Supplemental Digital Content is Available in the Text.
Key Words: PrEP, adherence, electronic monitoring, young women, sub-Saharan Africa
Abstract
Objective:
To present detailed analyses of long-term pre-exposure prophylaxis (PrEP) use and associated behaviors and perceptions among young Kenyan women.
Design:
Prospective, observational cohort.
Methods:
The Monitoring PrEP among Young Adult women Study involved 18 to 24-year-old women at high HIV risk initiating PrEP in Kisumu and Thika, Kenya. Visits for PrEP counseling and dispensing, HIV testing, and socio-behavioral data collection occurred at Month 1 and quarterly for 2 years. PrEP adherence was measured with pharmacy refill and real-time electronic monitoring, plus tenofovir diphosphate levels in 15% of participants. HIV risk behavior and perception were assessed by self-report in weekly short message service surveys from Months 6–24. Predictors of adherence were assessed with multivariable logistic regression analysis.
Results:
Three hundred forty-eight women (median age 21, VOICE risk score 7) were followed for 617 person-years. Pharmacy refills steadily declined from 100% (Month 0–1) to 54% (Months 22–24). Average electronically monitored adherence similarly declined from 65% (Month 0–1) to 15% (Months 22–24). Electronically monitored adherence had moderately high concordance with tenofovir diphosphate levels (67%). High average adherence (5+ doses/week) was seen at 385/1898 (20%) participant-visits and associated with low baseline VOICE risk score, >1 current sexual partner, ≤1-hour travel time to clinic, and the Kisumu site. short message service-reported behavior and risk perception were not associated with adherence. Four women acquired HIV (incidence 0.7/100 person-years).
Conclusions:
PrEP adherence was modest and declined over time. HIV risk was inconsistently associated with adherence; clinic access and site-level factors were also relevant. Relatively low HIV incidence suggests participants may have achieved protection through multiple strategies.
Young women accounted for nearly 60% of all new HIV infections in sub-Saharan Africa in 2019, reflecting an estimated 7000 infections each week.1 This population is thus a high priority for HIV prevention. Until recently, HIV prevention tools for young women have primarily consisted of HIV education, delayed sexual debut, condom use, and a monogamous relationship with a partner known to be HIV-negative.2 Pre-exposure prophylaxis (PrEP) is an additional, highly effective HIV prevention tool when taken daily during periods of HIV exposure, yet adherence to PrEP has been low among young women in phase III clinical trials and in more recent studies, demonstration projects, and real world settings.3–8
Adherence behavior is challenging to understand, particularly in clinical programs where practical considerations limit measurement tools to self-report and pharmacy refills. Although potentially informative, these measures often overestimate adherence and do not describe daily adherence patterns.9,10 The latter is particularly important for PrEP, as high adherence is most important around periods of high risk for HIV acquisition. This concept, known as prevention-effective adherence,11 can best be explored through day-to-day measurement of adherence, such as can be accomplished with electronic monitors (ie, “smart” pill boxes that record a date-and-time stamp of each opening as a proxy for pill ingestion).
Knowledge of prevention-effective PrEP adherence also requires that adherence measures are accompanied by contemporaneous assessments of HIV risk behavior. Numerous approaches have been developed to assess this risk in various populations, including questions and scores based on behaviors (eg, condomless sex), knowledge (eg, understanding of HIV transmission), and perceptions (eg, belief in HIV prevention tools).12–14 The ability of these measures to provide accurate and meaningful information about potential HIV exposure, however, is unclear.15
To date, no studies have used electronic monitors in combination with measures of HIV risk to understand prevention-effective PrEP adherence among young women in sub-Saharan Africa. Here, we present multiple measures of adherence among young women offered PrEP over 2 years in Kenya. Our objective was to obtain a detailed understanding of PrEP adherence over time and identify predictors of adherence, including repeated measures of HIV risk behavior.
METHODS
Setting and Participants
The Monitoring PrEP among Young Adult women (MPYA, meaning “new” in Swahili) Study involved young women in Thika and Kisumu, Kenya at high risk for HIV acquisition. The estimated HIV prevalence among women in Thika (peri-urban) and Kisumu (rural) are 5.9% and 17.4%, respectively.16 We enrolled participants from December 21, 2016 through February 5, 2018. We identified potential participants from multiple locations: colleges/vocational institutions, informal settlements, and community-based organizations providing primary health care and other services to young women, including those engaged in sex work. We defined high risk as a VOICE risk score ≥5 (suggesting a risk of >5 infections/100 person-years)12 with points given for age <25 (2 points), being single or not living with a primary sexual partner (2 points), lacking financial or material support from a sexual partner (1 point), a sexual partner having or potentially having other partners (2 points), and alcohol use (1 point). We also recruited women whose sexual partner had known or suspected HIV infection. Other inclusion criteria were assigned female gender at birth, age 18–24 years at enrollment, wanting and being clinically safe to start PrEP (creatinine clearance >60 mL/min, no hepatitis B infection or other complicating medical condition), sexually active within the prior 3 months, ownership of a cellular phone, ability to send short message service (SMS), willingness to use an electronic adherence monitor, and intention to stay in the area for at least 1 year. Pregnancy and breast-feeding were exclusion criteria for study entry (reflecting evolving PrEP safety data at the time), but those who became pregnant had the option to continue PrEP. Continued PrEP use during breastfeeding was later allowed midway through the study, if desired, reflecting new evidence of safety.17 We excluded women concurrently participating in other HIV prevention studies.
