ABSTRACT
Adeno-associated viruses (AAV) serve as vectors for therapeutic gene delivery. AAV9 vectors have been FDA approved, as Zolgensma, for the treatment of spinal muscular atrophy and are being evaluated in clinical trials for the treatment of neurotropic and musculotropic diseases. A major hurdle for AAV-mediated gene delivery is the presence of preexisting neutralizing antibodies in 40 to 80% of the general population. These preexisting antibodies can reduce therapeutic efficacy through viral neutralization and the size of the patient cohort eligible for treatment. In this study, cryo-electron microscopy and image reconstruction were used to define the epitopes of five anti-AAV9 monoclonal antibodies (MAbs), ADK9, HL2368, HL2370, HL2372, and HL2374, on the capsid surface. Three of these, ADK9, HL2370, and HL2374, bound to or near the icosahedral 3-fold axes, HL2368 bound to the 2/5-fold wall, and HL2372 bound to the region surrounding the 5-fold axes. Pseudoatomic modeling enabled the mapping and identification of antibody contact amino acids on the capsid, including S454 and P659. These epitopes overlap previously defined parvovirus antigenic sites. Capsid amino acids critical for the interactions were confirmed by mutagenesis, followed by biochemical assays testing recombinant AAV9 (rAAV9) variants capable of escaping recognition and neutralization by the parental MAbs. These variants retained parental tropism and had similar or improved transduction efficiency compared to AAV9. These engineered rAAV9 variants could expand the patient cohort eligible for AAV9-mediated gene delivery by avoiding preexisting circulating neutralizing antibodies.
IMPORTANCE The use of recombinant adeno-associated viruses (rAAVs) as delivery vectors for therapeutic genes is becoming increasingly popular, especially following the FDA approval of Luxturna and Zolgensma, based on serotypes AAV2 and AAV9, respectively. However, high-titer anti-AAV neutralizing antibodies in the general population exempt patients from treatment. The goal of this study is to circumvent this issue by creating AAV variant vectors not recognized by preexisting neutralizing antibodies. The mapping of the antigenic epitopes of five different monoclonal antibodies (MAbs) on AAV9, to recapitulate a polyclonal response, enabled the rational design of escape variants with minimal disruption to cell tropism and gene expression. This study, which included four newly developed and now commercially available MAbs, provides a platform for the engineering of rAAV9 vectors that can be used to deliver genes to patients with preexisting AAV antibodies.
KEYWORDS: AAV9, adeno-associated virus, antibodies, capsids, clinical therapeutics, cryo-EM, gene therapy, neutralization, vectors, viral variants
INTRODUCTION
One of the most promising viral vectors in development for therapeutic gene delivery is based on the single-stranded DNA packaging adeno-associated viruses (AAV) (1–7). These small (∼260 Å), nonenveloped viruses can package and deliver therapeutic genes to a wide range of tissues to treat monogenic diseases. AAV is a member of the Parvoviridae in the genus Dependoparvovirus (8). They require helper viruses such as Adenoviruses or Herpesviruses for successful replication (9, 10). Thirteen human and nonhuman primate serotypes (AAV1-13) have been described, each with various transduction efficiency in different host tissues (11). A much larger number of clonal isolates (∼1,800) have been described in human and nonhuman primate tissues (12–18). AAV serotypes 1 to 13 are currently classified into 7 clades based on functional and serologic similarities. These clades are as follows: AAV1 and AAV6 (clade A), AAV2 (clade B), AAV2-AAV3 hybrid including AAV13 (clade C), AAV7 (clade D), AAV8 and AAV10 (clade E), AAV9 (clade F), and AAV4, AAV11, and AAV12 (clade G) (12, 19).
The AAVs possess an ∼4.7-kb genome containing two major open reading frames (ORFs), rep and cap, flanked by two inverted terminal repeats (ITRs) (15, 20, 21). Alternative reading frames present in cap contain aap and maap (15, 22). The cap ORF also encodes three overlapping capsid viral proteins (VPs) from mRNAs produced by alternative splicing. The larger transcript contains the entire cap coding region and produces VP1, while the shorter ones encode VP2 (ACG) and VP3 (AUG) from two separate start codons. As a result, the VP1 (87 kDa) and VP2 (72 kDa) capsid proteins share the same ∼530 C-terminal-amino acid sequence within VP3 but have additional amino acids at their N termini. A total of 60 VPs assemble a T=1 icosahedral virus capsid in a 1:1:10 ratio of VP1, VP2, and VP3, respectively, with VP3 constituting ∼90% of the capsid (11). For recombinant AAV (rAAV) vectors, transgenic genomes of up to 5 kb in size can be packaged into a capsid (23, 24). The rAAVs can be produced by transient transfection of HEK293 cells, the baculovirus/Sf9 cell system, the herpes simplex type 1 system, or other systems using different packaging and producer cell lines (25–27). The VP monomers assemble the AAV T=1 icosahedral capsid utilizing 2-, 3-, and 5-fold symmetry-related monomer-monomer interactions. The capsid surface topology exhibits depressions at the 2-folds, three protrusions offset from the 3-folds, and cylindrical channels flanked by depressions at the 5-folds. In addition, a region separates the 2- and 5-fold depressions, termed the 2/5-fold wall. Although all AAV serotypes share these major capsid features, differences in amino acid residues and loop conformations result in differential receptor usage, tissue tropism, transduction efficiency, and antigenic reactivity (28–35). These amino acid differences are located predominantly in the surface loops, particularly in the previously described variable regions (VRs) (32). These VRs are structurally defined as two or more amino acids where the Cα positions are greater than 1 Å apart when the VP structures of AAV serotypes are superposed (32).
Despite the success of using AAV as a delivery vector for gene therapy, several issues prevent optimal efficacy (36). For example, transduction and expression efficiency are hampered by several factors, including patient preexisting neutralizing antibodies to the AAV capsids (5, 29, 37–44). Epidemiology studies have shown high neutralizing antibody titers against naturally occurring AAVs, in up to 80% of the population for the most common serotype, AAV2 (45–52). Current strategies to circumvent the immune response of a patient during AAV-mediated therapeutic gene delivery include (i) the removal of AAV capsid-specific antibodies by plasmapheresis (53), (ii) the administration of immunosuppressive therapy (50), (iii) the use of antibody-cleaving endopeptidases (54), and (iv) the use of modified vectors, able to escape antibody recognition, developed by either directed evolution or rational capsid mutagenesis (37, 38). The first two strategies are designed to temporarily reduce the immune response to the AAV capsid, while the third and fourth are designed to help the capsids escape recognition from the humoral immune system. Design of low immunogenic capsids via rational capsid mutagenesis requires information on AAV antigenic epitopes.
There is interest in using AAV9 for the treatment of musculotropic and neurotropic diseases (55–57). At present, a clinical trial to administer rAAV9-hGAA to late-onset Pompe disease patients (ClinicalTrials.gov registration number NCT02240407) is under development, relying on muscle transduction by AAV9. Furthermore, there is currently an ongoing clinical trial utilizing AAV9 for the treatment of Duchenne muscular dystrophy (ClinicalTrials.gov registration number NCT04240314). The FDA-approved drug Zolgensma also utilizes the AAV9 capsid to deliver a functional copy of the smn1 gene to motor neurons of spinal muscular atrophy patients (58).
For clinical trials and therapeutics utilizing rAAV9 vectors, patients with preexisting antibody immunity to AAV9 are excluded from treatment and readministration. Thus, there is a need to develop novel rAAV9 variants capable of escaping preexisting antibodies while maintaining transduction efficiency.
In this study, cryo-electron microcopy (cryo-EM) and 3D reconstruction are used to identify the epitopes of five anti-AAV9 monoclonal antibodies (MAbs) on the AAV9 capsid. Pseudoatomic models, based on these structures, enabled identification of antibody contact amino acids on the capsid surface, specifically S454 and P659. Mutagenesis confirmed the critical contact residues for variants capable of escaping recognition and neutralization by the MAbs while retaining transduction efficiency. This study identifies three regions, the 2/5-fold wall, the top and wall of the 3-fold protrusions, and the DE and HI loops of the 5-fold axis, serving as antigenic epitopes for AAV9.
RESULTS
Anti-AAV9 MAbs are neutralizing in vivo.
