ABSTRACT
Human adenovirus serotype 26 (Ad26) is used as a gene-based vaccine against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and HIV-1. However, its primary receptor portfolio remains controversial, potentially including sialic acid, coxsackie and adenovirus receptor (CAR), integrins, and CD46. We and others have shown that Ad26 can use CD46, but these observations were questioned on the basis of the inability to cocrystallize Ad26 fiber with CD46. Recent work demonstrated that Ad26 binds CD46 with its hexon protein rather than its fiber. We examined the functional consequences of Ad26 for infection in vitro and in vivo. Ectopic expression of human CD46 on Chinese hamster ovary cells increased Ad26 infection significantly. Deletion of the complement control protein domain CCP1 or CCP2 or the serine-threonine-proline (STP) region of CD46 reduced infection. Comparing wild-type and sialic acid-deficient CHO cells, we show that the usage of CD46 is independent of its sialylation status. Ad26 transduction was increased in CD46 transgenic mice after intramuscular (i.m.) injection but not after intranasal (i.n.) administration. Ad26 transduction was 10-fold lower than Ad5 transduction after intratumoral (i.t.) injection of CD46-expressing tumors. Ad26 transduction of liver was 1,000-fold lower than that ofAd5 after intravenous (i.v.) injection. These data demonstrate the use of CD46 by Ad26 in certain situations but also show that the receptor has little consequence by other routes of administration. Finally, i.v. injection of high doses of Ad26 into CD46 mice induced release of liver enzymes into the bloodstream and reduced white blood cell counts but did not induce thrombocytopenia. This suggests that Ad26 virions do not induce direct clotting side effects seen during coronavirus disease 2019 (COVID-19) vaccination with this serotype of adenovirus.
IMPORTANCE The human species D Ad26 is being investigated as a low-seroprevalence vector for oncolytic virotherapy and gene-based vaccination against HIV-1 and SARS-CoV-2. However, there is debate in the literature about its tropism and receptor utilization, which directly influence its efficiency for certain applications. This work was aimed at determining which receptor(s) this virus uses for infection and its role in virus biology, vaccine efficacy, and, importantly, vaccine safety.
KEYWORDS: CAR, CD46, adenovirus, gene therapy, oncolytic viruses, receptors, vaccines
INTRODUCTION
Adenoviruses (Ads) are a genetically diverse family that cause a range of ocular, respiratory, digestive, and systemic infections (1). Human mastadenoviruses (HAdVs) are grouped into species A through G, with whole-genome sequence diversity as high as 40% from one end of the virome to the other (2). Despite this drastic genetic diversity, most Ad biology is extrapolated from the human species C adenovirus Ad5, possibly due to historical factors and the unavailability of other Ad serotypes commercially.
Human species D Ad26 (HAd-D26) is currently being investigated as a gene-based vaccine and as an oncolytic virus for use in humans (3–11). Ad26 has most recently been used as replication-defective adenovirus (RD-Ad) vaccines against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) (12, 13). It has been demonstrated that intramuscular (i.m.) vaccination with high doses (1011) viral particles (vp) of Ad26 expressing different spike proteins can generate protection against respiratory challenge with SARS-CoV-2 challenge in macaques. While Ad26 has utility as an HIV-1 and coronavirus disease 2019 (COVID-19) vaccine, there is debate in the literature about this virus’ receptor utilization and tropism.
Most Ad tropism is dictated by the interactions of the fiber and penton base proteins. Ads use their fiber proteins to bind several receptors, including the coxsackie and adenovirus receptor (CAR), sialic acid, and CD46 (reviewed in references 14 and 15). The archetype Ad5 virus and its species C family members Ad1, -2, -6, and -57 bind CAR. In contrast, many species B Ads, such as Ad21 and Ad35, bind CD46 (16–19). Most (but not all) adenoviruses also bind integrins as primary or secondary receptors using RGD motifs in their penton base (20–22). Furthermore, many species D and G Ads recognize sialic acid-decorated proteins or glycolipids. In most cases, fiber mediates initial docking to cells, and lower-affinity integrin interactions then mediate secondary binding and cell entry by endocytosis (23). Ads can exclusively use their penton-integrin interactions if the target cell lacks fiber receptors or when fiber affinity for receptors is low (23).
Species D Ads are currently the largest group of adenoviruses in the human virome. For most, it is unclear what primary disease they may cause. A subset of species D Ads, including Ad37, cause severe keratoconjunctivitis in humans (24, 25). Species D viruses access a diverse portfolio of receptors, including CAR, sialic acid, and CD46, but the specific arsenal varies between serotypes and is often difficult to assess (reviewed in references 14 and 15). Species D Ads have short, stiff fibers with only 8 repeats (Fig. 1) (26), making CAR interactions less efficient than in other species (14). The Ad37 fiber binds to sialic acid, with high selectivity for the GD1a glycan motif (24, 25). While Ad37 binds sialic acid, the affinity of this interaction is only 19 μM, which is approximately 1,000-fold lower than the affinity of the Ad5 fiber for CAR and the Ad11 fiber for CD46 (17, 27).
FIG 1.

Cryo-EM structures of Ad26. (A) Full virion structure; (B) fiber and penton base. R, fiber shaft repeats; RGD, arginine-glycine-aspartic acid integrin binding motifs in the penton base. The knob is the receptor binding portion of the Ad26 fiber trimer.
CD46, or membrane cofactor protein, is a widely expressed membrane-bound complement control protein (28, 29; reviewed in references 30 and 31). CD46 has an extracellular ectodomain containing four complement control protein domains (CCP1 to CCP4), also known as short consensus regions (SCR1 to SCR4), on its N terminus (Fig. 2) (30). The four CCPs are attached to an alternatively spliced variable-length serine-threonine-proline (STP)-rich region, a transmembrane domain, and two alternate cytoplasmic tails (28). The four CCPs form an extended “hockey stick” structure that allows different interactions with complement and pathogen proteins (Fig. 2) (32). This structure also allows CD46 to display N- and O-linked carbohydrates on its CCP and STP domains, respectively, that can be capped with sialic acids, potentially mediating additional interactions with sialic acid-binding Ads. (Fig. 2) (33). Most crystallographic data on Ad knob-CD46 interactions have been obtained using the N-terminal CCP1 and CCP2 domains of CD46 (known as CD46-D2) (19).
FIG 2.
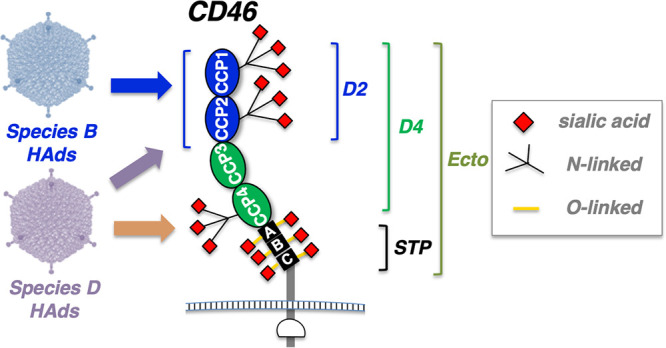
Schematic of potential adenovirus binding motifs on CD46. CCP, STP, D2, D4, and Ecto domains are described in the text. Species B Ads are known to bind the CCP1 and CCP2 domains on CD46.
The CD46 transcript is alternatively spliced to produce four predominant protein isoforms on most cells (28, 34, 35). All forms contain the four CCPs but vary in the number of STP domains. Most tissues express one or two STP domains (BC or C) and one of two different cytoplasmic tails (CYT-1 or CYT-2). The four common isoforms are named BC1, BC2, C1, and C2. All four isoforms coexist on most human tissues, but in different proportions. Many tissues express the BC and C isoforms (28, 34–36). BC2 isoforms predominate in the kidney and salivary glands (35). C2, in turn, is expressed in the brain (35), and a hypoglycosylated form is observed in spermatozoa (37, 38). A fifth CD46 variant including all three STP domains, named ABC, is rarely observed (28) and expressed at low levels in lung, ovary, placenta, and testes. Additionally, few tissues (brain and bladder) can express CD46 lacking all STP domains (36). While CD46 is broadly expressed on human cells, this is not the case in mice. Mice do not express CD46 in most tissues but instead express it only in the testes and in the retina (37, 39–42).
