Abstract
Introduction
Hypertension is the leading direct cause of death in the world and one of the most important risk factors for cardiovascular disease (CVD). Elevated blood pressure (BP) often coexists with lipid disorders and is an additional factor that increases CV risk. Nowadays, we are able to distinguish low density lipoproteins (LDL) and high density lipoproteins (HDL) subfractions. Except LDL also HDL small subfractions can increase the risk of CV events. Therefore, we aimed to investigate the associations between changes of lipoprotein subfractions and the risk of hypertension development.
Material and methods
In 2-year long study 200 volunteers with normal blood pressure at the age of 19–32 years were included. Each volunteer underwent detailed medical examination, 12-lead electrocardiogram was taken at rest, echocardiogram, lipid subfraction assessment (using Lipoprint®) and two 24-hour BP measurements.
Results
Mean total cholesterol concentration was 189 mg/dl (4.89 mmol/l), with mean LDL concentration of 107 mg/dl (2.77 mmol/l), HDL of 63 mg/dl (1.63 mmo/l), very low-density lipoprotein (VLDL) of 40 mg/dl (1.04 mmol/l) and triglycerides (TG) of 89 mg/dl (1.00 mmol/l). Subfractions LDL 1–3 were most abundant, LDL 4–5 making up a marginal portion and LDL 6–7 were not observed. Whereas, subfractions HDL 4–6 were most abundant, in lower concentration was present HDL 1–3 and HDL 8–10. We showed that increased systolic blood pressure coreclated significantly with HDL cholesterol concentrations (p = 0.0078), HDL intermediate subgractions (p = 0.0451), with HDL-3 subfraction (p = 0.0229), and intermediate density lipoprotein-A (IDL-A) (p = 0.038). A significant correlation between increased diastolic blood pressure and HDL lipoprotein levels (p = 0.0454) was only observed.
Conclusions
Obtained results indicating correlation between total HDL levels and HDL-3 subfraction concentration (for systolic BP) and the tendency to develop hypertension.
Key words: hypertension, high density lipoproteins, low density lipoproteins, lipid profile, lipoprotein subfractions
Introduction
Arterial hypertension is a major risk factor for cardiovascular disease (CVD) and is thus one of the most important causes of death worldwide, and the third most important cause of disability [1]. Since elevated blood pressure (BP) often co-exists with dyslipidaemia (irrespective of sex and age, as confirmed by the NATPOL 2011 registry) [2] and constitutes an additional CV risk factor, the combined use of antihypertensive and lipid-lowering agents is often appropriate.
Current studies indicate that the lipid profiles routinely reported by hospital laboratories may not differ substantially between patients with CVD and healthy subjects. Recent findings suggest that the evaluation of subfractions/subpopulations of the individual lipoproteins is likely to be of much greater relevance [1–3]. Low density lipoprotein (LDL) and high density lipoprotein (HDL) particles are known to be heterogeneous, with the subfractions defined based on particle size and density. Based on the gel electrophoresis LDL particles have been classified into seven subfractions (LDL 1–7). At least ten subfractions of HDL exist (HDL 1–10; though the most recent methods allow for the differentiation of as many as 26 subfractions). Anti-atherosclerotic effects of HDL appear to be mediated by two subfractions: HDL 2 and 3 (the so-called large particle HDL subpopulation) [3]. Heterogeneity of LDL and HDL particles is also associated with their various bioactivity levels. Subfractions LDL 1 and 2 (large LDL particles) have been associated with only a moderate risk of CVD, whereas small dense LDL (sdLDL) subfractions (LDL 3–7) increase the risk up to 4-fold [4–6]. The subfraction of larger HDL particles (HDL 2) might be responsible for the clinically beneficial effects that have been generally associated with HDL cholesterol [3]. On the other hand, the HDL 3 subfraction, and mostly other subpopulations of smaller HDL particles (intermediate and small HDL) may even have an undesirable atherogenic effect without inhibiting inflammation – however there are still many opposite results on this issue [3]. The described biological differences are likely to be the result of the fact that sdLDLs (subpopulations 3–7) easily penetrate vascular walls, undergo oxidation, and have a lower affinity for the LDL receptor; conversely, small dense HDL particles contain scarcely any apolipoproteins (Apo) AI and AII, are less effective in the reverse cholesterol transport from peripheral tissues to the liver and may be catabolised more rapidly and lose their endothelium-protective properties [3].
Several processes may be involved in structural and functional changes in LDL and HDL. Inflammation and oxidative stress, which often accompany an atherosclerotic CVD, seem to be particularly important [3]. A shift in balance in favour of one LDL or HDL atherogenic subfraction can play a role in developing obesity, metabolic syndrome, insulin resistance, and consequently – diabetes mellitus [7–10] as well as in developing hypertension. If dyslipidaemia is causally associated with the development of hypertension, evaluation of the lipid profile in normotensive patients would allow early targeted pharmacological intervention in susceptible patients. This approach could extend the time period before hypertension develops, or avoid hypertension (and its associated complications) altogether. This approach would be likely to result in substantial gains in public health, since hypertension is one of the greatest epidemiological challenges in Poland, Europe and around the world. Based on the data from NATPOL 2011 and POLSENIOR 2011 registries, there are nearly 11 million people affected by hypertension in Poland, and in the elderly population, this percentage can be as high as 75%. Moreover, hypertension is still undiagnosed in 30% affected patients and ineffectively treated in 36% of cases [2].
A study published in 2011 evaluated the risk of developing hypertension in 17,527 healthy women during an 8-year follow-up. In contrast to previously published research, no association was observed between baseline LDL cholesterol concentrations and the risk of developing hypertension. However, elevated sdLDL levels significantly increased this risk [11]. Similarly, risk was associated with variations in specific HDL subfractions: the risk was increased in women with a dominant HDL 3 subfraction and decreased in the group of women with dominant HDL 2 particles. Additionally, a higher risk of developing hypertension was associated with elevated levels of very low density lipoproteins (VLDL) rich in triglycerides [11].
Another study enrolled patients with hypertension – both women (41 patients) and men (66 patients) – into the study group and 150 healthy volunteers without hypertension, CVD or metabolic disorders into the control group. Both populations were assessed for the proportion of patients with an atherogenic lipid profile (predominant presence of lipoproteins: VLDL, IDL1 and IDL2 and sdLDL forming subfractions 3–7) with the use of Lipoprint. An atherogenic lipid profile was demonstrated in 93% of patients with associated hypertriglyceridemia, in 86% of patients with mixed hyperlipidaemia, in 52% of patients with hypercholesterolemia, but also in as many as 64% of patients with normolipidaemia. Atherogenic lipid profile was observed in only 7% of healthy volunteers without dyslipidaemia or hypertension, suggesting that disturbances in the proportion of individual lipid fractions are much more common in patients with hypertension than in the normotensive group. On the other hand, an atherogenic lipid profile significantly increases the risk of new-onset hypertension in healthy individuals. The limitations of the study included the relatively small study groups and a short follow-up period [12].