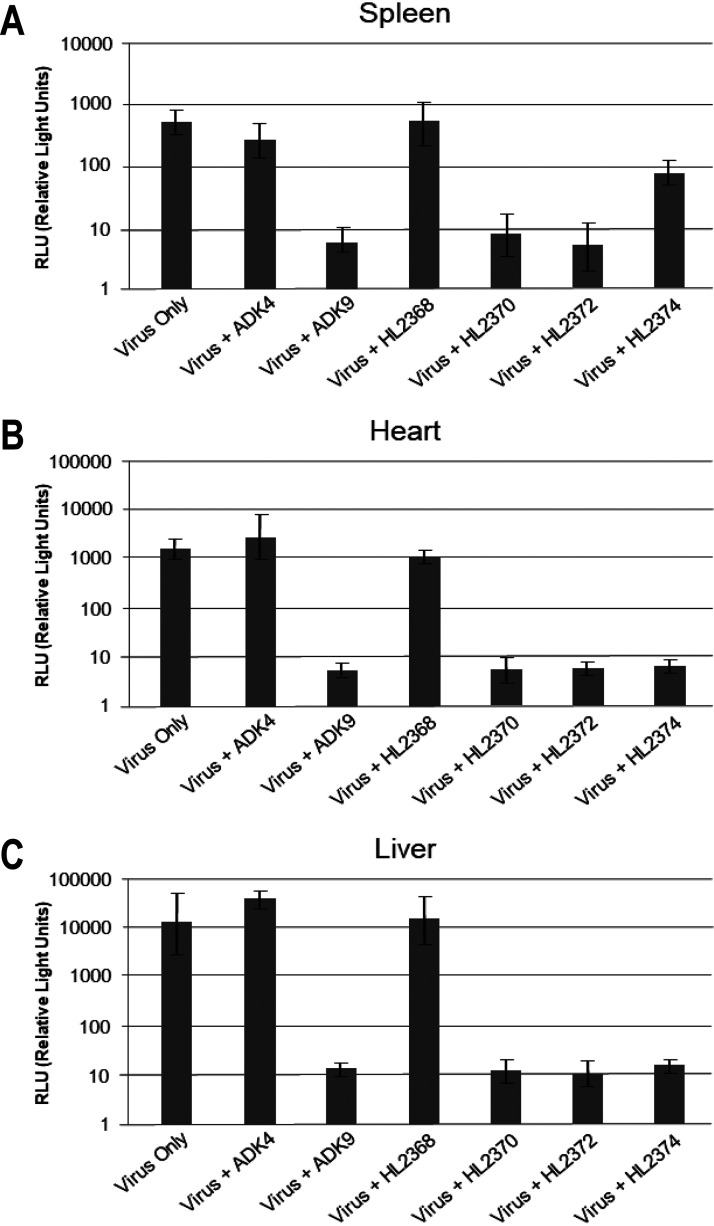
The ability of a panel of anti-AAV9 MAbs, HL2368, HL2370, HL2372, HL2374, and ADK9 (20, 59), to neutralize AAV9 infection in vivo was tested in BALB/c mice. The AAV9 vectors were preincubated with antibodies, and luciferase expression levels in spleen, heart, and liver were analyzed 7 days after intravenous injection. The luciferase activity in the AAV9 virus only (positive control) or the AAV9+ADK4 (ADK4 directed against the AAV4 capsid) groups (negative control) were high in all three tissues screened (Fig. 1). Luciferase expression of AAV9 vectors in the presence of ADK9, HL2370, and HL2372 were ∼100- to a 1,000-fold lower in all three tissues compared to AAV9 only, indicative of neutralization (Fig. 1). AAV9 transduction was also inhibited by HL2374 in the heart and liver, but transduction of the spleen was only reduced 10-fold (Fig. 1). It appears that HL2374 is a weak neutralizing antibody. In contrast to the other antibodies, the HL2368 MAb was nonneutralizing in vivo or in vitro (CHO-Lec2) (data not shown) despite its ability to neutralize viral infection in HeLa cells (59).
FIG 1.
In vivo neutralization of AAV9 by anti-AAV9 monoclonal antibodies. (A to C) Neutralization profiles of anti-AAV9 monoclonal antibodies in the (A) spleen, (B) heart, and (C) liver. The anti-AAV9 antibodies tested here are ADK9, HL2368, HL2370, HL2372, and HL2374, with ADK4 (anti-AAV4) as a negative control. ADK9, HL2370, and HL2372 are neutralizing to AAV9 in the tissues tested, however; HL2374 is nonneutralizing in the spleen. HL2368 is nonneutralizing in all tissues tested.
Anti-AAV9 antibodies recognize common AAV antigenic regions.
To determine the binding sites of these antibodies to the AAV9 capsids, the fragment antigen-binding (Fabs) or full-length antibodies of ADK9, HL2368, HL2370, HL2372, and HL2374 were mixed with AAV9 virus-like particles (VLPs). The structures of these complexes were determined by cryo-EM and reconstructed to between 3.9 and 14-Å resolution (Table 1, Fig. 2 and 3). At these resolutions, it was possible to build pseudoatomic models for the Fab and capsid into the density maps. This enabled the identification of the antigenic footprint, defined as capsid residues likely to be directly contacting or occluded by the antibody (Table 2, Fig. 2 and 3). In agreement with previously determined antibody epitopes mapped on other AAV serotypes (28, 38, 43, 60–64), the generated HL series of MAbs along with ADK9 recognize similar antigenic regions, namely, the 2/5-fold wall, 3-fold protrusions, and the area around the 5-fold channel. These observations further support the hypothesis that the AAVs have common antigenic regions and dominant epitopes on the capsid.
TABLE 1.
Summary of cryo-EM data collection and processing parameters
| Complex | Defocus range (μM) | No. of micrographs | No. of particles | Final map resolution (Å) |
|---|---|---|---|---|
| AAV9-ADK9 | 1.5–3.0 | 44 | 349 | 11.2 |
| AAV9-HL2368 | 1.5–3.0 | 59 | 202 | 12.5 |
| AAV9-HL2370 | 1.5–3.0 | 30 | 482 | 14 |
| AAV9-HL2372 | 1.5–3.0 | 101 | 496 | 11.1 |
| AAV9-HL2374 | 1.5–3.0 | 91 | 842 | 8.3 |
| AAV9-HL2370 | 0.5–3.0 | 241 | 5495 | 3.9 |
| AAV9-HL2372 | 0.5–3.0 | 145 | 2866 | 3.9 |
FIG 2.
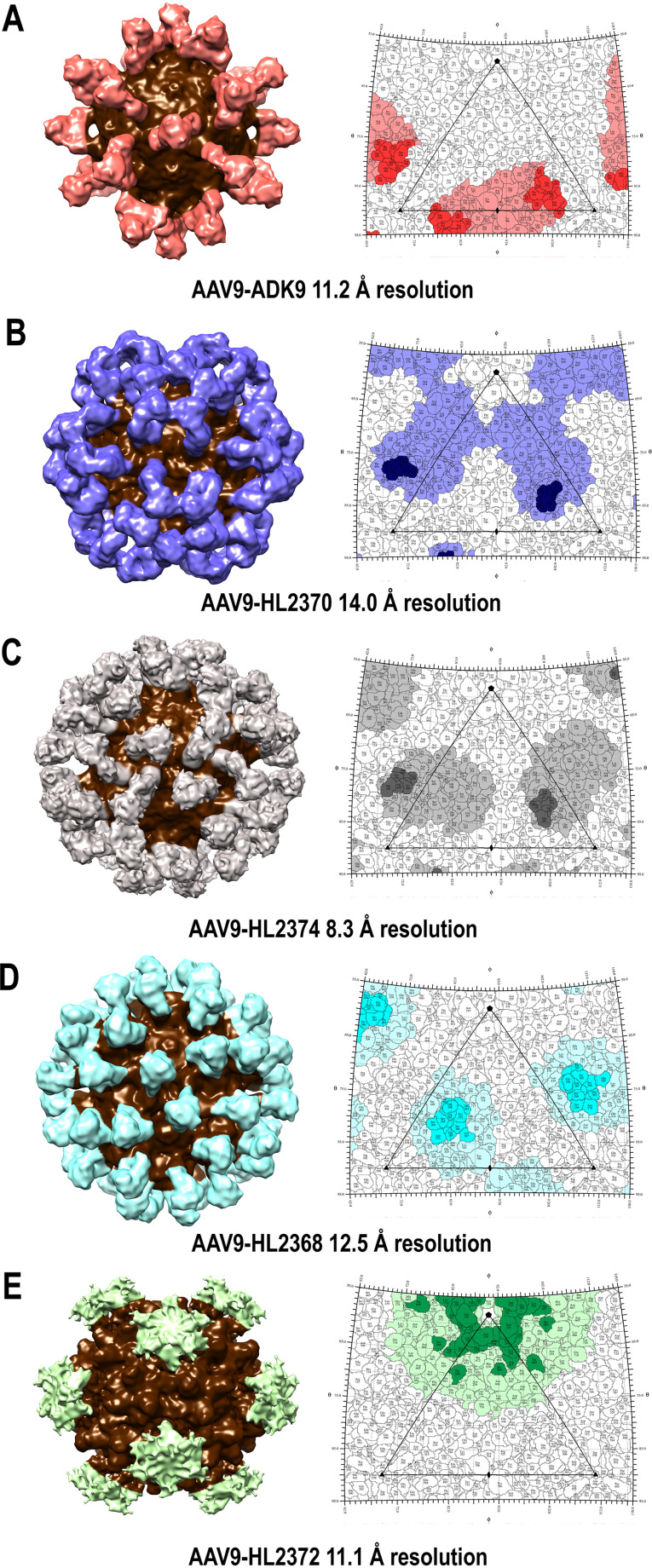
3D reconstruction of the AAV9-antibody complexes. (A to E) 3D surface representation and 2D surface projections (roadmaps) of the (A) AAV9-ADK9, (B) AAV9-HL2370, (C) AAV9-HL2374, (D) AAV9-HL2368, and (E) AAV9-HL2372 antibody complexes determined to 11.2, 14.0, 8.3, 12.5, and 11.1 Å resolution, respectively. In the 3D surface representations, the density corresponding to the AAV9 capsid structure is shown in brown, while that corresponding to the Fabs are shown in various colors. For the roadmaps, the antibody footprint (contact and occluded residues) is colored with dark and light shades of orange (ADK9), blue-purple (HL2370), gray (HL2374), cyan (HL2368), and green (HL2372). The icosahedral axes of symmetry are indicated by an ellipse (2-fold), triangle (3-fold), and pentagon (5-fold). ADK9, HL2370, and HL2374 bound to the 3-fold, HL2368 bound to the base of the 3-fold toward the 2/5-fold wall, and HL2372 bound to the 5-fold toward the 2/5-fold wall. The figure was generated using UCSF-Chimera (81) and the software RIVEM (69).
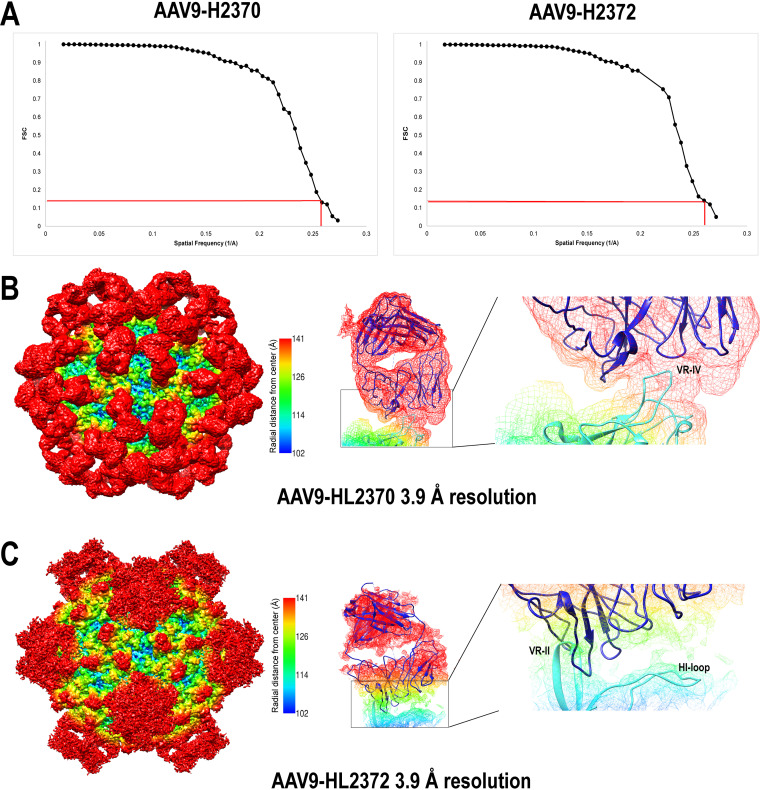
FIG 3.
