Abstract
Tuberculosis (TB), caused by Mycobacterium tuberculosis, is one of the most devastating bacterial diseases to affect humans. M. tuberculosis is a robust pathogen that has evolved the capacity to survive and grow inside macrophage phagosomes. A cocktail of antibiotics has long been successfully used against M. tuberculosis but is becoming less effective owing to the emergence of multidrug resistance. The only available preventive vaccine, using Mycobacterium bovis bacille Calmette–Guérin, is considered to be ineffective against adult pulmonary TB, the most prevalent form of the disease. Here, we review the potential use of biodegradable nanoparticle-based anti-TB drug delivery systems that have been shown to be more effective against M. tuberculosis in animal models than conventional antibiotic treatment regimens. This technology also has substantial potential for vaccination and other therapeutic strategies against TB and other infectious diseases.
Until the introduction of antibiotics in the 1940s, tuberculosis (TB), the disease caused by Mycobacterium tuberculosis, was a feared scourge that caused hundreds of millions of human deaths. The availability of antibiotics such as isoniazid (INH) and rifampicin (RIF) led to the widespread hope that the disease could eventually be eradicated. Alas, such optimism proved premature and the disease, which is also referred to as the ‘white plague’ or ‘consumption’, has gradually increased in severity to the point that it is now the bacterial infection that kills the most people worldwide1. A staggering one-third of the world’s population is latently infected and, according to the WHO, ~1.8 million people die every year, with an estimated 9.8 million new infections per year2. This pandemic is being driven by the additional complications of the emergence of multidrug-resistant (MDR) M. tuberculosis strains and the increase in patients with TB who are co-infected with HIV. For the past 80 years, one live attenuated bacterial vaccine based on Mycobacterium bovis bacilli Calmette–Guérin (BCG), which has lost several virulence genes, has been extensively used for the prevention of TB. Although this vaccine, given at birth, seems to offer some protection against childhood TB, especially tuberculous meningitis, a consensus is now emerging that it is not effective against adult TB. Therefore, the development of an improved vaccine is currently an international research priority2,3.
Drug-susceptible TB can be effectively treated with a cocktail of four ‘front-line’ drugs — RIF, INH, pyrazinamide (PZA) and ethambutol (ETB) — given daily for 6–8 months or longer by the oral route (usually, all four drugs are administered for 2 months, followed by INH and RIF for the remaining period). However, the fundamental problem in the treatment of TB is the long duration of therapy required to cure the patient, which can hamper patient lifestyle and induces patient non-compliance, treatment failure and development of drug-resistant strains4. Furthermore, the recalcitrance of M. tuberculosis to eradication by the current anti-TB drugs is thought to result from its ability to achieve a non-replicating state in the host. Because RIF, INH and ETB (but not PZA) require bacterial replication for their action, the non-replicating state is thought to render M. tuberculosis phenotypically resistant to otherwise bactericidal antibiotics3,4.
A few years after the introduction of streptomycin for TB therapy by Selman Waksam in 1944, the first signs of drug resistance were noted, and the same was seen later with RIF and INH. The situation deteriorated further when M. tuberculosis strains resistant to multiple drugs emerged; MDR M. tuberculosis is defined as being resistant to both RIF and INH4,5. Even more worrying now is the emergence of extensively drug-resistant (XDR) strains in many parts of the world. In addition to resistance to RIF and INH, XDR M. tuberculosis strains are resistant to at least one second-line, injectable drug (such as capreomycin, kanamycin or amikacin) and to any fluoroquinolone drug (for example, ciprofloxacin, levofloxacin, moxifloxacin or ofloxacin)4. These drugs are more expensive, inherently more toxic and less efficacious, need a higher dosage and must be used for up to 24 months. The use of second-line drugs is also a move towards broader-spectrum antibiotics, a strategy that can select for resistance among other, coexisting pathogens. The molecular mechanisms used by M. tuberculosis to induce the MDR and XDR state have been discussed recently5.
The fear of the spread of XDR strains and the diminishing arsenal of effective treatment options reinforce the need to develop new, effective anti-TB drugs to overcome the problem of drug resistance and to shorten the treatment course. However, there are currently fewer than ten compounds in clinical development — perhaps too few to guarantee even a single new anti-TB drug in the near future6 (see Further information for the Global Alliance for TB Drug Development). Conversely, current anti-TB drugs are still effective, and strategies allowing more efficient delivery of these drugs are called for. In this context, nanotechnology is one of the most promising approaches for the development of more effective and more compliant drug delivery systems for the treatment of TB. This technology also offers a potentially powerful strategy for the development and delivery of new-generation TB vaccines. Here, we discuss the potential use of biodegradable nanoparticle-based delivery systems for drug therapy and vaccination against TB.
The cell biology and immunology of TB
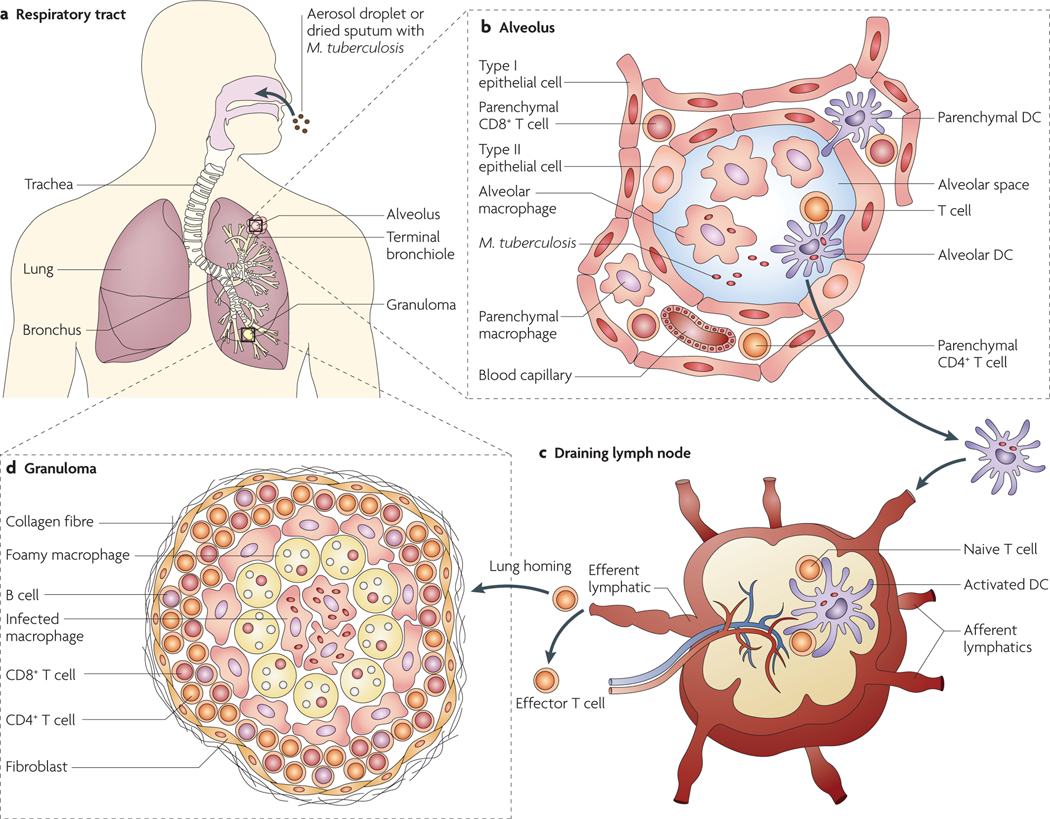
