Abstract
Background
Curcumin, a naturally occurring anti-inflammatory compound, has shown promise in preclinical studies to treat erectile dysfunction (ED) associated with type-1- diabetes (T1D). However, poor bioavailability following oral administration limits its efficacy. The present study evaluated the potential of topical application of curcumin loaded nanoparticles (curc-np) to treat ED in a rat model of type-2-diabetes (T2D).
Aim
Determine if topical application of curc-np treats ED in a T2D rat model and modulates expression of inflammatory markers.
Methods
Curc-np (4mg curcumin) or blank nanoparticles (blank-np) were applied every 2- days for 2-weeks to the shaved abdomen of 20-weeks old Zucker Diabetic Fatty male rats (ZDF) (N=5 per group). Lean ZDF controls were treated with blank-np (N=5). Penetration of nanoparticles and curcumin release were confirmed by two-photon fluorescence microscopy and histology. Erectile function was determined by measuring intracorporal blood pressure normalized to systemic blood pressure (ICP/BP) following cavernous nerve stimulation. Corporal tissue was excised and RT-qPCR used to determine expression of the following markers: nuclear factor-κB (nf-κb), NF-κB-activating protein (nkap), nuclear factor erythroid 2- related factor-2 (nrf2), Kelch-like ECH-associated protein-1 (keap1), heme oxygenase-1 (ho- 1), variable coding sequence-A1 (vcsa1), phosphodiesterase-5 (pde5), endothelial and neuronal nitric oxide synthase (enos, nnos), Ras homolog gene family member A (rhoA), and Rho-associated coiled-coil containing protein kinases-1 and -2 (rock1, rock2).
Outcomes
Erectile function by determination of ICP/BP and expression of molecular markers in corporal tissue by RT-qPCR.
Results
Nanoparticles penetrated the abdominal epidermis and persisted in hair follicles for 24-hours. Curc-np treated animals exhibited higher average ICP/BP than animals treated with blank-np at all levels of stimulation and was statistically significant (p<0.05) at 0.75mA. In corporal tissue, nkap expression decreased 60% and HO-1 expression increased 60% in curc-np compared to blank-np treated animals. ICP/BP values inversely correlated with nkap and directly correlated with ho-1 expression levels.
Clinical Translation
These studies demonstrate the potential for topical application of curcnp as a treatment for ED in T2D patients.
Strengths & Limitations
The T2D animal model of ED represents a more prevalent disease than the more commonly studied T1D model. Although there is improved erectile response in curc-np treated animals, only at the lower levels of stimulation (0.75mA) was this significant compared to the blank-np treated animals, suggesting more studies are needed to optimize protocols and evaluate toxicity.
Conclusions
Topical application of curc-np to a rat model of T2D can systemically deliver curcumin, treat ED and modulate corporal expression of inflammatory markers.
Keywords: Erectile dysfunction; curcumin; inflammation; nanoparticle, diabetes
INTRODUCTION
Orally administered PDE5 inhibitors (PDE5i) are commonly used as first-line therapy for men with erectile dysfunction (ED). However, certain patient groups, such as diabetics or men who have undergone radical prostatectomy (RP), are often refractory to this treatment1. In addition, a significant percentage of men have difficulty swallowing medications2 or cannot tolerate the side-effects associated with PDE5i’s. There is therefore a real need to identify novel strategies to treat ED.
Chronic inflammation, resulting from several disease conditions (such as obesity or diabetes), or following RP, is increasingly recognized as a factor in the development of ED.3, 4 Recent studies have also associated improved erectile function in patients treated with PDE5i with reduced levels of anti-inflammatory factors.5, 6 This has led a number of research groups to explore the use of agents modulating the inflammatory response to improve erectile function. For example, preclinical studies have provided evidence that oral administration of the anti-inflammatory, curcumin, is effective in treating ED in a type-1-diabetic (T1D) rat model.7, 8
Curcumin is a plant-derived natural phenol with anti-oxidant and anti-inflammatory properties and has been ascribed various putative preventive and therapeutic properties.9 Curcumin has been associated with the modulation of several signaling pathways/mechanisms associated with erectile function, such as downregulation of pro-inflammatory cytokines, reactive oxygen species, hyperglycemia, and insulin resistance; and upregulation of nitric oxide, cGMP, and insulin secretion.10 However, curcumin exhibits several chemical characteristics which limits its efficacy and clinical translation. It has low aqueous solubility, is rapidly degraded and has poor oral bioavailability.11 Transdermal drug delivery of curcumin could potentially overcome several of these limitations and allow sustained release into the systemic blood circulation.12
Our group recently developed a novel silane-based hydrogel nanoparticle platform which has been used for transdermal delivery of a variety erectogenic agents, such as nitric oxide, the opiorphin peptide and tadalafil across the penile dermis with significant improvement in erectile function in both aging and nerve-injured animal models of ED.13, 14 Topical application of curcumin loaded nanoparticles (curc-np) exhibit antimicrobial activity and promote burn-wound healing.15 Many of the limitations for clinical translation of curcumin as a treatment for ED could be overcome through the use of topically applied curc-np. These include sustained release of curcumin (extending the short systemic lifetime of orally administered curcumin), low frequency of application, avoidance of GI inactivation and non-invasive administration.16 Detailed characterization and subsequent optimization of nanoparticle-skin interaction and rate of drug release can be achieved through the use of imaging techniques, such as two-photon fluorescence microscopy (TPM), which enables both non-invasive, high-resolution, real-time imaging of treatment response and direct visualization of nanoparticle migration through the skin in live animals at up to approximately 200µm depth.17–19
Preliminary studies (unpublished) by our group have demonstrated a systemic effect by curc-np when topically applied to mouse models that is significantly more pronounced than animals treated with pure curcumin. Given published studies on a rat model of T1D demonstrating that oral administration of curcumin show a positive effect on erectile function, we performed the following studies to demonstrate in a T2D rat model of ED that: i) curc-np are able to penetrate abdominal skin with subsequent release of systemically active curcumin; ii) curcumin released from curc-np can treat ED; and iii) the impact on erectile function correlates with curcumin induced modulation of the inflammatory response.
