Abstract
T cells must migrate to encounter antigen-presenting cells and carry out their roles in host defense. Here, we showed that autocrine stimulation of the purinergic receptor P2Y11 regulate the migration of human CD4 T cells. P2Y11 receptors redistributed from the front to the back of polarized cells where they trigger intracellular cAMP/PKA signals that attenuate mitochondrial metabolism at the back. The absence of P2Y11 receptors at the front of cells resulted in hotspots of mitochondrial metabolism and localized ATP production that stimulated P2X4 receptors, Ca2+ influx, and pseudopod protrusion at the front. This regulatory function of P2Y11 receptors depended on their subcellular redistribution and autocrine stimulation by cellular ATP release and was perturbed by indiscriminate global stimulation. We conclude that excessive extracellular ATP, such as that which occurs in inflammation, sepsis, and cancer, disrupts this autocrine feedback mechanism, which results in defective T cell migration, impaired T cell function, and loss of host immune defense.
INTRODUCTION
Purinergic signaling mechanisms triggered by the release of cellular ATP have important roles in the regulation of immune cell functions (1–4). Stimulation of cells liberates ATP in a coordinated and localized fashion through various specialized ATP release mechanisms (5, 6). ATP release can lead to the activation of most of the purinergic receptors found on the surface of immune cells. These purinergic receptors comprise three major families, the P1, P2X, and P2Y receptors. P2X receptors are ionotropic ATP-gated cation channels that facilitate cellular Ca2+ influx in response to ATP stimulation (7). P2Y receptors are metabotropic G protein-coupled receptors (GPCR) that can elicit various downstream signaling events in response to stimulation by ATP and other nucleotides (8). P1 receptors also belong to the GPCR superfamily and recognize the ATP breakdown product adenosine (9).
T cells surround themselves with a “halo” of ATP that is caused by constitutive release of a portion of their cellular ATP content (10). This ATP halo changes its shape and intensity in response to T cell stimulation (11–13). T cells stimulated with chemokines rapidly release ATP through pannexin-1 (Panx1) channels (13–14). The ATP concentration released by such stimuli is sufficient to trigger purinergic receptors that elicit cell functions including T cell migration (13–16). T cell migration is an essential function that contributes to antimicrobial host defense, tumor control, autoimmunity, and allergic reactions (17). Cell migration enables naïve T cells to infiltrate lymph nodes and other lymphoid organs and to detect and respond to the antigens displayed by antigen-presenting cells (APCs) (18, 19). After their stimulation by APCs, naïve CD4 T cells proliferate and differentiate into effector T cells that are highly mobile and capable of infiltrating various host tissues where they coordinate immune responses (17).
Cell migration is a complex process that involves polarization of cells, protrusion of pseudopods at the front of cells, and retraction of the uropod at the back (20). These processes at the front and back of cells work hand-in-hand to regulate the cytoskeletal rearrangements that produce elongated cell shapes and promote the forward movement of cells. However, the exact mechanisms that regulate these events in T cells are still not well understood. We have previously reported that autocrine stimulation of P2X4 receptors helps define the front of polarized T cells (13). P2X4 receptors promote cellular Ca2+ influx that increases the metabolism of adjacent mitochondria by delivering Ca2+ that these mitochondria need for oxidative phosphorylation (OXPHOS) and for ATP production (21). These localized stimulatory events boost autocrine purinergic signaling and P2X4 receptor stimulation and deliver the ATP that is required to promote pseudopod protrusions at the front of cells (13). In the current study, we identified an inhibitory mechanism that limits mitochondrial ATP production to enforce P2X4 receptor signaling at the front while facilitating uropod retraction at the back of migrating T cells.
This mechanism involves P2Y11 receptors that T cells express in addition to their P2X4 receptors (22, 23). P2Y11 receptors are GPCRs that can respond to extracellular ATP by increasing intracellular cAMP levels and inducing downstream protein kinase A (PKA) signaling (24). Little information exists on the role of P2Y11 receptors in T cell activation and cell functions such as T cell migration. In this study, we found that P2Y11 receptors are important regulators of T cell migration. P2Y11 receptors retracted from the front of cells during cell polarization. Our results show that the absence of P2Y11 receptors at the front of cells permits localized mitochondrial ATP production that supplies P2X4 receptors with the ATP necessary to promote mitochondrial metabolism and enable pseudopod protrusion at the front of cells. We found that P2Y11 receptor accumulation at the back of cells suppressed mitochondria, prevented inappropriate pseudopod protrusions, and promoted uropod retraction at the back of cells. Based on these findings we conclude that P2Y11 receptors cooperate with P2X4 receptors to stabilize cell polarization and establish a push-pull signaling mechanism that promotes efficient T cell migration.
RESULTS
P2X4 and P2Y11 receptors are involved in promoting T cell migration
Adaptive immune responses depend on T cell migration, which allows T cells to scan APCs for suitable antigens (17, 25). We have previously identified that a feed-forward signaling mechanism consisting of mitochondrial ATP production, cellular ATP release, and autocrine stimulation of P2X4 receptors is indispensable for T cell migration (13). However, in addition to P2X4 receptors, T cells also abundantly express P2Y11 receptors (22, 23). In the current study, we examined whether and how P2Y11 receptors contribute to the regulation of T cell migration. Stromal-derived factor 1α (SDF-1α), also known as CXCL12, is a potent stimulator of T cell migration that activates CXCR4 chemokine receptors (26). Stimulation of CXCR4 with SDF-1α triggers rapid ATP release from T cells (13, 14). We found that blocking P2Y11 receptors with the specific antagonist NF340 or silencing P2Y11 receptors with siRNA impairs the migration of human CD4 T cells and Jurkat T cells in response to SDF-1α (Fig. 1, A and B; Movie S1). This effect was similar to that caused by silencing P2X4 receptors or inhibiting P2X4 receptors with the antagonist 5-BDBD (13). These findings suggest that P2X4 and P2Y11 receptors are both required for efficient T cell migration. P2Y11 receptors can increase intracellular cAMP levels and activate PKA-dependent signaling pathways that modulate T cell functions (24, 27, 28). Like P2Y11 receptor inhibition, inhibition of the PKA with H89 also suppressed SDF-1α-induced Jurkat cell migration (Fig. 1B). Inhibition of PKA or P2Y11 receptors also blocked the migration of effector CD4 T cells and of unstimulated Jurkat cells (Fig. 1C; fig. S1A; Movie S2). Thus, P2Y11 receptor signaling regulates the intrinsic motility of T cells and the migration of T cells in response to chemokine stimulation. These findings demonstrate that autocrine purinergic signaling mechanisms via P2X4 and P2Y11 receptors are both required for efficient T cell migration.
Fig. 1. P2X4 and P2Y11 receptors promote T cell migration.
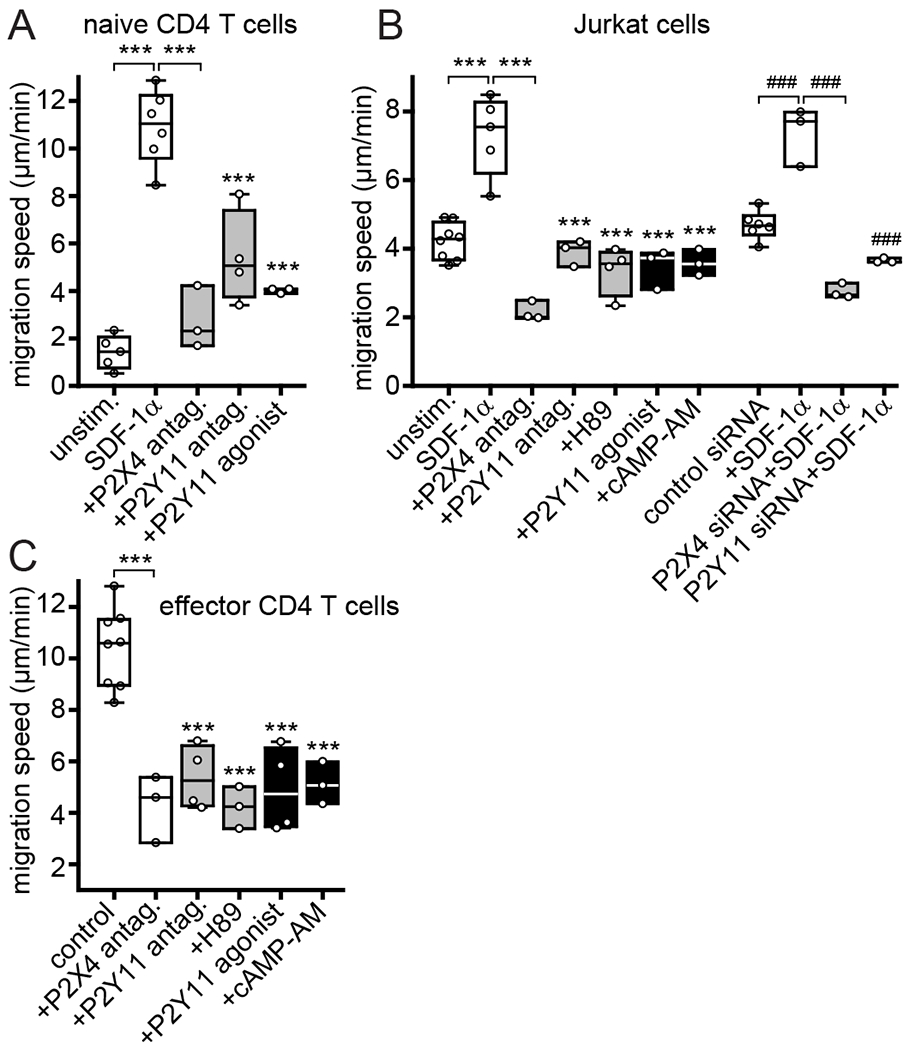
(A) Human PBMCs were treated for 20 min with P2X4 antagonist (antag.) (5-BDBD, 10 μM), P2Y11 antagonist (NF340, 10 μM), or P2Y11 agonist (agon.) (NF546, 1 μM). CD4 T cells were identified by CD4 labeling. CD4 T cell migration in response to SDF-1α (100 ng/ml) was monitored with video microscopy for 30 min and migration speeds of individual cells (n≥20 per experiment) were calculated. Box plots of the means of independent experiments (n=3-6; indicated by circles) are shown. ***P<0.001 compared to SDF-1α control (one-way ANOVA). (B) Jurkat T cells were treated with 5-BDBD, NF340, H89 (5 μM), NF546, or cell-permeable cAMP-AM (1 μM) or transfected with P2Y11-targeting, P2X4-targeting, or non-targeting control siRNA and cell migration in response to SDF-1α was analyzed. Mean migration speeds from at least 3 independent experiments (indicated by circles; n≥20 cells per experiment) are shown as box plots. ***P<0.001, ###P<0.001 compared to SDF-1α controls (one-way ANOVA). (C) Effect of 5-BDBD, NF340, H89, NF546, or cAMP-AM on the migration of CD4 effector T cells. Mean migration speeds from at least 3 independent experiments (indicated by circles; n≥20 cells per experiment) are shown as box plots. ***P<0.001 compared to control (one-way ANOVA).
