Abstract
Background:
Sleep dysfunction is common and disabling in persons with Parkinson’s Disease (PD). Exercise improves motor symptoms and subjective sleep quality in PD, but there are no published studies evaluating the impact of exercise on objective sleep outcomes.
Objective:
This study aimed to determine if high-intensity exercise rehabilitation combining resistance training and bodyweight interval training, compared to a sleep hygiene control, improved objective sleep outcomes in PD.
Methods:
Persons with PD (Hoehn & Yahr stage 2-3; age ≥45; not in a regular exercise program) were randomized to exercise (supervised 3x/week for 16 weeks) (N=27) or a sleep hygiene, no-exercise control (in-person discussion and monthly phone calls) (N=28). Participants underwent polysomnography at baseline and post-intervention. Change in sleep efficiency was the primary outcome, measured from baseline to post-intervention. Intervention effects were evaluated with general linear models with measurement of group x time interaction. As secondary outcomes, we evaluated changes in other aspects of sleep architecture and compared the effects of acute and chronic training on objective sleep outcomes.
Results:
The exercise group showed significant improvement in sleep efficiency compared to the sleep hygiene group (group x time interaction: F=16.0 p<0.001, d=1.08). Other parameters of sleep architecture also improved in exercise compared to sleep hygiene, including total sleep time, wake after sleep onset, and slow wave sleep. Chronic, but not acute exercise improved sleep efficiency compared to baseline.
Conclusion:
High-intensity exercise rehabilitation improves objective sleep outcomes in PD. Exercise is an effective non-pharmacological intervention to improve this disabling non-motor symptom in PD.
Keywords: Parkinson’s disease, Sleep, Exercise, Rehabilitation, Polysomnography
INTRODUCTION
Parkinson’s disease (PD) is a progressive neurodegenerative disorder with motor and non-motor symptoms, including sleep dysfunction. Non-motor symptoms adversely affect quality of life and are often more bothersome than motor symptoms1,2. Sleep disorders affect 74-98% of PD patients3,4 and include sleep fragmentation, REM sleep behavior disorder, daytime sleepiness, and insomnia5. PD patients also have alterations in sleep architecture, with reductions in sleep efficiency (percentage of time in bed that is actually spent asleep), total sleep time, and slow wave sleep6. In addition to negatively affecting quality of life, sleep disorders in PD are associated with depression, psychosis, autonomic dysfunction, worse motor disability, fatigue, and neuroinflammation5,7–9.
Despite the significant negative impact of sleep dysfunction in PD, few pharmacologic therapies effectively improve these symptoms and available treatments can have detrimental side effects10. Non-pharmacological therapies such as exercise are therefore promising alternatives for treatment of sleep dysfunction in PD. Studies investigating the influence of exercise on PD have shown beneficial effects on motor symptoms and quality of life and have been found to be safe and feasible11–14. Our prior work showed that high-intensity exercise rehabilitation combining resistance training with bodyweight interval training improves motor symptoms, quality of life, neuromuscular performance, motor unit integrity, and muscle mitochondrial function in PD11. Further, functional MRI showed that this intervention led to heightened resting state activity of the substantia nigra and the prefrontal cortex15. However, there are knowledge gaps in our understanding of the effects of exercise on sleep in PD.
In healthy adults, regular exercise improves objective sleep outcomes such as sleep efficiency, slow wave sleep, total sleep time, and latency to sleep onset while also improving subjective sleep quality16–18. In PD, exercise has been shown to improve subjective sleep quality, but there are no published studies documenting the effects of exercise on objective sleep outcomes, as measured by polysomnography19,20. This randomized, controlled, exercise rehabilitation clinical trial investigated the impact of high-intensity exercise rehabilitation on objective measures of sleep. We hypothesized that exercise training would increase sleep efficiency in PD compared to a no-exercise, sleep hygiene control.
METHODS
Participants:
This randomized, controlled, clinical trial randomized 71 participants. Using a per-protocol, efficacy design, fifty-five participants (27 in exercise (EX) group and 28 in sleep hygiene (SH-C) group) completed the protocol and were therefore included in the final analysis as described in the CONSORT flow diagram (Figure 1). Intention to treat analyses were also performed as secondary analyses. The study was registered at clinicaltrials.gov (NCT02593955) and was approved by the University of Alabama at Birmingham (UAB) Institutional Review Board. All participants gave written, informed consent prior to participation.
FIG. 1.
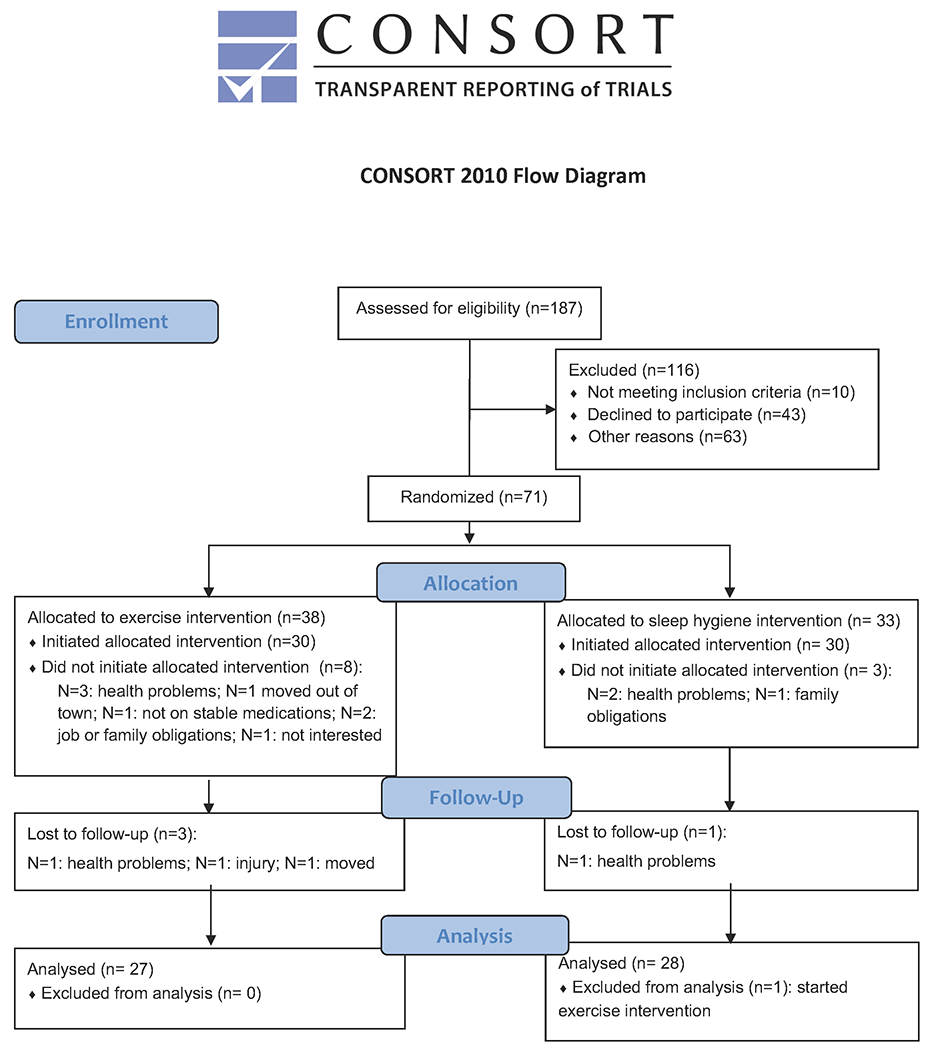
Consort Flow Diagram
Participants were recruited from the UAB Movement Disorders Center and local PD support groups between October 2015 and February 2018. Inclusion required age ≥45, clinical diagnosis of idiopathic PD, Hoehn and Yahr stage 2-3; and stable medication regimen for at least 4 weeks prior to study entry without anticipation of medication change during the study. Potential participants were excluded for: meeting or exceeding U.S. Health and Human Services physical activity guidelines (≥150 minutes/week of moderate-intensity aerobic activity or 75 minutes/week of vigorous-intensity aerobic activity and muscle strengthening activities involving all muscle groups 2 or more days/week)21; findings suggestive of atypical or secondary Parkinsonism, including cerebellar signs, supranuclear gaze palsy, apraxia, prominent autonomic failure, or other cortical signs; multiple strokes with stepwise progression of symptoms; neuroleptic treatment at time of study entry or time of onset of Parkinsonism; inability to walk without a cane or walker; deep brain stimulation; contraindication to an exercise program; Montreal Cognitive Assessment (MoCA) score <18; use of investigational drugs; or untreated sleep apnea. Study screening included home nocturnal pulse oximetry to assess sleep apnea risk. Participants with a desaturation index ≥5 events/hour had to undergo formal clinical sleep testing to evaluate for sleep apnea and, if diagnosed, had to be treated with continuous positive airway pressure (CPAP) for at least 6 weeks prior to study entry. If sleep apnea was diagnosed during the research polysomnography (PSG), the participant was removed from the study and allowed to reenter later following at least 6 weeks of CPAP treatment.