MATERIALS AND METHODS
Animal model
(All protocols using animals were approved by the Institutional Animal Care and Use Committee of the XXX). Ten Zucker Diabetic Fatty rats (diabetic ZDF; ZDF/Gmi-fa/fa) and 5 lean rats (ZDF/Gmi-fa/+), 11–12-weeks of age were procured from Charles River Laboratories (Willmington, MA, USA ) and housed until 20-weeks of age; all were fed with Purina #5008 diet as recommended to induce development of T2D. The purina #5008 diet was purchased from Labiet, inc, (St Louis, MO, USA) with the detailed composition available on their website: (http://www.labdiet.com/cs/groups/lolweb/@labdiet/documents/web_content/mdrf/mdi4/~edisp/ducm04_028444.pdf). Rats were divided into three groups (N=5 per group): diabetic ZDF rats treated with curc-np, diabetic ZDF rats treated with blank-np, and lean ZDF rats treated with blank-np. Animals were weighed and blood glucose determined at 18-, 20-, and 22-weeks of age using One Touch Ultra Blue test strips and a hand-held glucometer (Life Scan Inc., Milpitas, CA).
Treatment
On the day prior to the first treatment, the abdomen of each animal was shaved and depilated with Nair. Nanoparticles (200mg) were applied to the abdomen of a rat under isoflurane anesthesia as a coconut oil paste; the paste was gently massaged until the coconut oil melted and the nanoparticles were absorbed by the skin. The curc-np contained 4mg curcumin, equivalent to approximately 10mg curcumin/kg body weight. In prior studies free curcumin was given orally at a dose of 10 mg/ kg body weight. 7 The schedule consisted of 6 applications over a 2-week period.
Curcumin loaded nanoparticles
Porous, silane-based curcumin loaded nanoparticles (curcnp) were fabricated by modification of a previously published process,15, 20 wherein a curcumin doped silicate sol-gel was lyophilized and wet-milled to generate an aqueous dispersion of nanoparticles with average radius of 125nm. Nanoparticles were loaded with curcumin at 80µg/mg (blank-np were generated without curcumin loading). Although the major steps in the manufacturing process are as described in15, 20 minor modifications are outlined within a patent application: SOL-GEL/HYDROGEL THERAPEUTIC DELIVERY SYSTEM AND METHODS THEROF Inventors: Joel M. Friedman, Andrew Draganksi, Adam Friedman and Mahantesh Navati File: 96700/2568 (this information will become public upon award of the patent). Curc-np and blank-np were well dispersed in coconut oil at 4°C (1:3 by weight) to yield a soft paste, used as the treatment vehicle (stored at −20°C). C haracterization information (including particle size via Dynamic Light Scattering and Electron microscopy) and in vitro release kinetics have been previously described 15, 20. For fluorescence imaging experiments, nanoparticles were covalently labeled with Alexa Fluor 568 maleimide (Thermo Fisher Scientific, Waltham, MA), or alternatively loaded with NBD-AEA (20-[(7-nitro-2-1,3-benzoxadiazol-4-yl)amino] arachidonoyl ethanolamide, Avanti Polar Lipids, Inc., Alabaster, AL). During synthesis 0.1% of the TMOS precursor is replaced with mercaptopropyl trimethoxysilane (MPTS). This incorporates reactive thiol groups into the sol-gel matrix. Alexa fluor 568 maleimide readily reacts with these thiol groups at neutral pH. Probe and nanoparticles were incubated for 1 hour while mixing at room temperature. After incubation period particles were centrifuged at 13g and washed twice with PBS to remove any unreacted probe.
Imaging of curc-np skin penetration
TPM imaging of rat skin treated with curc-np was performed using an Olympus Inverted IX81 microscope with a 25x, 1.05 numerical aperture water immersion objective with excitation source femtosecond pulsed laser 690–1040 nm (Spectra Physics Mai Tai-DeepSee). Image detection was via point scanning with 4 photomultiplier tube channels that are simultaneously collected via a set of dichroic mirrors; the first channel detects second harmonics scattered light and is used to image collagen fibers in the dermis. The other three channels detect fluorescence (CFP, GFP, and mCherry). The heated microscope stage is designed to hold an anesthetized animal to allow imaging over several hours of both nanoparticle localization and release characteristics.
