ABSTRACT
In the age of COVID, nucleic acid vaccines have garnered much attention, at least in part, because of the simplicity of construction, production, and flexibility to adjust and adapt to an evolving outbreak. Orthopoxviruses remain a threat on multiple fronts, especially as emerging zoonoses. In response, we developed a DNA vaccine, termed 4pox, that protected nonhuman primates against monkeypox virus (MPXV)-induced severe disease. Here, we examined the protective efficacy of the 4pox DNA vaccine delivered by intramuscular (i.m.) electroporation (EP) in rabbits challenged with aerosolized rabbitpox virus (RPXV), a model that recapitulates the respiratory route of exposure and low dose associated with natural smallpox exposure in humans. We found that 4pox-vaccinated rabbits developed immunogen-specific antibodies, including neutralizing antibodies, and did not develop any clinical disease, indicating protection against aerosolized RPXV. In contrast, unvaccinated animals developed significant signs of disease, including lesions, and were euthanized. These findings demonstrate that an unformulated, nonadjuvanted DNA vaccine delivered i.m. can protect against an aerosol exposure.
IMPORTANCE The eradication of smallpox and subsequent cessation of vaccination have left a majority of the population susceptible to variola virus or other emerging poxviruses. This is exemplified by human monkeypox, as evidenced by the increase in reported endemic and imported cases over the past decades. Therefore, a malleable vaccine technology that can be mass produced and does not require complex conditions for distribution and storage is sought. Herein, we show that a DNA vaccine, in the absence of a specialized formulation or adjuvant, can protect against a lethal aerosol insult of rabbitpox virus.
KEYWORDS: DNA vaccines, aerosols, neutralizing antibodies, nucleic acid technology, poxvirus, rabbitpox, smallpox
INTRODUCTION
Orthopoxviruses are members of the Poxviridae, a family of enveloped viruses that encode large linear, double-stranded DNA genomes (>130-kb genome) (1). Several members of this family can cause significant human disease. In the genus Orthopoxvirus, variola virus (VARV) is the most significant human pathogen and the causative agent of smallpox. Despite the eradication of smallpox, concerns have been raised regarding use of VARV, or a genetically similar pathogenic orthopoxvirus, as a biological weapon (2–4). Additionally, there is concern that VARV may be accidentally released: for example, from leftover viral stocks. There is credence for the latter scenario, as VARV was discovered cold stored in a research laboratory in the United States (5). Orthopoxviruses are also emerging zoonoses throughout the world, including monkeypox virus (MPXV), cowpox virus (CPXV), and strains of vaccinia virus (VACV), which cause substantial disease in both humans and agricultural animals (6–11). In fact, both the number and intensity of human MPXV outbreaks have been on the rise (10, 12, 13). Accordingly, the continued development and refinement of orthopoxvirus countermeasures are warranted (14).
Early vaccines used to eradicate smallpox used calf lymph-produced live, unattenuated VACV strains, such as Dryvax (15). These vaccines are no longer produced and have been replaced by a cell culture-grown, live VACV vaccine called ACAM2000 (16), as well as a replication-deficient (in human cells) vaccine known as MVA (Modified Vaccinia Ankara). Both the calf lymph and cell culture vaccines can produce significant adverse events in vaccinated humans, including autoinoculation of the eye, generalized vaccinia, eczema vaccinatum, progressive vaccinia, myocarditis, and death (15, 17–19). These safety concerns, in the absence of a stronger global threat of orthopoxvirus disease, make widespread use of this vaccine unethical in the absence of an outbreak. Furthermore, due to safety concerns, a significant number of people are contraindicated for ACAM2000, including those with immune deficiencies and common skin conditions, such as eczema (20). The safety risks associated with ACAM2000 prompted production of more highly attenuated third-generation vaccines, including MVA and Lc16m8 (21, 22). The Barvarian Nordic version of the MVA vaccine, called JYNNEOS, has recently been approved by the U.S. Food and Drug Administration for the prevention of smallpox. JYNNEOS has an improved safety profile compared to ACAM2000 (23) and protects animals against lethal orthopoxvirus disease, including MPXV infection of nonhuman primates (NHPs), rabbitpox virus (RPXV) infection of rabbits, and VACV infection of mice (21, 24, 25). Similar to ACAM2000, protective targets of MVA are undefined, and both viruses express hundreds of gene products unlikely to contribute to protection (26). Several of these gene products are immunomodulatory proteins, whose function and impact in vaccinated humans are unknown.
To produce better-defined vaccines, subunit vaccines targeting protective antigens and delivered as purified protein or plasmid DNA or virally vectored vaccines have been developed that target one or both of the two immunologically distinct infectious forms of poxviruses, the mature virion (MV) and the enveloped virion (EV) (27–34). To date, protective immunogens used in subunit vaccines have included MV surface proteins L1, A27, D8, and H3 and EV surface proteins B5 and A33 (28–33, 35–37). Our group developed a DNA-based vaccine termed 4pox that targets L1, A27, B5, and A33 (35–40). This vaccine is immunogenic in mice and NHPs when delivered by gene gun (30, 35, 37, 41) or skin electroporation (EP) (37). Furthermore, it provides protection against lethal intranasal and intraperitoneal VACV infection of mice and intravenous MPXV infection of NHPs. In the intravenous NHP MPXV model, the 4pox subunit vaccine was equally as protective as MVA after two vaccinations, but unlike MVA, the DNA vaccine prevented viral shedding (41).
MPXV challenge of NHPs and VACV challenge of mice represent archetypal disease models used to demonstrate the protective efficacy of orthopoxvirus countermeasures. However, these model systems require the use of high doses of virus (>106 PFU/mL). In contrast, RPXV, another member of the Poxviridae family and genetically similar to VACV (42), is highly virulent in rabbits, with a 50% lethal dose (LD50) of ∼20 PFU, and similar to naturally acquired smallpox, RPXV can be transmitted by a respiratory route (43–45). Infection of rabbits closely mimics the patterns of natural transmission and signs of disease (lesions and rashes) observed with smallpox and human monkeypox (43, 44, 46, 47). Antiviral drugs such as ST-246 (TPOXX) (48, 49) and CMX001 (Tembexa) (50) have been shown to be effective in treating rabbits exposed to aerosol and/or intradermal (i.d.) RPXV. Here, we evaluated the ability of the 4pox DNA vaccine to protect rabbits against infection in the more stringent and highly lethal RPXV aerosol model. We report that vaccination of rabbits with the 4pox DNA vaccine by muscle electroporation elicited neutralizing antibodies and completely protected rabbits from a lethal challenge. Our findings demonstrate that the 4pox DNA-based vaccine prevented poxvirus disease against an aerosol challenge in highly susceptible animals. We also explored the immunogenicity of the 4pox DNA vaccine when delivered by less-invasive needle-free jet injector devices. Our findings will help guide the development of subunit antipoxvirus vaccines.
RESULTS
CpG oligodeoxynucleotides induce activation and proliferation of rabbit B cells.
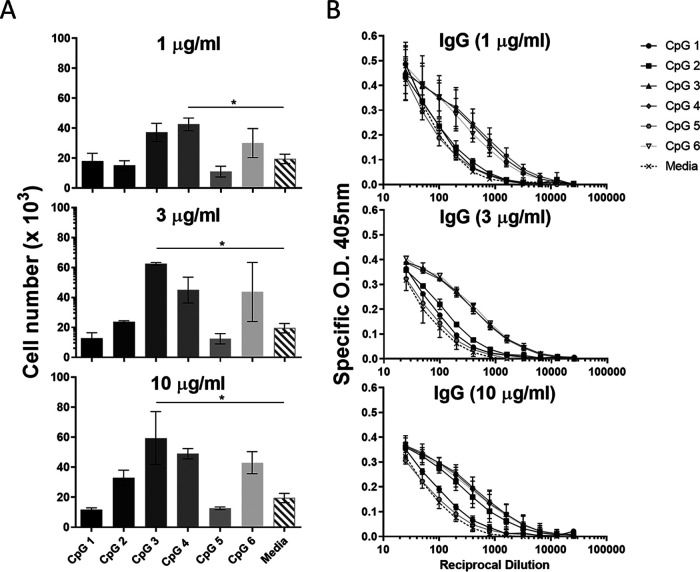
We previously demonstrated that 4pox DNA vaccine is immunogenic in rabbits (36). Here, we determined if the overall immunogenicity could be enhanced by the addition of CpG oligodeoxynucleotides (ODNs). Prior to vaccine studies, we first identified CpG oligodeoxynucleotides capable of enhancing B-cell responses in rabbits. To this end, six novel CpG oligodeoxynucleotides were evaluated for their ability to stimulate proliferation and antibody secretion by rabbit B cells. Rabbit splenocytes were stimulated in the presence or absence of various concentrations of CpG (1, 3, or 10 μg/mL), and 5 days after stimulation, the number of IgG+/IgM+ splenocytes was determined by flow cytometry. At each concentration tested, CpGs 3, 4, and 6 produced the greatest increases in cell number (Fig. 1A), increasing 2- to 3-fold compared to unstimulated cells or cells treated with CpG 1, 2, or 5. Cell culture supernatants from cells treated with all concentrations of CpG 3, 4, or 6 also contained higher concentrations of secreted IgG than unstimulated cells or cells treated with CpG 1, 2, or 5 (Fig. 1B). These results demonstrated the ability of CpG ODNs to differentially stimulate rabbit B cells and identified CpGs 3, 4, and 6 as potential adjuvant candidates to enhance DNA vaccination immunogenicity in rabbits.
FIG 1.
Evaluation of CpG ODNs as potential adjuvants for 4pox DNA vaccination in rabbits. (A) The number of IgM+/IgG+ splenocytes (“cell number × 103”) was determined 5 days after stimulation with individual CpG oligodeoxynucleotides numbered 1 to 6 (x axis) by flow cytometry. (B) Supernatants were analyzed by ELISA to determine titers of secreted IgG. O.D. optical density. Results are representative of three independent experiments. Symbols represent the mean ± standard error from each group. *, P < 0.05 by t test.
