Abstract
Background
The uptake of colonoscopy is low in individuals at risk of colorectal cancer (CRC). We constructed a risk-prediction score (RPS) in a large community-based sample at high risk of CRC to enable more accurate risk stratification and to motivate and increase the uptake rate of colonoscopy.
Methods
A total of 12,628 participants classified as high-risk according to positivity of immunochemical fecal occult blood tests or High-Risk Factor Questionnaire underwent colonoscopy. Logistic regression was used to derive a RPS and analysed the associations of the RPS with colorectal lesions, giving odds ratios (ORs) and 95% confidence intervals (CIs).
Results
Of the participants, men (OR = 1.73, 95% CI = 1.58–1.90), older age (≥65 years; 1.41, 1.31–1.53), higher body mass index (≥28 kg/m2; 1.22, 1.07–1.39), ever smoking (1.47, 1.31–1.65), and weekly alcohol use (1.28, 1.09–1.52) were associated with a higher risk of colorectal lesions. We assigned 1 point to each of the above five risk factors and derived a RPS ranging from 0 to 5, with a higher score indicating a higher risk. Compared with a RPS of 0, a RPS of 1, 2, 3, and 4–5 showed a higher risk of colorectal lesions, with the OR (95% CI) being 1.50 (1.37–1.63), 2.34 (2.12–2.59), 3.58 (3.13–4.10), and 3.91 (3.00–5.10), respectively. The area under the receiver-operating characteristic curve of RPS in predicting colorectal lesions was 0.62.
Conclusions
Participants with an increase in the RPS of ≥1 point had a significantly higher risk of colorectal lesions, suggesting the urgency for measuring colonoscopy in this very high-risk group. High-risk strategies incorporating RPS may be employed to achieve a higher colonoscopy-uptake rate.
Keywords: colorectal cancer, screening, risk-prediction score, colonoscopy
Introduction
Colorectal cancer (CRC) is one of the most common cancers worldwide with the third leading incidence and second-highest mortality rate of all types of cancer [1]. The incidence of CRC has had a continuously upward trend in recent decades [2]. In 2015, the incidence and mortality of CRC ranked the third and fifth, respectively, among all cancers in China [3]. The rates in Guangzhou were even higher (i.e. being 42.35/100,000 and 19.41/100,000 in 2015, respectively), ranking second and third, respectively, among all cancers according to the local annual report (Statistics of 2014–2015 in 2017–2018) [4].
Large-scale population screening can identify patients with early colorectal lesions to facilitate early and timely interventions. Currently, mass CRC screening has been carried out in large cities in China such as Shanghai and Tianjin [5, 6]. The City Authority of Guangzhou launched a mass CRC-screening program in 2015 [7]. Participants with positive results in immunochemical fecal occult blood tests (FOBTs) or the High-Risk Factor Questionnaire (HRFQ) were classified as at high risk and recommended to undergo colonoscopy [8]. However, no more than 30% of the high-risk individuals with positive results have undergone colonoscopy [7, 9, 10]. Hence, it is particularly important to further improve the colonoscopy-uptake rate in high-risk groups. We hereby explored risk factors predicting colorectal lesions, constructed a risk-prediction score (RPS) based on the identified risk factors, and analysed the associations of RPS with colorectal lesions, aiming at providing evidence for further risk stratification and setting up strategies to improve the colonoscopy-uptake rate in high-risk groups.
Materials and methods
Study participants
The data of this study were from the Guangzhou community-based CRC-screening program. Details of this city-wide CRC-screening program have been reported elsewhere [7]. Briefly, the mass CRC-screening program was launched in 2015. Permanent residents aged 50–74 years in Guangzhou (China) were eligible and invited to participate.
