Abstract
Breast cancer survival rates have markedly improved. Consequently, survivorship issues have received increased attention. One common sequel of treatment is chemotherapy-induced cognitive impairment (CICI). CICI causes a range of impairments that can have a significant negative impact on quality of life. Knowledge of the prevalence of this condition is required to inform survivorship plans, and ensure adequate resource allocation and support is available for sufferers, hence a systematic review of prevalence data was performed. Medline, Scopus, CINAHL and PSYCHInfo were searched for eligible studies which included prevalence data on CICI, as ascertained though the use of self-report, or neuropsychological tests. Methodological quality of included studies was assessed. Findings were synthesised narratively, with meta-analyses being used to calculate pooled prevalence when impairment was assessed by neuropsychological tests. The review included 52 studies. Time-points considered ranged from the chemotherapy treatment period to greater than 10 years after treatment cessation. Summary prevalence figures (across time-points) using self-report, short cognitive screening tools and neuropsychological test batteries were 44%, 16% and 21–34% respectively (very low GRADE evidence). Synthesised findings demonstrate that 1 in 3 breast cancer survivors may have clinically significant cognitive impairment. Prevalence is higher when self-report based on patient experience is considered. This review highlights a number of study design issues that may have contributed to the low certainty rating of the evidence. Future studies should take a more consistent approach to the criteria used to assess impairment. Larger studies are urgently needed.
Subject terms: Cancer, Cognitive neuroscience
Introduction
In women, breast cancer is the most frequently diagnosed cancer, with an estimated 2.3 million new cases globally per year1. Prognosis has significantly improved over time, with current 5-year survival rates of around 90% and 10-year survival at 80%1. This improvement has likely been brought about largely through the ability to characterise cancer subtypes, enabling development of targeted agents, increased use of personalised medicine and consequently improved treatment success1. As a result, the number of women with breast cancer living beyond a diagnosis has grown significantly, and survivorship issues are receiving more attention, with a particular focus on quality of life issues.
Breast cancer treatment generally uses a multi-modal treatment approach utilising combinations of surgery, chemotherapy, endocrine therapy and radiotherapy, dependant on disease stage and sub-type classification2. However, chemotherapy combined with surgery, especially in advanced stages of the disease, forms the mainstay of therapy. Cognitive impairment is commonly reported in breast cancer patients both during and after cessation of treatment, and is likely triggered by multiple factors, such as endocrine therapy, the cancer itself, stress, and the hormonal changes resulting from menopause, amongst others. However, chemotherapy has been implicated as a significant contributor to this impairment. The resultant condition has been termed chemotherapy-induced cognitive impairment (CICI), cancer treatment cognitive impairment (CTCI) or cancer related cognitive impairment (CRCI) or colloquially, chemofog or chemobrain3,4. Whilst the exact mechanisms for this are unclear, proposed mechanisms include direct neurotoxic injury, a reduction in neurogenesis (new neuron formation), central nervous system white matter abnormalities, and neuroinflammation5. Most of the evidence for these theories comes from the pre-clinical literature5–7, or neuro-imaging studies8,9.
The most common neuropsychological effects that arise in CICI are impairments to visual processing, visual motor function, executive function and attention10. Sufferers typically report side effects with a wide range of severity, from subtle to more severe impairment, which may persist for up to 20 years post-chemotherapy treatment11. Impairments typically manifest as survivors feeling ‘less-sharp’, being unable to recall words, having episodes of metal confusion, and requiring more mental effort to perform everyday activities10,12. As a result, CICI has the potential to significantly impact patient quality of life. Furthermore, it has been suggested that a lack of prior information, coupled with minimal validation and understanding from family and health care providers leads to patients feeling disempowered, and subsequently not receiving the emotional and rehabilitative support they may need13.
The reported prevalence of CICI following treatment for breast cancer is somewhat variable. A commonly cited range from the narrative review of Janelsins et al. 2014 suggests that 12–82% of women will experience impairment as a result of their treatment regime14. This variability may be attributable to a range of patient factors such as age15, IQ16, menopausal status16, and education level17. Alternately, study design factors such as the employment of cross-sectional, versus longitudinal study designs, with the latter able to account for baseline pre-treatment cognitive function, likely influences rates18. Furthermore, in spite of the name, it has been shown that cognitive impairment is often present before chemotherapy treatment has commenced, potentially arising as a result of cancer itself19,20. This may occur via direct effects of the tumour itself, as a result of associated co-morbidities, or due to psychological factors such as worry and fatigue21. Time since treatment, also likely contributes to the variance in prevalence reported. Neuroimaging has demonstrated structural and functional changes in various patient brain regions, which likely correlate with the corresponding changes seen in executive function and memory. However, these alterations also show partial recovery over time22,23, presumably leading to an improvement in symptoms. Finally, prevalence rates may be influenced by the method used to assess cognitive impairment, with the three common methods employed being neuropsychological testing24, short cognitive screening tools25,26 and self-report14.
A preliminary database search for previous systematic reviews on prevalence of CICI was conducted. One recent systematic review was sourced which reports prevalence data for cognitive impairment in breast cancer patients2. This review is limited in scope compared to the current review since the timeline of consideration was the breast cancer treatment period, rather than longer-term follow up points. The review authors also only included longitudinal studies, which utilized objective neuropsychological tests. The earlier systematic review of Hutchinson et al. 20128 compared objective and subjective cognitive impairments in patients with cancer but did not have a specific focus on prevalence rates. The present review differs from these previous studies by focusing specifically on CICI prevalence in patients with breast cancer as determined by self-report and objective test methods, and using meta-analytic methods and reporting guidelines designed for prevalence reviews. In the current review all study designs were eligible, not just longitudinal designs. This broad inclusion does not allow the teasing apart of the contribution of chemotherapy, as opposed to cancer itself, on impairment. However, from the point of view of the patient suffering cognitive difficulties, or health-care providers and policy makers needing evidence-based information, this distinction is of little consequence; external validity of the findings is assured. Understanding the burden of CICI will help inform survivorship strategies, and drive funding priorities for future research and targeted support, such as rehabilitation options. Furthermore, by identifying CICI incidence over the longer term this will indicate the need for longer-term support to inform cancer survivorship care plans.
Review question
The aim of this review was to synthesise the evidence on prevalence rates of cognitive impairment following chemotherapy treatment in breast cancer survivors, taking into consideration factors such as age and time since cessation of treatment.
Methods
The Joanna Briggs Institute guidelines on conducting prevalence reviews27 and the PRISMA guidelines28 were used to guide review performance and reporting. The objectives, inclusion criteria and methods of analysis for this review were specified in advance and documented in a protocol registered on PROSPERO; ref CRD42021228541.
Search strategy
The search strategy aimed to locate published studies in English. An initial search of Medline was undertaken to identify articles on the topic. Keywords used in the titles and abstracts of relevant articles, and the index terms used to describe the articles were used to develop a full search strategy for Medline via Pubmed using MeSH and free text terms. The four databases were searched in December 2020 using the developed search strategies (see supplementary material S1). Key concepts used for searching were “cancer”, “chemotherapy” and “cognitive impairment”. Hand searching of reference lists was performed to identify additional studies with the same selection criteria being applied. Studies published from database inception were eligible for inclusion. Publications were excluded if they were conference abstracts, review articles and grey literature.
Study selection
Following the search, all identified citations were uploaded into EndNote X8.0.1 and duplicates removed. Potentially relevant studies were retrieved in full and their citation details imported into Covidence (Veritas Health Innovation, Melbourne, Australia). Title, abstract and full text screening for assessment against the inclusion criteria for the review (see below) was performed by one reviewer (A.W), with checking of the information performed by a second reviewer (R.P.G).
Eligibility criteria
Population
Females of any age treated with systemic chemotherapeutic agents for any form of breast cancer were considered for inclusion. Patients currently in treatment or that had ceased treatment (remission) were eligible for inclusion. Studies with patients receiving other concurrent drug therapy or with other co-morbidities were eligible for inclusion with noting (see exclusion criteria). Patients of any socioeconomic status or level of education were eligible for inclusion with noting of any differences reported. Patients that were either pre-or post-menopausal were eligible for inclusion with noting.
The following groups of patients were excluded:
patients with previous history of traumatic brain injury, neurodegenerative conditions such as Parkinson's or Alzheimer's or severe depressive symptoms, since these conditions affect cognition either directly or indirectly29.
patients who received palliative care (due to differences in the treatment pathways and treatment regimens)30.
patients with metastatic CNS cancers or who have previously received CNS targeted therapy for other conditions due to the risk of direct effect on cognition as a result of the cancer/treatment31,32.
Intervention
Studies evaluating patients administered chemotherapy for breast cancer treatment were included. This included a range of common chemotherapy regimens using either sole or combination chemotherapy across any number of treatment cycles. Studies evaluating radiation and newer targeted therapies for cancer treatment were excluded. However, patients who received hormone therapy, such as tamoxifen, for prophylactic purposes following the treatment of their cancer, or accompanying local radiation therapy were eligible for inclusion with noting. This represents a deviation from the published protocol since it was discovered on screening that many studies included these patients within their chemotherapy—treatment groups. Given that these treatment strategies are commonly used, this inclusion is justified in enhancing study external validity.
Condition
Studies were included if they were investigating cognitive impairment arising as a result of interventions administered for cancer as described above. This condition may be variably described as ‘chemobrain’, ‘chemofog’, chemotherapy-induced cognitive impairment (CICI), or cancer-related cognitive impairment (CRCI). Studies where cognitive decline was indicated by any one of several commonly used modalities were eligible for inclusion. These measurement modalities included self-reported measures, imaging, or objective neuropsychological testing33.
Context and study design
Studies from any country or geographic region were eligible for inclusion with noting of location. Patients from community or clinic settings were eligible for inclusion. All study designs were eligible for inclusion. Studies that selected participants into groups based on report of cognitive decline were excluded due to the risk of sampling bias limiting the generalisability of the results.
Data extraction
Data were extracted from the included studies by one reviewer (A.W), with checking performed on all studies by a second reviewer using a modified extraction template in Covidence (supplementary material S2). The template was piloted on three studies to confirm that all required information was being considered. Key data extracted included the population sampled, chemotherapy and other treatment administered, timeframe since chemotherapy treatment or remission, identified confounding factors, method of cognitive testing and criteria for classifying impairment. Prevalence (%) was extracted or calculated (using numerator/denominator) from the available data. Where necessary, data were extracted from figures using GetData Graph Digitiser (version 2.26, S. Federov, Moscow, Russia). Prevalence estimates reported by sub-group, such as cognitive domain affected, age, or treatment were also recorded.
Assessment of methodological quality
Articles included in the study were assessed for methodological quality by one reviewer (AW), with confirmation provided by a second reviewer, using the JBI critical appraisal checklist and guidance notes developed for prevalence reviews (supplementary material S3)34. A study was deemed to be of high quality if it scored greater than 70%, moderate quality with scores between 50 and 70% and low quality when scoring below 50%, as described in Dijkshoorn et al. 20212. Summary graphs were created in Review Manager (RevMan) ([Computer program], Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014).
Data synthesis
An initial descriptive analysis of the studies was undertaken by tabulating the study characteristics and comparing against the planned sub-groups of method of cognitive assessment and timepoint following therapy cessation. Synthesis was undertaken narratively where there was heterogeneity in these study characteristics. Studies were grouped for synthesis based on time point of cognitive assessment. Basic statistical analysis was performed using Megastat Excel Add-In (version 10.3, McGraw-Hill Higher Education, New York, NY).