Exogenous stimulation of P2Y11 receptors blocks T cell migration
The results shown above demonstrated that P2Y11 receptor antagonists can impair T cell migration. Next, we studied how exogenous stimulation of P2Y11 receptors affects T cell migration. Surprisingly, stimulation of P2Y11 receptors with the specific agonist NF546 also impaired T cell migration (Fig. 1, A–C; fig. S1A; Movie S2). P2Y11 receptor stimulation can induce cAMP accumulation, which impairs T cell migration (29). Treatment with the cell permeable cAMP analog cAMP-AM recapitulated the inhibitory effect of the P2Y11 receptor agonist, which suggests that exogenous stimulation of P2Y11 receptor signaling impairs T cell migration in a cAMP-dependent manner (Fig. 1, B and C; fig. S1A). Together, these results show that P2Y11 receptors are essential regulators of T cell migration and that P2Y11 receptors must be stimulated in a spatiotemporally defined fashion to induce autocrine purinergic signaling events that promote T cell migration. The importance of this autocrine signaling mechanism is further supported by our findings that indiscriminate stimulation of P2Y11 receptors or cAMP signaling with exogenous P2Y11 receptor agonist or cell-permeable cAMP, respectively, impaired T cell migration.
Autocrine P2Y11 receptor signaling regulates cell polarization and pseudopod formation
Cell migration involves several distinct steps that include the initial polarization of cells, the formation of elongated cell shapes, the protrusion of pseudopods at the front of cells, and the retraction of the uropod at the back of cells (30). Autocrine stimulation of P2X4 receptors promotes pseudopod protrusion at the front of migrating T cells (13). Based on the results shown above, we hypothesized that P2Y11 receptor signaling may help regulate T cell polarization. Silencing P2Y11 receptors or treatment with the P2Y11 receptor antagonist NF340 impaired cell polarization in response to SDF-1α (Fig. 2, A and B; fig. S1, B and C). At the same time, these treatments increased the surface area of naïve and effector T cells and the number of pseudopods these cells formed in response to SDF-1α stimulation (Fig. 2, C–G; fig. S1, D and E). Inhibition of PKA signaling with H89 had similar disruptive effects on the shape, polarization, and pseudopod formation of T cells (Fig. 2, A–D). Paracrine interference by indiscriminate stimulation of P2Y11 receptors with the agonist NF546 or global stimulation of cAMP/PKA signaling with cAMP-AM also impaired T cell polarization and increased the surface area of cells (Fig. 2, A–C, E and F). Although inhibition of autocrine P2Y11 receptor signaling increased pseudopod formation, global stimulation of P2Y11 receptors with the receptor agonist blocked the formation of pseudopods by T cells (Fig. 2, D and G). These findings demonstrate that T cells require autocrine feedback via P2Y11 receptors and corresponding downstream signaling via cAMP/PKA to establish and maintain cell polarity and to coordinate the processes involved in pseudopod formation. Exogenous stimulation of P2Y11 receptor signaling disrupted these processes. Together with our previous work, these findings indicate that the spatiotemporal distribution of P2Y11 receptors and the coordinated stimulation of P2X4 and P2Y11 receptors are essential for proper cell polarization and effective T cell migration.
Fig. 2. Autocrine P2Y11 receptor signaling regulates cell polarization and pseudopod formation.
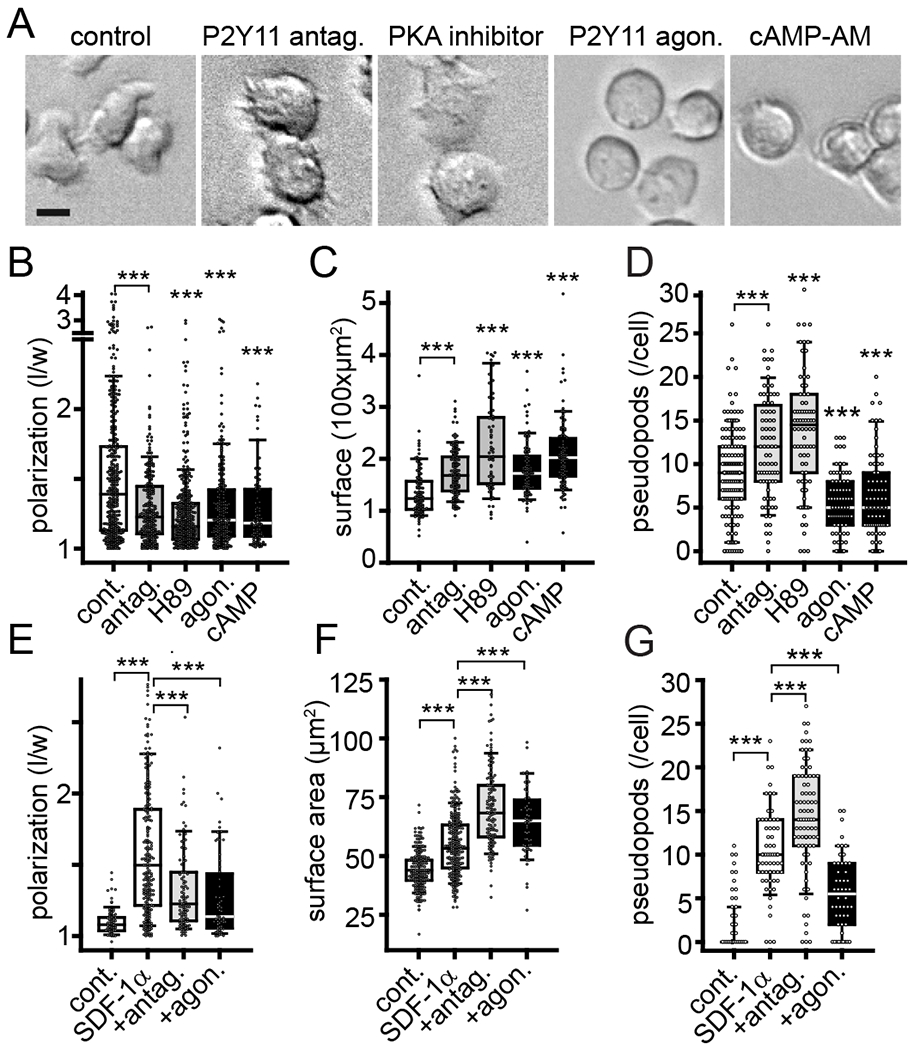
(A) Human effector CD4 T cells were obtained by stimulation for 3 days with anti-CD3/CD28 antibody-coated beads and then treated for 30 min with P2Y11 antagonist (NF340, 10 μM), PKA inhibitor (H89, 5 μM), P2Y11 agonist (NF546, 1 μM), or cell-permeable cAMP (cAMP-AM, 1 μM). Scale bar: 10 μm. (B-D) Cell polarization expressed as ratio between lengths and widths of individual cells (B), cell surface areas (C), and the number of pseudopods extended (D) were analyzed after treatment of effector T cells for 30 min with inhibitors and agonists. Box plots of n≥65 cells from at least 3 independent experiments are shown. Circles indicate single cells. ***P<0.001 compared to controls (Kruskal-Wallis test). (E-G) Freshly isolated naïve human CD4 T cells were treated with NF340 or NF546 for 10 min, stimulated with SDF-1α (100 ng/ml) or not (controls), and observed for 30 min by time-lapse video microscopy. Cell polarization (E), cell surface areas (F), and the number of pseudopods formed (G) were analyzed after 30 min. Box plots represent n≥50 cells (indicated by circles) from independent experiments (n≥3). ***P<0.001 compared to SDF-1α controls (Kruskal-Wallis test).
P2X4 and P2Y11 receptors jointly coordinate the activation of mitochondria in polarized cells
To study the subcellular distribution of P2Y11 and P2X4 receptors during cell polarization, we used live-cell imaging and Jurkat T cells that expressed YFP-tagged P2Y11 or EGFP-tagged P2X4 receptor fusion proteins. These experiments revealed uniform distribution of P2Y11 receptors across the cell surface of unstimulated cells (Fig. 3A). In response to cell stimulation, the distribution of P2Y11 receptors changed rapidly. P2Y11 receptors accumulated at the back of cells, rendering the leading edge of cells devoid of P2Y11 receptors (Fig. 3A). The distribution pattern of P2X4 receptors differed considerably from that of P2Y11 receptors (Fig. 3B). Although P2Y11 receptors were redistributed at the cell surface, P2X4 receptors associated predominantly with vesicular structures that shifted toward the leading edge during cell polarization. In response to cell stimulation, P2X4 receptors merged with the cell surface but remained clustered in patches distributed across the cell surface. P2X4 receptors initiate the polarization and migration of T cells in response to SDF-1α stimulation (13). Therefore, we wondered whether P2X4 receptors also influence the redistribution of P2Y11 receptors during T cell polarization. Indeed, inhibition of P2X4 receptors with the antagonist 5-BDBD prevented the redistribution of P2Y11 receptors to the back of stimulated cells (Fig. 3C). These findings indicate that P2X4 receptors are upstream regulators that initiate cell polarization and the accumulation of P2Y11 receptors at the back of cells. We found that the relative abundance of P2Y11 receptors that gathered at the back of polarized cells correlated with the speed of uropod retraction (Fig. 3D) and that the accumulation of P2Y11 receptors coincided with sites of localized membrane retractions that promote the forward movement of cells (fig. S2A, Movie S3). These findings indicate that P2Y11 receptor signaling regulates cell polarization and helps to define the direction of T cell migration.
Fig. 3. P2X4 receptor signaling defines the subcellular redistribution of P2Y11 receptors during T cell polarization.