Different pathogens use different domains of CD46 for cell binding (reviewed in references 15 and 30). Measles virus uses CCP1 and CCP2, human herpesvirus 6 uses CCP2 and CCP3, Streptococcus pyogenes uses CCP3 and CCP4, and Neisseria gonorrhoeae and Neisseria meningitidis use CCP3 and the STP domain. Species B adenoviruses bind the CCP1-CCP2 domain of CD46 (CD46-D2) using the knob domain of their fiber protein (Fig. 2) (14, 19).
Initial in vitro data on artificial CAR and CD46-modified B16F10 cells suggested that Ad26 did not use CAR but instead used CD46 as a receptor for infection (3). While Ad26 infection was increased on cells that artificially expressed CD46, infection by Ad26 of these cells was less than half as efficient as infection by the CD46-utilizing species B Ads Ad11 and Ad35 (3). Subsequent work by our laboratory on primary human patient B cell cancer cells indicated that Ad26 used a mixture of CD46 and integrin binding to infect these cells (7). CD46-blocking antibodies reduced Ad26 infection in these B cells by only 50%. This blockade could be increased to 100% by also blocking binding to integrins with cyclic RGD peptide (7). While species D Ads can use sialic acid as a receptor, Ad26 infection was unaffected by removing or blocking sialic acid on these cells. Ad26 is markedly more effective at infecting CD46 transgenic mice than normal mice after intramuscular injections (43).
These data suggested that Ad26 uses CD46 and integrins as receptors, albeit not as efficiently as other Ads. A more recent study reported that Ad26 does not use CD46 at all when infecting epithelial cells but instead uses αvβ3 integrin as its primary receptor (44). These well-controlled studies showed that knockdown of CD46 with small interfering RNA (siRNA) did not inhibit Ad26 infection. They also showed that CHO cells expressing the BC isoform of CD46 are not infected at higher efficiencies than CHO cells (44). This observation using CD46-BC expressing cells conflicts directly with earlier data obtained by testing Ad26 on B16F10-CD46 cells (3). It is unclear from this earlier report which isoform of CD46 is expressed on the B16F10 cells, so it is uncertain if this is a source of the discrepancy.
Another recent study from Baker et al. crystallized the Ad26 knob and performed structural and biological analyses of its interactions with CAR, CD46, and desmoglein-1 (45). This work showed that the Ad26 knob had a Kd of 20 μM for CAR and 50 μM for the CD46-BC1 ectodomain by surface plasmon resonance (SPR) (45). Cocrystals could be produced only with CAR, not with CD46 or desmoglein-1. Subsequent work by the same group showed that removing sialic acid from cells inhibits Ad26 infection and that its knob could be cocrystallized with sialic acid (46). From this, it was concluded that CD46 was not the receptor for Ad26.
The conundrum of observations of Ad26 using CD46 versus the failure of the Ad26 knob to bind or cocrystallize with CD46 was resolved by recent seminal work (47). This work demonstrated that Ad26 does indeed bind CD46, not with its fiber, but rather with its hexon protein.
Given the utility of Ad26 for vaccination and cancer therapy but the uncertainty regarding its receptor utilization, we further explored the ability of Ad26 to use CD46 as a receptor in vitro and importantly in vivo. This work also tested the possibility that the CD46 protein itself might not be the receptor for Ad26 but that instead, CD46 might simply serve as a scaffold to display sialic acid for the virus to bind (Fig. 2). In this case, we hypothesized that Ad26 might not only bind CCPs on CD46 but might also or instead bind to sialic acids on the CCPs or on the heavily O-glycosylated STP domain of the protein.
This work compares the in vivo activity of Ad26 with that of benchmark Ad5 virus in mice with and without CD46. Finally, given recent concerns about thrombotic thrombocytopenia in people vaccinated with Ad26 and ChAdOx1 (48, 49), this study also examined if intravenous delivery of high doses of Ad26 into CD46 transgenic mice can provoke changes in blood counts, platelets, and blood chemistry.
RESULTS
Ad26 infection via CD46 on cells.
New data demonstrate that Ad26 interacts with CD46 via its hexon protein rather than by its fiber protein (47). However, the question remains as to how the virus might use and balance interactions with CD46, integrins, or sialic acid in natural and therapeutic settings. To examine this further, replication-defective Ad26 (RD-Ad26) expressing green fluorescent protein (GFP)-luciferase was incubated with Chinese hamster ovary (CHO) cells, which naturally lack both CAR and CD46. This infection was compared to that in CHO-CD46-C1 cells, which express the C1 variant of human CD46, which includes only the single C STP domain (50), and in CHO cells expressing human CAR and human junctional adhesion molecule 1 (JAM1) (Fig. 3). In the absence of any added human receptors, Ad26 infected CHO cells at significant levels (P < 0.0001 by one-way ANOVA). This significant infection could occur by interactions with the hamster integrin RGD loop or by sialic acid interactions. Adding CAR or JAM1 did not increase this transduction over unmodified CHO cells. In contrast, Ad26 infected CHO-CD46-C1 cells 6- to 7-fold more extensively than all of the other cell lines (P < 0.0001). These data suggest that Ad26 infection is facilitated specifically by CD46.
FIG 3.

Infection of Ad26 on CHO cells displaying different human receptors. RD-Ad26-GL expressing GFP-luciferase was incubated with the indicated cells at an MOI of 10,000 vp/cell, and luciferase activity was measured 48 h later. ****, P < 0.0001 by one-way ANOVA.
Ad26 infection on cells expressing truncated CD46 variants.
To test if, analogous to species B Ads, Ad26 functionally uses the membrane-distal CCP1 and CCP2 domains of CD46 (14, 19), Ad26 was applied to CHO cells expressing CD46-BC1 with deletions of CCP1, CCP2, or the membrane-proximal BC1-STP domain (51, 52). Ad26 infection was significantly lower on CD46-BC1-ΔCCP1 and ΔCCP2 cells (Fig. 4). Infection was also lower on CD46-BC1-ΔSTP cells (P < 0.001). These data suggest that Ad26 binds to CCP1 and -2 of CD46 and that the presence of an STP domain also influences this interaction.
FIG 4.

Infection of Ad26 on CHO cells expressing CD46 with deletions of key domains. ΔCCP1, ΔCCP2, and ΔSTP indicate CD46-BC1 proteins with deletions in these domains. CD46-/- (shown on the graph) is a negative-control stable cell line with CD46 in the antisense orientation to the expression cassette. Ad26-GL was incubated with the indicated cells at an MOI of 10,000 vp/cell, and luciferase activity was measured 48 h later. **, P < 0.01, and ****, P < 0.0001, by one-way ANOVA.
Ad26 infection via CD46 with and without the presence of sialic acid.
We previously showed that a combination of CD46 antibody and cyclic RGD peptide can almost completely inhibit Ad26 infection of primary human B cells (7). In contrast, treating cells with neuraminidase or with wheat germ agglutinin to block sialic acid interactions did not inhibit Ad26 in our hands (7). This suggested that Ad26 uses CD46 and integrins, but not sialic acid, at least on these cells. In contrast, recent studies showed that Ad26 knob binds sialic acid at the same binding site as Ad37 (46).
Since the CCP1-2 and STP domains of CD46 are all heavily glycosylated, we hypothesized that Ad26 might not use CD46 as a protein receptor but might instead use the protein as a scaffold that displays sialic acid (Fig. 2) (33). In this model, overexpression of CD46 might simply increase the raw amount of sialic acid on the cell surface. Alternately, the extended hockey stick structure of CD46 (32) might simply provide an easily accessible landing pad to bind sialic acid on cells.