A meta-analysis of the prevalence rates of cognitive impairment was conducted for studies that utilised objective neuropsychological tests. Many of the included studies contained repeated observations where the unit of interest was the individual rather than observation. Consequently, these cannot be combined in a standard meta-analysis, with time-point as sub-group, since they introduce a unit-of-analysis error35. A number of approaches to perform meta-analyses in this situation have been proposed36. For this review, an All Time-points Meta-analysis (ATM) method was selected with studies at the time-points of interest being analysed separately, with a qualitative comparison being made with estimates at other time-points. Analysis was performed using the MetaXL (www.epigear.com) add-in for Microsoft Excel. A pooled prevalence figure was calculated with 95% CI. Overall estimates were calculated with random effects models employing the DerSimonian & Laird method as a between‐study variance estimator. Cochran's Q (verifying the presence of heterogeneity) and the I2 statistic (amount of heterogeneity) were calculated. A quality model37 was run as part of the meta-analysis where the weight of each study was calculated based on the previously calculated quality score. An I2 > 70% was considered substantial and these pooled data were subject to sensitivity analyses.
In a meta-analysis of prevalence, if the estimates for a study get closer to the limits of zero or one, the weight may be overestimated in the meta-analysis. Consequently, it has been recommended to transform the prevalence to a variable not constrained to the 0–1 range with an approximately normal distribution38. The double arcsine transformation is usually preferred38 and was applied here. For final presentation, the pooled transformed proportions and corresponding CIs were back transformed.
Publication bias was examined using doi plots and the Luis Furuya-Kanamori asymmetry index (LFK index) in MetaXL. The doi plot is suggested to be more sensitive than the funnel plot, particularly when small numbers of studies are involved39.
Potential influences on prevalence estimates were investigated using subgroup analyses and meta-regression. The criteria for performing a meta-regression were derived from Cochrane guidance35. Meta-regression was performed using Comprehensive Meta-Analysis (Version 3, Borenstein, M., Hedges, L., Higgins, J., and Rothstein, H. Biostat, Englewood, NJ 2013). Age, cognitive impairment criteria stringency, sample size, methodological quality and geographical location were identified a priori as potential sources of variation in the prevalence estimates.
Results
Search results
A total of 3010 titles were identified from the electronic searches, with 5 identified from reference lists (Fig. 1). Following removal of duplicates 2194 titles remained. After title screening, 127 records remained. A further 43 records were excluded after abstract examination. Eighty-nine full-text articles were screened. Fifty-two of these met the eligibility criteria for the review, with thirty-seven being excluded at this stage (refer to supplementary material S4).
Figure 1.
The PRISMA28 flow diagram for the systematic review detailing the databases searched, the number of abstracts screened and the full texts retrieved.
Included studies
The 52 included studies contributed 84 prevalence estimates across a series of time points in relation to chemotherapy treatment. It should be noted that the majority of studies did not seek to determine prevalence as a primary aim of the study, with the data being presented as one of a number of outcomes reported. Three studies did however have a focus on prevalence as expounded in their study aims40–42. The majority of studies adopted an observational study design (48 studies, 92%). The remainder were randomised controlled trials (RCT). The studies that employed RCT designs were evaluations where the primary study question related to differences between different chemotherapy regimes or alternate therapeutic strategies and there was no selection into groups based on presence of cognitive impairment (an exclusion criterion for this review). Out of the studies that were observational in nature, 25 (52%) were cohort designs, 20 (42%) cross-sectional and 13 (27%) prospective longitudinal. Sample sizes were generally small with a range from 20 to 1147. Studies with larger sample sizes tended to employ self-report measures rather than objective test modalities.
With the exception of two studies43,44, data came from countries with high-income economies (World Bank Classification). The vast majority of study data originated from the continents of North America and Europe (42% and 44% respectively). Age of study participants was generally similar across studies and to be expected given the breast cancer age risk. The pooled mean across the studies was age 52 (range of included study means: 45–71). Three studies15,45,46 were specifically interested in older patients and included only women over the age of 65.
Consistent with clinical practice, women received a range of chemotherapy drugs as part of their treatment cycles across the studies. Common combinations reported were FEC (5-fluorouracil, epirubicin, and cyclophosphamide), CMF (cyclophosphamide, methotrexate and 5-fluorouracil), doxorubicin + paclitaxel, doxorubicin + cyclophosphamide. This information has not been presented in the review due to a general lack of separation of outcome data based on treatment in the primary studies, necessitating assimilation as a group. In most studies (n = 43), a significant percentage of patients, especially at later follow-up points were receiving hormonal therapy such as tamoxifen. In the remaining studies, patients on hormonal therapies were either excluded from study design or studies were conducted early in the treatment phase (see Tables 1, 2 and 3). Hormonal therapies have been shown to influence cognition47,48. These agents are administered frequently and can be considered a standard part of treatment. Inclusion of these data hence supports the external validity of the review’s findings.
Table 1.
Summary of included studies that used self-report measures of cognitive impairment.
| Study | Study design | Location | Endocrine therapy use | Sample size | Sample age (range, mean) | Assessment method | Methodological quality | Definition of cognitive decline | Time pointsǂ | Prevalence % (95% CIs where available) |
|---|---|---|---|---|---|---|---|---|---|---|
| Buchanan 201554 | Cross-sectional | United States | Yes |
Chemotherapy = 288 Chemotherapy + Hormone = 859 |
34–82 | FACT-Cog | High | Reponses of cognitive complaints “sometimes”, “often”, or “always” | Mixed* |
74 Chemotherapy 78 Chemotherapy + Hormone |
| Debess 201050 | Cohort | Denmark | Yes | 75 | 29–59, 47.2 | Author-derived questions assessed on 7-point scale | High | Scores of 5–7 | T2 |
Memory: 29 Concentration: 13 Mental fatigue: 33 Vigour: 43 |
| Ganz 201355 | Cohort | United States | Yes | 189 | 51.8 | PAOFI | Moderate | Classified based on PAOFI score as high range versus normal/no complaints | Mixed* |
Memory: 23 High level cognition: 19 |
| Hurria 200645 | Prospective Longitudinal | United States | Yes | 45 | 65–84, 70 | Squire | Moderate | Unclear but based on score | T3 | 51 |
| Janelsins 201756 | Prospective Longitudinal | United States | Yes | 505 | 22–81, 53.4 | FACT-Cog | High | A 1/2 standard deviation representing a minimal clinically important difference | T2, T3 |
T2: 45 T3: 37 |
| Jenkins 200651 | Cohort | UK | Yes | 85 | 51.5 | Broadbent | High | Self-report of change | T2, T3 |
T2: Memory: 83 Concentration: 80 T3: Memory: 60 concentration: 45 |
| Koppelmans 201211 | Cohort | Netherlands | No | 196 | 50–80, 64.1 | Basic self-report | High | Yes response to question | T7 |
More problems remembering: 53 Forgetting (daily) pursuits: 43 Word-finding problems: 38 |
| Lange 201615 | Cohort | France | Yes | 58 | 65–81, 70 | FACT-Cog | High | A difference of more than 10% between baseline and T2 was considered clinically significant | T2 |
Perceived Cognitive Impairment: 34 Perceived Cognitive abilities: 49 (80 over 75 years) |
| Ng 201852 | Prospective Longitudinal | Singapore | Yes | 166 | 50.7 | FACT-Cog | High | A drop of 10.6 points in the global score from baseline | T1, T3 |
T1: 22 T3: 31 |
| Rey 201257 | Cohort | France | Yes | 222 | 18–40, 37 | Telephone Interview | High | Women who reported memory and⁄or attention troubles ‘‘very often’’ or ‘‘often’’ | T3, T4, T5 |
T3:37 T4: 37 T5: 42 |
| Schagen 199953 | Cohort | Netherlands | Yes | 39 | 47.1 | Interview with questions scored on a Likert scale | Moderate | A score of 2 (moderate) or more was considered a distinct complaint about the cognitive functioning in the domain concerned | T5 |
Concentration problems: 31 Memory problems: 21 Thinking: 8 Language: 8 |
| Schmidt 201658 | Cross-sectional | United States | Yes | 904 | 48.8 (across range of cancer types) | Based on Quality of Life in Adult Cancer Survivors (QLACS) with some amendment | High | An endorsement of the items was categorised as perceived cognitive decline | Unclear | 58 |
| Shilling 200759 | Cohort | UK | No | 85 | 51.7 | Interview | Moderate | An endorsement of the items | T2, T4 |
Memory T2: 83 T4: 60 Concentration: T2: 78 T3: 45 |
| Tager 201060 | Cohort | United States | No | 31 | 46.95–70.96, 60.7 | Participants asked to rate perceived memory abilities on a five-point Likert scale | Moderate | Ratings > 2 were coded as cognitive problems | T2,T3 |
T2: 43 T3: 46 |
| Van Dam 199842 | RCT | Netherlands | Yes |
FEC Ϯ:36 CTC≠: 34 |
FECϮ: 48.1 CTC≠: 45.5 |
Cognitive problems in daily life interview | High | An endorsement of the items was categorised as perceived cognitive decline | T5 |
Concentration: high-dose:38 Standard-dose: 31 Memory High-dose: 32 Standard-dose: 28 Thinking: High-dose: 21 Standard-dose: 11 Language: High-dose:12 Standard-dose: 11 |
*Time-points measured in study cross time-points used in this review preventing accurate assimilation.
ǂT1 = During chemotherapy treatment, T2 = Just after cessation of treatment, T3 = 6 months after treatment cessation, T4 = 1 year after treatment cessation, T5 = 2–3 years after treatment cessation, T6 = 5–10 years after treatment cessation, T7 = ≥ 10 years after treatment cessation.
ϮFluorouracil, epirubicin, cyclophosphamide (standard dose).
≠Four cycles of FEC followed by cyclophosphamide, thiotepa, and carboplatin (high dose).
Table 2.
Summary of included studies employing short cognitive screening tools to assess cognitive impairment.
| Study | Study design | Location | Endocrine therapy use | Sample size | Sample age (range, mean) | Assessment method | Methodological quality | Definition of cognitive decline | Time point(s)ǂ | Prevalence % (95% CIs where available) |
|---|---|---|---|---|---|---|---|---|---|---|
| Biglia 201249 | Prospective Longitudinal | Italy | No | 40 | 38–65, 51 | MMSE | Moderate | Score under the mean | T2 | 31 |
| Brezden 200025 | Cohort | Canada | Yes |
71 across two groups: (1) Currently receiving chemotherapy, and (2) one year after cessation |
34–70, 49 26–61, 46 |
HSCS | High | Classification as mild, moderate or severe according to pattern of scores as described by test authors | T1, T4 |
T1 Mild: 13 Moderate: 10 Severe:12/31 = 39 T4 Mild: 20 Moderate: 28 Severe:9/40 = 23 |
| Fan 200526 | Cohort | Canada | Yes | 81 | 48 (median) | HSCS | High | Scores from each item on the test were subject to an algorithm which generated bands of impairment | T2,T4,T5 |
T2 Mild: 35 Mod-Severe: 16 T4 Mild: 30 Mod-severe: 4 T5: Mild: 21 Mod-severe: 4 |
| Fontes 201640 | Prospective longitudinal | Portugal | Yes | 475 | 54.7 (median) | MoCA | High | A MoCA score at least 2.0 standard deviations below age- and education-adjusted cut-offs | T3, T5 |
T3: 7 T5: 8 |
| Ng 201852 | Prospective Longitudinal | Singapore | Yes | 166 | 50.7 | Headminder computerized test | High | Reliable change index (RCI) score calculated to determine cognitive decline in each cognitive domain. A RCI score of lower than − 1.5 was used as criteria for decline | T1, T3 |
T1: 6.1–21.6 T3: 1.2–14.6 |
| Pillai 201943 | Prospective longitudinal | India | Yes | 152 | 27–72, 47 | MMSE | Low | MMSE score of ≤ 24 (out of 30) | T2,T3,T4,T5 |
T2: 0 T3: 0 T4: 0 T5: 0 |
| Prokasheva 201161 | Cohort | Israel | Yes | 20 | 30–57, 49.3 | Doors and people test | Moderate | Below 1SD as indicative of mild impairment and below 2SD for moderate impairment | T5 | 40 |
| Ramalho 201762 | Cohort | Portugal | Yes | 418 | 27–87 | MoCA | High | MoCA score values at least 1.5 standard deviations below age- and education-adjusted cut-offs | T3 | 8 (5.8, 11) |
| Tchen 200363 | Cohort | Canada | No | 110 | 27–60, 48 | HSCS | High | Based on standard test criteria | T1 |
T1 Mild: 34 Moderate: 2 Severe: 14 |
ǂT1 = During chemotherapy treatment, T2 = Just after cessation of treatment, T3 = 6 months after treatment cessation, T4 = 1 year after treatment cessation, T5 = 2–3 years after treatment cessation.
