Abstract
OBJECTIVES:
Vasopressin is reported to retain vasoconstrictive activity in the setting of acidemia, but preclinical models are inconsistent and studies have not evaluated the clinical effectiveness of vasopressin based on arterial pH. This study sought to determine the association between arterial pH and blood pressure after vasopressin initiation in septic shock.
DESIGN:
This retrospective, multicenter, observational cohort study evaluated the association of arterial pH at the time of vasopressin initiation with hemodynamic response to vasopressin and change in catecholamine dose after vasopressin initiation. Hemodynamic response was defined as a catecholamine dose decrease with mean arterial pressure greater than or equal to 65 mm Hg at 6 hours after vasopressin initiation.
SETTING:
Patients from eight hospitals in a health system were evaluated.
PATIENTS:
Patients with septic shock initiated on vasopressin as a catecholamine adjunct between January 2012 and November 2017 were screened for inclusion.
INTERVENTIONS:
None.
MEASUREMENTS AND MAIN RESULTS:
A total of 1,350 patients were included. At the time of vasopressin initiation patients were severely ill with arterial pH 7.28 ± 0.13, Sequential Organ Failure Assessment 14.1 ± 3.5, lactate 5.6 ± 4.6 mmol/L, and norepinephrine-equivalent catecholamine dose 32.3 ± 25.4 µg/min. After adjusting for lactate and Sequential Organ Failure Assessment with multivariable logistic regression, lower arterial pH was independently associated with lower odds of hemodynamic response to vasopressin (for each 0.1 unit arterial pH was below 7.40, response odds ratio 0.79; 95% CI, 0.72–0.87). For each 0.1 unit the pH was below 7.40 at vasopressin initiation, the norepinephrine-equivalent catecholamine dose increased by 1.5 µg/min (95% CI, 0.5–2.5 µg/min) at 1 hour, and increased by 2.5 µg/min (95% CI, 1.4–3.5 µg/min) at 6 hours after vasopressin initiation.
CONCLUSIONS:
Compared with higher arterial pH, patients with septic shock and low arterial pH had lower odds of vasopressin response and higher catecholamine doses after vasopressin initiation. Similar to other vasopressors, the clinical effectiveness of vasopressin appears to be impaired in the setting of acidemia.
Keywords: acidosis, sepsis, septic shock, norepinephrine, vasoconstrictor agents, vasopressin
Sepsis is the leading cause of death in hospitalized patients (1). Septic shock, a subset of sepsis clinically identified by infection-induced hypotension requiring vasopressors with hyperlactatemia, has a mortality rate of 33% in North America (2, 3). Over half of patients with septic shock have concomitant acidemia, at least in part due to lactic acidosis (4–6). Acidemia causes a number of complications, including hemodynamic alterations due to decreased cardiac output and systemic vasodilation (7, 8). As arterial pH decreases, the effectiveness of endogenous and exogenous catecholamines to improve blood pressure is reduced (9, 10). Concomitantly, acidosis causes endogenous release of vasopressin, which depletes pituitary vasopressin stores and further contributes to vasodilation (11, 12). Additionally, vasopressin has been reported to retain vasoconstrictive activity in the setting of acidemia (13, 14). For these reasons, some clinicians initiate exogenous vasopressin in patients with septic shock specifically because of concomitant acidemia. However, preclinical models are inconsistent, with studies showing both retained and decreased activity of vasopressin in the setting of acidosis (15–17). Previous studies have reported that higher lactate concentrations were independently associated with lower odds of response to exogenous vasopressin in patients with septic shock, but studies have not evaluated the clinical effectiveness of vasopressin based on arterial pH (18, 19). This study was primarily designed to evaluate the association of arterial pH at vasopressin initiation with hemodynamic response in patients with septic shock. Secondarily, the study sought to evaluate the association of arterial pH at vasopressin initiation with catecholamine dose and blood pressure changes, and patient-centered outcomes.
MATERIALS AND METHODS
This retrospective, multicenter, observational cohort study evaluated patients admitted to a medical, surgical, or mixed medical/surgical ICU in one of eight hospitals in the Cleveland Clinic Health System between January 2012 and November 2017. Electronic health records of adult patients (≥ 18 yr) were electronically screened for the presence of septic shock based on the United States Centers for Disease Control and Prevention Adult Sepsis Event definition (20). Specifically, patients were identified as having septic shock if they met each of the following criteria: blood cultures obtained, received at least 4 days of broad-spectrum antibiotics (or until the time of their death, if sooner) initiated within 2 days of blood cultures, initiation of vasopressors within 2 days of blood cultures, and serum lactate concentration greater than 2 mmol/L at catecholamine initiation. This identification method for patients with septic shock is recommended for electronic health record-based studies (20). Notably, all patients who met this definition also met the definition for septic shock per the Sepsis-3 criteria (2). Identified patients with septic shock initiated on vasopressin as an adjunct to a catecholamine vasopressor were included in the study. Those with documented initiation of a catecholamine vasopressor and vasopressin simultaneously were not included in order to avoid inclusion of patients previously started on vasopressin at another hospital and subsequently transferred to a study hospital. Patients without a resulted pH from an arterial or venous blood gas within 6 hours of vasopressin start and those in whom vasopressin was titrated within the first 6 hours of therapy were excluded. All patients meeting study inclusion criteria without meeting an exclusion criterion during the study time frame were included. Only the first episode of exposure to vasopressin per patient was evaluated. Patients were treated according to the Surviving Sepsis Campaign guidelines available at the time (21, 22). The general approach to treating patients with septic shock, including timely antibiotic administration, adequate fluid resuscitation, and norepinephrine as the preferential initial vasopressor, did not change over the duration of the study.
The primary exposure variable was arterial pH at the time of vasopressin initiation, defined as an arterial pH value obtained within 6 hours before or after vasopressin initiation. In patients with multiple arterial pH values available, the value closest to vasopressin initiation was used. For patients in whom an arterial pH value was not available, arterial pH was calculated from venous pH by adding 0.04 to the venous pH (23). Alternative methods to calculate arterial pH from venous pH and an analysis restricted to only arterial pH samples were assessed in sensitivity analyses (e-Table 1, Supplemental Digital Content, http://links.lww.com/CCX/A913) (24–27).
