ABSTRACT
Background
In nonpregnant populations, higher serum ferritin, which reflects high iron stores, is associated with an increased risk of hypertension. We hypothesized that a dysregulated maternal iron status in early pregnancy may lead to impaired gestational hemodynamic adaptations, leading to an increased risk of gestational hypertensive disorders.
Objectives
We examined the associations of maternal iron status with maternal blood pressure, placental hemodynamic parameters, and the risks of gestational hypertensive disorders.
Methods
In a population-based prospective cohort study among 5983 pregnant women, we measured maternal serum ferritin, transferrin saturation, serum iron, and transferrin concentrations at a median of 13.2 weeks gestation (95% range, 9.6–17.6). Maternal blood pressure was measured in early pregnancy, mid pregnancy, and late pregnancy, and placental hemodynamic parameters in mid pregnancy and late pregnancy were measured by ultrasound. Information on gestational hypertensive disorders was collected from medical records. We examined the associations of maternal early pregnancy iron status with maternal systolic and diastolic blood pressure, placental hemodynamic parameters, and the risks of gestational hypertensive disorders using linear and logistic regression models.
Results
Higher maternal early pregnancy serum ferritin concentrations were associated with higher systolic and diastolic blood pressure throughout pregnancy in the basic models (P values < 0.05). After adjustment for maternal inflammation, sociodemographic and lifestyle factors, higher maternal early pregnancy serum ferritin concentrations were only associated with a higher early pregnancy diastolic blood pressure [0.27 (95% CI, 0.03–0.51) mmHg per SD score increase in serum ferritin] and with a higher mid pregnancy umbilical artery pulsatility index (P < 0.05). No associations with the risk of gestational hypertensive disorders were present.
Conclusions
No consistent associations were present of maternal iron status in early pregnancy with gestational hemodynamic adaptations or the risks of gestational hypertensive disorders. Further studies are needed to examine the potential role of iron metabolism in the development of gestational hypertensive disorders within higher-risk populations.
Keywords: pregnancy, iron, serum ferritin, placental hemodynamics, gestational hypertensive disorders
Introduction
Gestational hypertensive disorders, which include gestational hypertension and preeclampsia, are a leading cause of maternal and neonatal morbidity and mortality (1). Gestational hypertension is characterized by the late onset of hypertension in pregnancy in previously normotensive women, while preeclampsia is defined as gestational hypertension with the presence of high protein levels in the urine (2). Both high and low maternal hemoglobin concentrations in early pregnancy have been associated with elevated blood pressure levels during pregnancy, impaired placental function, and a higher risk of gestational hypertensive disorders (3–6). The underlying pathophysiological mechanisms for these associations are unclear, but it has been hypothesized that a dysregulated iron status may play a role.
A dysregulated iron status can cause oxidative stress. Iron overload leads to more production of reactive oxygen species (ROS), whereas iron deficiency can cause leakage of ROS through mitochondrial damage (7, 8). Oxidative stress leads to endothelial damage and impaired vasoreactivity, which may negatively affect placental development and gestational hemodynamic adaptations, predisposing women to the development of gestational hypertensive disorders (9–13). Already in nonpregnant populations, increased serum ferritin concentrations, which reflect high iron stores, have been associated with the risk of hypertension, increased arterial stiffness, and a higher risk of cardiovascular disease (14–24). In pregnant populations, far less is known about the influence of maternal iron status in early pregnancy on gestational hemodynamic adaptations and the risk of gestational hypertensive disorders (25–29). Two observational studies among 484 healthy Polish pregnant women and 57 healthy nulliparous American women reported that lower serum iron concentrations at 12 weeks gestation were associated with a higher risk of gestational hypertensive disorders (25, 26). In contrast, an observational study among 47 pregnant women with diabetes mellitus reported no association of serum iron concentrations at 12 weeks gestation with the risk of preeclampsia (27). On the contrary, iron supplementation in early pregnancy has been associated with a higher risk of gestational hypertensive disorders (28, 29).
We hypothesized that both decreased and increased maternal iron store concentrations are associated with higher maternal blood pressure and impaired placental vascular function throughout pregnancy, leading to a higher risk of gestational hypertensive disorders. Therefore, we examined the associations of maternal early pregnancy iron status with maternal systolic and diastolic blood pressure during pregnancy, placental hemodynamic parameters, and the risks of gestational hypertensive disorders within a population-based cohort of 5983 multi-ethnic pregnant women.
Methods
Study design and study sample
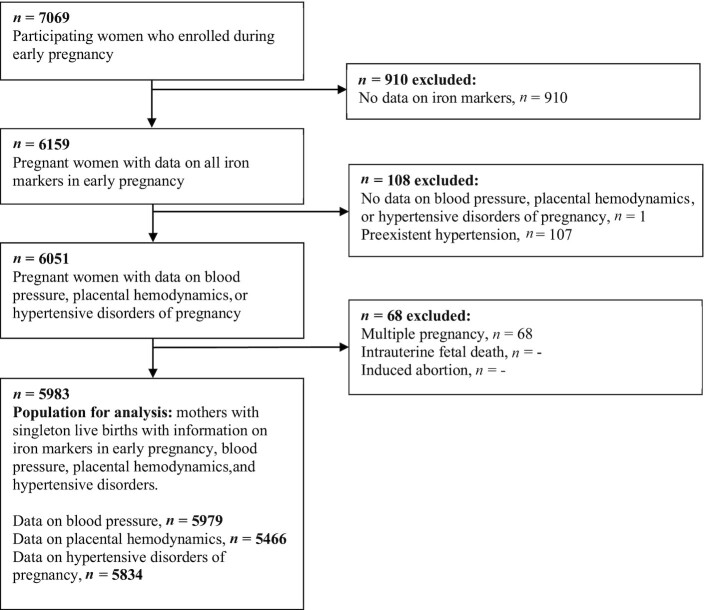
This study was embedded in the Generation R Study, a prospective, population-based cohort study from early pregnancy onwards in Rotterdam, the Netherlands (30). Written informed consent was obtained for all participants. The study was approved by the Medical Ethical Committee of the Erasmus MC, University Medical Center Rotterdam (MEC 198.782/2001/31). In total, 7069 women enrolled in early pregnancy. Data on maternal iron markers in early pregnancy was available for 6159 women. We excluded women with preexistent hypertension (n = 107), multiple pregnancies (n = 68), and absent outcome data (n = 1). The total population for analysis consisted of 5983 women (Figure 1).
FIGURE 1.
Flowchart of the study population.
Maternal iron status
Serum ferritin, serum iron, and transferrin concentrations were measured from maternal nonfasting venous blood samples that were collected during early pregnancy (median = 13.2 weeks gestation; 95% range, 9.6–17.6) (31). Ferritin was measured by electrochemiluminescence immunoassay on the Cobas e411 analyzer (Roche). Iron was measured by colorimetric assay and transferrin by immunoturbidimetric assay, both by the C502 module on the Cobas 8000 (Roche) (32). As serum ferritin is considered the gold standard to assess iron stores, we used serum ferritin concentrations as our primary exposure (33). To obtain further insight into maternal iron status, we additionally used transferrin saturation (TSAT), serum iron, and transferrin as secondary exposures. These measures provide additional information on the bioavailability of iron in the body. TSAT was calculated using serum iron and transferrin concentrations [TSAT = (serum iron × 100)/(transferrin × 25.1)] to reflect the iron-bound part of the total iron binding capacity.
We constructed quintiles of all iron markers to assess whether associations were restricted to lower or higher iron stores and to explore potential nonlinear effects. We also constructed standard deviation scores (SDS) of all iron markers to assess the continuous associations across the full range. Serum ferritin was log-transformed prior to the construction of the SDS due to its skewed distribution.