Antihypertensive drugs have interesting effects on concentrations of individual lipoprotein subfractions. In the 1990s, combined treatment with the angiotensin-converting enzyme (ACE) inhibitor captopril and the diuretic hydrochlorothiazide was shown to lead to BP lowering in patients with mild to moderate hypertension, with a simultaneous increase in HDL (due mainly to the increased HDL 3 subfraction) levels during a 16-week follow-up period. This treatment had no effect on concentrations of LDL-C; however, a transient triglyceride (TG) elevation was observed in week 8 [13]. Another study evaluated the effect of doxazosin added to the combined antihypertensive treatment with an ACE inhibitor and a calcium channel blocker in patients with hypertension and type 2 diabetes mellitus. The addition of an α1-blocker was shown not to be associated with a change in LDL levels, but affected the balance of LDL subfractions, with a shift towards the less atherogenic, large LDL particles (1–2) [14].
Another study in treatment-naive patients with grade 2 to 3 hypertension compared the effect of combination therapy with valsartan and amlodipine versus valsartan and hydrochlorothiazide on changes in lipid profiles during a 16-week follow-up period. The groups achieved a comparable reduction in BP; however, the valsartan/hydrochlorothiazide regimen was associated with an increase in small dense LDL particles, which was not observed in patients receiving a combined treatment with a sartan and a calcium channel blocker. This may suggest a higher risk of cardiovascular sequelae in the first group of patients despite the achieved reduction in BP [15].
By contrast, an evaluation of HDL subfractions in the group of patients diagnosed with hypertension and coronary artery disease revealed a significant decrease in large HDL particle levels and an increase in small HDL particle levels. Medium HDL particle levels were similar in both study groups. At the same time, the group with hypertension and coronary artery disease showed a shift in the LDL subpopulations in favour of small LDL particles. All differences were statistically significant [16].
The aim of the study was to assess the lipid profile and lipid subfractions in normotensive patients in order to evaluate the relationships between lipid disorders and developing hypertension in young healthy individuals.
Material and methods
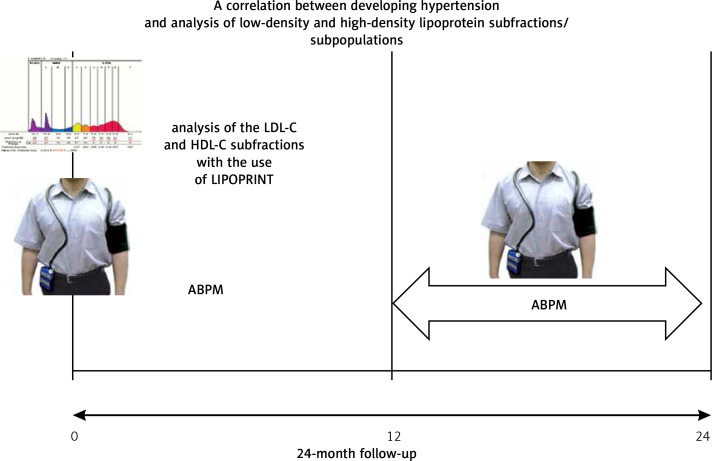
The study initially enrolled 200 healthy individuals (mainly university students) aged 19–32 years with normal BP. The follow-up time was 2 years. In this period, every patient underwent an interview and physical examination, 12-lead electrocardiogram taken at rest, echocardiogram, two 24-hour BP measurements (at least 1 year apart) made with a Holcard CR-07 recorder and lipid subfraction assessment with the use of Lipoprint® (Quantimetrix, Redondo Beach, CA, USA).
Lipoprint uses high resolution polyacrilamide gel electrophoresis and separate each LDL and HDL subfraction on the basis of size. Then, by using a special computer software, the amount of cholesterol in each subfraction can be calculated. Lipoprint separates and measures Very Low Density Lipoprotein (VLDL), VLDL remnants, Intermediate Density Lipoprotein (IDL), LDL 1–7 subfactions, and HDL in serum or plasma. Lipoprint measures cholesterol and area percent for each fraction and subfraction as well as mean LDL particle size. Lipoprint also separates and measures cholesterol in 10 HDL subfractions consisting of large HDL subfractions 1–3, intermediate subfractions 4–7, and small subfractions 8–10.
Out of the 200 enrolled individuals, finally 100 completed the study and were included in a statistical analysis aimed at assessing correlations between lipoprotein subfractions and developing hypertension or a tendency to develop this condition (Figure 1).
Figure 1.
The design of the study
The inclusion criteria were: (1) young and healthy adults; (2) office BP < 140/90 mm Hg at baseline using the auscultatory method in two separated measurements. The exclusion criteria included: (1) current or past treatment with any antihypertensive or lipid-lowering drugs; (2) triglyceride levels of > 500 mg/dl (5.65 mmol/l); (3) co-existing (at screening) secondary causes of hypercholesterolemia: hypothyroidism, National Kidney Foundation (NKF) stage 2–5 chronic kidney disease or nephrotic syndrome, cholestatic liver disease, the use of glucocorticosteroids or protease inhibitors in HIV-positive patients; (4) subject’s inability to comply with study requirements and/or provide his/her informed consent for study participation. Paradoxically, the last exclusion criterion in the group of young individuals resulted in losing almost half of the initially enrolled patients.
Ethics
The study was approved by the Bioethics Committee of the Medical University of Lodz (number of approval RNN/393/13/KB of 21 May 2013). Each person enrolled in the study obtained detailed information on the study design and was asked to sign an informed consent form.
Statistical analysis
Nominal data are presented as numbers and/or percentages. Continuous variables are presented as means ± standard deviations (SD). The analysis of risk factors for ambulatory BP monitoring (ABPMs) increase was carried out by the means of univariate logistic regression analysis, obtaining a set of assessments of regression parameters together with an assessment of their significance and the odds ratio (OR) calculated on their basis. The impact of investigated lipoprotein subfractions on ORs of the increase systolic (SBP) or diastolic BP (DBP) between the first and second visit was assessed. P < 0.05 was considered as significant. All analyzes were performed using the Statistica 12.0 package (StatSoft, Poland).
Results
The main information on the study characterists has been included in Table I.
Table I.