The primary outcome measure was the frequency of hemodynamic response to vasopressin, defined as a decrease from baseline in norepinephrine-equivalent catecholamine dose with a mean arterial blood pressure greater than or equal to 65 mm Hg at 6 hours after initiation of vasopressin. This definition of hemodynamic response to vasopressin was selected as the primary outcome because it has been independently associated with lower ICU mortality (18). Patients who died within 6 hours of vasopressin initiation were adjudicated as nonresponders. This outcome was first assessed by examining the association of arterial pH as a continuous variable with hemodynamic response using univariate logistic regression. Next, the histogram of arterial pH values was visually inspected, and four groups of arterial pH were developed to approximate quartiles with clinical applicability. These four groups of arterial pH were pH less than or equal to 7.19, pH 7.20–7.29, pH 7.30–7.39, and pH greater than or equal to 7.40. The frequency of hemodynamic response across the four pH groups was assessed with chi-square testing. Secondary outcomes included the change in norepinephrine-equivalent catecholamine dose at 1, 3, and 6 hours after vasopressin initiation; change in the ratio of mean arterial pressure to norepinephrine-equivalent catecholamine dose (MAP/NEQ) at 1, 3, and 6 hours after vasopressin initiation (28); 28-day mortality; days alive and free of the ICU, vasoactive medications, mechanical ventilation, and renal replacement therapy; and Sequential Organ Failure Assessment (SOFA) score change at 48 hours after vasopressin initiation. Norepinephrine-equivalent catecholamine dose change and MAP/NEQ change at 1, 3, and 6 hours after vasopressin initiation and SOFA score change at 48 hours after vasopressin initiation were only calculated for patients surviving for the respective time durations. Norepinephrine-equivalent catecholamine doses in µg/min were calculated as (norepinephrine [µg/min]) + (epinephrine [µg/min]) + (dopamine [µg/kg/min]/2) + (phenylephrine [µg/min]/10) (29). In a sensitivity analysis, norepinephrine-equivalent catecholamine doses in µg/min were calculated as (norepinephrine [µg/min]) + (epinephrine [µg/min]) + (dopamine [µg/kg/min] × weight [kg]/100) + (phenylephrine [µg/min]/10) (30). Exposure time for 28-day mortality was defined as the time from vasopressin initiation until death within 28 days, with censoring of alive patients at 28 days. Length of stay and therapy duration outcomes were calculated in the context of days alive and free from the index time point (31). Specifically, days alive and free were calculated as the number of days (up to day 28) alive after the last of ICU presence, vasopressors, mechanical ventilation, and renal replacement therapy. Broad spectrum antibiotic receipt was defined per the United States Centers for Medicare and Medicaid Services Early Management Bundle, Severe Sepsis/Septic Shock quality measure (32). Acute kidney injury was defined per the Kidney Disease: Improving Global Outcomes Acute Kidney Injury definition (33).
Categorical data are described as n (%) and compared with chi-square or Fisher exact test, as appropriate. Continuous data are described as mean ± sd or median (interquartile range), as appropriate, and compared with the Kruskal-Wallis rank test. Multivariable logistic regression was used to assess the independent association of arterial pH with hemodynamic response to vasopressin. Variables considered for confounder adjustment in the multivariable model were based on previous literature (18, 19, 34). A directed acyclic graph was used for model variable selection. Lactate concentration and SOFA score at vasopressin initiation were included in the model as confounders for the effect of arterial pH on hemodynamic response to vasopressin (e-Fig. 1, Supplemental Digital Content, http://links.lww.com/CCX/A913). Multicollinearity was assessed by variance inflation factors (threshold > 10) and Pearson correlation (threshold r > 0.8). Lactate, SOFA, and arterial pH were deemed to not be colinear (all variance inflation factors < 1.5 and r < 0.5). Additionally, because lactate may be both a confounder and a mediator of vasopressin response, the independent association of lactate with vasopressin response was assessed in the multivariable logistic regression model described above. The associations of arterial pH with change in catecholamine dose at 1, 3, and 6 hours after vasopressin initiation were evaluated with univariate linear regression models. Kaplan-Meier survival curves for arterial pH groups (with arterial pH ≥ 7.40 as the referent group) were constructed for 28-day mortality and evaluated with Cox proportional hazards regression, with a priori-specified adjustment for Acute Physiology and Chronic Health Evaluation III score, SOFA score, and lactate concentration at vasopressin initiation. The proportional hazards assumption was assessed by examining the log-minus-log survival plots, plotting Schoenfeld partial residuals, and introducing time-dependent covariates into the model (35). The proportional hazards assumption was not fulfilled; therefore, a time-varying coefficient for group allocation was retained in the model. A subgroup analysis of medical ICU patients was also performed because of a previously identified association of ICU type with vasopressin response (18). All analyses were performed using Stata (Version 14.2; StataCorp; College Station, TX) based on an overall significance level of 0.05. This study followed the Strengthening the Reporting of Observational Studies in Epidemiology reporting guideline (Supplemental Digital Content http://links.lww.com/CCX/A913) and was approved by the Cleveland Clinic Institutional Review Board (approval number 19-162) with a waiver of informed consent.
RESULTS
Patient Population
Of 4,417 patients with septic shock identified, 2,623 did not meet inclusion criteria and 444 were excluded, resulting in 1,350 patients included in the study (e-Fig. 2, Supplemental Digital Content, http://links.lww.com/CCX/A913). Most included patients were admitted to a medical ICU (n = 880; 65%), had received broad-spectrum antibiotics at the time of shock onset (n = 1278; 95%), and were severely ill at vasopressin initiation with SOFA score 14.1 ± 3.5, lactate 5.6 ± 4.6 mmol/L, and norepinephrine-equivalent catecholamine dose 32.3 ± 25.4 µg/min (Table 1). The majority (n = 1053; 78%) of patients had a pH value from an arterial blood gas sample; the remaining pH values were calculated from venous blood gas pH values. Eighty-five percent of patients exhibited acidemia, with arterial pH 7.28 ± 0.13 at the time of vasopressin initiation. When patients were categorized to clinical pH groups, characteristics differed, with patients in lower pH groups being more severely ill (Table 1). Notably, patients in lower pH groups received higher initial vasopressin doses.