4pox with or without CpGs delivered by muscle electroporation is highly immunogenic in rabbits.
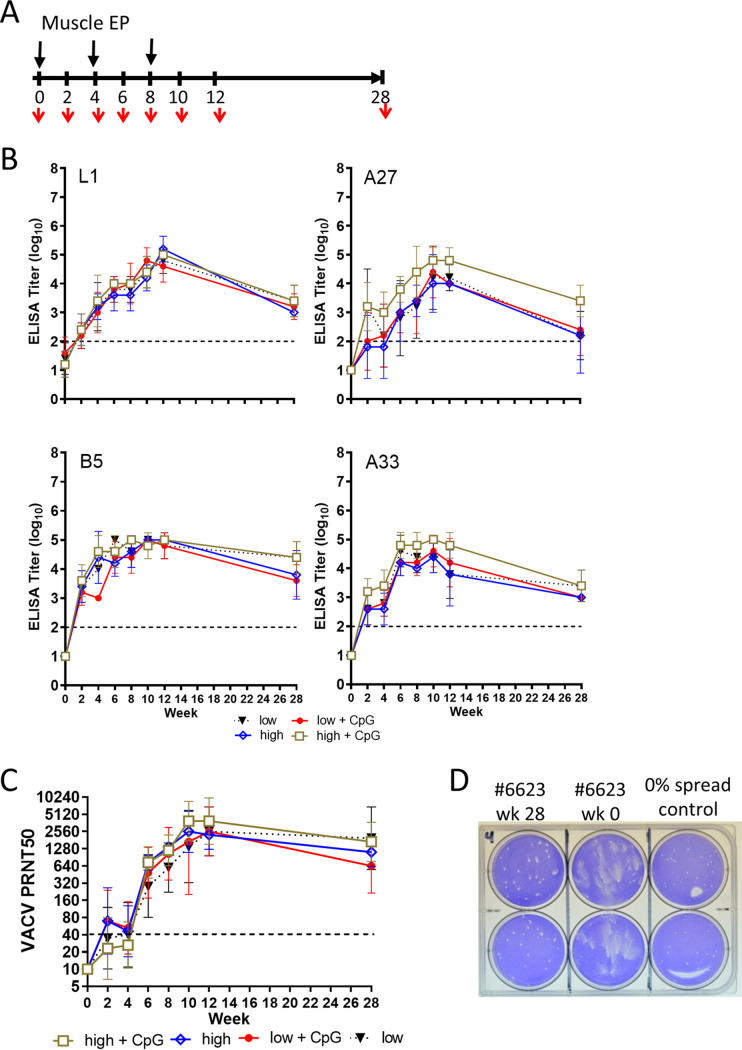
Two groups of rabbits (10 per group) were vaccinated with 4pox DNA three times at 1-month intervals by intramuscular (i.m.) EP (Fig. 2A). Rabbits in group 1 received a low dose of the 4pox DNA (0.4 mg/vaccination), and group 2 received a high dose of 4pox DNA (4.0 mg/vaccination). Subgroups of five rabbits from each group also received CpG adjuvant combination (CpGs 3, 4, and 6) as part of the inoculum. A third group consisted of unvaccinated rabbits and was used as a negative control. The presence of antibodies against the 4pox antigens (L1, A33, A27, and B5) was determined by enzyme-linked immunosorbent assay (ELISA) using sera collected on weeks 0, 2, 4, 6, 8, 10, 12, and 28. In all 4pox-vaccinated groups and subgroups, the levels of antibodies against all four target antigens increased steadily for each group following the initial vaccination. Each group produced a peak titer of ∼4 to 5 log10 at week 10 (Fig. 2B). This peak titer decreased on week 28 by approximately 1 log. The 50% plaque reduction/neutralization titers (PRNT50s) of the MV form of the virus correlated with the antibody ELISA titers for all groups. Following the initial vaccination, neutralizing antibody titers were at or slightly above the limit of detection (Fig. 2C). However, after three vaccinations, PRNT50s (expressed as geometric mean titers [GMTs]) for all four major groups at week 12 were between 2,560 and 5,120. PRNT50s (GMTs) remained near 2,560 for all groups 28 weeks after the initial vaccination. Serum from 4pox DNA-vaccinated rabbits also prevented spread of VACV in a comet inhibition assay, indicating that vaccination produced antibodies that inhibited EV particles (Fig. 2D). Although the high-dose CpG subgroup had A27 and A33 that trended slightly higher than all the other subgroups, there were no statistical differences in humoral responses between subgroups of animals receiving vaccine and the CpG adjuvant and those receiving only the 4pox vaccine based on antigen-specific ELISAs and the MV-based PRNTs. Furthermore, there were no significant differences between DNA vaccine doses. Taken together, these results demonstrated that the 4pox DNA vaccine elicits antibodies against the 4pox vaccine targets in rabbits and CpG adjuvant did not significantly increase immunogenicity.
FIG 2.
The 4pox DNA vaccine is immunogenic in rabbits. (A) Experimental layout showing the vaccination protocol in weeks. Rabbits were vaccinated three times by i.m. EP at 3-week intervals with the 4pox vaccine (low and high doses), where five animals from each 4pox-vaccinated dose group received a combination of adjuvant CpGs 3, 4, and 6. Serum was collected (red arrows) on the indicated weeks. (B) Purified 4pox antigens (L1, A33, B5, and A27) were plated in 96-well plates. Sera from rabbits vaccinated with the indicated vaccines or vaccine-adjuvant combination at the indicated time points were serially diluted 10-fold (from 1:100) and incubated with purified protein. Endpoint titers were calculated as described in the Materials and Methods. Data were plotted using Prism software. Symbols represent the mean ± standard deviation from each group. (C) Sera from vaccinated rabbits were serially diluted 2-fold and incubated with MV of VACV strain IHD-J. PRNT50s were calculated relative to the plaque count for virus that was not incubated with serum. Data were plotted as a geometric mean titer (GMT) from each group ± standard deviation. The dashed line indicates the limit of detection. (D) Comet inhibition was evaluated using serum from rabbit 6623, which received a low-dose 4pox vaccine without CpG. VACV strain IHD-J was adsorbed onto BSC-1 cells, and then medium containing week 0 and week 2 sera (1:20 dilution) was added to the wells. Plaques were visualized by staining with crystal violet 48 h after adsorption. As a positive control for comet inhibition, a semisolid methylcellulose overlay was added to control wells. Each sample was performed in duplicate and is representative of sera from other rabbits in the 4pox vaccine groups. EP, electroporation; “low,” 0.4-mg dose of 4pox vaccine; “low + CpG,” 0.4-mg dose of 4pox vaccine plus 0.5 mg CpGs (3, 4, and 6); “high,” 4-mg dose of 4pox vaccine; “high + CpG,” 4-mg dose of 4pox vaccine plus 0.5 mg CpGs (3, 4, and 6).
4pox DNA vaccination protects rabbits from lethal RPXV aerosol challenge.
Twenty-eight weeks after the initial 4pox DNA vaccination, rabbits were challenged with aerosolized RPXV. Because there were no differences between animals receiving adjuvant and those that did not, we will present and discuss the data from the 4pox-vaccinated animals based on the dose of vaccine the animals received and not further delineate into subgroups. All unvaccinated animals (5/5) were euthanized by day 7 due to severe disease (Fig. 3A). In marked contrast, 100% of rabbits (19/19) that received either the low or the high dose of the 4pox vaccine survived viral challenge. Severe disease in unvaccinated animals was proceeded by a period of weight loss, and by day 7, animals in the control group lost 14% of their body weight (Fig. 3B). Vaccinated rabbits also lost weight, but less than unvaccinated rabbits. 4pox DNA-vaccinated rabbits exhibited modest fluctuations in body temperature, but these levels were generally within the normal range and were sharply contrasted against unvaccinated control animals, whose temperatures were significantly elevated on day 3 (P < 0.05 by t test) (Fig. 3C).
FIG 3.
