initially treated conservatively.1 However, approximately 40% of patients will have persistent pain.11 Surgical repair may be an option in these cases. Rotator cuff repair (RCR) is one of the most commonly used surgical procedures1,4,12–21 and is becoming more frequent in advanced ages.13,22 In the United States alone, more than 300,000 RCRs are performed annualy.10
Rehabilitation plays a pivotal role in the recovery from RCR.18,20,21,23–29 The need for home-based digital solutions is now felt more acutely than ever, in the face of the current COVID-19 pandemic.30–32 Solutions enabling home-based rehabilitation without requiring real-time human supervision can be key to improving effectiveness and lowering costs, while keeping all stakeholders safe. Indeed, there are studies demonstrating the potential33–35 and cost-effectiveness36 of postoperative shoulder care and rehabilitation through telehealth.
However, although evidence is growing that digital therapeutics can improve outcomes, personalize care, and decrease costs,37 there is still much ground to be explored in this field after RCR.38,39 Several studies can be found on the validation/development of systems/algorithms for monitoring shoulder motion to assist clinicians on patient evaluation,40,41 but these do not meet the previously mentioned needs.
There have been some advances on new technologies for shoulder rehabilitation, namely, using wearable sensors39 and augmented reality.42 Although some of these are based on inertial motion trackers that can be used by the patient at home, under remote monitoring from the physical therapist (PT), they are either in very preliminary stages of development or validation.43–46
SWORD Health has developed a novel motion tracking–based digital biofeedback system for home-based physical rehabilitation—SWORD Phoenix—which is an Food and Drug Administration–listed class II medical device. Digital therapy (DT) programs using this device have demonstrated to be feasible, safe, and able to achieve comparable or, for rehabilitation after total hip and knee arthroplasty, superior outcomes than conventional physical therapy.47–49
The aim of this study was to evaluate the efficacy of a digitally assisted program for shoulder rehabilitation after arthroscopic RCR (ARCR), assessing its clinical outcomes in comparison with conventional home-based physical therapy. The hypothesis was that the digitally assisted program would be at least similar to conventional rehabilitation.
METHODS
Study Design
This was a single-center, prospective, nonblind, parallel-group, randomized controlled study.
Allocation
The study was restricted to patients living in a 20-km radius around the investigation center. Patient allocation to the two groups was performed using an online randomizer (https://www.randomizer.org), in permuted blocks of six, using a 1:1 ratio. Randomization was performed centrally by one investigator (MM) and communicated to those responsible for data acquisition only after patient enrollment. This investigator was not involved in data collection or in outcomes assessment.
Blinding
The nature of the study did not allow blinding of the patients regarding study groups. However, participants were blinded to the primary and secondary outcomes.
Participants
All consecutive patients admitted for ARCR between November 2018 and January 2020 were screened for eligibility at Hospital da Prelada, Porto, Portugal, by an orthopedic surgeon who oversaw the study (RS). Completion date for the 12-wk program was April 1, 2020. Completion date for the 12-mo follow-up was January 15, 2021.
Subjects were included if they were 18 yrs or older and younger than 70 yrs and had (a) shoulder pain and functional limitation with clinical examination compatible with rotator cuff tear; (b) imaging (magnetic resonance imaging or ultrasound) evidence of rotator cuff tear (supraspinatus and/or infraspinatus tendon tear inferior to 5 cm); (c) indication for RCR according to their orthopedic surgeon; and (d) ability to understand simple and complex motor commands.
Exclusion criteria were as follows: (a) indication for revision RCR; (b) complex cuff tears (involving more than one tendon besides supraspinatus and infraspinatus, or massive dimension tears, i.e., tears ≥5 cm); (c) glenohumeral arthritis; (d) irreparable tendon defect; (e) concomitant neurological disorders; (f) aphasia, dementia, or psychiatric comorbidity interfering with communication or compliance; (g) respiratory, cardiac, and metabolic conditions or others incompatible with at least 30 mins of light to moderate physical activity; and (h) blindness and/or illiteracy.
Subjects were also excluded postoperatively if they had the following: (a) irreparable tendon lesion; (b) major medical complications preventing discharge within 5 days; and (c) other medical and/or surgical complications that prevented them from complying with the program.
Intervention
Both groups received a 12-wk program, as outlined in Supplemental Digital Content 1 (http://links.lww.com/PHM/B291), consisting of five stages: (a) immediate postsurgery phase (weeks 0–2); (b) immobilization period (weeks 3–4); (c) passive mobilization (weeks 5–8); (d) active movement (weeks 9–10); and (e) strengthening (weeks 11–12). Face-to-face sessions schedule is depicted in Table 1.
TABLE 1.
Face-to-face session schedule according to the established protocol, not counting with the exceptions (i.e., patient who can proceed to the following phases earlier)
| Weeks | CT Group | DT Group | |
|---|---|---|---|
| Immediate postop phase | 1 | 0 | 0 |
| 2 | 0 | 0 | |
| Phase 1—immobilization, weeks 3–4 | 3 | 3 | 1 |
| 4 | 3 | 1 | |
| Total phase (accumulated) | 6 Sessions (6) | 2 Sessions (2) | |
| Phase 2—passive ROM, weeks 5–8 | 5 | 3 | 2 |
| 6 | 3 | 2 | |
| 7 | 3 | 2 | |
| 8 | 3 | 1 | |
| Total phase (accumulated) | 12 Sessions (18) | 7 Sessions (9) | |
| Phase 3—active ROM, weeks 9–10 | 9 | 3 | 1 |
| 10 | 3 | 1 | |
| Total phase (accumulated) | 6 Sessions (24) | 2 Sessions (11) | |
| Phase 4—strengthening, weeks 11–12 | 11 | 3 | 1 |
| 12 | 3 | 1 | |
| Total phase (accumulated) | 6 Sessions (30) | 2 Sessions (13) | |
In the absence of a criterion standard, the rehabilitation protocols were designed taking into account the following: (a) a recent systematic review on the subject4; (b) a review of the concepts and evidence-based guidelines on this subject by van der Meijden et al.21 (2012); (c) an overview of systematic reviews comparing early and conservative rehabilitation23; and (d) the protocol of the Massachusetts General Hospital for rehabilitation after RCR.
Participants in the DT group performed an exercise program using SWORD Phoenix. They were provided with a tablet computer with a SWORD mobile app installed, along with three inertial motion trackers to be placed on the chest, upper arm, and wrist, respectively (Fig. 1). These trackers enable precise movement quantification, feeding the mobile app, which guides the patient through the session, providing real-time audio and video biofeedback during exercise. Participants were instructed to perform digital exercise sessions at least 5 times per week. These sessions were set to last between 15 and 30 mins, depending on patient performance and program phase (see exercise protocol in Supplemental Digital Content 1, http://links.lww.com/PHM/B291). Patients had to complete at least 15 mins of session for the intervention to be counted. Sessions were evaluated on a daily basis and adjusted weekly by the assigned PT, through a web-based portal. These sessions were complemented with home-based one-to-one physical therapy sessions, for a total of thirteen 60-min sessions. Apart from the deployed session, where the PT assisted participants from the DT group setting up the digital therapist, either through phone or video call, no additional training was required to initiate the rehabilitation program.
FIGURE 1.
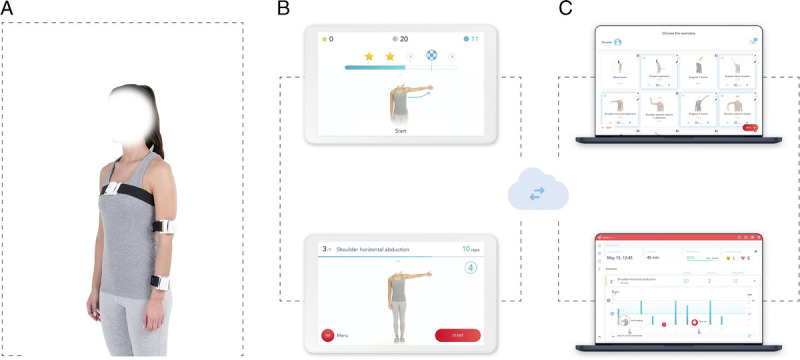
Digital therapy system components. A, Motion tracker setup. B, Mobile app: preparation screen (bottom image)—this screen is shown before each exercise and displays a video of the exercise, as well as audio instructions, and execution screen (top image)—this screen is shown during exercise execution, giving real-time audiovisual feedback on exercise performance. C, Web portal: results screen (bottom) and exercise prescription screen (top), for patient’s remote management by the PT.