TABLE 1.
Patient Characteristics by Arterial pH Group at Vasopressin Initiation
| Characteristics | Total (N = 1,350) | pH ≤7.19 (N = 325) | pH 7.20–7.29 (N = 359) | pH 7.30–7.39 (N = 421) | pH ≥ 7.40 (N = 245) | p |
|---|---|---|---|---|---|---|
| At shock onset | ||||||
| Age (yr), mean ± sd | 62.5 ± 15.5 | 62.1 ± 16.6 | 62.6 ± 15.3 | 62.3 ± 15.8 | 63.1 ± 13.9 | 0.93 |
| Male sex, n (%) | 697 (51.6) | 166 (50.8) | 187 (52.1) | 216 (51.3) | 129 (52.7) | 0.97 |
| Body weight (kg), mean ± sd | 88.6 ± 30.3 | 85.1 ± 27.7 | 92.9 ± 32.1 | 87.8 ± 31.3 | 88.4 ± 28.6 | < 0.01 |
| White/Caucasian race, n (%) | 955 (70.7) | 222 (68.3) | 264 (73.5) | 293 (69.6) | 176 (72.8) | 0.44 |
| Comorbid diseases, n (%) | ||||||
| Cirrhosis | 236 (17.5) | 55 (16.9) | 55 (15.3) | 75 (17.8) | 51 (20.8) | 0.37 |
| Chronic obstructive pulmonary disease | 292 (21.6) | 63 (19.4) | 92 (25.6) | 96 (22.8) | 41 (16.7) | 0.04 |
| Diabetes | 370 (27.4) | 91 (28.0) | 107 (29.8) | 108 (25.6) | 64 (26.1) | 0.58 |
| End-stage renal disease | 117 (8.7) | 14 (4.3) | 20 (5.6) | 52 (12.4) | 31 (12.7) | < 0.01 |
| Hepatic failure | 201 (14.9) | 45 (13.9) | 45 (12.5) | 63 (15.0) | 48 (19.6) | 0.11 |
| Immune suppression | 235 (17.4) | 51 (15.7) | 59 (16.4) | 81 (19.2) | 44 (17.9) | 0.59 |
| Leukemia, lymphoma, or myeloma | 108 (8.0) | 24 (7.4) | 28 (7.8) | 29 (6.9) | 27 (11.0) | 0.27 |
| No chronic diseases | 326 (24.2) | 76 (23.4) | 87 (24.3) | 108 (25.7) | 55 (22.5) | 0.80 |
| Medical ICU, n (%) | 880 (65.2) | 209 (64.3) | 235 (65.5) | 268 (63.7) | 168 (68.6) | 0.62 |
| Acute Physiologic and Chronic Health Evaluation III score, mean ± sd | 107.5 ± 36.1 | 121.0 ± 40.4 | 106.0 ± 33.9 | 101.7 ± 33.1 | 102.2 ± 33.7 | < 0.01 |
| Mechanical ventilation, n (%) | 645 (47.8) | 176 (54.2) | 176 (49.0) | 183 (43.5) | 110 (44.9) | 0.02 |
| Broad spectrum antibiotic receipt, n (%) | 1,278 (94.7) | 310 (95.4) | 343 (95.5) | 395 (93.8) | 230 (93.9) | 0.62 |
| Lactate concentration (mmol/L), mean ± sd | 5.2 ± 4.1 | 7.6 ± 5.7 | 5.0 ± 3.7 | 4.4 ± 3.0 | 3.9 ± 2.5 | < 0.01 |
| At vasopressin initiation | ||||||
| Arterial pH, mean ± sd | 7.28 ± 0.13 | 7.09 ± 0.09 | 7.25 ± 0.03 | 7.35 ± 0.03 | 7.44 ± 0.04 | < 0.01 |
| Fluid bolus volume given (L), mean ± sd | 2.1 ± 1.9 | 2.4 ± 2.1 | 2.1 ± 1.9 | 1.9 ± 1.7 | 1.9 ± 1.8 | < 0.01 |
| Norepinephrine -equivalent catecholamine dose (µg/min), mean ± sd | 32.3 ± 25.4 | 39.7 ± 34.0 | 32.3 ± 24.1 | 28.7 ± 18.9 | 28.6 ± 21.2 | < 0.01 |
| MAP (mm Hg), mean ± sd | 68.4 ± 14.6 | 67.0 ± 17.5 | 69.2 ± 13.6 | 67.9 ± 13.2 | 69.8 ± 13.7 | 0.09 |
| MAP/norepinephrine-equivalent catecholamine dose (mm Hg/µg/kg/min)a | 213 (132–345) | 167 (102–268) | 221 (143–353) | 230 (138–360) | 250 (163–381) | < 0.01 |
| Initial vasopressin dose (U/min), mean ± sd | 0.03 ± 0.02 | 0.04 ± 0.03 | 0.04 ± 0.01 | 0.03 ± 0.01 | 0.03 ± 0.01 | < 0.01 |
| Lactate concentration (mmol/L), mean ± sd | 5.6 ± 4.6 | 8.9 ± 6.1 | 5.2 ± 3.9 | 4.4 ± 3.1 | 4.1 ± 2.7 | < 0.01 |
| Time elapsed from catecholamine start (hr), mean ± sd | 14.6 ± 36.0 | 9.4 ± 27.1 | 10.3 ± 17.8 | 18.7 ± 48.0 | 20.4 ± 40.1 | < 0.01 |
| Sequential Organ Failure Assessment score, mean ± sd | 14.1 ± 3.5 | 14.7 ± 3.1 | 14.5 ± 3.3 | 13.9 ± 3.7 | 13.3 ± 3.6 | < 0.01 |
| Acute kidney injury, n (%) | 823 (61.0) | 216 (66.5) | 237 (66.0) | 241 (57.2) | 129 (52.7) | < 0.01 |
| Receiving continuous renal replacement therapy, n (%) | 112 (8.3) | 18 (5.5) | 30 (8.4) | 40 (9.5) | 24 (9.8) | 0.19 |
| Exposure during shock course, n (%) | ||||||
| Hydrocortisone | 748 (55.4) | 148 (45.5) | 218 (60.7) | 251 (59.6) | 131 (53.5) | < 0.01 |
| Epinephrine | 313 (23.2) | 103 (31.7) | 80 (22.3) | 88 (20.9) | 42 (17.1) | < 0.01 |
| Phenylephrine | 502 (37.2) | 136 (41.9) | 137 (38.2) | 151 (35.9) | 78 (31.8) | 0.09 |
| Dopamine | 54 (4.0) | 16 (4.9) | 14 (3.9) | 17 (4.0) | 7 (2.9) | 0.67 |
| Dobutamine | 107 (7.9) | 25 (7.7) | 27 (7.5) | 37 (8.8) | 18 (7.4) | 0.89 |
| Milrinone | 15 (1.1) | 1 (0.3) | 4 (1.1) | 7 (1.7) | 3 (1.2) | 0.35 |
| Continuous renal replacement therapy | 327 (24.2) | 72 (22.2) | 99 (27.6) | 108 (25.7) | 48 (19.6) | 0.10 |
MAP = mean arterial blood pressure.