Study Procedures
We followed participants prospectively for a 2-year period ending on March 27, 2020. Study visits occurred at month 1, month 3, and then quarterly thereafter. If HIV testing per Kenyan national guidelines18 was negative, PrEP was offered as open-label emtricitabine (200 mg)/tenofovir disoproxil fumarate (300 mg) to be taken as a single, once daily tablet. Participants were provided with a 30-day supply at enrollment and offered a 60-day supply at the month 1 visit and 90-day supplies thereafter. Consistent with the concept of prevention-effective adherence,11 all participants were encouraged to take PrEP for the first 6 months of the study based on high risk at enrollment and counseled thereafter to take PrEP if they remained at high risk and interested in PrEP. The study also involved a randomized controlled trial of SMS reminders to support PrEP adherence that showed no benefit.19
Participants completed demographic and socio-behavioral questionnaires at each study visit with data capture in REDCap.20 Potential factors influencing PrEP adherence focused primarily on the individual (ie, demographic characteristics, beliefs, preferences) and her partnership (ie, sexual behavior, relationship dynamics), but also included community (ie, PrEP disclosure, stigma) and structural (ie, study site, travel time) factors to reflect socio-ecological theory.21 We used scales validated in similar settings where possible. Specifically, we assessed for depression symptoms with the patient health questionnaire-2,22 perceived HIV stigma with a modified Berger scale,23 self-esteem with the Rosenberg scale,24 problematic alcohol use with the RAPS-4,25 perceptions of necessity for PrEP and concern about PrEP use by a modified Belief and Medicine Questionnaire (BMQ) with permission and input from Dr. Rob Horne,26 risk attitudes by eliciting preferences on a standard series of hypothetical lottery and gamble scenarios,27,28 intimate partner violence with a modified Conflict Tactics scale,29 and relationship power with the Sexual Relationship Power Scale (SRPS).30 We collected socio-behavioral data quarterly aside from necessity and concerns (collected semi-annually) and stigma, self-esteem, risk attitudes (collected annually); this schedule limited the questionnaire burden in this young population.
Adherence Measures
We measured PrEP adherence in all participants by pharmacy refill and with a real-time electronic monitor (Wisepill Technologies, Cape Town, South Africa). This device transmits a date-and-time stamp of each opening. It also demonstrates functionality through transmission of a daily electronic heartbeat (ie, battery life, signal strength). Records of device openings are stored for later transmission in the event of poor cellular network coverage. Devices have a battery life of ∼6 months. We encouraged participants to bring their devices to each study visit for battery replacement. Dried blood spots (DBS) were also collected at each quarterly visit and stored at −80 °C for batched processing. We tested a 15% random sample of DBS from nonpregnant participants receiving PrEP for tenofovir diphosphate levels (TFV-DP) using a validated liquid chromatography–tandem mass spectrometry assay31; TFV-DP levels reflect average adherence over several weeks before collection.32
We determined pharmacy refill adherence as the percent of participants picking up PrEP at each study visit. We used electronic monitoring to estimate the number of doses taken by summarizing data between study visits as the number of days on which an opening event was recorded divided by the number of days with functional monitoring and averaging individual values per visit.
SMS Surveys
SMS surveys included questions about sexual behavior, perceived HIV risk, and perceived protection from HIV risk according to the participant's PrEP use (see Appendix 1, Supplemental Digital Content, http://links.lww.com/QAI/B765). We used an automated platform (Ajua, Kenya) to send surveys to participants weekly from months 6 to 24. Participants received 50 Kenyan Shillings (∼US$0.50) for each completed survey. Surveys used branching logic, and questions on general health behaviors (eg, exercise, alcohol use) were used as needed to ensure all surveys would consist of 6 questions, thus promoting true responses versus rapid completion.
Socio-Behavioral Measures
We scored socio-behavioral scales as follows: depression (patient health questionnaire-2 Likert-response scores >3 indicated possible depression), stigma (modified Berger scores ranging 1–4 were summed and dichotomized at the median; higher numbers indicated more stigma), self-esteem (Rosenberg Likert-response scores ranging 0–3 were summed with higher scores indicating more self-esteem and dichotomized at 15), problematic alcohol use (a “yes” response to 1+ items on the RAPS-4 was considered positive), necessity and concern about PrEP use (BMQ scores ranging 1–5 were averaged and divided into tertiles; higher scores indicated stronger agreement with the necessity or concern for PrEP), risk attitudes (dividing lottery/gambling scores into tertiles), intimate partner violence (a “yes” response to 1+ items on the modified Conflict Tactics scale indicated the presence of violence), and relationship power (SRPS Likert-response scores ranging 1–4 were averaged with higher scores indicating less power).
Data Analysis
We considered participants retained in the study if they attended a study visit and/or their adherence continued to be monitored electronically. The latter criterion leveraged the data available with real-time technology and potential PrEP use obtained at study visits throughout the study period. We censored data for study-related drug holds or discontinuation (eg, during breastfeeding early in follow-up, seroconversion, death); we included data during pregnancy.
Participants from the SMS reminder and control arms were combined for this analysis given the lack of an intervention effect.
We categorized electronically monitored adherence over the prior 60 days and DBS TFV-DP levels as follows and compared them for concordance: high (5+ doses/week; >892 fmol/punch); moderate (3–4 doses/week; 537–892 fmol/punch; low (1–2 doses/week; 182–536 fmol/punch); and none. These TFV-DP thresholds reflect the 25th percentile of previously reported values.33 Of note, pharmacokinetic/pharmacodynamic modeling suggests the threshold for protection may be higher in cisgender women compared with men who have sex with men, for whom 4+ doses/week (700 fmol/punch) is considered adequate.34,35
We assessed predictors of high adherence (5+ doses/week) at each study visit initially with univariable logistic generalized estimated regression models with robust standard errors. Adherence was averaged over each week and summed at each study visit (ie, Month 1, Month 3, quarterly thereafter). Data on predictors not collected quarterly were carried forward. Those factors with statistical significance (P < 0.20) were included in a multivariable model. Given the current uncertainty regarding the optimal adherence needed for HIV protection among cisgender women and the ranges used in the literature,6,34 we also performed sensitivity analyses using a threshold of 4+ doses/week for moderately high adherence and 6+ doses/week for near perfect adherence. Predictors of persistence on PrEP and retention in the program will be published separately.
We examined the association between adherence and SMS survey responses at the participant-week level, with adherence defined over the week before the completed SMS survey. We again used logistic generalized estimated regression models to assess participants' weekly adherence with yes versus no responses to each survey question as predictor variables. Models included fixed effects at the individual level.