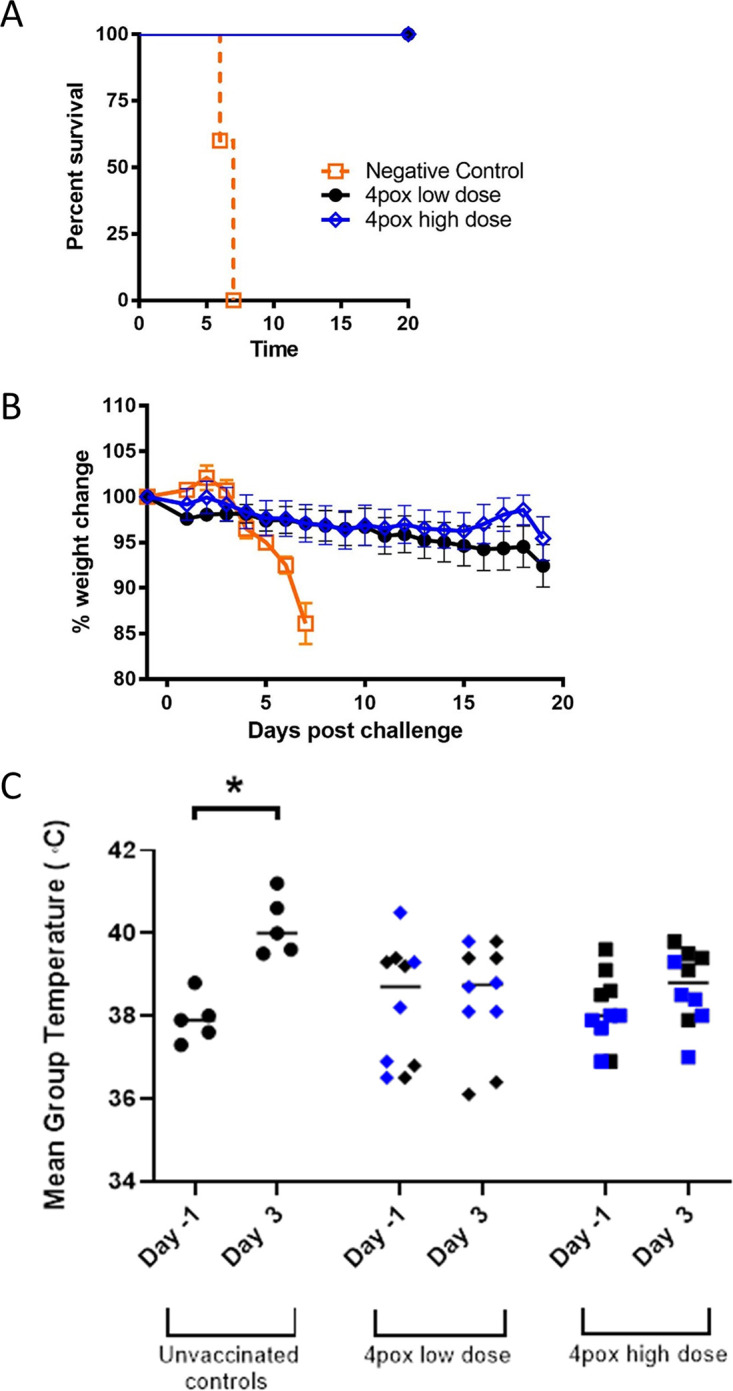
The 4pox vaccine administered i.m. by EP protects rabbits against lethal RPXV aerosol challenge. (A) Rabbits were aerosol challenged with a target dose of ∼1,000 PFU RPXV 28 weeks after the last DNA vaccination. Survival was plotted up to 20 days postinfection using Prism software. Symbols represent the mean ± standard error from each group. (B) Individual weights were calculated based on day 0 starting weight. Symbols represent the mean ± standard error from each group. (C) Mean temperatures on day −1 and day 3 were graphed for each group. Lines represent the geometric mean temperature for each group. The asterisk denotes statistical significance (P < 0.05 by t test). Blue data points represent animals that received CpG adjuvant with the 4pox vaccine.
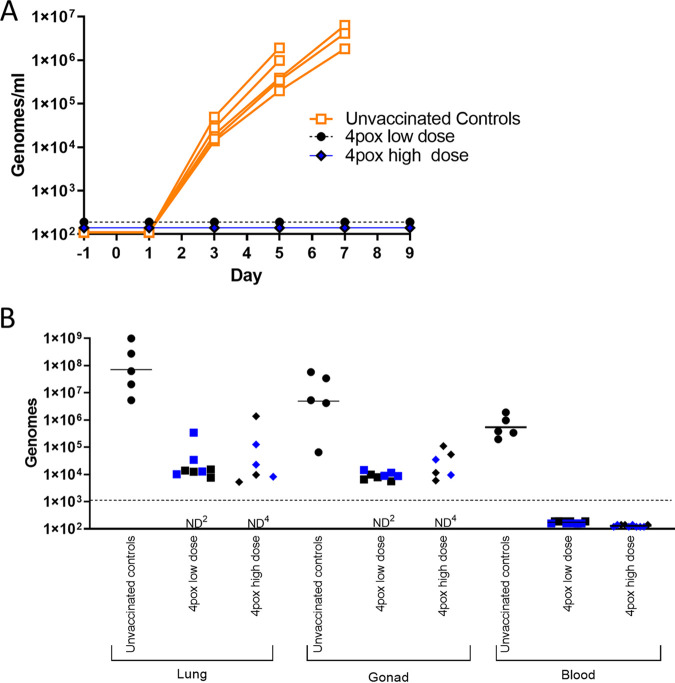
Consistent in rabbits exposed to aerosolized RPXV virus (24), all control rabbits developed gross respiratory and oral cavity lesions at the time of euthanasia (Table 1). No lesions were detected on rabbits vaccinated with either dose of the 4pox DNA vaccine. One vaccinated animal (rabbit 6621) was euthanized on day 22 from a condition unrelated to RPXV challenge. Based on pathological findings, this animal had a trichobezoar causing anorexia. Data from this animal were excluded from the study. As determined by quantitative real-time PCR (qPCR), viremia was not detected in any vaccinated rabbits throughout viral challenge (Fig. 4A). However, viremia was detected in all unvaccinated control animals starting on day 4 and increased until the animals succumbed to disease, with peak viremia between 105 and 106 genomes per mL of whole blood on days 6 and 7. RPXV genome was also detected in lung and gonads of all unvaccinated rabbits at the time of death. Vaccinated rabbits were euthanized following the in-life portion of the study (over 20 days postchallenge [Table 1]) and tested for the presence of RPXV genome in lung and gonads. Eight of 10 low-dose-vaccinated and 6/10 high-dose vaccinated rabbits had detectable RPXV genomes in lung and gonads (Fig. 4B). Overall, these data demonstrated that immune responses produced by the 4pox DNA vaccine can protect animals from RPXV.
TABLE 1.
Lesions present on RPXV-challenged rabbits
| Group | Rabbit no. | Day euthanized | Type of gross lesionsa | Presence of lip lesionsb |
|---|---|---|---|---|
| Unvaccinated controls | 7056 | 7 | Typical | Y |
| 7057 | 6 | Typical | N | |
| 7058 | 7 | Typical | Y | |
| 7059 | 7 | Typical | Y | |
| 7060 | 6 | Typical | N | |
| 4pox low dose | 6322 | 28 | NGL | N |
| 6620 | 28 | NGL | N | |
| 6621 | 22 | Atypical | N | |
| 6622 | 28 | NGL | N | |
| 6623 | 28 | NGL | N | |
| 6323c | 28 | NGL | N | |
| 6624c | 28 | NGL | N | |
| 6625c | 28 | NGL | N | |
| 6626c | 29 | NGL | N | |
| 6627c | 29 | NGL | N | |
| 4pox high dose | 6628 | 29 | NGL | N |
| 6629 | 29 | NGL | N | |
| 6630 | 27 | NGL | N | |
| 6631 | 27 | NGL | N | |
| 6632 | 27 | NGL | N | |
| 6633c | 28 | NGL | N | |
| 6634c | 27 | NGL | N | |
| 6635c | 27 | NGL | N | |
| 6636c | 29 | NGL | N | |
| 6637c | 29 | NGL | N | |
NGL, no gross lesions.
Y, lips had macular lesions; N, no lesions present.
CpG adjuvanted.
FIG 4.
The 4pox DNA vaccine delivered i.m. by EP prevents serum viremia and reduces viral load in peripheral tissues. (A) The presence of RPXV genomes was evaluated by qPCR in whole blood collected every other day following aerosol challenge. Each line represents a single rabbit. (B) Viral load in organs was determined by qPCR in organs collected from rabbits that succumbed to RPXV (unvaccinated controls) or from vaccinated rabbits that were euthanized at the conclusion of the study (days 27 to 29). Blood samples were collected from all rabbits on day 6 and are the same as those in panel A. Blue data points represent animals that received CpG adjuvant with the 4pox vaccine. ND, no genome was detected. (The superscript indicates the number of animals.) The limit of detection in the qPCR assay was 1,000 genomes.
4pox delivered by a DSJI device produces antibody responses in rabbits.
Here, our intentions were purely to explore the immunogenicity of the 4pox DNA vaccine in rabbits when delivered by different delivery methods, including disposable syringe jet injector (DSJI), relative to EP i.m. administration that provided protection against rabbitpox disease. These animals were not exposed to virus in an effort to show protection. Four groups of three rabbits were vaccinated with 0.4 mg 4pox DNA three times at 1-month intervals using i.m. EP, i.d. needle injection, the i.m. DSJI, or the i.d. DSJI (Fig. 5A). Serum was collected from rabbits in all groups prior to vaccination and 3, 7, and 10 weeks after vaccination, and the presence of antibodies against each 4pox antigen was quantified by ELISA. As observed above, i.m. EP resulted in the highest antibody titers against all four target antigens after three vaccinations, with log10 GMTs of >3.0 for all targets. In contrast, needle/syringe-delivered DNA produced the lowest antibody responses after three vaccinations (Fig. 5B). Differences in antibody ELISA titers between i.d. needle/syringe and i.m. EP delivery were statistically significant at week 10 (P < 0.05 by t test) for each antigen. Vaccination of rabbits with either i.m. or i.d. DSJI devices produced antibody responses against all four antigens, with week 10 GMTs of >2.5 log10 against all targets. With the exception of the B5 antigen, immune responses generated by DSJI were not significantly different from those by i.m. EP. ELISA titers in animals vaccinated with either i.d. or i.m. DSJI were statistically higher than i.d. needle/syringe delivery against A27, B5, and A33; however, only the i.d. DSJI device produced significantly higher responses against L1. DNA vaccination by each delivery method produced MV neutralizing antibodies with PRNT50s (GMTs) following vaccination about 3- to 4-fold higher by i.m. EP compared to those produced by the DSJI devices and a significant (P = 0.0352 by one-way analysis of variance [ANOVA]) 20- to 50-fold increase compared to i.d. needle/syringe delivery (Fig. 5C). Vaccination by i.m. EP also resulted in significant (P = 0.0043) PRNT50s at week 10 compared to unvaccinated controls. These results demonstrated that similar to muscle electroporation, the DSJI delivery of the 4pox DNA vaccine can produce high titers of functional antipoxvirus antibodies in rabbits.
FIG 5.
