Abstract
Background
Inappropriate use of antimicrobials is a key factor increasing antimicrobial resistance, a major global public health problem including in South Africa. Key drivers include antibiotics being dispensed without a prescription.
Objectives
To determine the accessibility of antibiotics without a prescription in community pharmacies in urban areas in South Africa and determine whether counselling was provided when antibiotics were dispensed.
Patients and methods
Prospective, observational study, employing simulated patients (SPs), presenting with upper respiratory tract infections (URTIs) and urinary tract infections (UTIs), undertaken to establish whether antibiotics can be obtained without a valid prescription in South Africa. This pilot study was conducted in privately owned (n = 20) and corporate (franchised, n = 14) community pharmacies in three regions in Gauteng Province.
Results
Antibiotics were sold in privately owned pharmacies without a prescription in 80% (16/20) of cases while no antibiotics were dispensed in corporate (franchised) pharmacies. Of the 16 pharmacies selling antibiotics without a prescription, pharmacist assistants were involved in 37.5% (n = 6) and counselling was not provided to 19% of SPs. Ciprofloxacin (42.9%) and metronidazole (28.6%) were the most common antibiotics dispensed. No antibiotics were dispensed for URTIs, only UTIs.
Conclusions
Dispensing antibiotics without prescriptions can be common among privately owned pharmacies in urban areas in South Africa. Corporate pharmacies, which probably have a greater income, appear to follow current legislation banning such activities. To limit selling with no prescription, community pharmacists and assistants especially in urban areas should be educated on appropriate patient care and legal requirements, with dispensing electronically monitored.
Introduction
Antibiotic sales without prescriptions have been observed across many countries especially low- and middle-income countries (LMICs).1–8 In Sub-Saharan Africa, we have seen in Zambia that all community pharmacists surveyed dispense antibiotics without a prescription,9 with dispensing of antibiotics without a prescription also common in Tanzania and Uganda, driven by limited confidence with the public healthcare system, easy access and porous medicine supply chains.1,10 This is of major concern as inappropriate dispensing of antimicrobials in community pharmacies and drug stores can account for up to 93% to 100% of all dispensed antibiotics among LMICs.2,5,9,11,12
Such high inappropriate utilization of antibiotics exacerbates inappropriate use increasing antimicrobial resistance (AMR) and associated morbidity, mortality and costs.13–17 The World Bank (2017) believes even conservatively by 2030, the loss on world output due to AMR could exceed US$1 trillion annually, and potentially up to US$3.4 trillion annually, unless key issues are addressed.18 This is equivalent to 3.8% of annual gross domestic product.18 These concerns have resulted in multiple activities especially in LMICs,13,19–23 and include the development and monitoring of national action plans (NAP) to reduce AMR.24–28
Published studies have suggested there are a number of reasons behind the dispensing of antibiotics without a prescription. These include greater accessibility and convenience of community pharmacists and drug stores; no co-payments to see a pharmacist unlike physicians in a number of countries; reduced travel costs and time off work; patient trust in pharmacists; patient pressure on pharmacists; the business orientation of pharmacists; porous supply chains; and that community pharmacists may be the principal healthcare person available to treat patients, especially in rural areas.1,7,8,11–13,29–33 However, concerns have been raised regarding the knowledge of pharmacists and their assistants regarding antibiotics and AMR in LMICs.1,6,13,34,35
Several strategies have been instigated by governments and others worldwide to reduce inappropriate dispensing of antibiotics without a prescription, potentially made worse by the recent COVID-19 pandemic.13,36–38 These range from educational initiatives through to enforcing legislation through fines and other activities, or a combination of these (Table 1).13,39–43 However, the monitoring of any regulations is particularly important to enhance their impact, building on the findings in other disease areas.44
Table 1.
Examples of initiatives to reduce self-purchasing of antibiotics in pharmacies predominantly in LMICs
| Activity | Outcome |
|---|---|
| Educational and other activities including regulations | |
| Chile68,69 | • Chile was one of the first countries in Latin America to introduce greater enforcement of the law banning the purchasing of antibiotics without a prescription—enhanced by antibiotics being removed from the list of medicines having sales incentives in pharmacies |
| • Antimicrobial consumption decreased from 12.3 DID before the intervention to 8.5 DID just after the enforcement (in 2000)—helped by public information campaigns before and during the implementation of enhanced enforcement of the regulations | |
| • However, there has been a slow increase in antimicrobial utilization since 2002 suggesting the impact of introduced regulations diminish over time unless pharmacists’ activities are continually monitored and further initiatives are introduced when pertinent | |
| China33 | • Multiple initiatives in Shaanxi Province in China—including stricter regulations for dispensing antibiotics, improving pharmacists’ education, a qualified pharmacist being present to dispense antibiotics and increased frequency of unannounced pharmacy inspections and punishments for misuse—decreased antibiotic sales between 2011 and 2017 |
| • For SPs acting as caregivers for a 5-year-old child with diarrhoea, dispensing of antibiotics without a prescription was reduced from 72.3% to 50.2% (P < 0.0001) | |
| • For SPs acting as a friend of a 20-year-male college student with a URTI, dispensing without an antibiotic was down from 95.8% to 69.5% (P < 0.0001) | |
| • Three demand levels were used to try and encourage antibiotics to be dispensed with the second level being ‘can you give me some antibiotics’ and the third level being ‘I would like some amoxicillin or cephalosporins’ | |
| Kenya40,58 | • Among pharmacists linked to the University of Nairobi, 94.1% of antibiotics were dispensed with a valid prescription with limited dispensing without a prescription |
| • There was no dispensing of antibiotics for ARIs, with OTC medicines such as cold and cough syrups and lozenges typically dispensed | |
| • There was no dispensing of either antimalarials or antibiotics without a prescription during a recent study conducted during the COVID-19 pandemic | |
| Namibia43,64,70 | • In a survey involving 100 households in Namibia, typically cold/flu medication, paracetamol and decongestants were used to treat adults or their children with ARIs including for common colds and influenza—helped by education among pharmacists, regulations banning the self-purchasing of antibiotics and the regular monitoring of community pharmacy activities |
| • There was a similar situation during the COVID-19 pandemic with no change in the utilization of antimicrobials compared with other African countries including Nigeria. This was helped by proactivity among pharmacists, knowledge regarding the current regulations banning self-purchasing and regular monitoring of pharmacy activities | |
| Republic of Srpska41,71 | • Education of pharmacists together with the production of guidelines including those for ARIs |