Baseline study group characteristics
| Gender | % | Age[years] | BMI[kg/m2] | TSH[μIU/ml] | fT3[pmol/l] | fT4[pmol/l] | eGFR[ml/min/ 1.73 m2] | GGTP[U/l] | Total bilirubin[μmol/l] | ALT[U/l] | AST[U/l] |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Females | 74 | 23 ±1.9 | 20.9 ±2.4 | 2.7 ±1.4 | 16.4 ±0.5 | 5.1 ±0.8 | 113 ±13 | 17 ±8 | 12.5 ±5 | 23 ±2 | 16 ±2 |
| Males | 26 | 23.5 ±3.2 | 24.0 ±2.4 | 2.6 ±0.9 | 17.0 ±3.1 | 5.3 ±0.8 | 108 ±14 | 19 ±8 | 15.6 ±7 | 33 ±23 | 27 ±19 |
BMI – body mass index, TSH – thyroid-stimulating hormone, fT3 – free triiodothyronine, fT4 – free thyroxine, eGFR – estimated glomerular filtration rate, GGTP – γ-glutamyl transpeptidase, ALT – alanine transaminase, AST – aspartate transaminase.
Mean total cholesterol (TC) concentration was 189 mg/dl (4.89 mmol/l), with mean LDL concentration of 107 mg/dl (2.77 mmol/l), HDL of 63 mg/dl (1.63 mmol/l), VLDL of 40 mg/dl (1.04 mmol/l) and TG of 89 mg/dl (1.00 mmol/l) (Table II).
Table II.
Baseline lipid profile (mean ± SD)
| Mean concentration | TC | LDL | HDL | VLDL | TG |
|---|---|---|---|---|---|
| mg/dl | 189 ±33 | 107 ±27 | 63 ±13 | 63 ±13 | 89 ±39 |
| mmol/l | 4.89 ±0.84 | 2.77 ±0.71 | 1.63 ±0.33 | 1.63 ±0.33 | 1.00 ±0.44 |
Among the LDL cholesterol particles, the large subfractions (LDL 1–3) were most abundant, with LDL subfractions 4–5 making up a marginal portion and the smallest LDL particles (subfractions 6–7) not observed at all (Table III).
Table III.
LDL and IDL subfraction concentrations
| Mean concentration | IDL-C | IDL-B | IDL-A | LDL-1 | LDL-2 | LDL-3 | LDL-4 | LDL-5 | LDL-6 | LDL-7 |
|---|---|---|---|---|---|---|---|---|---|---|
| mg/dl | 12 ±7 | 15 ±12 | 21 ±11 | 32 ±12 | 14 ±10 | 2 ±6 | 2 ±6 | 0.07 ±1 | 0 | 0 |
| mmol/l | 0.31 ±0.17 | 0.39 ±0.30 | 0.54 ±0.28 | 0.83 ±0.31 | 0.36 ±0.26 | 0.05 ±0.15 | 0.05 ±0.15 | 0.002 ±0.02 | 0 | 0 |
With respect to the distribution of HDL subfractions, intermediate lipoproteins (HDL 4–6) were most abundant, with a mean concentration of 29 mg/dl (0.75 mmol/l), followed by large HDL (HDL subfractions 1–3), with a mean concentration of 22 mg/dl (0.56 mmol/l). The smallest portion of HDL cholesterol was represented by small lipoproteins (HDL subfractions 8–10), with a mean concentration of 12 mg/dl (0.31 mmol/l) (Table IV).
Table IV.
HDL subfraction concentrations
| Mean concentration | HDL | HDL 1 | HDL 2 | HDL 3 | HDL- 4 | HDL 5 | HDL 6 | HDL 7 | HDL 8 | HDL 9 | HDL 10 | HDL large | HDL inter-mediate | HDL small |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| mg/dl | 63 ±13 | 7 ±4 | 9 ±5 | 6 ±4 | 6 ±4 | 6 ±2 | 12 ±6 | 4 ±2 | 4 ±2 | 3 ±1 | 5 ±4 | 22 ±11 | 29 ±7 | 12 ±7 |
| mmol/l | 1.64 ±0.33 | 0.17 ±0.10 | 0.24 ±0.13 | 0.16 ±0.10 | 0.16 ±0.10 | 0.16 ±0.05 | 0.31 ±0.15 | 0,10 ±0.05 | 0.10 ±0.05 | 0.08 ±0.03 | 0.12 ±0.10 | 0.56 ±0.30 | 0.75 ±0.18 | 0.31 ±0.17 |
Night-time and daytime mean SBP and DBP during the first measurement was 112/70 mm Hg: 116/74 mm Hg during the day and 104/60 mm Hg at night. After at least 12 months of follow-up, the following values were achieved: 113/70, 115/73 and 105/61 mm Hg (Tables V and VI).
Table V.
Baseline BP measurement results
| Parameter | SBP day and night | DBP day and night | SBP day | DBP day | SBP night | DBP night |
|---|---|---|---|---|---|---|
| Mean [mm Hg] | 112 ±13 | 70 ±6 | 116 ±10 | 74 ±7 | 104 ±8 | 60 ±7 |
| Median [mm Hg] | 113 ±9 | 71 ±7 | 115 ±10 | 73 ±7 | 104 ±8 | 59 ±6 |
SBP – systolic blood pressure, DBP – diastolic blood pressure.
Table VI.
BP measurement results during a follow-up examination (after at least 12 months)
| Parameter | SBP day and night | DBP day and night | SBP day | DBP day | SBP night | DBP night |
|---|---|---|---|---|---|---|
| Mean [mm Hg] | 113 ±9 | 70 ±7 | 115 ±9 | 73 ±7 | 105 ±10 | 61 ±8 |
| Median [mm Hg] | 112 ±10 | 70 ±7 | 114 ±9 | 72 ±7 | 103 ±11 | 60 ±7 |
Using a univariate logistic regression model, the risk of increased SBP between the first and second measurements, in relation to lipoprotein subfractions correlated significantly with HDL concentrations (and HDL intermediate subfractions) as well as also with IDL concentrations and – in relation to their subfractions – with HDL 3 and IDL-A (Table VII).
Table VII.