Histology
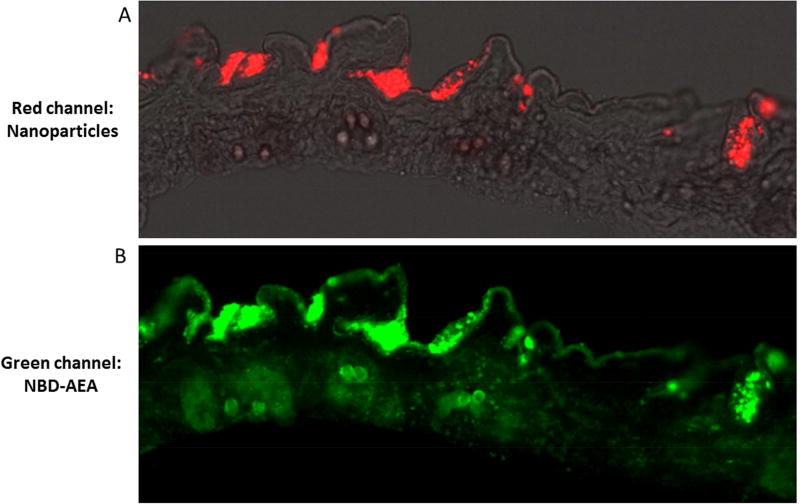
To further demonstrate the efficacy of transdermal drug delivery, red fluorescent nanoparticles were loaded with a green fluorescently labeled lipid molecule 20-[(7-nitro-2-1,3- benzoxadiazol-4-yl)amino] arachidonoyl ethanolamide (NBD-AEA). NBD-AEA was chosen because of its ability to diffuse out of nanoparticles with similar characteristics as curcumin (efforts to directly detect curcumin fluorescence in histological skin slices is hampered due to curcumin’s low fluorescent quantum yield and photostability). Figure 2 shows fluorescent microscopic images from an abdominal skin slice obtained from an animal sacrificed 1-hour after treatment with nanoparticles. Overlay of the phase and red fluorescent channels is shown in Figure 2A which demonstrates the penetration of nanoparticles through the epidermis as well as their localization in skin folds and hair follicles. Figure 2B shows the green fluorescent channel; the lipid NBD-AEA as diffused throughout the epidermis as well as deeper in the hair follicles. Furthermore, it is clear that not all of the lipid has yet diffused out from the np. After 24- hours particles that persist in the skin no longer fluoresce in the green channel indicating complete release of drug (not shown).
Figure 2. Epifluorescent histologic image of ZDF rat skin treated with fluorescent nanoparticles.
Images generated 1-hour post-application of red labeled nanoparticles loaded with green colored lipip in an 8µm thick slice of ZDF rat abdominal skin. (A) is an overlay of the phase and red channels showing labeled nanoparticles have penetrated the thin epidermis and have begun to collect in hair follicles. (B) is the green channel of the same image showing the labeled lipid diffusing out from nanoparticles across the epidermis as well as deeper at the base of hair follicles.
Erectile function monitoring
The erectile response was determined by electrostimulation of the cavernous nerve (CN) and by measuring intracavernous pressure/systemic blood pressure ratio (ICP/BP) as previously described.13 Briefly, through a lower midline abdominal incision, the CN was exposed and isolated. Cannulas were inserted into the carotid artery and the crura to measure the ICP and BP respectively, and a bipolar stainless-steel electrode, was used to directly stimulate the CN (probes were 2mm in diameter, separated by 1mm) via a signal generator and a custom-built constant-current amplifier generating monophasic rectangular pulses with stimulus parameters ranging from 0.75–10mA, 20 Hz, pulse width of 0.2ms, and duration of 50s. The ICP and BP were recorded in real time using a bioinformation acquisition system (LabChart; ADInstruments, Colorado Springs, CO). The maximal ICP/BP ratios in the experimental animals were calculated for each level of stimulation. The results were analyzed using GraphPad Prism software by comparing measures using two-tailed student’s t-test (alpha=0.05). The groups were considered to be significantly different at P<0.05. Data were reported as the mean ± standard deviation. Following these determinations, animals were euthanized.
Corporal tissue RNA expression
Animals were euthanized after surgery and corporal tissue was collected. Total RNA was isolated using TRIzol (Thermo Fisher Scientific, Waltham, MA) and 0.5 µg of total RNA was used to generate cDNA using SuperScript IV VILO Master Mix (Invitrogen; Carlsbad, CA). Quantitative RT-PCR was performed using the 7300 Real Time PCR system (Applied Biosystems, Carlsbad, CA). Primers included markers of inflammation: nf-κb, nkap, nrf2, keap1, ho-110; markers of erectile function: vcsa121, pde5, enos, nnos; and markers of smooth muscle calcium sensitization: rhoa, rock1, rock2. The primers not referenced herein were design using Primer Express 3.0.1software (Thermofisher, Waltham, MA). Details of primer sequences are provided in Table 1. The cycling conditions were 1 cycle at 95°C for 15min followed by 40 cycles at 95°C for 15s and 60°C for 1min. Dissociation curves were generated to verify the absence of nonspecific amplification. Data were analyzed using the Applied Biosystems, 7300 systems. Relative gene expression was determined by the ΔCt method and student’s t-test was performed on the results using GraphPad Prism software.
Table 1.