Ethics
This study was approved by the institutional regulatory boards at the Kenya Medical Research Institute, MassGeneral Brigham, and the University of Washington. All participants provided written informed consent.
RESULTS
Participant Characteristics
A total of 642 women were screened for study participation (see Appendix 2, Supplemental Digital Content, http://links.lww.com/QAI/B765). Two hundred ninety-two participants did not enroll for the following reasons: not interested (n = 179), pregnant or breast-feeding (n = 44), low VOICE risk score (n = 27), other medical issue (n = 20), HIV test was positive/indeterminant (n = 17), plans to relocate (n = 3), no phone (n = 1), and incomplete screening (n = 1). Two additional participants were excluded within 1 month of enrollment because of age <18 or concurrent study participation, leaving 348 for analysis. Participant characteristics are presented in Table 1. Retention was 82% over the 24-month follow-up period. The only significant difference in those participants not retained versus retained in the study was the baseline VOICE risk score, which was slightly higher in the former (mean 6.8 [SD, SD, 0.8] versus 6.6 [SD 0.9]; P = 0.03). Four women acquired HIV in the Kisumu site (incidence 0.7/100 person-years overall; 1.25/100 person-years in Kisumu specifically).
Table 1.
Participant Characteristics at Baseline
| Characteristic | Kisumu | Thika | Total |
| N (%) or median (IQR) | N (%) or median (IQR) | N (%) or median (IQR) | |
| Total | 174 (100%) | 174 (100) | 348 |
| Individual factors | |||
| Baseline VOICE risk score | 6 (6, 7) | 7 (6, 7) | 7 (6, 7) |
| Age (yr) | 20 (19, 22) | 21 (19, 22) | 21 (19, 22) |
| Education (yr) | 12 (9, 12) | 12 (10, 13) | 12 (10, 13) |
| Possible depression | 17 (10%) | 5 (3%) | 22 (6%) |
| Low self-esteem | 2 (1%) | 3 (2%) | 5 (1%) |
| Problem alcohol use | 24 (14%) | 42 (24%) | 66 (19%) |
| Other recreational drug use* | 2 (1%) | 16 (9%) | 18 (5%) |
| Prior daily medication use (other than PrEP) | 37 (22%) | 17 (10%) | 54 (16%) |
| Perceived necessity for PrEP† | |||
| Low (1.0–1.8) | 33 (19%) | 58 (34%) | 91 (26%) |
| Moderate (1.9–2.1) | 77 (44%) | 54 (31%) | 131 (38%) |
| High (2.2–4.0) | 64 (37%) | 61 (35%) | 125 (36%) |
| Concerns about taking PrEP† | |||
| Low (2.4–3.5) | 41 (23%) | 86 (50%) | 127 (37%) |
| Moderate (3.6–3.9) | 78 (45%) | 54 (31%) | 132 (38%) |
| High (4.0–5.0) | 55 (32%) | 33 (19%) | 88 (25%) |
| Risk attitude† | |||
| Low tolerance for risk | 55 (32%) | 55 (32%) | 110 (32%) |
| Moderate tolerance for risk | 77 (44%) | 67 (39%) | 144 (42%) |
| High tolerance for risk | 42 (24%) | 49 (29%) | 91 (26%) |
| Partnership factors | |||
| Current sero-different partnership | 9 (5%) | 4 (2%) | 13 (4%) |
| Median sex acts in prior mo | 2 (1, 4) | 3 (1, 7) | 2 (1, 5) |
| >1 total current sexual partner | 72 (41%) | 49 (28%) | 121 (35%) |
| Any condomless sex in past mo | 92 (53%) | 136 (79%) | 228 (66%) |
| Sexual relationship power†‡ | |||
| Low (1.0–2.4) | 33 (20%) | 28 (26%) | 61 (22%) |
| Moderate (2.5–2.8) | 80 (48%) | 52 (48%) | 132 (48%) |
| High (2.9–4.0) | 53 (32%) | 28 (26%) | 81 (30%) |
| Intimate partner violence in past 12 months | 38 (22%) | 17 (10%) | 55 (16%) |
| Community factors | |||
| PrEP disclosure§ | 122 (70%) | 65 (38%) | 187 (54%) |
| Perceived HIV stigma | |||
| Low (4–11) | 81 (47%) | 114 (66%) | 195 (56%) |
| High (12–16) | 93 (54%) | 60 (34%) | 153 (44%) |
| Structural factors | |||
| Travel to study site ≤ 1 hour | 93 (54%) | 139 (80%) | 232 (67%) |
Data were complete for 340 (98%) participants; some responses were missing for the remaining participants. IQR = interquartile range, N = number participants.
The most common “other recreational drug use” was cannabis (13/18, 72%); 1–4 participants reported khat, bhangi, heroin, kuber, and/or shisha use.
Because of the categorical nature of responses, tertiles were unevenly distributed.
Only reported for participants identifying a primary sexual relationship (N = 274).
Disclosure involved family members (108/157%, 58%), friends (78/18, 42%), and sexual partners (44/187, 18%); participants could report more than 1 type of disclosure.
PrEP Adherence
We analyzed 617 total person-years of follow-up. Among all study participants, most picked up PrEP early in follow-up, but rates steadily declined from 100% from Month 0%–1% to 54% from Months 21–24 (Fig. 1). Similarly, the average percent of doses taken decreased over time from 65% (month 0–1) to 15% (months 22–24). The percent of participants taking an average of 5+ doses/week declined from 53% in month 0–1 to ≤5% in months 13–24. Conversely, the percent of participants taking an average of <1 dose/week steadily increased from 8% in month 0%–1% to 71%–87% in months 13–24. Those taking an average of 1–4 doses/week was highest in Months 2–3 at 43% and decreased to 12% in Months 19–24. Electronically monitored adherence in the 90 days before detected seroconversion was 0% for 3 women and 6% for 1 woman.
FIGURE 1.
