Abstract
Background
Higher ultrafiltration (UF) rates are associated with numerous adverse cardiovascular outcomes among individuals receiving maintenance hemodialysis. We undertook this study to investigate the association of UF rate and incident atrial fibrillation in a large, nationally representative US cohort of incident, older hemodialysis patients.
Methods
We used the US Renal Data System linked to the records of a large dialysis provider to identify individuals ≥67 years of age initiating hemodialysis between January 2006 and December 2011. We applied an extended Cox model as a function of a time-varying exposure to compute adjusted hazard ratios (HRs) and 95% confidence intervals (CIs) for the association of delivered UF rate and incident atrial fibrillation.
Results
Among the 15 414 individuals included in the study, 3177 developed atrial fibrillation. In fully adjusted models, a UF rate >13 mL/h/kg (versus ≤13 mL/h/kg) was associated with a higher hazard of incident atrial fibrillation [adjusted HR 1.19 (95% CI 1.07–1.30)]. Analyses using lower UF rate thresholds (≤10 versus >10 mL/h/kg and ≤8 versus >8 mL/h/kg, separately) yielded similar results. Analyses specifying the UF rate as a cubic spline (per 1 mL/h/kg) confirmed an approximately linear dose–response relationship between the UF rate and the risk of incident atrial fibrillation: risk began at UF rates of ~6 mL/h/kg and the magnitude of this risk flattened, but remained elevated, at rates ≥9 mL/h/kg.
Conclusion
In this observational study of older individuals initiating hemodialysis, higher UF rates were associated with higher incidences of atrial fibrillation.
Keywords: atrial fibrillation, cardiovascular, hemodialysis, ultrafiltration rate
KEY LEARNING POINTS
What is already known about this subject?
Higher ultrafiltration (UF) rates are associated with numerous adverse cardiovascular outcomes among individuals receiving maintenance hemodialysis. However, the association between higher UF rates and incident atrial fibrillation is unknown.
What this study adds?
We showed that higher UF rates, defined as >13 (versus ≤13) mL/h/kg, are associated with a higher hazard of incident atrial fibrillation. Findings were similar when we considered the lower UF rate thresholds of ≤10 (versus >10) and ≤8 (versus >8) mL/h/kg, separately. Finally, we found that the elevated UF rate–related hazard for incident atrial fibrillation began at UF rates as low as 6 mL/h/kg and then rose in a near-linear dose–response fashion before leveling off.
What impact this may have on practice or policy?
Our results provide additional evidence regarding the harms of higher UF rates and underscore the need for trials investigating the impact of UF rate–lowering strategies on clinical and patient-reported outcomes.
INTRODUCTION
Atrial fibrillation is one of the most common arrhythmias in dialysis patients, affecting up to 25% of the hemodialysis population, with estimated incident rates ranging from 12.5 to 148 per 1000 patient-years [1–3]. The incidence of atrial fibrillation is rising [1, 4], and recent data suggest that paroxysmal atrial fibrillation can be detected in as many as 40% of individuals receiving in-center maintenance hemodialysis when using loop recorders [5]. Atrial fibrillation is associated with adverse outcomes, including a higher risk of ischemic stroke, heart failure, hospitalization and mortality [2, 3, 6–11]. Nonmodifiable risk factors including advanced age, non-Hispanic white race and underlying structural heart disease such as valvular abnormalities and heart failure are associated with the development of atrial fibrillation [12]. However, less is known about modifiable risk factors for the condition.
Hemodialysis patients have a 2-fold higher risk of incident atrial fibrillation compared with peritoneal dialysis patients in the first 90 days of dialysis therapy [13], suggesting that the hemodialysis procedure may play a role in provoking atrial fibrillation. Moreover, continuous electrocardiogram monitoring studies reveal that arrhythmias, including atrial fibrillation, peak in the peri- and intradialytic periods [5, 14, 15]. Lower dialysate potassium concentration, higher UF volume and lower predialysis systolic and diastolic blood pressures (BPs) are associated with incident atrial fibrillation in hemodialysis patients [14, 16]. Observational data support an association between more rapid fluid removal during hemodialysis (i.e. higher UF rates) and higher risks of cardiovascular morbidity and mortality [17–22]. UF rates that exceed plasma refill rates can induce hemodynamic instability, and repeat events may lead to end-organ ischemic damage such as cardiac remodeling and associated conduction system disruption, a precipitant for arrhythmias [23–26]. As UF rates are a modifiable aspect of hemodialysis treatment, it is essential to understand their association with the development of atrial fibrillation.
We undertook this study to investigate the association of UF rate with incident atrial fibrillation in a large, nationally representative cohort of older patients initiating hemodialysis for kidney failure. We selected an older-age cohort in order to study individuals qualifying for Medicare prior to hemodialysis initiation, facilitating exclusion of individuals with evidence of prevalent atrial fibrillation. We hypothesized that higher UF rates would be associated with a higher risk of incident atrial fibrillation.
MATERIALS AND METHODS
The study was approved by the institutional review boards at Stanford University (protocol IRB-17904) and Baylor College of Medicine (protocol H-36408) and was conducted under active Data Use Agreements with the National Institute of Diabetes and Digestive Diseases (NIDDK). A waiver of informed consent was granted due to the study’s retrospective design, size and data anonymity.
Data source
We previously published a detailed description of our study population [16]. In brief, we used data from the US Renal Data System (USRDS), the national registry for patients with kidney failure [27], linked with data from the electronic health records of a large US dialysis provider (DaVita, Denver, CO) using a crosswalk provided by the USRDS data coordinating center.
Study design and population
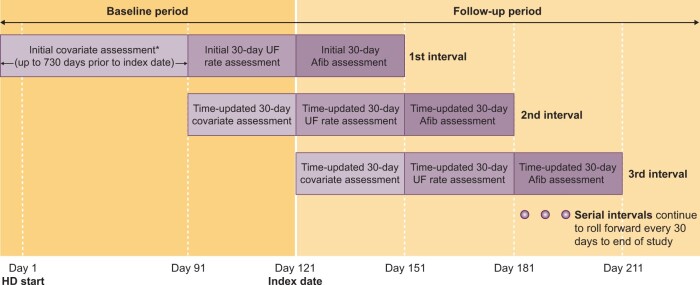
We conducted a retrospective cohort study using successive 30-day exposure intervals to investigate the association between delivered UF rates and the development of incident atrial fibrillation among incident, older, in-center hemodialysis patients. Figure 1 displays the study design. The index date was day 121 after hemodialysis initiation.
FIGURE 1.
Study design. The baseline period extends up to 730 days prior to the index date, which is designed as Day 121 after initiation of hemodialysis. Baseline covariates, UF rate and other dialysis parameters were assessed during the baseline period. The follow-up period begins at the index date and is divided into successive 30-day covariate assessment intervals, 30-day UF rate (exposure) intervals and 30-day atrial fibrillation (outcome) intervals. AFib, atrial fibrillation; UF rate, mean delivered UF rate (mL/h/kg).
