Abstract
Background
Constipation is highly prevalent in patients with chronic kidney disease (CKD), particularly among those with end-stage renal disease (ESRD), partly due to their dietary restrictions, comorbidities and medications. Laxatives are typically used for constipation management; however, little is known about laxative use and its associated factors in patients with advanced CKD transitioning to ESRD.
Methods
In a retrospective cohort of 102 477 US veterans transitioning to dialysis between October 2007 and March 2015, we examined the proportion of patients who filled a prescription for any type of laxative within each 6-month period over 36 months pre- and post-transition to ESRD. Factors associated with laxative use during the last 1-year pre-ESRD period were identified by multivariable logistic regression.
Results
The proportion of patients prescribed laxatives increased as patients progressed to ESRD, peaking at 37.1% in the 6 months immediately following ESRD transition, then remaining fairly stable throughout the post-ESRD transition period. Among laxative users, stool softeners were the most commonly prescribed (∼30%), followed by hyperosmotics (∼20%), stimulants (∼10%), bulk formers (∼3%), chloride channel activator (<1%) and several combinations of these. The use of anticoagulants, oral iron supplements, non-opioid analgesics, antihistamines and opioid analgesics were among the factors independently associated with pre-ESRD laxative use.
Conclusion
The use of laxatives increased considerably as patients neared transition to ESRD, likely mirroring the increasing burden of drug-induced constipation during the ESRD transition period. Findings may provide novel insight into better management strategies to alleviate constipation symptoms and reduce medication requirements in patients with advanced CKD.
Keywords: chronic kidney disease, constipation, end-stage renal disease, laxative, transition
KEY LEARNING POINTS
What is already known about this subject?
constipation is highly prevalent in patients with chronic kidney disease (CKD), particularly among those with end-stage renal disease (ESRD) receiving dialysis;
pharmacological interventions are often required for the management of constipation in advanced CKD patients, and a wide range of laxative agents are currently available; and
however, information is scarce on the prevalence and patterns of laxative use and its associated factors in patients with advanced CKD transitioning to ESRD.
What this study adds?
the use of laxatives increased considerably (up to ∼37%) as patients progressed to ESRD, and remained fairly stable after the transition to ESRD;
the use of anticoagulants, oral iron supplements, non-opioid analgesics, antihistamines and opioid analgesics were among the factors independently associated with laxative use during the last 1-year period before transition to ESRD; and
these results are likely mirroring the increasing burden of drug-induced constipation during the ESRD transition period, which may provide novel insight into better management strategies to alleviate constipation symptoms and reduce medication requirements in patients with advanced CKD.
What impact this may have on practice or policy?
the high prevalence of laxative use in advanced CKD may help raise the awareness of prevalent constipation in this population, along with its contribution to health and economic burden;
the identification of the medications associated with pre-ESRD laxative use may help detect previously under-recognized causes of drug-induced constipation and can help avoid unnecessary or inappropriate use of laxatives along with their unwanted adverse effects; and
the potential changes in practice habits to avoid unnecessary laxative use could contribute to a lower overall pill and economic burden in this relevant population.
INTRODUCTION
Constipation is the prototype of functional gastrointestinal disorders and one of the most prevalent conditions encountered in daily clinical practice [1]. Approximately 30% of the general population experiences problems with constipation during their lifetime, with women and elderly people being most affected [2]. In patients with chronic kidney disease (CKD), especially in its advanced stages, the prevalence of constipation is reported to be higher than in the general population [3–5], presumably due in part to their dietary restrictions (e.g. limited fiber and/or fluid intake), comorbidities, concomitant medications and altered gut microbiota [6–11]. Because of these predisposing factors, nonpharmacological treatments such as increased fiber supplements and physical activity may not always be practical and effective, and pharmacological interventions are often required for the management of constipation in this particular population [12].
Currently, a wide range of pharmacological agents are available, including commonly used laxative compounds (e.g. bulk formers, hyperosmotics, stimulants, stool softeners and lubricants) and relatively new laxatives with more physiological mechanisms of action (e.g. chloride channel activators, guanylate cyclase C-receptor agonists, selective serotonin 5-HT4 receptor agonists and ileal bile acid transporter inhibitors) [13, 14], some of which have been shown to have unique renoprotective properties [15–17]. Despite these therapeutic advances, no practice guidelines currently exist for constipation management in CKD; thus physicians may supposedly base their treatments largely on their clinical experience or habitual practice, which can sometimes be wasteful and harmful to patients [18]. Furthermore, the costs related to laxative administration (e.g. drug cost, pharmacy management and downstream investigations for laxative-induced adverse effects) are estimated to be strikingly high, contributing substantially to healthcare financial burden [19–21].
Given these problems with laxative use and the exceptionally high health and economic burden in patients with advanced CKD transitioning to end-stage renal disease (ESRD) [22], it is vital to better understand the real-world practice patterns of laxative use during this critical transition period toward efforts to improve patient-centered care and outcomes. However, information is scarce on the prevalence and patterns of laxative use in patients with advanced CKD. We therefore aimed to describe the prevalence and patterns of laxative use during the 36-month pre- and post-ESRD transition periods, and further examined the clinical factors independently associated with pre-ESRD laxative use.
MATERIALS AND METHODS
Study population
We analyzed longitudinal data from the US Renal Data System (USRDS) Transition of Care in CKD study, a nationally representative retrospective cohort study of US veterans who transitioned to ESRD [23–25]. In this study, a total of 102 477 US veterans who transitioned to ESRD from 1 October 2007 through 31 March 2015 were identified from the USRDS as a source population. In order to describe the trend in laxative use during the transition period (as detailed in the next subsection), we first identified 20 127 patients who had at least one prescription record for any medication within each 6-month time period over 36-months pre- and post-transition to ESRD. Prescribed medications were ascertained using both inpatient and outpatient prescriptions sourced from Centers for Medicare and Medicaid Services (CMS) Medicare Part D and Veterans Affairs (VA) pharmacy dispensation records [26].