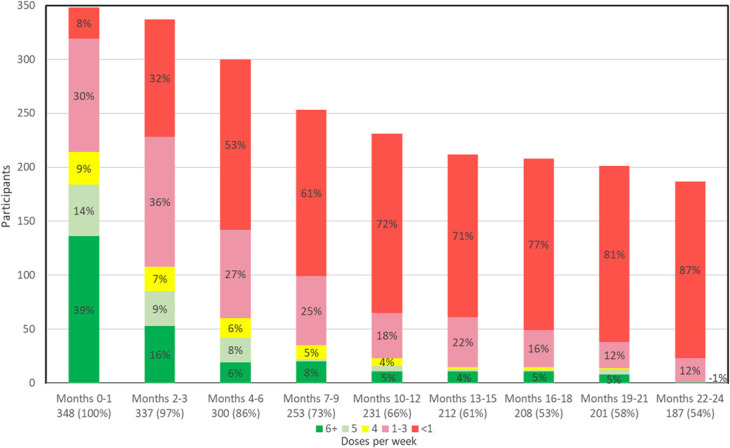
PrEP adherence over time. Each column indicates the number of participants picking up PrEP at each study visit (ie, pharmacy refill adherence) from months 0 through 21. Electronically monitored adherence over the period covered by each pharmacy refill is shown as the average number of doses per week. The green color indicates high adherence (5+ doses/week).
Concordance Between Electronically Monitored PrEP Adherence and TFV-DP Levels
Electronically monitored adherence data were available for 150 (85%) of the 177 TFV-DP samples with 67% concordance seen between the 2 measures (Table 2); most adherence levels were in the lowest categories. Most discordance was because of lower TFV-DP levels compared with electronically monitored adherence levels.
TABLE 2.
Concordance in Categories of Electronically Monitored Adherence and Tenofovir Diphosphate Levels in Dried Blood Spots (N = 150)
| Concordance = 67% | ||||
| Average Electronically Monitored Adherence over Prior 60 d | ||||
| TFV-DP (fmol/punch) | 0 to <1 Doses/week | 1 to <3 Doses/week | 3 to <5 Doses/week | 5+ Doses/week |
| Below quantification | 81 (54%) | 9 (6%) | 9 (6%) | 2 (1%) |
| Low (LLOQ-536) | 11 (7%) | 8 (5%) | 3 (2%) | 11 (7%) |
| Moderate (537–892) | 1 (1%) | 0 (0%) | 3 (2%) | 7 (5%) |
| High (>892) | 0 (0%) | 0 (0%) | 0 (0%) | 9 (6%) |
Bold indicates concordance between the two adherence measures.
LLOQ, lower level of quantification (25 fmol/sample).
Predictors of Electronically Monitored PrEP Adherence
The demographic and socio-behavioral factors assessed as potentially influencing high electronically monitored adherence (5+ doses/week) are shown in Table 3. A total of 1898 participant visits were considered for analysis. High adherence was significantly associated with a lower baseline VOICE risk score (adjusted odds ratio [aOR] 0.73 for each increasing point of the score), having >1 current sexual partner (aOR 1.82), the Kisumu site (aOR 1.57), and ≤1-hour travel time (aOR 1.39). Notable trends toward high adherence were seen with more sexual relationship power (moderate aOR 1.52, high aOR 1.26) and low perceived HIV stigma (aOR 1.45). When defining adherence as moderately high (4+ doses/week) and near perfect (6+ doses/week), significant associations were similar (Appendices 3 and 4, Supplemental Digital Content, http://links.lww.com/QAI/B765).
TABLE 3.
Predictors of High (5+ Doses/Week) Electronically Monitored PrEP Adherence
| N participant Visits (%) or Mean (SD) | Unadjusted Odds Ratio (95% CI) | P | Adjusted Odds Ratio (95% CI) | P | |
| Individual factors | |||||
| Baseline VOICE risk score | 6 (1) | 0.75 (0.62 to 0.92) | 0.005 | 0.73 (0.58 to 0.91) | 0.006 |
| Age at baseline (yr) | 21 (2) | 0.99 (0.91 to 1.10) | 0.95 | — | — |
| Education (yr) | 11 (2) | 0.92 (0.86 to 0.98) | 0.015 | 0.94 (0.87 to 1.02) | 0.12 |
| Possible depression | 6 (13%) | 0.64 (0.27,1.50) | 0.30 | — | — |
| Low self-esteem | 4 (10%) | 0.41 (0.06 to 2.6) | 0.35 | — | — |
| Problematic alcohol use | 20 (14%) | 1.04 (0.67 to 1.61) | 0.86 | — | — |
| Prior daily medication use | 55 (19%) | 0.80 (0.49 to 1.32) | 0.39 | — | — |
| Perceived necessity for PrEP | 0.82 | ||||
| Low (1–1.8) | 75 (21%) | Ref | |||
| Moderate (1.9–2.1) | 156 (22%) | 0.98 (0.67 to 1.43) | |||
| High (2.1–4.0) | 154 (19%) | 0.89 (0.58 to 1.37) | |||
| Concerns about taking PrEP | 0.30 | ||||
| Low (2.4–3.5) | 110 (20%) | Ref | |||
| Moderate (3.6–3.9) | 164 (22%) | 0.91 (0.67 to 1.25) | |||
| High (4.0–5.0) | 111 (18%) | 0.73 (0.49 to 1.09) | |||
| Risk attitude | 0.29 | ||||
| Low tolerance for risk | 133 (21%) | Ref | |||
| Moderate tolerance for risk | 152 (20%) | 1.07 (0.74 to 1.56) | |||
| High tolerance for risk | 99 (20%) | 1.19 (0.80 to 1.79) | |||
| Partnership factors | |||||
| Any HIV+ partner | 33 (32%) | 1.16 (0.64 to 2.13) | 0.62 | — | — |
| > 1 current sexual partner | 96 (21%) | 1.00 (0.99 to 1.01) | 0.90 | 1.82 (1.32 to 2.51) | <0.001 |
| Condomless sex | 203 (20%) | 1.11 (0.87 to 1.42) | 0.39 | — | — |
| Sexual relationship power* | 0.06 | 0.06 | |||
| Low (1.0–2.4) | 41 (14%) | Ref | |||
| Moderate (2.5–2.8) | 168 (23%) | 1.51 (1.06 to 2.14) | |||
| High (2.9–4.0) | 159 (21%) | 1.23 (0.81 to 1.86) | |||
| Intimate partner violence | 5 (16%) | 1.04 (0.46 to 2.36) | 0.92 | — | — |
| Community factors | |||||
| PrEP disclosure | 300 (21%) | 1.25 (0.92 to 1.71) | 0.16 | 1.07 (0.74 to 1.54) | 0.73 |
| Perceived HIV stigma | |||||
| Low (4–11) | 189 (21%) | 1.41 (0.99 to 2.00) Ref | 0.06 | 1.45 (1.00 to 2.09) Ref | 0.05 |
| High (12–16) | 196 (20%) | ||||
| Structural factors | |||||
| Site: Thika Kisumu |
129 (16%) 256 (23%) |
Ref 1.37 (1.10 to 1.56) |
0.01 |
Ref 1.57 (1.02 to 2.43) |
0.04 |
| Travel ≤1 hour to study | 199 (21%) | 1.41 (1.09 to 1.82) | 0.008 | 1.39 (1.05 to 1.84) | 0.02 |
N refers to the number of participant visits with each factor at which the average adherence was 5+ doses/week; the mean references those participants with 5+ doses/week at 1 or more of the participant visits. All measures were updated over time unless noted as baseline values. Bold indicates significance (P < 0.05) in the multivariable model.