In humans, TB is predominantly a disease of the lungs. M. tuberculosis infection starts with the inhalation of infectious bacteria and their deposition in the alveolar space of the lungs (FIG. 1). The bacteria are taken up by phagocytes in the lung, in particular by alveolar macrophages, and reside within intracellular phagosomes. Many receptors have been implicated in the uptake process, including mannose receptors, Toll-like receptor 2 (TlR2) and TlR4, surfactant protein A receptors, CD14, scavenger receptors, complement receptors and immunoglobulin receptors7.These receptors are all potentially interesting for targeting nanoparticles to TB-infected macrophages (see below). Under ideal conditions, pathogen-enclosing phagosomes fuse sequentially with early and then late endocytic organelles to become bactericidal phagolysosomes. However, M. tuberculosis can prevent phagosome maturation and phagosome–lysosome fusion, thereby avoiding exposure to the lower pH and hydrolytic environment of the phagolysosome8. Several mechanisms have been implicated in the ability of M. tuberculosis to arrest phagosome maturation, but our understanding of this process is far from complete. Macrophages are key effector cells in mycobacterial killing but can also provide a niche for M. tuberculosis multiplication. Dendritic cells (DCs) also engulf bacteria but lack killing ability and may serve as a ‘Trojan Horse’ (REF. 9). DCs engulfing M. tuberculosis migrate to the draining lymph nodes and prime the naive T cells that subsequently return to the lungs to control the infection (FIG. 1).
Figure 1 |. Mycobacterium tuberculosis infection and granuloma formation.
a | Mycobacterium tuberculosis infection starts with the inhalation of bacilli, either as an aerosol droplet generated by the cough of a patient with tuberculosis (TB) or as a dust microparticle of dried sputum, followed by deposition of the bacteria in the lung alveolar space. The lungs, where the major events of pulmonary TB are orchestrated, consist of the conducting airways, which are lined by mucosal tissue, and the lung parenchyma, which surrounds thin-walled alveoli that are specialized for gas exchange. b | The alveoli are lined by type I and type II epithelial cells and are separated by thin walls of interstitium containing pulmonary capillaries. in the alveolar cavity, the main hosts for the bacilli are alveolar macrophages. After initial bacterial multiplication in alveolar macrophages, the bacteria are taken up by dendritic cells (DCs), which carry M. tuberculosis to the draining thoracic lymph nodes70. Alternatively, DCs sampling the alveolar mucosa may carry bacilli to the lung parenchyma, leading to initiation of the local inflammatory foci. c | In the draining lymph nodes, DCs carrying bacilli undergo apoptosis, and the mycobacterial antigenic peptides that are released are presented by the activated lymph node-resident DCs to the specific naive cells through cross-presentation. On antigen presentation, activated T cells proliferate, become Effector T cells (which type depending on the cytokine milieu; for example, single-versus multiple-cytokine-producing polyfunctional helper CD4+ T cell subsets) and leave the lymph node to reach the blood circulation through the efferent lymphatics and the thoracic duct. d | effector T cells originating in the draining lymph nodes home back from the blood through pulmonary capillaries to the site of inflammation under the influence of chemokines and other mediators. extravasations of the mononuclear cells thus initiate the formation of signature ‘tubercle’ structures at the site of the infection, leading to containment of the infection. The classic TB granuloma is made up of a central core of infected macrophages surrounded by epithelioid and foamy macrophages and a peripheral rim of lymphocytes (B cells, CD4+ T cells and CD8+ T cells) in association with a fibrous cuff of extracellular matrix laid by fibroblasts.
These events lead to the formation of the granuloma at the site of infection, resulting in containment of the infection. This balanced status between host and mycobacterium is called ‘latency’, during which clinical signs of disease are absent and the bacteria persist in a non-transmissible form3. However, this balance is disturbed by factors such as malnutrition, immunosuppression, steroid use, anti-tumour necrosis factor (TNF) therapy or HIV infection, causing the bacteria to switch to high metabolic activity and initiate disease3.
It is generally accepted that a cell-mediated immune response involving both CD4+ (helper) and CD8+ (cytotoxic) T cells plays an important part in protection against TB. CD4+ T cells enhance the antibacterial activity of macrophages by releasing cytokines such as interferon-γ (IFNγ) and TNF, whereas CD8+ T cells kill infected macrophages — and, possibly, M. tuberculosis — by releasing cytotoxic mediators such as perforins, granzymes and granulysin9. Despite our improved knowledge of the complex cellular immune responses to M. tuberculosis, the type of immune response that is required to mediate protective immunity, and that should therefore be induced by vaccination, is not fully understood.
Nanobead-based therapies
Over the past 20 years, the potential has been explored for replacing the administration of antibiotics or other drugs in the ‘free’ form with an approach using drugs that are encapsulated in a nanoparticle (<1000 nm; some authors restrict this definition to <100 nm) or microparticle (>1000 nm) made up of a biodegradable polymer, allowing a slower, more sustained release of the drug than with conventional free-drug delivery.