For identifying factors associated with pre-ESRD laxative use, we separately identified 70 128 patients with at least one prescription for any medication during the last 1-year prior to dialysis initiation. In order to stringently define the laxative users and non-laxative users for this aim, we selected 11 667 out of 70 128 patients who had at least two laxative prescriptions 30 days apart during the 1-year pre-ESRD period (i.e. laxative users). Among the remaining 58 461 patients, we additionally identified 34 965 patients who did not have any laxative prescription during the entire pre-ESRD period and defined these as comparators (i.e. non-laxative users), resulting in the final analytical population of 46 632 patients (Supplementary data, Figure S1). Compared with patients in the final analytical population, those who were excluded from the analysis (i.e. n = 23 496) were younger, less likely to be married and more likely to be African American and use medications (Supplementary data, Table S1).
Laxative use prevalence and patterns
Laxative use during the transition period was described as the proportion of patients who used any type of laxative, which was defined as the ratio of the number of patients who filled at least one prescription of any laxative (i.e. numerator) to the 20 127 patients identified to have at least one prescription medication (i.e. denominator) within each 6-month period over the 36-month pre- and post-ESRD transition periods. Laxative agents were ascertained according to prescription information for the following six types of laxatives: stool softeners, hyperosmotics, stimulants, bulk formers, chloride channel activator and lubricants (Supplementary data, Table S2). Among patients with at least one laxative prescription, the proportion of each or combination of these types of laxatives was assessed for each 6-month period over the 72-month evaluation period. All proportions were reported as percentages. The relationship between the number of different types of laxatives and the number of prescribed drugs was additionally examined among patients with at least one prescription medication in the last 6-month pre-ESRD period.
Covariates
Patient demographic characteristics, including age, sex, and self-identified race and ethnicity, were ascertained from the following three national databases: the USRDS, VA and CMS. Data on marital status, smoking status and service connectivity (a measure indicating whether one or more of a patient’s comorbidities were caused by their military service, resulting in certain privileges, such as preferential access to care and lower copayments) were obtained from VA records only [27, 28]. Preexisting comorbidities were identified from the VA Inpatient and Outpatient Medical SAS Datasets, using the International Classification of Diseases, Ninth Revision, Clinical Modification diagnostic and procedure codes and Current Procedural Terminology codes, as well as from VA/CMS data [29]. The Charlson comorbidity index (CCI) score was calculated using the Deyo modification for administrative datasets, without including kidney disease [30]. Cardiovascular disease was defined as the presence of diagnostic codes for coronary artery disease, angina, myocardial infarction or cerebrovascular disease [31]. Bowel disorders were defined as the presence of diagnostic codes for inflammatory bowel disease, irritable bowel syndrome or diarrhea. Laboratory data were obtained from the VA research databases as previously described [32, 33], and their baseline values were defined as the average of each laboratory test during the 2-year baseline period (vide infra). Similarly, patients with at least one prescription over the 2-year baseline period were recorded as having been treated with the medication. Estimated glomerular filtration rate (eGFR) was calculated with the CKD Epidemiology Collaboration creatinine equation using outpatient serum creatinine and demographic data [34]. Intraindividual slope of eGFR was calculated using a linear mixed-effects model using all outpatient eGFR values available in the 2-year baseline period and, given the potential non-linear association of eGFR slope with laxative use, stratified into four a priori categories (i.e. less than −10, −10 to less than −5, −5 to <0 and ≥0 mL/min/1.73 m2/year) for the analysis [25].
Statistical analysis
Baseline patient characteristics were summarized by laxative users (n = 11 667) and non-laxative users (n = 34 965) and presented as number (percentages) for categorical variables and mean [standard deviation (SD)] for continuous variables with a normal distribution or median [interquartile range (IQR)] for those with a skewed distribution. In order to account for the temporality of the association between baseline clinical characteristics and laxative use status and not to miss the potential short-term effects of factors that might affect subsequent use of laxatives, the baseline was defined based on the 2-year time period immediately prior to the first date of laxative prescription during the last 1-year pre-ESRD period among laxative users. Among non-laxative users, the 2-year baseline period was anchored by an index date of 296 days prior to dialysis initiation, which corresponded to the median time interval from the first date of laxative prescription to dialysis initiation in laxative users.
We performed multivariable logistic regression to identify factors independently associated with laxative use during the last 1-year pre-ESRD period. Based on theoretical consideration and the availability in this study, the following variables were included in the main adjusted model: demographics [age, sex, race and marital status), smoking status, service connectivity, comorbidities (diabetes, hyperlipidemia, cardiovascular disease, congestive heart failure, cerebrovascular disease, lung disease, connective tissue disease, peptic ulcer disease, liver disease, human deficiency virus/acquired immunodeficiency syndrome (HIV/AIDS), malignancy, anemia, atrial fibrillation, depression and bowel disorders], CCI, medications [renin–angiotensin system inhibitors, β-blockers, calcium channel blockers, diuretics, phosphate binders (calcium acetate, sevelamer or lanthanum), sodium polystyrene sulfonate, antidepressants, non-opioid analgesics, opioids, antihistamines, anticholinergics, antiarrhythmics, anticoagulants, antipsychotics, anti-Parkinson drugs, antacids, anticonvulsants and oral iron supplements] and cumulative length of hospitalizations over the 2-year baseline period. Of the variables included in the multivariable model, data points were missing for race (0.01%), marital status (5.4%), service connectivity (1.9%), comorbidities (0.8%) and medications (7.6%). Of the 46 632 patients in the analytical cohort, 39 578 (88.7%) had complete data available for the multivariable model.