Among those with a primary sexual relationship.
SMS Surveys on HIV-Risk Behavior and Perception
We sent 84% of the SMS surveys (18,668/22,150) as planned; the primary reason for not sending SMS was a platform coding error early in the study. Overall, participants completed 77% (14,440/18,668) of the surveys; 304/348 (87%) participants provided at least one survey response. The only difference between those completing versus not completing the surveys was study site (96% in Kisumu vs 79% in Thika; P < 0.01). As shown in Table 4, we observed no significant difference in mean or high electronically measured adherence between weeks in which participants reported sexual activity versus those in which they reported no sexual activity, regardless of condom use or partner HIV status. Similarly, we saw no significant difference in adherence between weeks in which participants reported perceived HIV risk or perceived protection from HIV because of PrEP. In addition, dosing likely to protect against HIV infection (ie, 5+ doses/week) was seen in a minority of participant-weeks for all reported HIV risk behaviors: 32% (853/2638) for those reporting sexual activity, 30% (402/1342) for those reporting condomless sex, 32% (264/821) for those who reported having sex with a partner who may have had HIV, and 25% (87/351) for those reporting condomless sex with a partner who may have had HIV.
TABLE 4.
Summary of Weekly SMS Survey Data on Sexual Behavior and HIV Risk
| HIV Risk In the Prior Week, the Participant Reported… |
Participant Responded ‘Yes’ during at Least 1 Week, N (%) | Number of Weeks Participants Responded ‘Yes’, Mean (SD) | Survey Response for Prior Week | Mean (SD) Adherence in the Prior Week | P | N (%) Participant-wk with High Adherence in Prior Week (5+ Doses) | P |
| Sexual activity | 299 (86%) | 36 (22) | Yes | 32% (21, 51) | 0.49 | 853 (32%) | 0.07 |
| No | 34% (21, 56) | 565 (43%) | |||||
| Condoms not used with all sex acts | 268 (90%) | 21 (20) | Yes | 31% (19, 54) | 0.54 | 402 (30%) | 0.52 |
| No | 33% (19, 55) | 450 (35%) | |||||
| Sexual partner may have had HIV | 240 (80%) | 12 (16) | Yes | 33% (19, 62) | 0.94 | 264 (32%) | 0.76 |
| No | 30% (19, 53) | 584 (33%) | |||||
| Perceived HIV risk | 170 (49%) | 7 (12) | Yes | 29% (17, 62) | 0.58 | 87 (25%) | 0.52 |
| No | 33% (28, 43) | 177 (38%) | |||||
| Perceived protection from HIV because of PrEP* | 232 (67%) | 31 (22) | Yes | 31% (20, 53) | 0.79 | 768 (33%) | 0.16 |
| No | 31% (16, 60) | 50 (27%) |
Surveys were sent to all 348 participants weekly from Month 6–24 of the study (72 total weeks). Data reflect responses from 14,440/18,668 (77%) of the surveys completed. Adherence is defined as 5+ dose/week per electronic monitoring.
Among those picking up PrEP.
IQR, interquartile range; SD = standard deviation.
DISCUSSION
In this two-year study of young women offered PrEP in Kenya, we found high interest in PrEP, but steadily declining adherence according to multiple measures. Few participants consistently took PrEP at levels likely to achieve protection from HIV beyond the first few months. These findings replicate those of other studies among young women in sub-Saharan Africa.3,4,6–8 Although disappointing, many lessons can be learned from the factors associated with adherence and from the outcomes seen in this study.