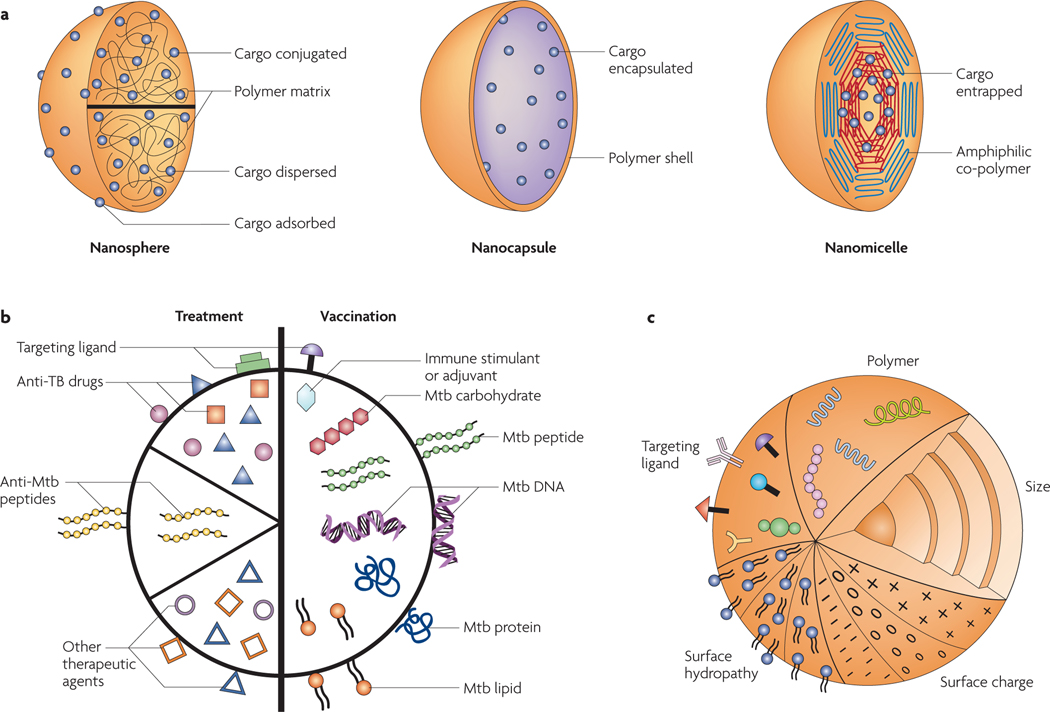
Polymeric nanobeads ( FIG. 2a ) are composed of solid matrices that can have various porosities, depending on the degree of physical or chemical cross-linking of the polymer network, and throughout which the drug molecules are homogeneously distributed. The most extensively used polymer, poly(lactic-co-glycolic acid) (PLGA), is soluble in organic solvents and has been used in many drug release applications10. Alternative water-soluble systems such as alginates or chitosans10 have also been described (see Supplementary information S1 (table)). Nanoparticles can be prepared by intramolecular cross-linking of the polymer chains or by adding a chemical agent. The density and size distribution of the particles can be controlled by manipulating the polymer concentration, the amount of cross-linking agent and the stirring rate of the solution during the preparation of the cross-linked particles.
Figure 2 |. Nanobead properties.
a | Polymeric nanoparticles (schematic transections are shown) are sub-μm colloidal particles. They include: nanospheres, in which the cargo is dissolved, adsorbed or dispersed throughout the matrix, attached to the surface or attached to the polymer matrix; nanocapsules, in which the cargo is in solution and surrounded by a shell-like wall; and nanomicelles, in which amphiphilic co-polymers with hydrophobic and hydrophilic blocks self assemble to entrap the cargo. b | The different types of nanoparticles and microparticles that have been used for tuberculosis (TB) treatment (such as those encapsulating first-line and second-line anti-TB drugs alone or in combination) and for vaccination (such as those encapsulating or adsorbing Mycobacterium tuberculosis (Mtb) immunogenic proteins, peptides and DNA with or without adjuvants), as well as other, potentially useful particles that have not yet been used for TB applications (such as those encapsulating or adsorbing anti-M. tuberculosis peptides, other unconventional drugs and immunostimulants, or immunogenic M. tuberculosis lipids and carbohydrates). c | The main nanoparticle properties that can influence the uptake and efficacy of nanoparticle-based vaccines and therapies. The natural or synthetic polymer used for nanoparticle engineering profoundly affects the characteristics of the particle, such as its biocompatibility and biodegradability, its encapsulation or adsorption efficiency, its internalization or cellular uptake and its release of cargo, as well as affecting its adjuvant and immunological properties and its eventual clearance. The size of a nanoparticle affects its uptake route and its clearance16 and also influences the type of immune response that is induced38,39. Positively charged particles are preferentially taken up by living cells owing to the negative charge of the cellular membrane. surface hydrophobicity also increases nanoparticle uptake, whereas hydrophilicity (resulting from, for example, surface modifications with polyethylene glycol and poloxamer polymers) decreases uptake and phagocytosis, increasing the systemic circulation of the particle. Targeting ligands can also be used to direct nanoparticles to cells of interest. Toll-like receptor ligands, adhesins and antibodies for specific cell surface receptors and molecules have been used to this end10,27,60.
The use of nanoparticles in this way is now considered to be increasingly important in biomedicine; an estimated two dozen nano-based therapeutics are approved for clinical use in cancer therapy (for example, poly-lactide-based particles enclosing taxol are used to treat breast cancer) and for treating infectious diseases (for example, polyethylene glycol (PEG)–interferon-α2a is used to treat hepatitis B and hepatitis C)10,11. Several other nanoparticle formulations are currently being evaluated in clinical or preclinical trials for the treatment of different diseases11,12. With the availability of several cheap, biodegradable natural and synthetic polymers approved for human use, both hydrophobic and hydrophilic molecules (including current anti-TB drugs) can be easily encapsulated in nanoparticles with almost no effect on drug shelf life and efficacy.
Nanoparticles and TB
Some of the most striking data have come from studies developing polymer-based antibiotic therapies against M. tuberculosis in animal models, including mouse, rat, guinea pig, rabbit and monkey models13. The caveat is that none of these models recapitulates all of the features of human TB. Most of these studies have used PLGA in combination with RIF, INH or both. Because of space limitations, we do not discuss alternative delivery systems that have shown promising results in the delivery of antibiotics targeting M. tuberculosis (for a brief summary, see BOX 1 and Supplementary information S2 (table)), such as liposomes14 and solid–lipid nanoparticles15; in general, these particles are less stable than their polymer-based counterparts.
Box 1 |. Alternative delivery systems for the treatment of tuberculosis.
During the past decade, liposomes have been extensively evaluated as a drug delivery system for the treatment of tuberculosis (TB) in animal models13,14 and have been approved for human use to treat fungal infections and breast cancer using amphotericin B and doxorubicin, respectively11,12. Liposomes are spherical vesicles with a bi-layered membrane composed of natural or synthetic amphiphilic lipid molecules. They can be coated with polymers for stabilization of the structure and to prolong circulation half-life, or functionalized with specific ligands for targeted cell or organ delivery. Their unique ability to encapsulate both hydrophobic and hydrophilic drugs makes them excellent as therapeutic carriers. However, liposomes are suitable for administration by limited routes (for example, intravenous injection and inhalation). Another class of polymeric substances that has attracted a great deal of interest for drug delivery applications is dendrimers68, although only one study has been reported for a TB application69. Dendrimers are highly branched, globular macromolecules with many arms emanating from a central core. Dendrimers have a very strong potential for anti-TB drug delivery and other applications, because their structure makes them suited for use in multivalent systems. In other words, one dendrimer molecule has hundreds of possible sites to couple to an active species. However, the production cost can be high. Alternative experimental delivery systems for drug delivery and other applications, such as fullerenes (for example, carbon spheres, or ‘buckyballs’, and carbon nanotubes) and metallic nanoshells (for example, gold nanoshells), are under development and have not yet been reported as having a TB application.