aPresented as median (interquartile range).
Patient Outcomes
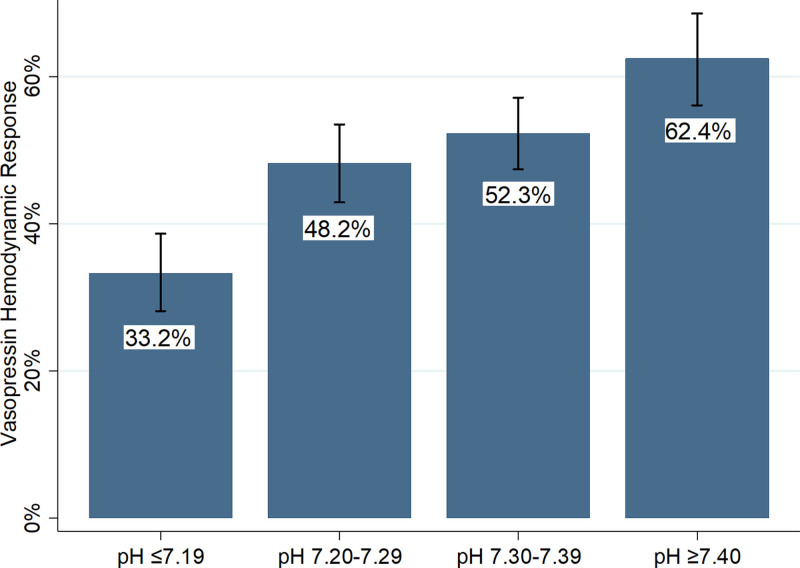
In the primary outcome evaluation, as arterial pH decreased the odds of hemodynamic response to vasopressin decreased (for each 0.1 unit, arterial pH was below 7.40; response odds ratio [OR], 0.72; 95% CI, 0.66–0.79; p < 0.01). After adjusting for lactate and SOFA with multivariable logistic regression, lower arterial pH was independently associated with lower odds of hemodynamic response to vasopressin (for each 0.1 unit, arterial pH was below 7.40; response OR, 0.79; 95% CI, 0.72–0.87; p < 0.01). Additionally, on multivariable logistic regression, a higher lactate concentration at vasopressin initiation was independently associated with lower odds of vasopressin response (for each 1 mmol/L lactate was above 2 mmol/L; response OR, 0.94; 95% CI, 0.91–0.97). These findings were comparable in the sensitivity analysis using different methods to calculate arterial pH from venous pH values (e-Table 1, Supplemental Digital Content, http://links.lww.com/CCX/A913). When patients were categorized to clinical pH groups, the results were similar. There was a between-group difference in the proportion of patients with a hemodynamic response to vasopressin, and on multivariable logistic regression, pH groups below 7.40 were independently associated with lower odds of hemodynamic response to vasopressin (Table 2 and Fig. 1).
TABLE 2.
Patient Outcomes by Clinical Arterial pH Group at Vasopressin Initiation
| Characteristics | Total (N = 1,350) | pH ≤ 7.19 (N = 325) | pH 7.20–7.29 (N = 359) | pH 7.30–7.39 (N = 421) | pH ≥ 7.40 (N = 245) | p |
|---|---|---|---|---|---|---|
| Primary outcome | ||||||
| Hemodynamic response, n (%) | 654 (48.4) | 108 (33.2) | 173 (48.2) | 220 (52.3) | 153 (62.5) | < 0.01 |
| Odds ratio (95% CI)a | 0.42 (0.29–0.61) | 0.64 (0.45–0.90) | 0.71 (0.51–0.99) | 1 (referent) | ||
| Secondary outcomes | ||||||
| Norepinephrine-equivalent catecholamine dose change at 1 hr after vasopressin start (µg/min), mean ± sd | –0.6 ± 24.3 | +2.8 ± 32.3 | –1.1 ± 30.7 | –1.6 ± 13.3 | –2.9 ± 13.1 | < 0.01 |
| Norepinephrine-equivalent catecholamine dose change at 3 hr after vasopressin start (µg/min), mean ± sd | +0.4 ± 23.1 | +5.7 ± 30.4 | +0.5 ± 24.1 | –1.5 ± 17.0 | –3.5 ± 18.2 | < 0.01 |
| Norepinephrine-equivalent catecholamine dose change at 6 hr after vasopressin start (µg/min), mean ± sd | –0.2 ± 25.6 | +5.0 ± 32.0 | –0.5 ± 24.3 | –2.0 ± 21.5 | –3.6 ± 23.7 | < 0.01 |
| MAP/NEQ change at 1 hr after vasopressin start (mm Hg/µg/kg/min)b | 29 (–16 to 103) | 9 (–28 to 60) | 21 (–27 to 91) | 40 (–1 to 121) | 56 (–5 to 166) | < 0.01 |
| MAP/NEQ change at 3 hr after vasopressin start (mm Hg/µg/kg/min)b | 29 (–35 to 128) | 3 (–47 to 83) | 26 (–44 to 125) | 37 (–18 to 134) | 56 (–30 to 264) | < 0.01 |
| MAP/NEQ change at 6 hr after vasopressin start (mm Hg/µg/kg/min)b | 38 (–44 to 184) | 12 (–63 to 132) | 37 (–50 to 178) | 43 (–28 to 187) | 86 (–18 to 355) | < 0.01 |
| 28-d mortality, n (%) | 802 (59.4) | 237 (72.9) | 210 (58.5) | 222 (52.7) | 133 (54.3) | < 0.01c |
| Days alive and free from ICUb | 0 (0–18.1) | 0 (0–7.3) | 0 (0–17.8) | 0 (0–19.6) | 0 (0–24.4) | < 0.01 |
| Days alive and free from vasoactive medicationsb | 0 (0–24.0) | 0 (0–5.0) | 0 (0–24.9) | 0 (0–25.0) | 2.0 (0–25.0) | < 0.01 |
| Days alive and free from mechanical ventilationb | 0.6 (0–22.4) | 0 (0–6.9) | 0.3 (0–18.7) | 2.8 (0–24.4) | 3.8 (0–25.8) | < 0.01 |
| Days alive and free from renal replacement therapyb | 1.7 (0–28.0) | 0.5 (0–7.6) | 1.8 (0–28.0) | 3.8 (0–28.0) | 3.8 (0.2–28.0) | < 0.01 |
| Sequential Organ Failure Assessment score change at 48 hr after vasopressin, mean ± sd | –1.8 ± 3.3 | –1.0 ± 3.1 | –1.7 ± 3.4 | –2.0 ± 3.0 | –2.3 ± 3.3 | < 0.01 |
HR = hazard ratio. MAP/NEQ = mean arterial pressure/norepinephrine-equivalent catecholamine dose.