To ensure balance across participants, computer-generated, stratified randomization was performed based on age and sex: 1) 10 females age 45-65; 2) 10 females age >65; 3) 20 males age 45-65; 4) 20 males age >65. If a randomized participant did not initiate the intervention, the participant was replaced within that stratum. Allocation sequence was concealed from the investigator enrolling and assessing eligibility (AWA), and randomization assignment was revealed sequentially after enrollment.
Assessments:
All assessments were performed at baseline and following the 16-week intervention period. Additionally, at the 16-week time point, EX participants underwent two PSGs to assess both chronic and acute effects of exercise: one on a non-exercise night (chronic exercise: CEX) (4-6 nights after final exercise training session) and one on an exercise night (acute exercise: AEX) (night of final exercise training session). The CEX PSG was chosen a priori as the primary outcome because 1) we were most interested in the effects of chronic exercise training on objective sleep outcomes; 2) acute exercise can have differential effects on sleep compared to chronic exercise17; and 3) this would be a better comparator to the sleep hygiene participants who did not exercise. As an exploratory outcome, we also evaluated the effects of acute exercise by comparing sleep architecture at baseline, AEX, and CEX.
Polysomnography:
Laboratory-based PSG recordings included electroencephalography (leads F3, F4, C3, C4, O1, and O2 referenced to contralateral mastoid), submental and bilateral anterior tibialis and extensor digitorum communis electromyograms, electrooculogram, airflow monitoring with thermocouple and nasal pressure, respiratory effort using polyvinylidene fluoride belts at the chest and abdomen, pulse oximetry, and video. PSGs were scored by a certified sleep technician and a board-certified Sleep Medicine physician (AWA). PSGs were labeled with a study code to allow blinding of PSG interpretation.
PSGs were started at approximately 10pm and duration of recording was 8 hours. Participants remained on their regular medication schedule. PSGs were evaluated for sleep architecture, including sleep efficiency, total sleep time (TST), wake after sleep onset (WASO) (amount of time spent awake after sleep onset), latency to sleep onset, time and percentage of each sleep stage (N1, N2, N3, and REM), latency to first REM period, arousal index, periodic limb movement (PLMS) index, apnea hypopnea index (AHI), and REM sleep without atonia. REM sleep without atonia was scored according to American Academy of Sleep Medicine Manual for the Scoring of Sleep and Associated Events22.
Additional Assessments:
Participants were also evaluated with the Movement Disorders Society-Unified Parkinson’s Disease Rating Scale (MDS-UPDRS)23, Pittsburgh Sleep Quality Index (PSQI), Epworth Sleepiness Scale (ESS), Fatigue Severity Scale (FSS), and the psychomotor vigilance task (PVT)24 (PVT-192; Ambulatory Monitoring, Inc., Ardsley, NY). The PVT is a handheld device that objectively measures participant reaction time to a light stimulus that appears at a random inter-stimulus interval over a 10-minute test. The PVT measures mean reciprocal reaction time (RRT) (response time) and lapses, both of which are sensitive to sleep deprivation25.
Intervention:
Exercise intervention (EX):
Participants randomized to EX had supervised exercise training 3x/week for 16 weeks at the UAB Center for Exercise Medicine (UCEM). All exercise sessions were performed prior to 2pm, and most were in the morning. Participants maintained their typical medication schedule and were encouraged to exercise at the time of day that they felt their PD medications were most effective. Exercise training consisted of a combination of resistance training (RT) and bodyweight functional mobility exercises with limited rest intervals that we previously used in PD to challenge strength, power, balance, and endurance11. After a familiarization session, resistance training volume and intensity progressed during a ramp-up phase over the first four sessions by increasing the number of sets (i.e. 1st day 1 set, 2nd day 1 set, 3rd day 2 sets, 4th day 3 sets). Thereafter, RT intensity/training loads targeted 10-repetition maximum (10RM) in sessions 1 and 3 each week. For session 2, resistance loads were reduced ~30% with greater emphasis on maximizing speed of movement during the concentric phase (eccentric phase was controlled/slowed) for 12 repetitions/set. The RT component of the prescription was adapted from our prior dose-response optimization trial in older adults, which we also implemented in our recent exercise-drug interaction trial26,27. The full volume exercise prescription consisted of: 1) five movements to improve strength and muscle mass (leg press, knee extension, chest press, overhead press, pull down), each performed for 3 sets of 8-12 repetitions (~30 repetitions at 10RM during sessions 1, 3; ~36 total repetitions during session 2); 2) trunk exercises to improve postural stability (trunk extension and flexion); and 3) 3-4 bodyweight exercises to improve power and balance (e.g. step up, squat, jump squat, lunge, side lunge, push-up, assisted pull-up, assisted dip). Bodyweight movements were modified as necessary to match abilities (e.g. weight assistance, bench or wall push-ups, etc. as necessary). For bodyweight movements, the goal was to accumulate at least 50 repetitions in each of 3-4 exercises/session. Resistance exercise movements and bodyweight movements were coupled/alternated while stressing different muscle groups (e.g. a set of chest press followed immediately by step ups, with the sequence repeated twice more before moving onto the next coupled combination, e.g., overhead press and lunge). Heart rate (HR) was recorded throughout each session via Polar HR monitor and helped determine the short rest intervals between sets. Experienced trainers certified by the American College of Sports Medicine and/or the National Strength and Conditioning Association supervised all sessions.
Sleep hygiene intervention (SH-C):
Participants randomized to SH-C received suggestions for improving sleep hygiene through discussion with a board-certified sleep medicine physician (AWA). The participants had an opportunity to express specific sleep complaints and had directed recommendations for improvement. They were provided a handout with tips for improving sleep hygiene and a recommendation for a book that describes sleep relaxation techniques and tips for improving symptoms of insomnia. The duration of these discussions was 30-60 minutes. Participants were also contacted by telephone every 4 weeks to address any questions about sleep hygiene measures and to maintain engagement in the study.
Statistical Analysis:
The study was a randomized, controlled, interventional design, with primary analysis performed per protocol. The primary outcome measure was the change in sleep efficiency within-participant, as measured by PSG, from baseline to post-16-week intervention (non-exercise night; CEX), compared between the two intervention groups. Sleep efficiency is defined as the percentage of time in bed actually spent asleep ([total sleep time/total time in bed] x 100). Based on a prior clinical trial28, we estimated SD of 7% and mean difference of 4.8%. Sample size of 27 per group would have 80% power to detect a change in sleep efficiency in EX compared to SH-C. The study was initially designed based on a one-sided test, but we report two-sided p-values to be more conservative. Secondary analyses included changes in other measures of sleep architecture and subjective sleep outcomes (PSQI, ESS, and FSS) compared between groups. As additional secondary analyses, the differences between the three PSGs (baseline, AEX, CEX) in the EX group were evaluated. Intention to treat (ITT) analysis were also performed with inclusion of five additional participants to determine if the results could be extrapolated to participants who did not complete the protocol. One SH-C participant had post-intervention data but was excluded from the per protocol analysis due to initiating a high-intensity exercise intervention outside of the study protocol. The other four participants (three EX and one SH-C) included in the ITT analysis did not have post-intervention data collected. Therefore, imputation was performed by using the mean post-intervention value for all participants who completed the protocol (N=55) for these missing objective and subjective sleep outcome values.