The inclusion criteria were as follows: (i) high-risk participants who had a positive FOBT result (test kits produced by W.H.P.M. Inc. Beijing, China) or the with presence of any of the following conditions assessed by a HRFQ [7, 11]: (a) a personal history of any cancer or colorectal polyps; (b) a first-degree-relative history of CRC in first-degree relatives; (c) at least two of the following symptoms/conditions: chronic constipation, chronic diarrhea, mucoid blood feces, traumatic events such as death among first-degree relatives, chronic appendicitis or appendectomy, and/or chronic cholecystitis or cholecystectomy; (ii) those who signed an informed consent form; and (iii) those who underwent colonoscopy after being classified as being in a high-risk group in the initial screening. Participants who were diagnosed with colorectal cancer prior to the initial screening were excluded from the study. For duplicated colonoscopy records (i.e. same report file or multiple reports of same participant), we retained the one with the most severe diagnosis.
Colonoscopy
All high-risk individuals were invited to designated hospitals to undergo colonoscopy with the cost covered by the basic medical insurance of Guangzhou. If any lesion was found on colonoscopy, a biopsy was performed and gastrointestinal pathologists evaluated all removed polyps. Colorectal lesions included ulcer, ulcerative colitis or Crohn’s disease, chronic inflammatory bowel disease, adenoma/polyp, and malignant tumors identified from colonoscopy [10]. Adenoma was defined as advanced adenoma (AA) if the diameter was ≥10 mm, villous component ≥25%, and/or presence of high-grade dysplasia [12]; the remaining were classified as non-advanced adenomas (NAAs). Non-adenomatous lesions included non-adenomatous polyps, chronic inflammatory bowel disease, ulcers, ulcerative colitis, and/or Crohn’s disease. When more than one lesion was present, the diagnosis was made according to the most severe lesion type present.
Risk factors
Risk factors considered included sex, age, body mass index (BMI), smoking status, alcohol use, marital status, education level, and occupation. As elderly is defined as a chronological age of ≥65 years according to the World Health Organization, we categorized age into two groups: ≥65 and <65 years. BMI was calculated as weight in kilograms divided by height in meters squared. According to the Chinese criteria for defining general obesity [13], BMI was categorized into two groups: ≥28 and <28 kg/m2. Smoking status was categorized into two groups: never and ever smoking (included former and current smoking). Alcohol use was categorized into two groups: never or occasional alcohol use and weekly alcohol use.
Data collection
The Guangzhou CRC-screening registration and management system has been established since 2015, before the CRC-screening program was launched. Data ports were designed for linkage with local community health centers, hospitals, and program-management organizations. Information on lifestyle factors was collected from 2016 to 2019. Data of demographic characteristics, the HRFQs, and FOBTs were stored in local community health centers. Results of colonoscopy and histopathology were assessed by qualified endoscopists from 2015 to 2020.
Statistical analysis
All statistical analysis was performed using Stata 16.0. Differences between groups with and without colorectal lesions were assessed by chi-square test. Multivariable logistic regression was fitted to identify significant risk factors associated with colorectal lesions, giving odds ratios (ORs) and 95% confidence intervals (CIs). Identified risk factors were used to construct a RPS. The area under the receiver-operating characteristic (ROC) curve (AUC) was calculated as an estimate for predictive performance. Two-sided P-values of <0.05 were considered statistically significant.
Ethics approval
All the procedures were performed in accordance with the Declaration of Helsinki and relevant policies in China. The Guangzhou Health Council and the Review Board of the Guangzhou Center for Disease Control and Prevention approved the study (GZCDC2014006). All participants who attended the screening program signed informed consent forms before participation.
Results
Of 22,867 high-risk individuals who underwent colonoscopy, 8,918 with missing data on the variables of interest, 704 aged <50 or >74 years, 182 with physician-diagnosed CRC, and 435 with duplicated colonoscopy records were excluded, leaving 12,628 participants in the current study. Of those, 7,278 (57.63%) participants had colorectal lesions. Men, older age, higher BMI, ever smoking, weekly alcohol use, and lower education were associated with the presence of colorectal lesions (P from <0.001 to 0.040). No specific pattern was found for occupation and colorectal lesions, although significant differences were identified (Table 1).
Table 1.