Table 3.
Summary of included studies employing objective neuropsychological testing to assess cognitive impairment.
| Study | Study design | Location | Endocrine therapy use | Sample size | Sample age (range, mean) | Number of tests in battery | Methodological quality | Definition of cognitive decline | Time point(s)ǂ | Prevalence % (95% CIs where available) |
|---|---|---|---|---|---|---|---|---|---|---|
| Andryszak 201864 | Cohort | Poland | No | 31 | 52.4 | 1 | Moderate | Scores lower than 2 SD compared to control group averages | T1,T2 |
T1:32 T2: 26 |
| Biglia 201249 | Prospective Longitudinal | Italy | No | 40 | 38–65, 51 | 10 | Moderate | “Unsatisfying score” not defined in report | T2 | 15 |
| Collins 200965 | Cohort | Canada | Yes | 53 | 50–65, 57.9 | 18 | High | SRB* scores of − 2 or less on at least two tests | T2, T4 |
T2: 34 T4: 11 |
| Collins 201366 | Prospective longitudinal | Canada | No | 60 | 52.4 | 17 | High | Scores lower than 2 SD on at least two tests | T1 (measured at end of each cycle) | 30 (mean across all cycles) |
| Collins 201467 | Cohort | Canada | Yes | 56 | 51.8 | 19 | High | SRB scores of − 2 or less on at least two tests |
T1 (measured at end of each cycle) T4 |
T1: 48 T4: 22 |
| Debess 201050 | Cohort | Denmark | Yes | 75 | 29–59, 47.2 | 4 | High | Significant changes (between 5 and 95th percentile in controls) on at least two tests | T2 | 4 |
| Hermelink 200768 | RCT | Germany | Yes | 101 | 48.6 | 12 | High | Reliable-change index (RCI) with a probability of error set at 10% | T1 | 27 |
| Hermelink 201769 | Cohort | Germany | Yes | 91 | 27.3–64.9, 52.3 | 18 | High | Five or more scores below 1.5 standard deviations and/or four below 2 standard deviations | T2, T4 |
T2: 6 T4: 18 |
| Hurria 200646 | Prospective longitudinal | United States | Yes | 28 | 65–84, 71 | 13 | Moderate | Scores lower than 2 SD on at least two tests | T3 | 39 |
| Jansen 201170 | Prospective longitudinal | United States | Yes | 71 | 30–65, 50.3 | 4 | High | Scores lower than 1 SD on at least two tests | T1, T2, T3 |
T1:23 T2:52 T3:20 |
| Jenkins 200651 | Cohort | UK | Yes | 85 | 51.5 | 7 | High | Reliable change index on at least two measurement | T2, T3 |
T2: 20 T3: 18 |
| Jim 200971 | Cohort | United States | Yes | 97 | 50 | 9 | High | Scores lower than 1.5 SD on at least two tests | T3 | 34 |
| Jung 201472 | Cross sectional study | Korea | Yes | 32 | 31–61, 46 | 3 | High | Cutoff as 5/4 on digit span-DS F; 4/3 on digit span B; 28–30/25 on Controlled Oral Word Association-COWA) | T2 |
DSF Mild: 32 Moderate: 13 DSB Mild: 32 Moderate: 42 COWA* (A) Mild: 3 Moderate: 58 COWA (B) Mild: 52 |
| Kesler 201773 | Cohort | United States | Yes | 31 | 34–65, 48.6 | 4 | Moderate | Scores lower than 1.5 SD on at least two tests or lower than 2SD on any one test | T4 | 55 |
| Kreukels 200874 | Cohort | Netherlands | Yes | 63 | 46.6 | 10 | High | Scores lower than 2 SD on at least three tests | T3/T4 | 33 |
| Lange 201615 | Cohort | France | Yes | 58 | 65–81, 70 | 8 | High | A significant change in a domain score | T2 | 64 |
| Mehlsen 200975 | Cross-sectional | Denmark | Yes | 36 | 48.6 | 13 | High | Decline on at least 3 of the cognitive measures | T2 | 29 |
| Mehnert 200776 | RCT | Germany | Yes | 23 | 33–65, 53 | 18 | Moderate | Scores lower than 1.4 SD on at least four tests | T6 | 13 |
| Menning 201677 | Cohort | Netherlands | Yes | 31 | 49.8 | 11 | High | Multivariate normative comparison comparing against distribution of scores in control group | T3 | 16 |
| Reid-Arndt 200978 | Cross sectional study | United States | Yes | 46 | 53.4 | 11 | High | Scores of below 1 SD | T2 |
Executive functioning: 22 Verbal fluency: 41 |
| Reid-Arndt 201079 | Prospective longitudinal | United States | Yes | 39 | 53.4 | 7 | High | Below 1SD as indicative of mild impairment and below 1.5 SD for moderate impairment | T3, T4 |
WMS Log Mem: T3: 6 T4: 33 RAVLT T3:13 T4: 6 WMS Log Mem II T3: 3 T4: 33 Trails A T3: 3 T4: 9 Trails B T3: 15 T4: 24 SCW T3: 3 T4: 3 COWA T3: 0 T4: 9 Category fluency: T3: 0 T4: 16 |
| Ruzich 200780 | Prospective longitudinal | Australia | Yes | 35 | 30–66, 53 | 15 | Moderate | Scores below 1 SD on at least two tests | T1,T2,T3 |
T1: 15 T2: 37 T3: 30 |
| Schagen 199953 | Cohort | Netherlands | Yes | 39 | 47.1 | 14 | Moderate | Scores below 2 SD on at least three tests | T5 | 28 |
| Schagen 200281 | Cross-sectional | Netherlands | Yes |
Two groups of different chemotherapy regimes: FECϮ: 23 CMFα: 31 |
50.3 | 13 | High | Number of tests with scores below 2SD | T5 |
FEC: 9 CMF: 13 |
| Schagen 200682 | RCT | Netherlands | Yes |
Two groups of different chemotherapy regimes: FECϮ: 39 CTC≠: 28 |
45 | 10 tests (not listed) | High | Scores below 2 SD on at least three tests | T3 |
FEC: 10 CTC: 20 |
| Schrauwen 202041 | Cohort | Belgium | No | 66 | 27–64, 46.7 | 5 | High | Standardized difference score exceeding − 2.5 SD on at least one test | T2 | 24 |
| Shilling 200583 | Cohort | UK | Yes | 50 | 51.1 | 8 | High | Reliable decline on two or more tests | T2 | 34 |
| Stewart 200884 | Cohort | Canada | Yes | 61 | 50–66, 57.5 | 18 | High | Two or more SRB scores of − 2.0 or less on at least two tests | T2 | 31 |
| Stouten-Kemperman 201585 | RCT | Netherlands | Yes |
Two groups of different chemotherapy regimes: Conventional dose = 24 High dose = 17 |
56.3–59.8 | 8 | Moderate | Score larger than 2SDs below the mean considered impaired on a test. The fifth percentile of the overall impairment score of healthy control scores was cutoff for impairment | T7 |
Conventional dose: 8 High dose: 11 |
| Syarif 201944 | Cross-sectional | Indonesia | Yes | 82 | 43 | 1 | Low | Not reported | Unclear | 87 (mild-serious impairment) |
| Van Dam 199842 | RCT | Netherlands | Yes |
FECϮ: 36 CTC≠: 34 |
FEC: 48.1 CTC: 45.5 |
13 | High | Scores below 2 SD on at least three tests | T5 |
FEC: 17 CTC: 32 |
| Van Dyk 201886 | Cross-sectional | United States | No | 20 | 47 | 17 | Moderate | Impaired by ICCTF guidelines | T2 | 45 |
| Vearncombe 200987 | Prospective longitudinal | Australia | No | 136 | 49.38 | 10 | High | Reliable change on at least 2 measures | T2 | 17 |
| Wefel 201088 | Prospective longitudinal | United States | Yes | 42 | 33–65, 48,4 | 6 | High | Scores below 2SD on one test | T2, T4 |
T2:65 T4:61 |
| Wieneke 199589 | Cross sectional | United States | Yes | 28 | 28–54, 42 | 15 | Moderate | Score below 2 SD on at least one test classified as moderate impairment | T2-T4 (varies across period) | 75 |
ǂT1 = During chemotherapy treatment, T2 = Just after cessation of treatment, T3 = 6 months after treatment cessation, T4 = 1 year after treatment cessation, T5 = 2–3 years after treatment cessation, T6 = 5 – 10 years after treatment cessation, T7 = ≥ 10 years after treatment cessation.
*Standardised regression based.
ϮFluorouracil, epirubicin, cyclophosphamide.
αCyclophosphamide, methotrexate, 5-fluorouracil.
≠Four cycles of FEC followed by cyclophosphamide, thiotepa, and carboplatin.
The included studies generally assessed cognition at similar time-points to coincide with typical phases of breast cancer treatment. Due to this similarity studies were grouped based on these time points for further assimilation, labelled T1–T7 (refer to supplementary material S5). Data from baseline, pre-chemotherapy assessments (T0), were not extracted as part of this review since we did not have as an inclusion criterion that studies had to be longitudinal. Further, the review question relates to prevalence of impairment after breast cancer treatment, not any measured decline as a result of treatment.
The included studies utilized a range of methods for assessment of cognitive impairment. It was considered that these differences could introduce substantial heterogeneity when assimilating prevalence estimates. Hence, assimilation was undertaken based on use of three different methods; self-report measures, short cognitive screening tools, and objective neuropsychological test batteries (supplementary material S6). Fifteen studies utilized self-report measures, nine employed short cognitive screening tools and a majority of 35 used neuropsychological test batteries. Seven studies utilized two of these testing modalities15,42,49–53. Studies using objective cognitive tests for the most part used tests which spanned the various cognitive domains of executive function, language function, motor, processing speed, verbal and visual learning and memory, visuo-spatial function and working memory. From here on, review data is presented based on these 3 groupings of outcome assessment method (refer to Table 1, 2, and 3).
Methodological quality
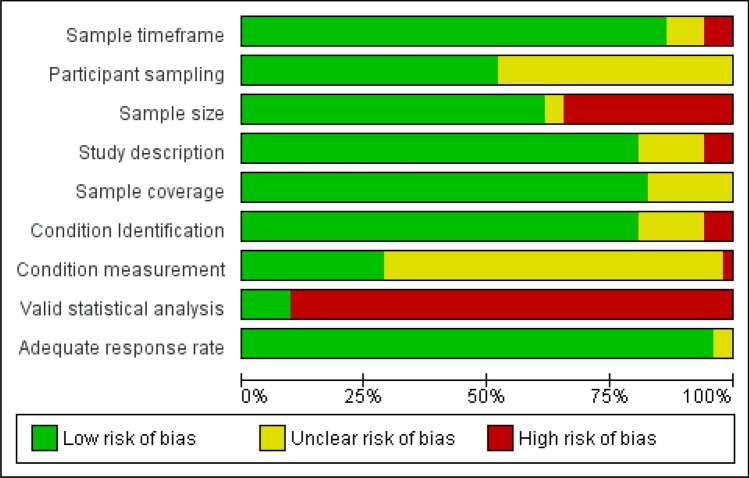
A summary of the methodological quality assessment for the included articles is provided in Fig. 2 and supplementary material S7. Thirty-five (67%) studies were considered to be of high quality, fifteen (29%) of moderate quality and only two43,44 (4%) studies of low quality (see Tables 1, 2 and 3). The main domains of concern were participant sampling, sample size, condition measurement and statistical analysis. Of these, the outcomes of assessment of risk in the domains of sample size and condition measurement are considered to have the most impact on the review outcomes. However, use of a quality model in meta-analysis and evaluating the impact of sample size by meta-regression minimizes this impact.