The inconsistent associations between multiple measures of HIV risk and PrEP adherence reveal the complexity in understanding risk behavior and perception. Lower baseline HIV risk according to the VOICE risk score was associated with higher adherence, which potentially indicates some degree of concordance between control over HIV exposure and ability to take a daily preventive medication. That said, over the course of the study, we saw increased odds of high adherence among those with >1 current sexual partner, signaling a recognition of increased risk and the need for PrEP. Yet, no difference in adherence was seen across degrees of underlying risk tolerance, nor by SMS-reported sexual activity, condom use, sex with some who may be living with HIV, or perceived HIV risk. Taken together, these findings suggest that young women may not recognize their risk and/or prioritize it above other concerns, as has been seen previously36,37; however, a straight-forward metric of multiple partners may be impactful in promoting HIV prevention behavior for some. Assessments of risk perception and prediction tools have had mixed results and warrant further research.38,39
Interestingly, the overall HIV incidence of 0.7/100 person-years was lower than the >5/100 person-years anticipated by the VOICE risk score and suggests that MPYA study participants achieved some degree of HIV prevention despite modest levels of PrEP adherence. This finding could be explained through well-aligned use of PrEP during periods of exposure that we were unable to measure. Alternatively, the VOICE risk score may not be an appropriate measure for all populations of young women.40 Although the VOICE risk score was developed from studies involving women from 3 countries in sub-Saharan Africa, it was largely based on women in southern Africa. Important differences are likely present in socio-cultural factors and behaviors, and HIV prevalence and incidence, in various settings. Nonetheless, the statistically significant association between the VOICE risk score and PrEP adherence suggests it has some relevance in this population. As another benchmark, young women participants in the Kisumu site of the Evidence for Contraceptive Options and HIV Outcomes Study41 (a randomized trial of multiple contraceptive options among women conducted concurrently with the MPYA Study who were not considered high risk for HIV) had an HIV incidence of 1.2/100 person-years. Both reference points thus suggest that MPYA participants achieved some HIV protection that warrants further investigation.
An alternate explanation of the low PrEP adherence and low HIV incidence is that protection was achieved through unmeasured use of other HIV prevention methods. Notably, participants reported perceived protection from HIV because of PrEP through the SMS surveys less than half of the time. Although participants indicated sexual activity and incomplete condom use throughout the study, we do not know the HIV risk behaviors among their sexual partners. The relatively high relationship power reported by MPYA participants (consistent with another study in Kenya30) suggests considerable agency, and a trend toward high adherence was seen with more sexual relationship power. Counseling may have been empowering and an important factor in achieving overall low HIV incidence. Qualitative assessment of these factors will be published separately. These findings suggest that young woman-focused programs (eg, DREAMS initiatives42) have potential for impact.
The detailed adherence data in this study have important implications for PrEP research and clinical delivery. First, the low electronically monitored adherence compared with pharmacy refill raises questions about the accuracy of PrEP adherence measurement reported in other studies relying solely on the latter5,7,8; true adherence may have been even lower. Other objective measures are therefore needed to assess PrEP use in routine care. One promising approach is the measurement of tenofovir levels in urine, which may be affordable at ∼$2/test.43 An important consideration, however, is that the test only comments on recent use and may be subject to “white coat dosing” (ie, taking a dose just before clinic but not at other times). Research on the validity of this measurement approach is ongoing.44 Second, the concordance of electronic adherence monitoring with TFV-DP in DBS suggests reasonable accuracy and utility of these measures. However, most adherence values were limited to the lowest categories; a broader range of adherence is needed to fully compare the measures and account for potential bias (eg, more device manipulation or pharmacokinetic variability at higher adherence levels). Notably, we did not see high TFV-DP levels concurrent with low electronically monitored adherence, suggesting that participants were not taking PrEP from alternative containers. The presence of high adherence by electronic monitoring with low TFV-DP levels could indicate device manipulation or curiosity openings (ie, openings without dosing) and/or inappropriate threshold TFV-DP levels for this population. Indeed, TFV-DP levels may be lower in the setting of anemia,45 which is common in this population; further research in this area is warranted. Third, this study highlights the utility of electronic adherence monitoring for assessing prevention-effective adherence. Although pharmacokinetic markers (eg, TFV-DP in DBS) provide useful objective data on pill ingestion, most are too blunt for such detailed analysis. Notably, novel approaches to drug levels in hair may allow for assessment of temporal trends.46
This study has important limitations. Principally, participants took PrEP in the context of a research study; behaviors may differ in routine care. Other limitations include the lack of generalizability beyond the setting studied and potential for social desirability bias, as well as the above-noted possibilities of adherence misclassification bias and inaccuracy of risk assessments. In addition, PrEP is being offered through other studies and programs near the study sites that may have influenced PrEP use, although we were not aware of any co-enrollments. The study also had many strengths, including high retention, robust and detailed adherence measurement, weekly assessment of HIV risk behavior and perception, and 2 years of follow-up.
In conclusion, PrEP adherence declined rapidly and remained low for most young women in this study, although a small proportion took PrEP at levels likely to provide protection from HIV acquisition. While the associations between HIV risk and PrEP adherence were complex, the straight-forward metric of having multiple current sexual partners may offer a promising counseling approach. Although the lower than anticipated HIV incidence could suggest targeted prevention-effective adherence, it may also indicate that young women gained other unmeasured benefits from participation in a PrEP program, such as the ability to have lower risk sexual partners. Attention to holistic HIV prevention, including PrEP and involvement of sexual partners, will be critical for this population going forward. Long-acting formulations47 may also be useful for those who desire PrEP but struggle with adherence to a daily pill. Importantly, the association of higher adherence with short travel time and other site-level factors (eg, local social norms and behaviors, staff interactions with clients) holds promise for better HIV prevention with less burdensome, user-friendly approaches to PrEP, such as may be achieved with community-based PrEP delivery. Ongoing studies in this area include PrEP My Way (NCT04408729) in which PrEP refills and other sexual and reproductive health services are being delivered to clients by peers on request and Pharmacy PrEP (NCT04558554) in which PrEP is being delivered by pharmacists.
ACKNOWLEDGMENTS
Gilead provided emtricitabine-tenofovir for use as PrEP. The authors thank the study participants, our community advisory boards, and the MPYA Study team:
Principal Investigators: Jessica E. Haberer, Jared M. Baeten, Elizabeth Bukusi, Nelly Mugo.
Co-Investigators: Kenneth Ngure, Ruanne Barnabas, Harsha Thirumurthy, Ingrid Katz.