Primer Sequences
| Vcsa1 | TGAATATCAATATCAGTGGCAGCTAA |
| TTGCTTGTTCTGTGGAATGAAGTT | |
| PDE5 | TGTTTCTGGCAATGCTGATGA |
| TTCTGCTATCCGTTGTTGAATAGG | |
| eNOS | GACTTTTAAGGAAGTAGCCAATGCA |
| TGTTTCTGGCAATGCTGATGAC | |
| nNOS | TTCTGCTATCCGTTGTTGAATAGG |
| CACTGTCATAGCTGAGGTCTACCAA | |
| Keap1 | CAGCGTGCTCGGGAGTATATC |
| AATATCAATATCAGTGGCAGCTAACTG | |
| Nrf2 | TAGCAGAGCCCAGTGGCGGT |
| TGCTCTGGGGATGCTCGGCT | |
| Nkap | AACGGCCTTCTGCACAGCGG |
| CCAGGTAACAGGGCGTGGCC | |
| Nf-kβ | AGCACCAAGACCGAAGCAATT |
| GAAAACCCACATCCTCTTCCTT | |
| HO-1 | GGCCTGGCTTTTTTCACCTT |
| TGCGAGCACGATAGAGCTGTT | |
| RhoA | CCCGCGTCTAGCTTGCA |
| CTCTGTGCTTCTGTCTCTGT | |
| ROCK1 | GCGGGTACGAAGGTATCGTC |
| TGTCCCCAGTGCACATGATG | |
| ROCK2 | ACAGTCCGTGGGTGGTTCA |
| CACCTGGCATGTACTCCATCA | |
| RPL19 | ACCCCAATGAAACCAACGAA |
| TCAGGCCATCTTTGATCAGCT |
RESULTS
Microscopy of nanoparticle skin penetration
Two-photon fluorescence microscopy
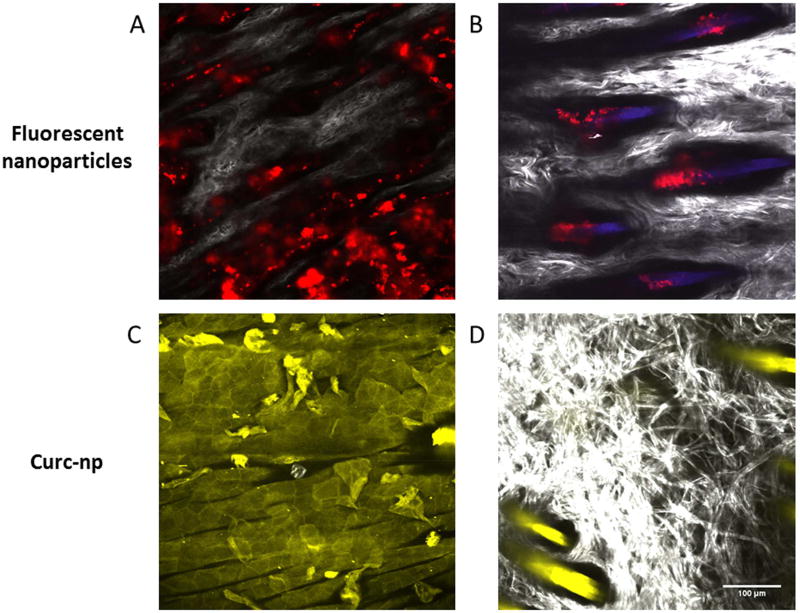
T2M images of rat abdominal skin at a depth of 45µm after application of fluorescently labeled nanoparticles are shown in Figure 1. In Figure 1A & B, the red color is due to the labeled nanoparticles, grayscale coloring is due to scattering of incident light by collagen and blue is due to tissue auto-fluorescence from hair follicles. After 1-hour nanoparticles have penetrated through the 15µm thick stratum corneum, the hydrophobic protective outer layer of the skin, and have collected in skin furrows at the bottom of the viable epidermis. After 24-hours nanoparticles exclusively persist deep in the hair follicles, where there is extensive vasculature, which likely provides for extended release into systemic circulation. Figure 1C & D shows fluorescence due to curcumin from the abdomen of a rat 2- hours after treatment with curc-np; upon two-photon excitation, curcumin emits a broad fluorescence from 475 to 600nm detected in the microscope’s green fluorescence (GF) channel. At a depth of 12µm from the skin surface, curcumin has largely diffused through the epidermal extracellular lipid matrix evident by the hexagonal traces of keratinocytes; at 45µm of depth, near the dermal junction it is readily apparent that curcumin has been heavily released into the hair follicles.
Figure 1. Two-photon microscopy of nanoparticles topically applied to rat abdomen.
(A) Image generated 1-hour post-application of nanoparticles to the shaved abdomen of a ZDF rat. Red fluorescently labeled nanoparticles are seen to reside in skin furrows between the white collagen fibers that make up dermal papillae at a depth of 45µm. (B) Image generated 24-hours post-application of nanoparticles show residence in hair follicles. (C) Image generated 2-hours post-application of curc-np at 12µm depth and (D) 45 µm depth; the yellow color is due to the fluorescence of curcumin.
Histology
To further demonstrate the efficacy of transdermal drug delivery, red fluorescent nanoparticles were loaded with a green fluorescently labeled lipid molecule (NBD-AEA). NBDAEA was chosen because of its ability to diffuse out of nanoparticles with similar characteristics as curcumin (efforts to directly detect curcumin fluorescence in histological skin slices is hampered due to curcumin’s low fluorescent quantum yield and photostability). Figure 2 shows fluorescent microscopic images from an abdominal skin slice obtained from an animal sacrificed 1-hour after treatment with nanoparticles. Overlay of the phase and red fluorescent channels is shown in Figure 2A which demonstrates the penetration of nanoparticles through the epidermis as well as their localization in skin folds and hair follicles. Figure 2B shows the green fluorescent channel; the lipid NBD-AEA as diffused throughout the epidermis as well as deeper in the hair follicles. Furthermore, it is clear that not all of the lipid has yet diffused out from the np. After 24-hours particles that persist in the skin no longer fluoresce in the green channel indicating complete release of drug (not shown).
General Animal Characteristics
The average weight of both diabetic and lean ZDF rat groups at 20-weeks of age was not significantly different, with an average weight of 408.3±16.9g, well within range of expected weight reported by the supplier. The blood glucose of the lean animals was significantly lower than the diabetic ZDF rats but did increased steadily throughout the 4- week period of measurement (16-weeks: 121.8 ± 3.4mg/dL; 18-weeks: 144.6 ± 16.5mg/dL; 20- weeks: 198.6 ± 18.4mg/dL). This increase was most likely due to the high energy diet all animal groups received during on-site housing. The diabetic ZDF rats treated with curc-np or blank-np exhibited significantly higher glucose levels than the lean animals at each time point considered (16-weeks: >532.6 ± 61.4mg/dL; 18-weeks: >510.9 ± 59.3mg/dL; 20 weeks: >567.5 ± 28.7mg/dL); there was no significant difference between diabetic ZDF rats treated with curc-np or blank-np.