Due to the relatively high proportion of missing information for body mass index (BMI; 33.3%), systolic blood pressure (30.4%), last eGFR and eGFR slope during the 2-year baseline period (37.7%), these variables were additionally included in the main model as a sensitivity analysis, which resulted in 57.8% of the population in the analytical cohort. A two-sided P < 0.05 was used as a threshold of statistical significance for all analyses. Due to the large sample size, the significance of differences in baseline characteristics by laxative use status was established based on considerations of biologically or clinically meaningful differences. All analyses were conducted in SAS Enterprise guide version 7.1 (SAS Institute, Cary, NC, USA) and STATA/MP version 15 (STATA Corporation, College Station, TX, USA). The study was approved by the Institutional Review Boards of the Memphis and Long Beach VA Medical centers, with exemption from informed consent.
RESULTS
Laxative use during the transition period
Within each 6-month period over the entire 72-month transition period, the proportion of patients with at least one laxative prescription ranged from 17.5% to 37.1% (Supplementary data, Table S3). As depicted in Figure 1, the use of laxatives gradually increased as patients progressed to ESRD, with a marked increase seen in the 6 months immediately prior to ESRD transition. Laxative use peaked in the first 6 months following dialysis initiation and remained fairly stable at ∼30% throughout the post-ESRD period (Figure 1). The proportion of patients who had continuous laxative prescriptions throughout the entire transition period was 2.8%.
FIGURE 1.
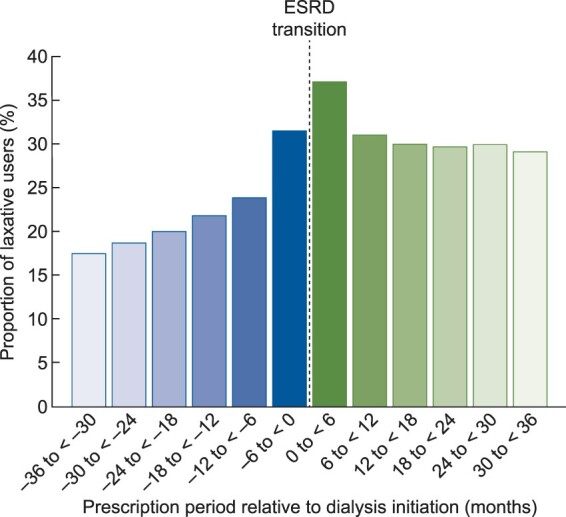
Proportion of laxative use within each 6-month period over 36 months pre- and post-ESRD transition.
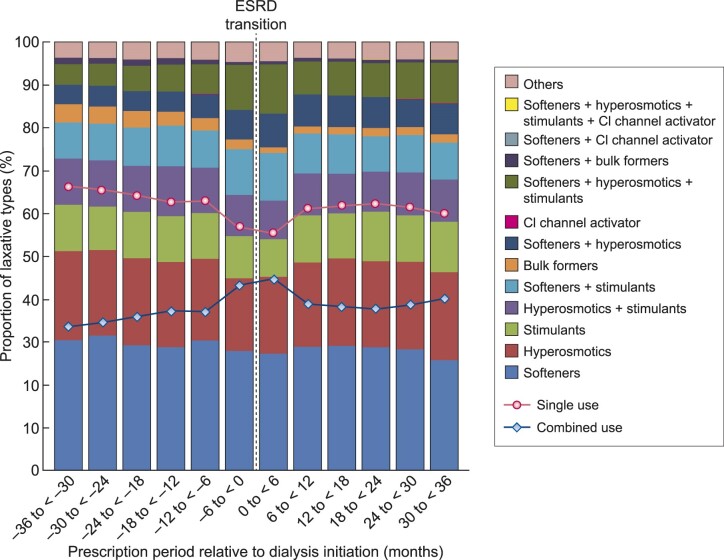
Among patients with at least one laxative prescription, the majority (≥55%) was prescribed a single type of laxative throughout the transition period, with stool softeners being the most commonly prescribed (25.8–31.5%), followed by hyperosmotics (16.9–20.8%), stimulants (8.8–11.7%), bulk formers (1.3–4.3%) and chloride channel activator (0–0.02%) (Figure 2 and Supplementary data, Table S4). There was a decreasing trend in the overall proportion of patients who used a single type of laxative over time, with a marked temporary drop seen in the 6 months immediately before and after the ESRD transition. A corresponding increase in the overall proportion of combined use of laxatives was observed. Among different laxative types, stool softeners, hyperosmotics and stimulants were commonly used in combination with each other (Figure 2 and Supplementary data, Table S4). There was a graded relationship between the number of different types of laxatives and the number of prescribed drugs (Supplementary data, Table S5).
FIGURE 2.
Proportion of laxative types used alone or in combination among patients with at least one laxative prescription within each 6-month period over 36 months pre- and post-ESRD transition. Solid (red) and dotted (blue) lines represent single and combined use of laxatives, respectively.