aOdds ratio and 95% CI from multivariable logistic regression analysis adjusted for lactate concentration and Sequential Organ Failure Assessment score at vasopressin initiation
bPresented as median (interquartile range)
cFor the comparison of the pH ≤ 7.19 group to the pH ≥ 7.40 group, HR(t) = e(0.63–0.12×t); at t = 0, HR, 1.88; 95% CI, 1.40–2.52.
Figure 1.
Vasopressin hemodynamic response by arterial pH group at vasopressin initiation. Proportion of patients with a hemodynamic response at 6 hr after vasopressin initiation by arterial pH group. Error bars represent 95% CIs. Overall p < 0.01 by χ2.
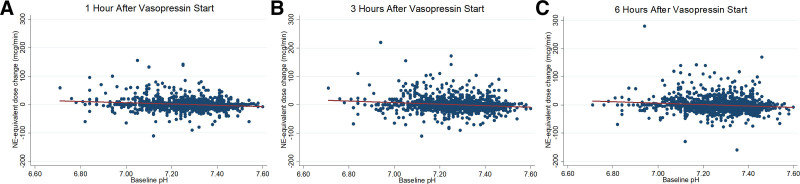
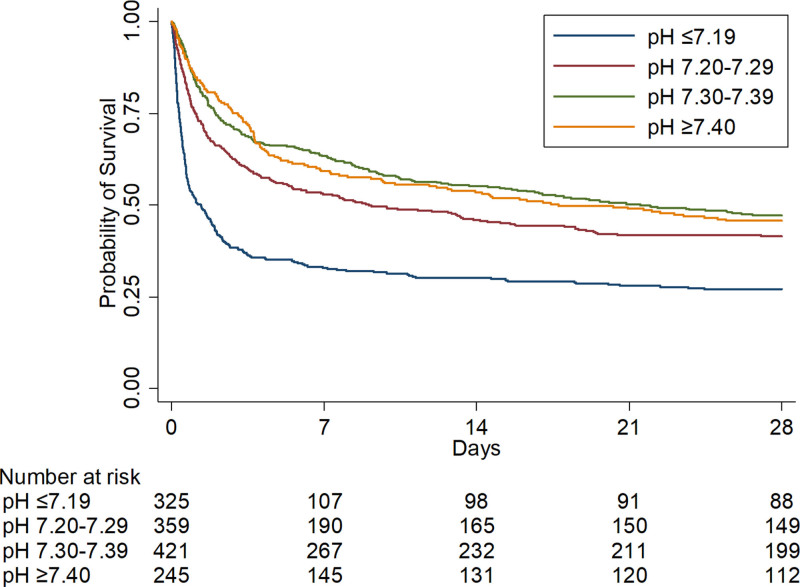
Lower arterial pH at vasopressin initiation was also associated with worse secondary outcomes. With decreasing arterial pH at vasopressin initiation, the norepinephrine-equivalent catecholamine dose increased at 1 hour (for each 0.1 unit, the arterial pH was below 7.40, catecholamine dose increased by 1.5 µg/min; 95% CI, 0.5–2.5 µg/min), 3 hours (for each 0.1 unit, the arterial pH was below 7.40, catecholamine dose increased by 2.7 µg/min; 95% CI, 1.8–3.6 µg/min), and 6 hours after vasopressin initiation (for each 0.1 unit, the pH was below 7.40, catecholamine dose increased by 2.5 µg/min; 95% CI, 1.4–3.5 µg/min) (Fig 2). When patients were categorized to clinical pH groups, catecholamine doses increased in lower pH groups and decreased in higher pH groups after vasopressin initiation (Table 2). Furthermore, change in MAP/NEQ after vasopressin initiation differed by clinical pH groups, with greater increases in higher pH groups (Table 2) (e-Fig. 3, Supplemental Digital Content, http://links.lww.com/CCX/A913). Patients in clinical groups with lower pH at vasopressin initiation had higher 28-day mortality and fewer days alive and free from the ICU, vasoactive medications, and renal replacement therapy (Table 2 and Fig. 3). Last, patients with lower pH at vasopressin initiation had less pronounced improvement in SOFA score 48 hours after vasopressin initiation. All findings were similar in the sensitivity analysis using an alternative norepinephrine-equivalent catecholamine dose equivalence (e-Table 2 and e-Fig. 4, Supplemental Digital Content, http://links.lww.com/CCX/A913) and the subgroup analysis of medical ICU patients (e-Table 3 and e-Fig. 5, Supplemental Digital Content, http://links.lww.com/CCX/A913).