To test this, we expressed several versions of CD46-BC1 on either sialylation-competent CHO cells or sialylation-deficient LEC2 cells. LEC2 cells are CHO cell mutants that have a 90% reduction in sialylation of cellular glycoproteins due to an inability to translocate CMP-sialic acid across Golgi vesicle membranes, and thus result in different glycosylation patterns (Fig. 5A, right) (53, 54). In addition to wild-type CD46-BC1, we generated constructs in which the complete ectodomain (Ecto) from CD46-BC1 (containing the 4 CCPs and 2 STP domains) were fused to a heterologous transmembrane domain from platelet-derived growth factor receptor (PDGFR). This allows display of all of the ectodomain of CD46, but fusion to PDGFR also removes the CD46 cytoplasmic domain that may also provide intracellular signaling functions (55). Another construct was made displaying only the STP domain from BC1 fused to PDGFR (STP) to display only this heavily glycosylated domain without native CD46 intracellular domain.
FIG 5.
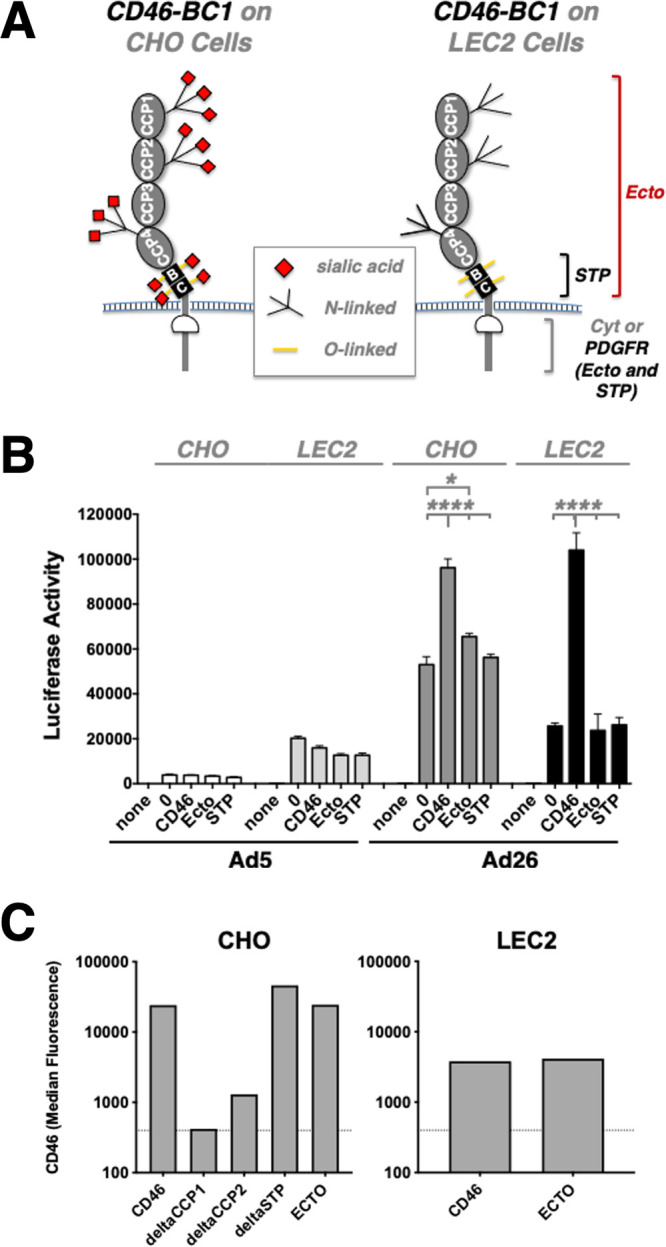
Effects of sialylation on Ad26 infection of CD46 mutant cells. (A) Schematic of CD46 glycosylation in CHO and LEC2 sialylation-deficient cells. (B) The indicated cells were incubated with Ad26-GL, and luciferase activity was measured 2 days later. (C) Flow cytometry of CD46 on cells used in the studies. The indicated cells were incubated with anti-CD46 antibody and analyzed by flow cytometry. Median fluorescence is shown. *, P < 0.05; ****, P < 0.0001.
When Ad5 and Ad26 were used to infect CHO cells expressing these proteins, Ad26 overall mediated significantly stronger gene delivery than CAR-utilizing Ad5, irrespective of whether CD46 was present (Fig. 5B). However, Ad26 once again infected CHO cells expressing wild-type CD46-BC1 cells more efficiently than unmodified CHO cells (P < 0.0001). Interestingly, Ad26 infection of the STP-PDGF and Ecto-PDGF chimeras was no better than the transduction of unmodified CHO cells.
The same CD46 plasmids were used to modify LEC2 cells (53). Indeed, Ad26 transduction of unmodified LEC2 cells resulted in more than 50% decreased luciferase levels compared to CHO cells, as was the case for LEC2 cells expressing Ecto-PDGF and STP-PDGF, which again yielded no discernible effect. However, when LEC2 cells overexpressing wild-type CD46-BC1 were infected with Ad26, the level of luciferase gene expression was virtually identical to that observed on CHO-CD46-BC1 cells (Fig. 5B). These results suggested that Ad26 does use sialic acid for some level of infection, perhaps on other cellular proteins, but that CD46 can functionally substitute for sialic acid when expressed at high levels, irrespective of its sialylation status. Ad26 clearly does not use CD46 as a mere scaffold for carbohydrate binding, and surprisingly, its transmembrane and cytoplasmic domains seem to be relevant for Ad26 transduction as well. Ad5 infection interestingly increased on all of the LEC2 cells.
In vivo infection by Ad26 in normal and CD46 transgenic mice.
CD46 is expressed on nearly all human cells (28). In contrast, normal mice express CD46 only in the testes and retina (39–41). We previously showed that in vivo intramuscular (i.m.) injection of RD-Ad26 into C57BL/6-CD46 transgenic mice mediated significantly stronger transduction than similar injections in normal BALB/c mice (43). While this supports the use of CD46 by the virus in vivo, this was not a direct comparison of Ad26 in mice with and without CD46.
To examine this more directly with viruses that are relevant to oncolytic virotherapy, replication-competent Ad5 (RC-Ad5) and replication-competent Ad26 (RC-Ad26) expressing GFP-luciferase were compared in vivo in C57BL/6 or C57BL/6-CD46 transgenic mice (56) (Fig. 6). These C57BL/6-CD46 transgenic mice contain a knock-in of the human CD46 gene as well as locus control regions from human chromosome 1, granting these mice near-ubiquitous expression of the human CD46 protein with expression patterns dictated by the human promoter sequence (52). To control for heterogeneity of mouse genetic backgrounds skewing transduction, the C57BL/6 mice used for these experiments were CD46− littermates of the CD46+ mice used, which were heterozygous for the transgene.
FIG 6.

In vivo luciferase activity after intramuscular, intranasal, intravenous, and intratumoral administration of Ad5 or Ad26 in CD46− and CD46+ mice. (A) Luciferase imaging 2 days after intranasal or intramuscular injection of 1 × 1010 vp of RC-Ad5-GL or RC-Ad26-GL. (B) Luciferase activity in livers 2 days after intravenous injection of 3 × 1010 vp of RC-Ad5-GL or RC-Ad26-GL into CD46 transgenic mice. (C) Luciferase activity in tumors 2 days after intratumoral injection of 3 × 1010 vp of RC-Ad5-GL or RC-Ad26-GL into B16-CAR-CD46 subcutaneous tumors. Graphs show the same data on a log scale (left) and a linear scale (right). *, P < 0.05; ****, P < 0.0001.