The conventional therapy (CT) group received home-based rehabilitation provided by a PT, 3 times a week, for a total of thirty 60-min sessions. Patients were also instructed to perform additional unsupervised sessions in at least two other days of the week.
Outcomes
Subjects were assessed preoperatively, at weeks 6 and 12 after surgery, and then at 12 mos after surgery for all outcome measures.
The primary outcome in the present study was the change in patient functional assessment at the end of the 12-wk rehabilitation period as compared with baseline, measured through the Constant-Murley shoulder outcome score (CM score).
The secondary outcomes were the change at the end of the 12-wk program compared with baseline regarding: (a) patient-reported function, measured through the QuickDASH score and (b) shoulder active pain-free range of motion (ROM) in the following exercises: scapular elevation (SE), flexion (SF), abduction (SA), and external rotation (ER).
The biofeedback device was used in both groups to measure active shoulder ROM, as it has been certified for use as an angle-measurement tool, with a reported root mean square error of 5.5 degrees compared with standard goniometry, using the various tracker placement settings.
Safety and Adverse Events
Patients from both groups were able to report any adverse events to their PT on every in-person visit or by phone call.
For patients in the DT group, pain and fatigue scores (graduated from 0 to 10) were collected at the end of each session by the digital system. These were available for remote monitoring through the portal. In case of excessive pain or fatigue, patients were contacted by their PT to ascertain the cause and change prescription if needed.
All adverse events were registered in the patient’s files (beginning date, resolution date [if applicable], resolution state, severity).
Sample Size Estimation and Statistical Analysis
Sample size estimation took into account the study by Arndt et al.50 (2012), which compared two different rehabilitation protocols after ARCR, using CM score as primary outcome. Considering a minimal clinically important difference (MCID) of 10.4,51 a power of 90%, a two-sided 0.05 significance level, and a 15% dropout rate, 68 patients would be necessary to detect a 10.4-point difference between groups.
To assess differences in clinical and demographic variables between study groups, independent samples t test or Mann-Whitney U test was used for quantitative variables. For qualitative variables, χ2 test or Fisher exact test was used. Outcome analysis was performed using a per-protocol analysis. Differences between study groups were performed using independent samples t test or Mann-Whitney U test. A repeated-measures analysis was also performed, using a 4 × 2 analysis of variance with group as an independent factor and time as a within-subjects factor.
An interim analysis was imposed because of several restrictions to the normal conduct of the study caused by the COVID-19 pandemic.
Ethics
The present study and the use of patient data for research purposes were approved by the ethics committee of Hospital da Prelada, Porto, Portugal, authorization number 42-26/06/2018, in accordance with the Declaration of the World Medical Association, and were registered at ClinicalTrials.gov (NCT03648047). In addition, a written informed consent from all participants was obtained as required. This study conforms to all Consolidated Standards of Reporting Trial guidelines and reports the required information accordingly (see Supplemental Digital Content 2, http://links.lww.com/PHM/B292).
Data Availability
All data relevant to the study are included in the article or are available as Supplemental Digital Content (including raw data in Supplemental Digital Content 3, http://links.lww.com/PHM/B293). Only deidentified individual participant data are provided. Other documents, namely, the study protocol, are available at ClinicalTrials.gov (NCT03648047).
RESULTS
Overall, 188 patients were assessed for eligibility between November 2019 and January 2020. At this point, as a consequence of the COVID-19 pandemic, participant recruitment had to be indefinitely postponed, forcing an unplanned interim analysis of results. The Consolidated Standards of Reporting Trial diagram for the study is presented in Figure 2.
FIGURE 2.
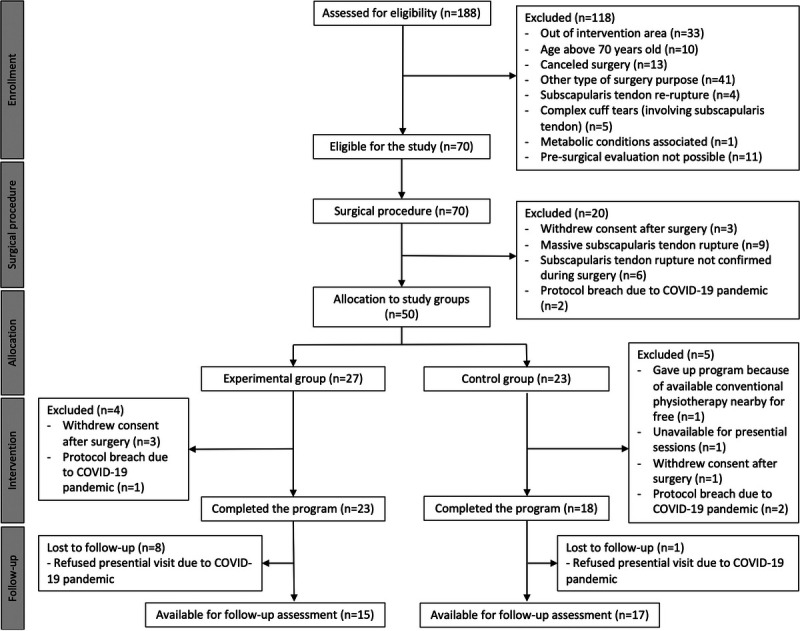
Study CONSORT diagram.
Baseline Sample Characterization
There were no differences at baseline between the two study groups regarding any population characteristics, as summarized in Table 2.
TABLE 2.
Baseline characteristics of study participants (N = 50)
| Variable | Total (N = 50) | DT Group (n = 27) | CT Group (n = 23) | P | |||
|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | ||
| Demographics | |||||||
| Sex | |||||||
| Female | 39 | 78 | 20 | 74 | 19 | 83 | 0.701a |
| Male | 11 | 22 | 7 | 26 | 4 | 17 | |
| Side of surgery | |||||||
| Left | 15 | 30 | 9 | 33 | 6 | 26 | 0.804a |
| Right | 35 | 70 | 18 | 67 | 17 | 74 | |
| Age, mean (SD) | 60.71 (6.9) | 61.30 (7.0) | 60.04 (6.8) | 0.353b | |||
| Comorbidities and known risk factors for adverse events | |||||||
| BMI, mean (SD) | 28.31 (4.9) | 28.77 (5.5) | 27.76 (4.1) | 0.463b | |||
| BMI > 40 | |||||||
| No | 49 | 98 | 26 | 96 | 23 | 100 | 1.00c |
| Yes | 1 | 2 | 1 | 4 | 0 | 0 | |
| Smoker | |||||||
| No | 46 | 92 | 25 | 93 | 21 | 91 | 1.00c |
| Yes | 4 | 8 | 2 | 7 | 2 | 9 | |
| Hypertension | |||||||
| No | 19 | 38 | 11 | 41 | 8 | 35 | 0.888a |
| Yes | 31 | 62 | 16 | 59 | 15 | 65 | |
| Diabetes | |||||||
| No | 37 | 74 | 20 | 74 | 17 | 74 | 1.00a |
| Yes | 13 | 26 | 7 | 26 | 6 | 26 | |
| Pulmonary disease | |||||||
| No | 47 | 94 | 24 | 89 | 23 | 100 | 0.240c |
| Yes | 3 | 6 | 3 | 11 | 0 | 0 | |
| Cardiac disease | |||||||
| No | 47 | 94 | 24 | 89 | 23 | 100 | 0.240c |
| Yes | 3 | 6 | 3 | 11 | 0 | 0 | |
| Renal disease | |||||||
| No | 48 | 96 | 26 | 96 | 22 | 96 | 1.00c |
| Yes | 2 | 4 | 1 | 4 | 1 | 4 | |
| Hematologic disease | |||||||
| No | 48 | 96 | 26 | 96 | 22 | 96 | 1.00c |
| Yes | 2 | 4 | 1 | 4 | 1 | 4 | |
| Steroid medication | |||||||
| No | 47 | 94 | 26 | 96 | 21 | 91 | 0.588c |
| Yes | 3 | 6 | 1 | 4 | 2 | 9 | |
| No. days from discharge to deploy, mean (SD) | 13.82 (4.5) | 13.41 (5.1) | 14.30 (3.6) | 0.945b | |||
| Postoperative clinical information | |||||||
| Involved tendon | |||||||
| Supraspinatus | 40 | 80 | 21 | 78 | 19 | 83 | 0.736c |
| Subscapularis | 10 | 20 | 6 | 22 | 4 | 17 | |
| Tear category | |||||||
| Partial tear | 7 | 14 | 4 | 15 | 3 | 13 | 1.00c |
| Full-thickness tear | 43 | 86 | 23 | 85 | 20 | 87 | |
| Tear size, mean (SD), cm | 1.99 (0.65) | 2.06 (0.60) | 1.90 (0.70) | 0.404b | |||
| Per protocol | n = 41 | n = 23 | n = 18 | ||||
| Adverse events during rehabilitation | |||||||
| No | 37 | 90 | 21 | 89 | 16 | 91 | 1.00c |
| Yes | 4 | 10 | 2 | 11 | 2 | 9 | |
| Extra visits | |||||||
| No | 34 | 83 | 16 | 70 | 18 | 100 | 0.012c |
| Yes | 7 | 17 | 7 | 30 | 0 | 0 | |
| Extra phone calls | |||||||
| No | 23 | 56 | 13 | 56 | 11 | 61 | 1.00a |
| Yes | 17 | 44 | 10 | 43 | 7 | 39 | |
a χ2 with continuity correction.