| • This coupled with greater enforcement of the regulations of guidelines banning the dispensing of antibiotics without a prescription resulted in the purchasing of antibiotics without a prescription for self-diagnosed URTIs significantly decreasing from 58% of requests to 18.5% for SPs | |
| • Encouragingly, the most common reason for not dispensing to SPs was that antibiotics cannot be dispensed without a prescription | |
| India, Malaysia and Vietnam36,72,73 | • There have been ongoing educational and other initiatives in recent years in India, Malaysia and Vietnam to try and reduce unnecessary dispensing of antimicrobials without a prescription |
| • These initiatives seem to be working, with no change or a decrease in the dispensing of antimicrobials among 83.3% to 100% of pharmacies surveyed in Malaysia and Vietnam in the initial months following the start of the COVID-19 pandemic despite the hype and concerns generally with increasing use of antibiotics. However, this may not always be the case | |
| • In India—no change in up to 57.7% of pharmacists surveyed | |
| Thailand74 | • Principally education involving a multidisciplinary intervention among grocery stores in a rural province in Thailand using trained community leaders |
| • There were 87% fewer antibiotics available postintervention compared with preintervention | |
| • Grocery stores in the control group saw only an 8% reduction in antibiotic availability between the two time periods | |
| Regulations/enforcement | |
| Brazil—private and public pharmacies75–77 | • In their study, Moura et al. (2015)75 found no difference in antibiotic utilization among public pharmacies between 2008 and 2012 where there had always been restrictions on the dispensing of antibiotics with a prescription and it is generally impossible for pharmacists to sell antibiotics without a prescription |
| • With respect to private pharmacists, Moura et al. (2015)75 showed a decrease in antibiotic use of 1.87 DID (P < 0.001) immediately after restrictions banning the sales of antibiotics without a prescription (2008 to 2012), with a greater decrease in the more developed regions as well as in the State Capitals | |
| • Lopes-Junior et al. (2015)76 also found that sales of amoxicillin (commonly sold antibiotic) among private pharmacies fell by approximately 30% post-legislation despite a general growth in the pharmaceutical market, with decreased sales of other popular antibiotics including tetracyclines (30.5% decrease), sulphonamides (28.5% decrease) and macrolides (25% decrease) | |
| • Mattos et al. (2017)77 also documented a decrease in the dispensing of cephalosporins (−19.4%), quinolones (−12.7%) and aminopenicillins (−11.1%) following restrictions in private pharmacies | |
| Colombia69,78 | • The initial enforcement of the law in 2005 had a modest impact on overall sales in the first three years (−1.00 DID) |
| • However, a follow-up study five years after implementation found a high number of pharmacies (80.3%) were still not complying with the law due to lax monitoring. This prompted calls for greater enforcement of the law | |
| Mexico68,79 | • The government implemented policies in 2010 to enforce existing laws whereby antibiotics could only be dispensed to patients presenting with a prescription. As part of this, the regulations require antibiotic prescriptions to be retained and registered in pharmacies, with fines imposed for non-compliance |
| • Antibiotic utilization decreased by 22.9% between 2007 and 2012, with the trend accelerating after greater enforcement of the legislation | |
| • There was also an appreciable seasonal reduction in the use of penicillins in Mexico after greater enforcement of the legislation | |
| Saudi Arabia42 | • The regulations and law concerning the purchasing of antibiotics without a prescription were enforced from May 2018 onwards alongside fines in Saudi Arabia |
| • 70.7% of pharmacies taking part in the study reported that purchasing of antibiotics without a prescription was common before the updated regulations and fines, with 96.6% and 87.7% of participating pharmacies dispensing antibiotics to SPs for pharyngitis and UTIs respectively | |
| • Following law enforcement and fines, only 12.9% of community pharmacists reported that the purchasing of antibiotics without a prescription was still a common practice | |
| • In addition, only 12.1% of pharmacies dispensed an antibiotic to SPs—and typically only at the third level of demand (57.1%), i.e. the SP directly asking for an antibiotic. This compares with 85.7% at the first level, i.e. just asking for something to relieve the symptoms, prior to the changes in regulations and fines | |
| • Similarly for UTIs, only 5.2% dispensed antibiotics without a prescription and typically only at the third level (66.7%). This compared with 74% at the first level prior to the changes | |
| Venezuela69 | • The government implemented policies to try and limit the dispensing of three antibiotic groups without a prescription |
| • However, there were no public awareness campaigns, and the ‘enforcement’ was only via government publications with no follow-up of the regulations | |
| • This resulted in no decrease of antibiotic utilization levels—in fact the opposite with an increase | |
Currently within South Africa, over 80% of the population are treated in the public healthcare system, which is a universal healthcare system, with the remainder treated in the private healthcare sector.45–47 Medicines contained within the South African Essential Medicine list are available on prescription without co-payments in the public system, and in rural areas of South Africa patients typically access their medicines free of charge through public healthcare facilities (PHCs).7,45 However, there can be medicine shortages in the public healthcare system in South Africa, and in many PHCs patients have to wait a long time to see either a nurse or physician as PHC facilities are under-resourced.48,49 This could be an issue for the economically disadvantaged if there are long waiting times resulting in these patients potentially seeking treatment elsewhere to ensure daily wages.47 This includes community pharmacists, who are typically open longer hours and with limited waiting times.48 However, this will be subject to 100% co-payment unless the patient has private insurance.
There are currently seven categories of pharmaceutical institutions (practice types) recorded by the South African Pharmacy Council. The majority (68.3%) are community pharmacies, all in the private sector. However, South Africa currently faces a shortage of healthcare professionals (HCPs), with maldistribution of HCPs between the public and private sectors and between urban and rural settings.50
South African regulations currently ban the dispensing of antibiotics in pharmacies without a prescription.51 Consequently, antibiotics should only be dispensed with a valid prescription signed by an authorized prescriber. The South African government requires that pharmacies operate under a license and the full-time management and supervision of a registered pharmacist. In addition to playing a central community education role advising patients on the correct use of medicines, pharmacists are also expected to assume a leadership role in the rational use of medicines including antibiotics. Two sets of ethical and professional conduct codes issued by the South African Pharmacy Council subsequently serve to guide pharmacists. They emphasize a practice philosophy, patient respect and the pharmacist’s role within a multidisciplinary team. Pharmacists may sell a greater or lesser quantity of antibiotics than prescribed. This though cannot be less or greater than 5% of that specified in the prescription.