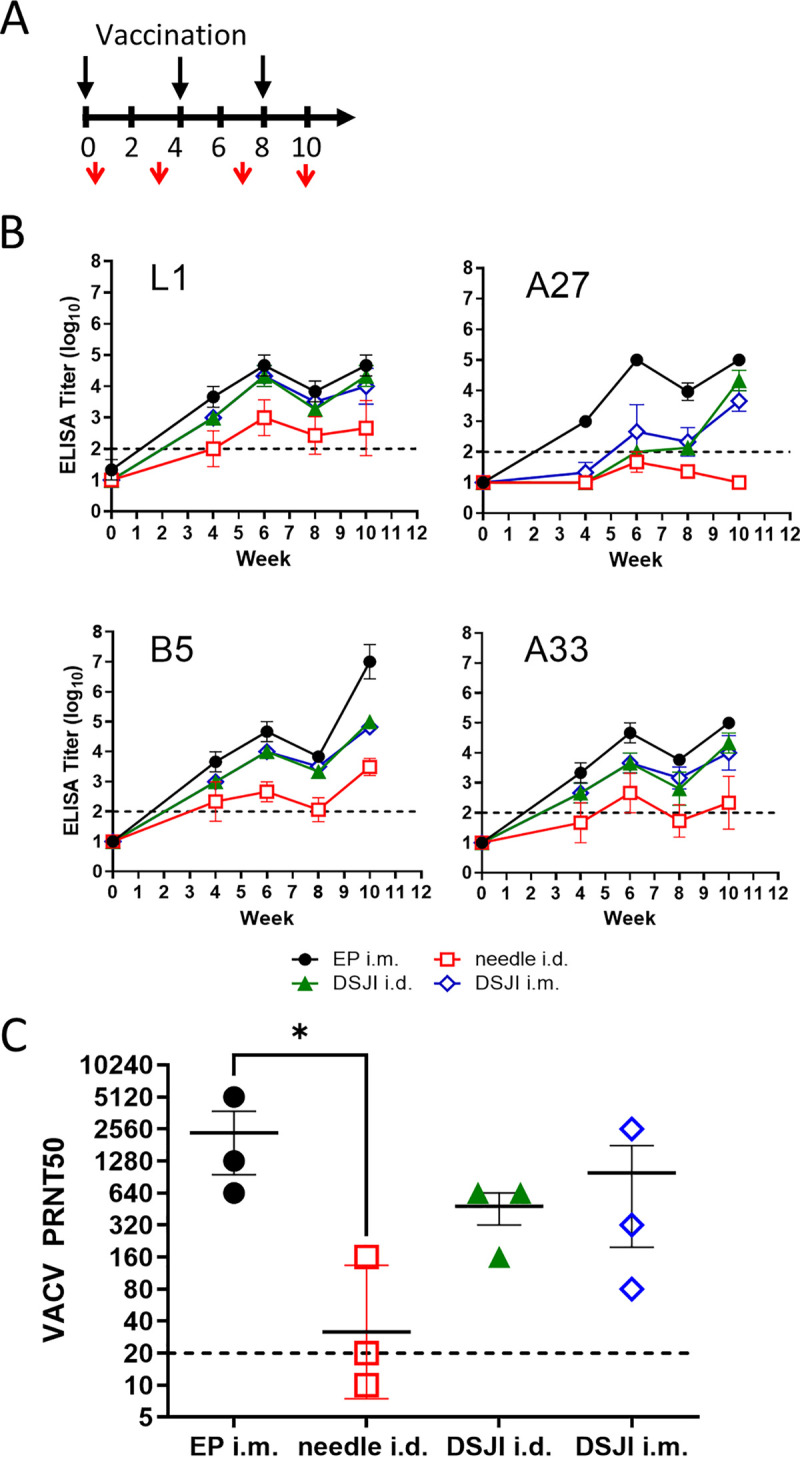
Comparison of 4pox DNA vaccine immunogenicity when delivered by multiple platforms in rabbits. (A) Experimental design. Four groups of rabbits (n = 3) were vaccinated three times at 1-month intervals (black arrows) with 4pox DNA by i.m. EP, i.d. needle/syringe, and the DSJI i.d. or i.m. device. Serum was collected at weeks 0, 4, 6, 8, and 10 (red arrows). (B) Protein-specific ELISAs were performed as in Fig. 2B (C) PRNT50s were determined with serum from the indicated group at the indicate time point as described in Fig. 2C. Geometric mean titers and standard deviations are shown.
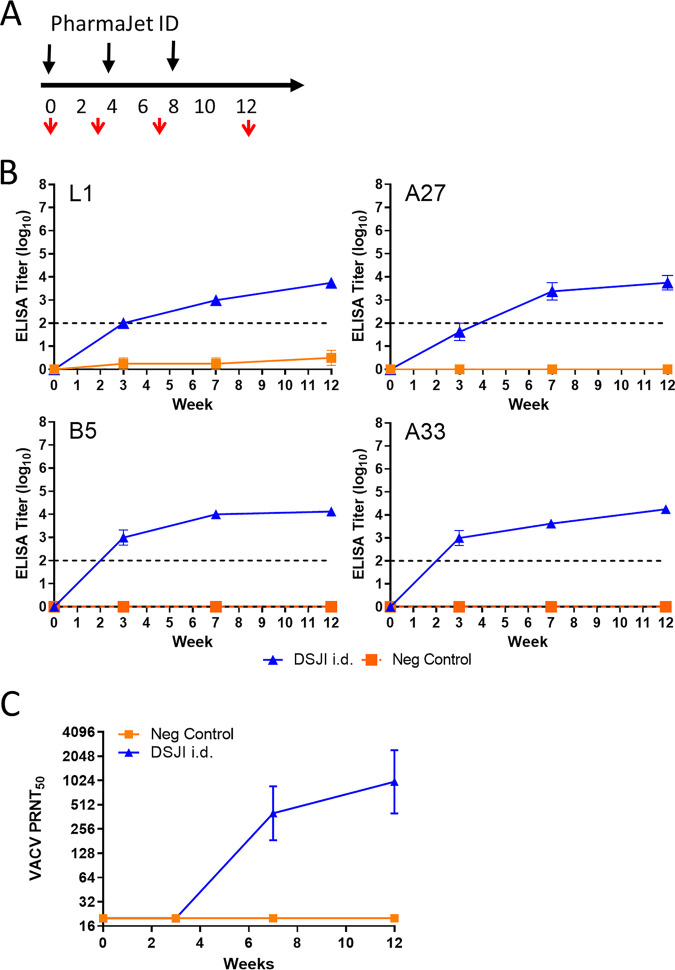
We next vaccinated a larger number of rabbits using the i.d. DSJI device to further explore the immunogenicity of DNA vaccines delivered by this method. Two groups of eight rabbits were vaccinated three times at 1-month intervals with the DSJI i.d. device with the 4pox DNA vaccine (Fig. 6A). A control group was included in which rabbits were vaccinated with a negative-control plasmid (pWRG/SN-M) mixed with empty vector pWRG/7077. Consistent with our prior study (Fig. 5), vaccination with the DSJI i.d. device produced antibody responses against all four target antigens. After the first vaccination (week 4), GMTs against B5 and A33 were ∼3 log10, and this titer increased after the booster vaccinations by about a log. The GMT for responses against L1 was 2.5 log10 after the priming vaccination, and these responses increased after boosting to ∼3.5 log10 observed at week 12 (Fig. 6B). Antibody responses against A27 antigen in rabbits vaccinated with the 4pox vaccine without adjuvant were ∼1.5 log10 after the first vaccination, and this level increased to ∼3 log10 on week 12. The PRNT50s correlated with the antibody titers determined by ELISA for all groups (Fig. 6C). By week 12, the PRNT50 (GMT) for 4pox-vaccinated rabbits was 987.0. These findings confirmed that the 4pox DNA vaccine can produce antibody responses when delivered by the i.d. DSJI device in vaccinated rabbits.
FIG 6.
Immunogenicity of 4pox DNA delivered by DSJI (PharmaJet ID) in rabbits. (A) Experimental design. Three groups of rabbits were vaccinated three times at 1-month intervals with the DSJI i.d. device (black arrows). Serum was collected at weeks 0, 3, 7, and 12 (red arrows). (B) Protein-specific ELISAs were determined as described in Fig. 2B. (C) PRNT50s were determined with serum from the indicated group at the indicated time point as described in Fig. 2C. Geometric mean titers and standard deviations are shown. The y axis is log base 2.
DISCUSSION
A successful vaccine must fulfill the minimum criteria of being safe and effective. The COVID pandemic is highlighting other important characteristics, such as manufacturing/scaling and logistical considerations (e.g., shipping and storage), but also the ability to adapt to novel or evolving viruses. For smallpox and/or human monkeypox, two vaccines are currently approved by the FDA: ACAM2000 and JYNNEOS. The best qualities of each of these vaccines are the efficacy and safety afforded, respectively, as well as storage and distribution. Other characteristics, such as efficient manufacturing processes and the ability to quickly (and safely) respond to a novel poxvirus, are relatively poor compared to current nucleic acid platforms. That being said, there are also limitations to current mRNA vaccines such as the COVID vaccines produced by Pfizer-BioNTech (BNT162b2) and Moderna (mRNA-1273). The lack of stability and related difficulties with distribution, as well as the increased reactogenicity/toxicity in part from the lipid nanoparticle (LNP) formulation, are a few of the issues associated with mRNA vaccines. Herein, we show that in the absence of formulation or additional adjuvants, DNA plasmids delivered by either EP or needless injection (DSJI) are sufficiently immunogenic as to provide protection against a stringent disease model of smallpox.
Protective efficacy of the 4pox DNA vaccine in rabbits.