Factors associated with laxative use
In a total of 46 632 patients included in this analysis, baseline characteristics overall and in those categorized by laxative use status are shown in Table 1. The overall mean (SD) age was 72.5 (11.3) years; 95.2% of patients were male; 22.7% were African American; and 72.9% were diabetic. The mean baseline eGFR was 32.5 mL/min/1.73 m2. Compared with nonlaxative users, laxative users were younger, more likely to be African American, a current smoker and service connected, and less likely to be married. They had a higher prevalence of diabetes as a primary cause of ESRD and comorbidities except cerebrovascular disease, connective tissue disease, peptic ulcer disease, malignancy and atrial fibrillation, and also had longer cumulative length of hospitalization during the 2-year baseline period. The use of medications except calcium channel blockers was more common among laxative users.
Table 1.
Baseline patient characteristics overall and stratified by laxative use status during the year prior to dialysis transition among 46 632 patients in the analytical cohort
| Characteristic | Total (N = 46 632) | Laxative users (n = 11 667) | Non-laxative users (n = 34 965) |
|---|---|---|---|
| Age, mean (SD), year | 72.5 (11.3) | 68.5 (10.4) | 73.8 (11.3) |
| Male sex, n (%) | 44 374 (95.2) | 11 264 (96.5) | 33 110 (94.7) |
| Race, n (%) | |||
| White | 34 528 (74.0) | 7165 (61.4) | 27 363 (78.3) |
| African American | 10 586 (22.7) | 4126 (35.4) | 6460 (18.5) |
| Others | 1515 (3.3) | 376 (3.2) | 1139 (3.2) |
| Married, n (%) | 27 278 (58.5) | 5675 (48.6) | 21 603 (61.8) |
| Smoking status, n (%) | |||
| Current | 12 221 (26.2) | 4352 (37.3) | 7869 (22.5) |
| Past | 13 401 (28.7) | 3581 (30.7) | 9820 (28.1) |
| Never | 11 268 (24.2) | 3284 (28.2) | 7984 (22.8) |
| Unknown | 9742 (20.9) | 450 (3.8) | 9292 (26.6) |
| Service connected, n (%) | 15 109 (32.4) | 5828 (49.9) | 9281 (26.5) |
| Systolic BP, mean (SD), mmHg | 141.7 (16.1) | 142.7 (14.7) | 141.2 (16.8) |
| BMI, mean (SD), kg/m2 | 29.4 (6.1) | 30.2 (6.7) | 28.9 (5.8) |
| Primary cause of ESRD, n (%) | |||
| Diabetes | 19 736 (42.3) | 5776 (49.5) | 13 960 (39.9) |
| Hypertension | 15 061 (32.3) | 2920 (25.0) | 12 141 (34.7) |
| Glomerulonephritis | 2546 (5.5) | 580 (5.0) | 1966 (5.6) |
| Cystic kidney disease | 564 (1.2) | 110 (1.0) | 454 (1.3) |
| Other urologic condition | 660 (1.4) | 141 (1.2) | 519 (1.5) |
| Other cause | 5148 (11.0) | 1285 (11.0) | 3863 (11.1) |
| Missing | 2917 (6.3) | 855 (7.3) | 2062 (5.9) |
| Renal replacement modality, n (%) | |||
| Hemodialysis | 38 224 (81.9) | 9890 (84.8) | 28 334 (81.0) |
| Peritoneal dialysis | 2511 (5.4) | 464 (3.9) | 2047 (5.9) |
| Other/uncertain | 5713 (12.3) | 1260 (10.8) | 4453 (12.7) |
| Missing | 184 (0.4) | 53 (0.5) | 131 (0.4) |
| Comorbidities, n (%) | |||
| Diabetes | 33 980 (72.9) | 9277 (79.5) | 24 703 (70.6) |
| Hypertension | 45 049 (96.6) | 11 485 (98.4) | 33 564 (95.9) |
| Hyperlipidemia | 39 578 (84.9) | 10 030 (85.9) | 29 458 (84.5) |
| Cardiovascular disease | 36 066 (77.3) | 9265 (79.4) | 26 801 (76.7) |
| Congestive heart failure | 30 870 (66.2) | 7972 (68.3) | 22 898 (65.5) |
| Cerebrovascular disease | 21 542 (46.2) | 5209 (44.6) | 16 333 (46.7) |
| Lung disease | 26 300 (56.4) | 7018 (60.2) | 19 282 (55.2) |
| Connective tissue disease | 4100 (8.8) | 826 (7.1) | 3274 (9.4) |
| Peptic ulcer disease | 5425 (11.6) | 1386 (11.9) | 4039 (11.6) |
| Liver disease | 9043 (19.4) | 2763 (23.7) | 6280 (17.9) |
| HIV/AIDS | 494 (1.1) | 172 (1.5) | 322 (0.9) |
| Malignancies | 15 242 (32.7) | 3761 (32.2) | 11 481 (32.8) |