Statistical analyses were performed using JMP Pro 14 (SAS Institute, Inc. Cary, NC). Summary statistics were calculated and tested for normality (Shapiro-Wilk). Group comparisons of baseline demographics and clinical characteristics (EX versus SH-C) were assessed with independent-sample t-tests for normally distributed data and with nonparametric tests (Mann-Whitney U) for non-normally distributed data. The primary statistical methods for the intervention effects were general linear models with measurement of group x time interaction. Effect sizes were evaluated with Cohen’s d. Because the objective sleep outcomes evaluated as secondary outcomes are not independent, we did not correct for multiple comparisons. To control for the potential contribution of change in motor symptoms to the sleep outcomes, a model was run with the dependent variable as changes in sleep outcome (i.e. sleep efficiency; N3 time, or total sleep time) and predictor variables as change in MDS-UPDRS part III and group. Sleep architecture differences across the time points (baseline, post-intervention exercise night (AEX) PSG, and post-intervention non-exercise night (CEX) PSG) were compared in EX with mixed model repeated measures ANOVA. If significant differences were found between the PSGs, Tukey’s HSD multiple comparison procedure was used to determine which nights were different.
RESULTS
Participant Characteristics and Exercise Adherence
Baseline demographics and clinical characteristics for participants are shown in Table 1. There were no significant group differences in age, sex, duration of disease, MDS-UPDRS scores, levodopa equivalent dose (LED), or dopamine agonist LED. Training progression and adherence was emphasized and adherence to EX averaged 92.2 ± 12.5% of sessions. Twenty-three out of 27 participants (85%) in EX had >90% adherence.
Table 1:
Baseline Demographics and Participant Characteristics
| Exercise Group | Sleep Hygiene Group | p-value | |
|---|---|---|---|
| N | 27 | 28 | -- |
| Randomization Strata: N | |||
| Males age 45-65 | 9 | 9 | |
| Females age 45-65 | 5 | 4 | X=0.72 |
| Males age >65 | 7 | 10 | p=0.87 |
| Females age >65 | 6 | 5 | |
| Age | |||
| Mean ± SD | 65.33 ± 8.17 | 65.82 ± 5.19 | t=0.26 |
| Range | 45-78 | 54-77 | p=0.79 |
| Sex N (%) | |||
| Male | 16 (59.3) | 19 (67.9) | X=0.44 |
| Female | 11 (40.7) | 9 (32.1) | p=0.51 |
| DOD (years) | z=−1.57 | ||
| Median (IQR) | 6.0 (3.0-9.0) | 3.0 (1.0-7.5) | p=0.12 |
| MDS-UPDRS part I | z=−0.96 | ||
| Median (IQR) | 7.0 (5.0-11.0) | 9 (6.0-12.5) | p=0.34 |
| MDS-UPDRS part II | t=−1.31 | ||
| Mean ± SD | 11.11 ± 5.88 | 9.14 ± 5.28 | p=0.20 |
| MDS-UPDRS part III | t=−1.45 | ||
| Mean ± SD | 33.48 ± 12.39 | 28.11 ± 15.02 | p=0.15 |
| MDS-UPDRS part IV | z=0.14 | ||
| Median (IQR) | 3.0 (0.75-5.0) | 3.0 (0.0-6.0) | p=0.88 |
| MDS-UPDRS Total | t=−1.20 | ||
| Mean ± SD | 56.46 ± 18.13 | 50.07 ± 20.91 | p=0.23 |
| LED | z=−1.08 | ||
| Median (IQR) | 640.0 (440.0-855.0) | 482.5 (300.0-748.8) | p=0.28 |
Normality tested with Shapiro-Wilks and non-parametric test reported (Wilcoxon z) if not normal. DOD: Duration of disease; LED: Levodopa Equivalent Dose; MDS-UPDRS: Movement Disorders Society-Unified Parkinson’s Disease Rating Scale.
Objective Sleep Outcomes
There were no group differences in sleep parameters at baseline. Participants in EX had a significant improvement in sleep efficiency compared to those in SH-C (i.e. significant group x time interaction) (F=16.04, p<0.001, d=1.08) (Table 2; Figure 2). To examine the potential contribution of changes in motor symptoms on sleep outcomes, change in MDS-UPDRS part III was included as a predictor variable for change in sleep efficiency and after adjustment, the model was significant (F=9.22, p=0.0004). However, MDS-UPDRS part III was not a significant predictor of change in sleep efficiency (p=0.44), but group remained significant (F=17.17, p=0.0001). Therefore, we conclude that the observed changes in sleep were due to the exercise intervention and not due to changes in motor symptoms.
Table 2:
Objective and Subjective Sleep Outcomes
| Exercise N=27 | Sleep Hygiene N=28 | Group x Time Interaction |
Δ betw. Groups** Effect size (d) 95% CI |
|||
|---|---|---|---|---|---|---|
|
| ||||||
| Pre-intervention | Post-intervention | Pre-intervention | Post-intervention | |||
| Sleep Efficiency * |
F=16.04
p<0.001 |
12.1 d=1.08 (5.1, 18.9) |
||||
| Median (IQR) | 76.8 (67.5-86.2) | 83.1 (76.9-90.7)# | 80.0 (73.2-86.7) | 75.7 (66.6-82.5)+ | ||
| Mean ± SD | 75.1 ± 15.3 | 82.2 ± 12.0 | 78.7 ± 10.2 | 73.8 ± 12.3 | ||
| WASO (min.) * |
F=12.56
p<0.001 |
−54.4 d=0.96 (−89.8, −19.0) |
||||
| Median (IQR) | 90.5 (61.5-147.6) | 67.8 (42.4-98.1)# | 78.1 (52.2-114.9) | 102.4 (65.5-145.9) | ||
| Mean ± SD | 108.1 ± 70.0 | 70.2 ± 83.9 | 89.8 ± 49.2 | 106.4 ± 55.4 | ||
| Total Sleep Time (min) * |
F=7.28
p=0.0093 |
44.5 d=0.73 (6.5, 82.5) |
||||