b Independent samples t test.
c Fisher exact test.
Treatment Intensity
Patients in the DT group completed on average 68 (SD = 6) sessions and median 13 in-person sessions (interquartile range [IQR] = 3, range = 9–17) with the PT. Patients in the CT group participated in median 27 in-person sessions (IQR = 3, range = 22–31).
Time spent on one-to-one sessions was different between groups (P < 0.001) with patients in the DT group spending, on average, less 11.6 hrs (95% confidence interval [CI] −13.6 to −9.5) with the PT (mean [SD] = 12.5 [2] vs. 24.1 [4] hrs).
Patients in the DT group completed median 33.5 hrs (IQR = 7 hrs, range = 22–57 hrs) of digitally assisted physical therapy at home.
The total mean (SD) treatment time for the DT group was 48.4 (8) hrs and for the CT group was 24.1 (4) hrs (mean difference between groups of 24.3 hrs, 95% CI = 20.4 to 28.2 hrs, P < 0.001).
Unscheduled Participant-PT Contacts
The number of unscheduled visits was different between groups (P = 0.012), with seven patients in the DT group requiring technical assistance—five due to issues with Internet connection and two requiring strap replacements (median 0 extra visits; IQR = 5, range = 0–5) and no occurrences in the CT group (Table 2).
In line with best clinical practices, patients from both groups were contacted by their assigned PT, either through text message or phone call, whenever they requested assistance or missed sessions (both groups) or reported intense pain or fatigue after a session (digital group). Given that the CT group had three face-to-face sessions a week versus one in the DT group, patients in the CT group naturally required less phone contacts than their counterparts. No further contacts occurred between the 12-wk program and the 1-yr follow-up.
Adherence to the Intervention
All participants in the DT group performed more exercise sessions than those initially protocoled (5 weekly sessions), with the majority (78%, 18/23) engaging on sessions 7 d/wk and 22% (5/23) on 6 d/wk.
There was a 4% rate of missed face-to-face sessions in the DT group (13 of the total 299 sessions protocoled for the 23 patients), either because of unavailability or an adverse event, against 11% in the CT group (52 of the total 540 sessions protocoled for the 18 patients), either because of patients’ or PT’s (three instances) unavailability or personal reasons. Two cases in the DT group missed four sessions each because of COVID-19 pandemic restrictions.
Outcomes Assessment
Baseline
Tables 3 and 4 present the per-protocol analysis of the study primary and secondary clinical outcomes. There were no differences between groups regarding all outcome measures at baseline, except for the pain subscale of the CM score patient-reported outcome measure (P = 0.04).
TABLE 3.
Primary outcome analysis of the CM shoulder outcome score
| Time Point | DT Group | CT Group | Pa | Estimate Difference (95% CI) |
|---|---|---|---|---|
| Pain (0–15 points) | ||||
| Baseline | 0 (5) | 5 (5) | 0.04 | 0 (0 to 5) |
| 6 wks | 10 (5) | 5 (5) | 0.41 | 0 (−5 to 0) |
| 12 wks | 10 (10) | 10 (10) | 0.91 | 0 (−5 to 5) |
| 12-mo follow-up | 10 (10) | 10 (10) | 0.33 | 0 (0 to 5) |
| Change baseline–6 wks | 5 (5) | 5 (5) | 0.03 | 0 (−5 to 0) |
| Change baseline–12 wks | 5 (10) | 7.5 (5) | 0.36 | 0 (−5 to 0) |
| Change baseline–12 mos | 10 (10) | 5 (10) | 0.08 | 5 (0 to 10) |
| Activities of daily living (0–20 points) | ||||
| Baseline | 8 (4) | 8 (2) | 0.51 | 0 (0 to 2) |
| 6 wks | 10 (2) | 10 (5) | 0.62 | 0 (−2 to 2) |
| 12 wks | 18 (10) | 20 (9) | 0.66 | 0 (0 to 2) |
| 12-mo follow-up | 20 (8) | 18 (9) | 0.43 | 0 (0 to 4) |
| Change baseline–6 wks | 0 (4) | 2 (4) | 0.89 | 0 (−2 to 2) |
| Change baseline–12 wks | 10 (12) | 10 (10) | 0.74 | 0 (−4 to 2) |
| Change baseline–12 mos | 12 (12) | 8 (7) | 0.35 | 2 (−2 to 6) |
| ROM (0–40 points) | ||||
| Baseline | 24 (8) | 29 (11) | 0.06 | 4 (0 to 8) |
| 6 wks | 30 (10) | 32 (6) | 0.16 | 2 (−2 to 6) |
| 12 wks | 36 (4) | 37 (4) | 0.50 | 0 (−2 to 2) |
| 12-mo follow-up | 38 (4) | 38 (7) | 0.94 | 0 (−2 to 2) |
| Change baseline–6 wks | 6 (16) | 4 (10) | 0.27 | -4 (−8 to 2) |
| Change baseline–12 wks | 12 (12) | 6 (8) | 0.17 | -4 (−8 to 2) |
| Change baseline–12 mos | 14 (14) | 10 (8) | 0.12 | 4 (−2 to 8) |
| Strength (0–25 points) | ||||
| Baseline | 0 (2.2) | 2.2 (2.5) | 0.08 | 0 (0 to 2.2) |
| 6 wks | 2.2 (2.2) | 2.2 (3.6) | 0.39 | 0 (0 to 2.2) |
| 12 wks | 4.4 (2.2) | 5.5 (2.7) | 0.39 | 0 (0 to 2.2) |
| 12-mo follow-up | 2.2 (2.2) | 2.2 (2.2) | 0.85 | 0 (−2 to 2.2) |
| Change baseline–6 wks | 0 (2.2) | 1.65 (4.4) | 0.63 | 0 (−1.1 to 2.2) |
| Change baseline–12 wks | 4.4 (4.4) | 4.4 (4.9) | 0.81 | 0 (−2.2 to 2.2) |
| Change baseline–12 mos | 2.2 (4.4) | 0 (2.2) | 0.28 | 1.11 (0 to 2.2) |
| CM score (0–100 points), mean (SD) | ||||
| Baseline | 33.81 (11.61) | 40.80 (12.92) | 0.08b | −6.99 (−14.89 to 0.91) |
| 6 wks | 46.03 (14.17) | 49.59 (11.88) | 0.39b | −3.55 (−11.79 to 4.68) |
| 12 wks | 65.51 (14.83) | 67.72 (11.57) | 0.60b | −2.2 (−10.81 to 6.38) |
| 12-mo follow-up | 67.63 (11.51) | 63.15 (14.92) | 0.35b | 4.47 (−5.25 to 14.19) |
| Change baseline–6 wks | 12.23 (16.55) | 8.79 (8.82) | 0.40b | 3.43 (−11.79 to 4.68) |
| Change baseline–12 wks | 31.70 (18.86) | 26.93 (13.92) | 0.36b | 5.13 (−4.99 to 15.25) |
| Change baseline–12 mos | 33.32 (18.03) | 23.95 (9.73) | 0.07b | 9.37 (−0.91 to 19.66) |
| Change 12 wks–12 mos | 4.76 (14.34) | −8.31 (22.57) | 0.07b | 12.98 (−0.9 to 26.87) |
Entries in boldface indicate that there was a statistically significant difference between the groups. Significance was set at P < 0.05.