Figure 2.
Change in norepinephrine-equivalent catecholamine dose after vasopressin start by pH. Change in norepinephrine-equivalent catecholamine dose at 1 hr (A; n = 1350), at 3 hr (B; n = 1345), and at 6 hr (C; n = 1344) after vasopressin start by arterial pH at vasopressin initiation. Lines represent the predicted change in norepinephrine-equivalent catecholamine dose from a linear regression of norepinephrine-equivalent catecholamine dose on arterial pH at vasopressin initiation. For each 0.1 unit, the pH was below 7.40 at vasopressin initiation, the norepinephrine-equivalent catecholamine dose increased by 1.5 µg/min (95% CI, 0.5–2.5 µg/min) at 1 hr, increased by 2.7 µg/min (95% CI, 1.8–3.6 µg/min) at 3 hr, and increased by 2.5 µg/min (95% CI, 1.4–3.5 µg/min) at 6 hr after vasopressin initiation. NE = norepinephrine.
Figure 3.
Kaplan-Meier curves for 28-d survival by pH group. Kaplan-Meier survival curves by clinical arterial pH group at vasopressin initiation. When compared with patients in the pH greater than or equal to 7.40 group, patients in the pH less than or equal to 7.19 group had an independently higher risk for 28-d mortality with a time-varying effect (HR(t) = e[0.63–0.12×t]; at t = 0, HR, 1.88; 95% CI, 1.40–2.52). HR = hazard ratio.
DISCUSSION
In this large, multicenter study of patients with septic shock receiving concomitant catecholamines and vasopressin, lower arterial pH at vasopressin initiation was associated with lower odds of hemodynamic response to vasopressin. Furthermore, lower arterial pH at vasopressin initiation was associated with a subsequent increase in catecholamine dose, less pronounced improvement in MAP/NEQ, and patient-centered outcomes were worse. If the vasoconstrictive activity of vasopressin is not affected by acidemia, blood pressure would be expected to consistently increase across the spectrum of pH values after vasopressin initiation with corresponding catecholamine dose decreases. However, we observed catecholamine doses increased, and MAP/NEQ increase was attenuated as pH was lower, suggesting the vasoconstrictive activity of vasopressin is impaired in the setting of acidemia. These findings were observed despite higher initial vasopressin doses in patients with lower arterial pH; higher vasopressin doses typically produce more pronounced blood pressure effects (36). To our knowledge, this is the first study to evaluate the clinical effectiveness of vasopressin in patients with septic shock based on arterial pH. Vasopressin is used to increase blood pressure with the intent to improve organ dysfunction and subsequent patient-centered outcomes (37). Similar to catecholamines, as acidemia worsens it appears vasopressin is less likely to achieve these goals.
The mechanism for attenuated blood pressure response to vasopressin in patients with lower pH is likely multifactorial. First, vasopressin binding to its receptor is reduced in the setting of acidosis. Both intracellular and extracellular acidosis decrease vasopressin-induced vascular smooth muscle cell contraction by decreasing the affinity of vasopressin for the V1a receptor (17). This mechanism for lessened vasopressin effect in the setting of acidosis differs from catecholamines, where β1 receptor cell surface expression is diminished as pH decreases (38). Furthermore, acidosis causes hyperpolarization of vascular smooth muscle cells by activation of adenosine triphosphate-sensitive potassium channels channels, intracellular calcium sequestration, and increased nitric oxide–mediated vasodilatory effects (8, 39). Together, these mechanisms decrease vascular responsiveness to endogenous and exogenously administered vasoactive agents in the setting of acidemia. Additionally, because cardiac output is reduced in the setting of acidemia, vasopressin may be a suboptimal vasopressor since it is a vasoconstrictor without inotropic properties (40). Evidence suggests that in patients with hyperdynamic septic shock, low-dose vasopressin infusion is not associated with a negative effect on inotropy (41). However, in patients with low baseline cardiac output, vasopressin can depress cardiac output further (42, 43). In summary, diminished blood pressure effects of vasopressin in patients with acidemia may be due to impaired vasoconstriction and/or negative inotropic effects.
We report higher lactate concentration to be associated with lower odds of vasopressin response, independent of arterial pH. This current finding is consistent with our prior observations and suggests lactate is part of a direct or indirect causal pathway for vasopressin response that is distinct from its effects on arterial pH (18, 19). Lactate is generated in the setting of tissue hypoxia; therefore, the association of higher lactate concentration with lower odds of vasopressin response could be reflective of inadequate oxygen delivery or uncoupling of the microcirculation and macrocirculation (44). Additionally, lactate is generated during the hyperinflammatory phase of sepsis due to shifted cellular metabolism from oxidative phosphorylation to aerobic glycolysis (45, 46). As such, hyperlactatemia may be a surrogate marker for exaggerated hyperinflammatory response and endothelial dysfunction leading to inadequate hemodynamic response to vasopressin. Further, lactate itself has immunosuppressive effects, causing additional immunomodulation in septic shock (47). Future studies should elucidate the mechanism for the association between hyperlactatemia and failure to respond to adjunctive vasopressin.
Our study is strengthened by its large size, multicenter nature, assessment of a wide range of clinically relevant arterial pH values, and evaluation of vasopressin response with multiple methods. However, there are several limitations. First, we designed the study specifically to evaluate the association of arterial pH with vasopressin response, and therefore, we only included vasopressin recipients. As such, we were unable to assess the relative effectiveness of various vasoactive agents in patients with acidemia (such as catecholamine vs vasopressin effectiveness). Additionally, our definition of vasopressin response was based on achievement of a mean arterial blood pressure greater than or equal to 65 mm Hg with a concomitant decrease in catecholamine dose. In patients with septic shock in our health system, catecholamines are almost universally titrated to a goal mean arterial blood pressure of 65–70 mm Hg, but we did not specifically evaluate this goal on a patient level. Therefore, the frequency of patients meeting bedside blood pressure goals after vasopressin initiation may be misspecified in our definition of hemodynamic response. Notably, though, it is unlikely there is a systematic difference in patients’ blood pressure target based on arterial pH. Therefore, the primary analysis of the association of different arterial pH values with vasopressin response is unlikely be affected by this limitation. Additionally, we evaluated the blood pressure effects of vasopressin as change in catecholamine dose and MAP/NEQ at 1, 3, and 6 hours after vasopressin initiation, which are also unlikely to be impacted by an individualized blood pressure goal.