Gestational hemodynamic adaptations
Systolic and diastolic blood pressure were measured in early pregnancy (median = 13.2 weeks gestation; 95% range, 9.6–17.6), mid pregnancy (median = 20.5 weeks gestation; 95% range, 18.7–23.1), and late pregnancy (median = 30.4 weeks gestation; 95% range, 28.6–32.8). An OMRON 907 automated digital oscillometric sphygmomanometer (OMRON Healthcare Europe BV) was used to perform the blood pressure measurements, as previously described (34, 35). Two blood pressure measurements were performed at a 60-second interval, from which the mean blood pressure was calculated (34, 35).
Ultrasounds were performed in mid pregnancy (median = 20.5 weeks gestation; 95% range, 18.7–23.1 weeks) and late pregnancy (median = 30.4 weeks gestation; 95% range, 28.6–32.8 weeks). Mid pregnancy and late pregnancy placental vascular resistance were evaluated with recorded flow velocity waveforms from the uterine and umbilical arteries (36). The umbilical artery pulsatility index (UmPI) was measured in a free‐floating loop of the umbilical cord. The uterine artery resistance index (UtRI) was measured in the uterine arteries near the crossover with the external iliac artery. Higher UmPI and UtRI values indicate increased placental vascular resistance. The presence of uterine artery notching was assessed. The presence of uterine artery notching reflects an increase in resistance to blood flowing into the placenta and is used for identification of high‐risk pregnancies.
Gestational hypertensive disorders
The gestational hypertensive disorder status was obtained from medical records, which were cross-checked with the original hospital charts (37). Gestational hypertension was defined as a diastolic blood pressure of at least 90 mmHg and/or systolic blood pressure of at least 140 mmHg after 20 weeks of gestation in women without previous hypertension. Preeclampsia was defined as gestational hypertension, including the presence of proteinuria (defined as a 24-hour urine sample containing at least 300 mg of protein, 1 catheter sample reading of at least 1+, or 2 or more dipstick readings of at least 2+) (2).
Covariates
Information on maternal age, ethnicity, prepregnancy weight, educational level, parity, folic acid supplementation, and maternal smoking was obtained from questionnaires. Ethnicity was categorized as European and non-European. European ethnicity included Dutch and other European ethnicities. Non-European ethnicity included Indonesian, Cape Verdian, Moroccan, Antillean, Surinamese, Turkish, African, American (Western and non-Western), Asian (Western and non-Western), and Oceanian ethnicities. Height was measured at the intake visit and was used to calculate the prepregnancy BMI. Maternal hemoglobin and C-reactive protein (CRP) concentrations were measured in the same nonfasting venous blood samples that were used for the measurement of the iron markers, as described previously (38).
Statistical analyses
First, we performed a nonresponse analysis comparing women with information on early pregnancy maternal iron markers to those without. Second, we used 1-way ANOVAs or chi-square tests to compare the participant characteristics across serum ferritin quartiles. Third, we examined the associations of maternal serum ferritin in categories (quintiles) and serum ferritin continuously (SDS) with systolic and diastolic blood pressure in early pregnancy, mid pregnancy, and late pregnancy; and UmPI and UtRI in mid pregnancy and late pregnancy using linear regression models. We examined the associations of maternal serum ferritin quintiles and SDS with the risk of bilateral uterine artery notching using logistic regression models. Fourth, we examined the associations of serum ferritin quintiles and SDS with the risk of gestational hypertensive disorders using logistic regression analyses. We constructed 2 different models: 1) a basic model that was adjusted for gestational age at the time of blood sampling and gestational age at the time of blood pressure measurement or ultrasound; and 2) a confounder model that was additionally adjusted for maternal sociodemographic factors, lifestyle factors, and inflammation, including maternal age, ethnicity, educational level, parity, prepregnancy BMI, folic acid supplementation, smoking, and CRP concentrations. Confounders were based on the literature and selected if they were associated with serum ferritin and the outcomes of interest or if they led to a change in the effect estimate of >10% when the covariate was included in the univariate model. As the effects of an impaired maternal iron status may be more pronounced among pregnant women who also have abnormal hemoglobin levels, we calculated the interaction terms of maternal serum ferritin with maternal hemoglobin for each outcome. Only the interaction terms for maternal systolic and diastolic blood pressure were significant, and we repeated these analyses with stratification by maternal hemoglobin levels, defined as low (≤11 g/dl), normal (>11 g/dl and <13.2 g/dl), and high (≥13.2 g/dl) (3).
Next, we performed several sensitivity analyses to examine the robustness of our findings. First, we repeated the analyses using maternal early pregnancy TSAT, serum iron, and transferrin concentrations as secondary exposures that further reflect the bioavailability of maternal iron. Second, as higher serum ferritin concentrations may be explained by acute inflammation, we performed a sensitivity analysis excluding all women with CRP concentrations >10 mg/ml, who may suffer from acute inflammation, while still additionally adjusting for CRP concentrations across the full range to consider the impact of chronic low-grade inflammation. Third, we repeated the analyses with maternal serum ferritin concentrations, additionally adjusted for transferrin concentrations, as physiological changes in transferrin concentrations occur to regulate the bioavailability of the iron stores (39).
We imputed missing data of the covariates using multiple imputation. The percentage of missing values was <10%, with the exception for smoking (11%), prepregnancy BMI (18%), and folic acid supplementation (24%). P values < 0.05 were considered significant. Analyses were performed using Statistical Package of Social Science version 25.0 for Windows (SPSS Inc).
Results
Participant characteristics
Table 1 shows the participant characteristics. Participating women were 30 years old on average, with a mean prepregnancy BMI of 23.5 kg/m2; 40% had higher education, and 57% were nulliparous. The median serum ferritin concentration was 52.2 μg/L (95% range, 9.9–203.7). Iron deficiency was observed in 7.2% (serum ferritin < 15 μg/L) and iron overload was observed in 6.7% (serum ferritin > 150 μg/L) of women (40). No differences in maternal blood pressure, placental hemodynamic parameters, or gestational hypertensive disorders were present for women with data on early pregnancy iron markers compared to those without (Supplementary Table S1).
TABLE 1.