Data are presented as median (IQR), unless otherwise stated. Per-protocol analysis (n = 41 during the 12-wk rehabilitation program; n = 32 at the 12-mo follow-up).
a Nonparametric independent samples Mann-Whitney U test and Hodges-Lehman median difference.
b Independent samples t test.
TABLE 4.
Secondary outcome analysis of the QuickDASH and shoulder ROM
| Time Point | DT Group | CT Group | Pa | Estimate Difference (95% CI) |
|---|---|---|---|---|
| QuickDASH, mean (SD) | ||||
| Baseline | 65.22 (14.41) | 55.41 (16.90) | 0.06 | 9.80 (−0.34 to 19.95) |
| 6 wks | 39.70 (13.73) | 37.47 (16.34) | 0.64 | 2.23 (−7.53 to 11.99) |
| 12 wks | 19.95 (19.47) | 17.79 (15.34) | 0.69 | 2.16 (−8.83 to 13.16) |
| 12-mo follow-up | 20.60 (19.17) | 28.75 (23.55) | 0.30 | −8.14 (−23.78 to 7.50) |
| Change baseline–6 wks | −25.51 (16.19) | −17.94 (11.23) | 0.09 | −7.57 (−16.25 to 1.11) |
| Change baseline–12 wks | −45.27 (24.55) | −37.62 (18.91) | 0.27 | −7.64 (−21.37 to 6.08) |
| Change baseline–12 mos | −45.61 (24.66) | −28.86 (20.04) | 0.04 | −16.7 (−32.90 to −0.60) |
| Change 12 wks–12 mos | −4.84 (17.0) | 9.91 (21.35) | 0.04 | −14.74 (−28.81 to −0.68) |
| Shoulder ROM, mean (SD) | ||||
| SE | ||||
| Baseline | 114.56 (26.38) | 123.28 (35.22) | 0.39 | −8.71 (−29.03; 11.61) |
| 6 wks | 141.65 (26.48) | 145.28 (23.40) | 0.64 | −3.63 (−19.42 to 12.17) |
| 12 wks | 164.48 (18.21) | 166.50 (13.11) | 0.68 | −2.02 (−11.93 to 7.88) |
| 12-mo follow-up | 156.47 (17.24) | 152.00 (25.25) | 0.34 | 7.47 (−8.36 to 23.30) |
| Change baseline–6 wks | 27.09 (37.13) | 22.00 (25.65) | 0.61 | 5.09 (−14.79 to 24.96) |
| Change baseline–12 wks | 49.91 (29.19) | 43.22 (37.05) | 0.53 | 6.69 (−15.00 to 28.38) |
| Change baseline–12 mos | 41.13 (31.36) | 32.88 (27.28) | 0.43 | 8.25 (−12.91 to 29.42) |
| Change 12 wks−12 mos | −1.8 (18.25) | −14 (26.55) | 0.15 | 12.2 (−4.48 to 28.88) |
| F | ||||
| Baseline | 122.91 (28.92) | 132.11 (31.30) | 0.34 | −9.20 (−28.54 to 10.14) |
| 6 wks | 144.48 (28.06) | 150.00 (21.25) | 0.48 | −5.52 (−21.10 to 10.05) |
| 12 wks | 164.57 (18.30) | 167.67 (13.24) | 0.53 | −3.10 (−13.07 to 6.87) |
| 12-mo follow-up | 157.40 (17.11) | 148.06 (24.38) | 0.22 | 9.34 (−6.07 to 24.75) |
| Change baseline–6 wks | 21.56 (35.76) | 17.89 (24.48) | 0.70 | 3.68 (−15.40 to 22.76) |
| Change baseline–12 wks | 41.65 (31.40) | 35.55 (29.72) | 0.53 | 6.10 (−13.32 to 25.52) |
| Change baseline–12 mos | 34.07 (34.25) | 18.00 (21.49) | 0.12 | 16.07 (−5.12 to 37.25) |
| Change 12 wks–12 mos | −4 (19.25) | −18.76 (24.18) | 0.07 | 14.76 (−1.16 to 30.69) |
| A | ||||
| Baseline | 110.52 (39.26) | 134.61 (37.23) | 0.05 | 24.09 (−48.40 to 0.22) |
| 6 wks | 146.35 (39.60) | 156.72 (33.76) | 0.37 | −10.37 (−33.57 to 12.82) |
| 12 wks | 176.61 (20.03) | 175.00 (12.95) | 0.76 | 1.61 (−8.86 to 12.08) |
| 12-mo follow-up | 177.00 (22.00) | 175.00 (24.50) | 0.41 | 4 (−8 to 18) |
| Change baseline–6 wks | 35.83 (56.65) | 22.11 (28.46) | 0.32 | 13.71 (−13.89 to 41.32) |
| Change baseline–12 wks | 66.09 (38.11) | 40.39 (35.93) | 0.03 | 25.70 (2.18 to 49.22) |
| Change baseline–12 mos | 56.53 (34.93) | 35.88 (34.41) | 0.10 | 20.65 (4.42 to 45.72) |
| Change 12 wks–12 mos | 0.4 (23.14) | −6.76 (28.49) | 0.44 | 7.16 (−11.74 to 26.07) |
| ER | ||||
| Baseline | 47.96 (13.11) | 48.72 (17.75) | 0.88 | −0.77 (−9.44 to 10.97) |
| 6 wks | 53.17 (13.22) | 50.67 (14.13) | 0.57 | 2.51 (−6.26 to 11.28) |
| 12 wks | 59.83 (16.02) | 57.67 (11.97) | 0.62 | 2.16 (−6.68 to 11.00) |
| 12-mo follow-up | 62.92 (14.90) | 61.65 (21.38) | 0.85 | 1.29 (−12.2 to 14.77) |
| Change baseline–6 wks | 5.22 (13.12) | 1.94 (12.16) | 0.41 | 3.27 (−4.75 to 11.29) |
| Change baseline–12 wks | 11.87 (14.55) | 8.94 (15.69) | 0.54 | 2.92 (−6.78 to 12.63) |
| Change baseline–12 mos | 15.87 (11.21) | 14.94 (17.34) | 0.86 | 0.92 (−9.78 to 11.63) |
| Change 12 wks–12 mos | 3 (15.25) | 4.18 (21.0) | 0.86 | −1.18 (−14.34 to 11.99) |
Entries in boldface indicate that there was a statistically significant difference between the groups. Significance was set at P < 0.05.
Results presented as mean (SD). Per-protocol analysis (n = 41 during the 12-wk rehabilitation program; n = 32 at the 12-mo follow-up).
a Independent samples t test.
Six-Week Assessment
At this time point, no statistically significant differences were found between groups for all outcome measures.
Change Between Baseline and the 6-Wk Assessment
No statistically significant differences were detected between groups in terms of the CM score (P = 0.4, mean difference = 3.43, 95% CI = −11.79 to 4.68 points). However, the change from baseline to week 6 attained by the DT group surpassed the MCID reference value of 10.4 points (3-mo mean change after rotator cuff surgery51), revealing an early clinically meaningful improvement (mean [SD] = 12.23 [16.55] vs. 8.79 [8.82]).