| Median (IQR) | 388.7 (322.5-414.0) | 403.0 (364.5-436.0)+ | 371.8 (336.9-415.5) | 361.5 (321.3-400.7) | ||
| Mean ± SD | 363.5 ± 75.5 | 393.1 ± 61.1 | 370.0 ± 54.9 | 355.1 ± 62.7 | ||
| Sleep Latency * | F=0.90 p=0.35 |
−9.0 d=0.26 (−30.9, 12.9) |
||||
| Median (IQR) | 7.3 (3.9-14.3) | 4.2 (2.3-14.2) | 9.6 (4.0-14.1) | 12.3 (7.2-25.0)+ | ||
| Mean ± SD | 12.4 ± 13.5 | 14.7 ± 39.2 | 10.5 ± 7.7 | 21.7 ± 28.3 | ||
| −N1 time* (min.) | F=0.27 p=0.60 |
11.7 d=0.31 (−11.6, 34.9) |
||||
| Median (IQR) | 38.0 (28.0-55.0) | 36.0 (24.5-53.5) | 35.0 (27.8-49.8) | 35.3 (23.8-46.9) | ||
| Mean ± SD | 40.0 ± 18.0 | 49.4 ± 47.5 | 42.4 ± 22.3 | 40.1 ± 27.2 | ||
| N1% * | F=0.008 p=0.93 |
−0.2 d=0.02 (−5.2, 4.8) |
||||
| Median (IQR) | 10.3 (7.6-15.2) | 8.9 (6.1-13.5) | 9.9 (6.4-14.0) | 9.1 (7.0-13.8) | ||
| Mean ± SD | 12.0 ± 7.3 | 11.1 ± 8.7 | 12.3 ± 8.6 | 11.6 ± 8.4 | ||
| N2 time* (min.) | F=0.007 p=0.93 |
1.3 d=0.02 (−35.3, 37.9) |
||||
| Mean ± SD | 199.6 ± 51.3 | 191.7 ± 47.8 | 215.9 ± 64.1 | 206.7 ± 61.6 | ||
| N2% * | F=2.88 p=0.096 |
−5.9 d=0.46 (−13.4, 2.1) |
||||
| Median (IQR) | 56.3 (48.0-62.3) | 51.5 (45.0-54.1)# | 56.4 (48.5-67.4) | 58.1 (48.8-66.2) | ||
| Mean ± SD | 55.0 ± 10.3 | 49.0 ± 10.6 | 57.8 ± 12.7 | 57.6 ± 12.3 | ||
| N3 time* (min) |
F=8.08
p=0.006 |
25.1 d=0.77 (4.7, 45.4) |
||||
| Median (IQR) | 50.5 (27.5-101.5) | 88.5 (54.0-130.5)# | 54.8 (16.8-85.9) | 56.8 (17.5-88.0) | ||
| Mean ± SD | 74.3 ± 62.3 | 99.4 ± 75.6 | 55.0 ± 42.6 | 55.0 ± 37.1 | ||
| N3% * | F=1.87 p=0.18 |
3.3 d=0.37 (−2.3, 8.9) |
||||
| Median (IQR) | 16.9 (8.7-28.4) | 23.6 (13.4-29.6)# | 17.0 (4.5-22.4) | 15.8 (5.1-24.4) | ||
| Mean ± SD | 19.7 ± 14.8 | 24.3 ± 15.7 | 14.7 ± 10.9 | 16.0 ± 11.1 | ||
| REM time* (min) | F=1.76 p=0.19 |
14.1 d=0.36 (−10.4, 38.6) |
||||
| Mean ± SD | 49.5 ± 24.3 | 60.2 ± 39.8 | 56.7 ± 30.3 | 53.3 ± 33.6 | ||
| REM % * | F=0.98 p=0.33 |
2.7 d=0.27 (−3.7, 9.1) |
||||
| Mean ± SD | 13.3 ± 6.4 | 15.6 ± 10.3 | 15.3 ± 7.9 | 14.9 ± 9.1 | ||
| REM latency (min.) * | F=0.022 p=0.88 |
4.2 d=0.04 (−66.5, 74.9) |
||||
| Median (IQR) | 141.8 (100.5-293.1) | 139.0 (96.8-256.0) | 147.5 (83.5-207.0) | 123.8 (86.4-184.3) | ||
| Mean ± SD | 187.2 ± 111.7 | 175.1 ± 97.5 | 148.9 ± 74.5 | 133.2 ± 71.5 | ||
| Arousal Index * | F=0.22 p=0.44 |
−0.7 d=0.21 (−2.8, 1.4) |
||||
| Median (IQR) | 3.9 (3.3-5.9) | 3.3 (2.5-5.6) | 4.3 (3.1-5.8) | 3.9 (3.2-7.5) | ||
| Mean ± SD | 5.0 ± 3.6 | 4.5 ± 3.2 | 5.0 ± 2.7 | 5.2 ± 3.0 | ||
| AHI (events/hour) * | F=0.25 p=0.57 |
−0.6 d=0.16 (−2.9, 1.7) |
||||
| Median (IQR) | 0.2 (0-1.1) | 0.3 (0-3.3) | 0.4 (0-1.4) | 0 (0-2.0) | ||
| Mean ± SD | 1.5 ± 3.2 | 1.6 ± 2.7 | 0.9 ± 1.3 | 1.6 ± 4.0 | ||
| PLMS Index * | F=0.189 p=0.67 |
−2.7 d=0.12 (−17.3, 11.8) |
||||
| Median (IQR) | 1.1 (0.2-7.4) | 1.6 (0.5-16.7) | 2.3 (0.6-30.9) | 8.2 (0.9-31.7) | ||
| Mean ± SD | 8.2 ± 14.8 | 11.5 ± 18.5 | 16.1 ± 22.5 | 22.2 ± 32.6 | ||
| % REM with RWA * | F=0.49 p=0.49 |
−0.04 d=0.20 −0.2, 0.1) |
||||
| Median (IQR) | 21.0 (1.8-68.0) | 25.8 (5.3-70.7) | 19.6 (3.9-57.4) | 34.1 (5.6-70.9) | ||
| Mean ± SD | 34.5 ± 35.6 | 39.1 ± 26.5 | 30.8 ± 31.3 | 39.6 ± 33.9 | ||
| RBD * ^ | ||||||
| N (%) | 11 (42.3) | 13 (50) | 12 (46.2) | 17 (60.7) | NA | NA |
| PVT Mean RRT * ↑ | F=0.048 p=0.83 |
0.02 d=0.06 (−0.2, 0.2) |
||||
| Median (IQR) | 3.4 (3.1-3.9) | 3.4 (3.2-3.8) | 3.5 (3.2-3.8) | 3.4 (3.0-4.0) | ||
| Mean ± SD | 3.5 ± 0.5 | 3.5 ± 0.5 | 3.4 ± 0.7 | 3.5 ± 0.8 | ||
| PVT Lapses * ↓ | F=0.001 p=0.98 |
0.03 d=0.01 (−3.0, 3.1) |
||||
| Median (IQR) | 2.0 (1.0-4.0) | 1.5 (0-4.25) | 2.0 (0-4.75) | 2.0 (0-7.5) | ||
| Mean ± SD | 3.2 ± 3.8 | 2.6 ± 2.8 | 6.7 ± 16.8 | 6.1 ± 13.9 | ||
|
| ||||||
| PSQI * ↓ |
F=4.38
p=0.041 |
1.8 d=0.57 (−0.2, 3.8) |
||||
| Median (IQR) | 6.0 (5.0-9.0) | 6.0 (4.0-10.0) | 8.0 (5.0-10.75) | 6.0 (4.0-8.0)+ | ||
| Mean ± SD | 6.9 ± 3.5 | 7.0 ± 3.5 | 8.1 ± 3.5 | 6.4 ± 2.9 | ||
| ESS * ↓ | F=0.60 p=0.44 |
−0.7 d=0.21 (−2.9, 1.4) |
||||
| Mean ± SD | 9.44 ± 4.65 | 9.46 ± 5.20 | 7.39 ± 5.06 | 8.14 ± 5.19 | ||
| FSS * ↓ | F=0.11 p=0.737 |
−1.1 d=0.09 (−8.9, 6.7) |
||||
| Mean ± SD | 36.23 ± 11.57 | 34.74 ± 12.85 | 33.67 ± 11.13 | 33.33 ± 10.59 | ||
For values not normally distributed, median (IQR) and mean ± SD are shown;
No significant difference between values at baseline (p>0.05);
p<0.01 for within group change from pre-intervention to post-intervention;
p<0.05 for within group change from pre-intervention to post-intervention;
Mean difference in change between groups. Within group mean change, effect size, and 95% CI are shown in Supplemental Table 1.
RBD: >27% epochs of REM with RWA;
=higher score is better;
=lower score is better;
AHI: apnea hypopnea index; ESS: Epworth Severity Scale; FSS: Fatigue Severity Scale; N1: stage N1 sleep; N2: stage N2 sleep; N3: stage N3 sleep; PLMS: periodic limb movements; PSQI: Pittsburgh Sleep Quality Index; PVT: psychomotor vigilance task; RBD: REM sleep behavior disorder; REM: rapid eye movement sleep; RWA: REM without atonia; WASO: wake after sleep onset
FIG. 2.