Figure 2.
Risk of bias graph: review authors' judgements about each methodological quality item presented as percentages across all included studies. Study number = 52.
Prevalence estimates based on self-report measures of cognitive impairment
It was considered that there was significant variation in the studies that used self-report measures in terms of method of assessment of cognitive impairment, timing of assessment in relation to chemotherapy treatment and form of report (based on cognitive domain rather than overall impairment). Therefore, these results are presented narratively.
Fifteen studies (Table 1) examined prevalence of cognitive impairment after breast cancer treatment contributing 22 prevalence estimates across all study time-points with the exception of T6. There were a number of studies with large sample sizes in this group54,56,58, however the median sample size for the group remained modest at 85 (range 31–1147). Studies utilized a variety of methods to assess impairment which ranged from validated self-report instruments such as FACT-Cog or PAOFI, to semi-structured interviews or Likert responses to questions on cognitive function. Seven (47%) of studies used the former. When the studies employing validated tests were contrasted with those using non-validated methods median global impairment across the time points was 46% and 39.5% respectively, with no significant difference in prevalence rate between the two subgroups (two tailed T-test; t (6,5) = -1.2, p = 0.27).
Prevalence rates for global impairment varied from 21 to 83% (mean 44%) across all time-points. Noting the paucity of data at T6 and T7, no obvious downward trend in impairment across time is evident (Fig. 3). Two studies in this sub-group focused on CICI in older women over the age of 6515,45 reporting prevalence rates of 51%45, and 34%15. Whilst, the former is clearly above the mean reported above of 44%, the value within Lange et al. 201615 actually falls under the calculated average.
Figure 3.

Boxplot of prevalence reported for cognitive impairment, via self-report methods, across the time-points included in the review (data from 12 studies in Tables 1, 3 studies were excluded due to mixed or unclear timing of assessment54,55,58).
Prevalence estimates based on use of short cognitive screening tools to measure cognitive impairment
There was considerably less variation in assessment method for cognitive impairment, and time points of measure for the studies that utilised short cognitive screening measures compared to those employing self-report. However, at each time point there were fewer than five studies. Study has suggested that at least 5 studies are needed to achieve statistical power from random effects meta-analyses that are superior to the individual contributing studies90. Studies within this group were therefore not subjected to meta-analytic techniques and are reported narratively.
Nine studies (Table 2) utilized short cognitive screening tools as part of this grouping, contributing 17 prevalence estimates. No studies performed longer-term follow up at the T6 and T7 time-points. Sample size ranged from 20 to 475 (mean = 170). Five different validated methods of assessing impairment were employed (Table 1). With the exception of the Doors and People test these methods assess a range of cognitive domains expected to be impacted by chemotherapy administration. In two studies25,26 test outcomes were reported based on a banding of scores as mild, moderate or severe. For the purposes of assimilation, only prevalence estimates for moderate-severe impairment have been considered.
In contrast to studies that used self-report none of these studies specifically examined older populations. However, three of these studies25,43,62 had a wider age range with patients above 65 years of age being included. This has not however led to a substantially increased mean participant age in comparison to the other studies.
Prevalence rates across all time-points ranged from 0 to 50. The mean prevalence was 16%. Exclusion of the four prevalence estimates of zero provided by Pilai et al. 201943 leads to a range of 4%-50%, with mean prevalence of 21%. This action may be justified given the low quality rating assigned to this study. There was a general downward trend in prevalence across time, but range at each time-point (with the exception of T3) was large (Fig. 4).
Figure 4.

Boxplot of prevalence reported for cognitive impairment, via short cognitive screening methods, across the time-points included in the review. Ng et al. 201852 was excluded from the calculation since a range was reported, and only considering moderate-severe classification of impairment from26 and25.
Prevalence estimates based on use of objective neuropsychological testing to measure cognitive impairment
Thirty-five studies (Table 3) utilized objective neuropsychological tests to screen for cognitive impairment. These studies contributed 46 prevalence estimates. Sample size ranged from 20 to 136 (median = 53) having the lowest range and average of all the groups. Studies reported prevalence across all time points of interest, although only one estimate was reported at each of the longer-term follow up points (T6 and T7). Two studies in the group evaluated women over the age of 6515,46. The neuropsychological tests used were all established, validated tests which when administered in a battery broadly encompass the major cognitive domains. Two studies only employed one test for cognitive assessment44,64, and as such did not evaluate all cognitive domains.
There were differences between the studies in the criteria used to determine cognitive impairment. In general a diagnosis of impairment was made based on two factors being met: (1) the level of decline in score relative to some reference value, for example healthy control patients (2) that this decline occurred in a pre-determined number of tests. Fourteen (40%) of the studies used 2 standard deviation declines to guide level but there was heterogeneity even between these in the number of tests with declining scores required to satisfy the diagnostic criteria (see Table 3). Studies that considered a one SD decline as criteria for impairment, or were not clear on the level used, were considered less stringent (see meta-analysis). Three studies72,78,79 reported test outcomes by cognitive domain or individual test, rather than global impairment rendering these data harder to assimilate with the other studies.
Meta-analysis
Pooled prevalence
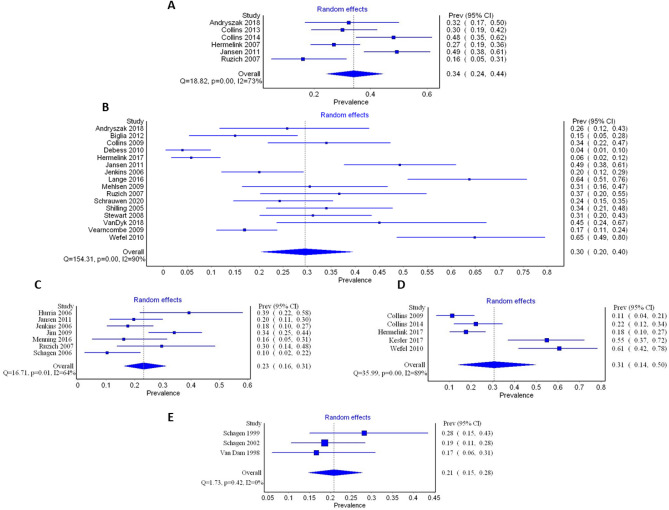
It was considered that meta-analysis was appropriate for further investigation of prevalence estimates derived from studies utilizing objective neuropsychological tests. Thirty-seven prevalence estimates from 27 articles were included in the meta-analysis, grouped based on time point of prevalence measure (from T1 to T5). Only one study contributed data at each of T676, and T785 and consequently these time points and studies were not included. Three studies were excluded from entry since prevalence was reported based on cognitive domain or test type72,78,79. A further three studies were excluded since the timing of measure was unclear44, or was performed across multiple time points as defined for this review74,89. In two studies, prevalence estimates were reported based on chemotherapy regime81,82. In order to ensure comparability with the other included studies only estimates for the standard dose chemotherapy regime from Schagen et al. 200682 were included in the meta-analysis. Prevalence estimates reported by chemotherapy regimen in Schagen et al. 200281 were pooled.
The overall random-effects pooled prevalence (95% CIs) of cognitive impairment from time points T1–T5 respectively was 34% (24–44), 30% (20–40), 23% (16–31), 31% (14–50%), and 21% (15–28) (Fig. 5). Figure 6 synthesises these findings narratively across the time-points with the inclusion of the single individual study estimate contributed from76 and85 at T6 and T7. There is a general downward trend in prevalence across time, although substantial imprecision in the effect at T4 with a slightly increased prevalence than might be expected if observing the trend.
Figure 5.
Forest plots of prevalence reported in studies utilising neuropsychological tests to diagnose cognitive impairment following chemotherapy treatment for breast cancer. (A) Cognitive assessment undertaken during chemotherapy treatment (T1), (B) Cognitive assessment undertaken just after cessation of chemotherapy treatment (T2), (C) Cognitive assessment undertaken 6 months after cessation of chemotherapy treatment (T3), (D) Cognitive assessment undertaken 1 year after cessation of chemotherapy treatment (T4), (E) Cognitive assessment undertaken 2–3 years after cessation of chemotherapy treatment (T5).
Figure 6.

Prevalence reported for cognitive impairment, via neuropsychological test methods, across the time-points included in the review. Data represent pooled prevalence estimates derived from meta-analysis, with 95% CIs. ∞—values are not pooled figures but represent individual values from one study.
Heterogeneity and publication bias
Heterogeneity as determined by the I2 statistic was universally high with the exception of T5 (where there were limited estimates) at 73%, 90%, 64%, 89% and 0 (T1–T5 respectively). Sensitivity analyses performed included removal of each study in turn, analysing untransformed data, considering the effect of the quality score and using a fixed effects model. In general, results were similar after these analyses (supplementary material S8). However, removal of Jansen et al. 201170 at T1 had a significant effect reducing I2 by 10% to 63% (potentially as a result of the impairment criteria used as described below). It is noteworthy that removal of the Andrysak 2018 study64, which only employed one cognitive test, did not impact the heterogeneity estimates, nor did removal of the studies with cross-sectional designs 75,86.
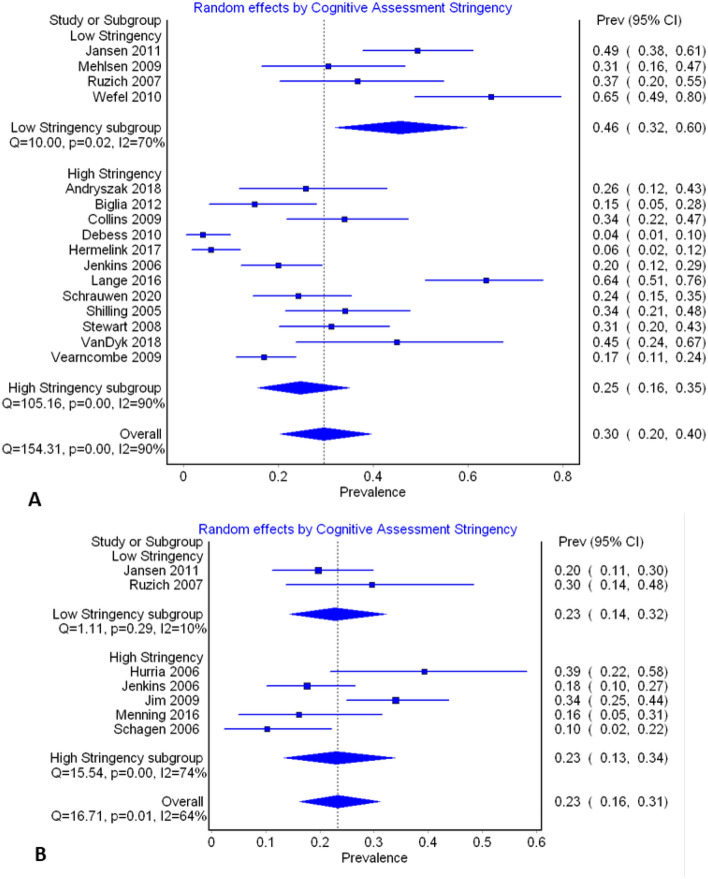
In order to explain the heterogeneity in the pooled effects sizes the influence of stringency of criteria for diagnosing cognitive impairment was explored via subgroup analysis. Only prevalence reports from T2 and T3 were subjected to subgroup analyses based on the number of studies contributing data at these time-points, and the number in each analysis when split into sub-groups. At these time-points, four studies were considered to have used less stringent test methods70,75,80,88. Heterogeneity remained high after sub-group analysis with the high stringency sub-group having a pooled prevalence of 25% (95% CIs: 16–35), I2 = 90% at T2, and 23% (95% CIs: 13–34), I2 = 74% at T3 (Fig. 7).