CONCLUSIONS
Compared with higher arterial pH, patients with septic shock and low arterial pH had lower odds of vasopressin response and worse patient-centered outcomes. Similar to other vasopressors, the clinical effectiveness of vasopressin appears to be impaired in the setting of severe acidemia.
ACKNOWLEDGMENTS
We would like to thank Eric Vogan, MSPH, and Vandana Mathur, MPH, for their assistance with data extraction from the electronic medical record and quality data registries, respectively.
Supplementary Material
Footnotes
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s website (http://journals.lww.com/ccejournal).
Dr. Bauer had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. Drs. Bauer, Sacha, and Siuba contributed substantially to the study design, data analysis and interpretation, and the writing of the article. Drs. Lam, Reddy, Duggal, and Vachharajani contributed substantially to data analysis and interpretation, and the writing of the article. All authors read and approved the final article.
Dr. Bauer reports receiving consultancy fees from Wolters Kluwer and is supported by a grant from the Cleveland Clinic Department of Pharmacy. Dr. Vachharajani is supported by a grant from the National Institutes of Health (grant number R01AA028763). The remaining authors have disclosed that they do not have any conflicts of interest.
Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health.
REFERENCES
- 1.Liu V, Escobar GJ, Greene JD, et al. : Hospital deaths in patients with sepsis from 2 independent cohorts. JAMA 2014; 312:90–92 [DOI] [PubMed] [Google Scholar]
- 2.Singer M, Deutschman CS, Seymour CW, et al. : The third international consensus definitions for sepsis and septic shock (sepsis-3). JAMA 2016; 315:801–810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vincent JL, Jones G, David S, et al. : Frequency and mortality of septic shock in Europe and North America: A systematic review and meta-analysis. Crit Care 2019; 23:196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.De Backer D, Biston P, Devriendt J, et al. ; SOAP II Investigators: Comparison of dopamine and norepinephrine in the treatment of shock. N Engl J Med 2010; 362:779–789 [DOI] [PubMed] [Google Scholar]
- 5.Asfar P, Meziani F, Hamel JF, et al. ; SEPSISPAM Investigators: High versus low blood-pressure target in patients with septic shock. N Engl J Med 2014; 370:1583–1593 [DOI] [PubMed] [Google Scholar]
- 6.Kraut JA, Madias NE: Lactic acidosis. N Engl J Med 2014; 371:2309–2319 [DOI] [PubMed] [Google Scholar]
- 7.Handy JM, Soni N: Physiological effects of hyperchloraemia and acidosis. Br J Anaesth 2008; 101:141–150 [DOI] [PubMed] [Google Scholar]
- 8.Celotto AC, Capellini VK, Baldo CF, et al. : Effects of acid-base imbalance on vascular reactivity. Braz J Med Biol Res 2008; 41:439–445 [DOI] [PubMed] [Google Scholar]
- 9.Campbell GS, Houle DB, Crisp NW, Jr, et al. : Depressed response to intravenous sympathicomimetic agents in humans during acidosis. Dis Chest 1958; 33:18–22 [DOI] [PubMed] [Google Scholar]
- 10.Weil MH, Houle DB, Brown EB, Jr, et al. : Vasopressor agents; influence of acidosis on cardiac and vascular responsiveness. Calif Med 1958; 88:437–440 [PMC free article] [PubMed] [Google Scholar]
- 11.Wang BC, Sundet WD, Goetz KL: Vasopressin in plasma and cerebrospinal fluid of dogs during hypoxia or acidosis. Am J Physiol 1984; 247:E449–E455 [DOI] [PubMed] [Google Scholar]
- 12.Holmes CL, Patel BM, Russell JA, et al. : Physiology of vasopressin relevant to management of septic shock. Chest 2001; 120:989–1002 [DOI] [PubMed] [Google Scholar]
- 13.Mutlu GM, Factor P: Role of vasopressin in the management of septic shock. Intensive Care Med 2004; 30:1276–1291 [DOI] [PubMed] [Google Scholar]
- 14.Dunser MW, Wenzel V, Mayr AJ, et al. : Management of vasodilatory shock: Defining the role of arginine vasopressin. Drugs 2003; 63:237–256 [DOI] [PubMed] [Google Scholar]
- 15.Fox AW, May RE, Mitch WE: Comparison of peptide and nonpeptide receptor-mediated responses in rat tail artery. J Cardiovasc Pharmacol 1992; 20:282–289 [DOI] [PubMed] [Google Scholar]
- 16.Kawano T, Tanaka K, Nazari H, et al. : The effects of extracellular pH on vasopressin inhibition of ATP-sensitive K+ channels in vascular smooth muscle cells. Anesth Analg 2007; 105:1714–1719, table of contents [DOI] [PubMed] [Google Scholar]
- 17.Okada K, Tsai P, Briner VA, et al. : Effects of extra- and intracellular pH on vascular action of arginine vasopressin. Am J Physiol 1991; 260:F39–F45 [DOI] [PubMed] [Google Scholar]
- 18.Sacha GL, Lam SW, Duggal A, et al. : Predictors of response to fixed-dose vasopressin in adult patients with septic shock. Ann Intensive Care 2018; 8:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sacha GL, Lam SW, Wang L, et al. : Association of catecholamine dose, lactate, and shock duration at vasopressin initiation with mortality in patients with septic shock. Crit Care Med 2021. Sep 24. [online ahead of print] [DOI] [PubMed] [Google Scholar]
- 20.United States Centers for Disease Control and Prevention: Hospital Toolkit for Adult Sepsis Surveillance. Available at: https://www.cdc.gov/sepsis/pdfs/Sepsis-Surveillance-Toolkit-Aug-2018_508.pdf. Accessed February 26, 2021
- 21.Dellinger RP, Levy MM, Rhodes A, et al. ; Surviving Sepsis Campaign Guidelines Committee including the Pediatric Subgroup: Surviving Sepsis Campaign: International guidelines for management of severe sepsis and septic shock: 2012. Crit Care Med 2013; 41:580–637 [DOI] [PubMed] [Google Scholar]