| Anemia | 37 926 (81.3) | 10 173 (87.2) | 27 753 (79.4) |
| Atrial fibrillation | 13 032 (27.9) | 2883 (24.7) | 10 149 (29.0) |
| Depression | 13 827 (29.6) | 5257 (45.1) | 8570 (24.5) |
| Bowel disordersa | 12 038 (25.8) | 3176 (27.2) | 8862 (25.4) |
| CCI, median (IQR) | 5 (3,7) | 5 (4,7) | 5 (3,7) |
| Cumulative length of hospitalization, median (IQR), days | 0 (0,7) | 4 (0,16) | 0 (0,5) |
| Medications, n (%) | |||
| RASi | 28 390 (60.9) | 8758 (75.1) | 19 632 (56.5) |
| β-blockers | 30 186 (64.7) | 9338 (80.0) | 20 848 (59.6) |
| Calcium channel blockers | 27 352 (58.7) | 8459 (30.9) | 18 893 (54.0) |
| Diuretics | 31 350 (67.2) | 9926 (85.1) | 21 424 (61.3) |
| Phosphate binders | 4226 (9.1) | 2168 (18.6) | 2058 (5.9) |
| Sodium polystyrene sulfonate | 4 483 (9.6) | 2289 (19.6) | 2194 (6.3) |
| Antidepressants | 12 529 (26.9) | 5216 (44.7) | 7313 (20.9) |
| Non-opioid analgesics | 7386 (15.8) | 4758 (40.8) | 2628 (7.5) |
| Opioids | 18 997 (40.7) | 7545 (64.7) | 11 452 (32.8) |
| Anticholinergics | 3875 (8.3) | 1827 (15.7) | 2048 (5.9) |
| Antihistamines | 1984 (4.3) | 1553 (13.3) | 431 (1.2) |
| Antiarrhythmics | 2123 (4.6) | 635 (5.4) | 1488 (4.3) |
| Anticoagulants | 5265 (11.3) | 4040 (34.6) | 1225 (3.5) |
| Antipsychotics | 2246 (4.8) | 1252 (10.7) | 994 (2.8) |
| Anti-Parkinson drugs | 1189 (2.5) | 346 (2.9) | 843 (2.4) |
| Antacids | 3444 (7.4) | 2253 (19.3) | 1191 (3.4) |
| Anticonvulsants | 8397 (18.0) | 3667 (31.4) | 4730 (13.5) |
| Oral iron supplements | 6147 (13.2) | 4311 (36.9) | 1836 (5.3) |
| Laboratory parameters | |||
| eGFR, mean (SD), mL/min/1.73 m2 | 32.5 (18.8) | 33.0 (19.4) | 32.2 (18.5) |
| Last eGFR, mL/min/1.73 m2 | 28.7 (19.0) | 27.6 (19.4) | 29.4 (18.8) |
| eGFR slope, n (%) | |||
| Less than −10 mL/min/1.73 m2/year | 5862 (12.6) | 2895 (24.8) | 2967 (8.5) |
| −10 to <−5 mL/min/1.73 m2/year | 9229 (19.8) | 3177 (27.2) | 6052 (17.3) |
| −5 to <0 mL/min/1.73 m2/year | 12 411 (26.6) | 3873 (33.2) | 8538 (24.4) |
| ≥0 mL/min/1.73 m2/year | 1552 (3.3) | 752 (6.5) | 800 (2.3) |
Data are presented as number (percentage), mean (SD) or median (IQR). All P-values except peptic ulcer disease and anti-Parkinson drugs for between-group comparison were statistically significant.
Bowel disorders include inflammatory bowel disease, irritable bowel syndrome and diarrhea.
RASi, renin–angiotensin system inhibitor.
Figure 3 shows the factors independently associated with pre-ESRD laxative use in descending order of the magnitude of the odds ratio (OR). In the multivariable-adjusted model, the use of certain medications such as anticoagulants [OR 4.24, 95% confidence interval (CI) 3.88–4.63], oral iron supplements (3.42, 95% CI 3.17–3.69), non-opioid analgesics (2.51, 95% CI 2.34–2.69), antihistamines (2.47, 95% CI 2.15–2.84) and opioid analgesics (2.11, 95% CI 1.98–2.23) were among the strongest factors positively associated with pre-ESRD laxative use. African American (versus white) race and the presence of anemia (OR 1.35, 95% CI 1.24–1.47), depression (1.32, 95% CI 1.16–1.49) and liver disease (1.11, 95% CI 1.03–1.20) were also associated with pre-ESRD laxative use. Meanwhile, married (versus unmarried) status (OR 0.69, 95% CI 0.65–0.73) and certain comorbidities such as HIV/AIDS (0.56, 95% CI 0.42–0.76), connective tissue disease (0.69, 95% CI 0.62–0.77) and hyperlipidemia (0.74, 95% CI 0.68–0.82) were negatively associated with pre-ESRD laxative use (Figure 3).
FIGURE 3.
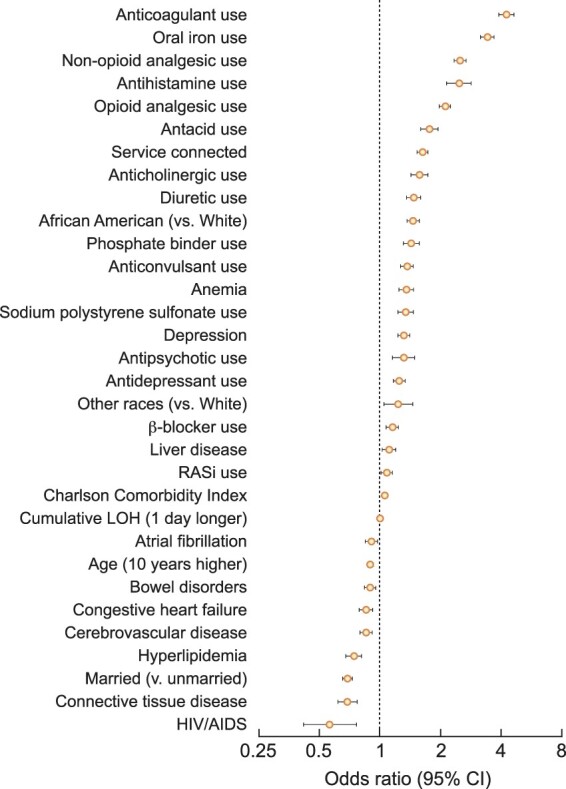
Factors independently associated with pre-ESRD laxative use. Pre-ESRD laxative use was defined as at least two laxative prescriptions 30-days apart during the 1-year pre-ESRD period.