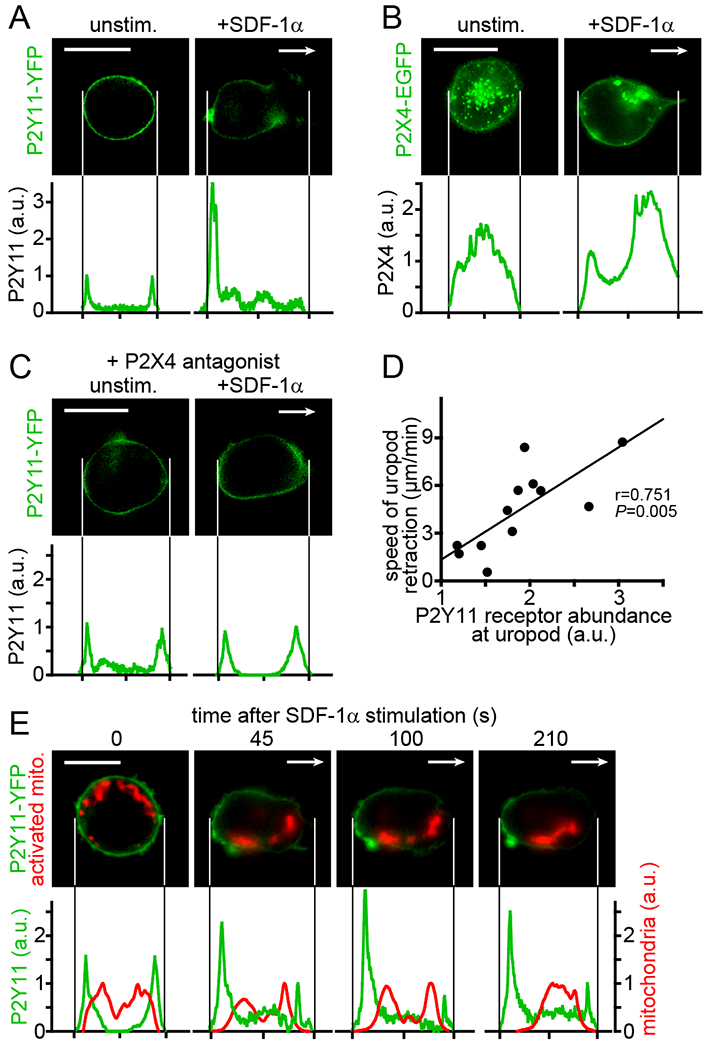
(A-C) P2Y11 and P2X4 receptor distribution patterns were analyzed in Jurkat cells expressing YFP-tagged P2Y11 or EGFP-tagged P2X4 receptors 10 min after the addition of SDF-1α (100 ng/ml) or vehicle control. In (C), cells were pre-incubated with the P2X4 receptor antagonist 5-BDBD (10 μM) for 10 min prior to stimulation. Arrows indicate the direction of cell migration. Images and corresponding receptor distribution profiles are representative of at least 20 (A, B) or 10 (C) cells from at least 3 separate experiments (scale bar: 10 μm). (D) Correlation between P2Y11 receptor accumulation at the back of cells and the uropod retraction speed in migrating cells. Uropod retraction was tracked for 2 min (n=12 cells from 3 independent experiments); r, Pearson’s correlation coefficient. (E) Jurkat cells expressing P2Y11-YFP were labeled with MitoTracker Red CM-H2Xros and the distribution of P2Y11 receptors and active mitochondria following stimulation with SDF-1α was recorded over time. Representative images of 10 cells from different experiments and corresponding distribution profiles of P2Y11-YFP and active mitochondria summed over the whole cell area are shown. Arrows indicate the direction of migration; scale bar: 10 μm.
Mitochondria are also involved in the activation and regulation of T cell migration (12, 31–33). P2X4 receptors contribute to the activation of mitochondria at the leading edge of polarized cells where they produce the ATP that fuels pseudopod protrusions during T cell migration (13). P2Y11 receptor agonists blocked pseudopod formation (Fig. 2, D and G). Therefore, we wondered whether P2Y11 receptors attenuate mitochondrial activation at the back of migrating T cells. In Jurkat cells expressing YFP-tagged P2Y11 receptors, we labeled mitochondria with MitoTracker Red CM-H2Xros, a fluorescent dye that senses mitochondrial ROS production as readout of mitochondrial ATP production (34). Redistribution of P2Y11 receptors to the back of SDF-1α-stimulated cells was accompanied by decreased mitochondrial activity at the back and a relative increase in mitochondrial activity near the front of polarized cells (Fig. 3E, fig. S2B). These findings suggest that P2Y11 receptors at the back of cells decrease the activity of adjacent mitochondria and that the redistribution of P2Y11 receptors favors mitochondrial activity at subcellular sites that are devoid of P2Y11 receptors, namely the front of polarized cells. Inhibition of P2X4 receptors with the antagonist 5-BDBD prevented the redistribution of P2Y11 receptors and consequently reduced the increase in mitochondrial activity at the front of polarized cells (fig. S2C). Together, these findings show that P2X4 and P2Y11 receptors must cooperate to optimize T cell migration. P2X4 receptors initiate the process of cell polarization that involves the translocation of P2Y11 receptors to the back of cells, while P2Y11 receptors amplify cell polarization by attenuating mitochondrial activity at the back and concentrating mitochondrial ATP production to the front of cells where ATP fuels the processes needed for T cell migration.
P2Y11 receptors restrict P2X4 receptor signaling to the front of migrating T cells
Activated mitochondria form excitatory signaling clusters at the front of migrating T cells where P2X4 receptors deliver Ca2+ influx to fuel mitochondrial ATP production and promote pseudopod formation (13). Because P2Y11 receptors seemed to contribute to this process, we examined whether P2Y11 receptors influence the interaction of P2X4 receptors with mitochondria. We expressed EGFP-tagged P2X4 receptors in Jurkat T cells, labeled mitochondria with MitoTracker Red CM-H2Xros, and examined the distribution of active mitochondria and P2X4 receptors with live-cell imaging. In unstimulated cells, P2X4 receptors and mitochondria were randomly dispersed throughout the cells. However, following SDF-1α stimulation, P2X4 receptors and active mitochondria accumulated near the front of polarized cells (Fig. 4, A and B). Inhibition of P2Y11 receptor signaling with NF340 disrupted the association of P2X4 receptors with mitochondria and resulted in inappropriate formation of pseudopods at the front and back of cells. Exogenous stimulation of P2Y11 receptors with the agonist NF546 also prevented the interaction of P2X4 receptors with mitochondria but abolished the ability of cells to form pseudopods. Global stimulation of P2Y11 receptors with this agonist resulted in rounded cell shapes and the progressive loss of mitochondrial activity throughout the cells (Fig. 4, A and B; Movie S4). Together, these findings demonstrate that autocrine signaling through P2Y11 receptors is needed to promote the interaction of mitochondria and P2X4 receptors at the front of cells, which results in amplified P2X4 receptor signaling. We conclude that P2Y11 receptors provide inhibitory signals that synergize with the excitatory signals generated by P2X4 receptors to confine ATP production and pseudopod protrusion to the front of migrating T cells.
Fig. 4. P2Y11 receptors restrict excitatory P2X4 receptor signaling to the front of migrating T cells.
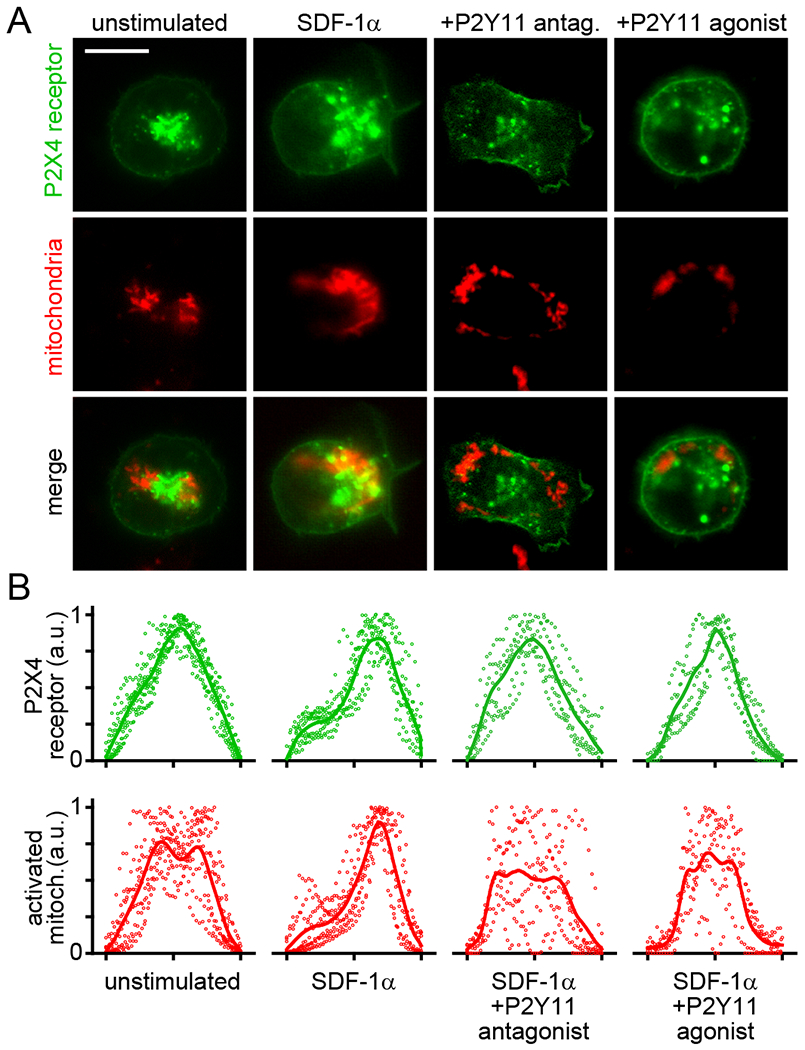
Jurkat cells expressing EGFP-tagged P2X4 receptors were labeled with MitoTracker Red CM-H2Xros and treated with P2Y11 antagonist (NF340, 10 μM), P2Y11 agonist (NF546, 1 μM), or vehicle control for 10 min. Then, cells were stimulated with SDF-1α (100 ng/ml) and redistribution of P2X4 receptors and active mitochondria was analyzed after 3 min. Representative images are shown in panel A. Summarized results of 6-10 separate experiments are shown in panel B. Dots indicate the distribution of single cell fluorescence values and lines represent averaged summarized line profiles of the whole cell areas. Scale bar: 10 μm.