A total of 1 × 1010 virus particles (vp) of RC-Ad5-GL or RC-Ad26-GL were injected intramuscularly (i.m.), intranasally (i.n.), intravenously (i.v.), or intratumorally (i.t.) into groups of 5 mice, and luciferase imaging was performed 2 days later (Fig. 6). Ad26 mediated 15-fold-higher transduction in CD46+ mice than CD46− mice by the i.m. route (P < 0.0001 by one-way analysis of variance [ANOVA]) (Fig. 6A). Ad26 transduction was also 2-fold higher than Ad5 transduction in the muscle of CD46+ mice. In contrast, Ad5 and Ad26 transduction was nearly equal in mice with and without CD46 when delivered intranasally. Overall, transduction by both vectors decreased approximately 10-fold by the i.n. route compared to the i.m. route. We therefore conclude that the usage of CD46 by Ad26 in vivo is dependent on the route of administration and that there seems to be preferred usage of CD46 following i.m. administration.
RC-Ad5-GL and RC-Ad26-GL were next injected into B16-CAR-CD46 subcutaneous tumors in CD46 transgenic mice, and luciferase expression was measured (Fig. 6B and C). After intravenous administration, Ad5 mediated >100-fold-higher luciferase expression in the liver than Ad26 (Fig. 6B). When the two vectors were injected i.t. into the B16-CAR-CD46 tumors, Ad5 mediated up to 10-fold-higher tumor infection than Ad26 (Fig. 6C). While this gives no indication of the specific usage of CD46, the results show how two different vectors can have vastly different efficacies depending on how they are administered.
Complete blood counts and blood chemistry after intravenous injection of Ad26 into CD46 transgenic mice.
A low frequency of thrombosis and thrombocytopenia have been reported after COVID-19 vaccination with the ChAdOx1 nCoV-19 vaccine (Oxford-AstraZeneca) and the Ad26.COV2.S vaccine (Johnson & Johnson/Janssen) (48, 49). While the COVID-19 vaccines have been injected intramuscularly, some fraction of injected vaccine will leak into the blood and could potentially induce effects on the clotting system.
We previously examined in detail the ability of Ad5 to provoke clotting side effects after intravenous injection (57). An i.v. dose of 1011 vp of Ad5 induces significant thrombocytopenia within 24 h. This response is rapid, as demonstrated by increases in D-dimer fibrin degradation products in the blood within 6 h of injection. Ad5 appears to induce this effect by binding and activating platelets and endothelial cells directly (57).
To examine if leakage of Ad26 into the blood might also perturb the clotting system, a similar large 1011-vp dose of RC-Ad26 was administered i.v. to CD46 transgenic mice, and complete blood counts (CBC) and blood chemistry analyses were performed 1 day later (Fig. 7). In contrast to Ad5, the i.v. bolus of Ad26 failed to provoke notable changes in platelets compared to control animals (Fig. 8A). In contrast, Ad26 did induce significant reductions in white blood cells, lymphocytes, and monocytes (P < 0.001) (Fig. 8B to D) after i.v. injection. Ad26 also produced significant elevations in liver enzymes, including alanine transaminase (ALT) and aspartate aminotransferase (AST) after i.v. injection (P < 0.01) (Fig. 8E and F) (58). These data suggest that direct exposure of blood proteins and cells does not provoke thrombocytopenia but does cause reductions in certain nucleated white blood cells and liver damage.
FIG 7.

Effects of intravenous administration of Ad26 in CD46 transgenic mice on CBCs and blood chemistry. Groups of 5 CD46 transgenic mice were injected with a high dose of 1 × 1011 vp of RC-Ad26-GL, and blood was collected 1 day later for CBC and blood chemistry analysis. Control values were collected from 5 uninjected CD46 mice.
FIG 8.
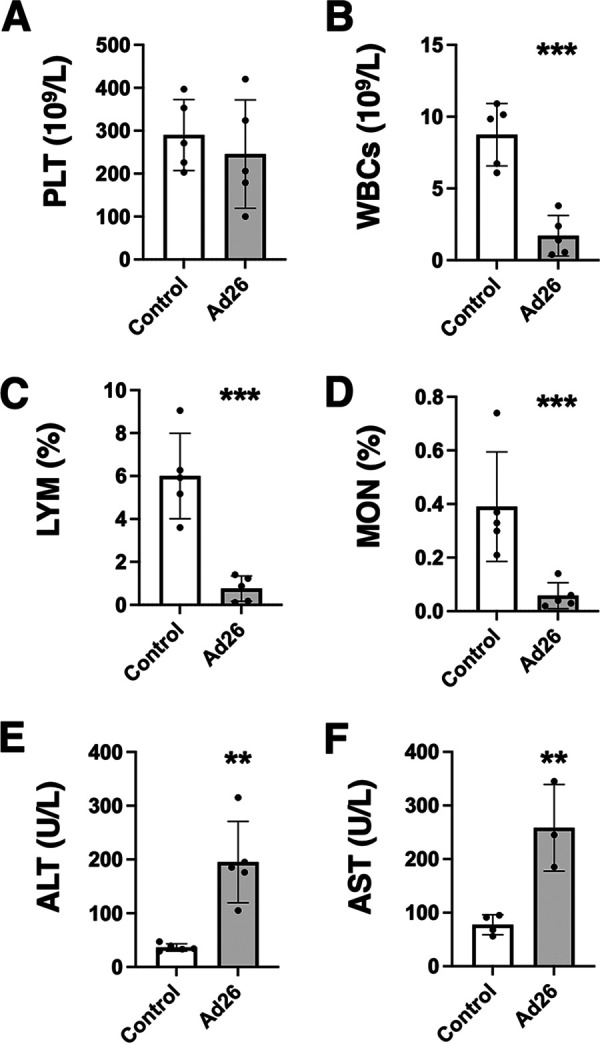
Effects of intravenous administration of Ad26 in CD46 transgenic mice on platelets, white blood cells, and liver enzymes. Selected parameters from Fig. 7 are shown for (A) platelets (PLT), (B) white blood cells (WBCs), (C) lymphocytes (LYM), (D) monocytes (MON), (E) alanine aminotransferase (ALT), and (F) aspartate aminotransferase (AST). **, P < 0.01, and ***, P < 0.001, by t test.
DISCUSSION
Our previous work found that species D human Ads can be potent oncolytic viruses against B cell cancers (6, 7) and have utility as mucosal gene-based vaccines (9, 43). Receptor utilization testing on primary patient B cell cancer cells showed that Ad26 uses CD46 and integrin binding to infect these cells (7). Blocking CD46 antibodies reduced Ad26 infection in these B cells, but this inhibition was not 100%. Combined blockade of CD46 and integrin could inhibit infection nearly 100% (7). Removing or blocking sialic acid on B cells had no effect.
More recent work reported that the Ad26 knob does not bind CD46 and instead uses αvβ3 integrin as its primary receptor on epithelial cells (44). Other work showed that the Ad26 knob could bind CAR but could not bind CD46 (45).
Given these conflicting results and analyses on different cell types, this study was performed to examine the functional use of receptors by Ad26 in different circumstances. During the course of our work, another group published seminal work showing as we did that Ad26 does indeed use CD46, not with its fiber but rather with its hexon (47).
We proceeded to functional testing on more controlled target cells than the primary B cell cancer cells used in our previous studies and to permutation of CD46 constructs that lack specific parts of the protein. Ad26 testing on well-defined CHO-CD46 cell lines (28, 29, 33) showed that Ad26 infected cells that expressed intact forms of CD46. Ad26 infected cells expressing CD46 lacking CCP1 and CCP2 significantly less efficiently than wild-type CD46. Ad26 infection was also less efficient when CD46 lacked its STP or transmembrane and cytoplasmic domain. Thus, like some species of adenovirus types B and D, Ad26 binds CD46 via CCP1 and -2 yet is also influenced by the STP and transmembrane/cytoplasmic domains. It is currently unclear whether this influence involves direct or allosteric effects.