Figure 7.
Forest plots of prevalence sub-grouped by stringency of cognitive assessment. (A) Cognitive assessment undertaken just after cessation of chemotherapy treatment (T2), (B) Cognitive assessment undertaken 6 months after cessation of chemotherapy treatment (T3).
Meta-regression was performed in an attempt to account for the remaining heterogeneity. The results of five individual meta-regression analyses performed at T2 based on (1) the continuous variables of age, methodological quality and sample size, and (2) categorical variables of cognitive impairment criteria stringency and geographical location (3) subsets; Europe (reference group), North America, Asia–Pacific) are included in Table 4. Cognitive impairment stringency explained some of the variance in prevalence, accounting for 16% of the heterogeneity at T2 (P = 0.04). Sample size also accounted for 16% of the heterogeneity (P = 0.04) with increased sample size tending to result in a lower prevalence estimate. The remainder of the covariates could not account for the variance observed.
Table 4.
Results of four individual meta-regression analyses at T2 based on age, quality score, location and cognitive impairment criterion stringency.
| T2- meta-regression | Meta-regression | ||
|---|---|---|---|
| Mean difference (95% CIs) | P | R2 | |
| Age | 0.06 (− 0.01 to 0.13) | 0.09 | 0.13 |
| Quality Score | − 0.25 (− 4.14 to 3.64) | 0.9 | 0 |
| Sample Size | − 0.02 (− 0.03 to 0) | 0.04 | 0.16 |
| Location | |||
| Europe (ref group) | |||
| North America | 1.04 (0.11–1.98) | 0.09 | 0.08 |
| Asia–Pacific | 0.16 (− 1.14 to 1.46) | ||
| Impairment criteria stringency | |||
| High stringency (ref group) | 0.97 (0.04–1.9) | 0.04 | 0.16 |
| Low stringency | |||
Meta-regression was then used to examine the relationship between sample size, cognitive impairment test stringency, and effect size. A test of this model yielded a Q-value of 6.99 with 2 degrees of freedom and corresponding p-value of 0.03. The test for heterogeneity yields a Q-value of 74.7 and a corresponding p-value of 0, implying that the variation of observed effects about the regression line falls within the range that cannot be explained by sampling error alone. The model is able to explain some 23% of the variance in true effects.
The doi plots for publication bias showed some asymmetry implying the presence of bias. The LF asymmetry index confirmed there was minor asymmetry at T2, and T5 and major asymmetry at T4. There was no evidence of publication bias at T1 and T3 (see supplementary material S9).
GRADE certainty assessment and results
The evidence presented across all the studies grouped by cognitive assessment methods was assessed using the GRADE (Grading of Recommendations Assessment, Development and Evaluation) approach (GRADEpro GDT: GRADEpro Guideline Development Tool [Software]). Results are presented in the Summary of Findings Table (Table 5). The certainty of evidence was graded as very low for all three-assessment types. Inconsistency was rated as serious for all groups of studies based on the wide variation in estimates and high calculated heterogeneity (neuropsychological testing). Imprecision was similarly rated as serious for all outcomes as a result of the wide confidence intervals. Publication bias was strongly suspected for the study group that used neuropsychological tests based on the doi plots and LFK index. Due to the method of synthesis used for the self-report and short cognitive screening tool groups, publication bias was not able to be assessed and was therefore rated as undetected.
Table 5.
GRADE Summary of Findings table for prevalence as determined through self-report, short cognitive screening tests and neuropsychological test batteries.
Discussion
This is the first systematic review on prevalence of CICI after treatment for breast cancer to have included and compared the three commonly used assessment modalities of self-report, short cognitive screen and objective neuropsychological tests. In contrast to the only other published systematic review of prevalence in CICI in this patient population 2, meta-analytic techniques were applied to allow reporting of pooled effects, and studies which performed cognitive assessment beyond the breast cancer treatment period were also included. Prevalence estimates derived ranged from 0 to 83% across all time-points and all cognitive test methods. This crude figure is highly comparable with the commonly cited range of 12–82% based on the narrative review of Janelsins et al. 201414. The current review allows greater examination of variability in these prevalence rates, through examination of the influence of factors such as test method, time since treatment and age on rates.
Impact of assessment tool choice on prevalence rates
There are striking differences in the average and maximum prevalence values reported based on method of cognitive assessment. Self-report of cognitive impairment yielded the highest values. There is substantially less variability between values obtained by short cognitive screen and neuropsychological test batteries. This difference in outcomes is to be expected for a number of reasons: (1) patient mood has been shown to have a relatively greater impact on subjective reports of impairment than actual cognitive performance. For example, aspects of mood such as anxiety, depression, poor quality of life and fatigue have all been shown to correlate with self-reported cognition complaints49,91–93; (2) expectations about treatment might influence self-report of cognitive symptoms. Study has shown that patients who were aware of the potential for cognitive side effects were more likely to report cognitive function issues than those who were unaware of the possibility94; (3) patients may have been functioning above the normal range of cognitive function prior to treatment, so whilst they experience a decline, their cognitive performance falls within the normal range95,96; (4) the battery of objective tests used are ineffective at diagnosing subtle impairment95,96, this may especially be of concern with the use of short cognitive screening tools97; (5) objective tests are ecologically invalid by failing to represent real-life situations where patients may experience the impairment95,96. Finally, based on the studies included in this review it could be argued that some of the assessment criteria are relatively crude, for example the endorsement of an item with no grading of response11,58,59 would likely lead to substantially higher reporting of issues, which may not actually differ from normal age-related memory loss experienced by healthy individuals11,58,59. In fact a cursory glance at the prevalence reports in these studies shows that reported prevalences are higher than the mean for the group. This perhaps highlights a future need to only use validated self-report instruments for cognitive assessment.
Our findings are in line with previous study where discrepancy between results of cognitive testing and clinical interview or self-report has been highlighted, with subjective perception of cognitive failure commonly not being able to be confirmed with objective tests8,98. Perceived impairment may actually be an indicator of psychological distress, brought about by anxiety, pain and depression, rather than a measure of cognitive functional capacity8. However, in clinical care patient perception matters. Addressing any of these individual factors through treatments or lifestyle change may bring about improvements in the others to provide an overall benefit. Disentangling the relative contribution of each of these elements is complex, and perhaps meaningless, in a clinical patient population98. Perhaps, there is a need to consider whether objective testing can be improved to reduce this discrepancy, for instance utilizing tests which cross cognitive domains and are therefore truer to real-life. Gamification may have a role in this regard. Alternatively, a hybrid model for diagnosing cognitive impairment may be superior, with the inclusion of objective tests and self-report in a standardized, validated protocol.
In spite of the reduced variability observed with objective tests, there was still significant statistical heterogeneity observed on meta-analysis. Approximately 16% of this heterogeneity could be explained by the use of less stringent test criteria for diagnosing impairment. Additionally, pooled prevalence was noticeably altered when subgroups based on test stringency were analysed. This finding provides strong evidence of the need for researchers to agree to, and use standardised impairment criteria, in order to make findings comparable, reproducible, and ultimately useful to practitioners. In fact, given that the International Cognition and Cancer Task Force advocated for such harmonisation in 201199, it is surprising that this has not yet occurred, but may reflect individual researcher differences in experience with, and available access to certain tests.
Impact of time since treatment on prevalence rates
Prevalence rates ascertained by short screening methods or larger batteries of tests showed a general downward trend over time, with a peak rate occurring during chemotherapy treatment. This finding is supported by pre-clinical and imaging studies in CICI, which demonstrate that, over time, partial resolution of the brain structural and functional impairments occurs22,23. However, it does contrast with the trend in prevalence determined by Dijkshoorn et al. 20212 which only included studies using objective tests, and found elevated (although perhaps not significantly so) prevalence rates at greater than one year after treatment, compared to just after treatment with chemotherapy. Notably however, actual prevalence values calculated in the current review for objective tests are remarkably similar to those in Dijkshoorn et al. 20212. Rates of 25%, 14% and 27% were reported in that review at time-points of just after treatment, up to 1 year following, and greater than 1 year after treatment respectively2. This corresponds with 30%, 23% and 31% at equivalent time points in the current review. This similarity in rates is in spite of the differences in methodology employed in the two reviews with the current review assimilating rates via meta-analytical techniques, and the previous review only including longitudinal studies such that impairment was based on a decline in cognitive function from baseline measures.
Conversely, the current review illustrates that prevalence rates as determined by self-report remain high (often above 40%), and show little evidence of decline over time. The previously discussed suggestions explaining the general elevation in self-reported cognitive decline over measured impairment may also be at play here. Furthermore, at late follow-up stages patients will have likely returned to normal daily-life activities, such as work, and may have a greater perception of impairments as they return to navigating everyday tasks. This fits with current cancer rehabilitation evidence that suggests that survivors commonly suffer disabilities relating to activities of daily living such as doing housework and shopping100.
Whilst fewer studies have evaluated longer-term follow-up points, greater than 5 years following treatment cessation, there is evidence from both self-report and objective testing that some individuals are cognitively impaired at these time-points. This is an important finding for survivorship care and deserves dedicated research attention to fully elucidate impact.
Impact of age on prevalence rates
There is recognition of the potential for increased significance of chemobrain in older adults with cancer due to higher levels of pre-existing cognitive impairment in this age group101. Furthermore, older adults may be more susceptible to cancer treatment toxicity leading to heightened symptoms15. Whilst it might therefore be predicted that CICI prevalence increases with age, the review did not find sufficient evidence to either support or refute this. Given there is wide variability in how people age even in the absence of medication effects studying this population group to come to reliable conclusions is likely problematic.
When meta-regression was applied, age in general was shown to have no effect on the high heterogeneity seen at T2. This lack of effect is unsurprising given that the studies included in the meta-regression had similar mean ages of the sample population, with relatively narrow ranges. Furthermore, for objective testing cognitive score results are usually compared against age-matched controls or some other age-based correction is applied, hence age is implicitly accounted for in experimental design.
Only three studies specifically focused on patients over the age of 6515,45,46. Two of these studies15,45 used self-report methods, with one reporting prevalence rates below the mean for the group45, and the other reporting rates high than the mean15. The third study46 utilized objective tests and reported a prevalence value higher than the reminder of the T3 subgroup. However, it also has the widest confidence intervals of the group suggesting the estimate to be imprecise. The study also used a low sample size, which may have influenced the estimate obtained. Noteworthy, is that the cohort study of Lange et al. 2016 also used cognitive tests, with diagnostic criteria categorized in this review as high stringency, and reported a high prevalence of 64% at T2. Moreover, confidence intervals were of similar width to other studies within the group15. This finding is perhaps the most suggestive of an effect of age on CICI prevalence and would be good grounds for dedicating future research effort to this question.
Strength of evidence on CICI prevalence
In order to account for any effect that low-quality studies might have had on the review findings, the meta-analyses incorporated a quality model, and a meta-regression using quality score as a variable was performed. These tools indicated that quality had minimal effect on prevalence rates or variances in the pooled estimates. As a result, there can be confidence that study quality has not affected review outcomes, at least for those studies that utilized objective cognitive assessment tools. Whilst, it is harder to make a formal determination of the impact of quality on the prevalence reports from studies using self-report and short cognitive screening tools, there is little to indicate that the results may have been unduly influenced by poor quality studies.