Higher-resolution Cryo-EM and 3D image reconstruction of the AAV9-HL2370 and HL2372 antibody complexes. (A) FSC plots of the final map (red lines indicate the 0.143 threshold used to estimate the resolution). (B and C) Surface density map of the cryo-reconstructed AAV9-HL2370 and AAV9-HL2372 antibody complex structures at 3.9-Å resolution. The view of the density maps is down the 2-fold axis of symmetry, and the maps are contoured at a sigma (σ) threshold level of 1.0 and 0.5, respectively. The mesh diagrams depict a closeup of the binding interaction of the HL2370 and HL2372 antibody density on the AAV9 capsid. A Fab molecule was fitted into the antibody density maps using Coot (82). The HL2370 Fab is situated on top of the 3-fold protrusions and specifically interacts with VR-IV, while HL2372 interacts with VR-II and protrudes toward the 2/5-fold wall at VR-IX. The density maps are colored radially such that regions closest to the center of the capsid are blue, and those farthest away are in red, indicated by the scale bar (center). The figure was generated using UCSF-Chimera (81).
TABLE 2.
Footprint residues of the monoclonal antibodies ADK9, HL2368, HL2370, HL2372, and HL2374 on the AAV9 capsid
| Antibody | Contact residues | Occluded residues | Variable region |
|---|---|---|---|
| ADK9 | 384, 389, 391 | III | |
| 456–459 | 452, 460, 462 | IV | |
| 492, 494–498 | 488, 491, 493 | V | |
| 528–534, 545 | VI | ||
| 554–556, 558–560 | VII | ||
| 584, 587–589 | VIII | ||
| 701–708, 712, 713 | IX | ||
| 724–728, 730, 731 | |||
| HL2368 | 253, 256, 258–260 | ||
| 262–270, 273 | 271, 272 | I | |
| 274, 275, 278, 293, 375, 376 | |||
| 385–387 | 382–384, 389, 391 | III | |
| 438–441, 443 | |||
| 446, 467–473 | IV | ||
| 488, 495, 503, 510–515 | V | ||
| 528–534 | VI | ||
| 572, 661, 662 | |||
| 703–718 | IX | ||
| 722 | |||
| HL2370 | 248, 250–255 | ||
| 265–269, 271, 272 | I | ||
| 332 | II | ||
| 334, 363–365, 370, 371, 373, 376, 438 | |||
| 438–441 | |||
| 454–458 | 446, 448, 449, 451–453, 459, 460, 462, 464–473 | IV | |
| 491, 493, 494, 496–500, 503, 504 | V | ||
| 547–560 | VII | ||
| 655–676 | |||
| 726 | IX | ||
| HL2372 | 221, 228, 246–248, 250–256, 258, 291, 324, 325 | ||
| 327–333 | II | ||
| 334, 335, 338, 341, 365, 369–373, 376 | |||
| 550 | VII | ||
| 659 | 651–653, 655, 656, 658, 661–668, 670–674, 678 | ||
| 718–720 | IX | ||
| HL2374 | 253, 258, 259, 260, 262 | ||
| 263–272 | I | ||
| 273–275, 278, 376 | |||
| 384–387 | III | ||
| 438, 439, 440, 441 | |||
| 449, 452–458 | 446, 448, 451, 457, 462, 464, 465, 467–473 | IV | |
| 497–501, 503–505, 506 | V | ||
| 515 | |||
| 529 | VI | ||
| 549–553 | VII | ||
| 579, 590 | VIII | ||
| 661, 662, 664 | |||
| 707–718 |
ADK9, HL2370, and HL2374 bind to the 3-fold region of the AAV9 capsid.
The AAV9-ADK9 complex structure was determined from 339 particle images to 11.2-Å resolution (Fig. 2A). ADK9, an IgA isotype MAb, was complexed with the capsid uncleaved. The MAb bounded at the side of the 3-fold protrusions facing the 2-fold axes with the Fab complementary determining region (CDRs) straddling the 2-fold axes and the constant region overlapping above the 2-fold. The AAV9 structure (PDB ID: 3UX1) and a generic Fab structure fit into the cryo-reconstruction density map with a correlation coefficient (CC) of 0.88 and showed occlusion of VR-III, IV, V, VI, VII, and IX (Table 2, Fig. 2A). The predicted contact residues are within VR-IV, V, and VIII (Table 2) assembling the protrusions.
The AAV9-HL2370 complex structure was determined from 482 particle images to 14.0-Å resolution (Fig. 2B). While the HL2370 Fab also bound the 3-fold protrusion, the constant regions of the Fab are extended outward to the 5-fold axis, unlike ADK9. This placement occluded the region between the protrusions and the 5-fold axis (Table 2, Fig. 2B) with a total area of ∼900 Å2. The AAV9 atomic model and generic Fab structure fit into the complex maps with a CC of 0.94. The HL2370 CDRs are located at the top of the 3-fold protrusions near the AAV9 residues 454 to 458, which are part of VR-IV.
The AAV9-HL2374 complex structure was determined from 842 particle images to 8.3-Å resolution (Fig. 2C). The AAV9 atomic model and generic Fab model fit into the density map with a CC of 0.83. The Fab bound to the capsid surface in a similar manner to HL2370, directly to the tips of the 3-fold protrusions, but with a marked difference in the orientation of the constant regions (Fig. 2C). For this antibody, the constant regions lean slightly over the 2/5-wall (Fig. 2C). The occluded region covers the three VRs assembling the 3-fold protrusions VR-IV, V, and VIII, in addition to VR-I, III, VI, and VII (Table 2, Fig. 2C). The predicted contact residues are within VR-IV (Table 2, Fig. 2C).
HL2368 binds near the 3-fold toward the 2/5-fold region of the AAV9 capsid.
The AAV9-HL2368 complex structure was determined from 202 particle images to 12.5-Å resolution (Fig. 2D). The Fab bound at the 2/5-fold wall and the side of the 3-fold protrusions, with its constant regions radiating outward from the capsid surface. The capsid-Fab pseudoatomic model, fitted to a CC of 0.94 as described above, identified residues in the valley surrounding each 3-fold protrusion, residues between the protrusions, and portions of the 2/5-fold wall as being part of the occluded epitope (Table 2, Fig. 2D). Contact residues are within VR-I and III (Table 2). Unlike the other AAV9-antibody structures, this complex structure was reconstructed with fewer particles and resulted in similar resolution compared to other complexes reconstructed from double the amount of particle images. This phenomenon may be a result of better sample and grid quality, as these properties also contribute to resolution.
HL2372 binds to the 5-fold region of the AAV9 capsid.
The AAV9:HL2372 complex structure was determined from 496 to 11.1 Å resolution (Fig. 2E). The antibody binds around the 5-fold axis of symmetry and interacts with the top of the protruding DE loops at VR-II residues 327 to 333, as well as with the HI loop, residue P659, that lies at the valley surrounding the 5-fold channel. Additional residues occluded by HL2372 include a larger section of the HI loop and VR-IX (Table 2, Fig. 2E).
High-resolution complex structures identified amino acid contact residues but did not improve antibody footprint information.
Higher-resolution complex structures, at ∼3.9 Å, were generated for AAV9-HL2370 and AAV9-HL2372 from 5,495 and 2,866 particles, respectively. This was done to determine if the footprint information could be better defined and compared to the pseudoatomic models built into the lower-resolution 14.0- and 11.1-Å density maps, respectively (Fig. 2 and 3). As expected, the density at the capsid-antibody interface was better resolved and showed direct contact with VR-IV and with VR-II and the HI loop for HL2370 and HL2372, respectively (Fig. 3). However, the comparison of the two sets of low- and high-resolution structures showed that the epitope footprint information can readily be obtained from low-resolution complex structures. The capsid structure of AAV9 has been previously determined, together with antibody structures, and these atomic models fit into the low-resolution maps. Thus, in these maps, the antibody epitopes can be identified without the need of additional higher-resolution data.
AAV9 variants can escape antibody recognition and neutralization while retaining transduction efficiency.
Three of the five MAbs contact amino acids in the 3-fold protrusions that are assembled by VR-IV, V, and VIII. To isolate the critical AAV9 capsid residues for MAb binding, loop swap (LS) variants to AAV1 amino acids were generated for these three loops. AAV1 residues were used, as the anti-AAV9 antibodies did not cross-react with AAV1 (59). The VR-IV LS variant consisted of residues 451-QNQSGSAQN-459 (VR-IV LS), and following production and purification, SDS-PAGE and negative stain EM showed that VR-IV LS expressed VP1, VP2, and VP3 in the wild-type 1:1:10 ratio and assembled capsids (Fig. 4A). Native dot immunoblots showed escape of the VR-IV LS variant from HL2370 and HL2374 recognition but not from ADK9 or HL2372 (Fig. 4B). In contrast, the VR-V LS (491-KTKTD-495) variant did not escape from any of the MAbs, and the VR-VIII LS (587-SSTDP-591) escaped only from ADK9 (data not shown). Analyzing the transduction efficiency of these variants showed that the VR-IV LS with a titer of 1 × 1010 vector genomes (vg)/μL, retained ∼2/3 of the infectivity compared to AAV9 (Fig. 4B), whereas VR-V (2.7 × 109 vg/μL) and VR-VIII LS (1 × 1010 vg/μL) were noninfectious (data not shown).