To construct the cohort, we first identified incident hemodialysis patients who were >67 years of age between 1 January 2006 and 1 December 2011 in the USRDS database. We required that individuals have uninterrupted Medicare Parts A and B coverage for at least 730 days prior to the date of their first hemodialysis treatment for kidney failure with at least one Medicare claim (Part A or B) in the 730–366 days and 365–0 days preceding incident dialysis-dependent kidney failure. This approach facilitated study of individuals with Medicare claims data prior to kidney failure designation. For non-US readers, Medicare is the public insurance system for older individuals in the USA providing near-universal healthcare coverage to the majority of its residents >65 years of age. We linked the national registry data to data from the electronic health records of a large US dialysis provider (DaVita) and selected patients who initiated dialysis with in-center hemodialysis (i.e. not home hemodialysis or peritoneal dialysis). We excluded patients who died, received a kidney transplant, recovered kidney function, received any peritoneal dialysis treatments or lost Medicare Part A or B coverage prior to the index date. To isolate the cohort to relatively stable outpatient hemodialysis patients, we further excluded those who had <10 or >16 clinic-based hemodialysis treatments in the 30 days prior to the index date (i.e. patients with numerous missed treatments and patients requiring numerous ‘extra’ treatments) and those with treatment times <2 h in >50% of their hemodialysis treatments during the initial UF rate ascertainment period (i.e. non-thrice-weekly treatment paradigms). Finally, to identify incident atrial fibrillation, we excluded patients with evidence of historical (i.e. prevalent) atrial fibrillation [any billing claim with an International Classification of Diseases, 9th Revision (ICD-9) diagnosis code of 427.3] prior to the index date [1].
Outcome: incident atrial fibrillation
The outcome was incident atrial fibrillation identified from any inpatient claim with an ICD-9 diagnosis code of 427.3 or an outpatient claim with an ICD-9 diagnosis code of 427.3 provided there was a second, subsequent inpatient or outpatient code of 427.3, in any position, separated by at least 1 day from the initial code appearance [1, 4, 16]. This definition has been shown to have a sensitivity of 81% and specificity of 99% [28].
Exposure: delivered UF rate
The exposure, delivered UF rate (mL/h/kg), was calculated as [(prehemodialysis weight – posthemodialysis weight, mL)/delivered treatment time, h/posthemodialysis weight, kg]. Starting at day 91, we considered the mean UF rate, defined as the average UF rate calculated across all available UF rate values for a given interval, in time-updated 30-day increments. In primary analyses we dichotomized the mean delivered UF rate at 13 (<13 versus ≥13) mL/h/kg, which was motivated by prior research and a US-based measure of dialysis care quality [18, 29]. In secondary analyses we dichotomized the mean delivered UF rate at 10 and 8 mL/h/kg; we also considered the mean delivered UF rate as a continuous variable modeled using splines.
Covariates
We assessed baseline comorbid medical conditions from 730 days prior to HD initiation until the start of the UF rate ascertainment window (day +90), baseline laboratory values in the 90 days prior to UF rate ascertainment and the baseline number of hospital days and hemodialysis treatment parameters in the 30 days prior to UF rate ascertainment. We used Medicare Parts A and B claims to ascertain comorbid medical conditions using ICD-9 diagnosis (requiring one inpatient or two outpatient encounters separated by at least 1 day) and procedure codes [30]. Comorbid conditions were then time-updated in 30-day intervals preceding each 30-day time-updated exposure period. We used the Medicare Evidence Form to obtain baseline age, sex, Medicaid dual eligibility status, year of incident dialysis-dependent kidney failure, race, ethnicity and estimated glomerular filtration rate (eGFR), as well as neighborhood-level socioeconomic data at the time of incident hemodialysis initiation. The number of recent hospital days was ascertained from Medicare Part A claims.
We used the dialysis electronic health record to ascertain laboratory (serum albumin, calcium and potassium) and dialysis-related (predialysis systolic and diastolic BPs and vascular access type) data. We used the most recent laboratory value in the 90-day laboratory assessment period and then time-updated these values in subsequent 30-day intervals preceding each 30-day time-updated exposure period. Vascular access type was designated as a central venous catheter versus never use of a central venous catheter during the 30 days prior to the UF rate exposure period and then time-updated in subsequent 30-day intervals.
Statistical analysis
We related the development of incident atrial fibrillation during period Pi with the UF rate ascertained during period Pi−1 and other covariates ascertained during period Pi−2, where i represents the period indicator. We censored follow-up time at the earliest occurrence of modality change (n = 1528), loss of Medicare Part A or B coverage (n = 349), discontinuation of dialysis care at the dialysis organization (n = 2302) or the end of the study period (31 December 31 2011; n = 5322). We assumed a cause-specific analysis of possible competing risks, where follow-up time was also censored for death (n = 2730) and kidney transplantation (n < 10) [31].
We present baseline characteristics by categories of mean delivered UF rate and summarized categorical variables as frequencies (percentages) and continuous variables as means [standard deviations (SDs)] or as medians [interquartile ranges (IQRs)]. We computed unadjusted incidence rates of atrial fibrillation by categories of mean delivered UF rate based on the number of events observed per person-year starting at day 121 after HD initiation. We applied an extended Cox model as a function of time-varying exposure to compute adjusted hazard ratios (HRs) and 95% confidence intervals (CIs) for the association of each delivered UF rate exposure and incident atrial fibrillation, separately. Estimates were adjusted in five nested multivariable models: Model 0, adjusted for year of incident dialysis-dependent kidney failure; Model 1 additionally adjusted for age, sex, race and Hispanic ethnicity; Model 2 additionally adjusted for census division, socioeconomic status variables and Medicaid dual eligibility; Model 3 additionally adjusted for comorbid conditions (Table 1), number of recent hospital days and vascular access type and Model 4 additionally adjusted for predialysis systolic and diastolic BP, number of hemodialysis treatments and serum laboratory values. We examined the correlation of the scaled Schoenfeld residuals with time and found no evidence that the log-HR changed with follow-up time for either the exposures or other Model 4 covariates (Schoenfeld test global P-value >0.05). We stratified all models by incidence year of dialysis-dependent kidney failure.
Table 1.