However, we are unaware of the impact of these combined regulations and codes on the self-purchasing of antibiotics among different pharmacy types. Consequently, we sought to address this building on the findings of Do et al. (2021).7 The South African study site in this study was rural.7,45 Consequently, it may not reflect the situation in urban areas where patients can readily access private pharmacies. We believe this is particularly important to assess at this time with planned reforms to allow pharmacists to prescribe and dispense medicines for HIV, including pre-exposure prophylaxis and agreed first-line antiretroviral medicines without a doctor’s prescription.52
In view of the paucity of data, especially in urban situations, and the ongoing challenges with accessing PHCs, we employed simulated patients (SPs) to establish whether antibiotics can be obtained without a valid prescription among both privately owned and corporate (franchise) community pharmacies in selected urban areas in one South African province.
Methods
Study design and period
We undertook a prospective, observational pilot study employing two SP scenarios to establish whether antibiotics can be obtained without a valid prescription among community pharmacies in urban, suburban and township areas in Gauteng Province, South Africa. The pharmacies consisted of both privately owned (independent sole proprietor) and corporate (franchised) pharmacies.
Study population and setting
A randomized sample of 34 pharmacies (14 urban, 14 suburban and 6 township) from one province was stratified by urban, suburban and township areas and by richer or poorer socioeconomic zones. Privately owned pharmacies (also known as independent pharmacies) are found mostly in the rural areas and corporate pharmacies in urban and suburban areas. In 2014 there were 914 pharmacies registered in South Africa, of which 472 were independent pharmacies, 290 were corporate pharmacies and the remainder registered as inactive. Consequently, this ‘pilot study’ study sampled 3.7% of the total pharmacy population.
The pharmacy personnel were identified according to their name and designation, as displayed on the nametag they were wearing and/or the display at the dispensary showing personnel on duty. In cases where the pharmacy personnel could not be identified, it was noted as such on the data collection sheet.
Data collection and training
Two different clinical cases were presented; each case type was always presented by the same SP (Table 2). The two scenarios focused on the authentic portrayal of patients in a pharmacy that require urgent assistance building on the considerable experience of the co-authors in pharmacy practice and undertaking SP research combined with previous studies with three levels of demand.33,41,42
Table 2.
Details of the two scenarios and the demand levels
| Content of the scenarios, backstories and demand levels | |
|---|---|
| SPs/scenarios | The scenarios were established to outline the name, age, gender and home address of the patient and the symptoms s/he presented with |
| The two SPs were given an opening line as well as a brief background regarding the patients they would portray | |
| To reduce the possibility of SPs feeling uncomfortable or being recognized: | |
| • They were accompanied by one of the researchers (R.N.M.) who acted as a niece in the case of a UTI and as a girlfriend for URTIs to add validity to the characters the SPs were portraying | |
| • The researcher, in her capacity as niece and/or girlfriend, would draw on her pharmaceutical knowledge in case the SPs were confronted by questions they cannot answer | |
| The researcher also accompanied the SPs in order to note any extra information, for example, if there is a name on the wall of the pharmacist on duty and the staff | |
| UTIs | |
| backstory | The ‘patient’ is aged 41 years and has come from Thabazimbi for her grandmother’s 80th birthday |
| She is married to a man who is 45 years old, who is the sole breadwinner and who is very traditional and very strict | |
| The female ‘patient’ contracted her UTI from using a public bathroom and fears that her husband will think she had contracted an STI through conducting an extramarital affair during the time she was away | |
| The fear exists that he will punish her by discontinuing to support her and their three children | |
| In addition, since the aunt’s husband also funds the niece’s studies, as well as most of her living expenses, the niece also has a vested interest in her ‘aunt’s’ recovery | |
| three levels of demand for antibiotics | • Level 1: the aunt needs to get better before returning home as she knows her husband will think that she slept around and contracted an STI—especially because she was visiting in Gauteng—to validate ‘I need help to alleviate these symptoms.’ |
| • Level 2: if she does not get better before returning home, her marriage will fall apart—to validate ‘Haven’t you got something stronger?’ | |
| • Level 3: she is very scared that her husband will leave. She grew up poor and she is so afraid that she will end in poverty again—especially due to the fact that her niece has not completed her studies yet and will not be able to look after her yet. She is also very scared that her husband will take the children—to further emphasize ‘I need antibiotics.’ | |
| URTIs | |
| backstory | The backstory was approached in the same way |
| The SP’s urgency lay in the fact that he would need to return to work within a week’s time | |
| His concern is that his coworkers would assume that he has AIDS-related TB and he might lose his job—losing his job would mean that he would also lose the possibility of saving for his envisaged wedding with his girlfriend | |
| three levels of demand for antibiotics | This was similar to UTIs, e.g.: |
| • Level 1: asking for something that will help alleviate the symptoms | |
| • Level 2: if the first level of demand was not effective in obtaining an antibiotic, the second level of demand was asking for something stronger | |
| • Level 3: if neither the first nor second level of demand was effective in obtaining an antibiotic, the SP asked explicitly for an antibiotic to help clear up their URTI | |
STI, sexually transmitted infection; URTI, upper respiratory tract infection; UTI, urinary tract infection.
The SPs were trained with the aid of one of the supervisors of the study (L.S.) with the assistance of the other supervisors (N.S. and E.B.) over 2 days to obtain standardization and reproducibility of the simulated disease. The two SPs who participated in the study had many years of experience acting as patients in tests for medical students and they also ‘look the part’ aiding their believability. Day 1 of the workshop focused on acting skills and Day 2 was the rehearsal of the simulated disease. The same SP and researchers were used in every study site to collect the data to enhance the robustness of the findings.
They memorized prepared responses for when the pharmacist asked about aspects relating to the infection that was being simulated.
