Abstract
Background
The relative anatomical understanding of the perirectal fasciae is of paramount importance for the proper performance of total mesorectal excision (TME). This study was to demonstrate the planes of TME and validates the intraoperative findings using cadaveric observations.
Methods
In this combined retrospective and prospective study, bilateral attachment of the rectosacral fascia (RSF) was observed in 28 cadaveric specimens (male, n = 14; female, n = 14). From January 2018 to December 2019, surgical videos of 67 patients who underwent laparoscopic TME at the Affiliated Union Hospital of Fujian Medical University (Fuzhou, China) were reviewed and interpreted with the cadaveric findings.
Results
The RSF (synonym: Waldeyer's fascia) is the end of the pre-hypogastric fascia at the level of S4 and comprises two layers (upper and lower). These two layers provide double fascial protection for the venous sacral plexus. It inserts into the fascia propria of the rectum along a broad horizontal arc that merges anterolaterally in an oblique downward direction until it meets the posterolateral merge of Denonvilliers' fascia at the lateral rectal ligament (LRL). This ligament does not look like a true ligament but is more likely to be a fascial combination that cushions the rectal innervation and middle rectal vessels.
Conclusions
Understanding the lateral attachment of RSF and its contribution to LRL provides invaluable surgical guidance to dissect this critical area. Therefore, lateral dissection is proposed from the anterior to the posterior direction to find the correct plane that guarantees an intact mesorectal envelope to protect the important nearby nerve structures.
Keywords: laparoscopy, anatomy, rectum, fascia, rectal neoplasms
Introduction
The concept of anatomical understanding is the basis of surgical interventions regardless of the type of operative intervention [1]. Therefore, anatomical research should be continually advanced to provide surgeons with more detailed anatomical descriptions [2]. Identification and dissection of the perirectal fasciae in the proper planes can prevent rectal perforation anteriorly, injury of the autonomic pelvic plexus laterally, or bleeding from the sacral plexus of veins posteriorly [3–5].
The total mesorectal excision (TME), which involves a sharp dissection within the perirectal fascial planes, decreases the local recurrence rate of rectal cancer to <10% [6]. Therefore, the relationship between the fascial planes around the rectum is vital in identifying the best plane for circumferential rectum dissections [7].
The mesorectum is covered by a visceral fascia [8] or the fascia propria of the rectum (FPR) [9]. Additionally, the pre-hypogastric fascia and the presacral fascia have been indicated as being dorsal to the FPR [10]. The rectosacral fascia (RSF) was described as a fascial layer connecting to the FPR at the level of the third/fourth sacral vertebrae (S3–S4) [11–13] and divides the retrorectal space (RRS) into the presacral space (PSS) and the precoccygeal space (PCS), representing the cranial and caudal portions, respectively [14].
Although the RSF is of paramount clinical significance [15, 16], some textbooks focusing on colorectal surgery are still deficient in its anatomical description [17, 18]. When the RRS is dissected, the RSF can be cut and the PSS connects to the PCS, without disruption of the caudal part of the FPR [13, 19, 20]. However, this definition only describes the morphology of the RSF behind the rectum; its lateral attachment is still not well described.
Despite being a critical anatomical area for rectal surgeons, surgical literature is still needed to describe the detailed surgical anatomy of the lateral fascial compartment and the relationship among fascial layers around the rectum. Thus, this study observed the topographic morphology of the RSF and the lateral fascial compartment during rectal dissection by high-definition laparoscopic/robotic TME operations. Furthermore, intraoperative findings were validated with cadaveric specimens to clarify the optimal safe surgical plane of this region.
Patients and methods
Clinical data collection
Surgical videos of 67 patients with rectal cancer who underwent TME between January 2019 and December 2019 at the Affiliated Union Hospital of Fujian Medical University (Fuzhou, China) were reviewed retrospectively. Videos of laparoscopic TME, in which dissection reached the terminal end of the mesorectum distally at the levator hiatus, were included. The operations were conducted by two of the most experienced surgeons in our department. Reviewing the operative videos was achieved by the surgical team to translate the findings into the applied clinical description. Accordingly, the surgical videos were blindly examined by two qualified surgeons (W.M.G. and X.W.) while the final results were checked and approved by the senior author (P.C.).
While dissecting the RRS, a monopolar hook is routinely used to dissect the inter-fascial plane until reaching the levator hiatus level. However, in some exceptional situations, when the RSF is relatively thick, the ultrasonic knife (harmonic) is used to cut the fascia carefully to avoid any chance of injury/bleeding from the sacral plexus of veins.
Cadaveric specimen examination
Twenty-eight fixed cadaveric specimens (male, n = 14; female, n = 14) were examined at the Laboratory of Clinical Applied Anatomy at Fujian Medical University (Fuzhou, China). The specimens were hemipelvis cut and viewed in the midsagittal plane. Only recently, fixed adult cadavers were included to reduce the bias that postmortem changes might cause. The fixation procedure was performed entirely under the supervision of the director of the human anatomy department. All cadavers were fixed by injection of 10% formalin into the femoral artery. The dissection was performed using common surgical instruments (tissue clamps, smooth forceps, scalpel, scissors) with the assistance of binocular loupes (Yiwu Suolan Appliance Company, China) to better examine anatomical details (magnification power up to 10×). The classical steps of top-to-bottom TME and complete rectal mobilization were performed. Specimens with a history of previous pelvic disease or pelvic surgery were excluded because the anatomical architecture might be disturbed in such cases.