Characteristics of the study population by early pregnancy serum ferritin quintiles (n = 5983)1
| Total group | Serum ferritin quintile 1 | Serum ferritin quintile 2 | Serum ferritin quintile 3 | Serum ferritin quintile 4 | Serum ferritin quintile 5 | ||
|---|---|---|---|---|---|---|---|
| n = 5983 | n = 1196 | n = 1197 | n = 1196 | n = 1198 | n = 1196 | P value2 | |
| Serum ferritin, range, μg/L | 2–390 | 2–26 | 26–42 | 42–63 | 63–96 | 96–390 | |
| Serum ferritin, μg/L | 52.2 [9.9, 203.7] | 17.3 [6.7, 25.9] | 34.2 [26.8, 42.1] | 52.2 [42.8, 62.4] | 76.2 [63.3, 94.2] | 128.4 [96.5, 295.7] | <0.001 |
| TSAT, % | 24.5 ± 10.5 | 18.4 ± 8.9 | 24.0 ± 8.9 | 26.1 ± 9.9 | 26.3 ± 10.4 | 28.0 ± 11.7 | <0.001 |
| Iron, μmol/L | 17.1 ± 6.6 | 14.7 ± 6.5 | 17.5 ± 6.0 | 17.9 ± 6.3 | 17.6 ± 6.5 | 18.0 ± 7.0 | <0.001 |
| Transferrin, g/L | 2.9 ± 0.5 | 3.3 ± 0.5 | 3.0 ± 0.4 | 2.8 ± 0.4 | 2.7 ± 0.4 | 2.6 ± 0.3 | <0.001 |
| Hemoglobin, g/dL | 12.3 ± 0.9 | 12.0 ± 1.0 | 12.3 ± 0.9 | 12.4 ± 0.9 | 12.4 ± 0.9 | 12.4 ± 0.9 | <0.001 |
| CRP, mg/L | 4.5 [0.6, 25.7] | 4.8 [0.7,23.6] | 4.4 [0.6,22.1] | 4.4 [0.7,24.4] | 4.3 [0.6,29.1] | 4.4 [0.5,32.8] | <0.001 |
| Maternal age at enrolment, years | 29.7 ± 5.1 | 29.0 ± 5.6 | 29.4 ± 5.3 | 29.8 ± 5.0 | 30.1 ± 4.8 | 30.5 ± 4.4 | <0.001 |
| Parity, nulliparous, n (%) | 3399 (56.8) | 481 (40.2) | 602 (50.3) | 682 (57.0) | 764 (63.8) | 870 (72.7) | <0.001 |
| Prepregnancy BMI, kg/m2 | 23.5 ± 4.2 | 23.5 ± 4.1 | 23.3 ± 4.2 | 23.3 ± 4.0 | 23.6 ± 4.2 | 23.9 ± 4.4 | <0.001 |
| Gestational age at blood sampling, weeks | 13.2 [9.6, 17.6] | 13.9 [7.6, 17.9] | 13.5 [9.5, 17.7] | 13.2 [9.6, 17.4] | 12.9 [9.5, 17.4] | 12.8 [9.5, 17.1] | <0.001 |
| Higher education, n (%) | 2501 (41.8) | 369 (30.9) | 467 (39.0) | 533 (44.6) | 546 (45.6) | 586 (49.0) | <0.001 |
| European ethnicity,3 n (%) | 3500 (61.0) | 470 (39.3) | 668 (55.8) | 740 (61.9) | 797 (66.5) | 825 (69.0) | <0.001 |
| Continued smoking during pregnancy, n (%) | 990 (16.5) | 188 (15.7) | 228 (19.0) | 212 (17.7) | 195 (16.3) | 167 (14.0) | <0.05 |
| Folic acid supplement use, n (%) | 3413 (57.0) | 532 (44.5) | 634 (53.0) | 703 (58.8) | 755 (63.0) | 789 (66.0) | <0.001 |
| Systolic blood pressure, mmHg | |||||||
| Early pregnancy | 115.3 ± 12.0 | 113.5 ± 11.9 | 114.8 ± 11.8 | 115.1 ± 11.8 | 115.9 ± 12.0 | 116.9 ± 12.3 | <0.001 |
| Mid pregnancy | 116.9 ± 11.8 | 115.5 ± 11.6 | 116.1 ± 11.6 | 116.9 ± 11.5 | 117.2 ± 12.1 | 118.9 ± 11.9 | <0.001 |
| Late pregnancy | 118.3 ± 11.8 | 116.5 ± 11.6 | 117.3 ± 11.4 | 118.6 ± 12.2 | 119.0 ± 12.0 | 119.7 ± 11.8 | <0.001 |
| Diastolic blood pressure, mmHg | |||||||
| Early pregnancy | 67.9 ± 9.3 | 66.8 ± 9.2 | 67.2 ± 9.0 | 67.7 ± 9.2 | 68.6 ± 9.4 | 69.4 ± 9.4 | <0.001 |
| Mid pregnancy | 67.0 ± 9.2 | 66.2 ± 9.3 | 66.4 ± 8.6 | 66.6 ± 9.1 | 67.4 ± 9.5 | 68.5 ± 9.3 | <0.001 |
| Late pregnancy | 68.9 ± 9.2 | 67.7 ± 9.5 | 68.2 ± 8.7 | 68.6 ± 9.2 | 69.6 ± 9.2 | 70.3 ± 9.0 | <0.001 |
| Uterine artery resistance index | |||||||
| Mid pregnancy | 0.54 ± 0.09 | 0.54 ± 0.08 | 0.54 ± 0.09 | 0.54 ± 0.09 | 0.55 ± 0.09 | 0.53 ± 0.09 | 0.08 |
| Late pregnancy | 0.48 ± 0.08 | 0.49 ± 0.08 | 0.49 ± 0.08 | 0.48 ± 0.08 | 0.48 ± 0.08 | 0.48 ± 0.08 | 0.26 |
| Umbilical artery pulsatility index | |||||||
| Mid pregnancy | 1.20 ± 0.18 | 1.18 ± 0.18 | 1.20 ± 0.18 | 1.20 ± 0.18 | 1.21 ± 0.18 | 1.22 ± 0.18 | <0.05 |
| Late pregnancy | 0.98 ± 0.17 | 0.98 ± 0.17 | 0.98 ± 0.17 | 0.98 ± 0.17 | 0.98 ± 0.17 | 0.98 ± 0.17 | 0.94 |
| Bilateral uterine artery notching | 108 ± 1.8 | 19 ± 1.6 | 25 ± 2.1 | 22 ± 1.8 | 20 ± 1.7 | 22 ± 1.8 | 0.90 |
| Gestational hypertensive disorders | |||||||
| Preeclampsia, n (%) | 123 (2.1) | 20 (1.7) | 32 (2.7) | 25 (2.1) | 20 (1.7) | 26 (2.2) | 0.38 |
| Gestational hypertension, n (%) | 228 (3.8) | 26 (2.2) | 35 (2.9) | 44 (3.7) | 47 (3.9) | 76 (6.4) | <0.05 |
Values are shown as the mean ± SD, median [95% range], or count (%). CRP, C-reactive protein; TSAT, transferrin saturation.
Differences between characteristics in serum ferritin quintiles were tested with a 1-way ANOVA for continuous variables and χ2 test for categorical variables.
In the full study population, European ethnicity consisted of 52.5% Dutch and 8.5% other European ethnicities. Non-European ethnicity (39.0%) consisted of 8.5% Surinamese, 8.1% Turkish, 6.0% Moroccan, 4.2% Cape Verdian, 3.0% Indonesian, 2.9% Antillean, 2.7% Asian, 1.9% American, 1.7% African, and 0.1% Oceanian.
Early pregnancy serum ferritin concentrations and blood pressure throughout pregnancy
In the basic models, higher serum ferritin concentrations across the full range were associated with higher systolic and diastolic blood pressure throughout pregnancy (all P values < 0.05; Supplementary Table S2). After adjustment for maternal sociodemographic factors, lifestyle factors, and inflammation, no associations of serum ferritin concentrations in quintiles or across the full range with systolic blood pressure throughout pregnancy were present (Table 2). Higher serum ferritin concentrations across the full range were only associated with higher early pregnancy diastolic blood pressure [0.27 (95% CI, 0.03–0.51) mmHg per SDS increase in serum ferritin], but not with mid pregnancy or late pregnancy diastolic blood pressure. No associations were present for the serum ferritin quintiles with diastolic blood pressure. Analyses stratified for maternal hemoglobin concentrations showed that the strongest effect for serum ferritin concentrations across the full range with early pregnancy diastolic blood pressure was present for women with high hemoglobin concentrations (P value < 0.05; Supplementary Table S3).
TABLE 2.