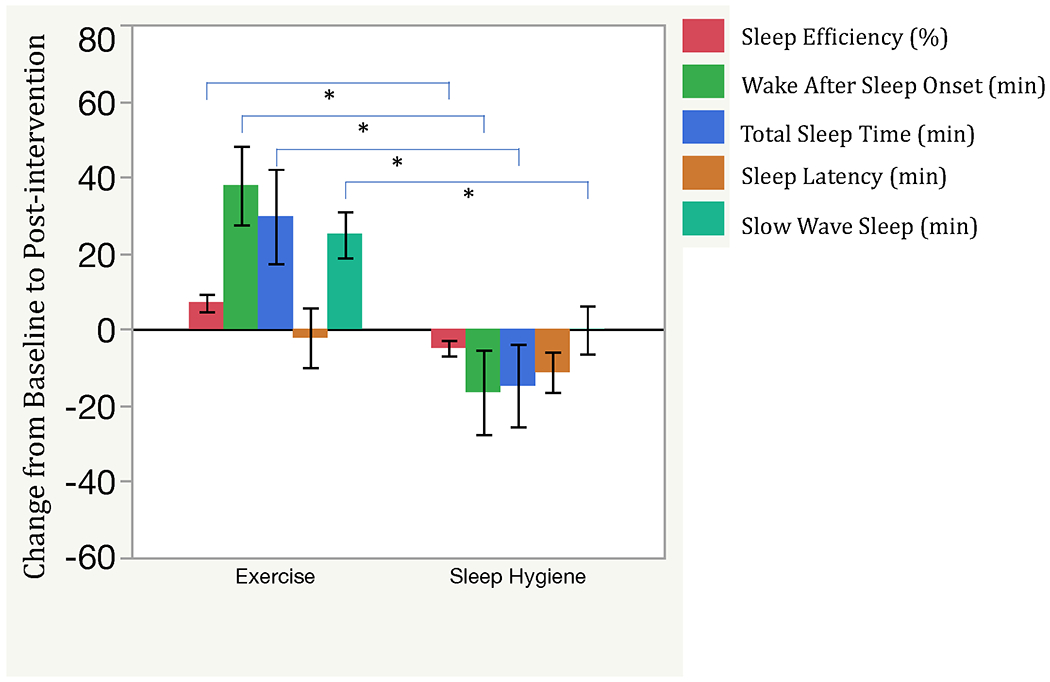
Objective Sleep Outcomes in Exercise and Sleep Hygiene Participants.
In order to show improvement as a positive change, outcomes for which a lower score is better (wake after sleep onset and sleep latency) were multiplied by −1 for this figure.
*Significant group x time interaction (p < 0.01).
As shown in Table 2 and Figure 2, other measures of sleep architecture also improved in EX compared to SH-C. These include significant group x time interactions for WASO, TST, and time spent in N3 (slow wave sleep). There were no significant changes between groups for REM sleep without atonia or PVT-assessed objective vigilance.
Acute and Chronic Exercise Effects
Participants in EX had 3 PSGs: at baseline; post-intervention on an exercise night (acute exercise: AEX); and post-intervention on a non-exercise night (chronic exercise: CEX). Comparison of sleep efficiency at baseline, AEX, and CEX showed significant differences among nights (F=4.04, p=0.0235) (Figure 3A). Tukey’s HSD multiple comparison procedure showed that sleep efficiency was significantly higher with CEX compared to baseline, without significant difference between baseline and AEX. There were also significant differences among the three nights for TST (F=3.66, p=0.0328) (Figure 3B), WASO (F=5.31, p=0.008) (Figure 3C), N3 time (F=9.29, p<0.001) (Figure 3D), N2% (F=3.96; p=0.025), and N3% (F=4.21, p=0.020). Tukey’s HSD multiple comparison procedure showed that TST was significantly higher with CEX compared to AEX; WASO was significantly better (lower) with CEX compared to baseline PSG and compared to AEX; N2% was significantly lower with CEX compared to baseline, and N3% and time spent in N3 were significantly higher with CEX night compared to baseline. There were no significant differences among the three nights (p>0.05) for other measures of sleep architecture. These outcomes are shown in Supplemental Table 2.
FIG. 3.
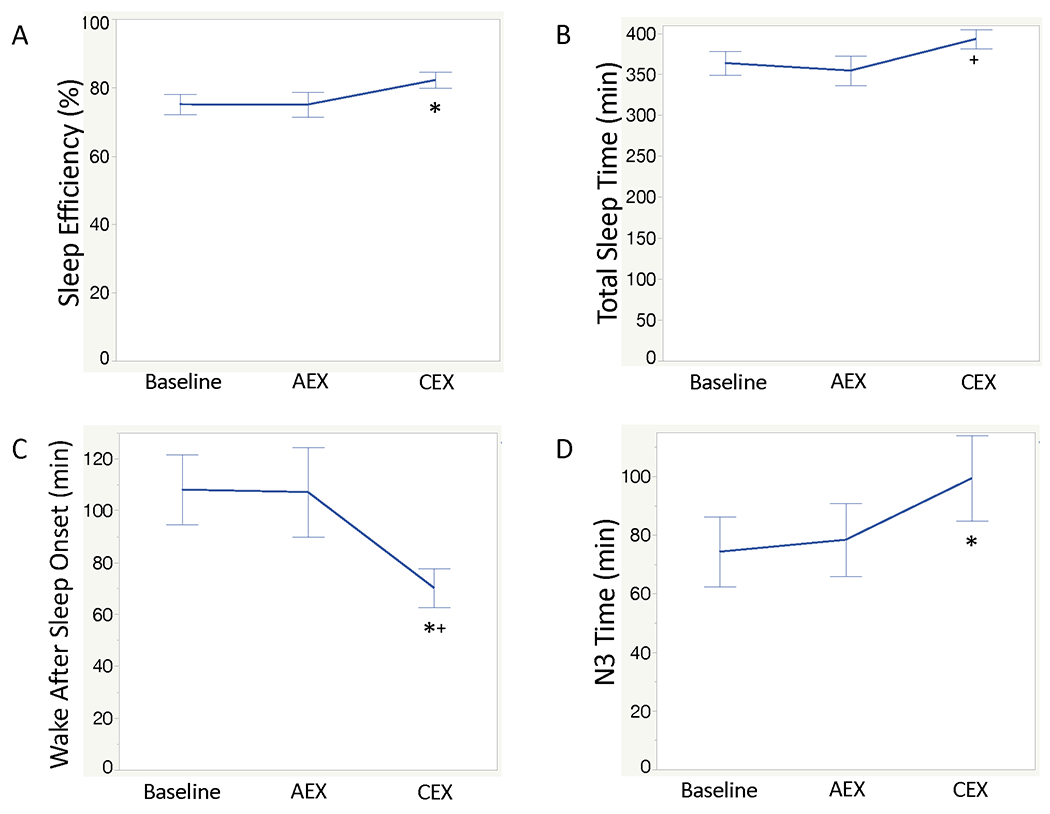
Objective sleep outcomes on PSGs recorded at baseline, in the trained state on an exercise night (AEX), and in the trained state on a nonexercised night (CEX).
A: Sleep Efficiency; B: Total Sleep Time; C: Wake after Sleep Onset; D: Time spent inN3.
*Significant difference from Baseline based on Tukey’s HSD multiple comparisons procedure; +Significantly different from AEX based on Tukey’s HSD multiple comparisons procedure.