Factors significantly associated with pre-ESRD laxative use were fairly similar after additional adjustment for BMI, systolic blood pressure, last eGFR and eGFR slope in a sensitivity analysis. Of note, eGFR slopes less than −10 and ≥0 (versus −5 to <0) mL/min/1.73 m2/year were both associated with pre-ESRD laxative use (ORs 1.56, 95% CI 1.35–1.81 and 1.41, 95% CI 1.28–1.54, respectively) (Supplementary data, Figure S2). The odds of pre-ESRD laxative use associated with all examined variables are summarized in Supplementary data, Table S6.
DISCUSSION
In this large national cohort of US veterans transitioning to dialysis, we described the patterns of laxative use during 36 months pre- and post-transition to ESRD and identified clinical factors associated with pre-ESRD laxative use. Laxative use increased as patients progressed to ESRD, peaked at 37.1% in the first 6 months following dialysis initiation and remained fairly stable thereafter throughout the post-ESRD period. While the majority of laxatives were used alone, with stool softeners (∼30% of all laxatives), hyperosmotics (∼20%) and stimulants (∼10%) being most commonly prescribed, there was an increasing trend in combined use of laxatives over time, peaking at ∼45% in the 6 months immediately before and after the transition to ESRD. The use of medications, such as anticoagulants, oral iron supplements, non-opioid analgesics, antihistamines and opioid analgesics, was associated with higher odds of pre-ESRD laxative use.
Several studies have examined the prevalence of laxative use and reported its wide variation ranging from 6 to 67% depending on the studied population, including the general population [35], community-dwelling elderly [36], hospitalized patients [20] and people living in nursing homes [37]. However, as with the paucity of data on the prevalence of constipation among patients with advanced stages of CKD, information on the prevalence of laxative use in the advanced CKD population is very limited. In a recent study including 21 patients with nondialysis-dependent CKD (NDD-CKD) with eGFR <15 mL/min/1.73 m2, 98 on hemodialysis and 21 on peritoneal dialysis, the prevalence of self-reported laxative use was 23.8, 30.6 and 42.9%, respectively [38]. The study also showed that, among different types of laxatives, docusate (a stool softener) was the most commonly used laxative in all patient groups [38]. In another study investigating the relationship between laxative use and clinical parameters among 136 hemodialysis patients, 66.2% of them used laxatives, and female sex, older age, diabetes and hyperhomocysteinemia were shown to be independently associated with laxative use [39]. These studies, however, were small in size and cross-sectional, focusing separately on NDD-CKD or ESRD populations. In this study, we therefore extended the previous observations to a large and unique cohort of patients with advanced NDD-CKD transitioning to dialysis, and for the first time described temporal changes in laxative use during the ESRD transition period and identified various factors independently associated with pre-ESRD laxative use.
Patients with CKD typically suffer from an immense burden of medications, comorbidities, metabolic abnormalities and altered gut microbiota, particularly in the most advanced stages of CKD [8, 40], all of which are suggested as predisposing factors for constipation [12]. In line with this evidence, our results showed a sustained increase in laxative use as patients progressed to ESRD, with a marked increase seen in the 6 months immediately preceding dialysis initiation. A similar increase observed in the combined use of laxatives might additionally suggest that the severity of constipation symptoms also increased with worsening kidney function during the pre-ESRD period. In this context, our findings regarding the factors associated with pre-ESRD laxative use may be of particular value, with potential clinical and research implications. Among various clinical characteristics, the majority of factors significantly associated with pre-ESRD laxative use were the use of medications, most of which are known to induce constipation as a side effect (e.g. oral iron, opioid analgesics and anticholinergics) [12]. Although it is unclear why the use of anticoagulants, which themselves seem unlikely to significantly affect gastrointestinal motility, showed the strongest association with pre-ESRD laxative use, it is possible that patients with anticoagulant use were prescribed laxatives for the purpose of preventing bleeding complications associated with constipation (e.g. lower gastrointestinal bleeding) [41, 42], albeit we cannot conclude any causal relationship. It is also possible that patients on warfarin adjust their diet to limit foods high in vitamin K, which could lead to constipation. The identification of these medications may help detect previously under-recognized causes of drug-induced constipation and, perhaps more importantly, can help avoid unnecessary or inappropriate use of laxatives along with their unwanted adverse effects. Specifically, for those taking both iron supplements and laxatives, for example, switching from oral to intravenous iron supplementation might be helpful to ameliorate their symptom of constipation and reduce laxative requirements. These changes in practice habits could also contribute to a lower overall pill burden in this relevant population. Whether the use of laxatives has any beneficial effects beyond conventional defecation management in this unique population (e.g. disposal of uremic toxins, maintaining mineral homeostasis or retaining commensal gut microbiota) may deserve further investigation.
Despite the advantages of this study including its large sample size of patients with advanced CKD, our results must be interpreted in light of some limitations. Most of our patients were male US veterans, and hence results may not apply to women or patients from other geographic areas. Information about over-the-counter use of laxatives was not available; therefore, it is possible that we underestimated the proportion of patients with laxative use and/or misclassified those who used only over-the-counter laxatives as nonlaxative users. It is also important to note that the use of laxatives did not necessarily reflect a person's constipation status, especially given the lack of information about subjective symptoms of constipation and the fact that only a minority of patients with constipation seek medical care [43]. In addition, prescription does not necessarily mean that patients actually took the drugs. Lastly, as with all observational studies, we cannot eliminate the possibility of unmeasured confounders (e.g. diet and lifestyle) that might have potentially affected pre-ESRD laxative use.