Associations of early pregnancy serum ferritin with systolic blood pressure and diastolic blood pressure during pregnancy (n = 5979)1
| Early pregnancy | Mid pregnancy | Late pregnancy | ||||
|---|---|---|---|---|---|---|
| Early pregnancy serum ferritin | β (95% CI) | n | β (95% CI) | n | β (95% CI) | n |
| Differences in systolic blood pressure (mmHg) | ||||||
| Quintile 12, 2–26 μg/L | –0.07 (–0.99 to 0.85) | 1189 | –0.09 (-1.02 to 0.83) | 1121 | –0.64 (–1.59 to 0.31) | 1110 |
| Quintile 22, 26–42 μg/L | 0.33 (–0.57 to 1.24) | 1190 | –0.33 (–1.24 to 0.57) | 1141 | –0.75 (–1.67 to 0.17) | 1146 |
| Quintile 3, 42–63 μg/L | reference | 1187 | reference | 1132 | reference | 1127 |
| Quintile 42, 63–96 μg/L | 0.14 (–0.76 to 1.04) | 1190 | –0.36 (–1.26 to 0.54) | 1156 | –0.12 (–1.03 to 0.80) | 1144 |
| Quintile 52, 96–390 μg/L | 0.35 (–0.56 to 1.26) | 1184 | 0.64 (–0.26 to 1.55) | 1159 | –0.08 (–1.01 to 0.84) | 1150 |
| SDS3 | –0.01 (–0.31 to 0.30) | 5940 | 0.20 (–0.10 to 0.51) | 5709 | 0.18 (–0.13 to 0.49) | 5709 |
| Differences in diastolic blood pressure, mmHg | ||||||
| Quintile 12, 2–26 μg/L | –0.23 (–0.94 to 0.48) | 1189 | 0.16 (–0.56 to 0.89) | 1120 | –0.06 (–0.79 to 0.67) | 1110 |
| Quintile 22, 26–42 μg/L | –0.02 (–0.72 to 0.68) | 1190 | 0.07 (–0.64 to 0.78) | 1141 | 0.0 (–0.72 to 0.72) | 1146 |
| Quintile 3, 42–63 μg/L | reference | 1187 | reference | 1132 | reference | 1126 |
| Quintile 42, 63–96 μg/L | 0.53 (–0.16 to 1.23) | 1190 | 0.38 (–0.33 to 1.08) | 1155 | 0.56 (–0.15 to 1.28) | 1144 |
| Quintile 52, 96–390 μg/L | 0.68 (–0.03 to 1.38) | 1184 | 0.90 (0.18–1.61)4 | 1159 | 0.62 (–0.10 to 1.34) | 1150 |
| SDS3 | 0.27 (0.03–0.51)4 | 5940 | 0.23 (–0.01 to 0.47) | 5707 | 0.23 (–0.02 to 0.47) | 5676 |
Models are adjusted for maternal age, ethnicity, educational level, parity, prepregnancy BMI, folic acid supplementation, smoking, gestational age at time of blood sampling, gestational age at time of blood pressure measurements, and CRP levels. CRP, C-reactive protein; SDS, standard deviation score.
Values are regression coefficients (95% CIs) and reflect the difference in mmHg blood pressure per serum ferritin quintile. Groups are compared to women in quintile 3 (serum ferritin: 42 μg/L–63 μg/L) as a reference. Estimates are from multiple imputed data.
Values are regression coefficients (95% CI) and reflect the difference in mmHg blood pressure per log serum ferritin SDS.
4 P value < 0.05.
Early pregnancy serum ferritin concentrations and placental hemodynamic parameters
In the confounder model, as compared to the third serum ferritin quintile, the lowest serum ferritin quintile was associated with a lower midpregnancy UmPI, whereas the highest serum ferritin quintile was associated with a higher mid pregnancy UmPI (all P values < 0.05; Table 3). Higher serum ferritin concentrations across the full range were also associated with a higher mid pregnancy UmPI [0.010 (95% CI, 0.005–0.016) per SDS increase in serum ferritin]. No associations were present with the UtRI or the risk of bilateral uterine artery notching. In the basic models, similar findings were present (Supplementary Table S4).
TABLE 3.
Associations of early pregnancy serum ferritin with umbilical artery pulsatility index, uterine artery resistance index, and third trimester bilateral uterine artery notching (n = 5466)1
| Umbilical artery pulsatility index | Uterine artery resistance index | Bilateral uterine artery notching | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Early pregnancy serum ferritin | Mid pregnancy | Late pregnancy | Mid pregnancy | Late pregnancy | Late pregnancy | |||||
| β (95% CI) | n | β (95% CI) | n | β (95% CI) | n | β (95% CI) | n | OR (95% CI)2 | n cases | |
| Quintile 13, 2–26 μg/L | –0.019 (–0.036 to –0.002)4 | 914 | –0.005 (–0.020 to 0.011) | 925 | 0.003 (–0.007 to 0.012) | 673 | 0.002 (–0.007 to 0.010) | 593 | 0.87 (0.46–1.65) | 19 |
| Quintile 23, 26–42 μg/L | –0.002 (–0.018 to 0.015) | 922 | –0.006 (–0.021 to 0.010) | 953 | 0.005 (–0.004 to 0.015) | 677 | 0.003 (–0.005 to 0.012) | 638 | 1.13 (0.63–2.05) | 25 |
| Quintile 3, 42–63 μg/L | reference | 922 | reference | 957 | reference | 699 | reference | 655 | reference | 22 |
| Quintile 43, 63–96 μg/L | 0.009 (–0.007 to 0.026) | 912 | –0.006 (–0.021 to 0.009) | 974 | 0.009 (0.000–0.018) | 682 | 0.000 (–0.008 to 0.008) | 682 | 0.88 (0.48–1.63) | 20 |
| Quintile 53, 96–390 μg/L | 0.017 (0.000–0.033)4 | 925 | –0.006 (–0.022 to 0.009) | 973 | –0.001 (–0.010 to 0.08) | 699 | –0.005 (–0.013 to 0.004) | 695 | 0.94 (0.51–1.73) | 22 |
| SDS4 | 0.010 (0.005–0.016)4 | 4595 | –0.001 (–0.006 to 0.005) | 4782 | –0.000 (–0.004 to 0.003) | 3430 | –0.002 (–0.005 to 0.001) | 3263 | 1.02 (0.83–1.25) | 108 |
Models are adjusted for maternal age, ethnicity, educational level, parity, prepregnancy BMI, folic acid supplementation, smoking habits, gestational age at time of blood sampling, gestational age at time of ultrasound measurements, and CRP levels. CRP, C-reactive protein; SDS, standard deviation score.
Values are ORs (95% CIs) that reflect differences in risks of third trimester bilateral uterine artery notching per serum ferritin quintile. Groups are compared to women in quintile 3 (serum ferritin: 42 μg/L–63 μg/L) as a reference. Estimates are from multiple imputed data.
Values are regression coefficients (95% CIs) and reflect differences in the umbilical artery pulsatility index and uterine artery resistance index per serum ferritin quintile. Groups are compared to women in quintile 3 (serum ferritin: 42 μg/L–63 μg/L) as a reference. Estimates are from multiple imputed data.
P value < 0.05.
Values are regression coefficients (95% CIs) that reflect the difference in the umbilical artery pulsatility index and uterine artery resistance index per log serum ferritin SDS or ORs (95% CI) that reflect differences in risks of third trimester bilateral uterine artery notching per log serum ferritin SDS.
Early pregnancy serum ferritin concentrations and risks of gestational hypertensive disorders
Table 4 shows that serum ferritin quintiles and serum ferritin concentrations across the full range were not associated with the risks of any gestational hypertensive disorder in the confounder model. In the basic model, the higher serum ferritin quintiles, as compared to the third quintile, and serum ferritin concentrations across the full range were associated with a higher risk of gestational hypertensive disorders and gestational hypertension, but not preeclampsia (all P values < 0.05; Supplementary Table S5).
TABLE 4.