A correlation of increase in SBP and lipoprotein concentration
| Variable | Odds ratio (OR) | 95% CI | P-value – significance level |
|---|---|---|---|
| LDL [mmol/l] | 1.086 | 0.607–1.944 | 0.7799 |
| LDL [mg/dl] | 1.002 | 0.987–1.017 | 0.7799 |
| VLDL [mg/dl] | 0.972 | 0.933–1.014 | 0.1862 |
| IDL-C [mg/dl] | 0.999 | 0.939–1.063 | 0.9805 |
| IDL-B [mg/dl] | 1.016 | 0.979–1.055 | 0.4014 |
| IDL-A [mg/dl] | 1.047 | 1.003–1.094 | 0.038 |
| IDL 1 [mg/dl] | 1.005 | 0.97–1.04 | 0.7959 |
| LDL 2 [mg/dl] | 0.97 | 0.928–1.013 | 0.1669 |
| LDL 3 [mg/dl] | 1.003 | 0.934–1.076 | 0.9422 |
| LDL 4 [mg/dl] | 1.117 | 0.883–1.413 | 0.3564 |
| HDL [mg/dl] | 1.06 | 1.016–1.107 | 0.0078 |
| HDL 1 [mg/dl] | 1.051 | 0.948–1.167 | 0.3447 |
| HDL 2 [mg/dl] | 1.063 | 0.975–1.158 | 0.1658 |
| HDL 3 [mg/dl] | 1.152 | 1.02–1.301 | 0.0229 |
| HDL 4 [mg/dl] | 1.108 | 0.985–1.247 | 0.088 |
| HDL 5 [mg/dl] | 1.146 | 0.93–1.412 | 0.1996 |
| HDL 6 [mg/dl] | 1.035 | 0.959–1.117 | 0.3722 |
| HDL 7 [mg/dl] | 1.052 | 0.847–1.305 | 0.648 |
| HDL 8 [mg/dl] | 1.033 | 0.828–1.289 | 0.7743 |
| HDL 9 [mg/dl] | 1.112 | 0.797–1.552 | 0.5329 |
| HDL 10 [mg/dl] | 0.963 | 0.865–1.072 | 0.4942 |
| HDL large [mg/dl] | 1.033 | 0.994–1.73 | 0.096 |
| HDL intermediate [mg/dl] | 1.076 | 1.002–1.156 | 0.0451 |
| HDL small [mg/dl] | 0.99 | 0.93–1.055 | 0.7681 |
Increased diastolic BP (DBP) also correlated with HDL lipoprotein levels. However, none of the subfractions, including the significantly increased IDL-A, demonstrated statistical significance (Table VIII).
Table VIII.
A correlation of increase in DBP and lipoprotein concentration
| Variable | Odds ratio (OR) | 95% CI | P-value – significance level |
|---|---|---|---|
| LDL [mmol/l] | 0.676 | 0.366–1.246 | 0.209 |
| LDL [mg/dl] | 0.99 | 0.974–1.006 | 0.209 |
| VLDL [mg/dl] | 0.989 | 0.951–1.029 | 0.5937 |
| IDL-C [mg/dl] | 0.996 | 0.936–1.061 | 0.9006 |
| IDL-B [mg/dl] | 1.007 | 0.972–1.043 | 0.6952 |
| IDL-A [mg/dl] | 1.002 | 0.965–1.041 | 0.9115 |
| IDL 1 [mg/dl] | 0.986 | 0.952–1.021 | 0.439 |
| LDL 2 [mg/dl] | 0.96 | 0.917–1.005 | 0.078 |
| LDL 3 [mg/dl] | 1.0 | 0.931–1.074 | 0.996 |
| LDL 4 [mg/dl] | 1.126 | 0.888–1.427 | 0.3276 |
| HDL [mg/dl] | 1.041 | 1.001–1.083 | 0.0454 |
| HDL 1 [mg/dl] | 1.03 | 0.928–1.142 | 0.5786 |
| HDL 2 [mg/dl] | 1.084 | 0.992–1.186 | 0.0757 |
| HDL 3 [mg/dl] | 1.115 | 0.992–1.254 | 0.0689 |
| HDL 4 [mg/dl] | 1.115 | 0.99–1.257 | 0.0736 |
| HDL 5 [mg/dl] | 1.157 | 0.935–1.431 | 0.1793 |
| HDL 6 [mg/dl] | 1.012 | 0.942–1.088 | 0.7414 |
| HDL 7 [mg/dl] | 0.943 | 0.756–1.176 | 0.5998 |
| HDL 8 [mg/dl] | 0.883 | 0.701–1.111 | 0.2877 |
| HDL 9 [mg/dl] | 1.032 | 0.74–1.44 | 0.8533 |
| HDL 10 [mg/dl] | 0.952 | 0.853–1.063 | 0.3837 |
| HDL large [mg/dl] | 1.031 | 0.993–1.071 | 0.1113 |
| HDL intermediate [mg/dl] | 1.046 | 0.98–1.116 | 0.1775 |
| HDL small [mg/dl] | 0.974 | 0.913–1.04 | 0.4347 |
Discussion
Changes in the lipid profile in patients with hypertension have been described for years, what is more these conditions – hypertension and lipid disorders – udually coexist in most ofour patients. Most reports have indicated that hypertensive patients have decreased LDL cholesterol, triglyceride and VLDL cholesterol concentrations and – to a lower extent – total cholesterol concentrations [17–19].
Our results, which indicate a positive correlation between the total HDL and HDL-3 concentrations a tendency to develop hypertension, seem surprising (despite relatively small effect size) – suffice it to mention the definition of metabolic syndrome, in which increased BP (defined as values ≥ 130/85 mm Hg) often coexists with lipid metabolism disorders in the form of increased fasting triglycerides concentrations (> 150 mg/dl (1.69 mmol/l)) and decreased HDL cholesterol concentrations (< 40 mg/dl (1.03 mmol/l) in men and < 50 mg/dl (1.29 mmol/l) in women); there are also publications which unequivocally associate decreased HDL concentrations with hypertension [19, 20]. On the other hand, there are studies available suggesting that in healthy individuals different risk factors, including obsesity, smoking, diabetes and hypertension might impair HDL functions, and increase the level of large (including HDL 3) and intermediate HDL what might have pro-atherogenic effect. And the observed increase of HDL-C should not be considered as a potentially protective effect in those patients [21–25].
The HDL particle is perhaps the most mysterious of all lipoproteins. The results of Framingham Heart Study (FHS) demonstrated an inverse relationship between HDL concentrations and cardiovascular events, thus attaching the long-lasting opinion of “good cholesterol”, with Dr Jekyll’s gentle face, to HDL-C. However, recent studies describing a complex, often detrimental role of this lipoprotein in the body, revealed its other face – that of Mr Hyde’s [26, 27].
However, pharmacological approaches to increasing the concentration of HDL to reduce the risk of cardiovascular events have been called into question based on a series of further studies [21–25, 28]. As a result, the 2016 ESC/EAS (European Society of Cardiology/European Society of Hypertension) guidelines do not directly recommend using HDL-C concentrations as a therapeutic target – it is a class III recommendation [29–32].