In all of the meta-analyses performed, with the exception of T5 where there were limited studies, moderate to high statistical heterogeneity was found. Sensitivity testing failed to account for any meaningful heterogeneity, and the subgroup analyses failed to reduce I2 values substantially. Meta-regression did reveal an effect of cognitive impairment test stringency and sample size on variance. These factors accounted for 23% of the variance in effect seen at T2 in a combined model. The remainder of the heterogeneity could possibly be accounted for by methodological diversity due to the differing test batteries used, and further considerations relating to defining impairment levels that were not considered by the sub-group analysis. Additionally, clinical heterogeneity may have arisen as a result of treatments administered, patient menopausal status, or race.
There was evidence of publication bias at a number of time-points included in the meta-analysis, which lowers certainty in the findings from this analysis. However, there are a few considerations regarding assessment of publication bias in prevalence studies. Firstly, there is no specific guidance on assessment of publication bias when proportional data are involved. The issue being that funnel plots may be imprecise at the extremes of a proportion102, and this may similarly apply to doi plots. It could be envisaged that the risk of publication bias should be lower in studies of prevalence since there is no favourable hypothesized outcome that may influence decision to publish. However, in the current review it is worth noting that in all of the studies the study objective was not solely around obtaining prevalence data on CICI. Taking this into consideration the possibility of publication bias likely exists, and was evidenced in this study.
Certainty of the evidence contributing to the findings of this review was assessed using the GRADE approach. It was determined that there was low certainty in the body of evidence contributing to the review. However, there is a lack of formal guidance from the GRADE working group on use of the methodology for reviews of prevalence, and the guidance criteria are not always applicable to these types of data. In spite of the lack of standardization, based on the assessment, conclusions should be made tentatively.
Study limitations
The review does have a number of limitations. First, due to the methodological diversity of the included studies, it was only possible to perform meta-analytic techniques on the studies that used neuropsychological tests. As a result, study weight has not been accounted for in the summary prevalence figures reported arising from self-report and short cognitive screening methods. This necessitates a need for extra circumspection around these values. Furthermore, even when meta-analytical techniques were used there was a resultant high statistical heterogeneity. Subgroup analyses and meta-regressions failed to account for most of this heterogeneity leaving the source(s) unknown, although some suggestions have been provided.
Second, due to the nature of the included studies with many having a repeated measures design it was not possible to use meta-analytical techniques to statistically compare prevalence across time-points; this being an aim of this review. Furthermore, in the current study using the All Time Points Meta-analysis approach assumption of independence between time-points was violated since some subjects contributed data at more than one time-point, whilst others only contribute at one time-point. This can lead to an ecological fallacy with a trend being seen on aggregate, that is not present at an individual level36. As a result there can only be low confidence in the direction of trends across time reported. Alternate approaches may be preferred when trends across time are of interest; for example, the use of a trend meta-analysis which uses regression modelling, or a change-in time meta-analysis where the primary study data is used to calculate differences between successive time-points or compared to baseline36. These alternate methods were not employed in this review since they require certain data to be provided in the primary studies such as a slope estimate for trend, or assume a longitudinal study design.
A few points regarding the conclusions that can be taken from this review are pertinent. The focus of the review was on estimating prevalence of CICI. However, as previously described cognition deficits may be caused by a range of factors associated with the cancer experience14. These may include the cancer itself and associated psychological distress and fatigue, or other administered treatments such as hormonal therapies. A further pertinent point is that study has suggested that race/ethnicity may influence prevalence of cognitive impairment, especially when self-report methods are used for assessment. 56 This review did not specifically consider impact of ethnicity on rates of impairment reported. Moreover, whilst included studies commonly reported ethnicity data as part of the patient characteristics, results were rarely stratified by ethnic group. The impact of race on CICI prevalence therefore appears to be understudied, potentially leading to disparate health outcomes. It would be valuable in future evidence synthesis to consider this question specifically, allowing article selection to specifically address the influence of ethnicity. Furthermore, there is a clear need for researchers conducting primary research to consider, and address this issue in data collection and reporting. Additionally, studies eligible for inclusion in the review were not restricted to those with a longitudinal design, thus allowing for the contrast of findings with a baseline cognitive score. Based on these two points, it is impossible to say whether the cognitive deficits reported were wholly the result of chemotherapy, as opposed to a combination of cancer and multiple treatment-related effects. This concern is academic. From the perspective of patients and health-care providers it is the nature of cognitive decline that is important, not the origin of it. Since, these combinations of factors are inherent, and common to most women with breast cancer, it is considered that the review findings are generalizable.
Conclusions
This review is the first systematic comparison of prevalence rates for cognitive impairment in women with breast cancer that has considered all methods used to ascertain impairment, and evaluates long-term prevalence. Mean prevalence rates for CICI across all time-points were 44% using self-report and 6% using short cognitive screening. Pooled prevalence rates of between 21 and 34% were derived for impairment diagnosed by objective neuropsychological tests. For all three assessment modalities the GRADE certainty in the evidence was rated as very low. Results therefore need to be interpreted with caution since the actual prevalence may be substantially different from this estimate.
Recommendations for practice
The findings of this review suggest that cognitive impairment may impact up to 1 in 3 patients at a level that is clinically significant. This impairment also extends beyond the treatment period and is often still apparent 2–3 years post treatment cessation. Health practitioners should consider the potential for cognitive impairment in breast cancer survivors, and guide them and their families towards sources of information and support. They may also like to discuss with patients some of the minimally invasive, non-therapeutic interventions that might ameliorate symptoms, for example cognitive training or physical activity32.
Recommendations for research
The uncertainty around the true prevalence of cognitive impairment following cancer treatment leads to under-consideration of the condition, and consequently a lack of support being available for survivors. This leads to reduced patient quality of life through less meaningful engagement with work, family and daily activities, as well as economic impacts for both the individual and society. This review has highlighted a number of considerations for future research to provide greater certainty in the effect estimates. This includes increasing study sample sizes, utilising standardised cognitive test methods and batteries, and applying accepted, clinically relevant criteria for diagnosing impairment. With the increasing number of older cancer survivors, there is also a need for targeted research investigating this group. Furthermore, in order to tease apart the impact of relative impact of cancer alone, it would be valuable to perform further evidence synthesis to determine the prevalence of cognitive impairment in women with breast cancer, who have not received chemotherapy treatment. Acquiring accurate prevalence data will better inform survivorship strategies by allowing appropriate delivery of targeted resources and services, and informed resource allocation for this effort. Access to accurate prevalence estimates will also inform, and perhaps encourage, the development of evidence-based practice guidelines for CICI.
Supplementary Information
Author contributions
A.L.W conceptualised the project, carried out the searches, performed data selection and extraction and quality appraisal as well as writing the manuscript. R.P.G validated the work performed by A.L.W in data selection, extraction and quality appraisal. L.O’M was involved in development of the project and supervision. All of the authors reviewed and edited the manuscript.
Funding
This research did not receive any specific Grant from funding agencies in the public, commercial, or not-for-profit sectors. ALW was supported by a NHMRC Peter Doherty Biomedical Research Fellowship (APP1140072). RPG was supported by an Australian Government Research Training Program Scholarship.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-022-05682-1.
References
- 1.Nardin S, et al. Breast cancer survivorship, quality of life, and late toxicities. Front. Oncol. 2020 doi: 10.3389/fonc.2020.00864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dijkshoorn ABC, et al. Prevalence of cognitive impairment and change in patients with breast cancer: A systematic review of longitudinal studies. Psychooncology. 2021;30:635–648. doi: 10.1002/pon.5623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ahles TA, Root JC, Ryan EL. Cancer- and cancer treatment-associated cognitive change: An update on the state of the science. J. Clin. Oncol. 2012;30(30):3675–3686. doi: 10.1200/JCO.2012.43.0116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mounier NM, Abdel-Maged AE, Wahdan SA, Gad AM, Azab SS. Chemotherapy-induced cognitive impairment (CICI): An overview of etiology and pathogenesis. Life Sci. 2020;258:118071. doi: 10.1016/j.lfs.2020.118071. [DOI] [PubMed] [Google Scholar]
- 5.Seigers R, Schagen S, Van Tellingen O, Dietrich J. Chemotherapy-related cognitive dysfunction: Current animal studies and future directions. Brain Imaging Behav. 2013;7:453–459. doi: 10.1007/s11682-013-9250-3. [DOI] [PubMed] [Google Scholar]
- 6.George RP, Semendric I, Hutchinson MR, Whittaker AL. Neuroimmune reactivity marker expression in rodent models of chemotherapy-induced cognitive impairment: A systematic scoping review. Brain Behav. Immun. 2021 doi: 10.1016/j.bbi.2021.01.021. [DOI] [PubMed] [Google Scholar]
- 7.Nguyen LD, Ehrlich BE. Cellular mechanisms and treatments for chemobrain: Insight from aging and neurodegenerative diseases. EMBO Mol. Med. 2020;12:e12075. doi: 10.15252/emmm.202012075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hutchinson AD, Hosking JR, Kichenadasse G, Mattiske JK, Wilson C. Objective and subjective cognitive impairment following chemotherapy for cancer: A systematic review. Cancer Treat. Rev. 2012;38:926–934. doi: 10.1016/j.ctrv.2012.05.002. [DOI] [PubMed] [Google Scholar]
- 9.Scherling CS, Smith A. Opening up the window into "chemobrain": A neuroimaging review. Sensors (Basel) 2013;13:3169–3203. doi: 10.3390/s130303169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wigmore P. The effect of systemic chemotherapy on neurogenesis, plasticity and memory. Curr. Top. Behav. Neurosci. 2013;15:211–240. doi: 10.1007/7854_2012_235. [DOI] [PubMed] [Google Scholar]
- 11.Koppelmans V, et al. Neuropsychological performance in survivors of breast cancer more than 20 years after adjuvant chemotherapy. J. Clin. Oncol. 2012;30:1080–1086. doi: 10.1200/JCO.2011.37.0189. [DOI] [PubMed] [Google Scholar]
- 12.Kanaskie ML, Loeb SJ. The experience of cognitive change in women with breast cancer following chemotherapy. J. Cancer Surviv. 2015;9:375–387. doi: 10.1007/s11764-014-0387-x. [DOI] [PubMed] [Google Scholar]
- 13.Mitchell T, Turton P. ‘Chemobrain’: concentration and memory effects in people receiving chemotherapy—A descriptive phenomenological study. Eur. J. Cancer Care. 2011;20:539–548. doi: 10.1111/j.1365-2354.2011.01244.x. [DOI] [PubMed] [Google Scholar]
- 14.Janelsins MC, Kesler SR, Ahles TA, Morrow GR. Prevalence, mechanisms, and management of cancer-related cognitive impairment. Int. Rev. Psychiatry. 2014;26:102–113. doi: 10.3109/09540261.2013.864260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lange M, et al. Decline in cognitive function in older adult swith early-stage breast cancer after adjuvant treatment. Oncologist. 2016;21:1337–1348. doi: 10.1634/theoncologist.2016-0014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Greendale GA, Derby CA, Maki PM. Perimenopause and Cognition. Obstet. Gynecol. Clin. North Am. 2011;38:519–535. doi: 10.1016/j.ogc.2011.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vardy J. Cognitive Function in Breast Cancer Survivors. Springer; 2009. pp. 387–419. [DOI] [PubMed] [Google Scholar]
- 18.Wefel JS, Lenzi R, Theriault RL, Davis RN, Meyers CA. The cognitive sequelae of standard-dose adjuvant chemotherapy in women with breast carcinoma: Results of a prospective, randomized, longitudinal trial. Cancer. 2004;100:2292–2299. doi: 10.1002/cncr.20272. [DOI] [PubMed] [Google Scholar]
- 19.Lange M, et al. Baseline cognitive functions among elderly patients with localised breast cancer. Eur. J. Cancer. 2014;50:2181–2189. doi: 10.1016/j.ejca.2014.05.026. [DOI] [PubMed] [Google Scholar]
- 20.Jansen CE, Dodd MJ, Miaskowski CA, Dowling GA, Kramer J. Preliminary results of a longitudinal study of changes in cognitive function in breast cancer patients undergoing chemotherapy with doxorubicin and cyclophosphamide. Psychooncology. 2008;17:1189–1195. doi: 10.1002/pon.1342. [DOI] [PubMed] [Google Scholar]
- 21.Lange M, Joly F. How to identify and manage cognitive dysfunction after breast cancer treatment. J. Oncol. Pract. 2017;13:784–790. doi: 10.1200/jop.2017.026286. [DOI] [PubMed] [Google Scholar]
- 22.Inagaki M, et al. Smaller regional volumes of brain gray and white matter demonstrated in breast cancer survivors exposed to adjuvant chemotherapy. Cancer. 2007;109:146–156. doi: 10.1002/cncr.22368. [DOI] [PubMed] [Google Scholar]
- 23.McDonald BC, Conroy SK, Ahles TA, West JD, Saykin AJ. Gray matter reduction associated with systemic chemotherapy for breast cancer: A prospective MRI study. Breast Cancer Res. Treat. 2010;123:819–828. doi: 10.1007/s10549-010-1088-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lv L, Mao S, Dong H, Hu P, Dong R. Pathogenesis, assessments, and management of chemotherapy-related cognitive impairment (CRCI): An updated literature review. J. Oncol. 2020;2020:3942439. doi: 10.1155/2020/3942439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brezden CB, Phillips KA, Abdolell M, Bunston T, Tannock IF. Cognitive function in breast cancer patients receiving adjuvant chemotherapy. J. Clin. Oncol. 2000;18:2695–2701. doi: 10.1200/jco.2000.18.14.2695. [DOI] [PubMed] [Google Scholar]
- 26.Fan HGM, et al. Fatigue, menopausal symptoms, and cognitive function in women after adjuvant chemotherapy for breast cancer: 1- and 2-Year follow-up of a prospective controlled study. J. Clin. Oncol. 2005;23:8025–8032. doi: 10.1200/JCO.2005.01.6550. [DOI] [PubMed] [Google Scholar]
- 27.Munn Z, M. S., Lisy K, Riitano D, Tufanaru C. Chapter 5: Systematic reviews of prevalence and incidence. In: JBI Manual for Evidence Synthesis (eds Aromataris, E., & Munn, Z.) (JBI, 2020). Available from https://synthesismanual.jbi.global. 10.46658/JBIMES-20-06.