FIG 4.
Analysis of AAV9 antibody escape variants. (A) SDS-PAGE and negative stain EM of WT AAV9 and variants. (B to E) Native dot immunoblots of AAV9 variants against AAV9 antibodies and transduction analysis compared to WT AAV9. Substitutions in variants of (B) VR-IV LS and (C) S454A are localized to the 3-fold axis of symmetry and exhibit escape from HL2370 and HL2374, while (D) P659K includes a substitution in the HI-loop and escapes HL2372. Panel E shows a combination S454A/P659K and escapes the three MAbs, HL2370, HL2372, and HL2374. The absence of a black dot in the native dot blots indicates loss of recognition of that variant by the antibody. WT AAV9 serves as a positive control and is recognized by all the cognate antibodies, while WT AAV5 serves as a negative control and is only recognized by its cognate antibody, ADK5b. ADK9 is as a positive native capsid loading control. B1 is a loading control and recognizes the C-terminal residues of denatured viral proteins (VP1, VP2, and VP3).
To isolate the minimal critical residues in the VR-IV region and create a MAb escape variant, the single S454A amino acid substitution variant was made. This residue, S454, was one of the five residues identified as making contact with HL2370 from the high-resolution structures (Table 2, Fig. 3A). This single variant also produced VPs in the expected 1:1:10 ratio and assembled capsids (Fig. 4A). Like VR-IV LS, this variant was able to evade binding from both HL2370 and HL2374 (Fig. 4C). Interestingly, however, S454A also exhibited enhanced transduction efficiency compared to AAV9 in vitro. Since S454 is not at the epitope of HL2372, this variant did not evade binding from HL2372.
To create an HL2372 escape variant, initially, the DE loop of the AAV9 capsid was targeted, namely, residues 327 and 329; however, this capsid variant did not escape binding from HL2372 (data not shown). The high-resolution cryo-EM map from the AAV9-HL2372 complex also identified residue P659 as a potential binding residue for this antibody; thus, the P659K single amino acid substitution variant was made, which also produced the VPs in the expected 1:1:10 ratio and assembled capsids (Fig. 4A). Native dot immunoblot analysis against our panel of anti-AAV9 antibodies indicated escape of this variant from only HL2372, and this P659K variant also showed similar transduction efficiency compared to AAV9 in vitro (Fig. 4D).
Based on the accumulated observed data, the two single variants were combined to produce the double S454A/P659K variant. Like the individual amino acid substitutions, this variant produced the three viral proteins in the expected 1:1:10 ratio and assembled capsids (Fig. 4A). Also as expected, via dot immunoblot analysis, the S454A/P659K variant escaped recognition from HL2370, HL2372, and HL2374 (Fig. 4E). However, transduction efficiency was impaired for this double variant compared to AAV9 in vitro. This reduction in transduction may be a consequence of these two variants together hindering important viral trafficking steps; however, singly they are not. Regardless of this decrease in transduction, immune escape does pose a more relevant phenotype, as neutralization would essentially lead to 0% transduction compared to the 50% from this double variant.
To confirm the escape phenotype observed via dot immunoblot analysis, in vitro neutralization assays were conducted. In these experiments AAV9 and the variant capsids were exposed to increasing concentrations of antibodies, and their transduction efficiency at these conditions was determined. In the presence of increasing concentrations of MAbs, AAV9 was neutralized by the anti-AAV9 MAbs but not by ADK5b, an antibody that recognizes AAV5 (Fig. 5A). However, the variants VR-IV LS, S454A, P659K, and S454A/P659K remained infectious even in increasing concentrations of HL2370 and HL2374 for VR-IV LS and S454A, HL2372 for P659K, and HL2370, HL2372, and HL2374 for S454A/P659K (Fig. 5B to D).
FIG 5.
Neutralization assays of AAV9 variants. (A to E) Normalized transduction units of (A) WT AAV9 and AAV9 variants (B) VR-IV LS, (C) S454A, (D) P659K, and (E) S454A/P659K in the presence of increasing concentrations of anti-AAV9 monoclonal antibodies, as determined by a luciferase gene reporter assay. Ratios indicate AAV9 capsids to number of antibodies; e.g., 1:30 indicates 1 AAV9 capsid to 30 antibodies. All experiments were carried out in triplicate and are displayed as the means ± standard deviation (n = 3).
DISCUSSION
The presence of neutralizing antibodies targeting the capsids of AAV vectors poses a challenge for the effectiveness of AAV-mediated gene delivery. Therefore, the development of novel rAAV vectors to circumvent the detrimental effects of neutralizing antibodies is essential to the success of the AAV viral vector gene delivery system. A superposition of all five AAV9-Fab complex structures mimics the polyclonal response of the adaptive immune system toward the AAV9 capsid (Fig. 6). With this superposition, the AAV9 capsid has no exposed surface area, other than the center of the 3-fold between the protrusions and a small region at the 2/5-fold wall. However, the center of the 3-fold protrusions has also been identified as the epitope for another previously described anti-AAV9 antibody, PAV9.1 (62). Thus, in vivo and in the presence of preexisting antibodies, the AAV9 capsid will have significantly reduced transduction efficiency due to the inability to interact with cellular factors required for infection. In addition to the center of the 3-fold protrusion, another study identified amino acid residues 453 to 457, located at VR-IV, as an antigenic region (62, 65). In addition, antigenic mapping for other AAV serotypes has identified regions proximal to the 2-, 3-, and 5-fold symmetry axes as implicated in antigenic epitopes (28, 38, 43, 60–64). The need for a more complete description of the antigenic epitopes for AAV9 led to the generation of four new MAbs, HL2368, HL2370, HL2372, and HL2374 (59), and the structural mapping of their epitopes in this study (Fig. 2 and 4). In addition, the epitope of a previously described MAb, ADK9 (20), was determined (Fig. 2).
FIG 6.
AAV9 capsid fully decorated by Fabs. This figure illustrates the interaction of the AAV9 monoclonal antibodies covering all accessible epitopes and recapitulates a polyclonal antibody response. On the top is a combination of the AAV9-Fab cryo-reconstruction density maps. The capsid is in brown and the Fab fragment densities are peach, cyan, blue-purple, green, and gray for ADK9, HL2368, HL2370, Hl2372, and HL2374, respectively. On the bottom is a stereographic roadmap projection of the epitopes for all the Fabs, colored similarly to the combination density map but including lighter shades as the occluded regions. All the anti-AAV9 monoclonal antibodies analyzed here (ADK9, HL2368, HL2370, HL2372, and HL2374) are shown with the color key identified below. The figure was generated using UCSF-Chimera (81) and RIVEM (69).
The footprint of antibody binding to the capsid surface of the AAVs had previously been classified into groups based on the location of the epitopes (64); HL2368, binding to the 2/5-fold wall, has a footprint that overlaps the A20 MAb against AAV2, ADK4 for AAV4, and ADK6 for AAV6 and would be placed in group III. The 2/5-fold wall, including the side of the 3-fold protrusions, has been shown to contain critical residues for receptor binding of AAV9. AAV9 has been described to use glycan moieties with terminal galactoses as an attachment factor to facilitate cellular entry. The galactose binding pocket was found to localize at residues 270, 271, 446, 470, 472, 473, and 503, located on the base of the 3-fold protrusions, using structure-guided docking (66). Furthermore, as AAV9 is dependent on the adeno-associated virus receptor (AAVR) for transduction, binding of this protein likely also occurs in this region like for AAV1 and AAV2 (67–69). Thus, it is very surprising that HL2368 does not neutralize AAV9 in vivo or in vitro using CHO-Lec2 cells (Fig. 1). It is plausible that the lack of neutralization is due to AAVR having a higher affinity for the AAV9 capsid, leading to competitive binding of AAVR over HL2368.
The immune dominance of the 3-fold region of the AAVs was evident in this study, with 3 of the 5 antibody footprints, ADK9, HL2370, and HL2374, mapping to the protrusions (Fig. 2). Capsid-Fab contact residues, based on pseudoatomic models, identified a common stretch of amino acids (453 to 457) with the epitope of these three antibodies, which was previously also identified by in vivo screening (65). This AAV capsid region was also described as a binding epitope for antibodies ADK1a, 4E4, and 5H7 against AAV1 (28), C37-B against AAV2, HL2476 against AAV5 (63), and ADK8, HL2381, and HL2383 against AAV8 (70).
The three 3-fold binding antibodies showed neutralization both in vitro and in vivo (59). While the footprint (occluded residues) of all these antibodies overlaps with the galactose binding site (66), other steps of the vector life cycle might be affected as well. Due to the tilt of the HL2370 Fab, the 5-fold region might be inaccessible and might interfere with suggested externalization of VP1u and its phospholipase enzymatic activity during endosomal trafficking (71) of the incoming AAV vectors. The HL2374 Fab occludes a large region of the 2/5-fold wall and, thus, might also interfere with galactose and AAVR binding like HL2368. However, given that HL2368 is not neutralizing, preventing galactose and AAVR binding might not be the reason for neutralizing ability of HL2374. ADK9’s binding across the 2-fold symmetry axis also occludes conserved residues of the AAV serotypes that were reported to be involved in transcription of AAV vectors (72, 73). Furthermore, ADK9, being an IgA antibody with the ability to dimerize, might aggregate AAV capsids and cause AAV9 neutralization.
In contrast to the other antibodies, HL2372 bound to the 5-fold region. HL2372 is the only antibody among the five MAbs showing cross-reactivity to another AAV serotype, AAV8. The binding pattern in AAV8 is identical (70) to the one described here for AAV9 and equally led to AAV8 neutralization by HL2372. Furthermore, mutation of a stretch of amino acids in the HI-loop of AAV8 led to evasion of HL2372 neutralization (70). The AAV9 5-fold region is also the binding site of a nanobody, capture select affinity ligand for AAV9 (CSAL9), utilized specifically for AAV9 purification (74). Preincubation of AAV9 vectors with the CSAL9 nanobody also resulted in their neutralization. HL2372’s binding at the 5-fold pore may abrogate VP1u externalization or prevent the conformational change that needs to take place before VP1u is externalized, leading to viral neutralization.