The HDL particle, composed like other lipoproteins of lipids and proteins called apolipoproteins, plays complex roles in the body which are not fully understood. Although it is known that the main apolipoprotein A1 (apoA1) is involved in reverse cholesterol transport and has antioxidative properties, the function of more than 100 other proteins that can be found in the HDL structure remains unclear. Recent research has focussed on so-called dysfunctional HDL, i.e. particles devoid of their beneficial effects. Dysfunctional lipoproteins appear during systemic inflammation, glycation or oxidative stress, i.e. in a situation when free radicals dominate over antioxidants [33–37], and their dysfunction results from activation of myeloperoxidase during inflammation, an enzyme capable of inhibiting oxidative enzymes, such as glutathione peroxidase or paraoxonase-1 (PON-1) whose functions include inhibition of lipid oxidation. It is also caused by a decrease in the amount of apoA1 and modification of its function, making it unable to activate the ABCA1 transporter (ATP-binding cassette transporter) responsible for direct cholesterol transfer in reverse transport from macrophages to HDL particles [38–43].
Recently published results suggests that oxidative stress might play a major role in the pathogenesis of hypertension [44, 45]. It has been observed that in patients with newly diagnosed or untreated hypertension, the function of the most important cellular free radical scavenging enzymes (superoxide dismutase and glutathione peroxidase) is impaired [46–48]. Intracellular enzyme dysfunction combined with an extracellular deficiency in free radical scavengers such as vitamins C and E, leads to increased cncentrations of reactive oxygen species (ROS), leading to the stimulation of signalling cascades, activation of NADPH oxidase (nicotinamide adenine dinucleotide phosphate oxidase), production of even more ROS, such as hydrogen peroxide and superoxide anion which produces peroxynitrite as a result of reaction with nitric oxide, at the same time reducing the available amount of the vasorelaxant nitric oxide. This results in impaired vessel relaxation and a shift in balance towards vasoconstriction. ROS – modulating the function of many secondary transmitters – are used by angiotensin II in the process of binding to receptor AT1. All these elements may lead to the development of hypertension [49–51].
Oxidative stress described above could explain, to an extent, the results of our study – if the development of hypertension is associated with an excess in ROS, which in turn are associated with the creation of dysfunctional HDL devoid of the antioxidative properties of PON-1, then their increased concentrations in people (especiall those considered as pro-atherogenic – large HDL subfraction) with a tendency to hypertension is a symptom indicating that the body attempts to sustain balance by producing normal particles, especially of the large or intermediate subfractions. On the other hand, it is worth emphasizing that there are also some opposite results, suggesting that large HDL might not have pro-atherogenic properties, and can be atheroprotective [52, 53].
Our results are consistent with those of two studies, both conducted by Japanese researchers, which demonstrated a positive correlation between HDL concentrations and hypertension. Oda and Kawai conducted a study in healthy individuals with no history of cardiovascular diseases (and thus without hypertension): 1803 men aged 49.9 ±9.0 years and 1150 women aged 49.5 ±9.0 years were enrolled. A positive relationship was observed between higher HDL values and decreased BP values — an increase in HDL of 1 mg/dl (0.026 mmol/l) was associated with an increase in BP of 1.03 (1.02–1.04; p < 0.001) in men and 1.03 (1.01–1.05; p = 0.002) in women. This was the first observation of such a correlation in the world. Like our study, these investigators excluded potential participants taking lipid-lowering or antihypertensive pharmacotherapy – thereby removing the possibility that the results were affected by lipid-lowering drugs. The authors suggested that their observed relationship may be a result of dysfunctional HDL particles, however they were not be able to measure HDL subfractions [54].
In 2017, a paper by another group of Japanese investigators was published [55]. The authors, aware of the previous study, decided to extend the research. Not only did they demonstrate a relationship between increased HDL concentrations and hypertension, but they also associated it with multipotent CD34 cells. A total of 477 men aged 60–69 years were enrolled in the cross-sectional study. It was demonstrated that HDL concentrations were statistically significantly associated with hypertension, but only in persons with a high count of circulating CD34 cells. A low number of these cells made the relationship between HDL and hypertension statistically non-significant. This means that with minor damage to the endothelium caused by HDL, the need for endothelial repair is low, which results in low concentrations of circulating CD34-positive cells and, and the same time, HDL stimulates the production of NO in epithelial cells and epithelial repair mechanisms. However, in individuals with major endothelial damage, the function of HDL, even in increased concentrations, is insufficient. As a result, the repair of the damaged endothelium causes increased the production of CD34 cells [56, 57]. These results in fact confirmed that even in healthy individuals, increased blood proeasure might be associated with the endothelium damage and impairment of HDL function.
An interesting extension of the concept of oxidative stress as the cause of hypertension would be the possibility of reversing this condition by using an antioxidant-rich diet, such as the DASH or Mediterranean diet. Based on the above data, it is possible that sugh a diet would also normalise the lipid profile [58]. In February 2019, a paper by Chiavaroli et al. was published – encompassing both meta-analyses and reviews and summarising the effect on the DASH diet on hypertension and dyslipidaemia. As expected, food restrictions recommended in the diet reduced BP values – systolic by 5.2 mm Hg and diastolic by 2.6 mm Hg. However, the effect of the diet on the lipid profile was modest: TC was reduced by 0.2 mmol/l and LDL by 0.1 mmol/l, with HDL and TG concentrations unchanged [59]. A meta-analysis by Rees et al. assessing the Mediterranean diet demonstrated that, when used in primary prevention, the diet was effective in reducing hypertension compared with lack of intervention (reduction in systolic pressure of –2.99 mm Hg and diastolic of –2.0 mm Hg). However, the effect of the Mediterranean diet on the lipid profile was minimal and manifested mainly in a TC level reduction of –0.16 mmol/l, with virtually no effect on LDL, HDL and TG concentrations [60].
Similarly modest effects of reduction diets on the lipid profile was described by Stelmach-Mardas and Walkowiak based on an analysis of studies from the years 1958–2016 in obese individuals (BMI > 30 kg/m2) – only TG concentration reduction was achieved in spite of significantly reduced BP (systolic by –4.73 mm Hg and diastolic by –2.75 mm Hg) [61]. Chiu et al. compared in 36 persons the effect of two diets – the typical DASH with a modified DASH, high in fat and low in carbohydrates (HF-DASH), on hypertension and lipid profile. After 3 weeks of follow-up, they observed that the typical DASH, unlike its modified version, reduced IDL and LDL cholesterol concentrations, including concentrations of large LDL particles, as well as HDL cholesterol and apolipoprotein AI. This is indirect evidence that high HDL concentrations are not associated with low BP values and, perhaps, the complete opposite is true – they may be associated with hypertension. On the other hand, the HF-DASH diet also reduced BP, while lipid profile changes included only decreased triglyceride concentrations and increased concentration of large LDL particles, with no effect on HDL-C [62–64].
Our study has some obvious limitations. One of the limitations of this study is, first and foremost, its group size, what cause that these results should be treated as preliminary. Secondly, this paper pertains only to a population of young individuals, mostly women. Thirdly, the publication does not include the influence of other factors, such as tobacco smoking or BMI, on the relationship in question, although they seem irrelevant given the low number of overweight individuals or smokers. We cannot obviously exclude the possibility of potential residual confounding.