P2X4 and P2Y11 receptors are both required for mitochondrial metabolism
The findings above indicate that P2X4 and P2Y11 receptors jointly regulate mitochondrial metabolism in migrating T cells. To elucidate the underlying mechanisms, we assessed how inhibition of P2X4 and P2Y11 receptors affects mitochondrial Ca2+ uptake, a prerequisite for ATP production by OXPHOS (21), and ATP release in response to T cell activation. We loaded purified CD4 T cells with the mitochondrial Ca2+ indicator Rhod-2 and assessed mitochondrial Ca2+ uptake in response to SDF-1α stimulation using live-cell imaging. Inhibition of P2Y11 receptor signaling with the P2Y11 antagonist NF340 or the PKA inhibitor H89 impaired mitochondrial Ca2+ uptake and abolished mitochondrial activation in response to SDF-1α stimulation (Fig. 5, A and B). This suppressive effect was similar to that caused by P2X4 receptor inhibition (Fig. 5, A and B), which indicates that autocrine signaling through both P2Y11 and P2X4 receptors is essential to trigger, amplify, and sustain mitochondrial ATP production during T cell migration. Chemotactic stimuli induce intracellular ATP accumulation by increasing mitochondrial metabolism (35). SDF-1α increased intracellular ATP production in T cells (fig. S3A). This increase was paralleled by enhanced cellular ATP release that was significantly reduced by inhibition of either P2X4 or P2Y11 receptor signaling (Fig. 5C). Global stimulation of P2Y11 receptors with the agonist or globally increasing cAMP concentrations with cAMP-AM also completely blocked mitochondrial activity and ATP release. These findings indicate that spatiotemporally defined stimulation of P2Y11 receptors by autocrine purinergic signaling is required for proper cell metabolism (Fig. 5, A–C). We conclude that purinergic signaling through P2X4 as well as through P2Y11 receptors is essential to regulate the mitochondrial metabolism needed to induce and maintain T cell migration.
Fig. 5. P2X4 and P2Y11 receptor signaling regulates mitochondrial activity.
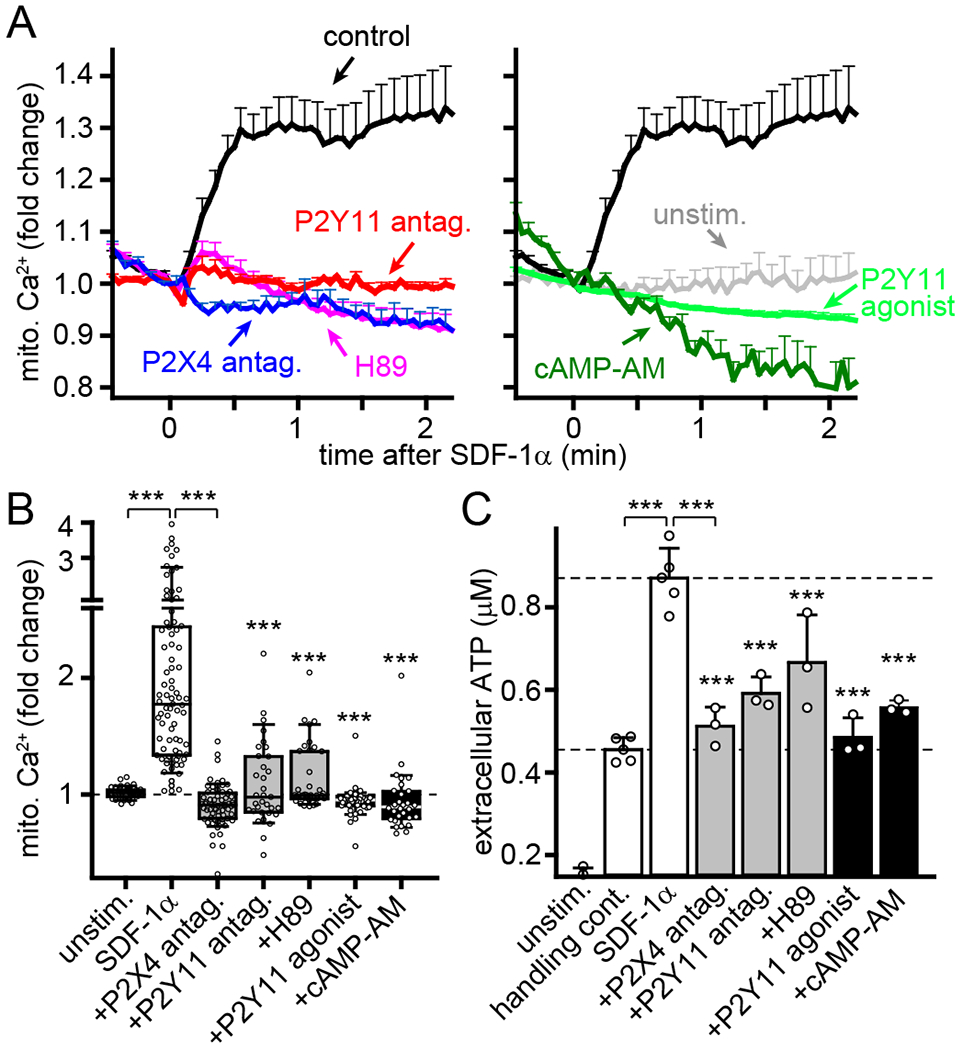
(A-B) Human CD4 T cells were labeled with Rhod-2 and treated for 20 min with P2X4 antagonist (5-BDBD, 10 μM), P2Y11 antagonist (NF340, 10 μM), PKA inhibitor (H89, 5 μM), P2Y11 agonist (NF546, 1 μM), or cell permeable cAMP (cAMP-AM, 1 μM). Mitochondrial Ca2+ levels following SDF-1α (100 ng/ml) stimulation were analyzed by fluorescence microscopy. Results show fold changes of mean Rhod-2 fluorescence values ± SEM (A) or peak fluorescence values (B) from 30-85 cells (indicated by circles) derived from separate experiments (n≥3); ***P<0.001 compared to SDF-1α-stimulated control (Kruskal-Wallis test). (C) Jurkat T cells were treated for 30 min with 5-BDBD, NF340, H89, NF546, or cAMP-AM, stimulated or not (unstim.) with SDF-1α (100 ng/ml) or vehicle (handling control), and ATP levels in the supernatants were determined after 5 min. Data are means ± SD, circles indicate independent experiments (n=3-5); *** P<0.001 compared to SDF-1α-stimulated control (one-way ANOVA).
P2X4 and P2Y11 receptor signaling is required for cellular Ca2+ signaling
The findings above demonstrate that P2Y11 receptors promote the interaction of P2X4 receptors with mitochondria to promote ATP production at the leading edge of polarized cells. Therefore, inhibition of P2Y11 receptor signaling should impair P2X4 receptor-induced cellular Ca2+ influx in response to SDF-1α stimulation. In agreement with a previous report (13), we found that inhibition of P2X4 receptors with the antagonist 5-BDBD prevented cytosolic Ca2+ signaling following SDF-1α stimulation (Fig. 6, A and B). Inhibition of P2Y11 receptor signaling with NF340 or PKA signaling with H89 also blocked Ca2+ signaling, which suggests that autocrine stimulation of P2Y11 receptors is required for full T cell activation. In addition to Gαs proteins, P2Y11 receptors can also couple to Gq proteins that mobilize Ca2+ signaling through PLC (24, 36). However, stimulation of P2Y11 receptors with the agonist NF546 failed to induce Ca2+ signaling in our assay systems (fig. S3, B and C). Therefore, we conclude that P2Y11 receptor signaling in T cells involves primarily the cAMP/PKA pathway. This conclusion is consistent with a previous report that P2Y11 receptor agonists increase intracellular cAMP but not Ca2+ levels in CD4 T cells (37). Global stimulation of P2Y11 receptors with the agonist or globally increasing cAMP concentrations with cAMP-AM abolished Ca2+ signaling in response to SDF-1α stimulation (Fig. 6, A and B). These findings provide further support for the concept that organized autocrine purinergic signaling through P2Y11 receptors is essential for T cell activation in response to SDF-1α. Together, these data suggest a multistep process by which P2X4 and P2Y11 receptors regulate T cell activation (Movie S5). Chemokine stimulation induces ATP release that initiates P2X4 receptor signaling and cell polarization, which involves P2Y11 receptor translocation to the back of cells (Fig. 7A). P2Y11 receptors amplify cell signaling by redirecting mitochondrial activity to the front of cells where localized P2X4 receptor stimulation and ATP production promote pseudopod protrusions. At the same time, P2Y11 receptors prevent aberrant cell activation and cell movement at the back of polarized cells (Fig. 7B). Spatiotemporally inappropriate stimulation of P2Y11 receptors perturbs the balanced actions of these signaling mechanisms by interfering with Ca2+ signaling and mitochondrial metabolism, which results in disrupted cell polarization and impaired T cell migration. This model can explain why both the agonist and antagonist of P2Y11 receptors impair T cell migration.
Fig. 6. Interference with P2Y11 receptor signaling impairs cytosolic Ca2+ signaling.
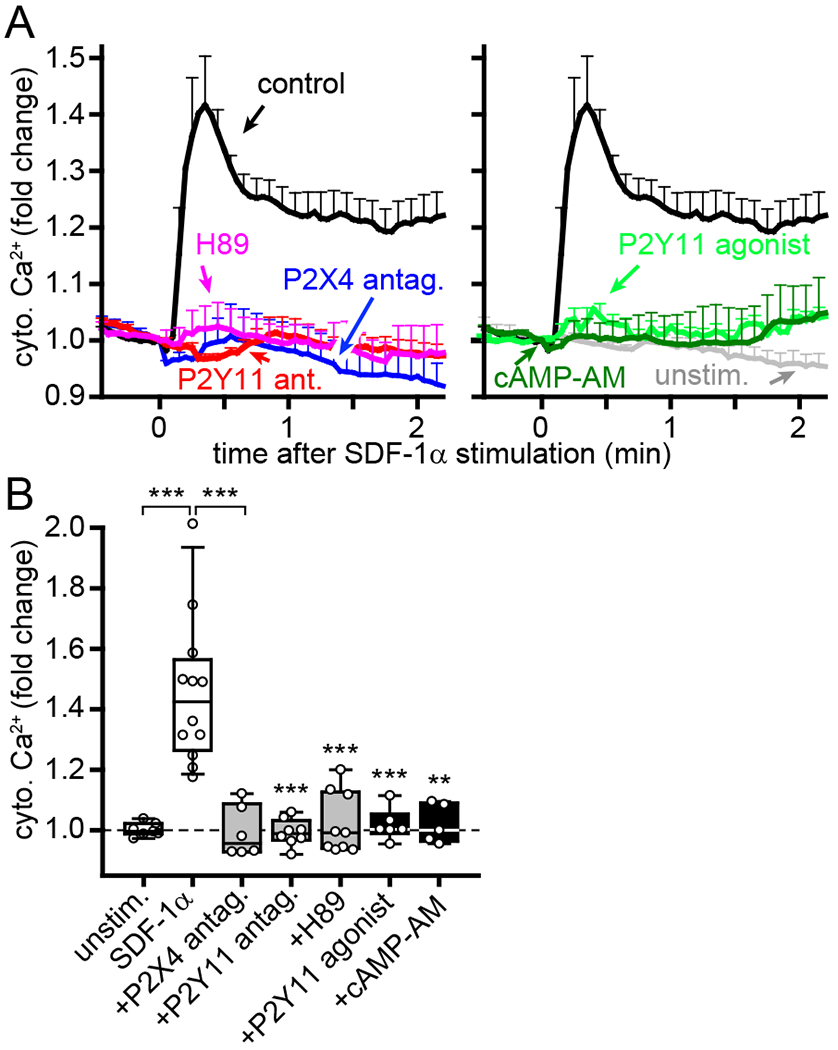
(A-B) Human CD4 T cells were labeled with Fluo-4 and treated for 20 min with P2X4 antagonist (5-BDBD, 10 μM), P2Y11 antagonist (NF340, 10 μM), PKA inhibitor (H89, 5 μM), P2Y11 agonist (NF546, 1 μM), or global cAMP stimulation (cAMP-AM, 1 μM). Cytosolic Ca2+ levels after stimulation with SDF-1α (100 ng/ml) were analyzed by fluorescence microscopy. Results show fold changes of mean Fluo-4 fluorescence values ± SEM (A) or box plots of averaged peak fluorescence values (B) from 5-12 separate experiments (indicated by circles; n≥15 cells per experiment). **P<0.01, ***P<0.001 compared to SDF-1α-stimulated control (one-way ANOVA).