In all cases, the SPs were instructed to be very polite and entered the pharmacies presenting with one of two conditions: urinary tract infections (UTIs) or upper respiratory tract infections (URTIs). There were both male and female SPs and they both presented either a URTI or UTI, but not both, at the same pharmacy (Tables 2 and 3). More focus was placed on the integration of a ‘backstory’ for the SPs as the backstories serve to convey a realistic sense of urgency regarding the need for medication in line with the recommendations of Amaratunge et al. (2021).53 Issues with sexually transmitted diseases are a real concern in Southern Africa with considerably higher prevalence rates seen in PHCs compared with European and other countries.54,55 The same is true for patients with URTIs with high rates of TB/HIV co-infections.56
Table 3.
Symptoms presented by the SP during the consultation
| Scenario 1: Urinary tract infection | Scenario 2: Upper respiratory tract infection |
|---|---|
| 1. Discomfort on urination with a burning sensation | 1. Cough for about 2 weeks |
| 2. Frequent urination | 2. Productive cough |
| 3. Foul smelling urine | 3. Sputum is yellowish |
| 4. Blood in urine | 4. Fatigue |
| 5. Dark urine | 5. Slight fever and chills |
| 6. Persistent urge to urinate | 6. Chest discomfort |
| 7. Vaginal irritation |
These parts of the role portrayal need not be verbalized but help the verbal and non-verbal communication process to make the portrayal realistic and believable. In addition, the SPs visited two pharmacies prior to the full study in which some of the researchers and authors of the study accompanied the SPs as part of the validation process,53 to enhance consistency and believability in the full study.
The SPs were accompanied by one of the researchers acting as niece in the case of a UTI and as girlfriend for URTIs. The researcher, in her capacity as niece and/or girlfriend, could also draw on her pharmaceutical knowledge if needed to add robustness and validity to the SP research.53
The SPs neither asked for a particular antibiotic nor insisted if an antibiotic was refused after Level 3 in order not to influence the pharmacist’s activity or arouse suspicion (Table 2).
Three levels of demand (Table 2) were presented in each of the two cases. Three levels were included as we believed this would better reflect real life, with such approaches used before in similar circumstances.33,42
If the pharmacist refused to sell an antibiotic without a prescription at any level, the reasons for the refusal were asked. A response was administrative if the reason given only referred to the regulations or law, i.e. the antibiotic cannot be sold without a prescription. A response was health related if the pharmacist expressed concern that it was not good for the patient’s health to sell them this medication, that antibiotics cannot be given for viral infections or that selling an antibiotic in this case could lead to the spread of resistance. The responses were subsequently collated into key themes.
The data were transferred from the data collection sheet onto a Microsoft Excel™ spreadsheet. The data captured was descriptively summarized and expressed as percentages using Microsoft Excel™. Descriptive statistical analyses of the results were undertaken. The qualitative data related to the open-ended question, i.e. ‘reasons for not dispensing antibiotics’, were thematically coded manually.
Ethical considerations
Permission granted by Sefako Makgatho University Research Ethics Committee (SMUREC/P/110/2019: PG) ensured that the need for informed consent on the research project and/or sub-study level was not necessary for the community pharmacies included in the study. The researcher, SPs and research supervisors signed a consent that no community pharmacy names would be published after the study.
Results
A total of 34 community pharmacies were visited. Of these, 14 (14/34, 41%) were corporately owned (franchised) and 20 (20/34, 59%) were privately owned.
Oral antibiotics were sold without a prescription in 16 (16/34, 47%) of the community pharmacies in which a UTI and URTI were presented by two different SPs in the different pharmacies (Table 4). Antibiotics were only sold in private pharmacies, which were mostly found in townships. No corporate (franchised) pharmacies dispensed an antibiotic without a prescription, pharmacy personnel only recommended over-the-counter (OTC) medication for both UTIs and URTIs.
Table 4.
Sale of antibiotics according to the clinical scenario and level of demand
| Level of demand | Clinical case presented; antibiotic obtained in n (%) of pharmacies visited |
||
|---|---|---|---|
| UTI (n = 34) | URTI (n = 34) | Total | |
| 1. (Can you give me something to alleviate the symptoms of the infection?) | 9 (26.4) | 0 | 9 |
| 2. (Can’t you give me something stronger?) | 0 | 0 | 0 |
| 3. (I would like an antibiotic.) | 7 (20.5) | 0 | 7 |
URTI, upper respiratory tract infection; UTI, urinary tract infection.
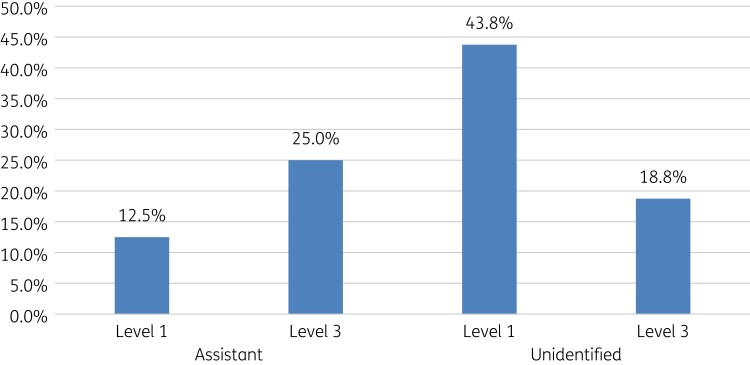
Among these 16 community pharmacies where antibiotics were dispensed without a prescription, pharmacist assistants in 6 of these (37.5%, 6/16) dispensed antibiotics to SPs with UTIs, and unidentified staff who assisted the SPs dispensed antibiotics in 10 (62.5%, 10/16). Overall, 18 pharmacist assistants were involved among the community pharmacies visited. Antibiotics were not issued for URTIs in any visited pharmacies, with antibiotics only issued for SPs presenting with UTIs among community pharmacies (Table 4).
Antibiotics were dispensed based on level of demand. Pharmacist assistants typically dispensed antibiotics more in Demand Level 3 (Table 2), whereas unidentified pharmacy staff dispensed more antibiotics on Level 1 for SPs with UTIs (Figure 1).
Figure 1.
Response to demand level among SPs presenting with UTIs in community pharmacies.