Guided by the cryo-EM structures, together with native dot immunoblots, and neutralization assays, S454 was identified as the key residue necessary for HL2370 and HL2374 binding and neutralization, while P659 was important for HL2372. The enhancement of transduction by the S454A variant further indicates the possibility that disrupting the AAV9 capsid at antigenic sites may not be detrimental to transduction. Therefore, it is possible to disrupt antibody binding while retaining transduction efficiency at residue positions that may be important to the capsid’s functioning. As for the enhancement observed with S454A, the S454 residue may be part of a degron site on the AAV9 capsid used for degradation of viral capsids during cellular trafficking (75). Thus, switching the serine to an alanine prevents its potential phosphorylation of the capsid and its subsequent ubiquitination, which will target the vector for degradation by the proteasome (75). The degron site has been mapped for another AAV serotype, AAV2 (75).
In summary, the 2/5-fold wall, 3-fold, and 5-fold of AAV9 act as epitopes for the binding of five monoclonal antibodies, HL2368, HL2370, HL2372, HL2374, and ADK9. Mutation of VR-IV residues 450 to 458 conferred escape from HL2370 and HL2374. Single amino acid substitution S454A conferred escape from HL2370 and HL2374, and P659K conferred escape from HL2372. This work provides a possibility for resolving the complex 3D structure of human sera-derived anti-AAV9 antibodies bound to AAV viral capsids to help create variants that escape human sera antibodies while retaining parental tropism and transduction efficiency. These variant capsids may be used to treat patients with preexisting anti-AAV9 neutralizing antibodies.
MATERIALS AND METHODS
Cell culture.
HEK293 cells were used for virus production via triple transfection as described in the vector production subsection. These cells were cultured in Dulbecco’s modified essential medium (DMEM) and supplemented with 10% fetal bovine serum (FBS) and 1% antimycotic/antimicrobial at 37°C and 5% CO2. CHO-Lec2 cells, a cell line defective for the generation of terminal sialic acids and, thus, displaying terminal N-linked galactoses on their cell surfaces (76), were used for all infectivity and neutralization assays. CHO-Lec2 cells were cultured in minimum essential medium (MEM) supplemented with 10% FBS and 1% antimycotic/antimicrobial.
MAb purification and Fab generation.
The University of Florida Interdisciplinary Center for Biotechnology Research (UF ICBR) hybridoma core produced hybridoma cell supernatants for monoclonal antibodies ADK9, HL2368, HL2370, HL2372, and HL2374 as previously described (59). Whole IgGs and IgA (ADK9) from these supernatants were purified on HiTrap protein G HP columns (GE Healthcare), eluted with glycine-HCl (pH 2.8), and neutralized with 1 M Tris-HCl at pH 9.0. Purified antibodies were concentrated and buffer-exchanged into 1× phosphate-buffered saline (PBS) for use in subsequent experiments. Fragment antigen-binding (Fab) fragments were cleaved from whole IgGs by incubating purified IgGs overnight with immobilized papain (Pierce) according to the manufacturer’s recommendations. After separation from the papain resin, the digested sample was passed through a HiTrap protein A column (GE Healthcare) to separate the fragment crystallizable (Fc) and undigested IgGs from the Fabs. These Fabs were collected from the flow through, concentrated, and buffer-exchanged into 1× PBS for complexing to virus capsid for epitope mapping studies.
VLP production and purification.
AAV virus-like particles (VLPs) were expressed using a recombinant baculovirus expressing the viral proteins of the desired AAV serotype (77). These VLPs were purified as previously described (78) and buffer-exchanged into 20 mM Tris, 250 mM NaCl, pH 7.5 for AAV5 and 20 mM Tris, 350 mM NaCl, 2 mM MgCl2, pH 7.5 for AAV9. The VLPs were concentrated using 150 kDa molecular weight cutoff (MWCO) Apollo spin columns, and their concentrations determined by optical density readings at 280 nm, utilizing an extinction coefficient of 1.7. Virus purity and assembled capsids were verified by SDS-PAGE and negative stain EM, respectively.
Negative stain electron microscopy (EM).
The integrity of all purified virus samples was confirmed using transmission electron microscopy. For each sample, 5 μL of purified virus was applied to glow-discharged carbon-coated copper grids (Electron Microscopy Sciences, Hatfield, PA, USA) for 5 min followed by blotting off excess buffer with filter paper (GE Healthcare Life Sciences). The grids were washed with 20 μL of filtered water three times, excess liquid was blotted off, and then the grids were stained two times with 20 μL 1% uranyl acetate for 3 s. Excess stain was removed with filter paper, and grids were imaged on a 120-keV Tecnai Spirit microscope (Thermo Fisher Scientific).
Virus-Fab or Ig complexing and Cryo-EM data collection.
Purified VLPs were mixed with Fab fragments or IgA (ADK9) at a ratio of 1:1, one VP-binding site to one Fab fragment molecule, producing AAV9 complexes with ADK9, HL2370, and HL2372. In the case of AAV9-HL2368 and AAV9-HL2374, the ratio was 1:2. The number of capsid and antibody particles per μL of sample was calculated using the molecular weight and Avogadro’s number. These complexes were incubated for 10 min at 4°C prior to vitrification. To obtain higher-resolution structures for AAV9-HL2370 and -HL2372, purified AAV9 capsids were mixed with IgG at a ratio of 1:1 for AAV9-HL2372 and 1:3 for AAV9-HL2370 and then incubated at 4°C for approximately 30 min prior to vitrification. C-Flat (CF-2/2-4c-50; Protochips, Inc.) holey carbon grids were prepared via glow discharge in a PELCO easiGlow system for 1 min prior to vitrification. Then, 3 μL of virus-antibody complexes were pipetted onto charged grids and plunge-vitrified into liquid ethane with a Vitrobot Mark IV (FEI) instrument. Vitrified grids were transferred into liquid nitrogen, where they were stored until data collection. For data collection, grids were loaded into Gatan 626 cryo transfer holders under liquid nitrogen. Data were collected on an FEI Tecnai F20 microscope using 0.5 to 3.0 μm defocus range under low-dose conditions, ∼20 electrons/Å2 with a Gatan UltraScan 4000 charge-coupled device (CCD) camera, or ∼60 electrons/Å2 with a Gatan K2 Summit direct electron detector (DED), resulting in sampling of 1.91 Å/pixel for the ADK9:AAV9 data set, 1.78 Å/pixel for HL2368:AAV9, HL2374:AAV9, HL2372:AAV9 (low res), and HL2370:AAV9 (low res), and 1.22 Å/pixel for HL2370:AAV9 (high resolution) and HL2372:AAV9 (high resolution).
Cryo-EM structure determination.
For each of the AAV:Ab data sets, ROBEM, a program within the Auto3DEM software package (https://bakerlab.ucsd.edu/wikis/software/start.php), was used to isolate individual particle images from each micrograph, while preprocessing and defocus level estimation for these particle images were performed as previously described (79). Initial models were generated using a random-model computation protocol from ∼150 particle images and used to screen ligand binding before refinement. Using this model, orientation, origin determination, and refinement of all the particle images were carried out with Auto3DEM (80). CTF-correction was used to correct the effects of phase reversal in the contrast transfer functions (CTFs) of images, as previously described (79). The resolutions of the final reconstructions were calculated by Fourier shell correlation (FSC) at 0.143. All graphical representations of these reconstructions and pseudoatomic modeling were carried out using the UCSF Chimera software package (81) and Coot (82).
Pseudoatomic model building.
The VIPERdb online server (83) was used to generate 60mers from the AAV9 capsid structure (PDB ID: 3UX1). Each AAV9-antibody complex reconstruction was fitted with these 60mers via the rigid-body rotation and translation functions of the “Fit in map” option in Chimera. This program was utilized to generate difference maps, in which the model map of the fitted 60mer was subtracted from the scaled and normalized complex map. These positive difference maps were used for further docking and analysis. As the sequences, and thus structure, of the panel of monoclonal antibodies are not available, a generic Fab (PDB ID: 2FBJ) or an anti-AAV5 Fab (HL2476) (63) was fitted into the antibody density for each reconstruction. The PDBe “Protein Interfaces, Surfaces, and Assemblies” (PISA) service at the European Bioinformatics Institute (http://www.ebi.ac.uk/pdbe/prot_int/pistart.html) was used to identify potential direct-interacting residues at the antibody-capsid interface (84). Utilizing this information, 2D representations of the capsid surface and their interacting residues were created using the RIVEM software package (85). Chimera was used to identify additional capsid surface areas occluded by the binding antibody and encompassed by the complex density attributed to the Fab.
Mutagenesis.
AAV9 variants were made by swapping the amino acids of AAV9 to those of AAV1 or single and double amino acid substitutions. Each set of mutations was carried out by site-directed mutagenesis PCR. Primers were designed to contain the nucleotide substitutions that would result in the amino acid being substituted. The mutagenized plasmid PCR product was DpnI digested to digest any parental plasmids before transforming into DH5alpha or top10 Escherichia coli cells for plating and colony selection. Small-scale cultures were grown from colonies, and DNA isolated via mini prep was subjected to sequencing to confirm the presence of each mutation. Once confirmed, large-scale DNA maxi preps were carried out for these plasmid variants for triple transfections.
Vector production.
Wild-type and variant rAAV9-Luciferase (rAAV9-Luc) vectors were produced via triple transfection as previously described (63). HEK293 cells were transfected with three plasmids, the pTRUF3-Luciferase plasmid, the WT or variant AAV9 capsid plasmid pXR9, containing the AAV2 rep and AAV9 cap genes, and the pHelper plasmid (containing the adenoviral helper genes required for replication). Plates were harvested at 72 h post-transfection, and capsids were purified by affinity column using POROS CaptureSelect affinity ligand for AAV9 (CSAL9) (Thermo Fisher) (78). Viral genome titers were determined by quantitative PCR (qPCR) with primers specific to the luciferase gene.
Native dot immunoblot.