Fig. 7. Paracrine interference with P2Y11 receptor signaling impairs T cell functions.
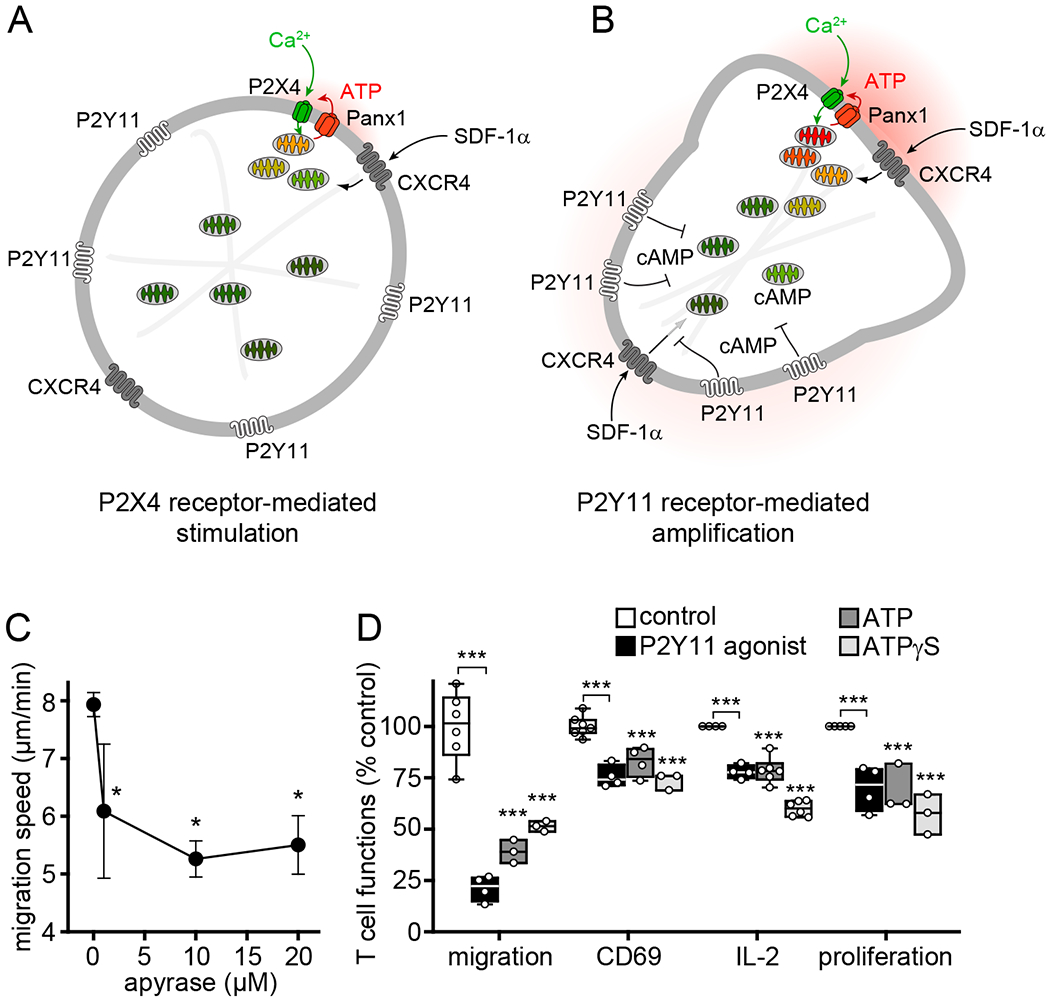
(A-B) Proposed model by which autocrine purinergic signaling regulates the polarization and migration of T cells. (A) Stimulation of CXCR4 by SDF-1α induces the release of ATP through pannexin-1 (Panx1) channels and P2X4 receptor-induced Ca2+ influx that initiates cell polarization, including the translocation of P2Y11 receptors to the back of cells. (B) Autocrine P2Y11 receptor signaling at the back shuts down the activation of nearby mitochondria and confines mitochondrial activity to the front of cells where mitochondrial ATP production amplifies P2X4 receptor signaling and energizes the cytoskeletal rearrangements needed for pseudopod protrusion and T cell migration. (C) Effect of apyrase on cell migration speed of CD4 effector T cells. Data are means ± SD of 3-6 independent experiments (n=20-40 cells per experiment). *P<0.05 compared to control (one-way ANOVA). (D) Human PBMCs were treated with P2Y11 agonist (NF546, 1 μM), exogenous ATP (10 μM), non-hydrolysable ATP analogue (ATPγS, 10 μM), or vehicle control and stimulated with SDF-1α (100 ng/ml; migration) or anti-CD3 antibodies (0.25 μg/ml; CD69, IL-2, proliferation). Migration speed of CD4 T cells (n≥20 cells per experiment) was analyzed by time-lapse video microscopy. CD69 expression in CD4 T cells was analyzed by flow cytometry after 4 h. IL-2 in the supernatant was measured after 6 h with ELISA. Proliferation of CD4 T cells was assessed 72 h after cell stimulation by CFSE dye dilution. Data are from at least 3 independent experiments (indicated by circles) with cells from different donors. ***P<0.001 compared to controls (one-way ANOVA).
Excessive stimulation of P2Y11 receptors disrupts cell polarization and T cell functions
The data described above demonstrate that autocrine P2Y11 receptor signaling is essential for T cell migration. In agreement, disruption of autocrine purinergic signaling by removing extracellular ATP with the ATP hydrolyzing enzyme apyrase impaired T cell migration (Fig. 7C). T cell migration is required for subsequent functional T cell responses. Because P2Y11 receptor stimulation must occur in a spatiotemporally regulated fashion, interference with autocrine P2Y11 receptor signaling is expected to block T cell migration and functions. In agreement with this concept, global stimulation of P2Y11 receptors by addition of the agonist NF546, exogenous ATP, or the non-hydrolysable ATP analogue ATPγS impaired T cell migration and subsequent T cell functions (Fig. 7D, Movie S6). These treatments interfered with the ability of CD4 T cells to express the early activation marker CD69, to release IL-2, and to proliferate in response to cell stimulation with anti-CD3 antibodies under experimental conditions that require T cells to migrate to obtain costimulatory CD28 signals from APCs (Fig. 7D). We found that T cell migration was not inhibited by the ATP metabolite adenosine or antagonists of the Gαs protein-coupled A2a or A2b adenosine receptors, which suggests that ATP and P2Y11 receptors rather than adenosine and P1 receptors are responsible for the inhibitory effects of exogenous ATP on human T cell functions (fig. S4, A and B).
DISCUSSION
Extracellular ATP and its metabolites are critical modulators of immune cell functions (1, 3, 4). In T cells, purinergic receptors regulate cell activation, cytokine production, proliferation, and T cell differentiation (15, 16, 38–40). Cellular ATP release and autocrine feedback through purinergic receptors are involved in the regulation of many of these T cell functions (2, 11). Autocrine stimulation of P2X4 receptors is particularly important because P2X4 receptors facilitate Ca2+ influx and initiate mitochondrial ATP production that provide excitatory triggers to elicit functional T cell responses (13, 16). Our current study revealed that P2Y11 receptors are also critical regulators that amplify excitatory signaling through P2X4 receptors. We showed that P2Y11 receptors help restrict pseudopod formation to the front of cells and promote uropod retraction at the back. These functions of P2Y11 receptors depend on cAMP/PKA signaling that restricte localized excitatory P2X4 receptor signaling and activation of mitochondria to the front of cells where increased mitochondrial metabolism generates the ATP that powers actin cytoskeleton remodeling during T cell migration (41).
Cell polarization is an essential prerequisite for cell migration. Mathematical models such as the local-excitation-global-inhibition (LEGI) model aim to explain the complex pull-push mechanisms that regulate the polarization and movements of migrating cells (42). The LEGI model suggests that cell migration is the result of local excitatory feedback signals that promote cell protrusions at the front of cells and that those mechanisms couple to global inhibitory signals that suppress pseudopod protrusions at sites other than the leading edge of cells (30, 43). The cAMP/PKA signaling pathway may represent the inhibitory signal proposed in the LEGI model (44, 45). P2Y11 receptors can increase intracellular cAMP levels in many cell types including in human T cells (24, 46–48). Our results demonstrate that cAMP/PKA signaling downstream of P2Y11 receptors is essential for the regulation of T cell migration. Thus, autocrine stimulation of P2X4 and P2Y11 receptors endows T cells with the pull-push signals that induce local excitation and global inhibition as proposed in the LEGI model. Other immune cells use different purinergic receptor combinations to achieve the same purpose. Chemotaxis of human neutrophils, for example, depends on autocrine signaling through P2Y2 and A2a receptors that generate the pull-push forces needed for cell migration (35). Like P2Y11 receptors, A2a receptors are Gαs protein-coupled GPCRs that inhibit chemotactic signaling and thereby promote uropod retraction at the back of neutrophils (49).