Ethical approval
The affiliated ethical committee has approved our study (No. 2020KY051). The cadaveric specimens were all treated according to the ethical regulations of Fujian Medical University to be used only for educational and research purposes.
Results
Intraoperative observation
The structure of the RSF was successfully recognized during surgery in all patients operated on for rectal cancer (100%) and its anatomical course was also observed.
During the dissection of the presacral portion of the RRS, there is a very well-defined surgical plane between the FPR and the parietal pelvic fascia (synonym: pre-hypogastric fascia [PHF]). Moving distally below the level of the peritoneal reflection, the surgical plane gradually becomes ill-defined and the loose connective tissue disappears suddenly. This molded part of the fascia exhibits some resistance to dissection and the plane becomes unclear accordingly. At that level, the RSF exists. However, with experienced surgeons, if dissection continued in direct contact with the FPR, the pneumoperitoneum is sufficient to reveal the RRS. By remaining in direct contact with the FPR, it is not necessary to identify the RSF (Supplemental video).
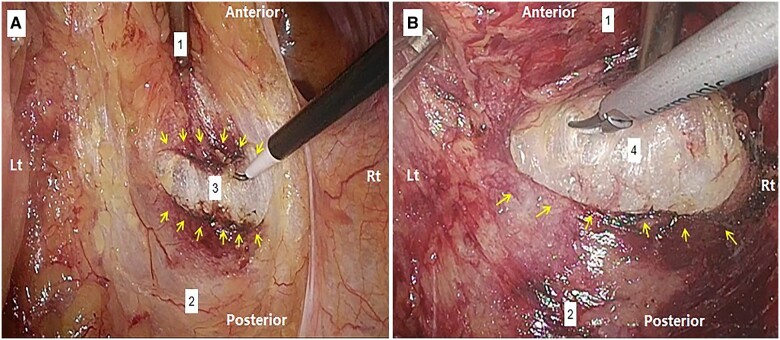
The rectum should be moved up and down during surgery to allow the surgeon to recognize the faint plane between the RSF and the FPR to cut it accurately from the posterior aspect (at 6 o'clock). The RSF was observed to have two well-defined layers, namely the upper and lower layers, in 35/67 (52.2%) patients. The recognition of those two layers depends, to a great extent, on the speed and dissection depth. The slower and more superficial the dissection of the upper layer, the easier it is to recognize the lower layer. At that level, slow dissection allows time for the laparoscopic air to enter between the two layers, causing pneumodissection that pushes the lower layer slightly deeper. Thus, the layers separate, becoming clearer and more recognizable. After that, once the lower layer is cut, a loose piece of connective tissue with a snowy appearance is seen and the PCS can be entered thereafter (Figure 1). Thereafter, the distal dissection is advanced in front of the presacral fascia until the pelvic floor is reached. The RSF posteriorly attaches to the FPR. Upon merging anterolaterally on both sides, the RSF seems to join a dense fascial component, which is the site of the lateral rectal ligament (LRL). Therefore, it is a bit risky to dissect this area continuously from posterior to anterior, as it may obscure the surgical plane and cause a likelihood of perforating the FPR, resulting in the mesorectal residue.
Figure 1.
The dissection of the posterior attachment of the rectosacral (Waldeyer’s) fascia (RSF) to the fascia propria of the rectum (FPR); entry into the precoccygeal space (PCS) (note the snowy appearance of the loose connective tissue) (the figure picked from the same patient). (A) Upper layer of the RSF; (B) lower layer of the RSF. Yellow arrows: the broad horizontal arc-like insertion of the RSF; 1: FPR enveloping mesorectum; 2: pre-hypogastric fascia (PHF); 3: space between the two layers of RSF; 4: PCS.
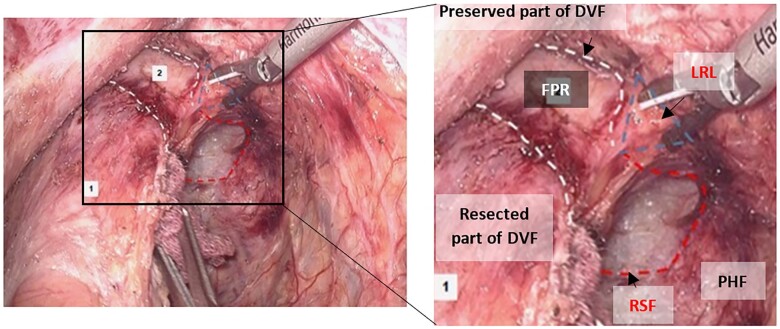
The relation among the RSF, Denonvilliers’ fascia (DVF), and the LRL was observed in patients who underwent TME with partial preservation of the DVF. In this technique, the DVF is approached through the following: (i) incision 1 cm above and anterior to the peritoneal reflection; (ii) U-shaped excision of the DVF 0.5 cm above the seminal vesicle base; (iii) dissection behind the DVF. At the site where the DVF is transected, the edge of the preserved part of the DVF (anterior) seems to meet the edge of the RSF (posterior) at a lateral zone with a bulky fascial composition (synonym: LRL).