Native dot immunoblots were performed via vacuum-assisted application of intact virus particles onto nitrocellulose membranes. Virus-containing membranes were blocked in 10% milk and 0.05% Tween 20 PBS for 1 h at room temperature (RT) and then incubated with primary antibodies (purified MAb), diluted in 1% milk/0.05% Tween 20 PBS, and allowed to rock overnight. The membranes were then washed three times for 5 min each in 0.05% Tween 20 PBS, before being incubated with horseradish peroxidase (HRP)-conjugated secondary antibody for 1 h at RT. After a second washing step as described above, the blots were developed by incubating the membranes with HRP development solution for 2 min at RT, and chemiluminescence was captured on X-ray film. Dark circles for each blot indicate recognition of the applied virus by the primary antibody. This method was used to test binding of antibodies against our WT and variant capsids.
Infectivity assays.
CHO-Lec2 cells were seeded in 24-well plates and allowed to grow overnight to ∼50% confluence (1.6 × 105 cells) the following day. Viral vectors were diluted into nonsupplemented MEM medium to be used at a multiplicity of infection (MOI) of 105 vector genomes per cell and added directly to cells. The infection persisted for 48 h before a luciferase assay was conducted per the manufacturer’s instructions (Promega luciferase assay system).
In vitro neutralization assays.
In vitro neutralization assays were carried out in CHO-Lec2 cells by doing viral infections at an MOI of 105 vector genomes per cell in the presence of antibodies. Capsid to antibody ratios of 1:0, 1:30, 1:60, 1:120, and 1:240 were tested. Capsids were combined with antibody at appropriate ratios and allowed to incubate for 30 min at 37°C. Infections were performed with antibody-bound viral capsids and incubated at 37°C for 48 h before a luciferase assay was conducted per the manufacturer’s instructions (Promega luciferase assay system).
In vivo neutralization assays.
Female BALB/c mice at 6 to 8 weeks old were intravenously injected with rAAV9 vectors packaging a chicken beta-actin (CBA) promoter-driven firefly luciferase (CBA-Luc) transgene at a dose of 5 × 1010 vector genomes per mouse, premixed with or without the antibodies. After a week, spleen, heart, and liver tissues were harvested, and the luciferase activities were assayed, as described previously (86). Brain and muscle tissues were not tested, because a dose of 5 × 1010 vg/mouse is insufficient to achieve biodistribution in these tissues.
In vitro luciferase assays.
Cells were washed 3 times with 1× PBS and then lysed with lysis buffer for 20 min at RT (Promega luciferase assay system). Luciferin substrate was added just before relative luminescence units (RLU) were recorded for each well in the plate, using a plate reader (BioTek). Readings were normalized to WT AAV9 infections.
ACKNOWLEDGMENTS
We thank the University of Florida Interdisciplinary Center for Biotechnology Research (UF-ICBR) Monoclonal Antibody Core Facility (RRID:SCR_019147) for generation of the monoclonal HL2368, HL2370, HL2372, and HL2374 antibodies and the UF-ICBR Electron Microscopy Core Facility (RRID:SCR_019146) for access to electron microscopes utilized for negative-stain electron microscopy and cryo-EM data collection. The FEI Spirit and TF20 cryo-electron microscopes were provided by the UF College of Medicine (COM) and Division of Sponsored Programs (DSP). We also thank Lavanya Rao and Travis Corriher from the University of North Carolina at Chapel Hill for technical assistance with mouse studies.
The University of Florida COM, NIH (GM082946) (to M.A.-M. and R.M.) and NIH (HL089221) (to A.A.) provided funds for the research efforts.
Contributor Information
Robert McKenna, Email: rmckenna@ufl.edu.
Colin R. Parrish, Cornell University
REFERENCES
- 1.Daya S, Berns KI. 2008. Gene therapy using adeno-associated virus vectors. Clin Microbiol Rev 21:583–593. 10.1128/CMR.00008-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Flotte TR, Carter BJ. 1995. Adeno-associated virus vectors for gene therapy. Gene Ther 2:357–362. [PubMed] [Google Scholar]
- 3.Hauswirth WW, Aleman TS, Kaushal S, Cideciyan AV, Schwartz SB, Wang L, Conlon TJ, Boye SL, Flotte TR, Byrne BJ, Jacobson SG. 2008. Treatment of Leber congenital amaurosis due to RPE65 mutations by ocular subretinal injection of adeno-associated virus gene vector: short-term results of a phase I trial. Hum Gene Ther 19:979–990. 10.1089/hum.2008.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Herzog RW, High KA. 1999. Adeno-associated virus-mediated gene transfer of factor IX for treatment of hemophilia B by gene therapy. Thromb Haemost 82:540–546. 10.1055/s-0037-1615877. [DOI] [PubMed] [Google Scholar]
- 5.Mingozzi F, High KA. 2011. Therapeutic in vivo gene transfer for genetic disease using AAV: progress and challenges. Nat Rev Genet 12:341–355. 10.1038/nrg2988. [DOI] [PubMed] [Google Scholar]
- 6.Nathwani AC, Tuddenham EGD, Rangarajan S, Rosales C, McIntosh J, Linch DC, Chowdary P, Riddell A, Pie AJ, Harrington C, O'Beirne J, Smith K, Pasi J, Glader B, Rustagi P, Ng CYC, Kay MA, Zhou J, Spence Y, Morton CL, Allay J, Coleman J, Sleep S, Cunningham JM, Srivastava D, Basner-Tschakarjan E, Mingozzi F, High KA, Gray JT, Reiss UM, Nienhuis AW, Davidoff AM. 2011. Adenovirus-associated virus vector-mediated gene transfer in hemophilia B. N Engl J Med 365:2357–2365. 10.1056/NEJMoa1108046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Simonelli F, Maguire AM, Testa F, Pierce EA, Mingozzi F, Bennicelli JL, Rossi S, Marshall K, Banfi S, Surace EM, Sun J, Redmond TM, Zhu X, Shindler KS, Ying G-S, Ziviello C, Acerra C, Wright JF, McDonnell JW, High KA, Bennett J, Auricchio A. 2010. Gene therapy for Leber’s congenital amaurosis is safe and effective through 1.5 years after vector administration. Mol Ther 18:643–650. 10.1038/mt.2009.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cotmore SF, Agbandje-McKenna M, Canuti M, Chiorini JA, Eis-Hubinger A-M, Modha S, Ogliastro M, Pénzes JJ, Pintel DJ, Qiu J, Soderlund-Venermo M, Tattersall P, Tijssen P, ICTV Report Consortium. 2019. ICTV virus taxonomy profile: Parvoviridae. J Gen Virol 100:367–368., 10.1099/jgv.0.001212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Berns KI, Linden RM. 1995. The cryptic life style of adeno-associated virus. Bioessays 17:237–245. 10.1002/bies.950170310. [DOI] [PubMed] [Google Scholar]
- 10.Muzyczka N, Berns KI. 2001. Parvoviridae: the viruses and their replication, p 2327–2360. In Fields virology, 4th ed. Lippincott, Williams and Wilkins, Philadelphia, PA. [Google Scholar]
- 11.Agbandje-McKenna M, Kleinschmidt J. 2011. AAV capsid structure and cell interactions. Methods Mol Biol 807:47–92. [DOI] [PubMed] [Google Scholar]
- 12.Gao G, Vandenberghe LH, Alvira MR, Lu Y, Calcedo R, Zhou X, Wilson JM. 2004. Clades of adeno-associated viruses are widely disseminated in human tissues. J Virol 78:6381–6388. 10.1128/JVI.78.12.6381-6388.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gao G-P, Alvira MR, Wang L, Calcedo R, Johnston J, Wilson JM. 2002. Novel adeno-associated viruses from rhesus monkeys as vectors for human gene therapy. Proc Natl Acad Sci USA 99:11854–11859. 10.1073/pnas.182412299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mori S, Wang L, Takeuchi T, Kanda T. 2004. Two novel adeno-associated viruses from cynomolgus monkey: pseudotyping characterization of capsid protein. Virology 330:375–383. 10.1016/j.virol.2004.10.012. [DOI] [PubMed] [Google Scholar]
- 15.Naumer M, Sonntag F, Schmidt K, Nieto K, Panke C, Davey NE, Popa-Wagner R, Kleinschmidt JA. 2012. Properties of the adeno-associated virus assembly-activating protein. J Virol 86:13038–13048. 10.1128/JVI.01675-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schmidt M, Grot E, Cervenka P, Wainer S, Buck C, Chiorini JA. 2006. Identification and characterization of novel adeno-associated virus isolates in ATCC virus stocks. J Virol 80:5082–5085. 10.1128/JVI.80.10.5082-5085.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schmidt M, Voutetakis A, Afione S, Zheng C, Mandikian D, Chiorini JA. 2008. Adeno-associated virus type 12 (AAV12): a novel AAV serotype with sialic acid- and heparan sulfate proteoglycan-independent transduction activity. J Virol 82:1399–1406. 10.1128/JVI.02012-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schnepp BC, Jensen RL, Clark KR, Johnson PR. 2009. Infectious molecular clones of adeno-associated virus isolated directly from human tissues. J Virol 83:1456–1464. 10.1128/JVI.01686-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mietzsch M, Jose A, Chipman P, Bhattacharya N, Daneshparvar N, McKenna R, Agbandje-McKenna M. 2021. Completion of the AAV structural atlas: serotype capsid structures reveals clade-specific features. Viruses 13:101. 10.3390/v13010101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sonntag F, Köther K, Schmidt K, Weghofer M, Raupp C, Nieto K, Kuck A, Gerlach B, Böttcher B, Müller OJ, Lux K, Hörer M, Kleinschmidt JA. 2011. The assembly-activating protein promotes capsid assembly of different adeno-associated virus serotypes. J Virol 85:12686–12697. 10.1128/JVI.05359-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sonntag F, Schmidt K, and, Kleinschmidt JA. 2010. A viral assembly factor promotes AAV2 capsid formation in the nucleolus. Proc Natl Acad Sci USA 107:10220–10225. 10.1073/pnas.1001673107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ogden PJ, Kelsic ED, Sinai S, Church GM. 2019. Comprehensive AAV capsid fitness landscape reveals a viral gene and enables machine-guided design. Science 366:1139–1143. 10.1126/science.aaw2900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Grieger JC, Samulski RJ. 2005. Packaging capacity of adeno-associated virus serotypes: impact of larger genomes on infectivity and postentry steps. J Virol 79:9933–9944. 10.1128/JVI.79.15.9933-9944.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wu Z, Yang H, Colosi P. 2010. Effect of genome size on AAV vector packaging. Mol Ther 18:80–86. 10.1038/mt.2009.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Aucoin MG, Perrier M, Kamen AA. 2008. Critical assessment of current adeno-associated viral vector production and quantification methods. Biotechnol Adv 26:73–88. 10.1016/j.biotechadv.2007.09.001. [DOI] [PubMed] [Google Scholar]