However, CD46 (over)expression on CHO cells increased Ad26 infection only 6-fold. This weak utilization of CD46 is consistent with earlier observations that Ad26 infects CD46-expressing cells only half as well as known CD46-targeting Ad11 and Ad35 (3).
When we tested Ad26 in vivo in CD46− and CD46+ transgenic mice, Ad26 infected CD46+ mice 15 times more efficiently than CD46− littermates after intramuscular injection. However, CD46 expression had little effect on Ad26 by the intranasal route. This suggests that Ad26 interactions with CD46 may be important during intramuscular vaccination but may be less relevant during natural respiratory infections.
When Ad5 and Ad26 were put to the stringent test of transduction after intravenous injection, Ad26 was 100- to 100,000-fold less effective than Ad5 at transducing the liver or other sites. When the two vectors were injected intratumorally into CD46-expressing B16 melanoma tumors to model oncolytic therapy, Ad5 was again more robust than Ad26.
Considering observations of clotting disorders during Ad26 COVID-19 vaccinations, we examined whether RC-Ad26 might provoke similar effects in CD46 transgenic mice in a worst-case scenario of injecting the virus directly into the bloodstream. Ad26 failed to provoke notable changes in platelets but did induce significant reductions in white blood cells, lymphocytes, and monocytes. This is consistent with observations that RC-Ad26 can reduce the viability of human CD14+ monocytes and CD3+ lymphocytes ex vivo (7). Ad26 also produced significant liver damage in the CD46 transgenic mice, as demonstrated by elevating liver enzymes without effectively transducing the liver. These data suggest that Ad26 virions themselves do not appear to directly induce thrombocytopenia but that it does damage white blood cells and hepatocytes at least when delivered at high doses as a replication-competent virus.
In summary, these data indicate that Ad26 can functionally interact with CD46 for infection in vitro and in vivo. It should be noted that these are observed under conditions where CD46 is ectopically expressed in cells or in mice. While this is ectopic expression in mice, this expression is driven by the natural locus control regions for CD46 from human chromosome 1, so it should mimic to some degree expression in human tissues.
While it is clear that Ad26 can use CD46 in vivo, this appeared to be important only by the intramuscular route. This is important for the virus’ efficacy as an intramuscular vaccine but less relevant during natural respiratory mucosal infections. Further examination of Ad26’s ability to interact with CD46 and other receptors is warranted to better understand the implications of these interactions for its effective use in vaccines and therapies.
MATERIALS AND METHODS
Cell lines.
Cells were maintained in HyClone Dulbecco’s high-glucose modified Eagle medium (Thermo Scientific, Waltham, MD) supplemented with 10% fetal bovine serum, 100 U/ml penicillin, and 100 μg/ml streptomycin. 293 and 293-IX-E4 cells were purchased from Microbix (Mississauga, Ontario, Canada). Chinese hamster ovary (CHO) and Lec2 cells were purchased from the American Type Culture Collection (ATCC, Manassas, VA). CHO-CD46-C1, CHO-CD46-BC-1, CHO-CD46-BC-1-ΔCCP1, CHO-CD46-BC-1-ΔCCP2, CHO-CD46-ΔSTP, CHO-CD46-C2, and CHO-CD46-BC-2 cells were generously provided by Kah-Whye Peng (Mayo Clinic) or by Kathy Liszewski and John Atkinson. Human CD46-BC1 cDNA (GenBank accession no. BC030594) was purchased from Transomic Technologies (Huntsville, AL, USA). pCDNA-CAR and pCDNA-JAM1 plasmids were generously supplied by Terrence Dermody (University of Pittsburgh). CHO and Lec2 cells were transfected with plasmids expressing human CD46-BC1 (accession no. BC030594), CAR, or junctional adhesion molecule 1 (JAM1) using PolyFect (Qiagen, CA, USA) and selected in 1000 μg/ml G418 for stable transfectants. The CD46 ectodomain (Ecto) was subjected to PCR from the human CD46-BC1 (BC030594) cDNA using CD46 5′ SCR (CCAGATCTCTGTGAGGAGCCACCAACATTTGAAG) and CD46 3′ STP (CTCTCTGGGCCCTCTAGATGAGACTGGAGGCTTGTAAGTAGG). The CD46 BC STP domain was subjected to PCR with CD46 5′ STP (CCAGATCTCAAAGTGTCGACTTCTTCCACTACAAAATC) and CD46 3′ STP. These were cut with BglII and ApaI and cloned into pHook2-PSTCD (59) in frame and between the α1-antitrypsin secretory leader and the PDGFR transmembrane domain from pHook2 (Invitrogen), which allows cell surface display of fusion proteins. CHO and Lec2 stable cell lines were selected with G418 as described above. Stable cells were stained with MEM-258 CD46 antibody and verified on a flow cytometer for CD46 expression before use (Fig. 5C). B16 mouse melanoma cells were purchased from ATCC. These cells were first modified with pCDNA3-CAR expressing human CAR by transient transfection and selection with G418. B16 cells were also transduced with a lentivirus expressing human CD46 followed by selection in puromycin. CAR and CD46 expression in the resulting B16-CAR-CD46 cells was verified by flow cytometry.
Adenoviruses.
Replication-defective Ad5 (RD-Ad5) and replication-competent Ad5 (RC-Ad5) expressing the green fluorescent protein-luciferase (GFP-Luc) fusion protein were produced as described in reference 60. RD-Ad6, RD-Ad26, and RC-Ad26 expressing GFP-Luc were generated as described in reference 61. RD-Ad5, RD-Ad6, and RC-Ad26 were grown in 293 cells. The Ad5 E1 in 293 cells supports E1 deletion-containing C viruses but not other Ad species (62). Therefore, E1 deletion-containing RD-Ad26 was grown in 293-IX-E4 cells, which also provide Ad5 E4 proteins, as described in reference 43. All viruses were purified by double CsCl banding, and viral particle (vp) concentrations were calculated from the optical density at 260 nm (OD260).
Mice.
Female C57BL/6 were purchased from Charles River Laboratories (Wilmington, MA, USA). C57BL/6-CD46 transgenic mice were generously provided by Roberto Cattaneo and Kah-Whye Peng at Mayo Clinic. The CD46 transgenic mouse colony was sustained by backcrossing with C57B/6 mice, and offspring were genotyped for hCD46 exon 14 (5′-GCCAGTTCATCTTTTGACTCTATTAA-3′ and 5′-AGCACTTCGACCTAAAAATAGAGAT-3′). Mice were housed in the Mayo Clinic Animal Facility under the Association for Assessment and Accreditation of Laboratory Animal Care (AAALAC) guidelines with animal use protocols approved by the Mayo Clinic Animal Use and Care Committee. All animal experiments were carried out according to the provisions of the Animal Welfare Act, PHS Animal Welfare Policy, the principles of the NIH Guide for the Care and Use of Laboratory Animals (63), and the policies and procedures of Mayo Clinic.
Cryo-electron microscopy of Ad26.
We recently described the first structure of species D Ad26 by cryo-electron microscopy (cryo-EM) at 3.7 Å resolution, and details of these analyses can be found in reference 26. Figure 1A shows the radially color-coded Ad26 virion down the icosahedral 2-fold axis, and Fig. 1B shows a representation of the short-shafted Ad26 fiber and penton base using coordinates from reference 26.
In vitro virus infections.
Cells were plated in 96-well black cell culture plates and infected at 50% confluence with the indicated viruses. Cells were infected at indicated multiplicities of infection (MOI) in terms of virus particles per cell. At indicated time points, medium was removed, Bright-Glo luciferase reagent (Promega, Madison, WI) diluted 1:1 with phosphate-buffered saline (PBS) was added, and luciferase activity was measured using the Beckman Coulter DTX 880 Multimode detector system. The indicated viral particles were then added to the well for 48 h before luciferase assay.