Erectile function
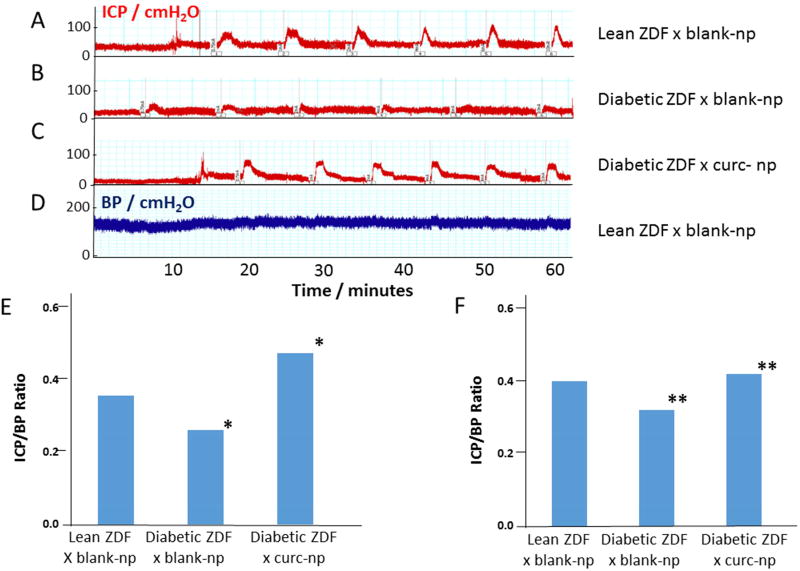
In Figure 3A-C representative traces of ICP response to different levels of electrostimulation of the cavernous nerve (CN) are shown for each of the animal groups. Figure 3D shows a representative trace of systemic BP measured simultaneously with ICP; although this trace was derived from lean ZDF rats treated with blank-np the basal BP was not significantly different between the groups of animals or during the CN stimulation experiments (data not shown). Visual inspection of the top trace (A) reveals that in the lean ZDF rats treated with blank-np, electrostimulation of the CN resulted in an increase in the ICP even at the lowest level of stimulation (0.75mA), with a trend towards a progressively increased ICP response with higher levels of CN stimulation. In the second trace (B), ZDF fatty rats treated with blank-np, there was a much reduced ICP response across all levels of CN electrostimulation, indicative of impaired erectile function in these animals. The third trace (C), represents the ICP response of ZDF fatty rats treated with curc-np showing a much improved response on CN stimulation. Figure 3E shows the average ICP/BP ratio for the 5 animals in each group at the 0.75mA stimulation. There is significant (P<0.05) improvement in ICP/BP ratio of curc-np compared to blank-np treated diabetic ZDF rats, as well as no significant difference between curc-np treated diabetic ZDF and blank-np treated lean rats. As shown in Figure 3F, the ICP/BP ratio averaged across all levels of stimulation demonstrated a significant (P<0.01) improvement in erectile function in the curc-np compared to the blank-np treated diabetic ZDF rats.
Figure 3. Erectile function: intracavernosal pressure (ICP) normalized by systemic blood pressure (BP).
Representative traces of ICP in response to electrostimulation of the cavernous nerve at 0.75, 1, 2, 4, 6, and 10mA current for (A) lean rats treated with blank-np, (B) Diabetic ZDF treated with blank-np, and (C) Diabetic ZDF treated with curc-np. The lean rats exhibited high ICP response to electrostimution, not seen in the ZDF fatty animals; this loss was recovered in the curc-np treated ZDF fatty rats. (D) Shows a representative trace of systemic BP (BP was not significantly different between the different groups of animals). The average ICP/BP ratio is shown for 0.75 mA (E) and for all level of stimulation (F), demonstrating improvement in erectile function in ZDF rats treated with curc-np (*P<0.05; **P <0.01).
RT-qPCR
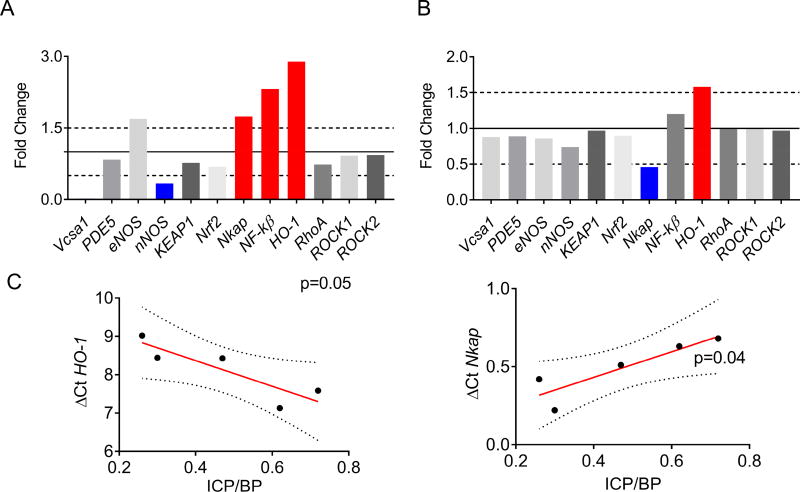
Comparative mRNA levels of various genetic markers important to erectile function are presented in Figure 4. Figure 4A shows expression of markers for blank-np treated diabetic ZDF rats compared to their lean counterparts; ho-1, nkap and nf-κβ, markers of endothelial activation, were upregulated; also vcsa1 and nnos, markers of erectile function, were downregulated. When the topical administration of curc-np was compared to blank-np diabetic ZDF rats ho-1 expression was increased by ~60% whereas nkap expression decreased by ~60%. Diabetic ZDF rats treated with curc-np or blank-np showed similar mRNA levels of nf-κβ, nrf2, keap1, vcsa1, pde5, enos, nnos, rhoA, rock1, and rock2 (Figure 4B). In order to further investigate the association of erectile function and gene expression, the ICB/BP measurements were correlated with mRNA level of specific genes using linear regression (Figure 4C). This analysis showed a direct correlation of ICP/BP with the expression levels of ho-1 mRNA level (P<0.05) and an inverse correlation of ICP/BP with nkap mRNA level (P<0.04). No correlation was observed between erectile function and mRNA expression levels of the other genes investigated in this study.