Antibiotics were not dispensed in 18 (53%) pharmacies. The pharmacy personnel stated various reasons why they could not dispense antibiotics. These included that a prescription is needed before an antibiotic can be dispensed (66.7%, 12/18), and the SP needs to visit the nurse on the premise for a urinary test first (33.3%) before an antibiotic can be dispensed for a UTI. Recommended OTC medicines for URTIs included paracetamol, codeine, acetylcysteine, doxylamine/diphenhydramine/diphenylpyraline/cetirizine, and guaifenesin in cold and cough remedies. Recommended OTC medicines for UTIs included sodium bicarbonate/citric acid/tartaric acid and Hibiscus sabdariffa.
Ciprofloxacin (42.9%) and metronidazole (28.6%) were the antibiotics that were mostly sold in community pharmacies without a prescription for patients with UTIs (Figure 2). In a minority of situations (25%), more than one antibiotic was dispensed for a UTI.
Figure 2.
Names of antibiotics that were dispensed without a prescription.
Pharmacy personnel in three (3/16, 18.8%) of the pharmacies dispensing antibiotics did not provide counselling to the SP when dispensing. They only gave them antibiotics and told them the cost. The remaining SP patients did receive some form of counselling and information (Table 5).
Table 5.
Type of counselling and frequency received by SPs when dispensed an antibiotic
| Type of counselling | Number |
|---|---|
| Explained how often to take the antibiotic | 13 |
| Explained how long the antibiotic should be taken | 8 |
| Asked patient about other symptoms | 12 |
| Asked patient whether she might be pregnant | 3 |
Of concern though is that no pharmacy staff member recommended that the patient must go and see a physician if there was no improvement in their condition. In addition, no pharmacy staff member asked the SP about any drug (penicillin) allergy when dispensing an antibiotic.
Discussion
The results of this pilot study demonstrate that antibiotics can be sold without a prescription in South Africa, which is a concern given the current regulations and concerns with the extent of AMR within South Africa.57 This contrasts with the findings of Do et al. (2021)7 in South Africa with their selective population.
This may be because, to the best of our knowledge, this is the first study conducted in South Africa to explore differences in community pharmacy behaviour for the management of both URTIs and UTIs. We appreciate that we used SPs and not actual patients. However, we are confident of the findings in view of the selection of the SPs, their training, the backstories and accompanying personnel in case of any problems (Table 2).
Encouragingly, no pharmacy personnel dispensed an antibiotic without a prescription for a URTI. This is welcomed as URTIs are the most prevalent infections in ambulatory care and are typically viral in origin.4 We have seen a similar situation in Kenya and Namibia in pharmacies involving trained pharmacists and those aware of the current laws banning the dispensing of medicines without a prescription, which recently included patients with suspected or actual COVID-19.40,43,58 However, we were concerned that antibiotics were dispensed for patients with UTIs. This may reflect greater sympathy for the SP presenting with these symptoms (Table 2), especially with the implications for her future and that of her niece.
Interestingly, our study showed that the location of the pharmacy does appear to play a key role in antibiotics dispensing for patients with UTIs. The findings that community pharmacy personnel are more likely to dispense an antibiotic for UTIs than URTIs is similar to the findings of Llor and Cots (2009).59 However, they are different to the findings of Alrasheedy et al. (2020)42 before changes in the law coupled with more aggressive monitoring and fining of pharmacists (Table 1).
Privately owned pharmacies in townships appeared more likely to dispense antibiotics for UTIs without a prescription (Table 4), reflecting the fact that adults attending these pharmacies may do so out of convenience and perhaps greater anonymity. In addition, we are aware that pharmacists are often the first point of contact within healthcare systems when patients experience illness, especially in rural areas in LMICs, and are a trusted source of information and treatment.31,41 For many patients in LMICs, the lack of the necessary resources and time to consult a physician makes community pharmacists their first (and, at times, only) point of contact within healthcare systems, with patient pressure adding to the potential for dispensing an antibiotic without a prescription.11–13,60,61
However, whilst patients in a number of LMICs, including Sub-Saharan African countries, may benefit from increased access to antibiotics from pharmacies there are drawbacks. This includes potential misdiagnosis of possible bacterial infections enhanced during the COVID-19 pandemic.36,62 In addition, there are concerns with allergies if not properly discussed, or courses of treatment not properly explained (Table 5) as well as increases in adverse drug-reactions and AMR.
There are a number of approaches that key stakeholder groups could undertake to reduce the extent of purchasing of antibiotics without a prescription (Table 1). Potential ways forward in the short term in South Africa could include the rapid development and dissemination of treatment guidelines coupled with increased monitoring of pharmacies especially in urban areas, building on the findings in the Republic of Srpska (Table 1).41,63 Alongside this, and also in the medium term, there could be improved training of pharmacists and their personnel regarding antibiotics, AMR and the regulations, building on the experiences in Kenya and Namibia (Table 1).40,43,64 There could also be increased monitoring of utilization and dispensing rates through mobile technologies and other approaches to encourage greater compliance with the regulations.12,33 If this does not produce the desired results, potentially fining pharmacists, as seen in Saudi Arabia, in addition to other measures, has been successful in other countries.13,39,42 Whatever approaches are chosen, these need to be followed through for maximum impact. The situation may be different in rural areas in Sub-Saharan African where there are limited facilities to access physicians. In this case, it may be necessary to agree a limited list of possible antibiotics to be dispensed based on the WHO AWaRe list, similar to the concept in Tanzania for infectious diseases among different pharmacies.65–67
We are aware that there are a number of limitations with this study. As mentioned, these include the fact that we used simulated patients rather than actual patients. However, this is a well-recognized technique to ascertain dispensing behaviour within pharmacies. In addition, we tried to make the backstories as realistic as possible for the situation in South Africa, with back up from one of the researchers in case of difficulties. We are also aware that we only approached 34 community pharmacies. However, we believe our findings are robust based on our approach providing guidance for the future.
Conclusions
This study shows that dispensing antibiotics without prescription for managing UTIs appears to be common practice among privately owned pharmacies (even with different ownership structures) in this region of South Africa. This needs to be addressed, with the findings from this study potentially used to design interventions to reduce future inappropriate dispensing of antibiotics without a prescription. Pertinent interventions could include greater education and dissemination of guidelines as well as greater monitoring of activities within pharmacies. We will continue to monitor the situation.
Acknowledgements
We would like to acknowledge the simulated patients that contributed greatly to the success of the project.
Funding
The analysis and write-up of the paper was self-funded by the authors. The School of Pharmacy Sefako Makgatho Health Sciences Publication Fund covered the cost of the actors who were the simulated patients.