Our current study showed that localized ATP release induces autocrine signaling through P2X4 and P2Y11 receptors, which synergize to regulate T cell migration. This process begins with the stimulation of CXCR4 receptors that trigger an initial burst of localized ATP release through Panx1 channels in response to SDF-1α stimulation (13, 14). Although the mechanistic details of Panx1 channel opening have yet to be defined, increased cytoplasmic Ca2+ levels could be involved (50). The pericellular ATP that stimulates P2X4 receptors in stimulated T cells promotes Ca2+ influx that may accomplish this process in addition to promoting the activation of nearby mitochondria. Together, these processes result in a second, more pronounced round of ATP release that is sufficiently intense to stimulate more distant P2Y11 receptors and to induce inhibitory cAMP/PKA signaling that prevents mitochondrial activity at the back of cells. The retraction of P2Y11 receptors from the front to the back of polarized T cells during cell polarization focuses the activity of mitochondria to the front of migrating T cells (Movie S5).
Together, we conclude that ATP release and autocrine signaling through P2Y11 and P2X4 receptors act in synergy to orchestrate the metabolic program that regulates T cell polarization and migration. Blocking or interfering with these purinergic signaling mechanisms, for example with excessive exogenous ATP, disrupts T cell migration. These findings have clinical implications because increased extracellular ATP levels can inhibit T cell functions (51, 52). Elevated extracellular ATP levels are hallmarks of inflammation, sepsis, and cancer (53–54). In critically ill patients, inflammation, hypoxia, and cell damage release large amounts of ATP into the systemic circulation (53, 55), which likely impairs T cell function by inappropriate P2Y11 and P2X4 receptor stimulation. High extracellular ATP levels are also characteristic of the tumor microenvironment (54). Enzymatic breakdown of ATP by the ectonucleotidases CD39 and CD73 and subsequent accumulation of adenosine can suppress T cells by stimulating A2a and A2b receptors and contributing to T cell exhaustion (54, 56). The adenosinergic pathway is being intensively studied as a possible checkpoint inhibitor target (57). Our findings suggest that elevated ATP itself might be involved in T cell suppression by interfering with P2Y11 and P2X4 receptors and preventing the ability of T cells to infiltrate tumors where they can elicit their cytotoxic T cell responses (58). P2Y11 receptor signaling and immune interference by systemic ATP may therefore be a possible therapeutic target to improve T cell function and host immune defense in sepsis and in other clinical settings including cancer. The importance of P2Y11 receptors for T cell function is supported by evidence that single nucleotide polymorphisms of the P2Y11 receptor gene are associated with inflammatory disorders that cause acute myocardial infarction and narcolepsy (60–61). These observations support our conclusion that P2Y11 receptors are important regulators of T cells. Mice and other rodents do not possess P2Y11 receptors (62, 63), suggesting that rodent T cells depend on other purinergic receptors to deliver the cAMP/PKA signal they need for cell migration. This species difference should be kept in mind when interpreting findings of preclinical studies using rodent models to explore treatments for human diseases such as infections, sepsis, and cancer.
MATERIALS AND METHODS
Reagents
Fluo-4 AM, Rhod-2 AM, MitoTracker Red CM-H2Xros, and carboxyfluorescein succinimidyl ester (CFSE) were purchased from Molecular Probes (Thermo Fisher Scientific). Recombinant human SDF-1α, NF340 (P2Y11 antagonist), NF546 (P2Y11 agonist), 5-BDBD (P2X4 antagonist), CSC (A2a antagonist), MRS1754 (A2b antagonist), and H89 (PKA antagonist) were from R&D Systems, and cAMP-AM was from BIOLOG Life Science Institute. In all experiments, 5-BDBD, NF340, and NF546 were used at concentrations (1-10 μM) at which they were reported to be specific for the respective receptor (36, 64, 65). Anti-human CD4-allophycocyanin (clone: OKT4) and anti-human CD69 FITC (clone: FN50) antibodies were purchased from Biolegend. Mouse anti-human CD3 (clone: HIT3a) antibodies were from BD Pharmingen. All other reagents were purchased from Sigma-Aldrich unless stated otherwise.
Cell isolation and cell culture
All studies involving human subjects were approved by the Institutional Review Board of the Beth Israel Deaconess Medical Center and written informed consent was obtained prior to blood draws. Peripheral blood mononuclear cells (PBMCs) were isolated from heparinized blood of healthy volunteers by Ficoll-Paque density centrifugation. CD4 T cells were purified from PBMCs by positive selection using immunomagnetic beads (Miltenyi Biotec).
Jurkat T cells (clone E6-1; ATCC) were cultured in fully supplemented RPMI-1640 medium as previously described (12). Phoenix-Ampho cells (ATCC) were maintained in Dulbecco’s Modified Eagle’s Medium supplemented with 10% heat-inactivated fetal bovine serum (FBS), 2 mM L-glutamine, 100 U/ml penicillin, and 100 μg/ml streptomycin (Gibco).
T cell polarization, pseudopod formation, and T cell migration were studied in three groups of cells: (i) freshly isolated human PBMCs or human CD4 T cells purified from healthy volunteers that were stimulated with SDF-1α; (ii) Jurkat T cells that were stimulated or not with SDF-1α; and (iii) CD4 effector T cells that were generated by culturing purified CD4 T cells from healthy volunteers with anti-CD3/CD28 antibody-coated beads (Dynabeads, Thermo Fisher Scientific) at a bead to cell ratio of 1:1 in RPMI-1640 medium (ATCC) supplemented with 10% heat-inactivated FBS and recombinant human IL-2 (0.25 ng/ml) for a period of 3 days.
T cell motility, cell shape, and pseudopod formation
T cells were seeded into fibronectin-coated (40 μg/ml) glass-bottom chamber slide dishes (Lab-Tek). The chambers were placed into a temperature-controlled stage incubator and maintained at 37°C in a humidified atmosphere containing 5% CO2 and 21% O2 (Live Cell Instrument). The cells were pretreated for 20 min with inhibitors or agonists as indicated. Cell migration in the presence or absence of SDF-1α (100 ng/ml) was analyzed with time-lapse microscopy using a Leica DMIRB inverted microscope using a 20x objective (numerical aperture (NA) 0.4) and capturing sequential images at 45-s intervals for 30 min with a Hamamatsu Orca II camera. CD4 positive PBMCs were identified by labeling with anti-CD4 antibodies and cells were imaged with a Leica DMI6000B microscope equipped with Cy5 excitation/emission filters. Bright field and fluorescence images were captured with a Leica DFC365 FX camera through a 40x objective (NA 0.75). The migration paths of individual cells were tracked with ImageJ software (NIH; MTrackJ plugin) and the length of these tracks was used to calculate migration speeds. Surface area and cell polarization, defined as the ratio of cell length versus cell width, were determined with ImageJ. Formation of pseudopods was analyzed as previously described (13).
Transfection and retroviral infection
The enhanced green fluorescent protein (EGFP)-tagged P2X4 receptor construct was generated as previously described (16). The pBMN-P2Y11-YFP plasmid was a kind gift from Severin Mühleder and Wolfgang Holnthoner from the Ludwig Boltzmann Institute for Clinical and Experimental Traumatology, Vienna, Austria (66). Expression plasmids were verified by sequencing. A mixture of four siRNA constructs targeting the P2Y11 receptor (SMARTpool® siRNA) was obtained from Dharmacon and pre-validated P2X4 receptor siRNA was purchased from Thermo Fisher Scientific. Jurkat cells were transfected with the EGFP-P2X4 plasmid, siRNA or non-targeting control siRNA (Qiagen) with a Neon electroporation transfection system (Invitrogen) as previously described (13, 16). Transfected cells were cultured for 5 h (plasmid) or 24 h (siRNA) in cell culture medium without antibiotics. Phoenix-Ampho cells were transfected with 10 μg of the pBMN-P2Y11-YFP plasmid using Lipofectamine 2000 (Invitrogen) according to the manufacturer’s instructions. Virus supernatants were collected after 48 and 72 h. CD4 T cells stimulated with anti-CD3/CD28 antibody-coated beads for 2 days or Jurkat cells were resuspended in fresh virus supernatants containing 2 μg/ml polybrene (Santa Cruz Biotechnology), centrifuged at 400 x g and room temperature for 1.5 h, and incubated overnight. After 24 h, cells were infected a second time and cultured for another 48 to 72 h before imaging experiments were performed. Infected CD4 T cell cultures were maintained with anti-CD3/CD28 antibody-coated beads (1 bead per cell) and IL-2 (0.25 ng/ml).
Live-cell imaging of purinergic receptor distribution and mitochondrial activity
Jurkat cells expressing YFP-tagged P2Y11 or EGFP-tagged P2X4 receptor fusion proteins were allowed to attach to fibronectin-coated glass-bottom chamber slides, reconstituted in cell culture medium without phenol red, and placed into a temperature-controlled (37°C) stage incubator. To analyze the distribution of mitochondrial activity, cells were labeled with MitoTracker Red CM-H2Xros dye (100 nM, 10 min). Fluorescence imaging was performed with the Leica DMI6000B microscope described above, using a 100x oil objective (NA 1.4), FITC and TRITC filter sets, and Leica LAS X imaging software. Image analysis was done with ImageJ. The impact of P2Y11 receptor accumulation at the back of cells on uropod retraction was assessed by correlating the P2Y11 receptor fluorescence intensities at the uropod and the retraction speed of the back, which was determined by tracking uropod movements for 2 min. Distribution profiles of fluorescence intensities summed up over the whole cell area were calculated with ImageJ.
Calcium measurements
Purified CD4 T cells were placed into fibronectin-coated glass-bottom dishes, labeled with Rhod-2 AM to detect mitochondrial Ca2+ or with Fluo-4 AM to detect cytosolic Ca2+, and treated for 20 min with inhibitors or agonists of P2Y11 receptor signaling as indicated. Cells were stimulated with SDF-1α (100 ng/ml), and changes in fluorescence were recorded (20 frames per min) with the Leica DMI6000B microscope described above using a 63x (NA 1.4; Rhod-2) or a 40x (NA 0.75; Fluo-4) objective. Images were analyzed with ImageJ software.
ATP measurements
Jurkat cells (5x105, suspended in 150 μl RPMI medium) were incubated for 30 min with or without 5-BDBD (10 μM), NF340 (10 μM), H89 (5 μM), NF546 (1 μM), or cAMP-AM (1 μM) in a 37°C water bath on a vibration isolation table to avoid mechanical stimulation. Then, cells were stimulated with SDF-1α (100 ng/ml) or RPMI medium (handling control) for up to 5 min, chilled in an ice bath to stop reactions, and the supernatants were collected by centrifugation at 0°C. Cell lysates for assessing intracellular ATP concentrations were prepared by ultrasonication in the presence of 4 mM perchloric acid to prevent enzymatic breakdown of ATP. ATP concentrations in supernatants or neutralized cell lysates were determined using a luciferin/luciferase-based ATP bioluminescence assay kit (Invitrogen) according to the manufacturer’s instructions.