- 22.Rhodes A, Evans LE, Alhazzani W, et al. : Surviving Sepsis Campaign: International guidelines for management of sepsis and septic shock: 2016. Crit Care Med 2017; 45:486–552 [DOI] [PubMed] [Google Scholar]
- 23.Kelly AM, McAlpine R, Kyle E: Venous pH can safely replace arterial pH in the initial evaluation of patients in the emergency department. Emerg Med J 2001; 18:340–342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kelly AM, Kyle E, McAlpine R: Venous pCO(2) and pH can be used to screen for significant hypercarbia in emergency patients with acute respiratory disease. J Emerg Med 2002; 22:15–19 [DOI] [PubMed] [Google Scholar]
- 25.Treger R, Pirouz S, Kamangar N, et al. : Agreement between central venous and arterial blood gas measurements in the intensive care unit. Clin J Am Soc Nephrol 2010; 5:390–394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Adrogué HJ, Rashad MN, Gorin AB, et al. : Assessing acid-base status in circulatory failure. Differences between arterial and central venous blood. N Engl J Med 1989; 320:1312–1316 [DOI] [PubMed] [Google Scholar]
- 27.Middleton P, Kelly AM, Brown J, et al. : Agreement between arterial and central venous values for pH, bicarbonate, base excess, and lactate. Emerg Med J 2006; 23:622–624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bosch NA, Teja B, Wunsch H, et al. : Characterization and validation of a novel measure of septic shock severity. Intensive Care Med 2020; 46:135–137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Russell JA, Walley KR, Singer J, et al. ; VASST Investigators: Vasopressin versus norepinephrine infusion in patients with septic shock. N Engl J Med 2008; 358:877–887 [DOI] [PubMed] [Google Scholar]
- 30.Goradia S, Sardaneh AA, Narayan SW, et al. : Vasopressor dose equivalence: A scoping review and suggested formula. J Crit Care 2021; 61:233–240 [DOI] [PubMed] [Google Scholar]
- 31.Russell JA, Lee T, Singer J, et al. : Days alive and free as an alternative to a mortality outcome in pivotal vasopressor and septic shock trials. J Crit Care 2018; 47:333–337 [DOI] [PubMed] [Google Scholar]
- 32.United States Centers for Medicare & Medicaid Services: Specifications Manual for National Hospital Inpatient Quality Measures. Available at: https://qualitynet.cms.gov/inpatient/specifications-manuals. Accessed November 30, 2020
- 33.Kidney Disease: Improving Global Outcomes (KDIGO) Acute Kidney Injury Work Group: KDIGO Clinical practice guideline for acute kidney injury: Section 2: AKI definition. Kidney Int Suppl 2012; 2:19–36 [Google Scholar]
- 34.Lederer DJ, Bell SC, Branson RD, et al. : Control of confounding and reporting of results in causal inference studies. Guidance for authors from editors of respiratory, sleep, and critical care journals. Ann Am Thorac Soc 2019; 16:22–28 [DOI] [PubMed] [Google Scholar]
- 35.Hess KR: Graphical methods for assessing violations of the proportional hazards assumption in Cox regression. Stat Med 1995; 14:1707–1723 [DOI] [PubMed] [Google Scholar]
- 36.Torgersen C, Dünser MW, Wenzel V, et al. : Comparing two different arginine vasopressin doses in advanced vasodilatory shock: A randomized, controlled, open-label trial. Intensive Care Med 2010; 36:57–65 [DOI] [PubMed] [Google Scholar]
- 37.Evans L, Rhodes A, Alhazzani W, et al. : Surviving Sepsis Campaign: International guidelines for management of sepsis and septic shock 2021. Crit Care Med 2021; 49:e1063–e1143 [DOI] [PubMed] [Google Scholar]
- 38.Marsh JD, Margolis TI, Kim D: Mechanism of diminished contractile response to catecholamines during acidosis. Am J Physiol 1988; 254:H20–H27 [DOI] [PubMed] [Google Scholar]
- 39.Landry DW, Oliver JA: The pathogenesis of vasodilatory shock. N Engl J Med 2001; 345:588–595 [DOI] [PubMed] [Google Scholar]
- 40.Bauer SR, Lam SW: Arginine vasopressin for the treatment of septic shock in adults. Pharmacotherapy 2010; 30:1057–1071 [DOI] [PubMed] [Google Scholar]
- 41.Gordon AC, Wang N, Walley KR, et al. : The cardiopulmonary effects of vasopressin compared with norepinephrine in septic shock. Chest 2012; 142:593–605 [DOI] [PubMed] [Google Scholar]
- 42.Arnolda L, McGrath BP, Cocks M, et al. : Vasoconstrictor role for vasopressin in experimental heart failure in the rabbit. J Clin Invest 1986; 78:674–679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Müller S, How OJ, Hermansen SE, et al. : Vasopressin impairs brain, heart and kidney perfusion: An experimental study in pigs after transient myocardial ischemia. Crit Care 2008; 12:R20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dünser MW, Dubin A: There is more to septic shock than arterial hypotension and elevated lactate levels: Another appeal to rethink current resuscitation strategies! Ann Intensive Care 2018; 8:55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cheng SC, Scicluna BP, Arts RJ, et al. : Broad defects in the energy metabolism of leukocytes underlie immunoparalysis in sepsis. Nat Immunol 2016; 17:406–413 [DOI] [PubMed] [Google Scholar]
- 46.Taylor DJ, Faragher EB, Evanson JM: Inflammatory cytokines stimulate glucose uptake and glycolysis but reduce glucose oxidation in human dermal fibroblasts in vitro. Circ Shock 1992; 37:105–110 [PubMed] [Google Scholar]
- 47.Nolt B, Tu F, Wang X, et al. : Lactate and immunosuppression in sepsis. Shock 2018; 49:120–125 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.