In conclusion, we showed a possible positive correlation between the total HDL and HDL-3 concentrations a tendency to develop hypertension in young individuals. Taking into account that these results might be treated as preliminary only, they need to be confirmed in larger studies to make sure that BP increase itself might be responsible for the impairment of proatherogenic HDL subfractions, and in the consequence might lead to the endothelium damage and progression of atherosclerosis.
Acknowledgments
The study was financed by the Polish National Science Centre (OPUS Grant, contract No. DEC-2013/09/B/NZ5/02746).
Conflict of interest
The authors declare no conflict of interest.
References
- 1.Banach M, Aronow WS. Hypertension therapy in the older adults – do we know the answers to all the questions? The status after publication of the ACCF/AHA 2011 expert consensus document on hypertension in the elderly. J Hum Hypertens. 2012;26:641–3. doi: 10.1038/jhh.2012.3. [DOI] [PubMed] [Google Scholar]
- 2.Zdrojewski T, Rutkowski M, Bandosz P, et al. Rozpowszechnienie i kontrola czynników ryzyka sercowo-naczyniowego w Polsce. Cele i sposób realizacji badania NATPOL 2011. Kardiol Pol. 2013;71:381–92. [Google Scholar]
- 3.Otocka-Kmiecik A, Mikhailidis DP, Nicholls SJ, Davidson M, Rysz J, Banach M. Dysfunctional HDL: a novel important diagnostic and therapeutic target in cardiovascular disease? Prog Lipid Res. 2012;51:314–24. doi: 10.1016/j.plipres.2012.03.003. [DOI] [PubMed] [Google Scholar]
- 4.Sesso HD, Buring JE, Chown MJ, Ridker PM, Gaziano JM. A prospective study of plasma lipid levels and hypertension in women. Arch Intern Med. 2005;165:2420–7. doi: 10.1001/archinte.165.20.2420. [DOI] [PubMed] [Google Scholar]
- 5.Halperin RO, Sesso HD, Ma J, Buring JE, Stampfer MJ, Gaziano JM. Dyslipidemia and the risk of incident hypertension in men. Hypertension. 2006;47:45–50. doi: 10.1161/01.HYP.0000196306.42418.0e. [DOI] [PubMed] [Google Scholar]
- 6.de Simone G, Devereux RB, Chinali M, et al. Strong Heart Study Investigators Risk factors for arterial hypertension in adults with initial optimal blood pressure: the strong heart study. Hypertension. 2006;47:162–7. doi: 10.1161/01.HYP.0000199103.40105.b5. [DOI] [PubMed] [Google Scholar]
- 7.Han SH, Quon MJ, Koh KK. Reciprocal relationships between abnormal metabolic parameters and endothelial dysfunction. Curr Opin Lipidol. 2007;18:58–65. doi: 10.1097/MOL.0b013e328012b627. [DOI] [PubMed] [Google Scholar]
- 8.Sacks FM, Campos H. Clinical review 163: cardiovascular endocrinology: low-density lipoprotein size and cardiovascular disease: a reappraisal. J Clin Endocr Metab. 2003;88:4525–32. doi: 10.1210/jc.2003-030636. [DOI] [PubMed] [Google Scholar]
- 9.Urbina EM, Srinivasan SR, Kieltyka RL, et al. Correlates of carotid artery stiffness in young adults: the Bogalusa Heart Study. Atherosclerosis. 2004;176:157–64. doi: 10.1016/j.atherosclerosis.2004.04.023. [DOI] [PubMed] [Google Scholar]
- 10.Sesso HD, Buring JE, Rifai N, Blake GJ, Gaziano JM, Ridker PM. C-reactive protein and the risk of developing hypertension. JAMA. 2003;290:2945–51. doi: 10.1001/jama.290.22.2945. [DOI] [PubMed] [Google Scholar]
- 11.Paynter NP, Sesso HD, Conen D, Otvos JD, Mora S. Lipoprotein subclass abnormalities and incident hypertension in initially healthy women. Clin Chem. 2011;57:1178–87. doi: 10.1373/clinchem.2011.167544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Oravec S, Dukat A, Gavornik P, Caprnda M, Kucera M, Ocadlik I. Contribution of the atherogenic lipoprotein profile to the development of arterial hypertension. Bratisl Lek Listy. 2011;112:4–7. [PubMed] [Google Scholar]
- 13.Perani G, Martignoni A, Muggia C, et al. Metabolic effects of the combination of captopril and hydrochlorothiazide in hypertensive subjects. J Clin Pharmacol. 1990;30:1031–5. doi: 10.1002/j.1552-4604.1990.tb03590.x. [DOI] [PubMed] [Google Scholar]
- 14.Tamasawa N, Matsui J, Ogawa Y, et al. Effect of doxazosin on the size of LDL particle in the type 2 diabetic patients with hypertension. J Diabetes Complications. 2000;14:135–9. doi: 10.1016/s1056-8727(00)00071-4. [DOI] [PubMed] [Google Scholar]
- 15.Christogiannis LG, Kostapanos MS, Tellis CC, Milionis HJ, Tselepis AD, Elisaf MS. Distinct effects of fixed combinations of valsartan with either amlodipine or hydrochlorothiazide on lipoprotein subfraction profile in patients with hypertension. J Hum Hypertens. 2013;27:44–50. doi: 10.1038/jhh.2011.108. [DOI] [PubMed] [Google Scholar]
- 16.Oravec S, Dostal E, Dukát A, Gavorník P, Kucera M, Gruber K. HDL subfractions analysis: a new laboratory diagnostic assay for patients with cardiovascular diseases and dyslipoproteinemia. Neuro Endocrinol Lett. 2011;32:502–9. [PubMed] [Google Scholar]
- 17.Flesch M, Sachinidis A, Ko YD, Kraft K, Vetter H. Plasma lipids and lipoproteins and essential hypertension. Clin Investig. 1994;72:944–50. doi: 10.1007/BF00577733. [DOI] [PubMed] [Google Scholar]
- 18.Lepira FB, M’Buyamba-Kabangu JR, Kayembe KP, Nseka MN. Correlates of serum lipids and lipoproteins in Congolese patients with arterial hypertension. Cardiovasc J S Afr. 2005;16:249–55. [PubMed] [Google Scholar]
- 19.Rubies-Prat J, Ordóñez-Llanos J, Martin S, et al. Low-density lipoprotein particle size, triglyceride-rich lipoproteins, and glucose tolerance in non-diabetic men with essential hypertension. Clin Exp Hypertens. 2001;23:489–500. doi: 10.1081/ceh-100104240. [DOI] [PubMed] [Google Scholar]
- 20.Halperin RO, Sesso HD, Ma J, Buring JE, Stampfer MJ, Gaziano JM. Dyslipidemia and the risk of incident hypertension in men. Hypertension. 2006;47:45–50. doi: 10.1161/01.HYP.0000196306.42418.0e. [DOI] [PubMed] [Google Scholar]