In conclusion, laxative use increased considerably as patients progressed to ESRD and remained fairly stable after the transition to ESRD, likely mirroring the increasing burden of drug-induced constipation during the ESRD transition period. Although further studies are needed to identify optimal practice patterns in the use of laxatives, our findings may provide novel insight into better management strategies to alleviate constipation symptoms and reduce medication requirements in patients with advanced CKD, potentially contributing to the improvement of patient-centered care and outcomes in this population.
SUPPLEMENTARY DATA
Supplementary data are available at ndt online.
Supplementary Material
ACKNOWLEDGEMENTS
C.P.K., K.K.-Z., E.S. and M.Z.M. are employees of the US Department of VA. The interpretation and reporting of these data are the responsibility of the authors and in no way should be seen as official policy or interpretation of the Department of VA or the US government. The results of this article have not been published previously in whole or part.
FUNDING
This study is supported by grant 5U01DK102163 from the National Institute of Health (NIH) to K.K.-Z. and C.P.K., and by resources from the US Department of Veterans Affairs. The data reported here have been supplied in part by the United States Renal Data System (USRDS). Support for VA/CMS data is provided by the Department of Veterans Affairs, Veterans Health Administration, Office of Research and Development, Health Services Research and Development, VA Information Resource Center (Project Numbers SDR 02-237 and 98-004).
AUTHORS’ CONTRIBUTIONS
K.S., A.A.D., P.K.P., F.T. and C.P.K. were responsible for study concept and design. K.S., A.A.D., P.K.P., M.Z.M., E.S., K.K.-Z. and C.P.K. were involved in data acquisition. K.S., A.A.D. and P.K.P. carried out data analyses. K.S., A.A.D., P.K.P., F.T., Y.O., M.Z.M., J.D.G., E.S., K.K.-Z. and C.P.K. were involved in data interpretation. K.K.-Z. and C.P.K. supervised the study. Each author contributed important intellectual content during manuscript drafting or revision and agreed to be accountable for all aspects of the work in ensuring that questions pertaining to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All authors reviewed and approved the final version of this manuscript.
CONFLICT OF INTEREST STATEMENT
None of the authors has relevant conflicts of interest.
REFERENCES
- 1. Sumida K, Molnar MZ, Potukuchi PK et al. Constipation and risk of death and cardiovascular events. Atherosclerosis 2019; 281: 114–120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bharucha AE, Pemberton JH, Locke GR III. American gastroenterological association technical review on constipation. Gastroenterology 2013; 144: 218–238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Strid H, Simren M, Johansson AC et al. The prevalence of gastrointestinal symptoms in patients with chronic renal failure is increased and associated with impaired psychological general well-being. Nephrol Dial Transplant 2002; 17: 1434–1439 [DOI] [PubMed] [Google Scholar]
- 4. Yasuda G, Shibata K, Takizawa T et al. Prevalence of constipation in continuous ambulatory peritoneal dialysis patients and comparison with hemodialysis patients. Am J Kidney Dis 2002; 39: 1292–1299 [DOI] [PubMed] [Google Scholar]
- 5. Cano AE, Neil AK, Kang JY et al. Gastrointestinal symptoms in patients with end-stage renal disease undergoing treatment by hemodialysis or peritoneal dialysis. Am J Gastroenterol 2007; 102: 1990–1997 [DOI] [PubMed] [Google Scholar]
- 6. Shirazian S, Radhakrishnan J. Gastrointestinal disorders and renal failure: exploring the connection. Nat Rev Nephrol 2010; 6: 480–492 [DOI] [PubMed] [Google Scholar]
- 7. Wu MJ, Chang CS, Cheng CH et al. Colonic transit time in long-term dialysis patients. Am J Kidney Dis 2004; 44: 322–327 [DOI] [PubMed] [Google Scholar]
- 8. Ramezani A, Massy ZA, Meijers B et al. Role of the gut microbiome in uremia: a potential therapeutic target. Am J Kidney Dis 2016; 67: 483–498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Quigley EM. The enteric microbiota in the pathogenesis and management of constipation. Best Pract Res Clin Gastroenterol 2011; 25: 119–126 [DOI] [PubMed] [Google Scholar]
- 10. Sumida K, Molnar MZ, Potukuchi PK et al. Constipation and Incident CKD. J Am Soc Nephrol 2017; 28: 1248–1258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sumida K, Kovesdy CP. The gut-kidney-heart axis in chronic kidney disease. Physiol Int 2019; 106: 195–206 [DOI] [PubMed] [Google Scholar]
- 12. Sumida K, Yamagata K, Kovesdy CP. Constipation in CKD. Kidney Int Rep 2019; 5: 121–134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Andresen V, Layer P. Medical therapy of constipation: current standards and beyond. Visc Med 2018; 34: 123–127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ford AC, Moayyedi P, Lacy BE et al. American College of Gastroenterology monograph on the management of irritable bowel syndrome and chronic idiopathic constipation. Am J Gastroenterol 2014; 109 (Suppl 1): S2–S26 [DOI] [PubMed] [Google Scholar]
- 15. Mishima E, Fukuda S, Shima H et al. Alteration of the intestinal environment by lubiprostone is associated with amelioration of adenine-induced CKD. J Am Soc Nephrol 2015; 26: 1787–1794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Nanto-Hara F, Kanemitsu Y, Fukuda S et al. The guanylate cyclase C agonist linaclotide ameliorates the gut-cardio-renal axis in an adenine-induced mouse model of chronic kidney disease. Nephrol Dial Transplant 2019; 35: 250–264 [DOI] [PubMed] [Google Scholar]