Because of its similarity to smallpox, such as susceptibility to low doses and respiratory mode of transmission, the RPXV model is particularly attractive (47). To that end, pinnacle studies to support tecovirimat (TPOXX; ST-246) and brincidofovir (Tembexa; CMX-001) efficacy utilized the intradermal infection model of RPXV in rabbits to help satisfy the U.S. FDA Animal Rule (21 CFR 314.600 through 314.650) (51, 52). Infection in rabbits requires an infectious RPXV dose at least 10,000-fold lower than those of MPXV NHP and VACV mouse models, although it should be noted that a low-dose, respiratory and intravenous MPXV model utilizing marmosets has been recently published (53, 54). Rabbits are also naturally susceptible to RPXV via the respiratory route, which is similar to human transmission and the likely route of infection by a biological weapon. Furthermore, the antigenic requirement for protection may depend on the route of viral insult (55): hence, the respiratory/RPXV model would supplement and strengthen confidence in the data obtained by the systemic (intravenous) macaque model (discussed below). For these reasons, we used the rabbit/RPXV test system to demonstrate that the 4pox DNA-based vaccine administered via intramuscular electroporation can protect rabbits against aerosol exposure. For comparison, Garza et al. examined the efficacy of MVA against aerosolized RPXV by vaccinating rabbits with a single low dose (1 × 107 50% tissue culture infective doses [TCID50s]) of MVA (Imvamune; Bavarian Nordic), a single high dose (1 × 108 TCID50s), or two high doses 4 weeks apart by the subcutaneous route (24). Rabbits vaccinated with MVA produced lower PRNT50 responses against VACV (VACV was used in PRNTs and not RPXV), with PRNT50s (GMTs) below 25 in animals receiving a single dose and below 100 in animals receiving two high doses. This response is ∼10- to 30-fold lower than that observed in rabbits vaccinated with a comparable regimen of 4pox vaccine (Fig. 1C). Furthernore, the experiments performed by Garza et al. exposed animals to 10,000 PFU of aerosolized RPXV, compared to a dose of 1,000 PFU in our report. Nevertheless, all MVA-vaccinated animals were protected against lethal infection, and no vaccinated animals developed viremia. Weight loss was not observed in any MVA-vaccinated rabbit regardless of vaccine dose, but control animals lost ∼8% of their day 0 body weight prior to succumbing to disease. In our study, several 4pox-vaccinated animals lost weight, with a maximum weight loss of 9% in the low-dose group. However, this was significantly different from the control group, which lost ∼14% of their body weight (P < 0.05 by ANOVA). Similar to the high dose of MVA given twice, gross lesions were not detected in any 4pox DNA-vaccinated rabbits, whereas control animals all developed gross lesions. We conclude that similar to MVA, the 4pox DNA vaccine protects animals from lethal RPXV infection.
We previously reported that the 4pox vaccine protects NHPs from lethal MPXV challenge after only two doses when delivered by gold particle dermal bombardment (gene gun) (41). In that study, protective efficacy of the 4pox vaccine was directly compared to ACAM2000 and MVA (ACAM3000) in the intravenous MPXV macaque model of disease. An identical vaccination schedule of two doses 4 weeks apart was followed for NHPs receiving the DNA vaccine (4pox vaccine at 8 μg/dose) or the MVA vaccine (1 × 108 PFU/dose). The DNA vaccine and MVA both protected 100% of the animals from lethality. However, 60% (3/5) of MVA-vaccinated NHPs developed severe disease, compared to only 20% (1/5) of the 4pox DNA-vaccinated animals. Contrary to MVA, the 4pox vaccine prevented throat shedding of virus, and viremia was markedly lower (18,363 versus 335,505 genomes/mL). Together with the RPXV model data presented in this current study, it is clear that the 4pox DNA vaccine is highly protective against systemic and respiratory routes of orthopoxvirus exposure in both high- and low-dose animal challenge models. Furthermore, the DNA vaccine provides protection similar to MVA, but may be better at preventing shedding of virus, which may be an added benefit to prevent viral transmission during an outbreak.
Needle-free vaccination devices and the immunogenicity of the 4pox vaccine.
The 4pox vaccine has previously been delivered by several modalities, including intradermal (i.d.) gold-particle bombardment (gene gun) or i.d. skin electroporation (35–37, 39). It has also been delivered in a Venezuelan equine encephalitis virus replicon (VRP), where it protected both mice and NHPs from lethal VACV and MPXV infections (40). Here, we compared both i.m. EP and i.m. and i.d. DSJI delivery systems to i.d. needle injection with the sole intention to determine which was more immunogenic based on humoral responses. Overall, i.m. EP provided the most robust responses against each target as measured by ELISA. However, i.m. EP and both i.m. and i.d. DSJI produced superior immune responses compared to i.d. needle/syringe, including over 5-fold increases in PRNT50. Given these data, our plan to only evaluate immunogenicity may have been overly cautious and shortsighted. Although somewhat inferior to i.m. EP, both DSJI methods most likely would have provided some level of protection. Future studies will involve optimizing the vaccination schedule and dosing using both i.m. and i.d. DSJI before challenging with virus.
While i.m. EP is clearly effective at delivering the vaccine, it requires complex equipment and highly specialized training. DSJI technology is ideally suited for field use because, contrary to EP and gene gun delivery technology, it does not require complex formulation, compressed gases, electricity, or circuitry. Furthermore, the PharmaJet i.m. DSJI is needle-free, portable, and FDA 510(k) approved to deliver any vaccine that can be delivered by needle injection and is routinely used in humans (56–58). It is currently used in humans to deliver several different vaccines, including the influenza vaccine (56). We have also demonstrated DSJI produces robust immune responses against several viral antigens, including hantaviruses, arenaviruses and Zika virus in multiple hosts (59–62). Moreover, DSJI has been shown to be compatible for i.m. administration of lipid nanoparticle (LNP)-formulated DNA, a modification that greatly enhances the kinetics, efficiency, and immunogenicity of the delivered vaccine or immunotherapy (encoded antipoxvirus L1 monoclonal antibody) (59). These characteristics accentuate the benefit for DSJI technology, considering a primary use of a poxvirus vaccine would be to mitigate an outbreak of virus resulting from terrorism and rapid responses will be needed to contain the event. Because orthopoxviruses, in particular MPXV, cause occasional infections in remote areas of Africa, a needle-free, technology-free device would provide a significant advantage.
Protection against orthopoxviruses in the post-smallpox eradication world.
Despite the eradication of smallpox over 3 decades ago, VARV or genetically modified orthopoxviruses pathogenic to humans remain threats as a biological weapon or as accidently released agents (14). Emerging orthopoxvirus zoonoses, including MPXV, CPXV, and VACV-like viruses, continue to infect both people and agricultural animals throughout the world (6–11); together these factors support the continued need for orthopoxvirus countermeasures. The highly defined and optimized 4pox DNA vaccine protects NHPs, mice, and in this current study, rabbits against lethal infections by MPXV, VACV, and RPXV, respectively (39, 41). At the same time, the DNA platform is very malleable and allows for the rapid transition from discovery to licensed product in the event of a novel poxvirus that may be encountered in the future. Approval of ACAM2000 and JYNNEOS was largely based on noninferiority of the neutralizing antibody responses versus calf hind-limb-derived vaccine (63). A similar approach could be used for the 4pox DNA vaccine, in addition to the demonstration of protective efficacy in appropriate animal models. Due to their rapid production possibility and thermostablility, next-generation DNA vaccines may provide a particular advantage during an orthopoxvirus outbreak and, in the case of the 4pox vaccine, could be administered prior to an outbreak to ensure that first responders and the medical community have preexisting immunity without fear of potential adverse events presented by the current second-generation vaccines.
MATERIALS AND METHODS
Virus and cells.
VACV strain IHD-J was maintained in Vero cell (ATCC CRL-1587) monolayers grown in Eagle’s minimal essential medium (EMEM) containing 5% heat-inactivated fetal bovine serum (FBS) (HyClone; Logan, UT), 1% antibiotics (100 U/mL penicillin, 100 μg/mL streptomycin, and 50 μg/mL gentamicin), and 10 mM HEPES (cEMEM). RPXV strain Utrecht was obtained from ATCC (Manassas, VA) and propagated in CV-1 cells in EMEM supplemented with 1% nonessential amino acids (NEAA), 1% 200 nM l-glutamine, and 7.5% FBS, as described previously (24). BSC-1 cells (ATCC CCL-26) were maintained in cEMEM and were used for plaque reduction/neutralization titer (PRNT) assays and EV spread inhibition assays.
Animals.
Female specific-pathogen-free New Zealand White (NZW) rabbits (Oryctolagus cuniculus) ∼11 weeks of age and weighing approximately 2.5 to 3.0 kg were purchased from Charles River Laboratories (Wilmington, MA). The rabbits were maintained on a 12-h light/12-h dark cycle and fed standard rabbit food supplemented with fresh leafy vegetables and water ad libitum. Each animal was implanted subcutaneously between its scapulae with a programmable temperature transponder chip (Bio Medic Data Systems, Seaford, DE) to determine rabbit identification and subcutaneous body temperature.
In vitro stimulation of rabbit lymphocytes and FACS analysis.
Dissociated rabbit spleens were mixed with peripheral blood cells from the same animal. Red blood cells (RBCs) were removed by Percoll gradient centrifugation. Cells were labeled with carboxyfluorescein succinimidyl ester (CFSE) (Molecular Probes) and then cultured in RPMI 1640 medium with 10% fetal calf serum (FCS), 4 mM l-glutamine, 0.1 mM nonessential amino acids, 1 mM sodium pyruvate, 100 U/mL penicillin and streptomycin, 10 mM HEPES, and 5 × 10−6 M 2-mercaptoethanol (2-ME) (RP-10). Bulk lymphocytes were then placed in microtiter wells (5 × 105 per well) containing the indicated CpG or lipopolysaccharide (LPS) and were incubated at 37°C for 5 days. After 5 days, the supernatant from each well was collected to determine by ELISA the amounts of IgG and IgM secreted. The cell layer was then collected and washed twice in phosphate-buffered saline (PBS) containing 2% FBS. Approximately 106 cells were stained with a goat anti-rabbit IgG (human adsorbed; Beckman Coulter) antibody. Stained cells were then were fixed in Cytofix buffer (BD Biosciences) for 15 min at 4°C before being analyzed on a FACSCalibur flow cytometer (BD Biosciences) using CellQuest software (BD Biosciences) or FACSCanto II flow cytometer (BD Biosciences) using FACsDiva software (BD Biosciences). Data were analyzed using FlowJo software (Treestar).