Associations of early pregnancy serum ferritin with hypertensive disorder of pregnancy, gestational hypertension and preeclampsia (n = 5834)1
| Gestational Hypertensive Disorder | Gestational hypertension | Preeclampsia | ||||
|---|---|---|---|---|---|---|
| Early pregnancy serum ferritin | OR (95% CI) | n cases | OR (95% CI) | n cases | OR (95% CI) | n cases |
| Quintile 12, 2–26 μg/L | 0.85 (0.57–1.27) | 46 | 0.86 (0.52–1.44) | 26 | 0.84 (0.45–1.55) | 20 |
| Quintile 22, 26–42 μg/L | 1.10 (0.77–1.57) | 67 | 0.95 (0.60–1.52) | 35 | 1.36 (0.80–2.34) | 32 |
| Quintile 3, 42–63 μg/L | reference | 69 | reference | 44 | reference | 25 |
| Quintile 42, 63–96 μg/L | 0.85 (0.60–1.21) | 67 | 0.90 (0.59–1.39) | 47 | 0.75 (0.41–1.37) | 20 |
| Quintile 52, 96–390 μg/L | 1.20 (0.86–1.66) | 102 | 1.35 (0.91–2.01) | 76 | 0.94 (0.54–1.65) | 26 |
| SDS3 | 1.08 (0.96–1.22) | 351 | 1.15 (0.99–1.34) | 228 | 0.98 (0.81–1.18) | 123 |
Models are adjusted for maternal age, ethnicity, educational level, parity, prepregnancy BMI, folic acid supplementation, smoking, gestational age at time of blood sampling, and CRP levels. CRP, C-reactive protein; SDS, standard deviation score.
Values are ORs (95% CIs) that reflect differences in risks of gestational hypertensive disorder, gestational hypertension, and preeclampsia per serum ferritin quintile. Groups are compared to women in quintile 3 (serum ferritin: 42 μg/L–63 μg/L) as the reference. Estimates are from multiple imputed data.
Values are ORs (95% CIs) that reflect differences in risks of gestational hypertensive disorder, gestational hypertension, and preeclampsia per log serum ferritin as SDS.
Sensitivity analyses
A higher TSAT was associated with a lower systolic and diastolic blood pressure in early pregnancy (P values < 0.05), but not in mid pregnancy or late pregnancy, in the confounder model (Supplementary Table S6). Higher serum iron concentrations were associated with a lower systolic blood pressure in early pregnancy (P value < 0.05), but not in mid pregnancy or late pregnancy, nor with diastolic blood pressure throughout pregnancy, in the confounder model (Supplementary Table S7). Higher transferrin concentrations were associated with higher systolic and diastolic blood pressure throughout pregnancy (P values < 0.05) in the confounder model (Supplementary Table S8). No consistent associations were found for TSAT, serum iron, and transferrin concentrations with placental hemodynamic parameters or the risks of any gestational hypertensive disorder (Supplementary Tables S9–S14). When we excluded women with acute inflammation (CRP > 10mg/L), we found stronger associations for serum ferritin concentrations across the full range with diastolic blood pressure throughout pregnancy as compared to the main analysis for serum ferritin (all P values < 0.05; Supplementary Tables S15–S17). We observed that higher serum ferritin concentrations across the full range were associated with higher systolic and diastolic blood pressure throughout pregnancy (all P values < 0.05) when we additionally adjusted the confounder model for transferrin concentrations, but no associations were present for placental hemodynamic parameters or the risks of gestational hypertensive disorders (Supplementary Tables S18–S20).
Discussion
In this population-based, prospective cohort study, we found no consistent associations of maternal early pregnancy iron status with maternal blood pressure and placental vascular function throughout pregnancy or the risks of gestational hypertensive disorders after considering maternal inflammatory, sociodemographic, and lifestyle factors.
Gestational hypertensive disorders are a leading cause of maternal and neonatal morbidity and mortality (1). Increased oxidative stress has been suggested to play a role in impaired placental development and the pathophysiology of gestational hypertensive disorders (41). Both iron overload and iron deficiency can induce increased levels of oxidative stress (7, 8). High levels of oxidative stress can lead to endothelial damage and impaired vasoreactivity, which may negatively affect placental development and gestational hemodynamic adaptations, predisposing women to the development of gestational hypertensive disorders (9–13). We hypothesized that both higher and lower serum ferritin concentrations in early pregnancy might lead to higher maternal blood pressure, impaired placental hemodynamics, and an increased risk of gestational hypertensive disorders.
Already in nonpregnant populations, it has been suggested that higher serum ferritin concentrations are associated with the risk of hypertension, increased arterial stiffness, and a higher risk of cardiovascular disease, but findings are inconsistent (14–24). Two large prospective observational studies showed that higher serum ferritin concentrations were associated with an increased risk of hypertension (14, 16). However, 2 large prospective observational studies among 4509 Chinese and 2895 French adult men and women showed no associations of serum ferritin concentrations with the risk of hypertension after a more thorough adjustment for potential confounding factors (42, 43). In line with these studies in nonpregnant populations, we did not find consistent associations of maternal iron status in early pregnancy with maternal blood pressure development or placental vascular function throughout pregnancy, after adjustment for maternal inflammation, sociodemographic, and lifestyle-related factors. In the basic models, we observed consistent associations of higher early pregnancy serum ferritin concentrations with higher maternal systolic and diastolic pressure throughout pregnancy, a higher UmPI in mid pregnancy, and a higher risk of gestational hypertensive disorders. After adjustment for maternal inflammation, sociodemographic, and lifestyle factors, only the associations of higher maternal early pregnancy serum ferritin concentrations with a higher diastolic blood pressure in early pregnancy and a higher UmPI in mid pregnancy remained. This indicates that the associations of serum ferritin with our outcomes are, for a large part, explained by sociodemographic and lifestyle-related factors, such as maternal age, BMI, and parity. These observed tendencies may be more pronounced in early pregnancy, since the iron measurements were conducted close to these time points. A high iron status may lead to higher blood pressure during pregnancy and an impaired uteroplacental vascular function due to increased levels of oxidative stress (10, 11, 13). A high iron status may also lead to higher hemoglobin levels, which have been previously associated with higher blood pressure throughout pregnancy (3). In the current study, we indeed observed the strongest effects of high serum ferritin concentrations on early pregnancy diastolic blood pressure among pregnant women who also had a high hemoglobin level. Iron availability is strongly regulated through physiological feedback, which may be even more pronounced in pregnancy, since iron stores are increasingly being mobilized during the course of pregnancy to facilitate placental and fetal development (44). Iron availability in the body is largely influenced by levels of transferrin, which is the main iron carrier in the blood. Transferrin concentrations decrease in the presence of iron overload, but they increase in the presence of iron deficiency to make it readily available for cells to use. We observed that the associations of serum ferritin concentrations with systolic and diastolic blood pressure during pregnancy were stronger when additionally adjusting for transferrin, which may further suggest that relatively higher serum ferritin concentrations negatively affect maternal blood pressure development in pregnancy. However, the observed effect is only small from a clinical perspective, but may be considered important on a population level and from an etiological perspective. We observed no consistent associations for other secondary measures of maternal iron status with blood pressure development in pregnancy. A higher early pregnancy TSAT was associated with lower systolic and diastolic blood pressure in early pregnancy, and higher early pregnancy serum iron was associated with lower systolic blood pressure in early pregnancy only. We cannot explain these findings. They may reflect a chance finding or be related to the measurement of these iron markers. Both TSAT and serum iron are influenced by recent dietary intake and diurnal variation (45). Thus, special care is needed in the interpretation of these findings, since these iron markers were determined from nonfasting blood samples and might not reflect the overall iron status accurately. To summarize, suboptimal maternal iron status in early pregnancy within the normal range was not consistently associated with maternal gestational hemodynamic adaptations after adjustment for inflammatory, sociodemographic, and lifestyle factors. Further studies are needed to explore whether more pronounced iron overload or iron deficiency affects gestational hemodynamic adaptations.