Accordingly, this zone was looked at like a triangle with its apex pointed toward the mesorectum and its base toward the pelvic sidewall. It seems to be an area where three fasciae meet the first layer of the DVF (anterior), RSF (posterior), and PHF (the base of the triangle). At that level, the pelvic plexus emerging from S2:S4 is covered by a gray-white fascia (PHF), while the first layer of the DVF traverses the fusion/meeting zone and attaches to it. When the rectum is laterally mobilized, the first layer of the DVF is encountered as a tough fascial layer that must be cut gradually (Figure 2). Dissection should first be performed from the anterior aspect in a downward direction and then from the posterior part in an upward direction until the neurovascular bundle (NVB) of Walsh [21] protection is ensured.
Figure 2.
The right margin of the rectosacral fascia (RSF) (laparoscopic view). Red dashes: RSF; white dashes: Denonvilliers’ fascia (DVF) (partially preserved); blue triangle: fascial-fusion zone (lateral rectal ligament [LRL]); 1: the rectum (covered by the resected part of DVF); 2: fascia propria of the rectum (FPR) (intact); PHF, pre-hypogastric fascia.
The FPR is dissected toward the RRS posteriorly, and the mesorectum is observed from the left side and checked for FPR integrity. The left and right fascial zones have symmetrical anatomical compositions.
Cadaveric observation
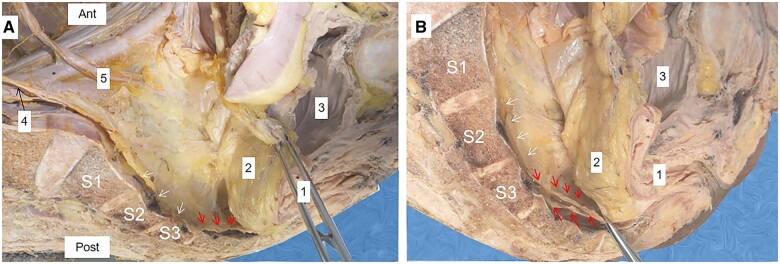
The RSF was observed in the RRS of 28 cadaveric hemipelvis specimens (lateral sagittal view). It was visible the end of the PHF, which holds the hypogastric nerve and the ureter (Figure 4A). At S4 of 26 (93%) cadaveric specimens, the RSF produces two layers (upper and lower) (Figure 3) and both have a broad arc-like insertion around the posterior and posterolateral aspects of the FPR (Figure 6A and C) (Supplemental video).
Figure 4.
Rectosacral fascia (RSF) at the S3 level (individual variation). (A) Before separation of the two layers of the RSF; (B) after separation of the two layers of the RSF. Red arrows: upper and lower layers of the RSF; white arrows: pre-hypogastric fascia; 1: the rectum; 2: the mesorectum; 3: the urinary bladder; 4: the hypogastric nerve; 5: the ureter.
Figure 3.
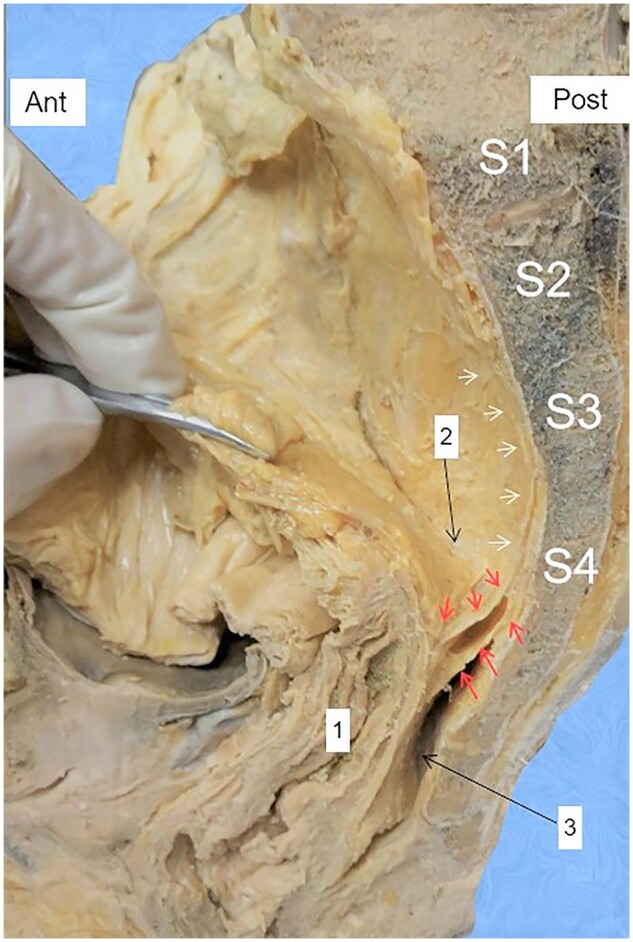
The rectosacral fascia (RSF) dividing the retrorectal space (RRS). Red arrows: upper and lower layers of the RSF; white arrows: pre-hypogastric fascia; 1: rectum; 2: presacral space; 3: precoccygeal space; 2 + 3: RRS.