CD69 expression and IL-2 production
PBMCs (1.5x105 per well in 96-well cell culture plates) were stimulated with anti-CD3 antibodies (0.25 μg/ml) in the presence or absence of purinergic receptor agonists as indicated. After 4 h, CD69 expression of CD4 T cells was measured by flow cytometry. CD4 T cells were identified based on forward and side scatter properties and anti-CD4 staining. IL-2 production by PBMCs was determined 6 h after cell stimulation using a commercially available ELISA assay kit (R&D Systems).
T cell proliferation
PBMCs were labeled with CFSE following the manufacturer’s instructions, placed into 48-well culture plates at low density (2.5 x 105 cells per well), and stimulated for 72 h with soluble anti-CD3 antibodies (0.25 μg/ml) in the presence or absence of purinergic receptor agonists as indicated. Under these conditions, T cells were forced to migrate to interact with antigen-presenting cells that provided the costimulatory signals needed for T cell receptor/CD28 signaling. CD4 T cells were identified based on forward and side scatter properties and anti-CD4 antibody labeling, and the percentage of proliferating (CFSElow) CD4 T cells was determined with flow cytometry.
Statistical analysis
Two groups were compared with the unpaired two-tailed Student’s t test. The one-way analysis of variance (ANOVA) was used for multiple group comparisons followed by the Holm-Sidak post hoc test to identify groups that differed significantly from the control group. Data that were not normally distributed were analyzed with the Mann-Whitney U test (two groups) or the Kruskal-Wallis test and post hoc Dunn’s test (multiple group comparisons). Correlations between two parameters were analyzed with Pearson’s test. Differences were considered statistically significant at P<0.05.
Supplementary Material
Movie S1. P2Y11 receptor silencing impairs Jurkat T cell migration.
Movie S2. P2X4 and P2Y11 receptors regulate T cell migration.
Movie S3. P2Y11 receptors direct cell protrusions during T cell migration.
Movie S4. P2Y11 receptors restrict P2X4 receptor signaling and mitochondrial activation to the front of migrating T cells.
Movie S5. Proposed model of how P2X4 and P2Y11 receptors synergize to regulate T cell migration.
Movie S6. Extracellular ATP impairs T cell migration.
Fig. S1. P2Y11 receptors regulate the polarization and migration of Jurkat T cells.
Fig. S2. Redistribution of P2Y11 receptors defines the subcellular distribution of active mitochondria and cell protrusions during T cell migration.
Fig. S3. P2Y11 receptor stimulation does not increase intracellular Ca2+ levels in human CD4 T cells.
Fig. S4. Adenosine does not affect human T cell migration.
Acknowledgments:
We thank S. Mühleder and W. Holnthoner (Ludwig Boltzmann Institute for Experimental and Clinical Traumatology, Vienna, Austria) for kindly providing the pBMN-P2Y11-YFP plasmid. This work was conducted with support from Harvard Catalyst | The Harvard Clinical and Translational Science Center (National Center for Research Resources and National Center for Advancing Translational Sciences, National Institutes of Health Award UL1 TR002541) and financial contributions from Harvard University and its affiliated academic health care centers. The content is solely the responsibility of the authors and does not necessarily represent the official views of Harvard Catalyst, Harvard University and its affiliated academic health care centers, or the National Institutes of Health.
Funding:
This study was supported in part by grants R01 GM-51477, R01 GM-116162, R01 HD-098363, R35 GM-136429, and T32 GM-103702 from the National Institutes of Health (to W.G.J). S.B. was supported by a scholarship from the Austrian Marshall Plan Foundation.
Footnotes
Competing interests: The authors declare that they have no competing interests.
Data and materials availability: All data needed to evaluate the conclusions in the paper are present in the paper or the Supplementary Materials.
REFERENCES AND NOTES
- 1.Burnstock G, Boeynaems JM, Purinergic signalling and immune cells. Purinergic Signal. 10, 529–564 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Junger WG, Immune cell regulation by autocrine purinergic signalling. Nat. Rev. Immunol 11, 201–212 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Di Virgilio F, Vuerich M, Purinergic signaling in the immune system. Auton. Neurosci 191, 117–123 (2015). [DOI] [PubMed] [Google Scholar]
- 4.Cekic C, Linden J, Purinergic regulation of the immune system. Nat. Rev. Immunol 16, 177–192 (2016). [DOI] [PubMed] [Google Scholar]
- 5.Lazarowski ER, Vesicular and conductive mechanisms of nucleotide release. Purinergic Signal. 8, 359–373 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Taruno A, ATP release channels. Int. J. Mol. Sci 19, 808 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Coddou C, Yan Z, Obsil T, Huidobro-Toro JP, Stojilkovic SS, Activation and regulation of purinergic P2X receptor channels. Pharmacol. Rev 63, 641–683 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Abbracchio MP, Burnstock G, Boeynaems JM, Barnard EA, Boyer JL, Kennedy C, Knight GE, Fumagalli M, Gachet C, Jacobson KA, Weisman GA, International Union of Pharmacology LVIII: update on the P2Y G protein-coupled nucleotide receptors: from molecular mechanisms and pathophysiology to therapy. Pharmacol. Rev 58, 281–341 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Haskó G, Antonioli L, Cronstein BN, Adenosine metabolism, immunity and joint health. Biochem. Pharmacol 151, 307–313 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yegutkin GG, Mikhailov A, Samburski SS, Jalkanen S S, The detection of micromolar pericellular ATP pool on lymphocyte surface by using lymphoid ecto-adenylate kinase as intrinsic ATP sensor. Mol. Biol. Cell 17, 3378–3385 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yip L, Woehrle T, Corriden R, Hirsh M, Chen Y, Inoue Y, Ferrari V, Insel PA, Junger WG, Autocrine regulation of T-cell activation by ATP release and P2X7 receptors. FASEB J 23, 1685–1693 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ledderose C, Bao Y, Lidicky M, Zipperle J, Li L, Strasser K, Shapiro NI, Junger WG, Mitochondria are gate-keepers of T cell function by producing the ATP that drives purinergic signaling. J. Biol. Chem 289, 25936–25945 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ledderose C, Liu K, Kondo Y, Slubowski CJ, Dertnig T, Denicoló S, Arbab M, Hubner J, Konrad K, Fakhari M, Lederer JA, Robson SC, Visner GA, Junger WG, Purinergic P2X4 receptors and mitochondrial ATP production regulate T cell migration. J. Clin. Invest 128, 3583–3594 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Velasquez S, Malik S, Lutz SE, Scemes E, Eugenin EA, Pannexin1 channels are required for chemokine-mediated migration of CD4+ T lymphocytes: role in inflammation and experimental autoimmune encephalomyelitis. J. Immunol 196, 4338–4347 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schenk U, Westendorf AM, Radaelli E, Casati A, Ferro M, Fumagalli M, Verderio C, Buer J, Scanziani E, Grassi F, Purinergic control of T cell activation by ATP released through pannexin-1 hemichannels. Sci. Signal 1, ra66 (2008). [DOI] [PubMed] [Google Scholar]
- 16.Woehrle T, Yip L, Elkhal A, Sumi Y, Chen Y, Yao Y, Insel PA, Junger WG, Pannexin-1 hemichannel-mediated ATP release together with P2X1 and P2X4 receptors regulate T-cell activation at the immune synapse. Blood 116, 3475–3484 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Masopust D, Schenkel JM, The integration of T cell migration, differentiation and function. Nat. Rev. Immunol 13, 309–320 (2013). [DOI] [PubMed] [Google Scholar]
- 18.Krummel MF, Bartumeus F, Gérard A, T cell migration, search strategies and mechanisms. Nat. Rev. Immunol 16, 193–201 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cahalan MD, Parker I, Choreography of cell motility and interaction dynamics imaged by two-photon microscopy in lymphoid organs. Annu. Rev. Immunol 26, 585–626 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ridley AJ, Schwartz MA, Burridge K, Firtel RA, Ginsberg MH, Borisy G, Parsons JT, Horwitz AR, Cell migration: integrating signals from front to back. Science 302, 1704–1709 (2003). [DOI] [PubMed] [Google Scholar]
- 21.Glancy B, Balaban RS, Role of mitochondrial Ca2+ in the regulation of cellular energetics. Biochemistry 51, 2959–2973 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Moore DJ, Chambers JK, Wahlin JP, Tan KB, Moore GB, Jenkins O, Emson PC, Murdock PR, Expression pattern of human P2Y receptor subtypes: a quantitative reverse transcription-polymerase chain reaction study. Biochim. Biophys. Acta 1521, 107–119 (2001). [DOI] [PubMed] [Google Scholar]
- 23.Manohar M, Hirsh MI, Chen Y, Woehrle T, Karande AA, Junger WG, ATP release and autocrine signaling through P2X4 receptors regulate γδ T cell activation. J. Leukoc. Biol 92, 787–794 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Communi D, Robaye B, Boeynaems JM, Pharmacological characterization of the human P2Y11 receptor. Br. J. Pharmacol 128, 1199–1206 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.von Andrian UH, Mackay CR, T-cell function and migration. Two sides of the same coin. N. Engl. J. Med 343, 1020–1034 (2000). [DOI] [PubMed] [Google Scholar]
- 26.Bleul CC, Fuhlbrigge RC, Casasnovas JM, Aiuti A, Springer TA, A highly efficacious lymphocyte chemoattractant, stromal cell-derived factor 1 (SDF-1). J. Exp. Med 184, 1101–1119 (1996). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mosenden R, Taskén K, Cyclic AMP-mediated immune regulation--overview of mechanisms of action in T cells. Cell Signal. 23, 1009–1016 (2011). [DOI] [PubMed] [Google Scholar]