- 17. Sueyoshi M, Fukunaga M, Mei M et al. Effects of lactulose on renal function and gut microbiota in adenine-induced chronic kidney disease rats. Clin Exp Nephrol 2019; 23: 908–919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. McKee KY, Widera E. Habitual prescribing of laxatives-it’s time to flush outdated protocols down the drain. JAMA Intern Med 2016; 176: 1217–1219 [DOI] [PubMed] [Google Scholar]
- 19. Menees SB, Guentner A, Chey SW et al. How do US gastroenterologists use over-the-counter and prescription medications in patients with gastroesophageal reflux and chronic constipation? Am J Gastroenterol 2015; 110: 1516–1525 [DOI] [PubMed] [Google Scholar]
- 20. Lee TC, McDonald EG, Bonnici A et al. Pattern of inpatient laxative use: waste not, want not. JAMA Intern Med 2016; 176: 1216–1217 [DOI] [PubMed] [Google Scholar]
- 21. Rantis PC Jr, , Vernava AM III, Daniel GL et al. Chronic constipation–is the work-up worth the cost? Dis Colon Rectum 1997; 40: 280–286 [DOI] [PubMed] [Google Scholar]
- 22. Saran R, Robinson B, Abbott KC et al. US renal data system 2018 annual data report: epidemiology of kidney disease in the United States. Am J Kidney Dis 2019; 73: A7–A8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Sumida K, Molnar MZ, Potukuchi PK et al. Blood pressure before initiation of maintenance dialysis and subsequent mortality. Am J Kidney Dis 2017; 70: 207–217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Sumida K, Molnar MZ, Potukuchi PK et al. Association between vascular access creation and deceleration of estimated glomerular filtration rate decline in late-stage chronic kidney disease patients transitioning to end-stage renal disease. Nephrol Dial Transplant 2017; 32: 1330–1337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Sumida K, Molnar MZ, Potukuchi PK et al. Association of slopes of estimated glomerular filtration rate with post-end-stage renal disease mortality in patients with advanced chronic kidney disease transitioning to dialysis. Mayo Clin Proc 2016; 91: 196–207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. US Department of Veterans Affairs VIRCV. VIReC Research User Guide: VHA Pharmacy Prescription Data. 2nd edn. Hines, IL: VIReC, 2008
- 27. Streja E, Gosmanova EO, Molnar MZ et al. Association of continuation of statin therapy initiated before transition to chronic dialysis therapy with mortality after dialysis initiation. JAMA Netw Open 2018; 1: e182311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. McGinnis KA, Brandt CA, Skanderson M et al. Validating smoking data from the Veteran’s affairs health factors dataset, an electronic data source. Nicotine Tob Res 2011; 13: 1233–1239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Sumida K, Molnar MZ, Potukuchi PK et al. Prognostic significance of pre-end-stage renal disease serum alkaline phosphatase for post-end-stage renal disease mortality in late-stage chronic kidney disease patients transitioning to dialysis. Nephrol Dial Transplant 2018; 33: 264–273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol 1992; 45: 613–619 [DOI] [PubMed] [Google Scholar]
- 31. Sumida K, Molnar MZ, Potukuchi PK et al. Pre-end-stage renal disease visit-to-visit systolic blood pressure variability and post-end-stage renal disease mortality in incident dialysis patients. J Hypertens 2017; 35: 1816–1824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kovesdy CP, Norris KC, Boulware LE et al. Association of race with mortality and cardiovascular events in a large cohort of US veterans. Circulation 2015; 132: 1538–1548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kovesdy CP, Alrifai A, Gosmanova EO et al. Age and outcomes associated with BP in patients with incident CKD. Clin J Am Soc Nephrol 2016; 11: 821–831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Levey AS, Stevens LA, Schmid CH et al. A new equation to estimate glomerular filtration rate. Ann Intern Med 2009; 150: 604–612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Wald A, Scarpignato C, Mueller-Lissner S et al. A multinational survey of prevalence and patterns of laxative use among adults with self-defined constipation. Aliment Pharmacol Ther 2008; 28: 917–930 [DOI] [PubMed] [Google Scholar]
- 36. Werth BL, Williams KA, Pont LG. A longitudinal study of constipation and laxative use in a community-dwelling elderly population. Arch Gerontol Geriatr 2015; 60: 418–424 [DOI] [PubMed] [Google Scholar]
- 37. Gustafsson M, Lamas K, Isaksson U et al. Constipation and laxative use among people living in nursing homes in 2007 and 2013. BMC Geriatr 2019; 19: 38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Lee A, Lambert K, Byrne P et al. Prevalence of constipation in patients with advanced kidney disease. J Ren Care 2016; 42: 144–149 [DOI] [PubMed] [Google Scholar]
- 39. Ikee R, Toyoyama T, Endo T et al. Clinical factors associated with constipation in hemodialysis patients. Int Urol Nephrol 2016; 48: 1741–1742 [DOI] [PubMed] [Google Scholar]
- 40. Zoccali C, Tripepi G, Mallamaci F. Predictors of cardiovascular death in ESRD. Semin Nephrol 2005; 25: 358–362 [DOI] [PubMed] [Google Scholar]
- 41. Chait MM. Lower gastrointestinal bleeding in the elderly. World J Gastrointest Endosc 2010; 2: 147–154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Ozdil B, Akkiz H, Sandikci M et al. Massive lower gastrointestinal hemorrhage secondary to rectal hemorrhoids in elderly patients receiving anticoagulant therapy: case series. Dig Dis Sci 2010; 55: 2693–2694 [DOI] [PubMed] [Google Scholar]
- 43. Galvez C, Garrigues V, Ortiz V et al. Healthcare seeking for constipation: a population-based survey in the Mediterranean area of Spain. Aliment Pharmacol Ther 2006; 24: 421–428 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.