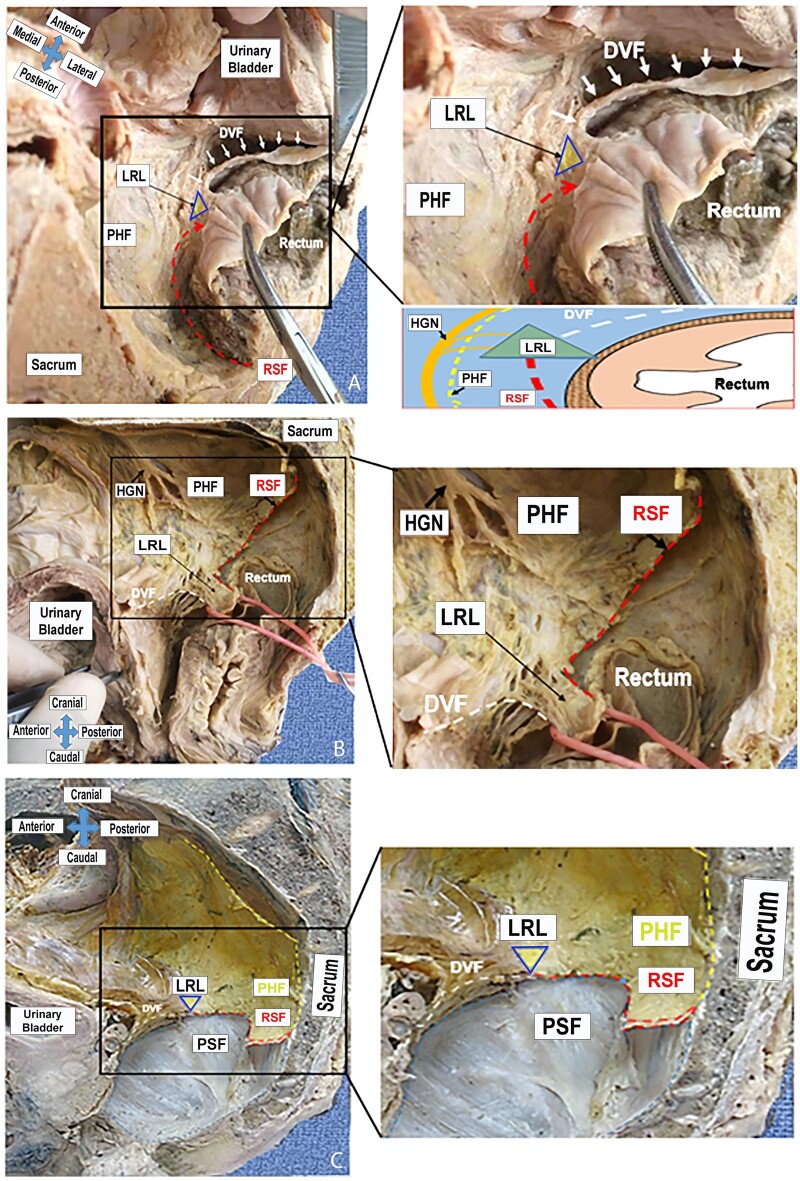
Figure 6.
Bilateral attachment of the posterior and anterior rectal fasciae. (A) Transverse view; (B) lateral view; (C) lateral view (the rectum is resected). White dashes: Denonvilliers’ fascia (DVF); red dashes: the rectosacral (Waldeyer’s) fascia (RSF); yellow dashes: pre-hypogastric fascia (PHF); triangle: lateral rectal ligament (LRL); HGN, hypogastric nerve; PSF, presacral fascia.
The appearance of the two layers was found in all cadavers. However, two types of individual normal variations were discovered in two cadaveric specimens. In the type-one variation, both layers of the RSF originated from the base of the S3 vertebral body (3.5%) (Figure 4B). In the type-two variation, which was found in one specimen (3.5%), the upper and lower layers originated from the S4 and S5 levels, respectively. Meanwhile, the PHF extends downward and attaches to the FPR near the level of the levator hiatus (Figure 5).
Figure 5.