Baseline characteristics of the study participants by colorectal lesions based on colonoscopy
| Characteristic | Colorectal lesions, n (%) |
χ 2 | P-value | |
|---|---|---|---|---|
| No | Yes | |||
| Number (%) | 5,350 (42.37) | 7,278 (57.63) | – | – |
| Sex | 408.49 | <0.001 | ||
| Women | 3,656 (68.34) | 3,666 (50.37) | ||
| Men | 1,694 (31.66) | 3,612 (49.63) | ||
| Age, years | 135.40 | <0.001 | ||
| <65 | 3,658 (68.37) | 4,238 (58.23) | ||
| ≥65 | 1,692 (31.63) | 3,040 (41.77) | ||
| BMI, kg/m2 | 10.35 | 0.001 | ||
| <28 | 4,915 (91.87) | 6,565 (90.20) | ||
| ≥28 | 435 (8.13) | 713 (9.80) | ||
| Smoking | 295.38 | <0.001 | ||
| Never | 4,634 (86.62) | 5,393 (74.10) | ||
| Ever | 716 (13.38) | 1,885 (25.90) | ||
| Alcohol use | 83.61 | <0.001 | ||
| Never or occasional | 5,121 (95.72) | 6,668 (91.62) | ||
| Weekly | 229 (4.28) | 610 (8.38) | ||
| Marital status | 1.52 | 0.217 | ||
| Married | 4,958 (92.67) | 6,786 (93.24) | ||
| Other | 392 (7.33) | 492 (6.76) | ||
| Education level | 6.51 | 0.040 | ||
| Illiteracy and primary school | 1,225 (22.90) | 1,807 (24.83) | ||
| Secondary school | 3,189 (59.61) | 4,251 (58.41) | ||
| University or higher | 936 (17.50) | 1,220 (16.76) | ||
| Occupation | 19.16 | 0.001 | ||
| Unemployed | 716 (13.38) | 1,009 (13.86) | ||
| Enterprise | 1,563 (29.21) | 2,364 (32.48) | ||
| Government or public institution | 573 (10.71) | 741 (10.18) | ||
| Peasant | 1,114 (20.82) | 1,397 (19.19) | ||
| Other | 1,384 (25.87) | 1,767 (24.28) | ||
BMI, body mass index; kg/m2, kilogram/meter2; n, number.
After mutual adjustment and adjustment for marital status, education, and occupation, men (OR = 1.73, 95% CI = 1.58–1.90), older age (OR = 1.41, 95% CI = 1.31–1.53), higher BMI (OR = 1.22, 95% CI = 1.07–1.39), ever smoking (OR = 1.47, 95% CI = 1.31–1.65), and weekly alcohol use (OR = 1.28, 95% CI = 1.09–1.52) were significantly associated with a higher risk of colorectal lesions. Compared with participants who had low education (illiteracy and primary school), those with higher education (secondary school and university or higher) had a lower risk of colorectal lesions (OR = 0.83, 95% CI = 0.75–0.91 and OR = 0.75, 95% CI = 0.66–0.85, respectively). Moreover, peasants, vs those who were unemployed, had a lower risk of colorectal lesions (OR = 0.84, 95% CI = 0.74–0.95) (Table 2).
Table 2.