- 26.Kotin RM. 2011. Large-scale recombinant adeno-associated virus production. Hum Mol Genet 20:R2–R6. 10.1093/hmg/ddr141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mietzsch M, Hering H, Hammer E-M, Agbandje-McKenna M, Zolotukhin S, Heilbronn R. 2017. OneBac 2.0: Sf9 cell lines for production of AAV1, AAV2, and AAV8 vectors with minimal encapsidation of foreign DNA. Hum Gene Ther Methods 28:15–22. 10.1089/hgtb.2016.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gurda BL, DiMattia MA, Miller EB, Bennett A, McKenna R, Weichert WS, Nelson CD, Chen W-j, Muzyczka N, Olson NH, Sinkovits RS, Chiorini JA, Zolotutkhin S, Kozyreva OG, Samulski RJ, Baker TS, Parrish CR, Agbandje-McKenna M. 2013. Capsid antibodies to different adeno-associated virus serotypes bind common regions. J Virol 87:9111–9124. 10.1128/JVI.00622-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gurda BL, Raupp C, Popa-Wagner R, Naumer M, Olson NH, Ng R, McKenna R, Baker TS, Kleinschmidt JA, Agbandje-McKenna M. 2012. Mapping a neutralizing epitope onto the capsid of adeno-associated virus serotype 8. J Virol 86:7739–7751. 10.1128/JVI.00218-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Raupp C, Naumer M, Müller OJ, Gurda BL, Agbandje-McKenna M, Kleinschmidt JA. 2012. The threefold protrusions of adeno-associated virus type 8 are involved in cell surface targeting as well as postattachment processing. J Virol 86:9396–9408. 10.1128/JVI.00209-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lochrie M, Tatsuno GP, Christie B, McDonnell JW, Zhou S, Surosky R, Pierce GF, Colosi P. 2006. Mutations on the external surfaces of adeno-associated virus type 2 capsids that affect transduction and neutralization. J Virol 80:831–824. 10.1128/JVI.80.2.821-834.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Govindasamy L, Padron E, McKenna R, Muzyczka N, Kaludov N, Chiorini JA, Agbandje-McKenna MS. 2006. Structurally mapping the diverse phenotype of adeno-associated virus serotype 4. J Virol 80:11556–11570. 10.1128/JVI.01536-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.DiMattia MA, Nam H-J, Van Vliet K, Mitchell M, Bennett A, Gurda BL, McKenna R, Olson NH, Sinkovits RS, Potter M, Byrne BJ, Aslanidi G, Zolotukhin S, Muzyczka N, Baker TS, Agbandje-McKenna M. 2012. Structural insight into the unique properties of adeno-associated virus serotype 9. J Virol 86:6947–6958. 10.1128/JVI.07232-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Govindasamy L, DiMattia MA, Gurda BL, Halder S, McKenna R, Chiorini JA, Muzyczka N, Zolotukhin S, Agbandje-McKenna M. 2013. Structural insights into adeno-associated virus serotype 5. J Virol 87:11187–11189. 10.1128/JVI.00867-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wu Z, Asokan A, Samulski RJ. 2006. Adeno-associated virus serotypes: vector toolkit for human gene therapy. Mol Ther 14:316–327. 10.1016/j.ymthe.2006.05.009. [DOI] [PubMed] [Google Scholar]
- 36.Colella P, Ronzitti G, Mingozzi F. 2018. Emerging issues in AAV-mediated in vivo gene therapy. Mol Ther Methods Clin Dev 8:87–104. 10.1016/j.omtm.2017.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mingozzi F, High KA. 2013. Immune responses to AAV vectors: overcoming barriers to successful gene therapy. Blood 122:23–36. 10.1182/blood-2013-01-306647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tseng YS, Agbandje-McKenna M. 2014. Mapping the AAV capsid host antibody response toward the development of second generation gene delivery vectors. Front Immunol 5:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Corti M, Elder ML, Falk DJ, Lawson L, Smith BK, Nayak S, Conlon TJ, Clément N, Erger K, Lavassani E, Green M, Doerfler PA, Herzog RW, Byrne BJ. 2014. B-cell depletion is protective against anti-AAV capsid immune response: a human subject case study. Mol Ther Methods Clin Dev 1:14033. 10.1038/mtm.2014.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Harbison CE, Weichert WS, Gurda BL, Chiorini JA, Agbandje-McKenna M, Parrish CR. 2012. Examining the cross-reactivity and neutralization mechanisms of a panel of mAbs against adeno-associated virus serotypes 1 and 5. J Gen Virol 93:347–355. 10.1099/vir.0.035113-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Li C, Narkbunnam N, Samulski RJ, Asokan A, Hu G, Jacobson LJ, Manco-Johnson MJ, Monahan PE, Joint Outcome Study Investigators. 2012. Neutralizing antibodies against adeno-associated virus examined prospectively in pediatric patients with hemophilia. Gene Ther 19:288–294. 10.1038/gt.2011.90. [DOI] [PubMed] [Google Scholar]
- 42.Tellez J, Van Vliet K, Tseng Y-S, Finn JD, Tschernia N, Almeida-Porada G, Arruda VR, Agbandje-McKenna M, Porada CD. 2013. Characterization of naturally-occurring humoral immunity to AAV in sheep. PLoS One 8:e75142. 10.1371/journal.pone.0075142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tseng Y-S, Gurda BL, Chipman P, McKenna R, Afione S, Chiorini JA, Muzyczka N, Olson NH, Baker TS, Kleinschmidt J, Agbandje-McKenna M. 2015. Adeno-associated virus serotype 1 (AAV1)- and AAV5-antibody complex structures reveal evolutionary commonalities in parvovirus antigenic reactivity. J Virol 89:1794–1808. 10.1128/JVI.02710-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hurlbut GD, Ziegler RJ, Nietupski JB, Foley JW, Woodworth LA, Meyers E, Bercury SD, Pande NN, Souza DW, Bree MP, Lukason MJ, Marshall J, Cheng SH, Scheule RK. 2010. Preexisting immunity and low expression in primates highlight translational challenges for liver-directed AAV8-mediated gene therapy. Mol Ther 18:1983–1994. 10.1038/mt.2010.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Calcedo R, Vandenberghe LH, Gao G, Lin J, Wilson JM. 2009. Worldwide epidemiology of neutralizing antibodies to adeno-associated viruses. J Infect Dis 199:381–390. 10.1086/595830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Boutin S, Monteilhet V, Veron P, Leborgne C, Benveniste O, Montus MF, Masurier C. 2010. Prevalence of serum IgG and neutralizing factors against adeno-associated virus (AAV) types 1, 2, 5, 6, 8, and 9 in the healthy population: implications for gene therapy using AAV vectors. Hum Gene Ther 21:704–712. 10.1089/hum.2009.182. [DOI] [PubMed] [Google Scholar]
- 47.Blacklow NR, Hoggan MD, Kapikian AZ, Austin JB, Rowe WP. 1968. Epidemiology of adenovirus-associated virus infection in a nursery population. Am J Epidemiol 88:368–378. 10.1093/oxfordjournals.aje.a120897. [DOI] [PubMed] [Google Scholar]
- 48.Blacklow NR, Hoggan MD, Sereno MS, Brandt CD, Kim HW, Parrott RH, Chanock RM. 1971. A seroepidemiologic study of adenovirus-associated virus infection in infants and children. Am J Epidemiol 94:359–366. 10.1093/oxfordjournals.aje.a121331. [DOI] [PubMed] [Google Scholar]
- 49.Erles K, Seböovà P, Schlehofer JR. 1999. Update on the prevalence of serum antibodies (IgG and IgM) to adeno-associated virus (AAV). J Med Virol 59:406–411. . [DOI] [PubMed] [Google Scholar]
- 50.Mingozzi F, Chen Y, Edmonson SC, Zhou S, Thurlings RM, Tak PP, High KA, Vervoordeldonk MJ. 2013. Prevalence and pharmacological modulation of humoral immunity to AAV vectors in gene transfer to synovial tissue. Gene Ther 20:417–424. 10.1038/gt.2012.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chirmule N, Propert K, Magosin S, Qian Y, Qian R, Wilson J. 1999. Immune responses to adenovirus and adeno-associated virus in humans. Gene Ther 6:1574–1583. 10.1038/sj.gt.3300994. [DOI] [PubMed] [Google Scholar]
- 52.Murphy SL, Li H, Mingozzi F, Sabatino DE, Hui DJ, Edmonson SA, High KA. 2009. Diverse IgG subclass responses to adeno-associated virus infection and vector administration. J Med Virol 81:65–74. 10.1002/jmv.21360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bertin B, Veron P, Leborgne C, Deschamps J-Y, Moullec S, Fromes Y, Collaud F, Boutin S, Latournerie V, van Wittenberghe L, Delache B, Le Grand R, Dereuddre-Bosquet N, Benveniste O, Moullier P, Masurier C, Merten O, Mingozzi F. 2020. Capsid-specific removal of circulating antibodies to adeno-associated virus vectors. Sci Rep 10:864. 10.1038/s41598-020-57893-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Leborgne C, Barbon E, Alexander JM, Hanby H, Delignat S, Cohen DM, Collaud F, Muraleetharan S, Lupo D, Silverberg J, Huang K, van Wittengerghe L, Marolleau B, Miranda A, Fabiano A, Daventure V, Beck H, Anguela XM, Ronzitti G, Armour SM, Lacroix-Desmazes S, Mingozzi F. 2020. IgG-cleaving endopeptidase enables in vivo gene therapy in the presence of anti-AAV neutralizing antibodies. Nat Med 26:1096–1101. 10.1038/s41591-020-0911-7. [DOI] [PubMed] [Google Scholar]