Subjective Sleep Outcomes
There was significant improvement in subjective sleep quality assessed by the PSQI in SH-C compared to EX (significant group x time interaction) (Table 2). SH-C showed improvement in sleep quality (p=0.041), while EX did not change. To investigate the aspect of subjective sleep quality driving these changes, we evaluated the PSQI subscores (Sleep Quality, Sleep Latency, Sleep Duration, Sleep Efficiency, Sleep Disturbance, Use of Sleep Medications, and Daytime Dysfunction. These results are shown in Supplemental Table 3. There was a significant improvement in the Sleep Disturbance subscore in SH-C compared to the EX (group x time interaction: F=5.84, p=0.019). Additionally, there was a reduction in the Use of Sleep Medications subscore in EX compared to SH-C (group x time interaction: F=6.60, p=0.013). None of the other subscores had significant differences over time between groups. Similar to the lack of change in the Daytime Dysfunction subscore of the PSQI, there were no changes in subjective sleepiness as measured by the Epworth Sleepiness Scale, or fatigue as measured by the Fatigue Severity Scale in either group (Table 2).
Intention to Treat Analysis
Outcomes from ITT analysis are shown in Supplemental Table 4. EX had significant improvement in sleep efficiency compared to SH-C (group x time interaction; F=5.35, p=0.024). Significant group x time interactions were also noted for WASO and time spent in N3, with improvement in EX compared to SH-C. In contrast to the per protocol analysis, the group x time interactions for TST and subjective sleep quality were no longer significant.
DISCUSSION
This randomized, controlled trial is the first to investigate the effects of exercise rehabilitation on objective sleep outcomes in PD. High-intensity exercise training, when compared to a no-exercise, sleep hygiene control, improved sleep efficiency, total sleep time, time spent in N3 (slow wave sleep) and WASO. In contrast, the sleep hygiene intervention improved subjective sleep quality compared to exercise. Further, the observed effects of exercise on objective sleep were not influenced by changes in motor symptoms (MDS-UPDRS part III). Because pharmacological therapies for sleep dysfunction are often ineffective or have intolerable side effects29, our findings demonstrate an important step forward in identifying non-pharmacological therapies for this common and disabling non-motor symptom.
Prior work investigating the impact of exercise on sleep in PD has been limited30. One controlled study showed that resistance training over 12 weeks improved subjective sleep quality and these self-reported improvements in sleep correlated with improvements in muscle strength20. Two other controlled studies, one using a multimodal exercise intervention and another a Qigong meditative movement intervention, also showed subjective sleep quality improvement19,31. In healthy older adults, exercise training improves objective sleep measures, including increases in sleep efficiency and total sleep time and reductions in latency to sleep onset16,17. To our knowledge, this is the first study to demonstrate exercise-induced objective sleep improvement, measured with PSG, in PD.
Several potential mechanisms underlying exercise-induced changes in sleep have been proposed, and the effects are likely multifactorial. For example, there is a significant bidirectional relationship between sleep and mood, and exercise can improve mood in PD, which may contribute to sleep improvement32. Further, exercise can increase brain-derived neurotrophic factor (BDNF), which is decreased in sleep dysfunction and important for regulation of slow wave sleep (stage N3)33–35. Exercise may also improve sleep by increasing body temperature, thus increasing slow wave sleep, which has been proposed to be important for thermoregulation16,17,36. This mechanism seems less likely an influence in the current study since sleep improved on CEX but not AEX and temperature effects due to exercise are more likely to have acute effects. Additional potential mechanisms of chronic exercise-induced benefits on sleep include reduction of inflammation, increases in growth hormone, alterations in autonomic function/heart rate variability, and changes in neurotransmitters important for sleep regulation16,17,37,38. In light of the prevalence of sleep dysfunction, mood disorders, autonomic dysfunction, and neuroinflammation in PD, the influence of exercise may be particularly relevant for this patient population1,39.
In comparing objective sleep outcomes in the untrained state (baseline) to chronic and acute effects of exercise, sleep architecture was improved with CEX compared to baseline, while the same effects were not seen with AEX. In healthy adults, prior work has shown that both acute and chronic exercise can improve sleep16,17,40. However, other studies have demonstrated no difference in total sleep time due to an acute bout of exercise41. Importantly, no prior work has evaluated objective sleep outcomes in PD in the trained state (i.e. CEX) following a single bout of high intensity exercise (i.e. AEX). Therefore, the cause of the lack of improvement following acute exercise in the trained PD participants is unclear. This effect could be due to the tendency of acute strenuous exercise to increase pro-inflammatory cytokines, including IL-1β, TNF-α, and IL-6, while chronic exercise promotes down-regulation of these pro-inflammatory cytokines42. Thus, alterations in levels of cytokines, which play important roles in sleep regulation, could result in relative worse sleep on a night of acute exercise compared to chronic training43. Another possibility is that there are differential effects of acute and chronic exercise on sleep specific to PD. For example, two proposed mechanisms for the beneficial effects of exercise on sleep are alterations in heart rate variability and increases in body temperature16,17. Perhaps the autonomic dysfunction of PD (including impairments in thermoregulation and cardiac autonomic function) alters the potential beneficial effects of acute exercise in these patients44. Another consideration is that a first-night effect (worse sleep on the first night in the sleep lab) could have influenced sleep at baseline and AEX, with CEX not influenced by this effect due to being performed soon after AEX. However, in our prior studies in PD, the first night effect has not adversely affected objective sleep outcomes45. Further study using different exercise prescriptions and intensities followed by PSG longitudinally is required to elucidate the underlying mechanisms.
We were surprised by the improvement in subjective sleep quality in SH-C compared to EX despite only EX showing objective sleep improvement. This disconnect between objective and subjective sleep in PD has been observed in prior work and therefore subjective sleep outcomes in PD should be interpreted with caution5,46. While it is possible that the sleep hygiene intervention is more beneficial for subjective sleep than the high-intensity exercise intervention, another potential explanation is that our inability to blind participants to their intervention group led to participant bias. For example, the focus of interactions with research staff was on sleep for SH-C. Therefore, it is possible that SH-C participants were unconsciously biased to report improvement in sleep quality. In contrast, many interactions with study personnel for EX were related to exercise in the supervised intervention. The subjective improvement in SH-C could thus be due to placebo effect. Another possibility is that the lack of objective improvement in sleep with AEX may have led to a perception of less improvement in sleep overall among the exercise group. An additional potential contribution is that, although there was no significant difference in PSQI at baseline between groups, the median baseline PSQI was 6.0 in EX and 8.0 in SH-C. Therefore, there may have been less room for improvement in the EX group due to a floor effect. In evaluation of the change in PSQI subscores over time, the Sleep Disturbance subscore improved in SH-C relative to EX. Therefore, while exercise improved objective sleep efficiency measured by PSG, the sleep hygiene intervention seems to have improved the subjective experience of nighttime sleep disturbances. This supports the idea of benefits of sleep hygiene for subjective sleep in PD and certainly the subjective experience is an important one for overall quality of life. There may be sleep benefits in the home environment due to sleep hygiene that are not detectable in the sleep laboratory. These potential improvements in sleep at home may also explain why some aspects of sleep architecture worsened post-intervention in SH-C, in that improvement at home may have reduced sleep drive (due to less sleep deprivation), thus leading to lower sleep efficiency by PSG. Interestingly, participants in EX reported reduced use of sleep medications following the intervention, suggesting that there was some subjective recognition of sleep improvement due to exercise. Perhaps future studies can investigate the utility of a combination of exercise and sleep hygiene to improve both objective and subjective sleep outcomes. In light of the importance of adequate sleep time on overall health, cognition, and mortality, the findings of beneficial effects of exercise on objective sleep remains important47,48.
One interesting and new finding was that chronic exercise training increased slow wave sleep (N3) in PD. This is intriguing because N3 has been proposed to be important for cognition, language, and memory consolidation in non-PD populations49,50. Further, increased slow wave sleep is important for executive function and selective slow wave sleep disruption leads to reduced performance on visuospatial testing51,52. Our own work also showed a relationship between cognitive performance and slow wave sleep in PD53. This raises the possibility that exercise interventions can improve cognition in PD by enhancing slow wave sleep.