In women that manifest preeclampsia during later stages of pregnancy, apparent differences in iron biomarkers are present when compared to healthy pregnant women (46). A few previous studies investigated the association of serum iron in early pregnancy with the risk of gestational hypertensive disorders (25–27). A case-control study from Poland among 484 healthy pregnant women and an observational study from the United States among 57 healthy nulliparous women found that lower serum iron concentrations from fasting samples at 12 weeks gestation were associated with higher risks of gestational hypertensive disorders (25, 26). However, adjustments for confounders were limited in these studies. In contrast, an observational study among 47 pregnant women with pregestational type 1 diabetes mellitus found no association of serum iron concentrations at 12 weeks gestation with preeclampsia (27). As the gestational age at the time of blood sampling was quite similar in our and previous studies, it seems unlikely that differences in gestational age at measurement of iron status explained any discrepancies between our and previous studies. With regards to iron overload, a retrospective study from Thailand among 400 pregnant women showed that iron supplementation in early pregnancy was associated with an increased risk of gestational hypertensive disorders (28). Similarly, a randomized, placebo-controlled trial among 727 Iranian nonanemic pregnant women reported that iron supplementation in early pregnancy was associated with an increased risk of gestational hypertensive disorders, but the baseline iron status was not determined in either of these studies (29). We did not find consistent associations of maternal early pregnancy iron status with the risk of gestational hypertensive disorders. Different findings in these previous studies and our study may be explained by our nonfasting blood samples, which influence the measurement of serum iron, and our relatively healthy population, since in comparison most of these previous studies had a higher percentage of gestational hypertensive disorders cases. Thus, our findings suggest that within our population, iron status in early pregnancy is not associated with the risk of gestational hypertensive disorders.
The response rate for participating in the Generation R Study was 61% for all the eligible women in the study area at the time of enrollment. Biased estimates are unlikely since they are more commonly caused by loss to follow-up rather than from nonresponse at baseline (47). Moreover, selection on the availability of iron status is unlikely to have affected the generalizability of the results, since we observed no substantial differences in the characteristics of women with data on early pregnancy iron markers compared to those without. Due to the design of our study, the gestational age at the measurement of iron status in early pregnancy was relatively broad. During early placental development, the syncytiotrophoblast can adapt to small increases in ROS by producing antioxidants (48). A dysregulated iron status in early pregnancy may increase the risk of gestational hypertensive disorders through small increases in oxidative stress that negatively influence placental development. In our study, iron markers were measured at a median of 13.2 weeks, partly after early placentation. However, we still consider our markers as adequate proxies of iron status from early gestation onwards, since the relative trend of iron status among the participants is likely to remain similar from conception to early pregnancy. For example, women with relatively low iron levels at conception are likely to continue having lower iron levels in early pregnancy compared to the rest of the study population. As individual absolute iron levels might differ depending on gestational age at blood sampling, we adjusted all analyses for gestational age at the time of blood sampling. We were able to adjust our analyses for maternal sociodemographic and lifestyle factors, but due to the observational nature of this study, residual confounding might still be present due to unmeasured factors such as iron supplementation.
We did not observe consistent associations of maternal iron status in early pregnancy with gestational hemodynamic adaptations or the risk of gestational hypertensive disorders. Our study population was mainly composed of relatively young women with higher education and a prepregnancy BMI within the normal range. We excluded women with preexistent hypertension. Pregnant populations with older women with a higher BMI would be at a higher risk for gestational hypertensive disorders. Furthermore, all markers of iron metabolism were mostly within the normal range. The prevalence of iron deficiency (serum ferritin < 15 μg/L) in our study population was 7%, and was slightly lower than that in the general Dutch population (49). Together, these factors may reflect a selection towards a relatively healthy and lower-risk pregnant population. Effects on gestational hemodynamic adaptations might be more pronounced in women with evidently low or high iron stores or those at higher risk for the development of gestational hypertensive disorders. In addition, the relatively low numbers of cases of iron deficiency, iron overload, and gestational hypertensive disorders may also have led to reduced statistical power. Studies in higher-risk populations could help to consolidate these initial findings and assess the potential associations of more extreme dysregulations in iron metabolism with hemodynamic adaptations in pregnancy. Moreover, the interpretation of iron status is particularly difficult in pregnancy due to iron stores being increasingly mobilized with gestational age progression in a reaction to higher iron requirements to facilitate placental and fetal development (44). Repeated measurement of fasting blood samples within the same participant are needed to assess longitudinal changes of iron parameters in pregnancy and their effects on maternal gestational hemodynamic adaptations. Since serum ferritin concentrations can be influenced by an inflammatory state, other important factors involved in the regulation of iron status, such as hepcidin, IL-6, and erythropoietin, in combination with other markers of iron metabolism that are less affected by inflammation, like the soluble transferrin receptor, could have aided in the interpretation of our results (50, 51). This is especially important in the context of this study, since inflammatory processes are suggested to be involved in the pathophysiology of gestational hypertensive disorders (52). Unfortunately, these markers were not available in our cohort due to the considerable costs of these measurements. Further studies including these markers could provide a broader picture of the role of maternal iron metabolism in the development of gestational hypertensive disorders.
In conclusion, we found no consistent associations of early pregnancy serum ferritin concentrations with maternal blood pressure, placental hemodynamic parameters, or the risks of gestational hypertensive disorders after considering maternal inflammation, sociodemographic, and lifestyle-related factors. Further studies are needed to investigate the potential role of iron metabolism on gestational hemodynamic adaptations and the development of gestational hypertensive disorders within higher-risk populations.
Supplementary Material
ACKNOWLEDGEMENTS
The Generation R Study is conducted by the Erasmus Medical Center in close collaboration with the School of Law and Faculty of Social Sciences of the Erasmus University Rotterdam; the Municipal Health Service Rotterdam area, Rotterdam; the Rotterdam Homecare Foundation, Rotterdam; and the Stichting Trombosedienst and Artsenlaboratorium Rijnmond, Rotterdam. We gratefully acknowledge the contributions of participating mothers, general practitioners, hospitals, midwives, and pharmacies in Rotterdam.
The authors’ responsibilities were as follows – MJT, CJW, RG: designed the research and had primary responsibility for the final content; MJT: wrote the paper; CJW, RG: supervised writing of the paper; MJT: analyzed data; MJV, MUM, IKR, HGQ-P: contributed to the design of the study and interpretation of the results and were responsible for critical review of the manuscript; and all authors: read and approved the final manuscript.
Notes
The Generation R Study is financially supported by the Erasmus Medical Center, Rotterdam; the Erasmus University Rotterdam; and the Netherlands Organization for Health Research and Development (ZonMW); the Netherlands Organisation for Scientific Research (NWO); and the Ministry of Health, Welfare and Sport. This project has received funding from the European Union's Horizon 2020 research and innovation programme under the ERA-NET (European Research Area Network) Cofund action (no 727565), and the European Joint Programming Initiative “A Healthy Diet for a Healthy Life” (JPI HDHL, EndObesity project, ZonMW the Netherlands no. 529051026).
Author disclosures: RG received funding of the Dutch Heart Foundation (grant number 2017T013), the Dutch Diabetes Foundation (grant number 2017.81.002), and the Netherlands Organization for Health Research and Development (NWO, ZonMW, grant number 543003109). MUM acknowledges funding from the German Academic Exchange Service and the Dietmar Hopp Stiftung. All other authors report no conflicts of interest.
None of the supporting sources had any involvement in the design, implementation, analysis or interpretation of the study.
Supplemental Tables 1–20 are available from the “Supplementary data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/jn/.
Abbreviations used: CRP, C-reactive protein; ROS, reactive oxygen species; SDS, standard deviation scores; TSAT, transferrin saturation; UmPI, umbilical artery pulsatility index; UtRI, uterine artery resistance index.
Contributor Information
Minerva J Taeubert, The Generation R Study Group, Erasmus MC, University Medical Center Rotterdam, Rotterdam, The Netherlands; Department of Pediatric Oncology, Hematology and Immunology, University Medical Center, Heidelberg, Germany.
Clarissa J Wiertsema, The Generation R Study Group, Erasmus MC, University Medical Center Rotterdam, Rotterdam, The Netherlands; Department of Pediatrics, Sophia's Children's Hospital, Erasmus MC, University Medical Center Rotterdam, Rotterdam, The Netherlands.