Characteristics of the study cohort overall and by UF rate at baseline
| Patient characteristics | Overall (N = 15 414) | UF rate ≤13 mL/h/kga (n = 14 065) |
UF rate >13 mL/h/kga (n = 1312) |
|---|---|---|---|
| Demographics | |||
| Age (years), mean ± SD | 76.3 ± 6.3 | 76.3 ± 6.3 | 76.4 ± 6.3 |
| Female, n (%) | 7838 (50.8) | 7054 (50.2) | 763 (58.2) |
| Race, n (%) | |||
| White | 10 626 (68.9) | 9715 (69.1) | 881 (67.1) |
| Black | 4063 (26.4) | 3745 (26.6) | 312 (23.8) |
| Asian | 477 (3.1) | 382 (2.7) | 94 (7.2) |
| Native American | 91 (0.6) | 80 (0.6) | 11 (0.8) |
| Other/unknown | 157 (1.0) | 143 (1.0) | 14 (1.1) |
| Hispanic ethnicity, n (%) | 1237 (8.0) | 1097 (7.8) | 136 (10.4) |
| Missing | 48 (0.3) | 46 (0.3) | 2 (0.2) |
| Dual Medicare/Medicaid eligible, n (%) | 4151 (26.9) | 3718 (26.4) | 421 (32.1) |
| Catheter vascular access, n (%) | 9732 (63.1) | 8900 (63.3) | 808 (61.6) |
| Missing | 37 (0.2) | 57 (0.4) | 3 (0.3) |
| Census data | |||
| Unemployed, median (IQR) | 9.3 (6.8–12.4) | 9.3 (6.8–12.4) | 9.5 (7.0–12.7) |
| Missing, n (%) | 140 (0.9) | 129 (0.9) | 11 (0.8) |
| Below poverty line, median (IQR) | 13.9 (8.3–20.9) | 13.8 (8.2–20.8) | 15.0 (8.7–22.0) |
| Missing, n (%) | 141 (0.9) | 130 (0.9) | 11 (0.8) |
| Less than a high school education, median (IQR) | 14.0 (8.8–21.1) | 13.9 (8.8–21.0) | 15.1 (9.2–22.4) |
| Missing, n (%) | 140 (0.9) | 129 (0.9) | 11 (0.8) |
| Monthly rent (US$), median (IQR) | 859 (686–1069) | 857 (685–1065) | 874 (692–1105) |
| Missing, n (%) | 312 (2.0) | 282 (2.0) | 30 (2.3) |
| Annual household income (US$), median (IQR) | 48 125 (38 245–63 555) | 48 151 (38 397–63 653) | 47 639 (37 132–63 010) |
| Missing, n (%) | 144 (0.9) | 132 (0.9) | 12 (0.9) |
| Hospital days in the 30 days prior to UF rate ascertainment, mean ± SD | 0.9 ± 3.0 | 0.9 ± 2.9 | 1.2 ± 3.5 |
| Comorbid medical conditions, n (%) | |||
| Coronary artery disease | 8547 (55.4) | 7781 (55.3) | 747 (56.9) |
| Myocardial infarction | 2110 (13.7) | 1886 (13.4) | 216 (16.5) |
| Unstable angina | 2598 (16.9) | 2351 (16.7) | 239 (18.2) |
| Heart failure | 9540 (61.9) | 8575 (61.0) | 942 (71.8) |
| Coronary revascularization | 3337 (21.6) | 3058 (21.7) | 271 (20.7) |
| ICD or pacemaker | 950 (6.2) | 869 (6.2) | 80 (6.1) |
| Ventricular fibrillation or another arrhythmia | 2405 (15.6) | 2153 (15.3) | 245 (18.7) |
| Valvular heart disease | 4580 (29.7) | 4067 (28.9) | 498 (38.0) |
| Stroke or TIA | 2873 (18.6) | 2613 (18.6) | 243 (18.5) |
| Hypertension | 15276 (99.1) | 13 938 (99.1) | 1301 (99.2) |
| Peripheral artery disease | 5685 (36.9) | 5141 (36.6) | 525 (40.0) |
| Diabetes mellitus | 10 523 (68.3) | 9613 (68.3) | 884 (67.4) |
| Hyperlipidemia | 11 468 (74.4) | 10 512 (74.7) | 930 (70.9) |
| Liver disease | 1136 (7.4) | 996 (7.1) | 136 (10.4) |
| Chronic lung disease | 5889 (38.2) | 5317 (37.8) | 554 (42.2) |
| Depression | 2392 (15.5) | 2148 (15.3) | 234 (17.8) |
| Biometric measurements | |||
| eGFR at HD initiation (mL/min/1.73 m2), mean ± SD | 10.4 ± 4.4 | 10.4 ± 4.3 | 10.6 ± 4.5 |
| Missing, n (%) | 162 (1.1%) | 147 (1.0%) | 14 (1.1%) |
| HD treatments in the 30-day UF rate ascertainment period, mean ± SD | 12.3 ± 1.6 | 12.3 ± 1.6 | 12.2 ± 1.7 |
| Delivered UF rate (mL/h/kg)a, mean ± SD | 7.7 ± 4.0 | 7.0 ± 3.2 | 15.6 ± 2.8 |
| Predialysis systolic BP (mmHg), mean ± SD | 147.3 ± 19.7 | 147.1 ± 19.6 | 148.7 ± 20.0 |
| Missing, n (%) | 170 (1.1) | 142 (1.0) | 20 (1.5) |
| Delivered treatment time (min), mean ± SD | 208.7 ± 24.5 | 209.8 ± 24.3 | 196.5 ± 23.4 |
| UF volume (L), mean ± SD | 1.9 ± 1.0 | 1.8 ± 0.9 | 3.0 ± 0.7 |
| Postdialysis weight (kg), mean ± SD | 73.3 ± 17.9 | 74.5 ± 17.9 | 60.2 ± 12.3 |
| Interdialytic weight gain (kg), mean ± SD | 2.0 ± 1.2 | 1.9 ± 1.2 | 3.1 ± 1.1 |
| Prescribed ‘dry’ weight (kg), mean ± SD | 73.3 ± 18.0 | 74.6 ± 17.9 | 59.7 ± 12.2 |
| Body mass index (kg/m2), mean ± SD | 27.7 ± 7.0 | 28.1 ± 7.0 | 24.0 ± 5.3 |
| Predialysis diastolic BP (mmHg), mean ± SD | 72.3 ± 10.1 | 72.3 ± 10.0 | 72.5 ± 10.6 |
| Missing, n (%) | 170 (1.1) | 142 (1.0) | 20 (1.5) |
| Serum albumin (g/L), mean ± SD | 3.6 ± 0.5 | 3.6 ± 0.4 | 3.6 ± 0.5 |
| Missing, n (%) | 161 (1.0) | 141 (1.0) | 18 (1.4) |
| Serum potassium (mEq/L), mean ± SD | 4.5 ± 0.6 | 4.4 ± 0.6 | 4.6 ± 0.7 |
| Missing, n (%) | 169 (1.1) | 148 (1.1) | 18 (1.4) |
| Calcium (mg/L), mean ± SD | 8.9 ± 0.7 | 9.0 ± 0.7 | 8.8 ± 0.7 |
| Missing, n (%) | 149 (1.0%) | 130 (0.9%) | 17 (1.3%) |
Thirty-seven patients who were missing delivered UF rate (due to missing treatment time, UF volume or postdialysis weight) at baseline were excluded.