Transparency declarations
None to declare. Additional data are available from the authors upon reasonable request.
References
- 1. Ndaki PM, Mushi MF, Mwanga JRet al. . Dispensing antibiotics without prescription at community pharmacies and accredited drug dispensing outlets in Tanzania: a cross-sectional study. Antibiotics 2021; 10: 1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Nepal G, Bhatta S. Self-medication with antibiotics in WHO Southeast Asian Region: a systematic review. Cureus 2018; 10: e2428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Auta A, Hadi MA, Oga Eet al. . Global access to antibiotics without prescription in community pharmacies: a systematic review and meta-analysis. J Infect 2019; 78: 8–18. [DOI] [PubMed] [Google Scholar]
- 4. Godman B, Haque M, McKimm Jet al. . Ongoing strategies to improve the management of upper respiratory tract infections and reduce inappropriate antibiotic use particularly among lower and middle-income countries: findings and implications for the future. Curr Med Res Opin 2020; 36: 301–27. [DOI] [PubMed] [Google Scholar]
- 5. Sakeena MHF, Bennett AA, McLachlan AJ. Non-prescription sales of antimicrobial agents at community pharmacies in developing countries: a systematic review. Int J Antimicrob Agents 2018; 52: 771–82. [DOI] [PubMed] [Google Scholar]
- 6. Belachew SA, Hall L, Selvey LA. Non-prescription dispensing of antibiotic agents among community drug retail outlets in Sub-Saharan African countries: a systematic review and meta-analysis. Antimicrob Resist Infect Control 2021; 10: 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Do NTT, Vu HTL, Nguyen CTKet al. . Community-based antibiotic access and use in six low-income and middle-income countries: a mixed-method approach. Lancet Glob Health 2021; 9: e610–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Batista AD, Rodrigues DA, Figueiras Aet al. . Antibiotic dispensation without a prescription worldwide: a systematic review. Antibiotics 2020; 9: 786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kalungia AC, Burger J, Godman Bet al. . Non-prescription sale and dispensing of antibiotics in community pharmacies in Zambia. Exp Rev Anti Infect Ther 2016; 14: 1215–23. [DOI] [PubMed] [Google Scholar]
- 10. Kibuule D, Kagoya HR, Godman B. Antibiotic use in acute respiratory infections in under-fives in Uganda: findings and implications. Exp Rev Anti Infect Ther 2016; 14: 863–72. [DOI] [PubMed] [Google Scholar]
- 11. Torres NF, Chibi B, Middleton LEet al. . Evidence of factors influencing self-medication with antibiotics in low and middle-income countries: a systematic scoping review. Public Health 2019; 168: 92–101. [DOI] [PubMed] [Google Scholar]
- 12. Kalungia A, Godman B. Implications of non-prescription antibiotic sales in China. Lancet Infect Dis 2019; 19: 1272–3. [DOI] [PubMed] [Google Scholar]
- 13. Godman B, Egwuenu A, Haque Met al. . Strategies to improve antimicrobial utilization with a special focus on developing countries. Life 2021; 11: 528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Cassini A, Hogberg LD, Plachouras Det al. . Attributable deaths and disability-adjusted life-years caused by infections with antibiotic-resistant bacteria in the EU and the European Economic Area in 2015: a population-level modelling analysis. Lancet Infect Dis 2019; 19: 56–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hofer U. The cost of antimicrobial resistance. Nat Rev Microbiol 2019; 17: 3. [DOI] [PubMed] [Google Scholar]
- 16. Dadgostar P. Antimicrobial resistance: implications and costs. Infect Drug Resist 2019; 12: 3903–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Founou RC, Founou LL, Essack SY. Clinical and economic impact of antibiotic resistance in developing countries: a systematic review and meta-analysis. PLoS One 2017; 12: e0189621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. The World Bank . Final Report—Drug-Resistant Infections: A Threat to Our Economic Future. 2017. http://documents1.worldbank.org/curated/en/323311493396993758/pdf/final-report.pdf.
- 19. WHO . Antimicrobial Resistance. 2018. http://www.who.int/news-room/fact-sheets/detail/antimicrobial-resistance.
- 20. Tornimbene B, Eremin S, Escher Met al. . WHO Global Antimicrobial Resistance Surveillance System early implementation 2016-17. Lancet Infect Dis 2018; 18: 241–2. [DOI] [PubMed] [Google Scholar]
- 21. IACG . No Time To Wait: Securing the Future From Drug-Resistant Infections. Report to the Secretary-General of the United Nations. 2019. https://www.who.int/antimicrobial-resistance/interagency-coordination-group/IACG_final_report_EN.pdf. [Google Scholar]
- 22. Rogers Van Katwyk S, Grimshaw JM, Nkangu Met al. . Government policy interventions to reduce human antimicrobial use: a systematic review and evidence map. PLoS Med 2019; 16: e1002819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Laxminarayan R, Van Boeckel T, Frost Iet al. . The Lancet Infectious Diseases Commission on antimicrobial resistance: 6 years later. Lancet Infect Dis 2020; 20: e51–60. [DOI] [PubMed] [Google Scholar]
- 24. Saleem Z, Godman B, Azhar Fet al. . Progress on the national action plan of Pakistan on antimicrobial resistance (AMR): a scoping review and the implications. Exp Rev Anti Infect Ther 2021;. doi: 10.1080/14787210.2021.1935238. [DOI] [PubMed] [Google Scholar]
- 25. Sangeda RZ, Kibona J, Munishi Cet al. . Assessment of Implementation of antimicrobial resistance surveillance and antimicrobial stewardship programs in Tanzanian health facilities a year after launch of the national action plan. Front Public Health 2020; 8: 454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Republic of Kenya . National Action Plan on Prevention and Containment of Antimicrobial Resistance, 2017–2022. 2017. https://www.afro.who.int/publications/national-action-plan-prevention-and-containment-antimicrobial-resistance-2017-2022.