Regarding QuickDASH scores, considering the reported lower and upper boundary MCIDs (i.e., 15.9 and 20 points, respectively),52 both groups surpassed the lower boundary value already at week 6 of rehabilitation, with a nonstatistically significant (P = 0.086) mean difference between groups (95% CI = −16.25 to 1.11 points). However, only the DT group surpassed the upper boundary (more rigorous) value as well (mean [SD] = −25.51 [16.19] vs. −17.94 [11.23] points).
Twelve-Week Assessment
No statistically significant differences were found between groups for all outcome measures.
Change Between Baseline and the 12-Wk Assessment
Both groups attained clinically meaningful outcomes regarding the primary endpoint (according to MCID reference value) with the DT group presenting a mean (SD) CM score change from baseline of 31.70 (18.6) points against 26.93 (13.9) points from the CT group. This difference was not statistically significant (P = 0.36; 5.13 points, 95% CI = −4.99 to 15.25 points).
As for the QuickDASH, patient’s improvement from baseline was similar in both groups (P = 0.267), with a mean difference of −7.64 points (95% CI = −21.37 to 6.08 points).
There were also no differences in mean changes between groups in shoulder ROM, except for shoulder SA, favoring the DT group (P = 0.03; 25.7 points, 95% CI = 2.18 to 49.22 points).
Follow-up Assessment
No statistically significant differences were found between groups for all outcome measures among those who were available for the 12-mo follow-up assessment.
Change Between Baseline and Follow-up Assessment
Both groups presented clinically meaningful improvements regarding the primary endpoint 12 mos after the end of the rehabilitation program, with the DT group presenting a mean (SD) CM score change from baseline of 33.32 (18.03) points against 23.95 (9.73) points from the CT group. Although the mean difference was not statistically significant, the scores favor the DT intervention (P = 0.07; 9.37 points, 95% CI = −0.91 to 19.66 points).
In face of the reference values for the CM score recently retrieved from a study by Cvetanovich et al.53 (n = 288 RCR patients, 12 mos postoperatively), namely, the MCID (4.6 points), the substantial clinical benefit (SCB; 5.5 points), and the patient acceptable symptomatic state (23.3 points), most patients achieved the three indicators. Interestingly, a higher proportion of patients in the DT group was found to achieve both the MCID (100% [15/15] vs. 94.1% [16/17], P = 1.0, exact Fischer test) and the patient acceptable symptomatic state (66.7% [10/15] vs. 58.8% [10/17]. P = 1.0, exact Fischer test). A similar proportion of patients achieved the SCB threshold (93.3% [14/15] vs. 94.1% [16/17], P = 0.927, χ2 with continuity correction). Differences between groups in this analysis were, however, not statistically significant.
As for the QuickDASH, patient’s improvement from baseline in the DT group was higher than that attained by the CT group (P = 0.043), with a mean difference of −16.7 (95% CI = −32.90 to −0.60).
Regarding the different shoulder ROM measures, there were no differences in mean changes between groups at this time point.
Change Between End of Program and Follow-up Assessment
One year after end of program, patients in the DT group showed further improvement in CM and QuickDASH scores, as well as stable evolution of shoulder ROM values, whereas the CT group experienced a slight regression on all outcomes measured, except for shoulder ER (Tables 3, 4). Differences between groups were statistically significant regarding QuickDASH (P = 0.04) and clinically meaningful for the CM score (12.98 points, 95% CI = −0.9 to 26.87 points).
Repeated-Measures Analysis
A repeated-measures analysis of variance was performed for CM and QuickDASH scores and for all ROM measures after transformation of shoulder SA (which was not normally distributed), with randomization group as an independent factor and time as a within-subject factor. Results are presented in Table 5 and Figure 3 (estimated marginal means over time, divided by group). This analysis confirmed a main effect of time on patient’s recovery (P < 0.001) and an interaction between time and group regarding the primary endpoint (P = 0.047).
TABLE 5.
Repeated-measures analysis of variance for the normally distributed variables CM shoulder outcome score, QuickDASH, shoulder SE, F, and ER (n = 32, 15 DT group vs. 17 CT group), and for the transformed variable shoulder A (n = 31, 14 vs. 17)
| Variable | Time | Group | Time*Group | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| F | df 1 | df 2 | P | F | df 1 | df 2 | P | F | df 1 | df 2 | P | |
| CM scorea | 43.58 | 2.38 | 71.29 | <0.001 | 0.27 | 1 | 30 | 0.609 | 3.02 | 2.38 | 71.29 | 0.047 |
| QuickDASH | 58.76 | 2.25 | 67.46 | <0.001 | 0.49 | 1 | 30 | 0.488 | 2.55 | 2.25 | 67.46 | 0.079 |
| Shoulder ROM | ||||||||||||
| SEa | 32.45 | 2.39 | 71.72 | <0.001 | 0.06 | 1 | 30 | 0.809 | 0.90 | 2.39 | 71.72 | 0.426 |
| Fa | 22.24 | 1.99 | 59.60 | <0.001 | 0.17 | 1 | 30 | 0.681 | 1.46 | 1.99 | 59.60 | 0.241 |
| Aa,b | 27.55 | 2.52 | 73.21 | <0.001 | 0.88 | 1 | 29 | 0.355 | 2.03 | 2.52 | 73.21 | 0.126 |
| ERa | 14.43 | 2.41 | 72.42 | <0.001 | 0.14 | 1 | 30 | 0.712 | 0.11 | 2.41 | 72.42 | 0.923 |
a Greenhouse-Geisser correction.
b Rank-based inverse normal transformation.
FIGURE 3.
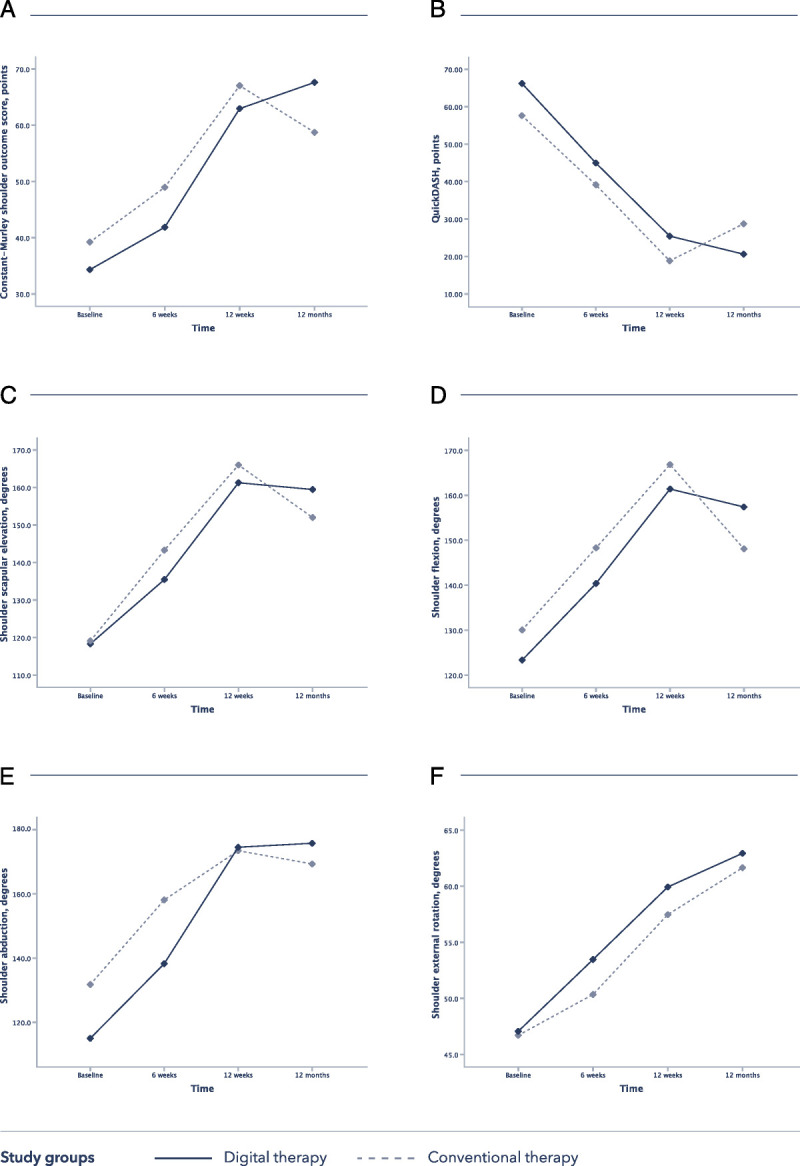
Evolution of the clinical outcomes over time in both groups, based on the repeated-measures analysis (estimated marginal means of transformed variables are presented). A, Constant-Murley shoulder outcome score. B, QuickDASH. C, Shoulder SE. D, Shoulder F. E, Shoulder A. F, Shoulder ER.