This study has several strengths, including the randomized, controlled design; being the only study in PD with PSG evaluation following an exercise intervention; the supervised nature of the intervention; and the excellent adherence to the protocol. There are also some limitations that should be discussed. First, due to the nature of the intervention, it was not possible to blind participants to group assignment, and this may have introduced potential bias. Second, PSG was performed on a single night at each time point, thus not accounting for potential influence of the first night effect. However, in our prior studies in PD, the first night effect has not adversely affected sleep45. Additionally, if the first night effect were playing a role in sleep improvement in the current study, it would have been expected to affect EX and SH-C groups equally. Another potential limitation is that, compared to EX, SH-C had less in-person contact with study staff. Thus, the social benefits of study participation in EX compared to SH-C could have influenced some results, although this would be expected to also influence subjective outcomes. Finally, we did not include a non-PD control group. While this may be useful in future studies, the focus of the current study was to identify effects of exercise on objective sleep outcomes in PD. Further, the effects of exercise on sleep architecture in healthy elderly is well-established16,17.
In conclusion, this is the first study to demonstrate the impact of high intensity exercise on objective sleep outcomes in PD. Specifically, PD participants showed improved sleep efficiency, total sleep time, stage N3 (slow wave sleep), and WASO following a 16-week exercise intervention compared to a sleep hygiene control group. Additionally, this is the first report of changes in sleep architecture comparing untrained, trained, and acute exercise (in the trained state) in PD. These findings have important therapeutic implications and are an exciting step forward in identifying non-pharmacological therapies for this common and disabling non-motor symptom. Further work is needed to better understand the mechanisms underlying the beneficial exercise-induced changes in sleep in PD.
Supplementary Material
Financial Support:
This study was funded by the NIH (K23NS080912, T32 HD071866), Parkinson’s Disease Foundation, The Manning Foundation, The Curry Foundation at the University of Virginia, UAB Center for Exercise Medicine, and the NIH National Rehabilitation Research Resource to Enhance Clinical Trials (REACT, P2CHD086851).
FINANCIAL DISCLOSURES:
AWA: Dr. Amara is a consultant for Grey Matter Technologies. She serves as site Principle investigator for studies sponsored by Michael J. Fox Foundation for Parkinson’s Research, Biogen Idec, Hoffman La Roche, Eli Lilly, and Jazz Pharmaceuticals, all unrelated to this project. She has grant funding from NIH.
KHW: None
AJ: None
RAM: None
JLP: None
SCT: None
JR: None
MJB: Dr. Barrett serves on an Advisory Board for Amneal and serves as site investigator for studies sponsored by Eisai and Acadia.
DAE: None
ALW: None
CPH: Dr. Hurt receives research grant funding from NIH, Michael J. Fox Foundation for Parkinson’s Research, and NIDILRR
GC: Dr. Cutter serves on the following Data and Safety Monitoring Boards: AMO Pharmaceuticals, Biolinerx, Brainstem, Galmed Pharmaceuticals, Horizon Pharmaceuticals, Hisun Pharmaceuticals, Merck, Merck/Pfizer, Opko Biologics, Neurim, Novartis, Ophazyme, Sanofi-Aventis, Reata Pharmaceuticals, Receptos/Celgene, Teva pharmaceuticals, NHLBI (Protocol Review Committee), NICHD (OPRU oversight committee). He serves on the following Consulting or Advisory Boards: Biogen, Argenix, Brainstorm Cell Therapeutics, Charleston Labs Inc, Click Therapeutics, Genzyme, Genentech, GW Pharmaceuticals, Klein-Buendel Incorporated, Medimmune, Medday, Novartis, Osmotica Pharmaceuticals, Perception Neurosciences, Roche, Scifluor, Somahlution, Teva pharmaceuticals, TG Therapeutics, UT Houston. He is President of Pythagoras, Inc. a private consulting company located in Birmingham AL.
MMB: Dr. Bamman receives research grant funding from the NIH, DOD, and VA.
Footnotes
Financial Disclosure/Conflicts of Interests: none
REFERENCES
- 1.Barone P, Antonini A, Colosimo C, et al. The PRIAMO study: A multicenter assessment of nonmotor symptoms and their impact on quality of life in Parkinson’s disease. Mov Disord. 2009;24(11):1641–1649. [DOI] [PubMed] [Google Scholar]
- 2.Politis M, Wu K, Molloy S, P GB, Chaudhuri KR, Piccini P. Parkinson’s disease symptoms: the patient’s perspective. Mov Disord. 2010;25(11):1646–1651. [DOI] [PubMed] [Google Scholar]
- 3.Lees AJ, Blackburn NA, Campbell VL. The nighttime problems of Parkinson’s disease. Clin Neuropharmacol. 1988;11(6):512–519. [DOI] [PubMed] [Google Scholar]
- 4.Nausieda PA, Weiner WJ, Kaplan LR, Weber S, Klawans HL. Sleep disruption in the course of chronic levodopa therapy: an early feature of the levodopa psychosis. Clin Neuropharmacol. 1982;5(2):183–194. [DOI] [PubMed] [Google Scholar]
- 5.Chahine LM, Amara AW, Videnovic A. A systematic review of the literature on disorders of sleep and wakefulness in Parkinson’s disease from 2005 to 2015. Sleep Med Rev. 2017;35:33–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bliwise DL, Trotti LM, Rye D. Movement Disorders Specific to Sleep and Sleep in Waking Movement Disorders. In: Standaert DG, Watts R, Obeso J, eds. Movement Disorders. 3rd ed. New York: McGraw Hill Medical; 2012. [Google Scholar]
- 7.Meindorfner C, Korner Y, Moller JC, Stiasny-Kolster K, Oertel WH, Kruger HP. Driving in Parkinson’s disease: mobility, accidents, and sudden onset of sleep at the wheel. Mov Disord. 2005;20(7):832–842. [DOI] [PubMed] [Google Scholar]
- 8.Neikrug AB, Maglione JE, Liu L, et al. Effects of sleep disorders on the non-motor symptoms of Parkinson disease. J Clin Sleep Med. 2013;9(11):1119–1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Amara AW, Chahine LM, Caspell-Garcia C, et al. Longitudinal assessment of excessive daytime sleepiness in early Parkinson’s disease. J Neurol Neurosurg Psychiatry. 2017;88(8):653–662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Amara AW, Chahine LM, Videnovic A. Treatment of Sleep Dysfunction in Parkinson’s Disease. Curr Treat Options Neurol. 2017;19(7):26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kelly NA, Ford MP, Standaert DG, et al. Novel, high-intensity exercise prescription improves muscle mass, mitochondrial function, and physical capacity in individuals with Parkinson’s disease. Journal of applied physiology. 2014;116(5):582–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schenkman M, Moore CG, Kohrt WM, et al. Effect of High-Intensity Treadmill Exercise on Motor Symptoms in Patients With De Novo Parkinson Disease: A Phase 2 Randomized Clinical Trial. JAMA Neurol. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Uc EY, Doerschug KC, Magnotta V, et al. Phase I/II randomized trial of aerobic exercise in Parkinson disease in a community setting. Neurology. 2014;83(5):413–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Corcos DM, Robichaud JA, David FJ, et al. A two-year randomized controlled trial of progressive resistance exercise for Parkinson’s disease. Mov Disord. 2013;28(9):1230–1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kelly NA, Wood KH, Allendorfer JB, et al. High-Intensity Exercise Acutely Increases Substantia Nigra and Prefrontal Brain Activity in Parkinson’s Disease. Med Sci Monit. 2017;23:6064–6071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kredlow MA, Capozzoli MC, Hearon BA, Calkins AW, Otto MW. The effects of physical activity on sleep: a meta-analytic review. J Behav Med. 2015;38(3):427–449. [DOI] [PubMed] [Google Scholar]
- 17.Uchida S, Shioda K, Morita Y, Kubota C, Ganeko M, Takeda N. Exercise effects on sleep physiology. Front Neurol. 2012;3:48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kubitz KA, Landers DM, Petruzzello SJ, Han M. The effects of acute and chronic exercise on sleep. A meta-analytic review. Sports Med. 1996;21(4):277–291. [DOI] [PubMed] [Google Scholar]
- 19.Nascimento CM, Ayan C, Cancela JM, Gobbi LT, Gobbi S, Stella F. Effect of a multimodal exercise program on sleep disturbances and instrumental activities of daily living performance on Parkinson’s and Alzheimer’s disease patients. Geriatrics & gerontology international. 2014;14(2):259–266. [DOI] [PubMed] [Google Scholar]
- 20.Silva-Batista C, de Brito LC, Corcos DM, et al. Resistance Training Improves Sleep Quality in Subjects With Moderate Parkinson’s Disease. J Strength Cond Res. 2017;31(8):2270–2277. [DOI] [PubMed] [Google Scholar]
- 21.Organization WH. Physical Activity and Older Adults. https://www.who.int/dietphysicalactivity/factsheet_olderadults/en/. Accessed.