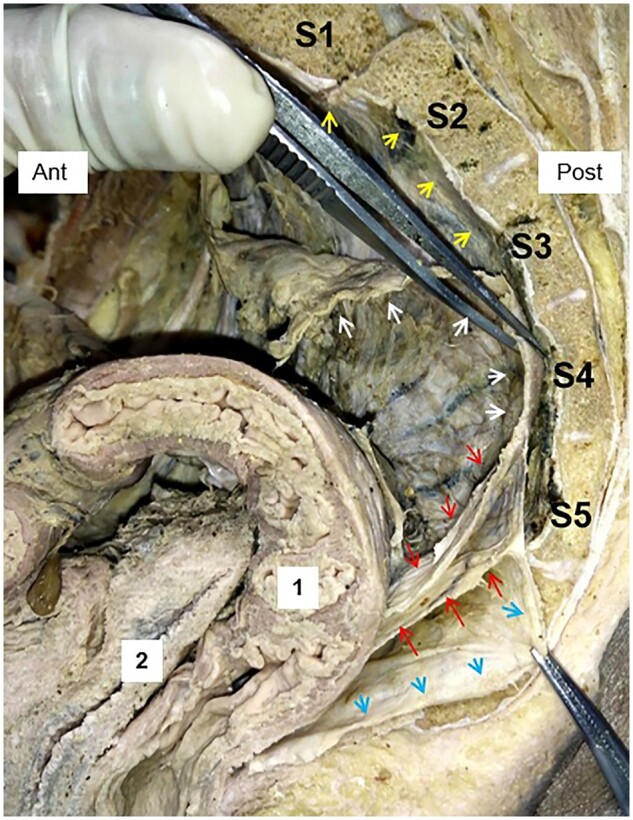
Downward extension of pre-hypogastric fascia (PHF) (individual variation). Red arrows: upper and lower layers of the rectosacral fascia (RSF); white arrows: PHF; blue arrows: the extended part of the PHF; yellow arrows: the presacral fascia (PSF); 1: the rectum; 2: the vagina.
As mentioned in the operative findings, the hypothesized bilateral fascial-fusion/meeting zone looks like a triangle in the horizontal position. The apex pointed toward the FPR, while the base faced the lateral pelvic sidewall formed by the PHF. The anterior fascial side of the triangle was formed by a merger with the posterolateral aspect of the DVF, the lateral fascial side was the PHF, and a merger with the RSF formed the posterior fascial side. With detailed dissection and transection of the rectum at the attachment site of the RSF, the RSF running from the superior and posterior to inferior and anterior was observed until it joined the lateral zone, where it met the PHF laterally and the anterior layer of the DVF anteriorly (Figure 6).
Discussion
The harmonization of cadaveric demonstration and intraoperative interpretation enhances anatomical understanding for surgeons and hastens the learning process accordingly [22]. However, there is a discrepancy between the dissection instruments used in cadaveric dissection and operative dissection. During real operations, dissection should be performed using monopolar or vessel sealing instruments, which may induce tissue fusion. Dealing with fascial layers of different thicknesses, this artificial fusion may mislead the surgical planes. While common surgical instruments used in cadaveric dissection (scalpel, scissors, etc.) can perform sharp dissection, and each distinct fascial layer can be dissected separately for research, thin fasciae need skillful and very cautious dissection otherwise they can be cut and missed easily.
This study delves into the anatomy of the perirectal fasciae, revealing how distinct fascial layers engage with the LRL. The awareness of the RSF architecture and the lateral fusion zones (LRL) allows a thorough understanding of the many perirectal anatomical concepts. As a result, surgeons will better understand the surgical planes for TME, which is of paramount importance for a better quality of surgical resection. Additionally, highlighting the surgical planes where the perirectal fascial layers combine would lower the unnecessary bleeding from the lateral and posterior adjacent vessels.
The RSF
In this study, the validation of cadaveric findings with intraoperative findings was potentially of great clinical value in pursuing high-quality TME. Understanding the concept of the two layers of the RSF may lower the potential risk of dissecting through the mesorectal tissue anteriorly or the presacral fascia posteriorly, causing inevitable venous bleeding. Moreover, these two layers provide double protection to the sacral venous plexus. Alternatively, the lower layer may pose challenges if it is not perpendicularly cut and the snowy connective tissue of the PCS appears. If the surgeon dissects the lower layer obliquely, part of the FPR may be peeled out.
Prof. H. Fritsch conducted anatomical and embryological investigations on the developmental changes of the RRS [14, 23, 24]. In 20-week-old embryos, the parietal pelvic fascia has been demonstrated, while dividing the RRS into the PSS and the PCS. However, it was not reported to have a certain number of layers. Compared with our findings, the traversed part of the parietal pelvic fascia may show the RSF described in this study, while the two layers of the RSF are perhaps formed later, sometime during the postnatal life.
This study highlights the concept of broad insertion of the RSF as a horizontal arc-like two-layered structure attached to the posterior and posterolateral aspects of the FPR as observed in the retrospective intraoperative video review (Figure 1) and confirmed with cadaveric dissection (Supplemental video). It resembles, to a great extent, what was reported by García-Armengol et al. [25] and Chen and Liang [26], who highlighted that the RSF originated from the presacral fascia dividing the RRS into upper and lower parts, while varying from what Wilhelm Waldeyer described [12, 27], as they claimed that the RSF traveled caudally with the FPR until 3–4 cm proximal to the anorectal junction. In the topographic view of laparoscopic and robotic surgery, the RSF may look like two distinct structures. Still, this study provides another view from the sagittal aspect through cadaveric dissection, which indicates that these two layers are related to each other and both represent the same fascia while attaching to the FPR.