Safety and Adverse Events
No differences were found between groups in terms of safety and adverse events (P = 1.00), and none of the occurrences were directly related to the intervention. Adverse event classification and description is available in Supplemental Digital Content 4 (http://links.lww.com/PHM/B294).
Interim Analysis Conclusions
No evident differences were detected in favor of any of the groups at the end of the 12-wk program. We could, however, conclude on the noninferiority of the DT intervention with regard to the primary outcome measure—change in CM score between baseline and 12 wks—given that the lower boundary of the 95% CI (−4.99 to 15.25) for this change is less than the 10.4 MCID for the CM score. Furthermore, given that this CI is not only within the (−MCID to MCID) interval, but that the higher boundary is above the MCID, equivalence between the two interventions can also be inferred at a minimum.
Despite the 5.13 points in the CM score in favor of the DT group, the P value was high (P = 0.36). Moreover, this difference was significantly lower than the MCID, and no effect was detected as per the repeated-measures analysis. Taken together, this seemed to indicate that superiority would not be demonstrated with bigger sample sizes. Hence, the decision to discontinue the study, motivated by COVID-19 pandemic–related barriers—with suspension of all nonessential clinical activities at the investigation center, still in effect on this article’s submission date—was further supported by this interim analysis. Despite this decision, all program completers were contacted 12 mos after surgery to schedule a follow-up reassessment.
DISCUSSION
As far as we are aware, no clinical validation exists on a fully integrated DT program for home-based shoulder rehabilitation after ARCR similar to the one we present herein. Thus, comparing results from the present study with similar studies is not possible. Instead, other studies published on conventional rehabilitation after ARCR or on telerehabilitation versus conventional rehabilitation were considered. Overall, clinical outcomes achieved were superior to those reported in the literature, either from conventional rehabilitation or from telerehabilitation programs.
Constant-Murley Shoulder Outcome Score
In both groups, the mean (SD) CM score value (70.6 [21.3] vs. 70.5 [16] points) at the 12-wk assessment was superior to that reported by Kukkonen et al.51 in a prospective cohort study of 802 consecutive shoulders with arthroscopically treated partial- or full-thickness rotator cuff tears (61.7 [16.4] points postoperatively at 12 wks) and equivalent to that previously reported for healthy shoulders (61–70 yrs old, female: 70 [4.0] points; male: 83 [4.2] points) in the same age group.54 This suggests that both rehabilitation programs (P = 0.36) provided full functional recovery. Interestingly, however, only patients in the DT group reached meaningful clinical improvement at week 6 (mean [SD] = 12.23 [16.55] vs. 8.79 [8.82]). We hypothesize that this could be a result of the higher therapeutic exercise dosage early after surgery.
Improvements in both groups were globally comparable with those reported for other telerehabilitation approaches, namely, those of a randomized controlled trial by Pastora-Bernal et al.34 (n = 18) comparing conventional with telerehabilitation. In this study, CM score changes from baseline to 12 wks were approximately 25 points, whereas in our study, changes were approximately 30 points (31.70 [18.86] vs. 26.93 [13.92] points).
Finally, comparing our results with those reported by Cvetanovich et al.,53 we found that higher proportions of patients in our study achieved clinically significant CM scores 1 yr postoperatively (MCID: 96.9% in our study vs. 75.8%; SCB: 93.8% vs. 75.8%; patient acceptable symptomatic state: 62.5% vs. 60.5%).
Disabilities of the Arm, Shoulder, and Hand (QuickDASH)
The results obtained in this study were far superior to the ones from Macdermid et al.55 (n = 132, mean age = 63 yrs). In our study, the changes from baseline to 12 wks were −45.27 (24.55) points in the digital group and −37.62 (18.91) points in the conventional group, in comparison with −9 points in the study by Macdermid et al.55 The total scores at 12 wks were also far superior in our study (19.95 [19.47] in the digital group and 17.79 [15.34] in the conventional group; Macdermid et al.’s55: 42.9 [22.4]).
In another study (n = 33 in 93, 35.38%, mean age = 48 yrs),56 patients reported a QuickDASH score of approximately 45 points at 1-mo postsurgery, far from their preoperative values (30.5 [17.2] points) and from our scores at week 6 (39.70 [13.73] vs. 37.47 [16.34]).
Range of Motion
A retrospective study by Kurowicki et al.57 (n = 627) on recovery after ARCR reported an improvement in SE and SA of 22% (137.9 [30.3] degrees) and 45% (90.9 [17.9] degrees), respectively, at 3 mos, and an aggravation in ER of −18% (44.2 [17.1] degrees). In our study, at 12 wks, patients in both the DT and CT groups achieved higher ROMs, especially in SA (SE: 164.48 [18.21] vs. 166.50 [13.11]; SA: 176.61 [20.03] vs. 175.00 [12.95]; ER: 59.83 [16.02] vs. 57.67 [11.97], respectively). In this study, the immobilization period was of 6 wks, and no strengthening exercises were prescribed for the first 3 mos. These results seem, therefore, to support earlier mobilization and earlier introduction of strengthening exercises.
Interestingly, our results were similar to the ones reported by Desrosiers et al.58 (n = 360), Walker et al.59 (n = 60), and McIntosh et al.60 (n = 41) for healthy subjects aged 50–69 yrs. In addition, in a recent systematic review (n = 36 studies) investigating the minimum ROM needed for activities of daily living, the authors established a benchmark of 130 degrees for SE and SA, which was far ensured for participants in this study.
Long-Term Outcomes
Although there was an overall convergence of clinical outcomes at the end of the 12-wk program, intervention groups evolved in opposite directions during subsequent 12 mos, the DT group attaining better functional outcomes. We hypothesize that this could be related to a greater empowerment of patients in the DT group, possibly leading them to maintain a treatment routine even after program completion, as has been observed in other cohorts of patients undergoing similar programs but for other musculoskeletal conditions (in publication route). However, this hypothesis was not formally tested and needs to be addressed in further studies.
Limitations and Strengths
The main limitation of this study is related to sample size, with the COVID-19 pandemic forcing the study to a halt. As mentioned, no differences were found between groups, and the probability of detecting a meaningful difference between them was very low. As such, the study was discontinued at 68% inclusion rate. Although we are aware that early discontinuation with low sample sizes yields low-powered studies, the tendency found was toward superiority of the digital group. Therefore, crucially, the decision to stop the study did not bias results toward the digital group.
The number of sessions as defined by protocol was not systematically respected in both groups. However, this reflects a real-world scenario where personal reasons, outside the influence of the PTs, often interfere with the course of treatment. Notwithstanding, the results obtained in the two groups demonstrate that clinical outcomes were not hindered because of this. Also reflective of real-world standards of care, time spent exercising at home by the CT group was not monitored, not allowing an accurate estimation of the difference in treatment intensity between groups. Still, factoring in time spent in these unsupervised sessions would reduce the difference in treatment intensity between groups and reinforce the argument in favor of long-lasting behavior change in the DT group as a driver of better long-term outcomes.
It is also important to highlight that most subjects in this study were female patients (78%). This could have affected compliance within the DT group, because findings indicated that male patients hold more favorable attitudes toward technology use than female patients.3 However, no such evidence could be found regarding the use of technology in rehabilitation. Eriksson et al.35 reported on a telerehabilitation program after shoulder joint replacement, showing an equivalent sex distribution (80% female) to ours, but no considerations were made regarding potential sex differences.