- 28.Page MJ, et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. doi: 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rodríguez Martín B, Fernández Rodríguez EJ, Rihuete Galve MI, Cruz Hernández JJ. Study of chemotherapy-induced cognitive impairment in women with breast cancer. Int. J. Environ. Res. Public Health. 2020;17:8896. doi: 10.3390/ijerph17238896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Addington-Hall J, McCarthy M. Dying from cancer: Results of a national population-based investigation. Palliat Med. 1995;9:295–305. doi: 10.1177/026921639500900404. [DOI] [PubMed] [Google Scholar]
- 31.Gehring K, Aaronson NK, Taphoorn MJ, Sitskoorn MM. Interventions for cognitive deficits in patients with a brain tumor: An update. Expert Rev. Anticancer Ther. 2010;10:1779–1795. doi: 10.1586/era.10.163. [DOI] [PubMed] [Google Scholar]
- 32.Treanor CJ, et al. Non-pharmacological interventions for cognitive impairment due to systemic cancer treatment. Cochrane Database Syst Rev. 2016 doi: 10.1002/14651858.CD011325.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Craig C, Monk B, Farley J, Chase D. Cognitive impairment in gynecologic cancers: A systematic review of current approaches to diagnosis and treatment. Support Care Cancer. 2013;22:279–287. doi: 10.1007/s00520-013-2029-7. [DOI] [PubMed] [Google Scholar]
- 34.Munn Z, Moola S, Lisy K, Riitano D, Tufanaru C. Methodological guidance for systematic reviews of observational epidemiological studies reporting prevalence and cumulative incidence data. Int. J. Evid. Based Healthc. 2015;13:147–153. doi: 10.1097/xeb.0000000000000054. [DOI] [PubMed] [Google Scholar]
- 35.Higgins. J. P. T. T. J., Chandler, J., Cumpston, M., Li, T., Page, M. J., Welch, V. A. (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 6.2 (updated February 2021) (Cochrane, 2021). Available from www.training.cochrane.org/handbook.
- 36.Peters JL, Mengersen KL. Meta-analysis of repeated measures study designs. J. Eval. Clin. Pract. 2008;14:941–950. doi: 10.1111/j.1365-2753.2008.01010.x. [DOI] [PubMed] [Google Scholar]
- 37.Doi SA, Thalib L. A quality-effects model for meta-analysis. Epidemiology. 2008;19:94–100. doi: 10.1097/EDE.0b013e31815c24e7. [DOI] [PubMed] [Google Scholar]
- 38.Barendregt J, Doi S, Lee YY, Pacella R, Vos T. Meta-analysis of prevalence. J. Epidemiol. Commun. Health. 2013;67:974–978. doi: 10.1136/jech-2013-203104. [DOI] [PubMed] [Google Scholar]
- 39.Furuya-Kanamori L, Barendregt JJ, Doi SAR. A new improved graphical and quantitative method for detecting bias in meta-analysis. Int. J. Evid. Based Healthc. 2018;16:195–203. doi: 10.1097/xeb.0000000000000141. [DOI] [PubMed] [Google Scholar]
- 40.Fontes F, Pereira S, Castro-Lopes JM, Lunet N. A prospective study on the neurological complications of breast cancer and its treatment: Updated analysis three years after cancer diagnosis. Breast. 2016;29:31–38. doi: 10.1016/j.breast.2016.06.013. [DOI] [PubMed] [Google Scholar]
- 41.Schrauwen W, et al. Heterogeneous response of chemotherapy-related cognitive decline in patients with breast cancer: A prospective study. J. Int. Neuropsychol. Soc. 2020;26:806–814. doi: 10.1017/S1355617720000296. [DOI] [PubMed] [Google Scholar]
- 42.van Dam FSAM, et al. Impairment of cognitive function in women receiving adjuvant treatment for high-risk breast cancer: High-dose versus standard-dose chemotherapy. J. Natl. Cancer Inst. 1998;90:210–218. doi: 10.1093/jnci/90.3.210. [DOI] [PubMed] [Google Scholar]
- 43.Pillai US, et al. Late effects of breast cancer treatment and outcome after corrective interventions. Asian Pac. J. Cancer Prev. 2019;20:2673–2679. doi: 10.31557/APJCP.2019.20.9.2673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Syarif H, Waluyo A, Afiyanti Y, Mansyur M. Executive function in breast cancer survivors and the influencing factors. Enferm. Clin. 2019;29(Suppl 2):280–285. doi: 10.1016/j.enfcli.2019.04.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hurria A, et al. Effect of adjuvant breast cancer chemotherapy on cognitive function from the older patient's perspective. Breast Cancer Res. Treat. 2006;98:343–348. doi: 10.1007/s10549-006-9171-6. [DOI] [PubMed] [Google Scholar]
- 46.Hurria A, et al. Cognitive function of older patients receiving adjuvant chemotherapy for breast cancer: A pilot prospective longitudinal study. J. Am. Geriatr. Soc. 2006;54:925–931. doi: 10.1111/j.1532-5415.2006.00732.x. [DOI] [PubMed] [Google Scholar]
- 47.Buwalda B, Schagen SB. Is basic research providing answers if adjuvant anti-estrogen treatment of breast cancer can induce cognitive impairment? Life Sci. 2013;93:581–588. doi: 10.1016/j.lfs.2012.12.012. [DOI] [PubMed] [Google Scholar]
- 48.Shilling V, Jenkins V, Fallowfield L, Howell T. The effects of hormone therapy on cognition in breast cancer. J. Steroid Biochem. Mol. Biol. 2003;86:405–412. doi: 10.1016/j.jsbmb.2003.07.001. [DOI] [PubMed] [Google Scholar]
- 49.Biglia N, et al. Objective and self-reported cognitive dysfunction in breast cancer women treated with chemotherapy: A prospective study. Eur. J. Cancer Care. 2012;21:485–492. doi: 10.1111/j.1365-2354.2011.01320.x. [DOI] [PubMed] [Google Scholar]
- 50.Debess J, Riis JØ, Engebjerg MC, Ewertz M. Cognitive function after adjuvant treatment for early breast cancer: A population-based longitudinal study. Breast Cancer Res. Treat. 2010;121:91–100. doi: 10.1007/s10549-010-0756-8. [DOI] [PubMed] [Google Scholar]
- 51.Jenkins V, et al. A 3-year prospective study of the effects of adjuvant treatments on cognition in women with early stage breast cancer. Br. J. Cancer. 2006;94:828–834. doi: 10.1038/sj.bjc.6603029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ng T, et al. Distinct and heterogeneous trajectories of self-perceived cognitive impairment among Asian breast cancer survivors. Psychooncology. 2018;27:1185–1192. doi: 10.1002/pon.4635. [DOI] [PubMed] [Google Scholar]
- 53.Schagen SB, et al. Cognitive deficits after postoperative adjuvant chemotherapy for breast carcinoma. Cancer. 1999;85:640–650. doi: 10.1002/(SICI)1097-0142(19990201)85:3<640::AID-CNCR14>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 54.Buchanan ND, et al. Post-treatment neurocognition and psychosocial care among breast cancer survivors. Am. J. Prev. Med. 2015;49:S498–S508. doi: 10.1016/j.amepre.2015.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ganz PA, et al. Cognitive complaints after breast cancer treatments: Examining the relationship with neuropsychological test performance. J. Natl. Cancer Inst. 2013;105:791–801. doi: 10.1093/jnci/djt073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Janelsins MC, et al. Cognitive complaints in survivors of breast cancer after chemotherapy compared with age-matched controls: An analysis from a nationwide, multicentre, prospective longitudinal study. J. Clin. Oncol. 2017;35:506–514. doi: 10.1200/jco.2016.68.5826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rey D, et al. Self-reported cognitive impairment after breast cancer treatment in young women from the ELIPPSE40 cohort: The long-term impact of chemotherapy. Breast J. 2012;18:406–414. doi: 10.1111/j.1524-4741.2012.01275.x. [DOI] [PubMed] [Google Scholar]
- 58.Schmidt JE, et al. Prevalence of perceived cognitive dysfunction in survivors of a wide range of cancers: Results from the 2010 LIVESTRONG survey. J. Cancer Surviv. 2016;10:302–311. doi: 10.1007/s11764-015-0476-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Shilling V, Jenkins V. Self-reported cognitive problems in women receiving adjuvant therapy for breast cancer. Eur. J. Oncol. Nurs. 2007;11:6–15. doi: 10.1016/j.ejon.2006.02.005. [DOI] [PubMed] [Google Scholar]
- 60.Tager FA, et al. The cognitive effects of chemotherapy in post-menopausal breast cancer patients: A controlled longitudinal study. Breast Cancer Res. Treat. 2010;123:25–34. doi: 10.1007/s10549-009-0606-8. [DOI] [PubMed] [Google Scholar]
- 61.Prokasheva S, Faran Y, Cwikel J, Geffen DB. Analysis of memory deficits following chemotherapy in breast cancer survivors: Evidence from the doors and people test. J. Psychosoc. Oncol. 2011;29:499–514. doi: 10.1080/07347332.2011.600751. [DOI] [PubMed] [Google Scholar]
- 62.Ramalho M, Fontes F, Ruano L, Pereira S, Lunet N. Cognitive impairment in the first year after breast cancer diagnosis: A prospective cohort study. Breast. 2017;32:173–178. doi: 10.1016/j.breast.2017.01.018. [DOI] [PubMed] [Google Scholar]
- 63.Tchen N, et al. Cognitive function, fatigue, and menopausal symptoms in women receiving adjuvant chemotherapy for breast cancer. J. Clin. Oncol. 2003;21:4175–4183. doi: 10.1200/JCO.2003.01.119. [DOI] [PubMed] [Google Scholar]
- 64.Andryszak P, Wiłkość M, Żurawski B, Izdebski P. Verbal memory in breast cancer patients treated with chemotherapy with doxorubicin and cyclophosphamide. Eur. J. Cancer Care. 2018;27(1):1–11. doi: 10.1111/ecc.12749. [DOI] [PubMed] [Google Scholar]
- 65.Collins B, Mackenzie J, Stewart A, Bielajew C, Verma S. Cognitive effects of chemotherapy in post-menopausal breast cancer patients 1 year after treatment. Psychooncology. 2009;18:134–143. doi: 10.1002/pon.1379. [DOI] [PubMed] [Google Scholar]