Paradoxically, this concept does not fit this knowledge. For instance, Sato and Sato [28] described the RSF in 36 pelvic halves. They found only a loose connection toward the LRL. Recently, the RSF was described as tissue condensation in the midline [29].
Jin et al. [6] reported that the RSF had two leaves that divided the RRS into two spaces and extended between the FPR and the presacral fascia. Some researchers have reported presacral (retrorectal) tumors in the potential space between the two components of the RSF. Still, they believed that the tumors originated from the presacral fascia rather than the PHF [30, 31].
The RSF was reported to start at the S2 vertebra in 15% of people, S3 in 38%, and S4 in 46% [25], while this study found that most of the cadaveric specimens had the fascia originating from S4 (93%). The RSF was reported as a rectosacral ligament that was claimed to divide the space behind the rectum into two non-communicating chambers: the retrorectal space and the supralevator space (synonym: the PSS and PCS described in this study, respectively) [32]. However, this traditional definition merely describes the shape of the RSF behind the rectum and does not describe its topographic lateral attachment.
Prof. Kinugasa and colleagues [10] described the difference in histological structure between the PHF and the presacral fascia, which was a novel description at that time. To a great extent, the authors depended on histological examination of cadaveric specimens, which made the clinical importance of their findings unclear. They were against the concept of the existence of any fascia between the presacral fascia and the FPR. Moreover, the two-layered structure of the RSF or its lateral attachment could not be well demonstrated.
The LRL
The LRL is defined differently in several textbooks of anatomy [33]. For instance, the bilateral ligaments were described by Gardner, Grey, and O’Rahilly as “two other condensations of connective tissue, in which are embedded the middle rectal arteries and plexuses, connect the rectum to the parietal pelvic fascia” [34], while Paulsen and Waschke have not defined the term of the LRL and it was stated that the mesorectal fascia (synonym: FPR mentioned in this study) forms a pathway for the NVB whereas a connective tissue envelops the pelvic plexus obtained from the parietal pelvic fascia [35].
This study hypothesizes that the lateral fascial-fusion zone, known as the “LRL,” is not an actual ligament. The pelvic plexus tends to send rectal innervation alongside the middle rectal vessels through this structure, which should be considered while performing TME (Supplemental Figure 1). This concept was compatible with Sato and Sato's study that clearly indicated that the so-called lateral ligament is not a true ligament, but rather a pathway for nerves and blood vessels on either side [28].
There is an embryologically based concept that the LRL has no definite borders. In young fetuses, the rectal adventitia grows from a layer of condensed mesenchyme to a thick connective tissue septa-divided layer. The rectum-supplying vessels are included within the perirectal compartment [23, 24]. Additionally, several studies have reported that the LRL is a composite of condensed fascia rather than an actual ligament [28, 36–39], which supports this study hypothesis.
Ishii et al. [40] reappraised the LRL through a cadaveric demonstration that was largely compatible with our findings. They claimed that the RSF is the structure connecting the PHF to the FPR and contributing to lateral fascial fusion (synonym: LRL). However, the anatomical details of the lateral attachment of the RSF and its two layers and clinical implications were not clearly discussed.
The anatomical site of the LRL is still a controversial point. Some studies have claimed that it was located on the anterolateral aspect of the rectum [38, 39], while other studies mentioned that it was found posterolaterally [41, 42]. Although some previous studies reported that the LRL was not a true ligament and appeared to be an extension of the mesorectum, as they claimed that the FPR and PHF (also called the parietal pelvic fascia) were the same layer [43], they overlooked the fascial-fusion zone hypothesized in this study.
This study examined fixed cadavers rather than fresh cadavers, depending on the availability at our institute. Thus, it has an inherent limitation that may be caused by postmortem degenerative changes. Additionally, this study focused mainly on gross topography relevant to the surgical anatomy so that histological examinations, similar to the previously published reports, were not conducted; this may be considered a limitation. However, it would be the foundation of future goals to be achieved for further histological investigations.
Conclusions
The RSF is commonly considered the end of the PHF at the level of S4 and consists of two layers (upper and lower). It attaches to the FPR along a horizontal arc that merges anterolaterally in an obliquely downward direction until it meets the posterolateral merger of the DVF at the hypothesized lateral fusion/meeting zone, known as the LRL. This ligament is not a true ligament but looks like a fascial combination that cushions the rectal innervation and middle rectal vessels. The PHF is a separate fascial layer between the FPR anteriorly and the presacral fascia posteriorly, holding the hypogastric nerve and the ureter within its fibers. The overall anatomical view of this region may provide invaluable surgical guidance for dissecting this difficult and critical part of the TME from anterior to posterior and finding the correct plane that guaranties intact FPR integrity and protects the essential nearby nerve structures.
Supplementary Data
Supplementary data is available at Gastroenterology Report online.
Authors’ Contributions
W.M.G., X.W., and P.C. conceived of and designed the project. W.M.G., X.W., and Z.Z. collected the data. W.M.G., X.W., and Z.Z. analysed and interpreted the data. W.M.G. drafted the manuscript. X.Z. supervised cadaveric dissection and revised the manuscript for intellectual content. All authors read and approved the final manuscript.