Finally, eligibility criteria were the main reason (86% of the total screening failures) behind the low inclusion rate in the present study (26%) versus consent withdrawal or participation refusal due to technology-drawn skepticism.47 This may indicate growing acceptance of new technologies even in an older population. Indeed, we found no differences in postrandomization dropout rates between groups. Notwithstanding, 30% (7/23) of patients in the DT group needed extra PT visits for technical assistance, reflecting that there is still room for technological refinement.
CONCLUSIONS
This is the first study comparing a digitally assisted home-based rehabilitation program after ARCR with conventional care. The results demonstrate that digital therapeutics can be used to maximize treatment intensity and long-term outcomes, while decreasing the dependency on human resources, without compromising clinical outcomes. These findings highlight the potential of digital therapeutics in this field and warrant larger multicentric randomized controlled studies to further validate this novel digital therapeutic.
Supplementary Material
ACKNOWLEDGMENTS
The authors thank Hospital da Prelada for partnering with SWORD Health in this research. The authors also thank all medical and nursing staff who were involved in patient recruitment, scheduling, and monitoring. The authors thank Jorge Malafaia for the work with figures and graphics setup and edition.
Footnotes
The study sponsor, SWORD Health, was involved in study design, data collection, and interpretation and writing of the manuscript.
MM, SL, DC, CC, and PC are employees at SWORD Health. FDC is the Chief Medical Officer of SWORD Health and owns stock in the company. JL and GF received a scientific councilor honorarium from SWORD Health. VB is the Chief Executive Officer of SWORD Health and owns stock in the company. RS declares that he has no conflict of interest.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s Web site (www.ajpmr.com).
Contributor Information
Fernando Dias Correia, Email: fanacorreia@gmail.com.
Maria Molinos, Email: mmolinos@swordhealth.com.
Diana Carvalho, Email: dcarvalho@swordhealth.com.
Carlos Carvalho, Email: ccarvalho@swordhealth.com.
Pedro Costa, Email: p.costa@swordhealth.com.
Rosmaninho Seabra, Email: a.rosmaninhoseabra@gmail.com.
Gerard Francisco, Email: Gerard.E.Francisco@uth.tmc.edu.
Virgílio Bento, Email: v@swordhealth.com.
Jorge Lains, Email: jorgelains@roviscopais.min-saude.pt.
REFERENCES
- 1.Edwards P Ebert J Joss B, et al. : Exercise rehabilitation in the non-operative management of rotator cuff tears: a review of the literature. Int J Sports Phys Ther 2016;11:279–301 [PMC free article] [PubMed] [Google Scholar]
- 2.Mitchell C Adebajo A Hay E, et al. : Shoulder pain: diagnosis and management in primary care. BMJ 2005;8(November):331–1124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lewis JS: Rotator cuff tendinopathy/subacromial impingement syndrome: is it time for a new method of assessment? Br J Sports Med 2009;43:259–64 [DOI] [PubMed] [Google Scholar]
- 4.Thomson S, Jukes C, Lewis J: Rehabilitation following surgical repair of the rotator cuff: a systematic review. Physiotherapy 2016;102:20–8 [DOI] [PubMed] [Google Scholar]
- 5.Hawkins RJ, Morin WD, Bonutti PM: Surgical treatment of full-thickness rotator cuff tears in patients 40 years of age or younger. J Shoulder Elbow Surg 1999;8:259–65 [DOI] [PubMed] [Google Scholar]
- 6.Mall NA Lee AS Chahal J, et al. : An evidenced-based examination of the epidemiology and outcomes of traumatic rotator cuff tears. Arthroscopy 2013;29:366–76 [DOI] [PubMed] [Google Scholar]
- 7.Tempelhof S, Rupp S, Seil R: Age-related prevalence of rotator cuff tears in asymptomatic shoulders. J Shoulder Elbow Surg 1999;8:296–9 [DOI] [PubMed] [Google Scholar]
- 8.Mather RC III Koenig L Acevedo D, et al. : The societal and economic value of rotator cuff repair. J Bone Jt Surg 2013;95:1993–2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Littlewood C, May S: A contractile dysfunction of the shoulder. Man Ther 2007;12:80–3 [DOI] [PubMed] [Google Scholar]
- 10.Aurora A McCarron J Iannotti JP, et al. : Commercially available extracellular matrix materials for rotator cuff repairs: state of the art and future trends. J Shoulder Elbow Surg 2007;16:S171–8 [DOI] [PubMed] [Google Scholar]
- 11.van der windt DA Koes BW Boeke AJ, et al. : Shoulder disorders in general practice: prognostic indicators of outcome. Br J Gen Pract 1996;46:519–23 [PMC free article] [PubMed] [Google Scholar]
- 12.Dickinson RN Kuhn JE Bergner JL, et al. : A systematic review of cost-effective treatment of postoperative rotator cuff repairs. J Shoulder Elbow Surg 2017;26:915–22 [DOI] [PubMed] [Google Scholar]
- 13.Gallagher BP Bishop ME Tjoumakaris FP, et al. : Early versus delayed rehabilitation following arthroscopic rotator cuff repair: a systematic review. Phys Sportsmed 2015;43:178–87 [DOI] [PubMed] [Google Scholar]
- 14.Lee BG, Cho NS, Rhee YG: Effect of two rehabilitation protocols on range of motion and healing rates after arthroscopic rotator cuff repair: aggressive versus limited early passive exercises. Arthroscopy 2012;28:34–42 [DOI] [PubMed] [Google Scholar]
- 15.Gladstone JN Bishop JY Lo IKY, et al. : Fatty infiltration and atrophy of the rotator cuff do not improve after rotator cuff repair and correlate with poor functional outcome. Am J Sports Med 2007;35:719–28 [DOI] [PubMed] [Google Scholar]
- 16.Kluger R Bock P Mittlböck M, et al. : Long-term survivorship of rotator cuff repairs using ultrasound and magnetic resonance imaging analysis. Am J Sports Med 2011;39:2071–81 [DOI] [PubMed] [Google Scholar]
- 17.Baumgarten KM, Vidal AF, Wright RW: Rotator cuff repair rehabilitation: a level I and II systematic review. Sports Health 2009;1:125–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Millett PJ Wilcox RB O’Holleran JD, et al. : Rehabilitation of the rotator approach. J Am Acad Orthop Surg 2006;14:599–609 [DOI] [PubMed] [Google Scholar]
- 19.Mall NA Tanaka MJ Choi LS, et al. : Factors affecting rotator cuff healing. J Bone Jt Surg Am 2014;96:778–88 [DOI] [PubMed] [Google Scholar]
- 20.Factor D, Dale B: Current concepts of rotator cuff tendinopathy. Int J Sports Phys Ther 2014;9:274–88 [PMC free article] [PubMed] [Google Scholar]
- 21.van der Meijden OA Westgard P Chandler Z, et al. : Rehabilitation after arthroscopic rotator cuff repair: current concepts review and evidence-based guidelines. Int J Sports Phys Ther 2012;7:197–218 [PMC free article] [PubMed] [Google Scholar]
- 22.Gulotta LV, Rodeo SA: Growth factors for rotator cuff repair. Clin Sports Med 2009;28:13–23 [DOI] [PubMed] [Google Scholar]
- 23.Mazuquin BF Wright AC Russell S, et al. : Effectiveness of early compared with conservative rehabilitation for patients having rotator cuff repair surgery: an overview of systematic reviews. Br J Sports Med 2018;52:111–21 [DOI] [PubMed] [Google Scholar]
- 24.Cuff DJ, Pupello DR: Prospective randomized study of arthroscopic rotator cuff repair using an early versus delayed postoperative physical therapy protocol. J Shoulder Elbow Surg 2012;21:1450–5 [DOI] [PubMed] [Google Scholar]
- 25.Kim YS Chung SW Kim JY, et al. : Is early passive motion exercise necessary after arthroscopic rotator cuff repair? Am J Sports Med 2012;40:815–21 [DOI] [PubMed] [Google Scholar]
- 26.Kokmeyer D, Dube E, Millett PJ: Prognosis driven rehabilitation after rotator cuff repair surgery. Open Orthop J 2016;10:339–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jackins S: Postoperative shoulder rehabilitation. Phys Med Rehabil Clin N Am 2004;15:643–82 [DOI] [PubMed] [Google Scholar]