TIA, transient ischemic attack.
In secondary analyses we constructed restricted cubic splines for delivered UF rate with knots at 6, 8, 10 and 12 mL/h/kg [17, 18]. We then plotted the HR of the delivered UF rate in restricted spline terms to compare two delivered UF rate values with 1 unit (1 mL/h/kg) apart where the comparator was lower. We additionally plotted the HR of the delivered UF rate in restricted spline terms with UF rate thresholds of 13, 10 and 8 mL/h/kg (reference). Analogous to primary analysis models, spline models were adjusted for the year of incident dialysis-dependent kidney failure; age; sex; race; Hispanic ethnicity; census division; socioeconomic status variables; Medicaid dual eligibility; comorbid conditions (Table 1); recent hospital days; vascular access type; predialysis systolic and diastolic BP; number of hemodialysis treatments and serum albumin, eGFR, potassium and calcium. In spline analyses we excluded delivered UF rates that were lower than the sample’s 5th percentile (2.5 mL/h/kg) or higher than the 95th percentile (14.5 mL/h/kg). In addition, we evaluated the association between UF rate (<13 versus ≥13 mL/h/kg) and incident atrial fibrillation in clinically relevant subgroups, including individuals with and without atrial fibrillation risk factors (male sex, advanced age, Hispanic ethnicity, black race and baseline diabetes). To investigate whether findings differed on the basis of delivered treatment time, UF volume or body size (i.e. the individual components of the UF rate calculation), we performed additional subgroup analyses considering individuals with body mass index <25th percentile (versus ≥25th percentile), UF volume >75th percentile (versus ≤75th percentile) and delivered treatment time <4 h (versus ≥4 h). This analysis was restricted to the 14 695 patients without missing UF rate–related data in the follow-up period.
Finally, to assess for influence from residual kidney function, we conducted two sensitivity analyses. In the first analysis we restricted the cohort to individuals who survived the first year of hemodialysis, ascertaining the initial UF rate exposure in the 360–390 days after hemodialysis initiation, and then repeated the primary analysis. In the second analysis we restricted the cohort to individuals with an eGFR ≤9.7 mL/min/1.73 m2 at hemodialysis initiation (i.e. ≤25th eGFR percentile based on the Medical Evidence Report Form) and then repeated the primary analysis.
Missing data
In the 15 414-patient cohort, 1515 patients (9.8%) had at least one covariate missing and 27 374 (10.4%) of 269 032 period records had missing data. eGFR was the most common missing baseline data (7.2%) and vascular access type was the most common missing time-varying data (0.9%). We handled missing data using multiple imputation by chained equations (MICE) as implemented in R (R Foundation for Statistical Computing, Vienna, Austria), with 11 imputed datasets obtained for each outcome [32, 33]. We assumed that the data were missing at random, conditional on observed variables. In addition to the exposure and all covariates included in the analysis model, the imputation model also included the event indicator, the Nelson–Aalen estimator of the cumulative marginal hazard [34] and auxiliary variables (patient’s weight at the time of incident dialysis-dependent kidney failure, predialysis nephrology care and a time-varying indicator for kidney transplant listing prior to or during the associated period Pi−1) [35]. We combined the estimates and standard errors obtained from the model applied to each imputed dataset using Rubin’s rules [36].
All statistical analyses were performed using SAS version 9.4 (SAS Institute, Cary, NC, USA), Stata version 15.1 (StataCorp, College Station, TX, USA) and R version 3.1.2.
RESULTS
Participant characteristics
Figure 2 displays a flow diagram of study cohort selection and Table 1 displays the baseline study cohort characteristics. A total of 15 414 incident hemodialysis patients were included in the study: 14 065 (91.2%) with mean delivered UF rates ≤13 mL/h/kg and 1312 (8.5%) with mean delivered UF rates >13 mL/h/kg. Overall, study patients had an average age of 76.3 ± 6.3 years, 50.8% were women, 26.4% were Black, 8.0% were Hispanic and 26.9% were dually Medicare and Medicaid eligible. On average, patients with UF rates >13 mL/h/kg were more often women and had a higher prevalence of most comorbid conditions, particularly coronary artery disease, heart failure, valvular heart disease and chronic lung disease, than patients with UF rates ≤13 mL/h/kg. Predialysis systolic BP and laboratory values were similar across the UF rate groups.
FIGURE 2.
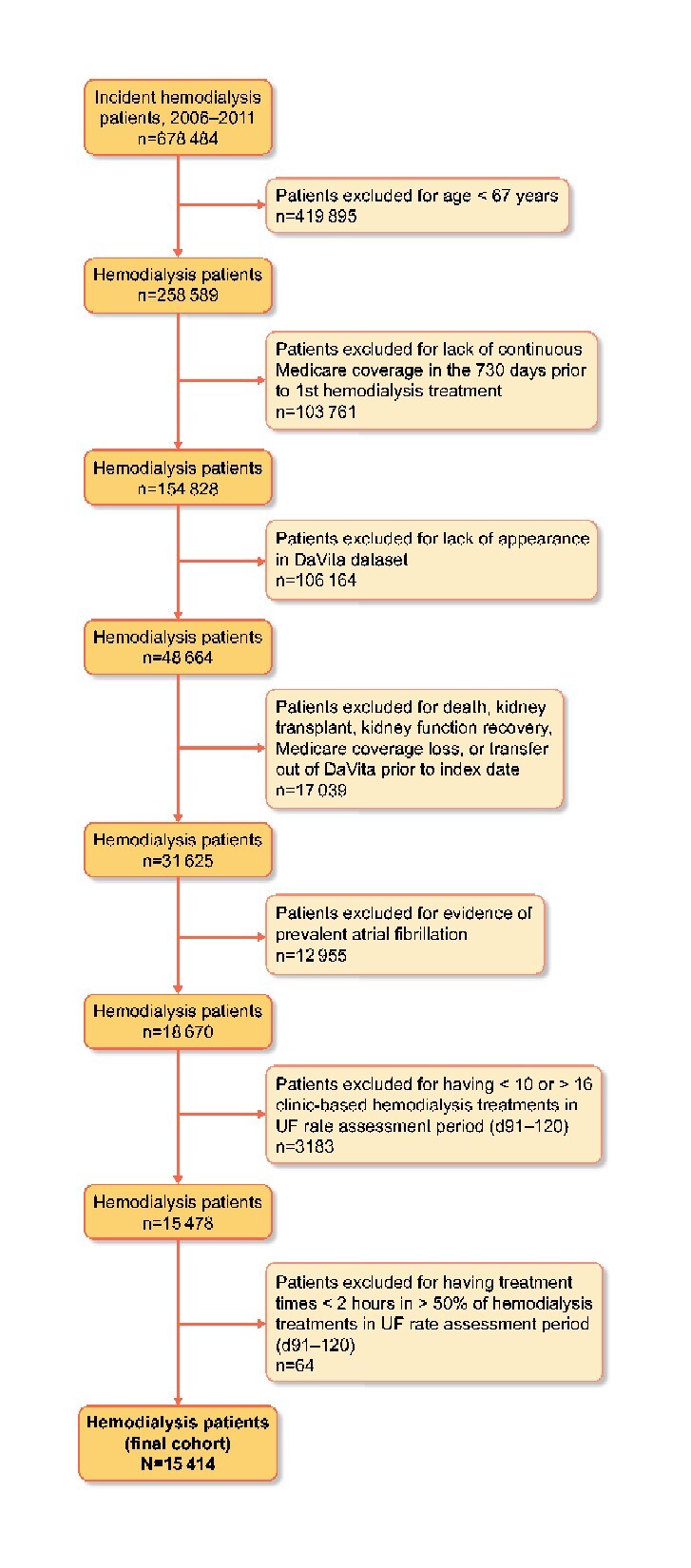
Flow chart depicting study cohort assembly. D, day.