Association of risk factors with colorectal lesions based on colonoscopy
| Factor | OR | SE | z | P-value | 95% CI | |
|---|---|---|---|---|---|---|
| Sex | ||||||
| Women | 1.00 | |||||
| Men | 1.73 | 0.08 | 11.60 | <0.001 | 1.58 | 1.90 |
| Age, years | ||||||
| <65 | 1.00 | |||||
| ≥65 | 1.41 | 0.06 | 8.75 | <0.001 | 1.31 | 1.53 |
| BMI, kg/m2 | ||||||
| <28 | 1.00 | |||||
| ≥28 | 1.22 | 0.08 | 3.04 | 0.002 | 1.07 | 1.39 |
| Smoking | ||||||
| Never | 1.00 | |||||
| Ever | 1.47 | 0.09 | 6.43 | <0.001 | 1.31 | 1.65 |
| Alcohol use | ||||||
| Never or occasional | 1.00 | |||||
| Weekly | 1.28 | 0.11 | 2.93 | 0.003 | 1.09 | 1.52 |
| Marital status | ||||||
| Married | 1.00 | |||||
| Other | 1.03 | 0.07 | 0.41 | 0.678 | 0.89 | 1.19 |
| Educational level | ||||||
| Illiteracy and primary school | 1.00 | |||||
| Secondary school | 0.83 | 0.04 | –3.79 | <0.001 | 0.75 | 0.91 |
| University or higher | 0.75 | 0.05 | –4.32 | <0.001 | 0.66 | 0.85 |
| Occupation | ||||||
| Unemployed | 1.00 | |||||
| Enterprise | 1.05 | 0.06 | 0.80 | 0.425 | 0.93 | 1.19 |
| Government or public institution | 1.02 | 0.08 | 0.27 | 0.786 | 0.87 | 1.19 |
| Peasant | 0.84 | 0.05 | –2.72 | 0.007 | 0.74 | 0.95 |
| Other | 0.92 | 0.06 | –1.31 | 0.192 | 0.81 | 1.04 |
BMI, body mass index; CI, confidence interval; kg/m2, kilogram/meter2; OR, odds ratio; SE, standard error.
A RPS was developed by integrating the above five variables, with 1 point being given for each (i.e. men, age ≥65 years, BMI ≥28 kg/m2, ever smoking, and weekly alcohol use). The RPS ranged from 0 to 5. The higher the score, the higher the risk of colorectal lesions.
Of 7,278 cases with colorectal lesions, 378 (2.99%) had CRC, 1,340 (10.61%) had AA, 2,001 (15.85%) had NAA, and 3,559 (28.18%) had a non-adenomatous lesion (Table 3). As very few participants (n = 11) had a score of 5, participants with a score of 4 and 5 were pooled together. Compared with those with a RPS of 0, those with a RPS of 1, 2, 3, and 4–5 had a higher risk of colorectal lesions, with OR (95% CI) being 1.50 (1.37–1.63), 2.34 (2.12–2.59), 3.58 (3.13–4.10), and 3.91 (3.00–5.10), respectively. Similar associations for CRC were also observed in those with a RPS of 1, 2, 3, and 4–5, with OR (95% CI) being 2.41 (1.76–3.32), 4.62 (3.35–6.39), 9.19 (6.49–13.02), and 10.30 (5.91–17.93), respectively (Table 3).
Table 3.
Association of risk-prediction scores (RPSs) with the different colonoscopy outcomes in high-risk groups
| RPS* | Normal, n (%) | Colorectal lesions |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Non-adenomatous lesions |
NAA |
AA |
CRC |
Colorectal lesions (all) |
|||||||
| n (%) | OR (95% CI) | n (%) | OR (95% CI) | n (%) | OR (95% CI) | n (%) | OR (95% CI) | n (%) | OR (95% CI) | ||
| Total | 5,350 (42.37) | 3,559 (28.18) | – | 2,001 (15.85) | – | 1,340 (10.61) | – | 378 (2.99) | – | 7,278 (57.63) | – |
| 0 | 2,362 (53.76) | 1,109 (25.24) | 1.00 | 562 (12.79) | 1.00 | 299 (6.80) | 1.00 | 62 (1.41) | 1.00 | 2,032 (46.24) | 1.00 |
| 1 | 1,705 (43.70) | 1,088 (27.88) | 1.36 (1.22, 1.51) | 618 (15.84) | 1.52 (1.34, 1.74) | 383 (9.82) | 1.77 (1.51, 2.09) | 108 (2.77) | 2.41 (1.76, 3.32) | 2,197 (56.30) | 1.50 (1.37, 1.63) |
| 2 | 865 (33.19) | 770 (29.55) | 1.90 (1.68, 2.14) | 504 (19.34) | 2.45 (2.12, 2.83) | 362 (13.89) | 3.31 (2.78, 3.93) | 105 (4.03) | 4.62 (3.35, 6.39) | 1,741 (66.81) | 2.34 (2.12, 2.59) |
| 3 | 344 (24.52) | 485 (34.57) | 3.00 (2.57, 3.51) | 249 (17.75) | 3.04 (2.52, 3.67) | 242 (17.25) | 5.56 (4.53, 6.81) | 83 (5.92) | 9.19 (6.49, 13.02) | 1,059 (75.48) | 3.58 (3.13, 4.10) |
| 4-5 | 74 (22.91) | 107 (33.13) | 3.08 (2.27, 4.18) | 68 (21.05) | 3.86 (2.74, 5.44) | 54 (16.72) | 5.76 (3.98, 8.35) | 20 (6.19) | 10.30 (5.91, 17.93) | 249 (77.09) | 3.91 (3.00, 5.10) |
AA, advanced adenoma; CI, confidence interval; CRC, colorectal cancer; n, number; NAA, non-advanced adenoma; OR, odds ratio; RPS, risk-prediction score.