- 28.Wehbi VL, Taskén K, Molecular mechanisms for cAMP-mediated immunoregulation in T cells - role of anchored protein kinase A signaling units. Front. Immunol 7, 222 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Clark AA, Nurmukhambetova S, Li X, Munger SD, Lees JR, Odorants specifically modulate chemotaxis and tissue retention of CD4+ T cells via cyclic adenosine monophosphate induction. J. Leukoc. Biol 100, 699–709 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rappel WJ, Edelstein-Keshet L, Mechanisms of cell polarization. Curr. Opin. Syst. Biol 3, 43–53 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Campello S, Lacalle RA, Bettella M, Mañes S, Scorrano L, Viola A, Orchestration of lymphocyte chemotaxis by mitochondrial dynamics. J. Exp. Med 203, 2879–2886 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Quintana A, Schwindling C, Wenning AS, Becherer U, Rettig J, Schwarz EC, Hoth M, T cell activation requires mitochondrial translocation to the immunological synapse. Proc. Natl. Acad. Sci. USA 104, 14418–14423 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Baixauli F, Martín-Cófreces NB, Morlino G, Carrasco YR, Calabia-Linares C, Veiga E, Serrador JM, Sánchez-Madrid F, The mitochondrial fission factor dynamin-related protein 1 modulates T-cell receptor signalling at the immune synapse. EMBO J. 30, 1238–1250 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kuznetsov AV, Kehrer I, Kozlov AV, Haller M, Redl H, Hermann M, Grimm M, Troppmair J, Mitochondrial ROS production under cellular stress: comparison of different detection methods. Anal. Bioanal. Chem 400, 2383–2390 (2011). [DOI] [PubMed] [Google Scholar]
- 35.Bao Y, Ledderose C, Graf AF, Brix B, Birsak T, Lee A, Zhang J, Junger WG, mTOR and differential activation of mitochondria orchestrate neutrophil chemotaxis. J. Cell Biol 210, 1153–1164 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Meis S, Hamacher A, Hongwiset D, Marzian C, Wiese M, Eckstein N, Royer HD, Communi D, Boeynaems JM, Hausmann R, Schmalzing G, Kassack MU, NF546 [4,4′-(carbonylbis(imino-3,1-phenylene-carbonylimino-3,1-(4-methyl-phenylene)-carbonylimino))-bis(1,3-xylene-alpha,alpha’-diphosphonic acid) tetrasodium salt] is a non-nucleotide P2Y11 agonist and stimulates release of interleukin-8 from human monocyte-derived dendritic cells. J. Pharmacol. Exp. Ther 332, 238–247 (2010). [DOI] [PubMed] [Google Scholar]
- 37.Duhant X, Schandené L, Bruyns C, Gonzalez NS, Goldman M, Boeynaems JM, Communi D, Extracellular adenine nucleotides inhibit the activation of human CD4+ T lymphocytes. J. Immunol 169, 15–21 (2002). [DOI] [PubMed] [Google Scholar]
- 38.Schenk U, Frascoli M, Proietti M, Geffers R, Traggiai E, Buer J, Ricordi C, Westendorf AM, Grassi F, ATP inhibits the generation and function of regulatory T cells through the activation of purinergic P2X receptors. Sci. Signal 4, ra12 (2011). [DOI] [PubMed] [Google Scholar]
- 39.Wang CM, Ploia C, Anselmi F, Sarukhan A, Viola A, Adenosine triphosphate acts as a paracrine signaling molecule to reduce the motility of T cells. EMBO J. 33, 1354–1364 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Borges da Silva H, Beura LK, Wang H, Hanse EA, Gore R, Scott MC, Walsh DA, Block KE, Fonseca R, Yan Y, Hippen KL, Blazar BR, Masopust D, Kelekar A, Vulchanova L, Hogquist KA, Jameson SC, The purinergic receptor P2RX7 directs metabolic fitness of long-lived memory CD8(+) T cells. Nature 559, 264–268 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Insall RH, Machesky LM, Actin dynamics at the leading edge: from simple machinery to complex networks. Dev. Cell 17, 310–322 (2009). [DOI] [PubMed] [Google Scholar]
- 42.Jilkine A, Edelstein-Keshet L, A comparison of mathematical models for polarization of single eukaryotic cells in response to guided cues. PLoS Comput. Biol 7, e1001121 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Levine H, Kessler DA, Rappel WJ, Directional sensing in eukaryotic chemotaxis: a balanced inactivation model. Proc. Natl. Acad. Sci. USA 103, 9761–9766 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Stepanovic V, Wessels D, Daniels K, Loomis WF, Soll DR, Intracellular role of adenylyl cyclase in regulation of lateral pseudopod formation during Dictyostelium chemotaxis. Eukaryot. Cell 4, 775–786 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Liu L, Das S, Losert W, Parent CA, mTORC2 regulates neutrophil chemotaxis in a cAMP- and RhoA-dependent fashion. Dev. Cell 19, 845–857 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wilkin F, Duhant X, Bruyns C, Suarez-Huerta N, Boeynaems JM, Robaye B, The P2Y11 receptor mediates the ATP-induced maturation of human monocyte-derived dendritic cells. J. Immunol 166, 7172–7177 (2001). [DOI] [PubMed] [Google Scholar]
- 47.Sakaki H, Tsukimoto M, Harada H, Moriyama Y, Kojima S, Autocrine regulation of macrophage activation via exocytosis of ATP and activation of P2Y11 receptor. PLoS One 8, e59778 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dreisig K, Sund L, Dommer MW, Kristensen NP, Boddum K, Viste R, Fredholm S, Odum N, Jäättelä M, Skov S, Kornum BR, Human P2Y(11) expression level affects human P2X7 receptor-mediated cell death. Front. Immunol 9, 1159 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bao Y, Chen Y, Ledderose C, Li L, Junger WG, Pannexin 1 channels link chemoattractant receptor signaling to local excitation and global inhibition responses at the front and back of polarized neutrophils. J. Biol. Chem 288, 22650–22657 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Locovei S, Wang J, Dahl G, Activation of pannexin 1 channels by ATP through P2Y receptors and by cytoplasmic calcium. FEBS Lett. 580, 239–244 (2006). [DOI] [PubMed] [Google Scholar]
- 51.Weiler M, Schmetzer H, Braeu M, Buhmann R, Inhibitory effect of extracellular purine nucleotide and nucleoside concentrations on T cell proliferation. Exp. Cell. Res 349, 1–14 (2016). [DOI] [PubMed] [Google Scholar]
- 52.Sueyoshi K, Ledderose C, Shen Y, Lee AH, Shapiro NI, Junger WG, Lipopolysaccharide suppresses T cells by generating extracellular ATP that impairs their mitochondrial function via P2Y11 receptors. J. Biol. Chem 294, 6283–6293 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Idzko M, Ferrari D, Eltzschig HK, Nucleotide signalling during inflammation. Nature 509, 310–317 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Di Virgilio F, Sarti AC, Falzoni S, De Marchi E, Adinolfi E, Extracellular ATP and P2 purinergic signalling in the tumour microenvironment. Nat. Rev. Cancer 18, 601–618 (2018). [DOI] [PubMed] [Google Scholar]
- 55.Sumi Y, Ledderose C, Li L, Inoue Y, Okamoto K, Kondo Y, Sueyoshi K, Junger WG, Tanaka H, Plasma adenylate levels are elevated in cardiopulmonary arrest patients and may predict mortality. Shock 51, 698–705 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Boison D, Yegutkin GG, Adenosine metabolism: emerging concepts for cancer therapy. Cancer Cell 36, 582–596 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Vijayan D, Young A, Teng MWL, Smyth MJ, Targeting immunosuppressive adenosine in cancer. Nat. Rev. Cancer 17, 709–724 (2017). [DOI] [PubMed] [Google Scholar]
- 58.Trujillo JA, Sweis RF, Bao R, Luke JJ, T cell-inflamed versus non-T cell-inflamed tumors: a conceptual framework for cancer immunotherapy drug development and combination therapy selection. Cancer Immunol. Res 6, 990–1000 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Amisten S, Melander O, Wihlborg AK, Berglund G, Erlinge D, Increased risk of acute myocardial infarction and elevated levels of C-reactive protein in carriers of the Thr-87 variant of the ATP receptor P2Y11. Eur. Heart. J 28, 13–18 (2007). [DOI] [PubMed] [Google Scholar]
- 60.Kornum BR, Kawashima M, Faraco F, Lin L, Rico TJ, Hesselson S, Axtell RC, Kuipers H, Weiner K, Hamacher A, et al. , Common variants in P2RY11 are associated with narcolepsy. Nat. Genet 43, 66–71 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Han F, Faraco J, Dong XS, Ollila HM, Lin L, Li J, An P, Wang S, Jiang KW, Gao ZC, Zhao L, Yan H, Liu YN, Li QH, Zhang XZ, Hu Y, Wang JY, Lu YH, Lu CJ, Zhou W, Hallmayer J, Huang YS, Strohl KP, Pollmacher T, Mignot E, Genome wide analysis of narcolepsy in China implicates novel immune loci and reveals changes in association prior to versus after the 2009 H1N1 influenza pandemic. PLoS Genet 9, e1003880 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Dreisig K, Kornum BR. A critical look at the function of the P2Y11 receptor. Purinergic Signal. 12, 427–437 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kennedy C, P2Y(11) receptors: properties, distribution and functions. Adv. Exp. Med. Biol 1051, 107–122 (2017). [DOI] [PubMed] [Google Scholar]
- 64.Balázs B, Dankó T, Kovács G, Köles L, Hediger MA, Zsembery A, Investigation of the inhibitory effects of the benzodiazepine derivative, 5-BDBD on P2X4 purinergic receptors by two complementary methods. Cell Physiol. Biochem 32, 11–24 (2013). [DOI] [PubMed] [Google Scholar]
- 65.Abdelrahman A, Namasivayam V, Hinz S, Schiedel AC, Köse M, Burton M, El-Tayeb A, Gillard M, Bajorath J, de Ryck M, Müller CE, Characterization of P2X4 receptor agonists and antagonists by calcium influx and radioligand binding studies. Biochem. Pharmacol 125, 41–54 (2017). [DOI] [PubMed] [Google Scholar]
- 66.Mühleder S, Fuchs C, Basílio J, Szwarc D, Pill K, Labuda K, Slezak P, Siehs C, Pröll J, Priglinger E, Hoffmann C, Junger WG, Redl H, Holnthoner W, Purinergic P2Y(2) receptors modulate endothelial sprouting. Cell. Mol. Life. Sci DOI: 10.1007/s00018-019-03213-2. [Epub ahead of print] (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Movie S1. P2Y11 receptor silencing impairs Jurkat T cell migration.
Movie S2. P2X4 and P2Y11 receptors regulate T cell migration.
Movie S3. P2Y11 receptors direct cell protrusions during T cell migration.
Movie S4. P2Y11 receptors restrict P2X4 receptor signaling and mitochondrial activation to the front of migrating T cells.
Movie S5. Proposed model of how P2X4 and P2Y11 receptors synergize to regulate T cell migration.
Movie S6. Extracellular ATP impairs T cell migration.
Fig. S1. P2Y11 receptors regulate the polarization and migration of Jurkat T cells.
Fig. S2. Redistribution of P2Y11 receptors defines the subcellular distribution of active mitochondria and cell protrusions during T cell migration.
Fig. S3. P2Y11 receptor stimulation does not increase intracellular Ca2+ levels in human CD4 T cells.
Fig. S4. Adenosine does not affect human T cell migration.