- 55.Pacak CA, Byrne BJ. 2011. AAV vectors for cardiac gene transfer: experimental tools and clinical opportunities. Mol Ther 19:1582–1590. 10.1038/mt.2011.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gray SJ, Matagne V, Bachaboina L, Yadav S, Ojeda SR, Samulski RJ. 2011. Preclinical differences of intravascular AAV9 delivery to neurons and glia: a comparative study of adult mice and nonhuman primates. Mol Ther 19:1058–1069. 10.1038/mt.2011.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Inagaki K, Fuess S, Storm TA, Gibson GA, Mctiernan CF, Kay MA, Nakai H. 2006. Robust systemic transduction with AAV9 vectors in mice: efficient global cardiac gene transfer superior to that of AAV8. Mol Ther 14:45–53. 10.1016/j.ymthe.2006.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Al-Zaidy SA, Mendell JR. 2019. From clinical trials to clinical practice: practical considerations for gene replacement therapy in SMA type 1. Pediatr Neurol 100:3–11. 10.1016/j.pediatrneurol.2019.06.007. [DOI] [PubMed] [Google Scholar]
- 59.Tseng Y-S, Vliet KV, Rao L, McKenna R, Byrne BJ, Asokan A, Agbandje-McKenna M. 2016. Generation and characterization of anti-adeno-associated virus serotype 8 (AAV8) and anti-AAV9 monoclonal antibodies. J Virol Methods 236:105–110. 10.1016/j.jviromet.2016.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bennett AD, Wong K, Lewis J, Tseng Y-S, Smith JK, Chipman P, McKenna R, Samulski RJ, Kleinschmidt J, Agbandje-McKenna M. 2018. AAV6 K531 serves a dual function in selective receptor and antibody ADK6 recognition. Virology 518:369–376. 10.1016/j.virol.2018.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.McCraw DM, O’Donnell JK, Taylor KA, Stagg SM, Chapman MS. 2012. Structure of adeno-associated virus-2 in complex with neutralizing monoclonal antibody A20. Virology 431:40–49. 10.1016/j.virol.2012.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Giles AR, Govindasamy L, Somanathan S, Wilson JM. 2018. Mapping an adeno-associated virus 9-specific neutralizing epitope to develop next-generation gene delivery vectors. J Virol 92:e01011-18. 10.1128/JVI.01011-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Jose A, Mietzsch M, Smith JK, Kurian J, Chipman P, McKenna R, Chiorini J, Agbandje-McKenna M. 2019. High-resolution structural characterization of a new adeno-associated virus serotype 5 antibody epitope toward engineering antibody-resistant recombinant gene delivery vectors. J Virol 93:e01394-18. 10.1128/JVI.01394-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Emmanuel SN, Mietzsch M, Tseng YS, Smith JK, Agbandje-McKenna M. 2021. Parvovirus capsid-antibody complex structures reveal conservation of antigenic epitopes across the family. Viral Immunol 34:3–17. 10.1089/vim.2020.0022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Adachi K, Enoki T, Kawano Y, Veraz M, Nakai H. 2014. Drawing a high-resolution functional map of adeno-associated virus capsid by massively parallel sequencing. Nat Commun 5:3075. 10.1038/ncomms4075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Shen S, Bryant KD, Brown SM, Randell SH, Asokan A. 2011. Terminal N-linked galactose is the primary receptor for adeno-associated virus 9. J Biol Chem 286:13532–13540. 10.1074/jbc.M110.210922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zhang R, Cao L, Cui M, Sun Z, Hu M, Zhang R, Stuart W, Zhao X, Yang Z, Li X, Sun Y, Li S, Ding W, Lou Z, Rao Z. 2019. Adeno-associated virus 2 bound to its cellular receptor AAVR. Nat Microbiol 4:675–682. 10.1038/s41564-018-0356-7. [DOI] [PubMed] [Google Scholar]
- 68.Zhang R, Xu G, Cao L, Sun Z, He Y, Cui M, Sun Y, Li S, Li H, Qin L, Hu M, Yuan Z, Rao Z, Ding W, Rao Z, Lou Z. 2019. Divergent engagements between adeno-associated viruses with their cellular receptor AAVR. Nat Commun 10:3760. 10.1038/s41467-019-11668-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Meyer NL, Hu G, Davulcu O, Xie Q, Noble AJ, Yoshioka C, Gingerich DS, Trzynka A, David L, Stagg SM, Chapman MS. 2019. Structure of the gene therapy vector, adeno-associated virus with its cell receptor, AAVR. Elife 8:e44707. 10.7554/eLife.44707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Havlik LP, Simon KE, Smith JK, Klinc KA, Tse LV, Oh DK, Fanous MM, Meganck RM, Mietzsch M, Kleinschmidt J, Agbandje-McKenna M, Asokan A. 2020. Coevolution of adeno-associated virus capsid antigenicity and tropism through a structure-guided approach. J Virol 94:e00976-20. 10.1128/JVI.00976-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Venkatakrishnan B, Yarbrough J, Domsic J, Bennett A, Bothner B, Kozyreva OG, Samulski RJ, Muzyczka N, McKenna R, Agbandje-McKenna M. 2013. Structure and dynamics of adeno-associated virus serotype 1 VP1-unique N-terminal domain and its role in capsid trafficking. J Virol 87:4974–4984. 10.1128/JVI.02524-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Salganik M, Aydemir F, Nam H-J, McKenna R, Agbandje-McKenna M, Muzyczka N. 2014. Adeno-associated virus capsid proteins may play a role in transcription and second-strand synthesis of recombinant genomes. J Virol 88:1071–1079. 10.1128/JVI.02093-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Aydemir F, Salganik M, Resztak J, Singh J, Bennett A, Agbandje-McKenna M, Muzyczka N. 2016. Mutants at the 2-fold interface of adeno-associated virus type 2 (AAV2) structural proteins suggest a role in viral transcription for AAV capsids. J Virol 90:7196–7204. 10.1128/JVI.00493-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Mietzsch M, Smith JK, Yu JC, Banala V, Emmanuel SN, Jose A, Chipman P, Bhattacharya N, McKenna R, Agbandje-McKenna M. 2020. Characterization of AAV-specific affinity ligands: consequences for vector purification and development strategies. Mol Ther Methods Clin Dev 19:362–373. 10.1016/j.omtm.2020.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Gabriel N, Hareendran S, Sen D, Gadkari RA, Sudha G, Selot R, Hussain M, Dhaksnamoorthy R, Samuel R, Srinivasan N, Srivastava A, Jayandharan GR. 2013. Bioengineering of AAV2 capsid at specific serine, threonine, or lysine residues improves its transduction efficiency in vitro and in vivo. Hum Gene Ther Methods 24:80–93. 10.1089/hgtb.2012.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Li E, Becker A, Stanley SL Jr.. 1989. Chinese hamster ovary cells deficient in N-acetylglucosaminyltransferase I activity are resistant to Entamoeba histolytica-mediated cytotoxicity. Infect Immun 57:8–12. 10.1128/iai.57.1.8-12.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sandro Q, Relizani K, Benchaouir R. 2019. AAV production using Baculovirus expression vector system. Methods Mol Biol 1937:91–99. 10.1007/978-1-4939-9065-8_5. [DOI] [PubMed] [Google Scholar]
- 78.Nass SA, Mattingly MA, Woodcock DA, Burnham BL, Ardinger JA, Osmond SE, Frederick AM, Scaria A, Cheng SH, O’Riordan CR. 2018. Universal method for the purification of recombinant AAV vectors of differing serotypes. Mol Ther Methods Clin Dev 9:33–46. 10.1016/j.omtm.2017.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Yan X, Sinkovits RS, Baker TS. 2007. AUTO3DEM: an automated and high throughput program for image reconstruction of icosahedral particles. J Struct Biol 157:73–82. 10.1016/j.jsb.2006.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Yan X, Dryden KA, Tang J, Baker TS. 2007. Ab initio random model method facilitates 3D reconstruction of icosahedral particles. J Struct Biol 157:211–225. 10.1016/j.jsb.2006.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Pettersen EF, Goddard TD, Huang CC, Couch GS, Greenblatt DM, Meng EC, Ferrin TE. 2004. UCSF Chimera: a visualization system for exploratory research and analysis. J Comput Chem 25:1605–1612. 10.1002/jcc.20084. [DOI] [PubMed] [Google Scholar]
- 82.Emsley P, Lohkamp B, Scott WG, Cowtan K. 2010. Features and development of Coot. Acta Crystallogr D Biol Crystallogr 66:486–501. 10.1107/S0907444910007493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Carrillo-Tripp M, Shepherd CM, Borelli IA, Venkataraman S, Lander G, Natarajan P, Johnson JE, Brooks CL, Reddy VS. 2009. VIPERdb2: an enhanced and web API enabled relational database for structural virology. Nucleic Acids Res 37:D436–D442. 10.1093/nar/gkn840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Krissinel E, Henrick K. 2007. Inference of macromolecular assemblies from crystalline state. J Mol Biol 372:774–797. 10.1016/j.jmb.2007.05.022. [DOI] [PubMed] [Google Scholar]
- 85.Xiao C, Rossmann MG. 2007. Interpretation of electron density with stereographic roadmap projections. J Struct Biol 158:182–187. 10.1016/j.jsb.2006.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Tse LV, Klinc KA, Madigan VJ, Castellanos Rivera RM, Wells LF, Havlik LP, Smith JK, Agbandje-McKenna M, Asokan A. 2017. Structure-guided evolution of antigenically distinct adeno-associated virus variants for immune evasion. Proc Natl Acad Sci USA 114:E4812–E4821. 10.1073/pnas.1704766114. [DOI] [PMC free article] [PubMed] [Google Scholar]