The basic idea is that the polymer provides a protective coat for the drug after its administration through injection or, more preferably, through oral or aerosol routes (FIG. 2a,b). After oral or aerosol administration, the particles bind to the apical surfaces of epithelial cells (for example, microfold (M) cells) and are actively transported across the epithelial layer by transcytosis before being taken up by phagocytic cells, such as macrophages. This is an attractive scenario for treating TB, because macrophages are the main cell type to harbour the bacteria, especially in the lungs. These cells can phagocytose any kind of particle in a certain size range (usually ~200 nm and up to ~10 μm)16, a property that is dependent on the broad range of ‘nonspecific’ receptors that are present on their surface17. Macrophages containing M. tuberculosis in a specialized phagosome can subsequently phagocytose an antibiotic-containing bead18,19, which will most likely be targeted to a distinct phagolysosome, such that the beads and the M. tuberculosis do not colocalize20. Once inside the phagolysosome, the bead polymer is degraded21, albeit slowly, and its contents are released in a sustained manner over several days, first locally, to kill the intramacrophage pathogens, and then systemically into the blood22. PLGA beads can be degraded non-enzymatically through ester hydrolysis in aqueous solutions, but they are degraded more rapidly in the low pH of the phagolysosome23.
In vitro experiments using macrophages show that the delivery of antibiotics such as RIF inside nanobeads leads to a substantial increase in the drug concentration inside the cells relative to the concentration outside (up to 20-fold) and relative to that observed when the drug is added in the free form13,20,24,25. Obviously, for a pathogen that lives inside the macrophage, such as M. tuberculosis, this is a crucial issue. A sustained and increased concentration of first-line drugs inside the cells harbouring M. tuberculosis has the potential to prevent the development of drug-resistant strains. In studies using PLGA beads, both in aqueous solution in the test tube and in macrophages, a burst of drug release (up to 40%) occurs in the first few hours followed by a slower, sustained release over the subsequent days. This rapid burst is considered to be due to drug adsorbed on the bead surface26.
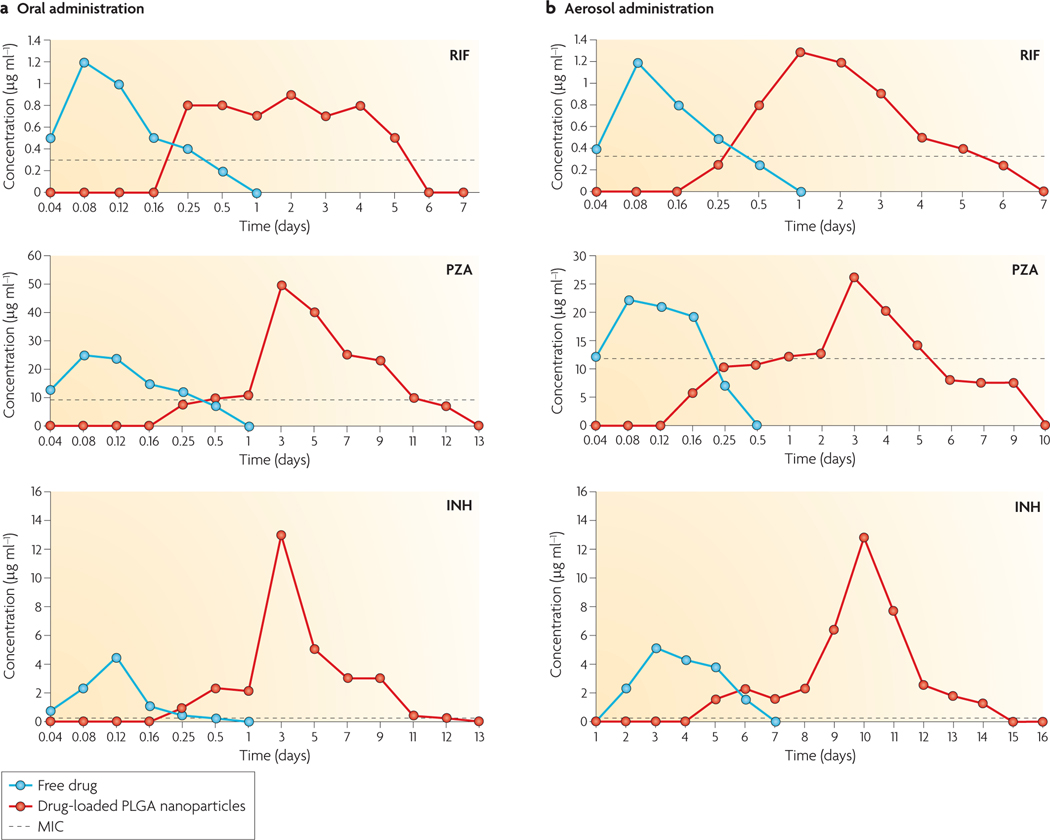
The concept of slow and sustained release from a biodegradable particle is a crucial aspect of nanobead delivery. When freely administered antibiotics are given orally or through inhalation into the lungs of M. tuberculosis-infected guinea pigs, the drugs reach a high concentration in the plasma in hours but are then rapidly degraded or excreted ( FIG. 3). By contrast, when the same overall dose of antibiotic is delivered through a single administration of PLGA beads by either delivery route, the plasma levels of the antibiotics remain above the minimum inhibitory concentration for up to 12 days27 ( FIG. 3). In a striking example using RIF and INH co-encapsulated into PLGA beads, only three oral applications of the beads gave the same therapeutic protection against M. tuberculosis in guinea pigs as 45 daily doses of the free antibiotics27. Similar data were shown using mice28. Thus, this technology has the potential to substantially reduce the dosing frequency and the ‘pill burden’ for patients with TB, leading to improved patient compliance, which would improve the treatment success rate and reduce the development of drug resistance.
Figure 3 |. The principle of slow drug release with nanoparticles.
The kinetics of the accumulation of rifampicin (RIF), pyrazinamide (PZA) and isoniazid (INH) in the sera of guinea pigs after drug administration in the free form or using drug-enclosed poly-(lactic-co-glycolic acid) (PLGA) nanoparticles. a | Drugs administered through the oral route. b | Drug administration through the aerosol (lung) route. Note the substantial extension of the elevated drug concentration in the plasma after the administration of drugs using the nanoparticle system. These levels are significantly higher than the minimum inhibitory concentration (MIC) of the antibiotics when used to treat Mycobacterium tuberculosis. Data from REF. 27.