Funding
This work was supported by the Chinese Scholarship Council (CSC) [No. 2017DFH010880] and the Fujian provincial health technology project (2021GGA013).
Supplementary Material
Acknowledgements
The authors are so grateful for the deceased people and their families who decided to encourage the research field by donation of their bodies after death.
Conflict of Interest
None declared.
References
- 1. Heald R, Husband E, Ryall R.. The mesorectum in rectal cancer surgery—the clue to pelvic recurrence? Br J Surg 1982;69:613–6. [DOI] [PubMed] [Google Scholar]
- 2. Heald R, Karanjia N.. Results of radical surgery for rectal cancer. World J Surg 1992;16:848–57. [DOI] [PubMed] [Google Scholar]
- 3. Quirke P, Dixon MF, Durdey P. et al. Local recurrence of rectal adenocarcinoma due to inadequate surgical resection: histopathological study of lateral tumour spread and surgical excision. Lancet 1986;328:996–9. [DOI] [PubMed] [Google Scholar]
- 4. Havenga K, DeRuiter MC, Enker WE. et al. Anatomical basis of autonomic nerve-preserving total mesorectal excision for rectal cancer. Br J Surg 1996;83:384–8. [DOI] [PubMed] [Google Scholar]
- 5. Sugihara K, Moriya Y, Akasu T. et al. Pelvic autonomic nerve preservation for patients with rectal carcinoma: oncologic and functional outcome. Cancer 1996;78:1871–80. [PubMed] [Google Scholar]
- 6. Jin Z-M, Peng J-Y, Zhu Q-C. et al. Waldeyer’s fascia: anatomical location and relationship to neighboring fasciae in retrorectal space. Surg Radiol Anat 2011;33:851–4. [DOI] [PubMed] [Google Scholar]
- 7. Heald R, Ryall R.. Recurrence and survival after total mesorectal excision for rectal cancer. Lancet 1986;327:1479–82. [DOI] [PubMed] [Google Scholar]
- 8. Heald RJ, Moran BJ.. Embryology and anatomy of the rectum. Semin Surg Oncol 1998;15:66–71. [DOI] [PubMed] [Google Scholar]
- 9. Bissett IP, Hill GL (eds). Extrafascial excision of the rectum for cancer: a technique for the avoidance of the complications of rectal mobilization. Semin Surg Oncol2000;18:207–15. [DOI] [PubMed] [Google Scholar]
- 10. Kinugasa Y, Murakami G, Suzuki D. et al. Histological identification of fascial structures posterolateral to the rectum. Br J Surg 2007;94:620–6. [DOI] [PubMed] [Google Scholar]
- 11. Waldeyer W, Becken D; Bonn: Cohen, 1899, 226–98.
- 12. Crapp A, Cuthbertson AJS.. Gynecology, obstetrics. Surg Gynecol Obstet 1974;138:252–6. [PubMed] [Google Scholar]
- 13. Church JM, Raudkivi PJ, Hill GL.. The surgical anatomy of the rectum—a review with particular relevance to the hazards of rectal mobilization. Int J Colorectal Dis 1987;2:158–66. [DOI] [PubMed] [Google Scholar]
- 14. Fritsch HJA. Developmental changes in the retrorectal region of the human fetus. Anat Embryol (Berl) 1988;177:513–22. [DOI] [PubMed] [Google Scholar]
- 15. Enker WE, Kafka NJ, Martz J (eds). Planes of sharp pelvic dissection for primary, locally advanced, or recurrent rectal cancer. Semin Surg Oncol2000;18:199–206. [DOI] [PubMed] [Google Scholar]
- 16. Atallah S, Albert M.. The neurovascular bundle of Walsh and other anatomic considerations crucial in preventing urethral injury in males undergoing transanal total mesorectal excision. Tech Coloproctol 2016;20:411–2. [DOI] [PubMed] [Google Scholar]
- 17. Gordon PH, Nivatvongs S.. Principles and Practice of Surgery for the Colon, Rectum, and Anus. Boca Raton, Florida: CRC Press, 2007. [Google Scholar]
- 18. Steele SR, Hull TL, Read TE. et al. The ASCRS Textbook of Colon and Rectal Surgery. New York: Springer, 2016. [Google Scholar]
- 19. Muntean VJS, Anatomy R.. The surgical anatomy of the fasciae and the fascial spaces related to the rectum. Surg Radiol Anat 1999;21:319–24. [DOI] [PubMed] [Google Scholar]
- 20. Kraima AC, West NP, Treanor D. et al. Understanding the surgical pitfalls in total mesorectal excision: investigating the histology of the perirectal fascia and the pelvic autonomic nerves. Eur J Surg Oncol 2015;41:1621–9. [DOI] [PubMed] [Google Scholar]
- 21. Walsh PC, Lepor H, Eggleston JC.. Radical prostatectomy with preservation of sexual function: anatomical and pathological considerations. Prostate 1983;4:473–85. [DOI] [PubMed] [Google Scholar]
- 22. Ghareeb WM, Wang X, Chi P. et al. The “multilayer” theory of Denonvilliers’ fascia: anatomical dissection of cadavers with the aim to improve neurovascular bundle preservation during rectal mobilization. Colorectal Dis 2020;22:195–202. [DOI] [PubMed] [Google Scholar]
- 23. Fritsch HJS, Anatomy R.. Topography and subdivision of the pelvic connective tissue in human fetuses and in the adult. Surg Radiol Anat 1994;16:259–65. [DOI] [PubMed] [Google Scholar]
- 24. Fritsch H, Hötzinger H.. Tomographical anatomy of the pelvis, visceral pelvic connective tissue, and its compartments. Clin Anat 1995;8:17–24. [DOI] [PubMed] [Google Scholar]
- 25. García‐Armengol J, García‐Botello S, Martinez‐Soriano F. et al. Review of the anatomic concepts in relation to the retrorectal space and endopelvic fascia: Waldeyer’s fascia and the rectosacral fascia. Colorectal Disease 2008;10:298–302. [DOI] [PubMed] [Google Scholar]
- 26. Chen TC, Liang JT.. Revisiting rectosacral and Waldeyer's fascia by laparoscopic or robotic approach—video vignette. Colorectal Dis 2018;20:254–5. [DOI] [PubMed] [Google Scholar]
- 27. Waldeyer W. Das Becken: Topographisch-Anatomisch mit Besonderer Berücksichtigung der Chirurgie und Gynäkologie. Bonn: Cohen, 1899.