Marijn J Vermeulen, Department of Pediatrics, Sophia's Children's Hospital, Erasmus MC, University Medical Center Rotterdam, Rotterdam, The Netherlands.
Hugo G Quezada-Pinedo, The Generation R Study Group, Erasmus MC, University Medical Center Rotterdam, Rotterdam, The Netherlands; Department of Pediatrics, Sophia's Children's Hospital, Erasmus MC, University Medical Center Rotterdam, Rotterdam, The Netherlands.
Irwin K Reiss, Department of Pediatrics, Sophia's Children's Hospital, Erasmus MC, University Medical Center Rotterdam, Rotterdam, The Netherlands.
Martina U Muckenthaler, Department of Pediatric Oncology, Hematology and Immunology, University Medical Center, Heidelberg, Germany.
Romy Gaillard, The Generation R Study Group, Erasmus MC, University Medical Center Rotterdam, Rotterdam, The Netherlands; Department of Pediatrics, Sophia's Children's Hospital, Erasmus MC, University Medical Center Rotterdam, Rotterdam, The Netherlands.
Data Availability
Data described in the manuscript, code book, and analytic code will be made available upon reasonable request.
References
- 1. Kintiraki E, Papakatsika S, Kotronis G, Goulis DG, Kotsis V. Pregnancy-induced hypertension. Hormones. 2015;14(2):211–23. [DOI] [PubMed] [Google Scholar]
- 2. Brown MA, Lindheimer MD, de Swiet M, Van Assche A, Moutquin JM. The classification and diagnosis of the hypertensive disorders of pregnancy: statement from the International Society for the Study of Hypertension in Pregnancy (ISSHP). Hypertens Pregnancy. 2001;20(1):ix– xiv. [DOI] [PubMed] [Google Scholar]
- 3. Gaillard R, Eilers PH, Yassine S, Hofman A, Steegers EA, Jaddoe VW. Risk factors and consequences of maternal anaemia and elevated haemoglobin levels during pregnancy: A population-based prospective cohort study. Paediatr Perinat Epidemiol. 2014;28(3):213–26. [DOI] [PubMed] [Google Scholar]
- 4. Murphy JF, O'Riordan J, Newcombe RG, Coles EC, Pearson JF. Relation of haemoglobin levels in first and second trimesters to outcome of pregnancy.Lancet North Am Ed. 1986;327(8488):992–5. [DOI] [PubMed] [Google Scholar]
- 5. Khoigani MG, Goli S, Hasanzadeh A. The relationship of hemoglobin and hematocrit in the first and second half of pregnancy with pregnancy outcome. Iran J Nurs Midwifery Res. 2012;17(2 Suppl 1):S165–70. [PMC free article] [PubMed] [Google Scholar]
- 6. Jung J, Rahman MM, Rahman MS, Swe KT, Islam MR, Rahman MO, Akter S. Effects of hemoglobin levels during pregnancy on adverse maternal and infant outcomes: A systematic review and meta-analysis. Ann NY Acad Sci. 2019;1450(1):69–82. [DOI] [PubMed] [Google Scholar]
- 7. Casanueva E, Viteri FE. Iron and oxidative stress in pregnancy. J Nutr. 2003;133(5):1700S–8S. [DOI] [PubMed] [Google Scholar]
- 8. Walter PB, Knutson MD, Paler-Martinez A, Lee S, Xu Y, Viteri FE, Ames BN. Iron deficiency and iron excess damage mitochondria and mitochondrial DNA in rats. Proc Natl Acad Sci. 2002;99(4):2264–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Naito Y, Hirotani S, Sawada H, Akahori H, Tsujino T, Masuyama T. Dietary iron restriction prevents hypertensive cardiovascular remodeling in Dahl salt-sensitive rats. Hypertension. 2011;57(3):497–504. [DOI] [PubMed] [Google Scholar]
- 10. Wang H, Li H, Hou Z, Pan L, Shen X, Li G. Role of oxidative stress in elevated blood pressure induced by high free fatty acids. Hypertens Res. 2009;32(2):152–8. [DOI] [PubMed] [Google Scholar]
- 11. Day SM, Duquaine D, Mundada LV, Menon RG, Khan BV, Rajagopalan S, Fay WP. Chronic iron administration increases vascular oxidative stress and accelerates arterial thrombosis. Circulation. 2003;107(20):2601–6. [DOI] [PubMed] [Google Scholar]
- 12. He H, Qiao Y, Zhou Q, Wang Z, Chen X, Liu D, Yin D, He M. Iron overload damages the endothelial mitochondria via the ROS/ADMA/DDAHII/eNOS/NO pathway. Oxid Med Cell Longev. 2019;2019:2340392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Mannaerts D, Faes E, Cos P, Briede JJ, Gyselaers W, Cornette J, Gorbanev Y, Bogaerts A, Spaanderman M, Van Craenenbroeck E et al. Oxidative stress in healthy pregnancy and preeclampsia is linked to chronic inflammation, iron status and vascular function. PLoS One. 2018;13(9):e0202919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kim MK, Baek KH, Song KH, Kang MI, Choi JH, Bae JC, Park CY, Lee WY, Oh KW. Increased serum ferritin predicts the development of hypertension among middle-aged men. Am J Hypertens. 2012;25(4):492–7. [DOI] [PubMed] [Google Scholar]
- 15. Jamshidi-Naeini Y, Bavil AK, Egal A, Oldewage-Theron W. Hemoglobin and ferritin concentrations are positively associated with blood pressure and hypertension risk in older adults: A retrospective cross-sectional study, Sharpeville, South Africa. Asia Pac J Clin Nutr. 2019;28(3):533–43. [DOI] [PubMed] [Google Scholar]
- 16. Ryoo JH, Kim SY, Oh CM, Park SK, Kim E, Park SJ, In Yu J, Kim MG, Choi YS, Ko TS. The incidental relationship between serum ferritin levels and hypertension. Int J Cardiol. 2015;183:258–62. [DOI] [PubMed] [Google Scholar]
- 17. Lee DH, Kang SK, Choi WJ, Kwak KM, Kang D, Lee SH, Lee JH. Association between serum ferritin and hypertension according to the working type in Korean men: The fifth Korean national health and nutrition examination survey 2010–2012. Ann Occup Environ Med. 2018;30(1):40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Valenti L, Maloberti A, Signorini S, Milano M, Cesana F, Cappellini F, Dongiovanni P, Porzio M, Soriano F, Brambilla M et al. Iron stores, hepcidin, and aortic stiffness in individuals with hypertension. PLoS One. 2015;10(8):e0134635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lin KC, Tsai MY, Chi CL, Yu LK, Huang LH, Chen CA. Serum ferritin is associated with arterial stiffness in hemodialysis patients: Results of a 3-year follow-up study. Int Urol Nephrol. 2015;47(11):1847–53. [DOI] [PubMed] [Google Scholar]
- 20. Ha JY, Kim MK, Kang S, Nam JS, Ahn CW, Kim KR, Park JS. Serum ferritin levels are associated with arterial stiffness in healthy Korean adults. Vasc Med. 2016;21(4):325–30. [DOI] [PubMed] [Google Scholar]
- 21. Lee KR, Sweeney G, Kim WY, Kim KK. Serum ferritin is linked with aortic stiffness in apparently healthy Korean women. Crit Pathw Cardiol. 2010;9(3):160–3. [DOI] [PubMed] [Google Scholar]
- 22. Salonen JT, Nyyssonen K, Korpela H, Tuomilehto J, Seppanen R, Salonen R. High stored iron levels are associated with excess risk of myocardial infarction in eastern Finnish men. Circulation. 1992;86(3):803–11. [DOI] [PubMed] [Google Scholar]
- 23. Xu H, Song Y, Xu J, Gu Y, Zhang Q, Liu L, Meng G, Wu H, Xia Y, Bao X et al. Increased serum ferritin levels are independently associated with carotid atherosclerosis in women. Br J Nutr. 2017;117(11):1623–30. [DOI] [PubMed] [Google Scholar]