Primary analysis: UF rate >13 mL/h/kg and incident atrial fibrillation
The cohort was followed for 21 767 person-years and had an average follow-up duration of 1.4 ± 1.3 years. During the nearly 4.5-year follow-up period, 3177 individuals developed incident atrial fibrillation (incidence rate of 15 cases of incident atrial fibrillation/100 person-years) with 296 outcomes among individuals with UF rates >13 mL/h/kg (incidence rate of 17 cases of incident atrial fibrillation/100 person-years) and 2881 outcomes among individuals with UF rates ≤13 mL/h/kg (incidence rate of 14 cases of incident atrial fibrillation/100 person-years).
The unadjusted hazard of atrial fibrillation was higher among individuals with UF rates >13 mL/h/kg compared with individuals with UF rates ≤13 mL/h/kg. In sequentially adjusted nested models (Table 2), a UF rate >13 mL/h/kg (versus ≤13 mL/h/kg) was associated with a higher hazard of incident atrial fibrillation [adjusted HR 1.15 (95% CI 1.04–1.25) in the fully adjusted model (Model 4)].
Table 2.
Unadjusted and adjusted associations between mean delivered UF rate and incident atrial fibrillation a
| HR (95% CI) |
|||
|---|---|---|---|
| Modelb | UF rate >13 (versus ≤13 mL/h/kg) | UF rate >10 (versus ≤10 mL/h/kg) | UF rate >8 (versus ≤8 mL/h/kg) |
| 0 | 1.21 (1.10–1.31) | 1.08 (1.00–1.15) | 1.08 (1.01–1.15) |
| 1 | 1.22 (1.12–1.33) | 1.10 (1.02–1.17) | 1.10 (1.03–1.17) |
| 2 | 1.22 (1.11–1.32) | 1.09 (1.02–1.17) | 1.10 (1.03–1.17) |
| 3 | 1.16 (1.06–1.27) | 1.06 (0.99–1.14) | 1.08 (1.01–1.15) |
| 4 | 1.15 (1.04–1.25) | 1.06 (0.99–1.14) | 1.09 (1.02–1.16) |
Extended Cox models with multiple imputation for missing data were used to compute adjusted HRs and 95% CIs for the association of each delivered UF rate exposure and incident atrial fibrillation. All models were stratified by year of incident end-stage kidney disease.
Model 0, unadjusted; Model 1, additionally adjusted for age, sex, race and Hispanic ethnicity; Model 2, additionally adjusted for census division, socioeconomic status variables and Medicare/Medicaid dual eligibility; Model 3, additionally adjusted for all comorbid conditions listed in Table 1, number of hospital days in 30 days prior to UF rate ascertainment period and vascular access type; Model 4, additionally adjusted for predialysis systolic and diastolic BP, number of dialysis sessions during UF rate ascertainment period and serum albumin, eGFR, potassium and calcium.
Secondary analyses
Similarly, compared with a UF rate ≤8 mL/h/kg, a UF rate >8 mL/h/kg was associated with a higher hazard of incident atrial fibrillation [Model 4 adjusted HR 1.09 (95% CI 1.02–1.16)]. When the UF rate was dichotomized at 10 mL/h/kg, the UF rate–atrial fibrillation association was attenuated and no longer reached statistical significance [Model 4 adjusted HR 1.06 (95% CI 0.99–1.14)] (Table 2).
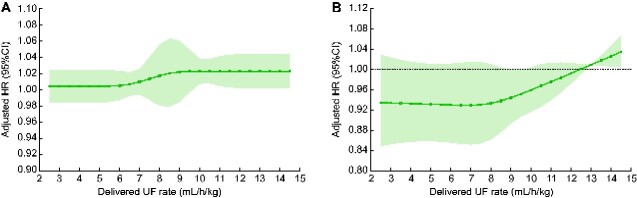
In order to examine the association of UF rate and incident atrial fibrillation in a more flexible manner, we conducted analyses in which we examined the association of UF rate as a cubic spline and incident atrial fibrillation. First, we specified the UF rate as per 1 mL/h/kg, comparing each value to the next lower value (Figure 3A). This analysis showed a linear increase in the risk of incident atrial fibrillation beginning at UF rates of ~6 mL/h/kg, which flattened but remained elevated at rates >9 mL/h/kg. Second, we specified the UF rate as per 1 mL/h/kg, comparing each value to a fixed UF rate of 13 mL/h/kg (Figure 3B). Once again, results showed a lower risk of incident atrial fibrillation with lower UF rates and a higher risk of incident atrial fibrillation with UF rates >13 mL/h/kg.
FIGURE 3.
Adjusted associations between mean delivered UF rate and incident atrial fibrillation when UF rate is modeled as a cubic spline (per 1 mL/h/kg). The solid black lines indicate multivariable-adjusted HRs for incident atrial fibrillation as a function of UF rate and the light gray shading represents the associated 95% CIs. (A) Adjusted HRs and 95% CIs comparing UF rate values 1 mL/h/kg apart where the comparator is lower. (B) Adjusted HRs and 95% CIs comparing UF rate values to a reference of 13 mL/h/kg. All models are adjusted for year of incident dialysis-dependent kidney failure, age, sex, race, Hispanic ethnicity, census division, socioeconomic status variables, Medicare/Medicaid dual eligibility, comorbid conditions (Table 1), number of hospital days in 30 days prior to UF rate ascertainment period, vascular access type, predialysis systolic and diastolic BP, number of hemodialysis treatments in the UF rate ascertainment period and serum albumin, eGFR, potassium and calcium. Estimates are presented for UF rates between 2.5 and 14.5 mL/h/kg (the 5th and 95th percentiles of delivered UF rate in the study sample, respectively). AFib, atrial fibrillation; UF rate, mean delivered UF rate (mL/h/kg).