- 28. Sato K, Sato T.. The vascular and neuronal composition of the lateral ligament of the rectum and the rectosacral fascia. Surg Radiol Anat 1991;13:17–22. [DOI] [PubMed] [Google Scholar]
- 29. Stelzner S, Heinze T, Nikolouzakis TK. et al. Perirectal fascial anatomy: new insights into an old problem. Dis Colon Rectum 2021;64:91–102. [DOI] [PubMed] [Google Scholar]
- 30. Marecik SJ, Al-Khamis A, Kochar K. et al. Robotic resection of presacral (retrorectal) tumors and Waldeyer’s fascia. Dis Colon Rectum 2018;61:e351–2. [DOI] [PubMed] [Google Scholar]
- 31. Messick CA. Presacral (retrorectal) tumors: optimizing the management strategy. Dis Colon Rectum 2018;61:151–3. [DOI] [PubMed] [Google Scholar]
- 32. Chen N, Min PQ, Liu ZY. et al. Radiologic and anatomic study of the extraperitoneal space associated with the rectum. AJR Am J Roentgenol 2010;194:642–52. [DOI] [PubMed] [Google Scholar]
- 33. Pak-Art R, Tansatit T, Mingmalairaks C. et al. The location and contents of the lateral ligaments of the rectum: a study in human soft cadavers. Dis Colon Rectum 2005;48:1941–4. [DOI] [PubMed] [Google Scholar]
- 34. Gardner E, J Gray D, O'Rahilly R.. Anatomy: A Regional Study of Human Structure. Philadelphia: WB Saunders, 1963. [Google Scholar]
- 35. Paulsen F, Waschke J.. Sobotta Atlas of Human Anatomy, Vol. 2, English. Internal Organs: Elsevier Health Sciences, 2013. [Google Scholar]
- 36. Takahashi T, Ueno M, Azekura K. et al. (eds). Lateral ligament: its anatomy and clinical importance. Semin Surg Oncol, 2000;19:386–95. [DOI] [PubMed] [Google Scholar]
- 37. Stelzner S, Holm T, Moran BJ. et al. Deep pelvic anatomy revisited for a description of crucial steps in extralevator abdominoperineal excision for rectal cancer. Dis Colon Rectum 2011;54:947–57. [DOI] [PubMed] [Google Scholar]
- 38. Runkel N, Reiser H.. Nerve-oriented mesorectal excision (NOME): autonomic nerves as landmarks for laparoscopic rectal resection. Int J Colorectal Dis 2013;28:1367–75. [DOI] [PubMed] [Google Scholar]
- 39. Zhou H, Ruan C, Sun Y. et al. Nerve-guided laparoscopic total mesorectal excision for distal rectal cancer. Ann Surg Oncol 2015;22:550–1. [DOI] [PubMed] [Google Scholar]
- 40. Ishii M, Shimizu A, Lefor AK. et al. Reappraisal of the lateral rectal ligament: an anatomical study of total mesorectal excision with autonomic nerve preservation. Int J Colorectal Dis 2018;33:763–9. [DOI] [PubMed] [Google Scholar]
- 41. Nano M, Dal Corso H, Lanfranco G. et al. Contribution to the surgical anatomy of the ligaments of the rectum. Dis Colon Rectum 2000;43:1592–7. [DOI] [PubMed] [Google Scholar]
- 42. Keighley MR, Williams NS.. Keighley & Williams' Surgery of the Anus, Rectum and Colon: Two-Volume Set. Boca Raton, Florida: CRC Press, 2018. [Google Scholar]
- 43. Zhang C, Ding Z-H, Li G-X. et al. Perirectal fascia and spaces: annular distribution pattern around the mesorectum. Dis Colon Rectum 2010;53:1315–22. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.