The RPS was developed by integrating five variables (i.e. men, older age of ≥65 years, BMI ≥28 kg/m2, ever smoking, and weekly alcohol use), with 1 point being given for each.
ROC analyses comparing the predictive capability of HRFQ, FOBT, and RPS showed a significantly higher AUC for RPS than either HRFQ or FOBT alone (AUC = 0.62 vs 0.53 and 0.55, respectively) (Figure 1).
Figure 1.
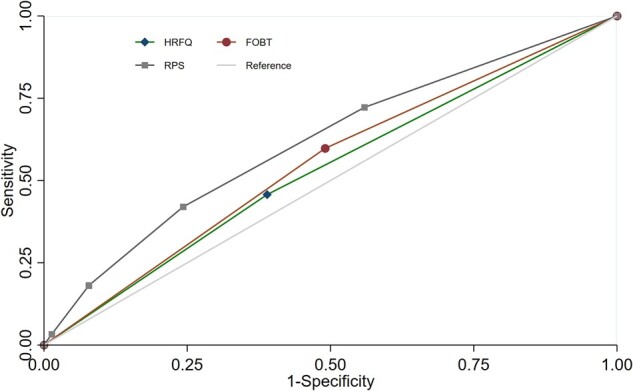
Receiver-operating characteristic curves of HRFQ, FOBT, and RPS in identifying colorectal lesions. FOBT, fecal occult blood test; HRFQ, High-Risk Factor Questionnaire; RPS, risk prediction score. The RPS was developed by integrating five variables (i.e. men, older age of ≥65 years, BMI ≥28 kg/m2, ever smoking, and weekly alcohol use), with 1 point being given for each.
Discussion
We constructed a RPS based on five simple risk factors (sex, age, BMI, smoking, and alcohol use) in individuals at risk of CRC to facilitate accurate risk classification. Participants with any one of the above five risk factors had a significantly higher risk of colorectal lesions and should be encouraged strongly to undergo colonoscopy immediately. Given the low colonoscopy-uptake rate even in high-risk groups in China and other settings, our results provided important evidence for establishing alternative strategies to motivate or increase colonoscopy uptake in high-risk groups.