Figure 4. Quantitative reverse transcription PCR analysis of corporal tissue.
(A) Comparison of gene expression by RT-qPCR between ZDF fatty and lean rats expressed in fold change. RPL19 was used as the reference gene. Red bars denote upregulated genes and blue bars denote downregulated genes. The solid line represents relative gene expression in the lean animals (control). (B) Comparison of fold-change in gene expression between curc-np and blank-np (control) treated diabetic ZDF rats. (C) Linear regression analysis between gene expression of ho-1 and (D) nkap and ICP/BP following CN stimulation between 0.75 and 1.0mA. The y-axis (ΔCt) represents the amount of target gene normalized by the reference gene (RPL19). Ct (threshold cycle) values are inverse to the amount of RNA in the sample. Dotted line represents 95% confidence intervals. P< 0.05 denotes statistical significance.
DISCUSSION
The studies presented here provide the first evidence that curcumin can protect erectile function in a T2D rat model of diabetes. Furthermore, the utility of silane-based nanoparticles as a transdermal delivery system for curcumin is demonstrated. We also correlate improved erectile function with modulation of the gene expression of certain inflammatory markers, demonstrating that the protective effect of curcumin on erectile function in the T2D rat model is associated with modulation of the inflammatory response.
Transdermal delivery of curcumin
It is clear from the microscopic analyses of skin samples treated with curc-np that the nanoparticles are able to penetrate across the stratum corneum down to the furrows in skin folds where they persist in the hair follicle. It is expected that hydrophobic compounds, such as curcumin, diffuse out of nanoparticles from the hair follicles into the systemic circulation via direct access into capillaries or the lymphatics by plasma membrane diffusion and transmembrane exchange.12, 16 Quantification of plasma levels of curcumin is challenging and are the subject of a paper “in preparation” which describes the pharmacokinetics of curc-np applied to skin by using micro-HPLC to measure the plasma level of curcuminoids at different time points after topical application. These studies show that plasma levels of curcumin when derived from topically appied curc-np can exceed 1×106-fold the levels resulting from i.v. injection (because the low solubility of curcumin limits amounts that can be injected). Although there was significant improvement in erectile function in animals treated with curc-np compared to controls treated with blank-np, and we have previously demonstrated improved efficacy of curc-np over free curcumin in different animal models of pathology15, 20 a potential limitation of these studies is the absence of an additional control consisting of a physical mixture of curcumin and silane.
A parallel study (manuscript in preparation) utilizing the same curc-np used in our experiments demonstrated that curc-np applied to the abdomen of obese C57BL/6 mice caused levels of key plasma inflammatory cytokines (MCP-1, TNF-α, and IL-6) to become normalized to levels observed in lean mice. However, application of pure curcumin to the skin did not achieve the same results. The curc-np treated fat mice also displayed lowered levels of adipose cytokines, plasma lipids, leptin, blood glucose and insulin. One difference to the studies reported here was that curc-np treated obese mice exhibited significantly lower blood glucose levels, whereas there was no effect on blood glucose levels of diabetic ZDF rats It is possible that this difference may be due to the diabetic ZDF rat model suffering from a more significant disease (i.e. non-functional leptin receptors).
Improvement in erectile function
The parameter used to determine treatment of ED in T2D animals was measurement of ICP/BP following CN stimulation, and maintenance of maximal ICP over the 50 s of CN stimulation. We used stimulation parameters that have previously been used for determination of ICP/BP in the commonly used rat models of ED (Fischer F344 and Sprague-Dawley) in our laboratory. As shown in Fig 3A, although this did result in an increasing ICP/BP response with increasing voltage stimulation in lean ZDF rats, in the diabetic ZDF rats the ICP/BP response at lower stimulation was already maximized and not increased with higher stimulations. A possible explanation for the effect on the stimulation/response curve in diabetic ZDF rats is that diabetes results in cavernous nerve frailty in the diabetic ZDF rats compared to the lean rats. However, as shown in Figure 3E, the average ICP/BP at 0.75 mA level of CN stimulation in diabetic ZDF rats treated with curc-np was 1.5-fold higher than those treated with blank-np (P<0.05); and collectively across all levels of stimulation there was a 1.3-fold improvement (P<0.01). Conventionally, a visible erection is believed to correspond with an ICP/BP ratio>0.6. However, this value is arbitrary, and predominantly based on observations of erectile function in chemically (streptozotocin) induced T1D animal models. In the current studies, even lean animals had a lower erectile function response, as determined by ICP/BP ratio, than would be expected in young heathy animals of different rodent species (for example Sprague-Dawley rats where as ICP/BP ratio >0.6 is assumed to correspond with a visible erection). However, several of the animals used in these studies achieved a visible erection with an ICP/BP ratio <0.6 and therefore future studies may be needed to determine the species specific ICP/BP ratio that can be deemed “normal”.