- 22.Berry R, Albertario C, Harding SM, et al. The AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology, and Technical Specifications. In: Darien, IL: American Academy of Sleep Medicine; 2018. [Google Scholar]
- 23.Goetz CG, Tilley BC, Shaftman SR, et al. Movement Disorder Society-sponsored revision of the Unified Parkinson’s Disease Rating Scale (MDS-UPDRS): scale presentation and clinimetric testing results. Mov Disord. 2008;23(15):2129–2170. [DOI] [PubMed] [Google Scholar]
- 24.Dinges DF. Microcomputer analysis of performance on a portable, simple visual RT task during sustained operations. Behavioral Research Methods, Instruments, & Computers. 1985;17(6):652–655. [Google Scholar]
- 25.Basner M, Dinges DF. Maximizing sensitivity of the psychomotor vigilance test (PVT) to sleep loss. Sleep. 2011;34(5):581–591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stec MJ, Thalacker-Mercer A, Mayhew DL, et al. Randomized, four-arm, dose-response clinical trial to optimize resistance exercise training for older adults with age-related muscle atrophy. Exp Gerontol. 2017;99:98–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Walton RG, Dungan CM, Long DE, et al. Metformin blunts muscle hypertrophy in response to progressive resistance exercise training in older adults: A randomized, double-blind, placebo-controlled, multicenter trial: The MASTERS trial. Aging cell. 2019;18(6):e13039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gillin JC, Rapaport M, Erman MK, Winokur A, Albala BJ. A comparison of nefazodone and fluoxetine on mood and on objective, subjective, and clinician-rated measures of sleep in depressed patients: a double-blind, 8-week clinical trial. J Clin Psychiatry. 1997;58(5):185–192. [DOI] [PubMed] [Google Scholar]
- 29.Schroeck JL, Ford J, Conway EL, et al. Review of Safety and Efficacy of Sleep Medicines in Older Adults. Clin Ther. 2016;38(11):2340–2372. [DOI] [PubMed] [Google Scholar]
- 30.Amara AW, Memon AA. Effects of Exercise on Non-Motor Symptoms in Parkinson’s Disease. Clin Ther. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xiao CM, Zhuang YC. Effect of health Baduanjin Qigong for mild to moderate Parkinson’s disease. Geriatrics & gerontology international. 2016;16(8):911–919. [DOI] [PubMed] [Google Scholar]
- 32.Cusso ME, Donald KJ, Khoo TK. The Impact of Physical Activity on Non-Motor Symptoms in Parkinson’s Disease: A Systematic Review. Front Med (Lausanne). 2016;3:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Deuschle M, Schredl M, Wisch C, et al. Serum brain-derived neurotrophic factor (BDNF) in sleep-disordered patients: relation to sleep stage N3 and rapid eye movement (REM) sleep across diagnostic entities. Journal of sleep research. 2017. [DOI] [PubMed] [Google Scholar]
- 34.Faraguna U, Vyazovskiy VV, Nelson AB, Tononi G, Cirelli C. A causal role for brain-derived neurotrophic factor in the homeostatic regulation of sleep. J Neurosci. 2008;28(15):4088–4095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Szuhany KL, Bugatti M, Otto MW. A meta-analytic review of the effects of exercise on brain-derived neurotrophic factor. Journal of psychiatric research. 2015;60:56–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McGinty D, Szymusiak R. Keeping cool: a hypothesis about the mechanisms and functions of slow-wave sleep. Trends Neurosci. 1990;13(12):480–487. [DOI] [PubMed] [Google Scholar]
- 37.Reynolds GO, Otto MW, Ellis TD, Cronin-Golomb A. The Therapeutic Potential of Exercise to Improve Mood, Cognition, and Sleep in Parkinson’s Disease. Mov Disord. 2016;31(1):23–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sellami M, Bragazzi NL, Slimani M, et al. The Effect of Exercise on Glucoregulatory Hormones: A Countermeasure to Human Aging: Insights from a Comprehensive Review of the Literature. Int J Environ Res Public Health. 2019;16(10). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Allen Reish HE, Standaert DG. Role of alpha-synuclein in inducing innate and adaptive immunity in Parkinson disease. J Parkinsons Dis. 2015;5(1):1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Youngstedt SD. Effects of exercise on sleep. Clin Sports Med. 2005;24(2):355–365, xi. [DOI] [PubMed] [Google Scholar]
- 41.Wang X, Youngstedt SD. Sleep quality improved following a single session of moderate-intensity aerobic exercise in older women: Results from a pilot study. J Sport Health Sci. 2014;3(4):338–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Steinacker JM, Lormes W, Reissnecker S, Liu Y. New aspects of the hormone and cytokine response to training. Eur J Appl Physiol. 2004;91(4):382–391. [DOI] [PubMed] [Google Scholar]
- 43.Krueger JM. The role of cytokines in sleep regulation. Curr Pharm Des. 2008;14(32):3408–3416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Coon EA, Low PA. Thermoregulation in Parkinson disease. Handb Clin Neurol. 2018;157:715–725. [DOI] [PubMed] [Google Scholar]
- 45.Amara AW, Walker HC, Joop A, et al. Effects of Subthalamic Nucleus Deep Brain Stimulation on Objective Sleep Outcomes in Parkinson’s Disease. Movement Disorders Clinical Practice. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dulski J, Schinwelski M, Konkel A, et al. The impact of subthalamic deep brain stimulation on sleep and other non-motor symptoms in Parkinson’s disease. Parkinsonism Relat Disord. 2019;64:138–144. [DOI] [PubMed] [Google Scholar]
- 47.Lim J, Dinges DF. A meta-analysis of the impact of short-term sleep deprivation on cognitive variables. Psychol Bull. 2010;136(3):375–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hublin C, Partinen M, Koskenvuo M, Kaprio J. Sleep and mortality: a population-based 22-year follow-up study. Sleep. 2007;30(10):1245–1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Diekelmann S, Born J. The memory function of sleep. Nat Rev Neurosci. 2010;11(2):114–126. [DOI] [PubMed] [Google Scholar]
- 50.Kim SJ, Lee JH, Lee DY, Jhoo JH, Woo JI. Neurocognitive dysfunction associated with sleep quality and sleep apnea in patients with mild cognitive impairment. Am J Geriatr Psychiatry. 2011;19(4):374–381. [DOI] [PubMed] [Google Scholar]
- 51.Landsness EC, Crupi D, Hulse BK, et al. Sleep-dependent improvement in visuomotor learning: a causal role for slow waves. Sleep. 2009;32(10):1273–1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wilckens KA, Hall MH, Nebes RD, Monk TH, Buysse DJ. Changes in Cognitive Performance Are Associated with Changes in Sleep in Older Adults With Insomnia. Behav Sleep Med. 2016;14(3):295–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Amara AW, Memon RA, Joop A, et al. Slow-Wave Sleep Is Associated with Cognitive Performance in Patients with Parkinson’s Disease. 142nd Annual Meeting of the American Neurological Association October 15, 2017, 2017; San Diego, CA. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