Several second-line antibiotics have also been successfully administered against M. tuberculosis through the nanobead approach, including moxifloxacin20 and capreomycin29. Also worth mentioning are studies showing that anti-M. tuberculosis drugs that cannot pass into the blood system when taken orally, such as streptomycin and econazole, can have effective therapeutic properties when delivered through PLGA or alginate beads, respectively30,31. These observations provide strong indirect evidence that the beads can cross the gut epithelial barrier, in agreement with the direct evidence from electron microscopy studies32. Furthermore, encapsulating the more toxic, second-line anti-M. tuberculosis drugs can substantially reduce overall systemic toxicity10, owing to their slow release. In general, the overall lethal dose of antibiotics in beads is many-fold lower than that for free drugs.
Work in this field is pushing towards the application of these promising therapeutic tools for use against human TB. Phase I clinical trials have been initiated at the Postgraduate Institute of Medical Education and Research (PGIMER), Chandigarh, India, using PLGA nanoparticles encapsulating first-line anti-TB drugs. PLGA has long been approved by the US Food and Drug Administration for human use for implants and sutures. Nevertheless, there is some concern that the organic solvents used to dissolve this polymer may have undesirable side effects, especially for vaccination trials (see below). For this reason, alternative, water-soluble polymers may be preferred, and alginate nanoparticles enclosing antibiotics have also been used successfully against M. tuberculosis through oral and aerosol administration in mice and guinea pigs33.
Polymers can be formulated by well-established recipes to make particles of different sizes; for example, PLGA beads can be made in sizes from 10 nm to many μm34. Most studies have reached the conclusion that 200–1000 nm particles are more effective for drug delivery than smaller or larger particles. However, the best results using different kinds of polymers and different animal models of M. tuberculosis have been achieved with particles of between 200 nm and 400 nm22. Earlier studies showed that some particles up to 10 μm in size can transverse epithelial cells and reach the underlying Peyer’s patches to access phagocytic and immune cells32. However, there is no consensus on the upper size limit35.
Nanoparticles in TB vaccine delivery
Given the lack of success of the M. bovis BCG vaccine, efforts are ongoing to develop more effective vaccines using a range of strategies36 (see Further information for details on the vaccine pipeline).The development of nanoparticle- and microparticle-based delivery systems for new-generation TB vaccines is an exciting emerging field, using proteins, peptides or DNA that are protected by encapsulation in the particles ( FIG. 2b); these particles can be administered by different mucosal and systemic routes. This process can also serve as a depot for the slow release of antigens, leading to a prolonged immune response10. In principle, multiple antigens, such as different stage-specific antigens of M. tuberculosis37, can be co-encapsulated with or without immune stimulants or adjuvants. The bead size has been shown to influence the type of immune response induced ( FIG. 2c), and nanoparticle vaccines can be formulated to induce cellular and/or humoral immunity, activating CD8+ and/or CD4+ T cells for improved efficacy38,39. One challenging problem is the delivery of exogenous protein subunit vaccine candidates to major histocompatibility complex (MHC) class I molecules to induce the cytotoxic T cell response, and nanoparticles using endosomal escape strategies have been engineered for this purpose40,41.
For subunit vaccine delivery, the idea of using a water-insoluble polymer such as PLGA, in which the antigen must be exposed to a harsh, probably denaturing solvent, is theoretically less attractive. Nevertheless, different M. tuberculosis protein or peptide subunit vaccine candidates have been evaluated in animal models using PLGA microparticle-based delivery systems through parenteral (injection) or respiratory routes, and these vaccines induced strong B cell and T cell responses42–46. In one study, mice that were immunized parenterally with the M. tuberculosis cell wall 71 kDa protein carried in PLGA microparticles exhibited a robust clearance of bacteria from the lungs and liver after challenge42. In another mouse study, immunization by the intranasal route using PLGA microparticles containing early secretory antigen target 6 (ESAT6) induced a strong immunogen-specific effector and memory T cell response in the lungs and thoracic lymph nodes44. Similarly, PLGA microparticles have been shown to enhance the in vitro T cell response to a TB10.4 (ESAT6 protein family member; also known as EsxH)–antigen 85B (Ag85B) fusion protein after aerosol adminstration46. Recently, an aerosol subunit vaccine using Ag85Bcontaining PLGA microparticles gave promising results in a mouse model47. Aerosol boost with this vaccine in M. bovis BCG-primed mice imparted protection against M. tuberculosis challenge and reduced the number and size of granulomas in the lungs47. In general, the aerosol route is especially attractive for nanoparticle delivery for TB vaccination and therapy, although microparticles have often been more effective than nanoparticles when delivered by this route48.
A few groups have attempted to formulate nanobead or microbead DNA vaccines against M. tuberculosis. An earlier study in mice showed promising vaccination using fbpA DNA (which encodes Ag85A) adsorbed to cationic PLGA microparticles49, and anti-mycobacterial immunity was also induced by a single injection of a Mycobacterium leprae heat shock protein 65-encoding plasmid in biodegradable PLGA microbeads50. A chitosan–DNA nanoparticle vaccine encoding human T cell epitopes of six M. tuberculosis proteins was evaluated in transgenic mice51, and in a more recent study, a DNA vaccine encoding latency antigen Rv1733c associated with PLGA–polyethyleneimine nanoparticles was evaluated using a DNA prime–protein boost vaccination regimen in a mouse model52. Both of these nanoparticle-based DNA vaccines induced DC maturation and robust T cell responses after aerosol delivery. Evidently, degradation of the DNA by nucleases before it reached the target cells was not a substantial problem in these studies.
An alternative, innovative approach was developed using genes of interest loaded into nanoparticles that are then attached to the surface of attenuated bacteria53. This approach could theoretically be used to target TB granulomas, as super-infecting M. tuberculosis bacilli have been shown to enter pre-existing granulomas in the zebrafish and mouse models of TB54. These ‘microbots’ (miniature robots) using attenuated mycobacterial strains attached to nanoparticles encapsulating TB vaccines, therapeutics or immune stimulants could be developed for granuloma-specific delivery. Attempts have also been made to adsorb M. bovis BCG onto nanoparticles55 or to encapsulate it in microparticles56 for improved delivery and efficacy. In a guinea pig model, aerosol delivery of M. bovis BCG adsorbed on leucine nanoparticles imparted much better protection against M. tuberculosis challenge than parenteral immunization55,57, whereas in mice oral delivery of M. bovis BCG encapsulated in alginate microparticles induced a stronger T cell response and greater protection than oral vaccination with free M. bovis BCG56.
The addition of specific ligands to the surface of nanobeads and microbeads is an attractive option to facilitate vaccination ( FIG. 2b,c ); for example, M. tuberculosis surface adhesin could enable a targeted delivery of TB vaccines to lung mucosa. Nanoparticles can be alternatively formulated into hollow, low-density, dried particles called porous nanoparticle-aggregate particles for effective delivery of TB vaccines or therapeutics to the lungs58, and recombinant viral vector-based TB vaccines can be improved into ‘smart’ nanoparticulate vaccines59. A nanoparticulate TB vaccine intended for intranasal administration would target nasal mucosa and nasal-associated lymphoid tissue (NALT), whereas a vaccine intended for oral vaccination would target gut-associated lymphoid tissue (GALT), including the Peyer’s patches, where M cell-, epithelial cell- and DC-targeting strategies could be used60.