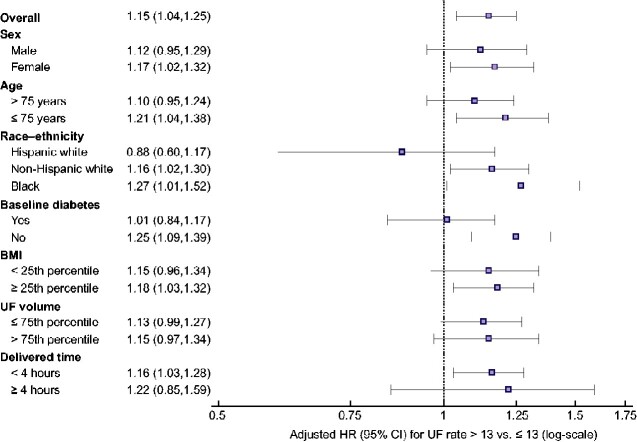
Finally, subgroup analyses showed that the association between a UF rate ≥13 (versus <13) mL/h/kg and incident atrial fibrillation was similar across patient subgroups (Figure 4).
FIGURE 4.
Association between UF rate >13 (versus ≤13) mL/h/kg and incident atrial fibrillation within clinically relevant subgroups. Extended Cox models with multiple imputation for missing data were used to compute adjusted HRs and 95% CIs for the association of delivered UF rate exposure (<13 versus ≥13 mL/h/kg) and incident atrial fibrillation. All models were stratified by year of incident end-stage kidney disease. Models were adjusted for age, sex, race, Hispanic ethnicity, census division, socioeconomic status variables, Medicare/Medicaid dual eligibility, all comorbid conditions listed in Table 1, number of hospital days in the 30 days prior to the UF rate ascertainment period, vascular access type, predialysis systolic and diastolic BP, number of dialysis treatments during the UF rate ascertainment period and serum albumin, eGFR, potassium and calcium. The subgroups of body mass index, UF volume and delivered treatment time were restricted to the 14 695 patients without missing UF rate–related data in the follow-up time period. UF rate, mean delivered UF rate (mL/h/kg).
Sensitivity analyses
Sensitivity analyses restricted to individuals who survived the first year of hemodialysis yielded results analogous to the primary findings: a UF rate >13 (versus ≤13) mL/h/kg was associated with a higher hazard of incident atrial fibrillation [adjusted HR 1.16 (95% CI 1.02–1.29) in the fully adjusted model (Model 4)]. Sensitivity analyses restricted to individuals with lower eGFRs at hemodialysis initiation (<9.7 mL/min/1.73 m2) yielded similar but non–statistically significant findings [adjusted HR 1.10 (95% CI 0.97–1.22) in the fully adjusted model (Model 4)].
DISCUSSION
Using a large, nationally representative cohort of older incident hemodialysis patients with linked data from administrative claims and electronic health records databases, we show an association between higher UF rates and the development of incident atrial fibrillation. Specifically, we found that UF rates >13 (versus ≤13), 10 (versus ≤10) and 8 (versus ≤8) mL/h/kg, separately, were all associated with higher hazards of incident atrial fibrillation. In addition, we found that the elevated UF rate–related hazard for incident atrial fibrillation began at UF rates as low as 6 mL/h/kg and then rose in a near-linear dose–response fashion before leveling off.
Our findings extend the existing evidence base supporting associations between overly rapid fluid removal and such untoward patient-reported and clinical outcomes as longer hemodialysis recovery times [37], loss of residual kidney function [38], higher hospitalization and mortality rates [17–21] and, now, higher risk of incident atrial fibrillation. Our results should not be surprising, as prior studies suggested that other volume-related aspects of the hemodialysis procedure can contribute to arrhythmias. In a 40-patient substudy of the ICD-2 Trial, Buiten et al. [14] found that both UF volumes and left atriums were larger among hemodialysis patients with atrial fibrillation than those without. Others have found that lower postdialysis diastolic BP is associated with atrial fibrillation [39]. Similarly, our group recently showed that lower systolic and diastolic BP and more intradialytic hypotensive episodes associated with incident atrial fibrillation [16].
The pathophysiology of atrial fibrillation is complex and involves a focal ectopic firing, most commonly triggered by delayed afterdepolarizations, in the setting of a vulnerable substrate that can maintain reentry [40]. Structural remodeling is a key contributor to reentry abnormalities and includes changes in atrial fibrosis, size and cellular ultrastructure [40]. Greater atrial fibrosis and larger atrial size are both structural risk factors for the development of atrial fibrillation [41]. Such structural heart disease is common in dialysis patients, particularly those with hypertension and heart failure, and the hemodialysis procedure itself can induce both transient and long-standing cardiac structural changes [23, 26, 42]. While some structural changes are volume overload dependent (e.g. dilation of right cardiac chambers) [43], others are volume depletion dependent [24, 25]. For example, intradialytic echocardiographs have revealed cardiac ‘stunning’ in the setting of higher UF rates that, over time, can induce cardiac remodeling and create a cardiac milieu conducive to arrhythmia development [23, 26, 42].
Importantly, the UF rate is a modifiable risk factor for atrial fibrillation that may be driven by either higher UF volumes or shorter treatment times. Our analyses are insufficient to determine if the observed association between higher UF rates and incident atrial fibrillation were driven by higher UF volumes or shorter treatment times. However, our subgroup analyses suggest that the observed association is similar among patients with higher (versus lower) UF volumes, shorter (versus longer) treatment times and lower (versus higher) body mass indices. Further disentangling these associations across the components of UF rate should be fodder for future analyses, as findings will be central to guiding selection of a UF rate mitigation strategy. Lowering UF rates can be accomplished by increasing hemodialysis treatment time or reducing UF volume. The latter is typically accomplished by lowering interdialytic weight gains through dietary restrictions or increasing treatment frequency. However, in cases where UF volumes are reduced without a simultaneous reduction in fluid gain or extension of treatment duration, patients may become volume expanded. Large observational studies using objective measures of volume status show an association between extracellular volume overload and mortality [44], providing caution that it may be imprudent to lower UF rates at the expense of residual volume expansion. When trying to prevent atrial fibrillation, this may be particularly true as volume expansion–induced cardiac remodeling is also a risk factor for the development of atrial fibrillation [40, 41]. Additional studies aimed at understanding the relative contributions of rapid UF rates and extracellular volume overload to clinical outcomes such as atrial fibrillation are needed.