Gene expression in cavernous tissue
It is widely accepted that chronic inflammation is associated with ED.3, 4, 22 Upregulation and activation of the NF-κβ pathway has long been considered a prototypical proinflammatory signaling pathway (largely based on the role of NF-κβ in regulating the expression of proinflammatory genes23, 24) and it has been shown that inhibiting NF-κβ improves erectile function in rats.25 Consistent with the literature, we observed increased mRNA expression of nf-κβ in the ZDF group compared with the lean group. Although in our studies nf-κβ mRNA levels were similar in both T2D animals treated with curc-np or blank-np, the recently identified protein, nkap (NF-κβ Activating Protein)26 was down-regulated in the curc-np treated T2D animals, suggesting that curcumin indirectly regulates NF-κβ activity by down-regulating NKAP. This contrasts with observations reported Aziz et al. where curcumin appears to directly downregulate NF-κβ in T1D diabetic rats.10
Another marker of inflammation, Heme Oxygenase 1(HO-1), is expressed in both endothelial and smooth muscle cells where it exerts anti-oxidant and anti-apoptotic actions27 and has been associated with improved erectile function in sildenafil citrate treated rats.28 It has been shown that HO-1 expression decreases in T1D and T2D.29 In our study, ho-1 was upregulated in the ZDF group treated with curc-np compared to animals treated with blank-np, and this increased expression of ho-1 correlated with improved erectile function (Figure 4C). Interestingly, despite a dramatic downregulation of Vcsa1 (which encodes sialorphin), a proposed marker of physiologic erectile function30, in the ZDF group compared to the lean one, there was no observed difference in its expression between curc-np or blank-np treated ZDF rats. The expression of the NF-κβ regulators keap1 and nrf2 were downregulated in the ZDF rats, but curc-np had no effect in their levels of expression. nnos was also downregulated in T2D rats compared to the lean group but curc-np didn’t change its expression in contrast with the finding of Aziz et al. in a T1D rat model where curcumin upregulates nnos expression in the treated group.10 There were no significant changes in expression of the other genes studied.
At present our studies have focused on gene expression; potentially post-translation modification of the proteins encoded by these genes could affect their activity and therefore protein expression and functional activity studies would be a focus of future work.
Although Figure 4C was restricted to showing the correlation between ho-1 or nkap, and ICP/BP in the curc-np treated ZDF fatty rats, this correlation remained true when compared across all 15 animals used in the study (data not shown). This suggests “naturally” occurring factors (such as aging, diet and underlying disease) which affect the inflammatory status of an animal can affect erectile function, reinforcing the emerging concept that erectile function is not only a sentinel marker of co-existing and undetected cardiovascular disease, but also that of a man’s overall well-being.31
CONCLUSIONS
Our results demonstrate that in a T2D rat model topical application of curc-np on the abdomen can treat ED. The primary effect on corporal gene expression appears to be on markers of inflammation. This suggests that the therapeutic effect of curcumin on erectile function in the T2D rat model is associated with modulation of the inflammatory response in the corporal tissue by a systemic delivery of curcumin from topical application of curc-np to the abdomen.
Acknowledgments
This work was supported by an SMSNA Pfizer Fellowship (PI: A. Draganski) and from the National Institutes of Health [NIH/NIDDK R01DK107807 PI: K. P. Davies).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest: “None”
References
- 1.Rendell MS, Rajfer J, Wicker PA, Smith MD. Sildenafil for treatment of erectile dysfunction in men with diabetes: a randomized controlled trial. Sildenafil Diabetes Study Group. JAMA. 1999;281:421–6. doi: 10.1001/jama.281.5.421. [DOI] [PubMed] [Google Scholar]
- 2.Bhattacharyya N. The prevalence of dysphagia among adults in the United States. Otolaryngol Head Neck Surg. 2014;151:765–9. doi: 10.1177/0194599814549156. [DOI] [PubMed] [Google Scholar]
- 3.Hannan JL, Maio MT, Komolova M, Adams MA. Beneficial impact of exercise and obesity interventions on erectile function and its risk factors. J Sex Med. 2009;6(Suppl 3):254–61. doi: 10.1111/j.1743-6109.2008.01143.x. [DOI] [PubMed] [Google Scholar]
- 4.Khoo J, Piantadosi C, Duncan R, et al. Comparing effects of a low-energy diet and a high-protein low-fat diet on sexual and endothelial function, urinary tract symptoms, and inflammation in obese diabetic men. J Sex Med. 2011;8:2868–75. doi: 10.1111/j.1743-6109.2011.02417.x. [DOI] [PubMed] [Google Scholar]
- 5.Santi D, Granata AR, Guidi A, et al. Six months of daily treatment with vardenafil improves parameters of endothelial inflammation and of hypogonadism in male patients with type 2 diabetes and erectile dysfunction: a randomized, double-blind, prospective trial. Eur J Endocrinol. 2016;174:513–22. doi: 10.1530/EJE-15-1100. [DOI] [PubMed] [Google Scholar]
- 6.Garcia LA, Hlaing SM, Gutierrez RA, et al. Sildenafil attenuates inflammation and oxidative stress in pelvic ganglia neurons after bilateral cavernosal nerve damage. Int J Mol Sci. 2014;15:17204–20. doi: 10.3390/ijms151017204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Abdel Aziz MT, Rezq AM, Atta HM, et al. Molecular signalling of a novel curcumin derivative versus Tadalafil in erectile dysfunction. Andrologia. 2015;47:616–25. doi: 10.1111/and.12309. [DOI] [PubMed] [Google Scholar]