Other potential uses in TB therapy
Several antimicrobial peptides have been shown to have bactericidal effects against M. tuberculosis in vitro and/or in vivo. These include ll37 (the 37-amino acid polypeptide cleaved from the precursor cathelicidin antimicrobial peptide)61,62, defensins and human neutrophil peptide 1 (HNP1; also known as neutrophil defensin 1)63. MDR M. tuberculosis strains are generally less fit than non-MDR M. tuberculosis strains, and they are more susceptible to killing by peptides derived from granulysin64. However, MDR M. tuberculosis strains do not necessarily need to lose fitness65. In addition to incorporating anti-M. tuberculosis peptides inside nanobeads, it may be even more interesting to test the cDNAs encoding these peptides, as well as the signal peptide sequence that is needed to direct the newly synthesized protein into the endoplasmic reticulum.
It has been shown that phenothiazines, especially thioridiazine, have interesting therapeutic effects against M. tuberculosis66. Although the molecular effects of thioridiazine and other phenothiazines are not clearly-defined, they seem to block the efflux pumps of mycobacteria and other bacteria; they show bacteriostatic effects at low concentrations and bactericidal effects at high concentrations against both M. tuberculosis and MDR M. tuberculosis. Moreover, they are somehow concentrated (up to 100-fold) in macrophages, and TZ shows impressive killing of M. tuberculosis and MDR M. tuberculosis in macrophages66. For such drugs, slow-release nanoparticles offer an attractive alternative, especially in combination with more conventional classes of antibiotics.
Finally, another idea would be to use nanobeads to enclose drugs that enhance the innate immune mechanisms of macrophages against M. tuberculosis. For example, when protein kinase B (PKB; also known as AKT1) is inhibited, M. tuberculosis (and Salmonella enterica subsp. enterica serovar Typhimurium) loses the ability to arrest phagosome–lysosome fusion, and the bacteria are effectively killed67. Using nanobeads to selectively target inhibitors of such kinases to infected macrophages is an attractive option to consider. Such drugs could also be combined with more traditional antibiotic therapy.
Conclusions and future directions
Nanobead and microbead technology has enormous potential for the different strategies that must be developed to both prevent and treat TB and other diseases in humans. The results summarized here for antibiotic delivery using nanoparticles make us optimistic that the nanoparticle approach will provide a substantial advantage over conventional therapy for human TB, owing to its enormous potential to reduce the ‘pill burden’ and improve patient compliance. This sustained delivery system can also be used to administer new TB drugs as they become available, to treat latent and active disease and to shorten the treatment course. Future efforts can also be focused on combining imaging agents and therapeutic agents in the same particle to revolutionize the treatment of TB. The technology is now being prepared for the transition from bench to clinic, with the initiation of Phase I therapeutic clinical trials in India. The use of nanoparticles and microparticles is one of many ‘promising’ approaches being followed at present for improving vaccination against TB; in this context, it is much more difficult to predict which approach (if any) will be successful in protecting the human population against the white plague.
Supplementary Material
Acknowledgements
We thank A. Maleki, M. Gutierrez, A. Haas, B. Plikaytis and J. Posey for critically evaluating this Review, and J. Husley and the creative services of the CDC for help with figures.
Footnotes
Competing interests statement
The authors declare no competing financial interests.
FURTHER INFORMATION
Global Alliance for TB Drug Development: http://www.tballiance.org/home/home.php
Stop TB Partnership tuberculosis vaccine candidates – 2009: http://www.stoptb.org/wg/new_vaccines/assets/documents/TB_vaccine_Pipeline_2009.pdf
Contributor Information
Gareth Griffiths, Department of Molecular Biosciences, University of Oslo, 0316 Oslo, Norway..
Bo Nyström, Department of Chemistry, University of Oslo, 0315 Oslo, Norway..
Suraj B. Sable, Division of Tuberculosis, Elimination, National Center for HIV/AIDS, Viral Hepatitis, STD, and TB Prevention, Centers for Disease Control and Prevention, 1600 Clifton Road, Atlanta, Georgia 30333, USA.
Gopal K. Khuller, Department of Biochemistry, Postgraduate Institute of Medical Education & Research, 160 012, Chandigarh, India.
References
- 1.Young D, Perkins M, Duncan K. & Barry CE 3rd. Confronting the scientific obstacles to global control of tuberculosis. J. Clin. Invest 118, 1255–1265 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dye C. & Williams BG The population dynamics and control of tuberculosis. Science 328, 856–861 (2010). [DOI] [PubMed] [Google Scholar]
- 3.Russell DG, Barry CE 3rd & Flynn JL Tuberculosis: what we don’t know can, and does, hurt us. Science 328, 852–856 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chan ED & Iseman MD Multidrug-resistant and extensively drug-resistant tuberculosis: a review. Curr. Opin. Infect. Dis 21, 587–595 (2008). [DOI] [PubMed] [Google Scholar]
- 5.Zhang Y. & Yew WW Mechanisms of drug resistance in Mycobacterium tuberculosis. Int. J. Tuberc. Lung Dis 13, 1320–1330 (2009). [PubMed] [Google Scholar]
- 6.Dye C. Doomsday postponed? Preventing and reversing epidemics of drug-resistant tuberculosis. Nature Rev. Microbiol 7, 81–87 (2009). [DOI] [PubMed] [Google Scholar]
- 7.Bhatt K. & Salgame P. Host innate immune response to Mycobacterium tuberculosis. J. Clin. Immunol 27, 347–362 (2007). [DOI] [PubMed] [Google Scholar]
- 8.Vergne I, Chua J, Singh SB & Deretic V. Cell biology of Mycobacterium tuberculosis phagosome. Annu. Rev. Cell Dev. Biol 20, 367–394 (2004). [DOI] [PubMed] [Google Scholar]