Injections in mice.
Animals were housed under the Association for Assessment and Accreditation of Laboratory Animal Care (AAALAC) guidelines in the Mayo Clinic Animal Facility. Experiments were performed according to animal use protocols approved by the Mayo Clinic Animal Use and Care Committee. All experiments were performed under the provisions of the Animal Welfare Act, PHS Animal Welfare Policy, and the principles of the NIH Guide for the Care and Use of Laboratory Animals (63). C57BL/6 or C57BL/6-CD46 transgenic mice were injected once by the indicated routes with the indicated amounts of Ad5 or Ad26 virus expressing GFP-Luc or with PBS. For tumor studies, 106 B16-CAR-CD46 cells were injected subcutaneously in Matrigel. Tumors were injected with RC-Ad5-GL or RC-Ad26-GL when the average size of tumors reached 200 mm3. The mice were imaged at the indicated time points on a Xenogen IVIS200 to measure in vivo luciferase activity.
CBCs and blood chemistry.
C57BL/6-CD46 transgenic mice were injected with 1011 vp of RC-Ad26. After 24 h, blood was collected via submandibular vein puncture and collected in BD lithium heparin and K2 EDTA tubes. Complete blood cell counts (CBCs) and blood chemistry were analyzed on VetScan HM5 and Piccalo Express instruments, respectively. Controls were completed following the same procedure, except that no virus was administered. These analyses were performed by Mayo Pharmacology and Toxicology Core.
Statistical analyses.
All analyses were performed using GraphPad Prism.
ACKNOWLEDGMENTS
This project was supported by a Developmental Project to M.A.B. from the University of Iowa/Mayo Clinic Lymphoma Specialized Program of Research Excellence (SPORE) in Lymphoma (P50 CA097274), the Walter & Lucille Rubin Fund in Infectious Diseases Honoring Michael Camilleri, M.D., and the COVID-19 Task Force at Mayo Clinic. Research reported in this publication was supported by the National Institute of General Medical Sciences of the National Institutes of Health under awards R01 GM099111 and R35 GM136352 for John P. Atkinson and M. Kathryn Liszewski.
Contributor Information
Michael A. Barry, Email: mab@mayo.edu.
Lawrence Banks, International Centre for Genetic Engineering and Biotechnology.
REFERENCES
- 1.Davison AJ, Benkő M, Harrach B. 2003. Genetic content and evolution of adenoviruses. J Gen Virol 84:2895–2908. 10.1099/vir.0.19497-0. [DOI] [PubMed] [Google Scholar]
- 2.Weaver EA, Hillestad ML, Khare R, Palmer D, Ng P, Barry MA. 2011. Characterization of species C human adenovirus serotype 6 (Ad6). Virology 412:19–27. 10.1016/j.virol.2010.10.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Abbink P, Lemckert AA, Ewald BA, Lynch DM, Denholtz M, Smits S, Holterman L, Damen I, Vogels R, Thorner AR, O'Brien KL, Carville A, Mansfield KG, Goudsmit J, Havenga MJ, Barouch DH. 2007. Comparative seroprevalence and immunogenicity of six rare serotype recombinant adenovirus vaccine vectors from subgroups B and D. J Virol 81:4654–4663. 10.1128/JVI.02696-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liu J, O'Brien KL, Lynch DM, Simmons NL, La Porte A, Riggs AM, Abbink P, Coffey RT, Grandpre LE, Seaman MS, Landucci G, Forthal DN, Montefiori DC, Carville A, Mansfield KG, Havenga MJ, Pau MG, Goudsmit J, Barouch DH. 2009. Immune control of an SIV challenge by a T-cell-based vaccine in rhesus monkeys. Nature 457:87–91. 10.1038/nature07469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mast TC, Kierstead L, Gupta SB, Nikas AA, Kallas EG, Novitsky V, Mbewe B, Pitisuttithum P, Schechter M, Vardas E, Wolfe ND, Aste-Amezaga M, Casimiro DR, Coplan P, Straus WL, Shiver JW. 2010. International epidemiology of human pre-existing adenovirus (Ad) type-5, type-6, type-26 and type-36 neutralizing antibodies: correlates of high Ad5 titers and implications for potential HIV vaccine trials. Vaccine 28:950–957. 10.1016/j.vaccine.2009.10.145. [DOI] [PubMed] [Google Scholar]
- 6.Senac JS, Doronin K, Russell SJ, Jelinek DF, Greipp PR, Barry MA. 2010. Infection and killing of multiple myeloma by adenoviruses. Hum Gene Ther 21:179–190. 10.1089/hum.2009.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen CY, Senac JS, Weaver EA, May SM, Jelinek DF, Greipp P, Witzig T, Barry MA. 2011. Species D adenoviruses as oncolytics against B-cell cancers. Clin Cancer Res 17:6712–6722. 10.1158/1078-0432.CCR-11-0968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Weaver EA, Chen CY, May SM, Barry ME, Barry MA. 2011. Comparison of adenoviruses as oncolytics and cancer vaccines in an immunocompetent B cell lymphoma model. Hum Gene Ther 22:1095–1100. 10.1089/hum.2011.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Weaver EA, Barry MA. 2013. Low seroprevalent species D adenovirus vectors as influenza vaccines. PLoS One 8:e73313. 10.1371/journal.pone.0073313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Baden LR, Walsh SR, Seaman MS, Tucker RP, Krause KH, Patel A, Johnson JA, Kleinjan J, Yanosick KE, Perry J, Zablowsky E, Abbink P, Peter L, Iampietro MJ, Cheung A, Pau MG, Weijtens M, Goudsmit J, Swann E, Wolff M, Loblein H, Dolin R, Barouch DH. 2013. First-in-human evaluation of the safety and immunogenicity of a recombinant adenovirus serotype 26 HIV-1 Env vaccine (IPCAVD 001). J Infect Dis 207:240–247. 10.1093/infdis/jis670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Barouch DH, Alter G, Broge T, Linde C, Ackerman ME, Brown EP, Borducchi EN, Smith KM, Nkolola JP, Liu J, Shields J, Parenteau L, Whitney JB, Abbink P, Ng’ang’a DM, Seaman MS, Lavine CL, Perry JR, Li W, Colantonio AD, Lewis MG, Chen B, Wenschuh H, Reimer U, Piatak M, Lifson JD, Handley SA, Virgin HW, Koutsoukos M, Lorin C, Voss G, Weijtens M, Pau MG, Schuitemaker H. 2015. Protective efficacy of adenovirus-protein vaccines against SIV challenges in rhesus monkeys. Science 349:320–324. 10.1126/science.aab3886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mercado NB, Zahn R, Wegmann F, Loos C, Chandrashekar A, Yu J, Liu J, Peter L, McMahan K, Tostanoski LH, He X, Martinez DR, Rutten L, Bos R, van Manen D, Vellinga J, Custers J, Langedijk JP, Kwaks T, Bakkers MJG, Zuijdgeest D, Rosendahl Huber SK, Atyeo C, Fischinger S, Burke JS, Feldman J, Hauser BM, Caradonna TM, Bondzie EA, Dagotto G, Gebre MS, Hoffman E, Jacob-Dolan C, Kirilova M, Li Z, Lin Z, Mahrokhian SH, Maxfield LF, Nampanya F, Nityanandam R, Nkolola JP, Patel S, Ventura JD, Verrington K, Wan H, Pessaint L, Van Ry A, Blade K, Strasbaugh A, Cabus M, et al. 2020. Single-shot Ad26 vaccine protects against SARS-CoV-2 in rhesus macaques. Nature 586:583–588. 10.1038/s41586-020-03100-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Logunov DY, Dolzhikova i.v., Zubkova OV, Tukhvatullin AI, Shcheblyakov DV, Dzharullaeva AS, Grousova DM, Erokhova AS, Kovyrshina AV, Botikov AG, Izhaeva FM, Popova O, Ozharovskaya TA, Esmagambetov IB, Favorskaya IA, Zrelkin DI, Voronina DV, Shcherbinin DN, Semikhin AS, Simakova YV, Tokarskaya EA, Lubenets NL, Egorova DA, Shmarov MM, Nikitenko NA, Morozova LF, Smolyarchuk EA, Kryukov EV, Babira VF, Borisevich SV, Naroditsky BS, Gintsburg AL. 2020. Safety and immunogenicity of an rAd26 and rAd5 vector-based heterologous prime-boost COVID-19 vaccine in two formulations: two open, non-randomised phase 1/2 studies from Russia. Lancet 396:887–897. 10.1016/S0140-6736(20)31866-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Arnberg N. 2009. Adenovirus receptors: implications for tropism, treatment and targeting. Rev Med Virol 19:165–178. 10.1002/rmv.612. [DOI] [PubMed] [Google Scholar]