Our results were generally consistent with those of previous studies on risk scores for CRC [14–17], although different studies included different risk factors. For example, the Physician’s Health Study developed a risk scoring system for CRC prediction based on age, smoking history, alcohol use, and BMI in a cohort of >21,000 men and showed that those with a score of 9–10 points had an OR of 15.29 (6.19–37.81) for CRC compared with those with the lowest estimated risk [14]. Another study constructing a score using age, sex, smoking status, family history, BMI, and diabetes found that participants with a higher score had a 2.37-fold higher risk of advanced neoplasia than those with average risk [15]. Furthermore, the Asia-Pacific Colorectal Screening score including risk factors of age, sex, family history, and smoking showed that the high-score group had a 4-fold higher risk of colorectal advanced neoplasia than the group with a lower score [16]. Another study developed a score including five risk factors (age, smoking, alcohol intake, height, and a variable combined sex/race/ethnicity) and showed that the risk of advanced neoplasia ranged from 3.2% in the low-risk group to 8.6% in the intermediate/high-risk group [17]. However, all the previous studies above investigated the association of predictive scores with the development of CRC or advanced neoplasia. Few studies investigated the association of a risk score with various stages of colorectal carcinogenesis [18]. Our results on the predictive capability of RPSs were consistent with those of the previous studies and added to the literature that high-risk individuals with any 1 point of the RPS had a significantly higher risk of colorectal lesions, i.e. by 1.50-fold for a RPS of 1 to 3.91-fold for a RPS of 4–5. As the risk factors included in the RPS were simple and readily accessible, the RPS could serve as a useful tool in further risk stratification and motivating colonoscopy uptake in high-risk groups immediately.
The risk factors identified in our model have been reported to be associated with CRC risk in a number of previous studies. For example, men and older age are well-documented risk factors of CRC [16, 19, 20]. BMI was associated with the risk of CRC in both men and women, with each 5-kg/m2 increase in BMI being associated with an 18% higher CRC risk [21]. An up-to-date systematic review from the World Cancer Research Fund/American Institute for Cancer Research reported a 6% higher risk of CRC associated with a per-5 kg/m2 increment in BMI [22]. Smoking was significantly associated with CRC incidence and mortality in previous studies [23]. A meta-analysis of 26 studies found that smokers had an 18% higher risk of CRC compared to non-smokers [23]. Another meta-analysis of 5 case–control studies and 11 nested case–control studies found that compared with occasional alcohol drinking or non-drinking, alcohol drinking of ≥42 gram/day was significantly associated with a higher risk of CRC (OR = 1.25, 95% CI = 1.11–1.40) [24]. In addition, our study also showed that peasants had a lower risk of colorectal lesions than those who were unemployed, which could be possibly due to the higher levels of physical activity in this group.
Most of the previous CRC-screening risk-assessment models were developed in asymptomatic or average-risk subjects [15, 25–27]. The current study based on high-risk groups of CRC was aimed at identifying individuals at “very high risk” for immediate colonoscopy intervention. As the colonoscopy-uptake rate is usually low even in high-risk individuals (i.e. with positive FOBT results and/or family history of CRC) [7], improving colonoscopy uptake can identify more individuals with early colorectal lesions for timely intervention. Healthcare practitioners in community settings usually fail to effectively mobilize high-risk groups to undergo colonoscopy. Hence, healthcare practitioners could provide strong recommendations (i.e. colonoscopy) to those who have a RPS of ≥1 with sound scientific evidence. In addition, more frequent follow-up and personalized health education should also be provided specifically to those at very high risk of colorectal lesions.
There were some limitations to the study. First, some potential risk factors of CRC were not included in the score because of the lack of data, such as diet [27, 28], genetic [29–31], or laboratory [32] variables. However, a simple score including factors that were readily accessible might be more practical and efficient than complex equations for application in community settings. Second, as all participants of this study were adults aged 50–74 years in Guangzhou, generalizability of this score to other settings or other age groups should be done with caution. Third, as this study only included participants who underwent colonoscopy and a number of high-risk individuals without colonoscopy were not included, selection bias may be a concern. Finally, the effectiveness of using a RPS in motivating immediate colonoscopy is unclear and needs to be tested in further randomized–controlled trials.
In conclusion, even a 1-point increment in the RPS was significantly associated with a higher risk of colorectal lesions, highlighting the urgency for colonoscopy immediately in this group. High-risk strategies incorporating a RPS may be used to achieve a higher colonoscopy-uptake rate.