- 9.Cooper AM Cell-mediated immune responses in tuberculosis. Annu. Rev. Immunol 27, 393–422 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Couvreur P. & Vauthier C. Nanotechnology: intelligent design to treat complex disease. Pharm. Res 23, 1417–1450 (2006). [DOI] [PubMed] [Google Scholar]
- 11.Zhang L. et al. Nanoparticles in medicine: therapeutic applications and developments. Clin. Pharmacol. Ther 83, 761–769 (2008). [DOI] [PubMed] [Google Scholar]
- 12.Davis ME, Chen ZG & Shin DM Nanoparticle therapeutics: an emerging treatment modality for cancer. Nature Rev. Drug Discov 7, 771–782 (2008). [DOI] [PubMed] [Google Scholar]
- 13.Sosnik A, Carcaboso AM, Glisoni RJ, Moretton MA & Chiappetta DA New old challenges in tuberculosis: potentially effective nanotechnologies in drug delivery. Adv. Drug Deliv. Rev 62, 547–559 (2010). [DOI] [PubMed] [Google Scholar]
- 14.Khuller GK, Kapur M. & Sharma S. Liposome technology for drug delivery against mycobacterial infections. Curr. Pharm. Des 10, 3263–3274 (2004). [DOI] [PubMed] [Google Scholar]
- 15.Pandey R. & Khuller GK Solid lipid particle-based inhalable sustained drug delivery system against experimental tuberculosis. Tuberculosis 85, 227–234 (2005). [DOI] [PubMed] [Google Scholar]
- 16.Desjardins M. & Griffiths G. Phagocytosis: latex leads the way. Curr. Opin. Cell Biol 15, 498–503 (2003). [DOI] [PubMed] [Google Scholar]
- 17.Areschoug T. & Gordon S. Scavenger receptors: role in innate immunity and microbial pathogenesis. Cell. Microbiol 11, 1160–1169 (2009). [DOI] [PubMed] [Google Scholar]
- 18.Yoshida A. et al. Selective delivery of rifampicin incorporated into poly(dl-lactic-co-glycolic) acid microspheres after phagocytotic uptake by alveolar macrophages, and the killing effect against intracellular Mycobacterium bovis Calmette–Guérin. Microbes Infect. 8, 2481–2491 (2006). [DOI] [PubMed] [Google Scholar]
- 19.Hirota K. et al. Delivery of rifampicin–PLGA microspheres into alveolar macrophages is promising for treatment of tuberculosis. J. Control. Release 142, 339–346 (2010). [DOI] [PubMed] [Google Scholar]
- 20.Kisich KO et al. Encapsulation of moxifloxacin within poly(butyl cyanoacrylate) nanoparticles enhances efficacy against intracellular Mycobacterium tuberculosis. Int. J. Pharm 345, 154–162 (2007). [DOI] [PubMed] [Google Scholar]
- 21.Onoshita T. et al. The behavior of PLGA microspheres containing rifampicin in alveolar macrophages. Colloids Surf. B Biointerfaces 76, 151–157 (2010). [DOI] [PubMed] [Google Scholar]
- 22.Pandey R. & Khuller GK Polymer based drug delivery systems for mycobacterial infections. Curr. Drug Deliv 1, 195–201 (2004). [DOI] [PubMed] [Google Scholar]
- 23.Shive MS & Anderson JM Biodegradation and biocompatibility of PLA and PLGA microspheres. Adv. Drug Deliv. Rev 28, 5–24 (1997). [DOI] [PubMed] [Google Scholar]
- 24.Anisimova YV, Gelperina SI, Peloquin CA & Heifets LB Nanoparticles as antituberculosis drugs carriers: effect on activity against Mycobacterium tuberculosis in human monocyte-derived macrophages. J. Nanopart. Res 2, 165–171 (2000). [Google Scholar]
- 25.Muttil P. et al. Inhalable microparticles containing large payload of anti-tuberculosis drugs. Eur. J. Pharm. Sci 32, 140–150 (2007). [DOI] [PubMed] [Google Scholar]
- 26.Singh R. & Lillard JW Jr. Nanoparticle-based targeted drug delivery. Exp. Mol. Pathol 86, 215–223 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sharma A, Sharma S. & Khuller GK Lectinfunctionalized poly (lactide-co-glycolide) nanoparticles as oral/aerosolized antitubercular drug carriers for treatment of tuberculosis. J. Antimicrob. Chemother 54, 761–766 (2004). [DOI] [PubMed] [Google Scholar]
- 28.Pandey R. et al. Poly (dl-lactide-co-glycolide) nanoparticle-based inhalable sustained drug delivery system for experimental tuberculosis. J. Antimicrob. Chemother 52, 981–986 (2003). [DOI] [PubMed] [Google Scholar]
- 29.Garcia-Contreras L. et al. Inhaled large porous particles of capreomycin for treatment of tuberculosis in a guinea pig model. Antimicrob. Agents Chemother 51, 2830–2836 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ahmad Z, Sharma S. & Khuller GK The potential of azole antifungals against latent/persistent tuberculosis. FEMS Microbiol. Lett 258, 200–203 (2006). [DOI] [PubMed] [Google Scholar]
- 31.Pandey R. & Khuller GK Nanoparticle-based oral drug delivery system for an injectable antibiotic – streptomycin. Evaluation in a murine tuberculosis model. Chemotherapy 53, 437–441 (2007). [DOI] [PubMed] [Google Scholar]
- 32.Hussain N, Jaitley V. & Florence AT Recent advances in the understanding of uptake of microparticulates across the gastrointestinal lymphatics. Adv. Drug Deliv. Rev 50, 107–142 (2001). [DOI] [PubMed] [Google Scholar]
- 33.Ahmad Z. & Khuller GK Alginate-based sustained release drug delivery systems for tuberculosis. Expert Opin. Drug Deliv 5, 1323–1334 (2008). [DOI] [PubMed] [Google Scholar]
- 34.Jain RA The manufacturing techniques of various drug loaded biodegradable poly(lactide-co-glycolide) (PLGA) devices. Biomaterials 21, 2475–2490 (2000). [DOI] [PubMed] [Google Scholar]
- 35.Gaumet M, Gurny R. & Delie F. Localization and quantification of biodegradable particles in an intestinal cell model: the influence of particle size. Eur. J. Pharm. Sci 36, 465–473 (2009). [DOI] [PubMed] [Google Scholar]
- 36.Skeiky YA & Sadoff JC Advances in tuberculosis vaccine strategies. Nature Rev. Microbiol 4, 469–476 (2006). [DOI] [PubMed] [Google Scholar]
- 37.Andersen P. Vaccine strategies against latent tuberculosis infection. Trends Microbiol. 15, 7–13 (2007). [DOI] [PubMed] [Google Scholar]
- 38.Fifis T. et al. Size-dependent immunogenicity: therapeutic and protective properties of nano-vaccines against tumors. J. Immunol 173, 3148–3154 (2004). [DOI] [PubMed] [Google Scholar]
- 39.Xiang SD et al. Pathogen recognition and development of particulate vaccines: does size matter? Methods 40, 1–9 (2006). [DOI] [PubMed] [Google Scholar]
- 40.Hu Y. et al. Cytosolic delivery of membraneimpermeable molecules in dendritic cells using pH-responsive core-shell nanoparticles. Nano Lett. 7, 3056–3064 (2007). [DOI] [PubMed] [Google Scholar]