- 28.Littlewood C, Bateman M: Rehabilitation following rotator cuff repair: a survey of current UK practice. Shoulder Elbow 2015;7:193–204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ross D Maerz T Lynch J, et al. : Rehabilitation following arthroscopic rotator cuff repair: a review of current literature. J Am Acad Orthop Surg 2014;22:1–9 [DOI] [PubMed] [Google Scholar]
- 30.Eccleston C Blyth FM Dear BF, et al. : Managing patients with chronic pain during the COVID-19 outbreak: considerations for the rapid introduction of remotely supported (eHealth) pain management services. Pain 2020;161:889–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Parisien RL Shin M Constant M, et al. : Telehealth utilization in response to the novel coronavirus (COVID-19) pandemic in orthopaedic surgery. J Am Acad Orthop Surg 2020;28:e487–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wosik J Fudim M Cameron B, et al. : Telehealth transformation: COVID-19 and the rise of virtual care. J Am Med Inform Assoc 2020;27:957–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kane LT Thakar O Jamgochian G, et al. : The role of telehealth as a platform for postoperative visits following rotator cuff repair: a prospective, randomized controlled trial. J Shoulder Elbow Surg 2020;29:775–83 [DOI] [PubMed] [Google Scholar]
- 34.Pastora-Bernal JM Martín-Valero R Barón-López FJ, et al. : Telerehabilitation after arthroscopic subacromial decompression is effective and not inferior to standard practice: preliminary results. J Telemed Telecare 2018;24:428–33 [DOI] [PubMed] [Google Scholar]
- 35.Eriksson L, Lindström B, Ekenberg L: Patients’ experiences of telerehabilitation at home after shoulder joint replacement. J Telemed Telecare 2011;17:25–30 [DOI] [PubMed] [Google Scholar]
- 36.Pastora-Bernal JM, Martín-Valero R, Barón-López FJ: Cost analysis of telerehabilitation after arthroscopic subacromial decompression. J Telemed Telecare 2018;24:553–9 [DOI] [PubMed] [Google Scholar]
- 37.Bain EE Shafner L Walling DP, et al. : Use of a novel artificial intelligence platform on Mobile devices to assess dosing compliance in a phase 2 clinical trial in subjects with schizophrenia. JMIR Mhealth Uhealth 2017;5:e18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Carnevale A Longo UG Schena E, et al. : Wearable systems for shoulder kinematics assessment: a systematic review. BMC Musculoskelet Disord 2019;20:546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang Q Markopoulos P Yu B, et al. : Interactive wearable systems for upper body rehabilitation: a systematic review. J Neuroeng Rehabil 2017;14:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mejia-Hernandez K Chang A Eardley-Harris N, et al. : Smartphone applications for the evaluation of pathologic shoulder range of motion and shoulder scores—a comparative study. JSES Open Access 2018;2:109–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chiensriwimol N Chan JH Mongkolnam P, et al. : Monitoring frozen shoulder exercises to support clinical decision on treatment process using smartphone. Procedia Comput Sci 2017;111:129–36 [Google Scholar]
- 42.Viglialoro RM Condino S Turini G, et al. : Review of the augmented reality systems for shoulder rehabilitation. Inf 2019;10:154 [Google Scholar]
- 43.Carbonaro N Lucchesi I Lorusssi F, et al. : Tele-monitoring and tele-rehabilitation of the shoulder muscular-skeletal diseases through wearable systems. Annu Int Conf IEEE Eng Med Bio Soc 2018;2018:4410–3 [DOI] [PubMed] [Google Scholar]
- 44.Burns DM Leung N Hardisty M, et al. : Shoulder physiotherapy exercise recognition: machine learning the inertial signals from a smartwatch. Physiol Meas 2018;39:075007. [DOI] [PubMed] [Google Scholar]
- 45.Pan JI, Chung HW, Huang JJ: Intelligent shoulder joint home-based self-rehabilitation monitoring system. Int J Smart Home 2013;7:395–404 [Google Scholar]
- 46.Ongvisatepaiboon K, Chan JH, Vanijja V: Smartphone-based tele-rehabilitation system for frozen shoulder using a machine learning approach. In: 2015 IEEE Symposium Series on Computational Intelligence. Cape Town, South Africa, IEEE, 2015:811–15. doi: 10.1109/SSCI.2015.120 [DOI] [Google Scholar]
- 47.Correia FD Nogueira A Magalhães I, et al. : Home-based rehabilitation with a novel digital biofeedback system versus conventional in-person rehabilitation after total knee replacement: a feasibility study. Sci Rep 2018;8:11299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Correia FD Nogueira A Magalhães I, et al. : Medium-term outcomes of digital versus conventional home-based rehabilitation after total knee arthroplasty: prospective, parallel-group feasibility study. JMIR Rehabil Assist Technol 2018;6:e13111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dias Correia F Nogueira A Magalhães I, et al. : Digital versus conventional rehabilitation after total hip arthroplasty: a single-center, parallel-group pilot study. JMIR Rehabil Assist Technol 2019;6:e14523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Arndt J Clavert P Mielcarek P, et al. : Immediate passive motion versus immobilization after endoscopic supraspinatus tendon repair: a prospective randomized study. Orthop Traumatol Surg Res 2012;98(suppl 6):S131–8 [DOI] [PubMed] [Google Scholar]
- 51.Kukkonen J Kauko T Vahlberg T, et al. : Investigating minimal clinically important difference for Constant score in patients undergoing rotator cuff surgery. J Shoulder Elbow Surg 2013;22:1650–5 [DOI] [PubMed] [Google Scholar]
- 52.Franchignoni F Vercelli S Giordano A, et al. : Minimal clinically important difference of the disabilities of the arm, shoulder and hand outcome measure (DASH) and its shortened version (QuickDASH). J Orthop Sport Phys Ther 2014;44:30–9 [DOI] [PubMed] [Google Scholar]
- 53.Cvetanovich GL Gowd AK Liu JN, et al. : Establishing clinically significant outcome after arthroscopic rotator cuff repair. J Shoulder Elbow Surg 2019;28:939–48 [DOI] [PubMed] [Google Scholar]
- 54.Constant CR Gerber C Emery RJH, et al. : A review of the constant score: modifications and guidelines for its use. J Shoulder Elbow Surg 2008;17:355–61 [DOI] [PubMed] [Google Scholar]
- 55.Macdermid JC Birmingham TB Khadilkar L, et al. : Validity of the QuickDASH in patients with shoulder-related disorders undergoing surgery. J Orthop Sport Phys Ther 2015;45:25–36 [DOI] [PubMed] [Google Scholar]
- 56.Nishimori M Warner JJP Gill TJ, et al. : Long term outcomes following discharge from shoulder surgery in an ambulatory setting. Ambul Surg 2007;13 [Google Scholar]
- 57.Kurowicki J Berglund DD Momoh E, et al. : Speed of recovery after arthroscopic rotator cuff repair. J Shoulder Elbow Surg 2017;26:1271–7 [DOI] [PubMed] [Google Scholar]
- 58.Desrosiers J Hébert R Bravo G, et al. : Shoulder range of motion of healthy elderly people: a normative study. Phys Occup Ther Geriatr 1995;13:101–14 [Google Scholar]
- 59.Walker JM Sue D Miles-Elkousy N, et al. : Active mobility of the extremities in older subjects. Phys Ther 1984;64:919–23 [DOI] [PubMed] [Google Scholar]
- 60.McIntosh L, McKenna K, Gustafsson L: Active and passive shoulder range of motion in healthy older people. Br J Occup Ther 2003;66:318–24 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data relevant to the study are included in the article or are available as Supplemental Digital Content (including raw data in Supplemental Digital Content 3, http://links.lww.com/PHM/B293). Only deidentified individual participant data are provided. Other documents, namely, the study protocol, are available at ClinicalTrials.gov (NCT03648047).