CpGs.
Phosphorothioate ODNs were synthesized at the Core Facility of the Center for Biologics Evaluation and Research (FDA, Bethesda, MD). The following ODNs were used: CpG ODN 1555 (GCTAGACGTTAGCGT) and control ODN 1612 (GCTAGAGCTTAGCGT). All ODNs were free of detectable protein or endotoxin contamination.
Vaccinations.
Four vaccine delivery technologies were used in this study: (i) intramuscular (i.m.) disposable syringe jet injector (DSJI) (PharmaJet; Golden, CO), (ii) intradermal (i.d.) DSJI, (iii) i.m. electroporation (EP) using the TriGrid delivery system (Ichor Medical Systems, San Diego, CA), and (iv) i.d. 1-mL syringe and 25G 5/8 needle delivery. The muscles of the lateral thigh were used for i.m. injection sites. Skin overlying the lateral thigh muscles was used as the i.d. injection sites. Initial vaccinations were performed on the left leg (week 0) and then alternated for subsequent boosts on weeks 4 and 8, respectively. Prior to vaccinations, fur at injection sites was removed using electric clippers. The i.m. EP vaccinations were performed as described by Dupuy et al. (64). Briefly, rabbits were injected with 1 mL of a 0.4-mg/mL DNA solution (low dose) or 4-mg/mL DNA solution (high dose) using a 3/10-cm3 U-100 insulin syringe (Becton, Dickinson) inserted into the center of a TriGrid electrode array with 2.5-mm electrode spacing. Subgroups of five rabbits in each of the low- and high-4pox-dosed groups also included 0.5 mg of CpG oligonucleotides 3, 4, and 6 within each vaccine dose. Injection of DNA was followed immediately by electrical stimulation at amplitude of 250 V/cm, and the total duration was 40 ms over a 400-ms interval. For i.d. DSJI device and needle/syringe vaccinations, rabbits received 0.1 mL of a 4-mg/mL DNA solution. For i.m. DSJI vaccinations, rabbits received 0.5 mL of a 0.8- or 8-mg/mL DNA solution. For each vaccine system, DNA was diluted in PBS at pH 7.4. Blood samples were obtained at the indicated time points.
Plasmids.
The 4pox genes were synthesized de novo after having been optimized for mRNA stability and codon usage in human cells (GeneArt, Burlingame, CA). Generation of the L1R plasmid was done essentially as described previously (39). A33R, B5R, and A27R were also optimized for expression, including the addition of a Kozak sequence on the 5′ untranslated region of the genes. To construct pWRG/A33Rkopt, pWRG/B5Rkopt, and pWRG/A27kopt, open reading frames from the de novo-synthesized genes were cloned into the NotI and BglII sites of the pWRG vector. Each construct was confirmed by sequencing and expression using monoclonal and polyclonal antibodies before use in vaccine studies. The construction and characterization of control plasmid pWRG/SN-M have been described elsewhere (65).
Antigen-specific ELISAs.
Antigen-specific ELISAs using VACV histidine-tagged antigens L1 (300 ng/well), A33 (50 ng/well), B5 (50 ng/well), and A27 (50 ng/well) have been described in detail previously (40). An irrelevant histidine-tagged protein purified from Escherichia coli (BotN) was used as a negative-control antigen. For rabbit IgG ELISAs, wells were coated with a goat anti-rabbit primary antibody (AbD Serotec; 100 ng/well) and then probed with a chicken anti-rabbit IgG secondary antibody (Abcam; 40 ng/well). Endpoint titers were calculated as the highest dilution with an absorbance value greater than the mean absorbance value from negative-control sera plus 3 standard deviations.
PRNT assays.
The plaque reduction/neutralization titer (PRNT) assay utilizing the mature viron (MV) form of the virus has been described previously (30, 66). Briefly, VACV strain IHD-J was diluted in cEMEM to give ∼250 PFU/mL. Heat-inactivated rabbit serum was serially diluted (starting at 1:40) and incubated with an equal volume of virus diluted in cEMEM for 1 h at 37°C. Virus-serum mixtures were then adsorbed to confluent BSC-1 cell monolayers in 6-well plates for 1 h in a 37°C 5% CO2 incubator. After adsorption, a 2-mL semisolid overlay (Earle's basal minimal essential medium, 1.5% methylcellulose, 5% heat-inactivated FBS, and antibiotics [100 U/mL penicillin], 100 μg/mL streptomycin, and 50 μg/mL gentamicin) was added to each well. After 4 days, cell monolayers were stained with staining solution (3% crystal violet and 15% ethanol in H2O) overnight. Monolayers were rinsed with water, and the plaques were counted. The percentage of neutralization was calculated relative to the number of plaques in the absence of antibody. Titers represent the reciprocal of the highest dilution resulting in a 50% reduction in the number of plaques in the absence of antibody.
Comet spread inhibition assay.
The comet reduction test was carried essentially as described by Fogg et al. (31) by infecting confluent BSC-1 cells in 12-well plates (Costar; Corning, Acton, MA) with VACV strain IHD-J, diluted in cEMEM to give approximately 40 plaques per well. After 2 h at 37°C, the inoculum was removed and the cells were washed. Rabbit serum diluted in cEMEM was added to duplicate wells, and the plates were incubated for 48 h at 37°C, then stained with crystal violet.
Viral challenge.
Rabbits were exposed to a target dose of 1,000 PFU of aerosolized RPXV, as previously described (24, 67). Briefly, the respiratory function of each rabbit was first measured using whole-body plethysmography (Buxco Systems, Sharon, CT) immediately prior to exposure. Rabbits were exposed to aerosolized RPXV using a dynamic muzzle-only (nose and mouth) inhalation chamber, operated within a class III biosafety cabinet maintained under negative pressure (48).
DNA isolation and qPCR.
The presence of RBVX genomes in blood and tissue was determined as previously described (24). Briefly, starting the day before exposure and continuing every second day after exposure until day 14, blood was collected for viral load analysis in whole blood. In addition, rabbits that succumbed to disease or those that survived virus challenged and were euthanized at the end of the study were necropsied, and selected tissue samples were collected for viral load determination by the quantitative real-time PCR (qPCR) assay. DNA was isolated from blood and tissue samples using Biorobot M48 (Qiagen, Valencia, CA) in accordance with the manufacturer’s instructions. A pan-orthopox hemagglutinin (HA) assay was used to measure viral load in whole blood and tissues (68). The limit of detection (LOD) for this assay was 1,000 genomes/mL. Data were analyzed by using the Roche LightCycler data analysis software (version 4.0).
Statistical analysis.
Differences in anti-neutralization ELISA titers were analyzed by ANOVA with Dunnett’s post hoc comparisons between groups. Survival analyses were done using the log rank test conducted with GraphPad Prism. Differences in neutralizing antibody titers were determined by the two-tailed, paired t test using GraphPad Prism.
Ethics.
All animal studies were conducted in compliance with the Animal Welfare Act and other federal statutes and regulations relating to animals and experiments involving animals and adhere to principles stated in the Guide for the Care and Use of Laboratory Animals of the National Research Council (69). All animal experimental protocols were approved by a preexisting internal Institutional Animal Care and Use Committee (IACUC). The facilities where this research was conducted are fully accredited by the Association for Assessment and Accreditation of Laboratory Animal Care International. Animals meeting the relevant criteria were humanely euthanized.
ACKNOWLEDGMENTS
We thank Rebecca Brocato for critically reviewing the manuscript. We also thank the Veterinary Medicine, Pathology and Aerobiology Divisions for technical assistance. Opinions, interpretations, conclusions, and recommendations are those of the authors and not necessarily endorsed by the U.S. Army or the Department of Defense.
The National Institutes of Health, Department of Health and Human Services, under IAA Y1-AI-9426-02, Appendix A120 B.19, provided funding to E.M.M., J.W.G., C.D.H., J.M.K., M.D.J., A.N., and J.W.H. Pharmajet LLC and Aldevron LLC provided funding to M.R. and J.B., respectively. The funders had no role in study design or data collection.
M.R. and J.B. are current or past employees of for-profit organizations. J.B. owns stock or holds stock options. J.W.H. has a patent for DNA vaccines against poxviruses. E.M.M., J.W.G., C.D.H., J.M.K., and M.D.K. declare no conflict of interest.
Contributor Information
Jay W. Hooper, Email: jay.w.hooper.civ@mail.mil.