- 66.Collins B, MacKenzie J, Tasca GA, Scherling C, Smith A. Cognitive effects of chemotherapy in breast cancer patients: A dose-response study. Psychooncology. 2013;22:1517–1527. doi: 10.1002/pon.3163. [DOI] [PubMed] [Google Scholar]
- 67.Collins B, Mackenzie J, Tasca GA, Scherling C, Smith A. Persistent cognitive changes in breast cancer patients 1 year following completion of chemotherapy. J. Int. Neuropsychol. Soc. 2014;20:370–379. doi: 10.1017/s1355617713001215. [DOI] [PubMed] [Google Scholar]
- 68.Hermelink K, et al. Cognitive function during neoadjuvant chemotherapy for breast cancer: Results of a prospective, multicenter, longitudinal study. Cancer. 2007;109:1905–1913. doi: 10.1002/cncr.22610. [DOI] [PubMed] [Google Scholar]
- 69.Hermelink K, et al. Chemotherapy and Post-traumatic Stress in the Causation of Cognitive Dysfunction in Breast Cancer Patients. J Natl Cancer Inst. 2017 doi: 10.1093/jnci/djx057. [DOI] [PubMed] [Google Scholar]
- 70.Jansen CE, Cooper BA, Dodd MJ, Miaskowski CA. A prospective longitudinal study of chemotherapy-induced cognitive changes in breast cancer patients. Support Care Cancer. 2011;19:1647–1656. doi: 10.1007/s00520-010-0997-4. [DOI] [PubMed] [Google Scholar]
- 71.Jim HSL, et al. Cognitive functioning in breast cancer survivors: A controlled comparison. Cancer. 2009;115:1776–1783. doi: 10.1002/cncr.24192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Jung MS, Cimprich B. Cognitive deficits in Korean women treated with chemotherapy for breast cancer. Cancer Nurs. 2014;37:E31–E42. doi: 10.1097/NCC.0b013e3182980383. [DOI] [PubMed] [Google Scholar]
- 73.Kesler SR, et al. Predicting long-term cognitive outcome following breast cancer with pre-treatment resting state fMRI and random forest machine learning. Front. Hum. Neurosci. 2017;11:555. doi: 10.3389/fnhum.2017.00555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kreukels BPC, Van Dam FSAM, Ridderinkhof KR, Boogerd W, Schagen SB. Persistent neurocognitive problems after adjuvant chemotherapy for breast cancer. Clin. Breast Cancer. 2008;8:80–87. doi: 10.3816/CBC.2008.n.006. [DOI] [PubMed] [Google Scholar]
- 75.Mehlsen M, Pedersen AD, Jensen AB, Zachariae R. No indications of cognitive side-effects in a prospective study of breast cancer patients receiving adjuvant chemotherapy. Psychooncology. 2009;18:248–257. doi: 10.1002/pon.1398. [DOI] [PubMed] [Google Scholar]
- 76.Mehnert A, et al. The association between neuropsychological impairment, self-perceived cognitive deficits, fatigue and health related quality of life in breast cancer survivors following standard adjuvant versus high-dose chemotherapy. Patient Educ. Couns. 2007;66:108–118. doi: 10.1016/j.pec.2006.11.005. [DOI] [PubMed] [Google Scholar]
- 77.Menning S, et al. Cognitive impairment in a subset of breast cancer patients after systemic therapy—results from a longitudinal study. J. Pain Symptom Manag. 2016;52:560–569.e561. doi: 10.1016/j.jpainsymman.2016.04.012. [DOI] [PubMed] [Google Scholar]
- 78.Reid-Arndt SA, Yee A, Perry MC, Hsieh C. Cognitive and psychological factors associated with early posttreatment functional outcomes in breast cancer survivors. J. Psychosoc. Oncol. 2009;27:415–434. doi: 10.1080/07347330903183117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Reid-Arndt SA, Hsieh C, Perry MC. Neuropsychological functioning and quality of life during the first year after completing chemotherapy for breast cancer. Psychooncology. 2010;19:535–544. doi: 10.1002/pon.1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ruzich M, Ryan B, Owen C, Delahunty A, Stuart-Harris R. Prospective evaluation of cognitive function in patients with early breast cancer receiving adjuvant chemotherapy. Asia Pac. J. Clin. Oncol. 2007;3:125–133. doi: 10.1111/j.1743-7563.2007.00109.x. [DOI] [Google Scholar]
- 81.Schagen SB, et al. Late effects of adjuvant chemotherapy on cognitive function: A follow-up study in breast cancer patients. Ann. Oncol. 2002;13:1387–1397. doi: 10.1093/annonc/mdf241. [DOI] [PubMed] [Google Scholar]
- 82.Schagen SB, Muller MJ, Boogerd W, Mellenbergh GJ, van Dam FSAM. Change in cognitive function after chemotherapy: A prospective longitudinal study in breast cancer patients. J. Natl. Cancer Inst. 2006;98:1742–1745. doi: 10.1093/jnci/djj470. [DOI] [PubMed] [Google Scholar]
- 83.Shilling V, Jenkins V, Morris R, Deutsch G, Bloomfield D. The effects of adjuvant chemotherapy on cognition in women with breast cancer–preliminary results of an observational longitudinal study. Breast. 2005;14:142–150. doi: 10.1016/j.breast.2004.10.004. [DOI] [PubMed] [Google Scholar]
- 84.Stewart A, et al. The cognitive effects of adjuvant chemotherapy in early stage breast cancer: A prospective study. Psychooncology. 2008;17:122–130. doi: 10.1002/pon.1210. [DOI] [PubMed] [Google Scholar]
- 85.Stouten-Kemperman MM, et al. Very late treatment-related alterations in brain function of breast cancer survivors. J. Int. Neuropsych. Soc. 2015;21:50–61. doi: 10.1017/S1355617714001015. [DOI] [PubMed] [Google Scholar]
- 86.Van Dyk K, Bower JE, Crespi CM, Petersen L, Ganz PA. Cognitive function following breast cancer treatment and associations with concurrent symptoms. npi Breast Cancer. 2018 doi: 10.1038/s41523-018-0076-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Vearncombe KJ, et al. Predictors of cognitive decline after chemotherapy in breast cancer patients. J. Int. Neuropsych. Soc. 2009;15:951–962. doi: 10.1017/S1355617709990567. [DOI] [PubMed] [Google Scholar]
- 88.Wefel JS, Saleeba AK, Buzdar AU, Meyers CA. Acute and late onset cognitive dysfunction associated with chemotherapy in women with breast cancer. Cancer. 2010;116:3348–3356. doi: 10.1002/cncr.25098. [DOI] [PubMed] [Google Scholar]
- 89.Wieneke MH, Dienst ER. Neuropsychological assessment of cognitive functioning following chemotherapy for breast cancer. Psychooncology. 1995;4:61–66. doi: 10.1002/pon.2960040108. [DOI] [Google Scholar]
- 90.Jackson D, Turner R. Power analysis for random-effects meta-analysis. Res. Synth. Methods. 2017;8:290–302. doi: 10.1002/jrsm.1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Castellon SA, et al. Neurocognitive performance in breast cancer survivors exposed to adjuvant chemotherapy and tamoxifen. J. Clin. Exp. Neuropsychol. 2004;26:955–969. doi: 10.1080/13803390490510905. [DOI] [PubMed] [Google Scholar]
- 92.Krolak D, Collins B, Weiss L, Harris C, Van der Jagt R. Cognitive function and its relationship to other psychosocial factors in lymphoma survivors. Support Care Cancer. 2017;25:905–913. doi: 10.1007/s00520-016-3480-z. [DOI] [PubMed] [Google Scholar]
- 93.Boscher C, et al. Perceived cognitive impairment in breast cancer survivors and its relationships with psychological factors. Cancers Basel. 2020;12:3000. doi: 10.3390/cancers12103000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Schagen SB, Das E, Vermeulen I. Information about chemotherapy-associated cognitive problems contributes to cognitive problems in cancer patients. Psychooncology. 2012;21:1132–1135. doi: 10.1002/pon.2011. [DOI] [PubMed] [Google Scholar]
- 95.Vardy JL, et al. Cognitive function in patients with colorectal cancer who do and do not receive chemotherapy: A prospective, longitudinal controlled study. J. Clin. Oncol. 2015;33:4085–4092. doi: 10.1200/jco.2015.63.0905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Bray VJ, Dhillon HM, Vardy JL. Systematic review of self-reported cognitive function in cancer patients following chemotherapy treatment. J. Cancer Surviv. 2018;12:537–559. doi: 10.1007/s11764-018-0692-x. [DOI] [PubMed] [Google Scholar]
- 97.Argyriou AA, Assimakopoulos K, Iconomou G, Giannakopoulou F, Kalofonos HP. Either called “Chemobrain” or “Chemofog”, the long-term chemotherapy-induced cognitive decline in cancer survivors is real. J. Pain Symptom Manage. 2011;41:126–139. doi: 10.1016/j.jpainsymman.2010.04.021. [DOI] [PubMed] [Google Scholar]
- 98.Bellens A, Roelant E, Sabbe B, Peeters M, van Dam PA. A video-game based cognitive training for breast cancer survivors with cognitive impairment: A prospective randomized pilot trial. Breast. 2020;53:23–32. doi: 10.1016/j.breast.2020.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Wefel JS, Vardy J, Ahles T, Schagen SB. International cognition and cancer task force recommendations to harmonise studies of cognitive function in patients with cancer. Lancet Oncol. 2011;12(7):703–708. doi: 10.1016/S1470-2045(10)70294-1. [DOI] [PubMed] [Google Scholar]
- 100.Neo J, Fettes L, Gao W, Higginson IJ, Maddocks M. Disability in activities of daily living among adults with cancer: A systematic review and meta-analysis. Cancer Treat. Rev. 2017;61:94–106. doi: 10.1016/j.ctrv.2017.10.006. [DOI] [PubMed] [Google Scholar]
- 101.Loh KP, et al. Chemotherapy-related cognitive impairment in older patients with cancer. J. Geriatr. Oncol. 2016;7:270–280. doi: 10.1016/j.jgo.2016.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Hunter JP, et al. In meta-analyses of proportion studies, funnel plots were found to be an inaccurate method of assessing publication bias. J. Clin. Epidemiol. 2014;67:897–903. doi: 10.1016/j.jclinepi.2014.03.003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.