Study team members (Kisumu): Kevin Kamolloh, Josephine Odoyo, Linda Aswani, Lawrence Juma, Elizabeth Koyo, Bernard Rono, Stanley Cheruiot, Vallery Ogello, Loice Okumu, Violet Kwach, Alfred Obiero, Stella Njuguna, Millicent Faith Akinyi, Lilian Adipo, Sylvia Akinyi
Study team members (Thika): Catherine Kiptiness, Nicholas Thuo, Stephen Gakuo Maina, Irene Njeru, Peter Mogere, Sarah Mbaire, Murugi Micheni, Lynda Oluoch, John Njoroge, Snaidah Ongachi, Jacinta Nyokabi
Program manager: Lindsey Garrison
Statisticians/analysts: Maria Pyra, Katherine K. Thomas, Nicholas Musinguzi, Susie Valenzuela.
Footnotes
Supported by the National Institute of Mental Health (R01MH109309); study medication was provided by Gilead (United States). Neither organization influenced the study conduct, analysis, or presentation of the results.
J.E.H. reports personal fees from Merck, outside the submitted work. K.N. reports personal fees from Gilead Sciences and Merck. P.L.A. reports personal fees and grants from Gilead Sciences. J.M.B. reports personal fees from Gilead Sciences, Janssen, and Merck, outside the submitted work. The remaining authors have no conflicts of interest to disclose.
Presented at the Retroviruses and Opportunistic Infections 27th Conference: 2020, Boston.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's Web site (www.jaids.com).
Trial registration: NCT02915367.
REFERENCES
- 1.UNAIDS. When Women Lead, Change Happens: Women Advancing the End of AIDS. Geneva, Switzerland: UNAIDS; 2017. [Google Scholar]
- 2.National Research Council Panel on data and research priorities for arresting AIDS in sub-saharan Africa. In: Cohen B, Trussell J, editors. Preventing and Mitigating AIDS in Sub-saharan Africa: Research and Data Priorities for the Social and Behavioral Sciences. Washington, DC: National Academies Press; 1996. [PubMed] [Google Scholar]
- 3.Van Damme L, Corneli A, Ahmed K, et al. Preexposure prophylaxis for HIV infection among African women. N Engl J Med. 2012;367:411–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Marrazzo JM, Ramjee G, Richardson BA, et al. Tenofovir-based preexposure prophylaxis for HIV infection among African women. N Engl J Med. 2015;372:509–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kyongo J, Kiragu M, Karuga R, et al. How Long Will They Take it? Oral Pre-exposure Prophylaxis (PrEP) Retention for Female Sex Workers, Men Who Have Sex with Men and Young Women in a Demonstration Project in Kenya. Amsterdam, Netherlands:International AIDS Society; 2018. [Google Scholar]
- 6.Celum C, Hosek S, Tsholwana M, et al. PrEP uptake, persistence, adherence, and effect of retrospective drug level feedback on PrEP adherence among young women in southern Africa: results from HPTN 082, a randomized controlled trial. Plos Med. 2021;18:e1003670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mugwanya KK, Pintye J, Kinuthia J, et al. Integrating preexposure prophylaxis delivery in routine family planning clinics: a feasibility programmatic evaluation in Kenya. PLoS Med. 2019;16:e1002885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Celum C, Bukusi E, Bekker L-G, et al. PrEP initiation, persistence, and HIV seroconversaion rates in African adolescent girls and young women (AGYW) from Kenya and South Africa: the POWER demonstration project. HIV Research for Prevention. 2021; Virtual. Abstract PE 16.19.
- 9.Berg KM, Arnsten JH. Practical and conceptual challenges in measuring antiretroviral adherence. J Acquir Immune Defic Syndr. 2006;43(suppl 1):S79–S87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kagee A, Nel A. Assessing the association between self-report items for HIV pill adherence and biological measures. AIDS Care. 2012;24:1448–1452. [DOI] [PubMed] [Google Scholar]
- 11.Haberer JE, Bangsberg DR, Baeten JM, et al. Defining success with HIV pre-exposure prophylaxis: a prevention-effective adherence paradigm. AIDS. 2015;29:1277–1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Balkus JE, Brown E, Palanee T, et al. An empiric HIV risk scoring tool to predict HIV-1 acquisition in african women. J Acquir Immune Defic Syndr. 2016;72:333–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Irungu EM, Heffron R, Mugo N, et al. Use of a risk scoring tool to identify higher-risk HIV-1 serodiscordant couples for an antiretroviral-based HIV-1 prevention intervention. BMC Infect Dis. 2016;16:571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.US Public Health Service. Preexposure Prophylaxis for the Prevention of HIV Infection in the United States - 2017 Update: A Clinical Practice Guideline. Centers for Disease Control and Prevention; 2017. Available at: https://www.cdc.gov/hiv/pdf/risk/prep/cdc-hiv-prep-guidelines-2021.pdf. [Google Scholar]
- 15.Peebles K, Palanee-Phillips T, Balkus JE, et al. Age-specific risk scores do not improve HIV-1 prediction among women in South Africa. J Acquir Immune Defic Syndr. 2020;85:156–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.National AIDS and STI Control Programme (NASCOP). Preliminary KENPHIA 2018 Report. Nairobi: NASCOP; 2020. [Google Scholar]
- 17.Mugwanya KK, John-Stewart G, Baeten J. Safety of oral tenofovir disoproxil fumarate-based HIV pre-exposure prophylaxis use in lactating HIV-uninfected women. Expert Opin Drug Saf. 2017;16:867–871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.National AIDS, STI Control Program, Ministry of Health. Guidelines on Use of Antiretroviral Drugs for Treating and Preventing HIV Infection in Kenya. 2018 Edition. Nairobi, Kenya: NASCOP; 2018. [Google Scholar]
- 19.Haberer JE, Bukusi EA, Mugo NR, et al. Effect of SMS reminders on PrEP adherence in young Kenyan women (MPYA study): a randomised controlled trial. Lancet HIV. 2021;8:e130-e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Harris PA, Taylor R, Thielke R, et al. Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42:377–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bronfenbrenner U. Ecological systems theory. Ann Child Dev. 1989;6:187–249. [Google Scholar]