Joanna L. Shisler, University of Illinois at Urbana Champaign
REFERENCES
- 1.Moss B. 2011. Smallpox vaccines: targets of protective immunity. Immunol Rev 239:8–26. 10.1111/j.1600-065X.2010.00975.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Koblentz GD. 2017. The de novo synthesis of horsepox virus: implications for biosecurity and recommendations for preventing the reemergence of smallpox. Health Secur 15:620–628. 10.1089/hs.2017.0061. [DOI] [PubMed] [Google Scholar]
- 3.Smith GL, McFadden G. 2002. Smallpox: anything to declare? Nat Rev Immunol 2:521–527. 10.1038/nri845. [DOI] [PubMed] [Google Scholar]
- 4.Noyce RS, Lederman S, Evans DH. 2018. Construction of an infectious horsepox virus vaccine from chemically synthesized DNA fragments. PLoS One 13:e0188453. 10.1371/journal.pone.0188453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Reardon S. 2014. 'Forgotten' NIH smallpox virus languishes on death row. Nature 514:544. 10.1038/514544a. [DOI] [PubMed] [Google Scholar]
- 6.Di Giulio DB, Eckburg PB. 2004. Human monkeypox: an emerging zoonosis. Lancet Infect Dis 4:15–25. 10.1016/s1473-3099(03)00856-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Essbauer S, Pfeffer M, Meyer H. 2010. Zoonotic poxviruses. Vet Microbiol 140:229–236. 10.1016/j.vetmic.2009.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Glatz M, Richter S, Ginter-Hanselmayer G, Aberer W, Mullegger RR. 2010. Human cowpox in a veterinary student. Lancet Infect Dis 10:288. 10.1016/S1473-3099(10)70054-2. [DOI] [PubMed] [Google Scholar]
- 9.Kroon EG, Mota BE, Abrahao JS, da Fonseca FG, de Souza Trindade G. 2011. Zoonotic Brazilian vaccinia virus: from field to therapy. Antiviral Res 92:150–163. 10.1016/j.antiviral.2011.08.018. [DOI] [PubMed] [Google Scholar]
- 10.Rimoin AW, Mulembakani PM, Johnston SC, Lloyd Smith JO, Kisalu NK, Kinkela TL, Blumberg S, Thomassen HA, Pike BL, Fair JN, Wolfe ND, Shongo RL, Graham BS, Formenty P, Okitolonda E, Hensley LE, Meyer H, Wright LL, Muyembe JJ. 2010. Major increase in human monkeypox incidence 30 years after smallpox vaccination campaigns cease in the Democratic Republic of Congo. Proc Natl Acad Sci USA 107:16262–16267. 10.1073/pnas.1005769107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Willemse A, Egberink HF. 1985. Transmission of cowpox virus infection from domestic cat to man. Lancet 325:1515. 10.1016/S0140-6736(85)92299-8. [DOI] [PubMed] [Google Scholar]
- 12.Nolen LD, Osadebe L, Katomba J, Likofata J, Mukadi D, Monroe B, Doty J, Hughes CM, Kabamba J, Malekani J, Bomponda PL, Lokota JI, Balilo MP, Likafi T, Lushima RS, Ilunga BK, Nkawa F, Pukuta E, Karhemere S, Tamfum JJ, Nguete B, Wemakoy EO, McCollum AM, Reynolds MG. 2016. Extended human-to-human transmission during a monkeypox outbreak in the Democratic Republic of the Congo. Emerg Infect Dis 22:1014–1021. 10.3201/eid2206.150579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kantele A, Chickering K, Vapalahti O, Rimoin AW. 2016. Emerging diseases—the monkeypox epidemic in the Democratic Republic of the Congo. Clin Microbiol Infect 22:658–659. 10.1016/j.cmi.2016.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Golden JW, Hooper JW. 2011. The strategic use of novel smallpox vaccines in the post-eradication world. Expert Rev Vaccines 10:1021–1035. 10.1586/erv.11.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lane JM, Goldstein J. 2003. Adverse events occurring after smallpox vaccination. Semin Pediatr Infect Dis 14:189–195. 10.1016/s1045-1870(03)00032-3. [DOI] [PubMed] [Google Scholar]
- 16.Nalca A, Zumbrun EE. 2010. ACAM2000: the new smallpox vaccine for United States Strategic National Stockpile. Drug Des Devel Ther 4:71–79. 10.2147/dddt.s3687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Casey CG, Iskander JK, Roper MH, Mast EE, Wen XJ, Torok TJ, Chapman LE, Swerdlow DL, Morgan J, Heffelfinger JD, Vitek C, Reef SE, Hasbrouck LM, Damon I, Neff L, Vellozzi C, McCauley M, Strikas RA, Mootrey G. 2005. Adverse events associated with smallpox vaccination in the United States, January–October 2003. JAMA 294:2734–2743. 10.1001/jama.294.21.2734. [DOI] [PubMed] [Google Scholar]
- 18.Copeman PW, Wallace HJ. 1964. Eczema vaccinatum. Br Med J 2:906–908. 10.1136/bmj.2.5414.906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lane JM, Ruben FL, Neff JM, Millar JD. 1969. Complications of smallpox vaccination, 1968. N Engl J Med 281:1201–1208. 10.1056/NEJM196911272812201. [DOI] [PubMed] [Google Scholar]
- 20.Acambis. 2008. ACAM2000 Smallpox (Vaccinia) Vaccine, Live. Package insert.
- 21.Kennedy JS, Greenberg RN. 2009. IMVAMUNE: modified vaccinia Ankara strain as an attenuated smallpox vaccine. Expert Rev Vaccines 8:13–24. 10.1586/14760584.8.1.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kenner J, Cameron F, Empig C, Jobes DV, Gurwith M. 2006. LC16m8: an attenuated smallpox vaccine. Vaccine 24:7009–7022. 10.1016/j.vaccine.2006.03.087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Frey SE, Winokur PL, Hill H, Goll JB, Chaplin P, Belshe RB. 2014. Phase II randomized, double-blinded comparison of a single high dose (5×108 TCID50) of modified vaccinia Ankara compared to a standard dose (1×108 TCID50) in healthy vaccinia-naive individuals. Vaccine 32:2732–2739. 10.1016/j.vaccine.2014.02.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Garza NL, Hatkin JM, Livingston V, Nichols DK, Chaplin PJ, Volkmann A, Fisher D, Nalca A. 2009. Evaluation of the efficacy of modified vaccinia Ankara (MVA)/IMVAMUNE against aerosolized rabbitpox virus in a rabbit model. Vaccine 27:5496–5504. 10.1016/j.vaccine.2009.06.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hatch GJ, Graham VA, Bewley KR, Tree JA, Dennis M, Taylor I, Funnell SG, Bate SR, Steeds K, Tipton T, Bean T, Hudson L, Atkinson DJ, McLuckie G, Charlwood M, Roberts AD, Vipond J. 2013. Assessment of the protective effect of Imvamune and Acam2000 vaccines against aerosolized monkeypox virus in cynomolgus macaques. J Virol 87:7805–7815. 10.1128/JVI.03481-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Antoine G, Scheiflinger F, Dorner F, Falkner FG. 1998. The complete genomic sequence of the modified vaccinia Ankara strain: comparison with other orthopoxviruses. Virology 244:365–396. 10.1006/viro.1998.9123. [DOI] [PubMed] [Google Scholar]
- 27.Boulter EA, Appleyard G. 1973. Differences between extracellular and intracellular forms of poxvirus and their implications. Prog Med Virol 16:86–108. [PubMed] [Google Scholar]
- 28.Davies DH, McCausland MM, Valdez C, Huynh D, Hernandez JE, Mu Y, Hirst S, Villarreal L, Felgner PL, Crotty S. 2005. Vaccinia virus H3L envelope protein is a major target of neutralizing antibodies in humans and elicits protection against lethal challenge in mice. J Virol 79:11724–11733. 10.1128/JVI.79.18.11724-11733.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sakhatskyy P, Wang S, Chou TH, Lu S. 2006. Immunogenicity and protection efficacy of monovalent and polyvalent poxvirus vaccines that include the D8 antigen. Virology 355:164–174. 10.1016/j.virol.2006.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hooper JW, Custer DM, Schmaljohn CS, Schmaljohn AL. 2000. DNA vaccination with vaccinia virus L1R and A33R genes protects mice against a lethal poxvirus challenge. Virology 266:329–339. 10.1006/viro.1999.0096. [DOI] [PubMed] [Google Scholar]
- 31.Fogg C, Lustig S, Whitbeck JC, Eisenberg RJ, Cohen GH, Moss B. 2004. Protective immunity to vaccinia virus induced by vaccination with multiple recombinant outer membrane proteins of intracellular and extracellular virions. J Virol 78:10230–10237. 10.1128/JVI.78.19.10230-10237.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Galmiche MC, Goenaga J, Wittek R, Rindisbacher L. 1999. Neutralizing and protective antibodies directed against vaccinia virus envelope antigens. Virology 254:71–80. 10.1006/viro.1998.9516. [DOI] [PubMed] [Google Scholar]
- 33.Fang M, Cheng H, Dai Z, Bu Z, Sigal LJ. 2006. Immunization with a single extracellular enveloped virus protein produced in bacteria provides partial protection from a lethal orthopoxvirus infection in a natural host. Virology 345:231–243. 10.1016/j.virol.2005.09.056. [DOI] [PubMed] [Google Scholar]
- 34.Mucker EM, Lindquist M, Hooper JW. 2020. Particle-specific neutralizing activity of a monoclonal antibody targeting the poxvirus A33 protein reveals differences between cell associated and extracellular enveloped virions. Virology 544:42–54. 10.1016/j.virol.2020.02.004. [DOI] [PubMed] [Google Scholar]
- 35.Hooper JW, Custer DM, Thompson E. 2003. Four-gene-combination DNA vaccine protects mice against a lethal vaccinia virus challenge and elicits appropriate antibody responses in nonhuman primates. Virology 306:181–195. 10.1016/s0042-6822(02)00038-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hooper JW, Thompson E, Wilhelmsen C, Zimmerman M, Ichou MA, Steffen SE, Schmaljohn CS, Schmaljohn AL, Jahrling PB. 2004. Smallpox DNA vaccine protects nonhuman primates against lethal monkeypox. J Virol 78:4433–4443. 10.1128/jvi.78.9.4433-4443.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hooper JW, Golden JW, Ferro AM, King AD. 2007. Smallpox DNA vaccine delivered by novel skin electroporation device protects mice against intranasal poxvirus challenge. Vaccine 25:1814–1823. 10.1016/j.vaccine.2006.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Golden JW, Zaitseva M, Kapnick S, Fisher RW, Mikolajczyk MG, Ballantyne J, Golding H, Hooper JW. 2011. Polyclonal antibody cocktails generated using DNA vaccine technology protect in murine models of orthopoxvirus disease. Virol J 8:441. 10.1186/1743-422X-8-441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Golden JW, Josleyn MD, Hooper JW. 2008. Targeting the vaccinia virus L1 protein to the cell surface enhances production of neutralizing antibodies. Vaccine 26:3507–3515. 10.1016/j.vaccine.2008.04.017. [DOI] [PubMed] [Google Scholar]