- 21.Barylski M, Toth PP, Nikolic D, Banach M, Rizzo M, Montalto G. Emerging therapies for raising high-density lipoprotein cholesterol (HDL-C) and augmenting HDL particle functionality. Best Pract Res Clin Endocrinol Metab. 2014;28:453–61. doi: 10.1016/j.beem.2013.11.001. [DOI] [PubMed] [Google Scholar]
- 22.Rizzo M, Otvos J, Nikolic D, Montalto G, Toth PP, Banach M. Subfractions and subpopulations of HDL: an update. Curr Med Chem. 2014;21:2881–91. doi: 10.2174/0929867321666140414103455. [DOI] [PubMed] [Google Scholar]
- 23.Sonmez A, Nikolic D, Dogru T, et al. Low- and high-density lipoprotein subclasses in subjects with nonalcoholic fatty liver disease. J Clin Lipidol. 2015;9:576–82. doi: 10.1016/j.jacl.2015.03.010. [DOI] [PubMed] [Google Scholar]
- 24.Rysz-Gorzynska M, Gluba-Brzozka A, Banach M. High-density lipoprotein and low-density lipoprotein subfractions in patients with chronic kidney disease. Curr Vasc Pharmacol. 2017;15:144–51. doi: 10.2174/1570161114666161003093032. [DOI] [PubMed] [Google Scholar]
- 25.Kidawa M, Gluba-Brzozka A, Zielinska M, Franczyk B, Banach M, Rysz J. Cholesterol subfraction analysis in patients with acute coronary syndrome. Curr Vasc Pharmacol. 2019;17:365–75. doi: 10.2174/1570161116666180601083225. [DOI] [PubMed] [Google Scholar]
- 26.Castelli WP. Cholesterol and lipids in the risk of coronary artery disease-the Framingham Heart Study. Can J Cardiol. 1988;4(Suppl A):5A–10A. [PubMed] [Google Scholar]
- 27.Zanoni P, Khetarpal SA, Larach DB, et al. CHD Exome+ Consortium; CARDIoGRAM Exome Consortium; Global Lipids Genetics Consortium Rare variant in scavenger receptor BI raises HDL cholesterol and increases risk of coronary heart disease. Science. 2016;351:1166–71. doi: 10.1126/science.aad3517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fendler W, Rizzo M, Borowiec M, et al. Less but better: cardioprotective lipid profile of patients with GCK-MODY despite lower HDL cholesterol level. Acta Diabetol. 2014;51:625–32. doi: 10.1007/s00592-014-0567-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Baigent C, Keech A, Kearney PM, et al. Cholesterol Treatment Trialists’ (CTT) Collaborators Efficacy and safety of cholesterollowering treatment: prospective meta-analysis of data from 90,056 participants in 14 randomised trials of statins. Lancet. 2005;366:1267–78. doi: 10.1016/S0140-6736(05)67394-1. [DOI] [PubMed] [Google Scholar]
- 30.Boekholdt SM, Arsenault BJ, Hovingh GK, et al. Levels and changes of HDL cholesterol and apolipoprotein A-I in relation to risk of cardiovascular events among statin-treated patients: a meta-analysis. Circulation. 2013;128:1504–12. doi: 10.1161/CIRCULATIONAHA.113.002670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Briel M Ferreira-Gonzalez I, You JJ, et al. Association between change in high density lipoprotein cholesterol and cardiovascular disease morbidity and mortality: systematic review and meta-regression analysis. BMJ. 2009;16:338. doi: 10.1136/bmj.b92. b92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Catapano AL, Graham I, De Backer G, et al. 2016 ESC Scientific Document Group ESC/EAS guidelines for the management of dyslipidaemias. Eur Heart J. 2016;37:2999–3058. doi: 10.1093/eurheartj/ehw272. [DOI] [PubMed] [Google Scholar]
- 33.Ragbir S, Farmer JA. Dysfunctional high-density lipoprotein and atherosclerosis. Curr Atheroscler Rep. 2010;12:343–8. doi: 10.1007/s11883-010-0091-x. [DOI] [PubMed] [Google Scholar]
- 34.Barter PJ, Nicholls S, Rye KA, Anantharamaiah GM, Navab M, Fogelman AM. Antiinflammatory properties of HDL. Circ Res. 2004;95:764–72. doi: 10.1161/01.RES.0000146094.59640.13. [DOI] [PubMed] [Google Scholar]
- 35.Shao B, Oda MN, Oram JF, Heinecke JW. Myeloperoxidase: an oxidative pathway for generating dysfunctional high-density lipoprotein. Chem Res Toxicol. 2010;23:447–54. doi: 10.1021/tx9003775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Khera AV, Cuchel M, de la Llera-Moya M, et al. Cholesterol efflux capacity, high-density lipoprotein function, and atherosclerosis. N Engl J Med. 2011;364:127–35. doi: 10.1056/NEJMoa1001689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Montuschi P, Barnes P, Roberts LJ., 2nd Insights into oxidative stress: the isoprostanes. Curr Med Chem. 2007;14:703–17. doi: 10.2174/092986707780059607. [DOI] [PubMed] [Google Scholar]
- 38.Patra SK, Singh K, Singh R. Paraoxonase 1: a better atherosclerotic risk predictor than HDL in type 2 diabetes mellitus. Diabetes Metab Syndr. 2013;7:108–11. doi: 10.1016/j.dsx.2013.02.007. [DOI] [PubMed] [Google Scholar]
- 39.Loued S, Berrougui H, Componova P, Ikhlef S, Helal O, Khalil A. Extra-virgin olive oil consumption reduces the age-related decrease in HDL and paraoxonase 1 anti-inflammatory activities. Br J Nutr. 2013;110:1272–84. doi: 10.1017/S0007114513000482. [DOI] [PubMed] [Google Scholar]
- 40.Huang Y, Wu Z, Riwanto M, et al. Myeloperoxidase, paraoxonase-1, and HDL form a functional ternary complex. J Clin Invest. 2013;123:3815–28. doi: 10.1172/JCI67478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang DX, Liu H, Yan LR, et al. The relationship between serum amyloid A and apolipoprotein A-I in high-density lipoprotein isolated from patients with coronary heart disease. Chin Med J. 2013;126:3656–61. [PubMed] [Google Scholar]
- 42.Sokolov AV, Kostevich VA, Runova OL, et al. Proatherogenic modification of LDL by surface-bound myeloperoxidase. Chem Phys Lipids. 2014;180:72–80. doi: 10.1016/j.chemphyslip.2014.02.006. [DOI] [PubMed] [Google Scholar]