Our analysis has several strengths, including the use of linked administrative claims and electronic health record databases, facilitating the study of hemodialysis treatment-level risk factors for atrial fibrillation; study of a nationally representative cohort of older incident hemodialysis patients and utilization of analytic methods that support evaluation of time-updated UF rates. However, our findings must be considered in the context of study limitations. First, we identified incident atrial fibrillation using administrative claims. While prior studies have demonstrated the validity of this approach [1, 28], some outcome misclassification may exist. However, such misclassification would likely be noninformative, biasing results to the null. Second, we do not have reliable data on residual kidney function and cannot account for potential confounding from residual kidney function or other unobserved differences between exposure groups. Third, we cannot exclude the possibility that UF rates are increased in response to the occurrence of atrial fibrillation. However, the use of a 30-day exposure assessment window prior to the 30-day outcome assessment window likely reduces this possibility. Fourth, models were not adjusted for dialysate composition, concomitant medications or time-varying changes in the dialysis prescription (i.e. estimated dry weight, dialysate composition), thus related confounding may remain. Fifth, we studied an older incident hemodialysis population to ensure Medicare coverage in the pre–kidney failure period. Our findings may not generalize to patients who are younger, have been receiving hemodialysis longer or who are receiving home hemodialysis or peritoneal dialysis.
In conclusion, we demonstrated that a higher UF rate, defined several ways, was associated with a higher hazard of incident atrial fibrillation. The UF rate–associated risk of atrial fibrillation began at rates lower than the Centers for Medicare and Medicaid Services quality reporting metric-specified UF rate level (i.e. 13 mL/h/kg) [29] and followed a dose–response pattern. Our results provide additional evidence regarding the harms of higher UF rates and underscore the need for trials investigating the impact of UF rate–lowering strategies on clinical and patient-reported outcomes.
ACKNOWLEDGEMENTS
The data reported here were provided by the USRDS. The interpretation and reporting of these data are the responsibility of the authors and in no way should be seen as official policy or interpretation of the US government.
FUNDING
J.E.F. and W.C.W. were supported by R01 HL152034 from the National Heart, Lung, and Blood Institute of the National Institutes of Health (NIH). J.E.F. was supported by K23 DK109401 from the National Institute of Diabetes and Digestive and Kidney Diseases of the NIH. S.L., M.E.M.-R., W.CW. and T.I.C. were supported by R01 DK 095024 from the NIDDK of the NIH, which specifically provided funding for this study.
AUTHORS’ CONTRIBUTIONS
J.E.F., W.C.W., and T.I.C. were responsible for conception and oversight. S.L. and M.E.M.-R. were responsible for analysis of data. All authors were involved in the interpretation of data, article drafting and final approval of the manuscript.
DATA AVAILABILITY STATEMENT
The data used in this study are available from DaVita Clinical Research and the U.S. Renal Data System (USRDS), but restrictions apply to the availability of these data, which were used under data use agreements for the current study, and are not publicly available without additional agreements.
CONFLICT OF INTEREST STATEMENT
In the last 3 years, J.E.F. received speaking honoraria from American Renal Associates, the American Society of Nephrology, Dialysis Clinic, the National Kidney Foundation and multiple universities, as well as investigator-initiated research funding from the Renal Research Institute, a subsidiary of Fresenius Medical Care, North America. J.E.F. is on the medical advisory board of NxStage Medical and has received consulting fees from Fresenius Medical Care, North America and AstraZeneca. W.C.W. has served as a consultant to Akebia, AstraZeneca, Bayer, Daichii-Sankyo, Janssen Pharmaceuticals, Merck, Relypsa and Vifor Fresenius Medical Care Renal Pharma. T.I.C. has received funding paid by Janssen Pharmaceuticals to Stanford University; served as a consultant for Bayer, Janssen Pharmaceuticals, Novo Nordisk, Fresenius Medical Care, Tricida, Gilead and AstraZeneca and has received grant support from Satellite Healthcare.
REFERENCES
- 1. Goldstein BA, Arce CM, Hlatky MA. et al. Trends in the incidence of atrial fibrillation in older patients initiating dialysis in the United States. Circulation 2012; 126: 2293–2301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Airy M, Chang TI, Ding VY. et al. Risk profiles for acute health events after incident atrial fibrillation in patients with end-stage renal disease on hemodialysis. Nephrol Dial Transplant 2018; 33: 1590–1597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Zimmerman D, Sood MM, Rigatto C. et al. Systematic review and meta-analysis of incidence, prevalence and outcomes of atrial fibrillation in patients on dialysis. Nephrol Dial Transplant 2012; 27: 3816–3822 [DOI] [PubMed] [Google Scholar]
- 4. Winkelmayer WC, Patrick AR, Liu J. et al. The increasing prevalence of atrial fibrillation among hemodialysis patients. J Am Soc Nephrol 2011; 22: 349–357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Roy-Chaudhury P, Tumlin JA, Koplan BA. et al. Primary outcomes of the Monitoring in Dialysis Study indicate that clinically significant arrhythmias are common in hemodialysis patients and related to dialytic cycle. Kidney Int 2018; 93: 941–951 [DOI] [PubMed] [Google Scholar]
- 6. Freeman JV, Wang Y, Akar J. et al. National trends in atrial fibrillation hospitalization, readmission, and mortality for medicare beneficiaries, 1999–2013. Circulation 2017; 135: 1227–1239 [DOI] [PubMed] [Google Scholar]
- 7. Airy M, Schold JD, Jolly SE. et al. Cause-specific mortality in patients with chronic kidney disease and atrial fibrillation. Am J Nephrol 2018; 48: 36–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chatterjee NA, Chae CU, Kim E. et al. Modifiable risk factors for incident heart failure in atrial fibrillation. JACC Heart Fail 2017; 5: 552–560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Pandey A, Kim S, Moore C. et al. Predictors and prognostic implications of incident heart failure in patients with prevalent atrial fibrillation. JACC Heart Fail 2017; 5: 44–52 [DOI] [PubMed] [Google Scholar]
- 10. Seliger SL, Gillen DL, Tirschwell D. et al. Risk factors for incident stroke among patients with end-stage renal disease. J Am Soc Nephrol 2003; 14: 2623–2631 [DOI] [PubMed] [Google Scholar]
- 11. Kumar N, Khera R, Garg N.. Atrial fibrillation associated hospitalizations in patients with end-stage renal disease in the United States, 2003–2012. Heart Rhythm 2016; 13: 2027–2033 [DOI] [PubMed] [Google Scholar]
- 12. Lau DH, Nattel S, Kalman JM. et al. Modifiable risk factors and atrial fibrillation. Circulation 2017; 136: 583–596 [DOI] [PubMed] [Google Scholar]