- 15.Liszewski MK, Atkinson JP. 2021. Membrane cofactor protein (MCP; CD46): deficiency states and pathogen connections. Curr Opin Immunol 72:126–134. 10.1016/j.coi.2021.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Persson BD, Reiter DM, Marttila M, Mei YF, Casasnovas JM, Arnberg N, Stehle T. 2007. Adenovirus type 11 binding alters the conformation of its receptor CD46. Nat Struct Mol Biol 14:164–166. 10.1038/nsmb1190. [DOI] [PubMed] [Google Scholar]
- 17.Wang H, Liaw YC, Stone D, Kalyuzhniy O, Amiraslanov I, Tuve S, Verlinde CL, Shayakhmetov D, Stehle T, Roffler S, Lieber A. 2007. Identification of CD46 binding sites within the adenovirus serotype 35 fiber knob. J Virol 81:12785–12792. 10.1128/JVI.01732-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Persson BD, Muller S, Reiter DM, Schmitt BB, Marttila M, Sumowski CV, Schweizer S, Scheu U, Ochsenfeld C, Arnberg N, Stehle T. 2009. An arginine switch in the species B adenovirus knob determines high-affinity engagement of cellular receptor CD46. J Virol 83:673–686. 10.1128/JVI.01967-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cupelli K, Muller S, Persson BD, Jost M, Arnberg N, Stehle T. 2010. Structure of adenovirus type 21 knob in complex with CD46 reveals key differences in receptor contacts among species B adenoviruses. J Virol 84:3189–3200. 10.1128/JVI.01964-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wickham TJ, Mathias P, Cheresh DA, Nemerow GR. 1993. Integrins avb3 or avb5 promote adenovirus internalization but not virus attachment. Cell 73:309–319. 10.1016/0092-8674(93)90231-E. [DOI] [PubMed] [Google Scholar]
- 21.Albinsson B, Kidd AH. 1999. Adenovirus type 41 lacks an RGD alpha(v)-integrin binding motif on the penton base and undergoes delayed uptake in A549 cells. Virus Res 64:125–136. 10.1016/s0168-1702(99)00087-8. [DOI] [PubMed] [Google Scholar]
- 22.Storm RJ, Persson BD, Skalman LN, Frangsmyr L, Lindstrom M, Rankin G, Lundmark R, Domellof FP, Arnberg N. 2017. Human adenovirus type 37 uses alphaVbeta1 and alpha3beta1 integrins for infection of human corneal cells. J Virol 91:e02019-16. 10.1128/JVI.02019-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Greber UF, Willetts M, Webster P, Helenius A. 1993. Stepwise dismantling of adenovirus 2 during entry into cells. Cell 75:477–486. 10.1016/0092-8674(93)90382-z. [DOI] [PubMed] [Google Scholar]
- 24.Arnberg N, Edlund K, Kidd AH, Wadell G. 2000. Adenovirus type 37 uses sialic acid as a cellular receptor. J Virol 74:42–48. 10.1128/JVI.74.1.42-48.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nilsson EC, Storm RJ, Bauer J, Johansson SM, Lookene A, Angstrom J, Hedenstrom M, Eriksson TL, Frangsmyr L, Rinaldi S, Willison HJ, Pedrosa Domellof F, Stehle T, Arnberg N. 2011. The GD1a glycan is a cellular receptor for adenoviruses causing epidemic keratoconjunctivitis. Nat Med 17:105–109. 10.1038/nm.2267. [DOI] [PubMed] [Google Scholar]
- 26.Yu X, Veesler D, Campbell MG, Barry ME, Asturias FJ, Barry MA, Reddy VS. 2017. Cryo-EM structure of human adenovirus D26 reveals the conservation of structural organization among human adenoviruses. Sci Adv 3:e1602670. 10.1126/sciadv.1602670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Henry LJ, Xia D, Wilke ME, Deisenhofer J, Gerard RD. 1994. Characterization of the knob domain of the adenovirus type 5 fiber protein expressed in Escherichia coli. J Virol 68:5239–5246. 10.1128/JVI.68.8.5239-5246.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Post TW, Liszewski MK, Adams EM, Tedja I, Miller EA, Atkinson JP. 1991. Membrane cofactor protein of the complement system: alternative splicing of serine/threonine/proline-rich exons and cytoplasmic tails produces multiple isoforms that correlate with protein phenotype. J Exp Med 174:93–102. 10.1084/jem.174.1.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lublin DM, Liszewski MK, Post TW, Arce MA, Le Beau MM, Rebentisch MB, Lemons LS, Seya T, Atkinson JP. 1988. Molecular cloning and chromosomal localization of human membrane cofactor protein (MCP). Evidence for inclusion in the multigene family of complement-regulatory proteins. J Exp Med 168:181–194. 10.1084/jem.168.1.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yamamoto H, Fara AF, Dasgupta P, Kemper C. 2013. CD46: the 'multitasker' of complement proteins. Int J Biochem Cell Biol 45:2808–2820. 10.1016/j.biocel.2013.09.016. [DOI] [PubMed] [Google Scholar]
- 31.Liszewski MK, Kemper C. 2019. Complement in motion: the evolution of CD46 from a complement regulator to an orchestrator of normal cell physiology. J Immunol 203:3–5. 10.4049/jimmunol.1900527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Persson BD, Schmitz NB, Santiago C, Zocher G, Larvie M, Scheu U, Casasnovas JM, Stehle T. 2010. Structure of the extracellular portion of CD46 provides insights into its interactions with complement proteins and pathogens. PLoS Pathog 6:e1001122. 10.1371/journal.ppat.1001122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ballard LL, Bora NS, Yu GH, Atkinson JP. 1988. Biochemical characterization of membrane cofactor protein of the complement system. J Immunol 141:3923–3929. [PubMed] [Google Scholar]
- 34.Ballard L, Seya T, Teckman J, Lublin DM, Atkinson JP. 1987. A polymorphism of the complement regulatory protein MCP (membrane cofactor protein or gp45-70). J Immunol 138:3850–3855. [PubMed] [Google Scholar]
- 35.Johnstone RW, Russell SM, Loveland BE, McKenzie IF. 1993. Polymorphic expression of CD46 protein isoforms due to tissue-specific RNA splicing. Mol Immunol 30:1231–1241. 10.1016/0161-5890(93)90038-d. [DOI] [PubMed] [Google Scholar]
- 36.Tang SJ, Luo S, Ho JX, Ly PT, Goh E, Roca X. 2016. Characterization of the regulation of CD46 RNA alternative splicing. J Biol Chem 291:14311–14323. 10.1074/jbc.M115.710350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lyzogubov V, Wu X, Jha P, Tytarenko R, Triebwasser M, Kolar G, Bertram P, Bora PS, Atkinson JP, Bora NS. 2014. Complement regulatory protein CD46 protects against choroidal neovascularization in mice. Am J Pathol 184:2537–2548. 10.1016/j.ajpath.2014.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Riley RC, Kemper C, Leung M, Atkinson JP. 2002. Characterization of human membrane cofactor protein (MCP; CD46) on spermatozoa. Mol Reprod Dev 62:534–546. 10.1002/mrd.10144. [DOI] [PubMed] [Google Scholar]