- 8.Zaahkouk AM, Abdel Aziz MT, Rezq AM, et al. Efficacy of a novel water-soluble curcumin derivative versus sildenafil citrate in mediating erectile function. Int J Impot Res. 2015;27:9–15. doi: 10.1038/ijir.2014.24. [DOI] [PubMed] [Google Scholar]
- 9.Noorafshan A, Ashkani-Esfahani S. A review of therapeutic effects of curcumin. Curr Pharm Des. 2013;19:2032–46. [PubMed] [Google Scholar]
- 10.Abdel Aziz MT, Motawi T, Rezq A, et al. Effects of a water-soluble curcumin protein conjugate vs. pure curcumin in a diabetic model of erectile dysfunction. J Sex Med. 2012;9:1815–33. doi: 10.1111/j.1743-6109.2012.02741.x. [DOI] [PubMed] [Google Scholar]
- 11.Flora G, Gupta D, Tiwari A. Nanocurcumin: a promising therapeutic advancement over native curcumin. Crit Rev Ther Drug Carrier Syst. 2013;30:331–68. doi: 10.1615/critrevtherdrugcarriersyst.2013007236. [DOI] [PubMed] [Google Scholar]
- 12.McConville J. Special Focus Issue: Transdermal, Topical and Folicular Drug Delivery Systems. Drug Dev Ind Pharm. 2016;42:845. doi: 10.3109/03639045.2016.1169634. [DOI] [PubMed] [Google Scholar]
- 13.Han G, Tar M, Kuppam DS, et al. Nanoparticles as a novel delivery vehicle for therapeutics targeting erectile dysfunction. J Sex Med. 2010;7:224–33. doi: 10.1111/j.1743-6109.2009.01507.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tar M, Cabrales P, Navati M, et al. Topically applied NO-releasing nanoparticles can increase intracorporal pressure and elicit spontaneous erections in a rat model of radical prostatectomy. J Sex Med. 2014;11:2903–14. doi: 10.1111/jsm.12705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Krausz AE, Adler BL, Cabral V, et al. Curcumin-encapsulated nanoparticles as innovative antimicrobial and wound healing agent. Nanomedicine. 2015;11:195–206. doi: 10.1016/j.nano.2014.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ita KB. Transdermal drug delivery: progress and challenges. J Drug Deliv Sci Tec. 2014;24:245–50. [Google Scholar]
- 17.Nooshabadi F, Yang HJ, Bixler JN, Kong Y, Cirillo JD, Maitland KC. Intravital Fluorescence Excitation in Whole-Animal Optical Imaging. PLoS One. 2016;11:e0149932. doi: 10.1371/journal.pone.0149932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fernandes R, Smyth NR, Muskens OL, et al. Interactions of skin with gold nanoparticles of different surface charge, shape, and functionality. Small. 2015;11:713–21. doi: 10.1002/smll.201401913. [DOI] [PubMed] [Google Scholar]
- 19.Tseng TY, Yang CS, Tsai TH, Chen YF, Dong CY. Dynamic characterization of hydrophobic and hydrophilic solutes in oleic-acid enhanced transdermal delivery using two-photon fluorescence microscopy. Appl Phys Lett. 2014;105 [Google Scholar]
- 20.Zhang Z, Leong DJ, Xu L, et al. Curcumin slows osteoarthritis progression and relieves osteoarthritis-associated pain symptoms in a post-traumatic osteoarthritis mouse model. Arthritis research & therapy. 2016;18:128. doi: 10.1186/s13075-016-1025-y. [DOI] [PMC free article] [PubMed] [Google Scholar] [Research Misconduct Found]
- 21.Calenda G, Tong Y, Tar M, et al. Vcsa1 acts as a marker of erectile function recovery after gene therapeutic and pharmacological interventions. The Journal of urology. 2009;181:2806–15. doi: 10.1016/j.juro.2009.01.096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Senn JJ, Klover PJ, Nowak IA, Mooney RA. Interleukin-6 induces cellular insulin resistance in hepatocytes. Diabetes. 2002;51:3391–9. doi: 10.2337/diabetes.51.12.3391. [DOI] [PubMed] [Google Scholar]
- 23.Lawrence T. The nuclear factor NF-kappaB pathway in inflammation. Cold Spring Harb Perspect Biol. 2009;1:a001651. doi: 10.1101/cshperspect.a001651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sun SC. The non-canonical NF-kappaB pathway in immunity and inflammation. Nat Rev Immunol. 2017;17:545–58. doi: 10.1038/nri.2017.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sahin K, Orhan C, Akdemir F, et al. Comparative evaluation of the sexual functions and NF-kappaB and Nrf2 pathways of some aphrodisiac herbal extracts in male rats. BMC Complement Altern Med. 2016;16:318. doi: 10.1186/s12906-016-1303-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen D, Li Z, Yang Q, Zhang J, Zhai Z, Shu HB. Identification of a nuclear protein that promotes NF-kappaB activation. Biochem Biophys Res Commun. 2003;310:720–4. doi: 10.1016/j.bbrc.2003.09.074. [DOI] [PubMed] [Google Scholar]
- 27.Abdel Aziz MT, El Asmer MF, Mostafa T, et al. Effects of losartan, HO-1 inducers or HO-1 inhibitors on erectile signaling in diabetic rats. J Sex Med. 2009;6:3254–64. doi: 10.1111/j.1743-6109.2009.01517.x. [DOI] [PubMed] [Google Scholar]
- 28.Aziz MT, Al-Asmar MF, Mostafa T, et al. Assessment of heme oxygenase-1 (HO-1) activity in the cavernous tissues of sildenafil citrate-treated rats. Asian J Androl. 2007;9:377–81. doi: 10.1111/j.1745-7262.2007.00241.x. [DOI] [PubMed] [Google Scholar]
- 29.Abraham NG, Rezzani R, Rodella L, et al. Overexpression of human heme oxygenase-1 attenuates endothelial cell sloughing in experimental diabetes. Am J Physiol Heart Circ Physiol. 2004;287:H2468–77. doi: 10.1152/ajpheart.01187.2003. [DOI] [PubMed] [Google Scholar]
- 30.Davies KP, Tar M, Rougeot C, Melman A. Sialorphin (the mature peptide product of Vcsa1) relaxes corporal smooth muscle tissue and increases erectile function in the ageing rat. BJU Int. 2007;99:431–5. doi: 10.1111/j.1464-410X.2006.06577.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Capogrosso P, Ventimiglia E, Boeri L, et al. Sexual functioning mirrors overall men's health status, even irrespective of cardiovascular risk factors. Andrology. 2017;5:63–69. doi: 10.1111/andr.12299. [DOI] [PubMed] [Google Scholar]