- 40.Hooper JW, Ferro AM, Golden JW, Silvera P, Dudek J, Alterson K, Custer M, Rivers B, Morris J, Owens G, Smith JF, Kamrud KI. 2009. Molecular smallpox vaccine delivered by alphavirus replicons elicits protective immunity in mice and non-human primates. Vaccine 28:494–511. 10.1016/j.vaccine.2009.09.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Golden JW, Josleyn M, Mucker EM, Hung CF, Loudon PT, Wu TC, Hooper JW. 2012. Side-by-side comparison of gene-based smallpox vaccine with MVA in nonhuman primates. PLoS One 7:e42353. 10.1371/journal.pone.0042353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Li G, Chen N, Roper RL, Feng Z, Hunter A, Danila M, Lefkowitz EJ, Buller RM, Upton C. 2005. Complete coding sequences of the rabbitpox virus genome. J Gen Virol 86:2969–2977. 10.1099/vir.0.81331-0. [DOI] [PubMed] [Google Scholar]
- 43.Nalca A, Nichols DK. 2011. Rabbitpox: a model of airborne transmission of smallpox. J Gen Virol 92:31–35. 10.1099/vir.0.026237-0. [DOI] [PubMed] [Google Scholar]
- 44.Roy CJ, Voss TG. 2010. Use of the aerosol rabbitpox virus model for evaluation of anti-poxvirus agents. Viruses 2:2096–2107. 10.3390/v2092096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Adams MM, Rice AD, Moyer RW. 2007. Rabbitpox virus and vaccinia virus infection of rabbits as a model for human smallpox. J Virol 81:11084–11095. 10.1128/JVI.00423-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chapman JL, Nichols DK, Martinez MJ, Raymond JW. 2010. Animal models of orthopoxvirus infection. Vet Pathol 47:852–870. 10.1177/0300985810378649. [DOI] [PubMed] [Google Scholar]
- 47.Perry MR, Warren R, Merchlinsky M, Houchens C, Rogers JV. 2018. Rabbitpox in New Zealand White rabbits: a therapeutic model for evaluation of poxvirus medical countermeasures under the FDA Animal Rule. Front Cell Infect Microbiol 8:356. 10.3389/fcimb.2018.00356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nalca A, Hatkin JM, Garza NL, Nichols DK, Norris SW, Hruby DE, Jordan R. 2008. Evaluation of orally delivered ST-246 as postexposure prophylactic and antiviral therapeutic in an aerosolized rabbitpox rabbit model. Antiviral Res 79:121–127. 10.1016/j.antiviral.2008.03.005. [DOI] [PubMed] [Google Scholar]
- 49.Merchlinsky M, Albright A, Olson V, Schiltz H, Merkeley T, Hughes C, Petersen B, Challberg M. 2019. The development and approval of tecoviromat (TPOXX), the first antiviral against smallpox. Antiviral Res 168:168–174. 10.1016/j.antiviral.2019.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rice AD, Adams MM, Lampert B, Foster S, Robertson A, Painter G, Moyer RW. 2011. Efficacy of CMX001 as a prophylactic and presymptomatic antiviral agent in New Zealand White rabbits infected with rabbitpox virus, a model for orthopoxvirus infections of humans. Viruses 3:63–82. 10.3390/v3020063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Antimicrobial Drugs Advisory Committee Meeting. 2018. Tecovirimat for the treatment of smallpox disease. FDA Advisory Committee briefing document. https://www.fda.gov/media/112808/download.
- 52.FDA. 2021. Brincidofovir. https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/214460s000,214461s000lbl.pdf.
- 53.Mucker EM, Chapman J, Huzella LM, Huggins JW, Shamblin J, Robinson CG, Hensley LE. 2015. Susceptibility of marmosets (Callithrix jacchus) to monkeypox virus: a low dose prospective model for monkeypox and smallpox disease. PLoS One 10:e0131742. 10.1371/journal.pone.0131742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mucker EM, Wollen-Roberts SE, Kimmel A, Shamblin J, Sampey D, Hooper JW. 2018. Intranasal monkeypox marmoset model: prophylactic antibody treatment provides benefit against severe monkeypox virus disease. PLoS Negl Trop Dis 12:e0006581. 10.1371/journal.pntd.0006581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kaufman DR, Goudsmit J, Holterman L, Ewald BA, Denholtz M, Devoy C, Giri A, Grandpre LE, Heraud JM, Franchini G, Seaman MS, Havenga MJ, Barouch DH. 2008. Differential antigen requirements for protection against systemic and intranasal vaccinia virus challenges in mice. J Virol 82:6829–6837. 10.1128/JVI.00353-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.McAllister L, Anderson J, Werth K, Cho I, Copeland K, Le Cam Bouveret N, Plant D, Mendelman PM, Cobb DK. 2014. Needle-free jet injection for administration of influenza vaccine: a randomised non-inferiority trial. Lancet 384:674–681. 10.1016/S0140-6736(14)60524-9. [DOI] [PubMed] [Google Scholar]
- 57.Nelson EA, Lam HS, Choi KC, Ho WC, Fung LW, Cheng FW, Sung RY, Royals M, Chan PK. 2013. A pilot randomized study to assess immunogenicity, reactogenicity, safety and tolerability of two human papillomavirus vaccines administered intramuscularly and intradermally to females aged 18–26 years. Vaccine 31:3452–3460. 10.1016/j.vaccine.2013.06.034. [DOI] [PubMed] [Google Scholar]
- 58.Resik S, Tejeda A, Mach O, Sein C, Molodecky N, Jarrahian C, Saganic L, Zehrung D, Fonseca M, Diaz M, Alemany N, Garcia G, Hung LH, Martinez Y, Sutter RW. 2015. Needle-free jet injector intradermal delivery of fractional dose inactivated poliovirus vaccine: association between injection quality and immunogenicity. Vaccine 33:5873–5877. 10.1016/j.vaccine.2015.06.071. [DOI] [PubMed] [Google Scholar]
- 59.Mucker EM, Karmali PP, Vega J, Kwilas SA, Wu H, Joselyn M, Ballantyne J, Sampey D, Mukthavaram R, Sullivan E, Chivukula P, Hooper JW. 2020. Lipid nanoparticle formulation increases efficiency of DNA-vectored vaccines/immunoprophylaxis in animals including transchromosomic bovines. Sci Rep 10:8764. 10.1038/s41598-020-65059-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kwilas S, Kishimori JM, Josleyn M, Jerke K, Ballantyne J, Royals M, Hooper JW. 2014. A hantavirus pulmonary syndrome (HPS) DNA vaccine delivered using a spring-powered jet injector elicits a potent neutralizing antibody response in rabbits and nonhuman primates. Curr Gene Ther 14:200–210. 10.2174/1566523214666140522122633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Golden JW, Maes P, Kwilas SA, Ballantyne J, Hooper JW. 2016. Glycoprotein-specific antibodies produced by DNA vaccination protect guinea pigs from lethal Argentine and Venezuelan hemorrhagic fever. J Virol 90:3515–3529. doi:10.1128/JVI.02969–15. 10.1128/JVI.02969-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Stein DR, Golden JW, Griffin BD, Warner BM, Ranadheera C, Scharikow L, Sloan A, Frost KL, Kobasa D, Booth SA, Josleyn M, Ballantyne J, Sullivan E, Jiao JA, Wu H, Wang Z, Hooper JW, Safronetz D. 2017. Human polyclonal antibodies produced in transchromosomal cattle prevent lethal Zika virus infection and testicular atrophy in mice. Antiviral Res 146:164–173. 10.1016/j.antiviral.2017.09.005. [DOI] [PubMed] [Google Scholar]
- 63.Emergent Product Development. 2007. ACAM2000, Smallpox (Vaccinia) Vaccine, Live. Package insert. Emergent Product Development Gaithersburg, Inc, Gaithersburg, MD.
- 64.Dupuy LC, Richards MJ, Ellefsen B, Chau L, Luxembourg A, Hannaman D, Livingston BD, Schmaljohn CS. 2011. A DNA vaccine for Venezuelan equine encephalitis virus delivered by intramuscular electroporation elicits high levels of neutralizing antibodies in multiple animal models and provides protective immunity to mice and nonhuman primates. Clin Vaccine Immunol 18:707–716. 10.1128/CVI.00030-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hooper JW, Josleyn M, Ballantyne J, Brocato R. 2013. A novel Sin Nombre virus DNA vaccine and its inclusion in a candidate pan-hantavirus vaccine against hantavirus pulmonary syndrome (HPS) and hemorrhagic fever with renal syndrome (HFRS). Vaccine 31:4314–4321. 10.1016/j.vaccine.2013.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Schmaljohn C, Vanderzanden L, Bray M, Custer D, Meyer B, Li D, Rossi C, Fuller D, Fuller J, Haynes J, Huggins J. 1997. Naked DNA vaccines expressing the prM and E genes of Russian spring summer encephalitis virus and Central European encephalitis virus protect mice from homologous and heterologous challenge. J Virol 71:9563–9569. 10.1128/JVI.71.12.9563-9569.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Pitt ML, Little SF, Ivins BE, Fellows P, Barth J, Hewetson J, Gibbs P, Dertzbaugh M, Friedlander AM. 2001. In vitro correlate of immunity in a rabbit model of inhalational anthrax. Vaccine 19:4768–4773. 10.1016/S0264-410X(01)00234-1. [DOI] [PubMed] [Google Scholar]
- 68.Kulesh DA, Baker RO, Loveless BM, Norwood D, Zwiers SH, Mucker E, Hartmann C, Herrera R, Miller D, Christensen D, Wasieloski LP, Jr, Huggins J, Jahrling PB. 2004. Smallpox and pan-orthopox virus detection by real-time 3'-minor groove binder TaqMan assays on the Roche LightCycler and the Cepheid Smart Cycler platforms. J Clin Microbiol 42:601–609. 10.1128/JCM.42.2.601-609.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.National Research Council. 2011. Guide for the care and use of laboratory animals, 8th ed. National Academies Press, Washington, DC. [Google Scholar]