- 27. Mendelson M, Matsoso M. The South African Antimicrobial Resistance Strategy Framework. AMR Control 2015; 54–61. [Google Scholar]
- 28. Khan MS, Durrance-Bagale A, Mateus Aet al. . What are the barriers to implementing national antimicrobial resistance action plans? A novel mixed-methods policy analysis in Pakistan. Health Policy Plan 2020; 35: 973–82. [DOI] [PubMed] [Google Scholar]
- 29. Manderson L. Prescribing, care and resistance: antibiotic use in urban South Africa. Humanit Soc Sci Commun 2020; 7: 77. [Google Scholar]
- 30. Rezal RS, Hassali MA, Alrasheedy AAet al. . Prescribing patterns for upper respiratory tract infections: a prescription-review of primary care practice in Kedah, Malaysia, and the implications. Exp Rev Anti Infect Ther 2015; 13: 1547–56. [DOI] [PubMed] [Google Scholar]
- 31. Chowdhury M, Stewart Williams J, Wertheim Het al. . Rural community perceptions of antibiotic access and understanding of antimicrobial resistance: qualitative evidence from the Health and Demographic Surveillance System site in Matlab, Bangladesh. Glob Health Action. 2019; 12: 1824383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Belachew SA, Hall L, Erku DAet al. . No prescription? No problem: drivers of non-prescribed sale of antibiotics among community drug retail outlets in low and middle income countries: a systematic review of qualitative studies. BMC Public Health 2021; 21: 1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Chang J, Xu S, Zhu Set al. . Assessment of non-prescription antibiotic dispensing at community pharmacies in China with simulated clients: a mixed cross-sectional and longitudinal study. Lancet Infect Dis 2019; 19: 1345–54. [DOI] [PubMed] [Google Scholar]
- 34. Hoxha I, Malaj A, Kraja Bet al. . Are pharmacists’ good knowledge and awareness on antibiotics taken for granted? The situation in Albania and future implications across countries. J Glob Antimicrob Resist 2018; 13: 240–5. [DOI] [PubMed] [Google Scholar]
- 35. Saleem Z, Hassali MA, Hashmi FKet al. . Antimicrobial dispensing practices and determinants of antimicrobial resistance: a qualitative study among community pharmacists in Pakistan. Fam Med Com Health 2019; 7: e000138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Langford BJ, So M, Raybardhan Set al. . Antibiotic prescribing in patients with COVID-19: rapid review and meta-analysis. Clin Microbiol Infect 2021; 27: 520–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Rawson TM, Moore LSP, Zhu Net al. . Bacterial and Fungal coinfection in individuals with coronavirus: a rapid review to support COVID-19 antimicrobial prescribing. Clin Infect Dis 2020; 71: 2459–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Hsu J. How Covid-19 is accelerating the threat of antimicrobial resistance. BMJ. 2020; 369: m1983. [DOI] [PubMed] [Google Scholar]
- 39. Jacobs TG, Robertson J, van den Ham HAet al. . Assessing the impact of law enforcement to reduce over-the-counter (OTC) sales of antibiotics in low- and middle-income countries; a systematic literature review. BMC Health Serv Res 2019; 19: 536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Mukokinya MMA, Opanga S, Oluka Met al. . Dispensing of antimicrobials in Kenya: a cross-sectional pilot study and its implications. J Res Pharm Pract 2018; 7: 77–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Markovic-Pekovic V, Grubisa N, Burger Jet al. . Initiatives to reduce nonprescription sales and dispensing of antibiotics: findings and implications. J Res Pharm Pract. 2017; 6: 120–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Alrasheedy AA, Alsalloum MA, Almuqbil FAet al. . The impact of law enforcement on dispensing antibiotics without prescription: a multi-methods study from Saudi Arabia. Exp Rev Anti Infect Ther 2020; 18: 87-–97. [DOI] [PubMed] [Google Scholar]
- 43. Kibuule D, Nambahu L, Sefah IAet al. . Activities in Namibia to limit the prevalence and mortality from COVID-19 including community pharmacy activities and the implications. Sch Acad J Pharm. 2021; 10: 82–92. [Google Scholar]
- 44. Godman B, Fadare J, Kwon H-Yet al. . Evidence-based public policy making for medicines across countries; findings and implications for the future. J Comp Eff Res 2021; 10: 1019–52 [DOI] [PubMed] [Google Scholar]
- 45. Meyer JC, Schellack N, Stokes Jet al. . Ongoing initiatives to improve the quality and efficiency of medicine use within the public healthcare system in South Africa; a preliminary study. Front Pharmacol 2017; 8: 751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Schellack N, Benjamin D, Brink Aet al. . A situational analysis of current antimicrobial governance, regulation, and utilization in South Africa. Int J Infect Dis 2017; 64: 100–6. [DOI] [PubMed] [Google Scholar]
- 47. Gordon T, Booysen F, Mbonigaba J. Socio-economic inequalities in the multiple dimensions of access to healthcare: the case of South Africa. BMC Public Health 2020; 20: 289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Stott BA, Moosa S. Exploring the sorting of patients in community health centres across Gauteng Province, South Africa. BMC Fam Pract 2019; 20: 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Modisakeng C, Matlala M, Godman Bet al. . Medicine shortages and challenges with the procurement process among public sector hospitals in South Africa; findings and implications. BMC Health Serv Res 2020; 20: 234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Gray A, Riddin J, Jugathpal J. Health care and pharmacy practice in South Africa. Can J Hosp Pharm 2016; 69: 36–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Torres N, Chibi B. Antibiotic Use and Resistance in South Africa: The Need for Better Data. 2019. http://www.hsrc.ac.za/en/review/hsrc-review-june-2019/antibiotic-use-and-resistance-in-sa.
- 52. Comins L. Pharmacies to Dispense ARVs and PEP Without a Prescription. 2021. https://www.msn.com/en-za/news/national/pharmacies-to-dispense-arvs-and-pep-without-a-prescription/ar-AAPbg5t?ocid=entnewsntp.
- 53. Amaratunge S, Harrison M, Perry Det al. . Assessing the reporting quality of simulated patient studies in pharmacy research using a novel checklist (CRiSP). Res Social Adm Pharm 2022; 18: 2301–2307. [DOI] [PubMed] [Google Scholar]
- 54. Mashalla Y, Setlhare V, Massele Aet al. . Assessment of prescribing practices at the primary healthcare facilities in Botswana with an emphasis on antibiotics: findings and implications. Int J Clin Pract 2017; 71: e13042. [DOI] [PubMed] [Google Scholar]
- 55. Matsitse TB, Helberg E, Meyer JCet al. . Compliance with the primary health care treatment guidelines and the essential medicines list in the management of sexually transmitted infections in correctional centres in South Africa: findings and implications. Exp Rev Anti Infect Ther 2017; 15: 963–72. [DOI] [PubMed] [Google Scholar]
- 56. CDC South Africa . Global Health - South Africa. 2021. https://www.cdc.gov/globalhealth/countries/southafrica/default.htm.