- 41.Verma A. et al. Surface-structure-regulated cellmembrane penetration by monolayer-protected nanoparticles. Nature Mater. 7, 588–595 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dhiman N. & Khuller GK Protective efficacy of mycobacterial 71-kDa cell wall associated protein using poly (dl-lactide-co-glycolide) microparticles as carrier vehicles. FEMS Immunol. Med. Microbiol 21, 19–28 (1998). [DOI] [PubMed] [Google Scholar]
- 43.Venkataprasad N. et al. Induction of cellular immunity to a mycobacterial antigen adsorbed on lamellar particles of lactide polymers. Vaccine 17, 1814–1819 (1999). [DOI] [PubMed] [Google Scholar]
- 44.Carpenter ZK, Williamson ED & Eyles JE Mucosal delivery of microparticle encapsulated ESAT-6 induces robust cell-mediated responses in the lung milieu. J. Control. Release 104, 67–77 (2005). [DOI] [PubMed] [Google Scholar]
- 45.Kirby DJ et al. PLGA microspheres for the delivery of a novel subunit TB vaccine. J. Drug Target 16, 282–293 (2008). [DOI] [PubMed] [Google Scholar]
- 46.Shi S. & Hickey AJ PLGA microparticles in respirable sizes enhance an in vitro T cell response to recombinant Mycobacterium tuberculosis antigen TB10.4-Ag85B. Pharm. Res 27, 350–360 (2010). [DOI] [PubMed] [Google Scholar]
- 47.Lu D. et al. Pulmonary immunization using antigen 85-B polymeric microparticles to boost tuberculosis immunity. AAPS J. 12, 338–347 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Muttil P, Wang C. & Hickey AJ Inhaled drug delivery for tuberculosis therapy. Pharm. Res 26, 2401–2416 (2009). [DOI] [PubMed] [Google Scholar]
- 49.Mollenkopf HJ et al. Enhanced protective efficacy of a tuberculosis DNA vaccine by adsorption onto cationic PLG microparticles. Vaccine 22, 2690–2695 (2004). [DOI] [PubMed] [Google Scholar]
- 50.Johansen P. et al. Anti-mycobacterial immunity induced by a single injection of M. leprae Hsp65-encoding plasmid DNA in biodegradable microparticles. Immunol. Lett 90, 81–85 (2003). [DOI] [PubMed] [Google Scholar]
- 51.Bivas-Benita M. et al. Pulmonary delivery of chitosanDNA nanoparticles enhances the immunogenicity of a DNA vaccine encoding HLA-A*0201-restricted T-cell epitopes of Mycobacterium tuberculosis. Vaccine 22, 1609–1615 (2004). [DOI] [PubMed] [Google Scholar]
- 52.Bivas-Benita M. et al. Pulmonary delivery of DNA encoding Mycobacterium tuberculosis latency antigen Rv1733c associated to PLGA–PEI nanoparticles enhances T cell responses in a DNA prime/protein boost vaccination regimen in mice. Vaccine 27, 4010–4017 (2009). [DOI] [PubMed] [Google Scholar]
- 53.Akin D. et al. Bacteria-mediated delivery of nanoparticles and cargo into cells. Nature Nanotech. 2, 441–449 (2007). [DOI] [PubMed] [Google Scholar]
- 54.Cosma CL, Humbert O, Sherman DR & Ramakrishnan L. Trafficking of superinfecting Mycobacterium organisms into established granulomas occurs in mammals and is independent of the Erp and ESX-1 mycobacterial virulence loci. J. Infect. Dis 198, 1851–1855 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Garcia-Contreras L. et al. Immunization by a bacterial aerosol. Proc. Natl Acad. Sci. USA 105, 4656–4660 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ajdary S. et al. Oral administration of BCG encapsulated in alginate microspheres induces strong TH1 response in BALB/c mice. Vaccine 25, 4595–4601 (2007). [DOI] [PubMed] [Google Scholar]
- 57.Wong YL et al. Drying a tuberculosis vaccine without freezing. Proc. Natl Acad. Sci. USA 104, 2591–2595 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sung JC, Pulliam BL & Edwards DA Nanoparticles for drug delivery to the lungs. Trends Biotechnol. 25, 563–570 (2007). [DOI] [PubMed] [Google Scholar]
- 59.Singh R. & Kostarelos K. Designer adenoviruses for nanomedicine and nanodiagnostics. Trends Biotechnol. 27, 220–229 (2009). [DOI] [PubMed] [Google Scholar]
- 60.Reddy ST, Swartz MA & Hubbell JA Targeting dendritic cells with biomaterials: developing the next generation of vaccines. Trends Immunol. 27, 573–579 (2006). [DOI] [PubMed] [Google Scholar]
- 61.Liu PT et al. Toll-like receptor triggering of a vitamin D-mediated human antimicrobial response. Science 311, 1770–1773 (2006). [DOI] [PubMed] [Google Scholar]
- 62.Liu PT, Stenger S, Tang DH & Modlin RL Cutting edge: vitamin D-mediated human antimicrobial activity against Mycobacterium tuberculosis is dependent on the induction of cathelicidin. J. Immunol 179, 2060–2063 (2007). [DOI] [PubMed] [Google Scholar]
- 63.Sharma S, Verma I. & Khuller GK Antibacterial activity of human neutrophil peptide-1 against Mycobacterium tuberculosis H37Rv: in vitro and ex vivo study. Eur. Respir. J 16, 112–117 (2000). [DOI] [PubMed] [Google Scholar]
- 64.Toro JC et al. Enhanced susceptibility of multidrug resistant strains of Mycobacterium tuberculosis to granulysin peptides correlates with a reduced fitness phenotype. Microbes Infect. 8, 1985–1993 (2006). [DOI] [PubMed] [Google Scholar]
- 65.Bottger EC, Springer B, Pletschette M. & Sander P. Fitness of antibiotic-resistant microorganisms and compensatory mutations. Nature Med. 4, 1343–1344 (1998). [DOI] [PubMed] [Google Scholar]
- 66.Amaral L, Martins M. & Viveiros M. Phenothiazines as anti-multi-drug resistant tubercular agents. Infect. Disord. Drug Targets 7, 257–265 (2007). [DOI] [PubMed] [Google Scholar]
- 67.Kuijl C. et al. Intracellular bacterial growth is controlled by a kinase network around PKB/AKT1. Nature 450, 725–730 (2007). [DOI] [PubMed] [Google Scholar]
- 68.Lee CC, MacKay JA, Frechet JM & Szoka FC Designing dendrimers for biological applications. Nature Biotechnol. 23, 1517–1526 (2005). [DOI] [PubMed] [Google Scholar]
- 69.Kumar PV, Agashe H, Dutta T. & Jain NK PEGylated dendritic architecture for development of a prolonged drug delivery system for an antitubercular drug. Curr. Drug Deliv 4, 11–19 (2007). [DOI] [PubMed] [Google Scholar]
- 70.Wolf AJ et al. Initiation of the adaptive immune response to Mycobacterium tuberculosis depends on antigen production in the local lymph node, not the lungs. J. Exp. Med 205, 105–115 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.