- 24. Silvestre OM, Goncalves A, Nadruz W Jr., Claggett B, Couper D, Eckfeldt JH, Pankow JS, Anker SD, Solomon SD. Ferritin levels and risk of heart failure-the atherosclerosis risk in communities study. Eur J Heart Fail. 2017;19(3):340–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lewandowska M, Sajdak S, Lubinski J. Can serum iron concentrations in early healthy pregnancy be risk marker of pregnancy-induced hypertension?. Nutrients. 2019;11(5):1086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Tande DL, Ralph JL, Johnson LK, Scheett AJ, Hoverson BS, Anderson CM. First trimester dietary intake, biochemical measures, and subsequent gestational hypertension among nulliparous women. J Midwifery Womens Health. 2013;58(4):423–30. [DOI] [PubMed] [Google Scholar]
- 27. Basu A, Yu JY, Jenkins AJ, Nankervis AJ, Hanssen KF, Henriksen T, Lorentzen B, Garg SK, Menard MK, Hammad SM et al. Trace elements as predictors of preeclampsia in type 1 diabetic pregnancy. Nutr Res. 2015;35(5):421–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Jirakittidul P, Sirichotiyakul S, Ruengorn C, Techatraisak K, Wiriyasirivaj B. Effect of iron supplementation during early pregnancy on the development of gestational hypertension and pre-eclampsia. Arch Gynecol Obstet. 2018;298(3):545–50. [DOI] [PubMed] [Google Scholar]
- 29. Ziaei S, Norrozi M, Faghihzadeh S, Jafarbegloo E. A randomised placebo-controlled trial to determine the effect of iron supplementation on pregnancy outcome in pregnant women with haemoglobin > or = 13.2 g/dl. BJOG. 2007;114(6):684–8. [DOI] [PubMed] [Google Scholar]
- 30. Kooijman MN, Kruithof CJ, van Duijn CM, Duijts L, Franco OH, van IMH, de Jongste JC, Klaver CC, van der Lugt A, Mackenbach JP et al. The Generation R Study: design and cohort update 2017. Eur J Epidemiol. 2016;31(12):1243–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Jaddoe VW, Bakker R, van Duijn CM, van der Heijden AJ, Lindemans J, Mackenbach JP, Moll HA, Steegers EA, Tiemeier H, Uitterlinden AG et al. The Generation R Study biobank: A resource for epidemiological studies in children and their parents. Eur J Epidemiol. 2007;22(12):917–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Quezada-Pinedo HG, Mensink-Bout SM, Reiss IK, Jaddoe VWV, Vermeulen MJ, Duijts L. Maternal iron status during early pregnancy and school-age, lung function, asthma, and allergy: The Generation R Study. Pediatr Pulmonol. 2021;56(6):1771–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. van den Broek NR, Letsky EA, White SA, Shenkin A. Iron status in pregnant women: Which measurements are valid?. Br J Haematol. 1998;103(3):817–24. [DOI] [PubMed] [Google Scholar]
- 34. El Assaad MA, Topouchian JA, Darne BM, Asmar RG. Validation of the Omron HEM-907 device for blood pressure measurement. Blood Press Monit. 2002;7(4):237–41. [DOI] [PubMed] [Google Scholar]
- 35. Gaillard R, Steegers EA, Hofman A, Jaddoe VW. Associations of maternal obesity with blood pressure and the risks of gestational hypertensive disorders. The Generation R Study. J Hypertens. 2011;29(5):937–44. [DOI] [PubMed] [Google Scholar]
- 36. Gaillard R, Arends LR, Steegers EA, Hofman A, Jaddoe VW. Second- and third-trimester placental hemodynamics and the risks of pregnancy complications: The Generation R Study. Am J Epidemiol. 2013;177(8):743–54. [DOI] [PubMed] [Google Scholar]
- 37. Coolman M, de Groot CJ, Jaddoe VW, Hofman A, Raat H, Steegers EA. Medical record validation of maternally reported history of preeclampsia. J Clin Epidemiol. 2010;63(8):932–7. [DOI] [PubMed] [Google Scholar]
- 38. Ernst GD, de Jonge LL, Hofman A, Lindemans J, Russcher H, Steegers EA, Jaddoe VW. C-reactive protein levels in early pregnancy, fetal growth patterns, and the risk for neonatal complications: The Generation R Study. Am J Obstet Gynecol. 2011;205(2):132.e1–132.e12. [DOI] [PubMed] [Google Scholar]
- 39. Hentze MW, Muckenthaler MU, Galy B, Camaschella C. Two to tango: Regulation of mammalian iron metabolism. Cell. 2010;142(1):24–38. [DOI] [PubMed] [Google Scholar]
- 40. WHO. Serum ferritin concentrations for the assessment of iron status and iron deficiency in populations. Vitamin and Mineral Nutrition Information System. Geneva: World Health Organization; 2011 (WHO/NMH/NHD/MNM/11.2). [Google Scholar]
- 41. Myatt L, Webster RP. Vascular biology of preeclampsia. J Thromb Haemost. 2009;7(3):375–84. [DOI] [PubMed] [Google Scholar]
- 42. Zhu Y, Chen G, Bo Y, Liu Y. Markers of iron status, blood pressure and incident hypertension among Chinese adults. Nutr Metab Cardiovasc Dis. 2019;29(8):830–6. [DOI] [PubMed] [Google Scholar]
- 43. Galan P, Vergnaud AC, Tzoulaki I, Buyck JF, Blacher J, Czernichow S, Hercberg S. Low total and nonheme iron intakes are associated with a greater risk of hypertension. J Nutr. 2010;140(1):75–80. [DOI] [PubMed] [Google Scholar]
- 44. Fisher AL, Nemeth E. Iron homeostasis during pregnancy. Am J Clin Nutr. 2017;106(Suppl 6):1567S–74S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Elsayed ME, Sharif MU, Stack AG. Transferrin saturation: A body iron biomarker. Adv Clin Chem. 2016;75:71–97. [DOI] [PubMed] [Google Scholar]
- 46. Toldi G, Stenczer B, Molvarec A, Takats Z, Beko G, Rigo J, Vasarhelyi B. Hepcidin concentrations and iron homeostasis in preeclampsia. Clin Chem Lab Med. 2010;48(10):1423–6. [DOI] [PubMed] [Google Scholar]
- 47. Nohr EA, Frydenberg M, Henriksen TB, Olsen J. Does low participation in cohort studies induce bias?. Epidemiology. 2006;17(4):413–8. [DOI] [PubMed] [Google Scholar]
- 48. Schoots MH, Gordijn SJ, Scherjon SA, van Goor H, Hillebrands JL. Oxidative stress in placental pathology. Placenta. 2018;69:153–61. [DOI] [PubMed] [Google Scholar]
- 49. Scholing JM, Olthof MR, Jonker FA, Vrijkotte TG. Association between pre-pregnancy weight status and maternal micronutrient status in early pregnancy. Public Health Nutr. 2018;21(11):2046–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Pagani A, Nai A, Silvestri L, Camaschella C. Hepcidin and anemia: A tight relationship. Front Physiol. 2019;10:1294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Beguin Y. Soluble transferrin receptor for the evaluation of erythropoiesis and iron status. Clin Chim Acta. 2003;329(1-2):9–22. [DOI] [PubMed] [Google Scholar]
- 52. Karumanchi SA, Granger JP. Preeclampsia and pregnancy-related hypertensive disorders. Hypertension. 2016;67(2):238–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data described in the manuscript, code book, and analytic code will be made available upon reasonable request.