- 22.Monahan PO, Shacham E, Reece M, et al. Validity/reliability of PHQ-9 and PHQ-2 depression scales among adults living with HIV/AIDS in Western Kenya. J Gen Intern Med. 2009;24:189–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kaai S, Bullock S, Sarna A, et al. Perceived stigma among patients receiving antiretroviral treatment: a prospective randomised trial comparing an m-DOT strategy with standard-of-care in Kenya. SAHARA J. 2010;7:62–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rosenberg M. Conceiving the Self. New York: Basic Books; 1979. [Google Scholar]
- 25.Cherpitel CJ, Ye Y, Bond J, et al. Cross-national performance of the RAPS4/RAPS4-QF for tolerance and heavy drinking: data from 13 countries. J Stud Alcohol . 2005;66:428–432. [DOI] [PubMed] [Google Scholar]
- 26.Horne R, Weinman J. Patients' beliefs about prescribed medicines and their role in adherence to treatment in chronic physical illness. J Psychosom Res. 1999;47:555–567. [DOI] [PubMed] [Google Scholar]
- 27.Brewer NT, Weinstein ND, Cuite CL, et al. Risk perceptions and their relation to risk behavior. Ann Behav Med. 2004;27:125–130. [DOI] [PubMed] [Google Scholar]
- 28.Eckel CC, Grossman PJ. Forecasting risk attitudes: an experimental study using actual and forecast gamble choices. J Econ Behav Org. 2008;68:1–17. [Google Scholar]
- 29.Young CR, Kaida A, Kabakyenga J, et al. Prevalence and correlates of physical and sexual intimate partner violence among women living with HIV in Uganda. PLoS One. 2018;13:e0202992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pulerwitz J, Mathur S, Woznica D. How empowered are girls/young women in their sexual relationships? Relationship power, HIV risk, and partner violence in Kenya. PLoS One. 2018;13:e0199733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zheng JH, Rower C, McAllister K, et al. Application of an intracellular assay for determination of tenofovir-diphosphate and emtricitabine-triphosphate from erythrocytes using dried blood spots. J Pharm Biomed Anal. 2016;122:16–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Castillo-Mancilla JR, Haberer JE. Adherence measurements in HIV: new advancements in pharmacologic methods and real-time monitoring. Curr HIV/AIDS Rep. 2018;15:49–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Castillo-Mancilla J, Zheng G, Rower JE, et al. Tenofovir, emtricitabine, and tenofovir disphosphate in dried blood spots for determining recent and cumulative drug exposure. AIDS Res Hum Retrovir. 2013;29:384–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cottrell ML, Yang KH, Prince HM, et al. A translational pharmacology approach to predicting outcomes of preexposure prophylaxis Against HIV in men and women using tenofovir disoproxil fumarate with or without emtricitabine. J Infect Dis. 2016;214:55–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Anderson PL, Glidden DV, Liu A, et al. Emtricitabine-tenofovir concentrations and pre-exposure prophylaxis efficacy in men who have sex with men. Sci Transl Med. 2012;4:151ra125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Carroll JJ, Heffron R, Mugo N, et al. Perceived risk among human immunodeficiency virus serodiscordant couples in east Africa taking oral pre-exposure prophylaxis. Sex Transm Dis. 2016;43:471–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bradley H, Tsui A, Hindin M, et al. Developing scales to measure perceived HIV risk and vulnerability among Ethiopian women testing for HIV. AIDS Care. 2011;23:1043–1052. [DOI] [PubMed] [Google Scholar]
- 38.Koss CA, Ayieko J, Mwangwa F, et al. Early adopters of human immunodeficiency virus preexposure prophylaxis in a population-based combination prevention study in rural Kenya and Uganda. Clin Infect Dis. 2018;67:1853–1860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gilkey MB, Marcus JL, Garrell JM, et al. Using HIV risk prediction tools to identify candidates for pre-exposure prophylaxis: perspectives from patients and primary care providers. AIDS Patient Care STDS. 2019;33:372–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Giovenco D, Pettifor A, MacPhail C, et al. Assessing risk for HIV infection among adolescent girls in South Africa: an evaluation of the VOICE risk score (HPTN 068). J Int AIDS Soc. 2019;22:e25359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Evidence for Contraceptive Options, HIV Outcomes Trial Consortium. HIV incidence among women using intramuscular depot medroxyprogesterone acetate, a copper intrauterine device, or a levonorgestrel implant for contraception: a randomised, multicentre, open-label trial. Lancet. 2019;394:303–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Abdool Karim Q, Baxter C, Birx D. Prevention of HIV in adolescent girls and young women: key to an AIDS-free generation. J Acquir Immune Defic Syndr. 2017;75(suppl 1):S17–s26. [DOI] [PubMed] [Google Scholar]
- 43.Spinelli MA, Haberer JE, Chai PR, et al. Approaches to objectively measure antiretroviral medication adherence and drive adherence interventions. Curr HIV/AIDS Rep. 2020;17:301–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Drain P, Ngure K, Mugo N, et al. Testing a real-time tenofovir urine adherence assay for monitoring and providing feedback to preexposure prophylaxis in Kenya (PUMA): protocol for a pilot randomized controlled trial. JMIR Res Protoc. 2020;9:e15029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pyra M, Bukusi E, Mugo N, et al. Comparison of TFV-DP and wisepill adherence among young women using PrEP. Conference on Retroviruses and Opportunistic Infections. 2020; Virtual. Abstract 1029.
- 46.Rosen EP, Thompson CG, Bokhart MT, et al. Analysis of antiretrovirals in single hair strands for evaluation of drug adherence with infrared-matrix-assisted laser desorption electrospray ionization mass spectrometry imaging. Anal Chem . 2016;88:1336–1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Delany-Moretlwe S, Hughes J, Bock P, et al. Long Acting Injectable Cabotegravir Is Safe and Effective in Preventing HIV Infection in Cisgender Women: Interim Results from HPTN 084. HIV Research for Prevention; 2020; Virtual. Abstract HY01.02.