- 13. Niu J, Shah MK, Perez JJ. et al. Dialysis modality and incident atrial fibrillation in older patients with ESRD. Am J Kidney Dis 2019; 73: 324–331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Buiten MS, de Bie MK, Rotmans JI. et al. The dialysis procedure as a trigger for atrial fibrillation: new insights in the development of atrial fibrillation in dialysis patients. Heart 2014; 100: 685–690 [DOI] [PubMed] [Google Scholar]
- 15. Wong MC, Kalman JM, Pedagogos E. et al. Temporal distribution of arrhythmic events in chronic kidney disease: highest incidence in the long interdialytic period. Heart Rhythm 2015; 12: 2047–2055 [DOI] [PubMed] [Google Scholar]
- 16. Chang TI, Liu S, Airy M. et al. Blood pressure and incident atrial fibrillation in older patients initiating hemodialysis. Clin J Am Soc Nephrol 2019; 14: 1029–1038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Assimon MM, Wenger JB, Wang L. et al. Ultrafiltration rate and mortality in maintenance hemodialysis patients. Am J Kidney Dis 2016; 68: 911–922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Flythe JE, Kimmel SE, Brunelli SM.. Rapid fluid removal during dialysis is associated with cardiovascular morbidity and mortality. Kidney Int 2011; 79: 250–257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Saran R, Bragg-Gresham JL, Levin NW. et al. Longer treatment time and slower ultrafiltration in hemodialysis: associations with reduced mortality in the DOPPS. Kidney Int 2006; 69: 1222–1228 [DOI] [PubMed] [Google Scholar]
- 20. Movilli E, Gaggia P, Zubani R. et al. Association between high ultrafiltration rates and mortality in uraemic patients on regular haemodialysis. A 5-year prospective observational multicentre study. Nephrol Dial Transplant 2007; 22: 3547–3552 [DOI] [PubMed] [Google Scholar]
- 21. Chazot C, Vo-Van C, Lorriaux C. et al. Even a moderate fluid removal rate during individualised haemodialysis session times is associated with decreased patient survival. Blood Purif 2017; 44: 89–97 [DOI] [PubMed] [Google Scholar]
- 22. Kim TW, Chang TI, Kim TH. et al. Association of ultrafiltration rate with mortality in incident hemodialysis patients. Nephron 2018; 139: 13–22 [DOI] [PubMed] [Google Scholar]
- 23. Burton JO, Korsheed S, Grundy BJ. et al. Hemodialysis-induced left ventricular dysfunction is associated with an increase in ventricular arrhythmias. Ren Fail 2008; 30: 701–709 [DOI] [PubMed] [Google Scholar]
- 24. Burton JO, Jefferies HJ, Selby NM. et al. Hemodialysis-induced cardiac injury: determinants and associated outcomes. Clin J Am Soc Nephrol 2009; 4: 914–920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Burton JO, Jefferies HJ, Selby NM. et al. Hemodialysis-induced repetitive myocardial injury results in global and segmental reduction in systolic cardiac function. Clin J Am Soc Nephrol 2009; 4: 1925–1931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. McIntyre CW, Odudu A.. Hemodialysis-associated cardiomyopathy: a newly defined disease entity. Semin Dial 2014; 27: 87–97 [DOI] [PubMed] [Google Scholar]
- 27. US Renal Data System. 2014 Researcher’s Guide to the USRDS Database. Bethesda, MD: National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, 2014.
- 28. Kokotailo RA, Hill MD.. Coding of stroke and stroke risk factors using International Classification of Diseases, Revisions 9 and 10. Stroke 2005; 36: 1776–1781 [DOI] [PubMed] [Google Scholar]
- 29. Centers for Medicare and Medicaid Services. ESRD QIP Summary: Payment Years 2019–2024. https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/ESRDQIP/Downloads/ESRD-QIP-Summary-Payment-Years-2019-2024.pdf (6 August 2020, date last accessed)
- 30. Rhee JJ, Zheng Y, Montez-Rath ME. et al. Associations of glycemic control with cardiovascular outcomes among US hemodialysis patients with diabetes mellitus. J Am Heart Assoc 2017; 6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Austin PC, Fine JP.. Practical recommendations for reporting fine-gray model analyses for competing risk data. Stat Med 2017; 36: 4391–4400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Van Buuren S, Boshuizen HC, Knook DL.. Multiple imputation of missing blood pressure covariates in survival analysis. Stat Med 1999; 18: 681–694 [DOI] [PubMed] [Google Scholar]
- 33. Van Buuren S, Brand JP, Groothuis-Oudshoorn C. et al. Fully conditional specification in multivariate imputation. J Stat Comput Simul 2006; 76: 1049–1064 [Google Scholar]
- 34. White IR, Royston P.. Imputing missing covariate values for the Cox model. Stat Med 2009; 28: 1982–1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Montez-Rath ME, Winkelmayer WC, Desai M.. Addressing missing data in clinical studies of kidney diseases. Clin J Am Soc Nephrol 2014; 9: 1328–1335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Little RJ, Rubin DB.. Statistical Analysis with Missing Data. Hoboken, NJ, USA: John Wiley & Sons, 2002 [Google Scholar]
- 37. Hussein WF, Arramreddy R, Sun SJ. et al. Higher ultrafiltration rate is associated with longer dialysis recovery time in patients undergoing conventional hemodialysis. Am J Nephrol 2017; 46: 3–10 [DOI] [PubMed] [Google Scholar]
- 38. Lee YJ, Okuda Y, Sy J. et al. Ultrafiltration rate, residual kidney function, and survival among patients treated with reduced-frequency hemodialysis. Am J Kidney Dis 2020; 75: 342–350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Franczyk B, Gluba-Brzózka A, Bartnicki P. et al. The occurrence of atrial fibrillation in dialysis patients and its association with left atrium volume before and after dialysis. Int Urol Nephrol 2017; 49: 1071–1077 [DOI] [PubMed] [Google Scholar]
- 40. Andrade J, Khairy P, Dobrev D. et al. The clinical profile and pathophysiology of atrial fibrillation: relationships among clinical features, epidemiology, and mechanisms. Circ Res 2014; 114: 1453–1468 [DOI] [PubMed] [Google Scholar]
- 41. Nattel S, Harada M.. Atrial remodeling and atrial fibrillation: recent advances and translational perspectives. J Am Coll Cardiol 2014; 63: 2335–2345 [DOI] [PubMed] [Google Scholar]
- 42. McIntyre CW. Haemodialysis-induced myocardial stunning in chronic kidney disease – a new aspect of cardiovascular disease. Blood Purif 2010; 29: 105–110 [DOI] [PubMed] [Google Scholar]
- 43. Loutradis C, Sarafidis PA, Papadopoulos CE. et al. The ebb and flow of echocardiographic cardiac function parameters in relationship to hemodialysis treatment in patients with ESRD. J Am Soc Nephrol 2018; 29: 1372–1381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Zoccali C, Moissl U, Chazot C. et al. Chronic fluid overload and mortality in ESRD. J Am Soc Nephrol 2017; 28: 2491–2497 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data used in this study are available from DaVita Clinical Research and the U.S. Renal Data System (USRDS), but restrictions apply to the availability of these data, which were used under data use agreements for the current study, and are not publicly available without additional agreements.