- 57. Engler D, Meyer JC, Schellack Net al. . Compliance with South Africa’s Antimicrobial Resistance National Strategy Framework: are we there yet? J Chemother 2021; 33: 21–31. [DOI] [PubMed] [Google Scholar]
- 58. Opanga SA, Rizvi N, Wamaitha Aet al. . Availability of medicines in community pharmacy to manage patients with COVID-19 in Kenya; pilot study and implications. Sch Acad J Pharm 2021; 10: 36–42. [Google Scholar]
- 59. Llor C, Cots JM. The sale of antibiotics without prescription in pharmacies in Catalonia, Spain. Clin Infect Dis 2009; 48: 1345–9. [DOI] [PubMed] [Google Scholar]
- 60. Abasaeed AE, Vlcek J, Abuelkhair MAet al. . A comparative study between prescribed and over-the-counter antibiotics. Saudi Med J 2013; 34: 1048–54. [PubMed] [Google Scholar]
- 61. Roque F, Soares S, Breitenfeld Let al. . Attitudes of community pharmacists to antibiotic dispensing and microbial resistance: a qualitative study in Portugal. Int J Clin Pharm 2013; 35: 417–24. [DOI] [PubMed] [Google Scholar]
- 62. Sefah IA, Ogunleye OO, Essah DOet al. . Rapid assessment of the potential paucity and price increases for suggested medicines and protection equipment for COVID-19 across developing countries with a particular focus on Africa and the implications. Front Pharmacol 2021; 11: 588106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Bojanic L, Markovic-Pekovic V, Skrbic Ret al. . Recent initiatives in the Republic of Srpska to enhance appropriate use of antibiotics in ambulatory care; their influence and implications. Front Pharmacol 2018; 9: 442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Kamati M, Godman B, Kibuule D. Prevalence of self-medication for acute respiratory infections in young children in Namibia: findings and implications. J Res Pharm Pract 2019; 8: 220–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Sharland M, Gandra S, Huttner Bet al. . Encouraging AWaRe-ness and discouraging inappropriate antibiotic use-the new 2019 Essential Medicines List becomes a global antibiotic stewardship tool. Lancet Infect Dis 2019; 19: 1278–80. [DOI] [PubMed] [Google Scholar]
- 66. Klein EY, Milkowska-Shibata M, Tseng KKet al. . Assessment of WHO antibiotic consumption and access targets in 76 countries, 2000-15: an analysis of pharmaceutical sales data. Lancet Infect Dis 2021; 21: 107–15. [DOI] [PubMed] [Google Scholar]
- 67. Mwita S, Jande M, Marwa Ket al. . Medicines dispensers’ knowledge on the implementation of an artemisinin-based combination therapy policy for the treatment of uncomplicated malaria in Tanzania. J Pharm Health Serv Res 2017; 8: 227–33. [Google Scholar]
- 68. Santa-Ana-Tellez Y, Mantel-Teeuwisse AK, Dreser Aet al. . Impact of over-the-counter restrictions on antibiotic consumption in Brazil and Mexico. PLoS One. 2013; 8: e75550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Wirtz VJ, Herrera-Patino JJ, Santa-Ana-Tellez Yet al. . Analysing policy interventions to prohibit over-the-counter antibiotic sales in four Latin American countries. Trop Med Int Health 2013; 18: 665–73. [DOI] [PubMed] [Google Scholar]
- 70. Haque M, Abubakar A, Ogunleye OOet al. . Changes in availability, utilization, and prices of medicines and protection equipment for COVID-19 in an urban population of Northern Nigeria. J Res Pharm Pract 2021; 10: 17–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Markovic-Pekovic V, Grubisa N. Self-medication with antibiotics in the Republic of Srpska community pharmacies: pharmacy staff behavior. Pharmacoepidemiol Drug Saf. 2012; 21: 1130–3. [DOI] [PubMed] [Google Scholar]
- 72. Haque M, Kumar S, Charan Jet al. . Utilisation, availability and price changes of medicines and protection equipment for COVID-19 in India: findings and implications. Front Pharmacol 2021; 11: 582154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Godman B, Haque M, Islam Set al. . Rapid assessment of price instability and paucity of medicines and protection for COVID-19 across Asia: findings and public health implications for the future. Front Public Health 2020; 8: 585832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Arparsrithongsagul S, Kulsomboon V, Zuckerman IH. Multidisciplinary perspective intervention with community involvement to decrease antibiotic sales in village groceries in Thailand. Asia-Pacific J Public Health 2015; 27: NP2480–8. [DOI] [PubMed] [Google Scholar]
- 75. Moura ML, Boszczowski I, Mortari Net al. . The impact of restricting over-the-counter sales of antimicrobial drugs: preliminary analysis of national data. Medicine 2015; 94: e1605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Lopes-Junior R, de Sa Del Fiol F, Araujo JLOet al. . Decrease in penicillin sales in Brazil after over-the-counter restrictions. Antimicrob Agents Chemother 2015; 59: 5862–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Mattos KPH, Visacri MB, Quintanilha JCFet al. . Brazil’s resolutions to regulate the sale of antibiotics: impact on consumption and Escherichia coli resistance rates. J Glob Antimicrob Resist 2017; 10: 195–9. [DOI] [PubMed] [Google Scholar]
- 78. Vacca CP, Nino CY, Reveiz L. [Restriction of antibiotic sales in pharmacies in Bogotá, Colombia: a descriptive study]. Revista Panam Salud Publica. 2011; 30: 586–91. [PubMed] [Google Scholar]
- 79. Santa-Ana-Tellez Y, Mantel-Teeuwisse AK, Leufkens HGet al. . Seasonal variation in penicillin use in Mexico and Brazil: analysis of the impact of over-the-counter restrictions. Antimicrob Agents Chemother 2015; 59: 105–10. [DOI] [PMC free article] [PubMed] [Google Scholar]