- 43.Shao B, Oda MN, Oram JF, Heinecke JW. Myeloperoxidase: an inflammatory enzyme for generating dysfunctional high density lipoprotein. Curr Opin Cardiol. 2006;21:322–8. doi: 10.1097/01.hco.0000231402.87232.aa. [DOI] [PubMed] [Google Scholar]
- 44.Montezano AC, Touyz RM. Oxidative stress, Noxs, and hypertension: experimental evidence and clinical controversies. Ann Med. 2012;44(Suppl 1):S2–16. doi: 10.3109/07853890.2011.653393. [DOI] [PubMed] [Google Scholar]
- 45.Rodrigo R, González J, Paoletto F. The role of oxidative stress in the pathophysiology of hypertension. Hypertens Res. 2011;34:431–40. doi: 10.1038/hr.2010.264. [DOI] [PubMed] [Google Scholar]
- 46.Pedro-Botet J, Covas MI, Martín S, Rubiés-Prat J. Decreased endogenous antioxidant enzymatic status in essential hypertension. J Hum Hypertens. 2000;14:343–5. doi: 10.1038/sj.jhh.1001034. [DOI] [PubMed] [Google Scholar]
- 47.Lip GY, Edmunds E, Nuttall SL, Landray MJ, Blann AD, Beevers DG. Oxidative stress in malignant and non-malignant phase hypertension. J Hum Hypertens. 2002;16:333–6. doi: 10.1038/sj.jhh.1001386. [DOI] [PubMed] [Google Scholar]
- 48.Touyz RM. Reactive oxygen species, vascular oxidative stress, and redox signaling in hypertension: what is the clinical significance? Hypertension. 2004;44:248–52. doi: 10.1161/01.HYP.0000138070.47616.9d. [DOI] [PubMed] [Google Scholar]
- 49.Touyz RM. Reactive oxygen species and angiotensin II signaling in vascular cells: implications in cardiovascular disease. Braz J Med Biol Res. 2004;37:1263–73. doi: 10.1590/s0100-879x2004000800018. [DOI] [PubMed] [Google Scholar]
- 50.Touyz RM, Chen X, Tabet F, et al. Expression of a gp91phox-containing leukocyte-type NADPH oxidase in human vascular smooth muscle cells: modulation by Ang II. Circ Res. 2002;90:1205–13. doi: 10.1161/01.res.0000020404.01971.2f. [DOI] [PubMed] [Google Scholar]
- 51.Taniyama Y, Griendling KK. Reactive oxygen species in the vasculature: molecular and cellular mechanisms. Hypertension. 2003;42:1075–81. doi: 10.1161/01.HYP.0000100443.09293.4F. [DOI] [PubMed] [Google Scholar]
- 52.Maeda S, Nakanishi S, Yoneda M, et al. Associations between small dense LDL, HDL subfractions (HDL2, HDL3) and risk of atherosclerosis in Japanese-Americans. J Atheroscler Thromb. 2012;19:444–52. doi: 10.5551/jat.11445. [DOI] [PubMed] [Google Scholar]
- 53.Kasko M, Gaspar L, Dukát A, Gavorník P, Oravec S. High-density lipoprotein profile in newly-diagnosed lower extremity artery disease in Slovak population without diabetes mellitus. Neuro Endocrinol Lett. 2014;35:531–5. [PubMed] [Google Scholar]
- 54.Oda E, Kawai R. High-density lipoprotein cholesterol is positively associated with hypertension in apparently healthy Japanese men and women. Br J Biomed Sci. 2011;68:29–33. doi: 10.1080/09674845.2011.11732838. [DOI] [PubMed] [Google Scholar]
- 55.Shimizu Y, Sato S, Koyamatsu J, et al. Association between high-density lipoprotein-cholesterol and hypertension in relation to circulating CD34-positive cell levels. J Physiol Anthropol. 2017;36:26. doi: 10.1186/s40101-017-0143-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Walter DH, Rittig K, Bahlmann FH, et al. Statin therapy accelerates reendothelialization: a novel effect involving mobilization and incorporation of bone marrow-derived endothelial progenitor cells. Circulation. 2002;105:3017–24. doi: 10.1161/01.cir.0000018166.84319.55. [DOI] [PubMed] [Google Scholar]
- 57.Shimizu Y, Sato S, Koyamatsu J, et al. Platelets and circulating CD34-positive cells as an indicator of the activity of the vicious cycle between hypertension and endothelial dysfunction in elderly Japanese men. Atherosclerosis. 2017;259:26–31. doi: 10.1016/j.atherosclerosis.2017.02.016. [DOI] [PubMed] [Google Scholar]
- 58.Dragan S, Serban C, Banach M. Can we change the functionality of HDL cholesterol with nonpharmacological and pharmacological agents? Curr Med Chem. 2014;21:2927–46. doi: 10.2174/0929867321666140303153829. [DOI] [PubMed] [Google Scholar]
- 59.Chiavaroli L, Viguiliouk E, Nishi SK, et al. DASH dietary pattern and cardiometabolic outcomes: an umbrella review of systematic reviews and meta-analyses. Nutrients. 2019;11 doi: 10.3390/nu11020338.E338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rees K, Takeda A, Martin N, et al. Mediterranean-style diet for the primary and secondary prevention of cardiovascular disease. Cochrane Database Syst Rev. 2019;3:CD009825. doi: 10.1002/14651858.CD009825.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Stelmach-Mardas M, Walkowiak J. Dietary interventions and changes in cardio-metabolic parameters in metabolically healthy obese subjects: a systematic review with meta-analysis. Nutrients. 2016;8 doi: 10.3390/nu8080455.E455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chiu S, Bergeron N, Williams PT, Bray GA, Sutherland B, Krauss RM. Comparison of the DASH (Dietary Approaches to Stop Hypertension) diet and a higher-fat DASH diet on blood pressure and lipids and lipoproteins: a randomized controlled trial. Am J Clin Nutr. 2016;103:341–7. doi: 10.3945/ajcn.115.123281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Banach M, Burchardt P, Chlebus K, et al. PoLA/CFPiP/PCS/PSLD/PSD/PSH guidelines on diagnosis and therapy of lipid disorders in Poland 2021. Arch Med Sci. 2021;17:1447–547. doi: 10.5114/aoms/141941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Miazgowski T, Kopec J, Widecka K, Miazgowski B, Kaczmarkiewicz A. Epidemiology of hypertensive heart disease in Poland: findings from the Global Burden of Disease Study 2016. Arch Med Sci. 2021;17:874–80. doi: 10.5114/aoms.2019.85222. [DOI] [PMC free article] [PubMed] [Google Scholar]