- 39.Kemper C, Leung M, Stephensen CB, Pinkert CA, Liszewski MK, Cattaneo R, Atkinson JP. 2001. Membrane cofactor protein (MCP; CD46) expression in transgenic mice. Clin Exp Immunol 124:180–189. 10.1046/j.1365-2249.2001.01458.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Holers VM, Kinoshita T, Molina H. 1992. The evolution of mouse and human complement C3-binding proteins: divergence of form but conservation of function. Immunol Today 13:231–236. 10.1016/0167-5699(92)90160-9. [DOI] [PubMed] [Google Scholar]
- 41.Oldstone MB, Lewicki H, Thomas D, Tishon A, Dales S, Patterson J, Manchester M, Homann D, Naniche D, Holz A. 1999. Measles virus infection in a transgenic model: virus-induced immunosuppression and central nervous system disease. Cell 98:629–640. 10.1016/S0092-8674(00)80050-1. [DOI] [PubMed] [Google Scholar]
- 42.Lyzogubov VV, Bora PS, Wu X, Horn LE, de Roque R, Rudolf XV, Atkinson JP, Bora NS. 2016. The complement regulatory protein CD46 deficient mouse spontaneously develops dry-type age-related macular degeneration-like phenotype. Am J Pathol 186:2088–2104. 10.1016/j.ajpath.2016.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Camacho ZT, Turner MA, Barry MA, Weaver EA. 2014. CD46-mediated transduction of a species D adenovirus vaccine improves mucosal vaccine efficacy. Hum Gene Ther 25:364–374. 10.1089/hum.2013.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nestic D, Uil TG, Ma J, Roy S, Vellinga J, Baker AH, Custers J, Majhen D. 2019. Alphavbeta3 integrin is required for efficient infection of epithelial cells with human adenovirus type 26. J Virol 93:e01474-18. 10.1128/JVI.01474-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Baker AT, Greenshields-Watson A, Coughlan L, Davies JA, Uusi-Kerttula H, Cole DK, Rizkallah PJ, Parker AL. 2019. Diversity within the adenovirus fiber knob hypervariable loops influences primary receptor interactions. Nat Commun 10:741. 10.1038/s41467-019-08599-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Baker AT, Mundy RM, Davies JA, Rizkallah PJ, Parker AL. 2019. Human adenovirus type 26 uses sialic acid-bearing glycans as a primary cell entry receptor. Sci Adv 5:eaax3567. 10.1126/sciadv.aax3567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Persson BD, John L, Rafie K, Strebl M, Frangsmyr L, Ballmann MZ, Mindler K, Havenga M, Lemckert A, Stehle T, Carlson LA, Arnberg N. 2021. Human species D adenovirus hexon capsid protein mediates cell entry through a direct interaction with CD46. Proc Natl Acad Sci USA 118:e2020732118. 10.1073/pnas.2020732118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Greinacher A, Thiele T, Warkentin TE, Weisser K, Kyrle PA, Eichinger S. 2021. Thrombotic thrombocytopenia after ChAdOx1 nCov-19 vaccination. N Engl J Med 384:2092–2101. 10.1056/NEJMoa2104840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Muir KL, Kallam A, Koepsell SA, Gundabolu K. 2021. Thrombotic thrombocytopenia after Ad26.COV2.S vaccination. N Engl J Med 384:1964–1965. 10.1056/NEJMc2105869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nakamura T, Peng KW, Vongpunsawad S, Harvey M, Mizuguchi H, Hayakawa T, Cattaneo R, Russell SJ. 2004. Antibody-targeted cell fusion. Nat Biotechnol 22:331–336. 10.1038/nbt942. [DOI] [PubMed] [Google Scholar]
- 51.Oglesby TJ, White D, Tedja I, Liszewski K, Wright L, Van den Bogarde J, Atkinson JP. 1991. Protection of mammalian cells from complement-mediated lysis by transfection of human membrane cofactor protein and decay-accelerating factor. Trans Assoc Am Physicians 104:164–172. [PubMed] [Google Scholar]
- 52.Oglesby TJ, Allen CJ, Liszewski MK, White DJ, Atkinson JP. 1992. Membrane cofactor protein (CD46) protects cells from complement-mediated attack by an intrinsic mechanism. J Exp Med 175:1547–1551. 10.1084/jem.175.6.1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Stanley P, Siminovitch L. 1977. Complementation between mutants of CHO cells resistant to a variety of plant lectins. Somatic Cell Genet 3:391–405. 10.1007/BF01542968. [DOI] [PubMed] [Google Scholar]
- 54.Deutscher SL, Nuwayhid N, Stanley P, Briles EI, Hirschberg CB. 1984. Translocation across Golgi vesicle membranes: a CHO glycosylation mutant deficient in CMP-sialic acid transport. Cell 39:295–299. 10.1016/0092-8674(84)90007-2. [DOI] [PubMed] [Google Scholar]
- 55.Lee SW, Bonnah RA, Higashi DL, Atkinson JP, Milgram SL, So M. 2002. CD46 is phosphorylated at tyrosine 354 upon infection of epithelial cells by Neisseria gonorrhoeae. J Cell Biol 156:951–957. 10.1083/jcb.200109005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mrkic B, Pavlovic J, Rulicke T, Volpe P, Buchholz CJ, Hourcade D, Atkinson JP, Aguzzi A, Cattaneo R. 1998. Measles virus spread and pathogenesis in genetically modified mice. J Virol 72:7420–7427. 10.1128/JVI.72.9.7420-7427.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hofherr SE, Mok H, Gushiken FC, Lopez JA, Barry MA. 2007. Polyethylene glycol modification of adenovirus reduces platelet activation, endothelial cell activation, and thrombocytopenia. Hum Gene Ther 18:837–848. 10.1089/hum.2007.0051. [DOI] [PubMed] [Google Scholar]
- 58.Chen CY, Weaver EA, Khare R, May SM, Barry MA. 2011. Mining the adenovirus virome for oncolytics against multiple solid tumor types. Cancer Gene Ther 18:744–750. 10.1038/cgt.2011.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Parrott MB, Barry MA. 2001. Metabolic biotinylation of secreted and cell surface proteins from mammalian cells. Biochem Biophys Res Commun 281:993–1000. 10.1006/bbrc.2001.4437. [DOI] [PubMed] [Google Scholar]
- 60.Khare R, May SM, Vetrini F, Weaver EA, Palmer D, Rosewell A, Grove N, Ng P, Barry MA. 2011. Generation of a Kupffer cell-evading adenovirus for systemic and liver-directed gene transfer. Mol Ther 19:1254–1262. 10.1038/mt.2011.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Weaver EA, Rubrum AM, Webby RJ, Barry MA. 2011. Protection against divergent influenza H1N1 virus by a centralized influenza hemagglutinin. PLoS One 6:e18314. 10.1371/journal.pone.0018314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Havenga M, Vogels R, Zuijdgeest D, Radosevic K, Mueller S, Sieuwerts M, Weichold F, Damen I, Kaspers J, Lemckert A, van Meerendonk M, van der Vlugt R, Holterman L, Hone D, Skeiky Y, Mintardjo R, Gillissen G, Barouch D, Sadoff J, Goudsmit J. 2006. Novel replication-incompetent adenoviral B-group vectors: high vector stability and yield in PER.C6 cells. J Gen Virol 87:2135–2143. 10.1099/vir.0.81956-0. [DOI] [PubMed] [Google Scholar]
- 63.National Research Council. 2011. Guide for the care and use of laboratory animals, 8th ed. National Academies Press, Washington, DC. [Google Scholar]