Authors’ Contributions
L.X.L., B.H.L., and L.X. conceived and designed the project. L.X.L., Y.R.L., K.L., P.Z.Q., G.Z.L., Y.L., H.X., S.X.W., and B.H.L. collected the data. L.X.L., Y.R.L., Q.L.J., and L.X. analysed and interpreted the data. L.X.L. and Y.R.L. drafted the manuscript. All authors read and approved the final manuscript.
Funding
This work was supported by the Special Foundation for Science and Technology Basic Research Program [2019FY101103].
Acknowledgements
We thank all the participants in the study and the healthcare workers of the Community Health Centers and the Center for Disease Control and Prevention.
Conflicts of Interest
The authors declare that there is no conflict of interests in this study.
References
- 1. Bray F, Ferlay J, Soerjomataram I. et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018;68:394–424. [DOI] [PubMed] [Google Scholar]
- 2. Brody H. Colorectal cancer. Nature 2015;521:S1. [DOI] [PubMed] [Google Scholar]
- 3. Zheng R, Sun K, Zhang S. et al. Report of cancer epidemiology in China, 2015. Chin J Oncol 2019;41:19–28 (in Chinese). [DOI] [PubMed] [Google Scholar]
- 4. Liu H, Lin G.. Guangzhou cancer registry annual report (2017–2018). Guangzhou: Yangcheng Evening News Publishing, 2018. (in Chinese). [Google Scholar]
- 5. Gong Y, Peng P, Bao P. et al. The implementation and first-round results of a community-based colorectal cancer screening program in Shanghai, China. Oncologist 2018;23:928–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Zhao L, Zhang X, Chen Y. et al. Does self-reported symptom questionnaire play a role in nonadherence to colonoscopy for risk-increased population in the Tianjin colorectal cancer screening programme? BMC Gastroenterol 2021;21:117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lin G, Feng Z, Liu H. et al. Mass screening for colorectal cancer in a population of two million older adults in Guangzhou, China. Sci Rep 2019;9:10424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Meng W, Cai SR, Zhou L. et al. Performance value of high risk factors in colorectal cancer screening in China. World J Gastroenterol 2009;15:6111–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wu Y, Liang Y, Zhou Q. et al. Effectiveness of a short message service intervention to motivate people with positive results in preliminary colorectal cancer screening to undergo colonoscopy: a randomized controlled trial. Cancer 2019;125:2252–61. [DOI] [PubMed] [Google Scholar]
- 10. Chen W, Zhang W, Liu H. et al. How spatial accessibility to colonoscopy affects diagnostic adherences and adverse intestinal outcomes among the patients with positive preliminary screening findings. Cancer Med 2020;9:4405–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Fang JY, Zheng S, Jiang B. et al. Consensus on the prevention, screening, early diagnosis and treatment of colorectal tumors in China: Chinese Society of Gastroenterology, October 14-15, 2011, Shanghai, China. Gastrointest Tumors 2014;1:53–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. CSDE. Chinese guideline for colorectal cancer screening and treatment by endoscopology (2014, Beijing). Chin J Degist Endoscopol 2015;32:341–60 (in Chinese). [Google Scholar]
- 13. Chen C, Kong L.. Guidelines for Prevention and Control of Overweight and Obesity in Chinese Adults. Beijing: People's Medical Publishing House, 2006. (in Chinese). [Google Scholar]
- 14. Driver JA, Gaziano JM, Gelber RP. et al. Development of a risk score for colorectal cancer in men. Am J Med 2007;120:257–63. [DOI] [PubMed] [Google Scholar]
- 15. Wong MC, Lam TY, Tsoi KK. et al. A validated tool to predict colorectal neoplasia and inform screening choice for asymptomatic subjects. Gut 2014;63:1130–6. [DOI] [PubMed] [Google Scholar]
- 16. Yeoh KG, Ho KY, Chiu HM. et al. ; Asia-Pacific Working Group on Colorectal Cancer. The Asia-Pacific Colorectal Screening score: a validated tool that stratifies risk for colorectal advanced neoplasia in asymptomatic Asian subjects. Gut 2011;60:1236–41. [DOI] [PubMed] [Google Scholar]
- 17. Schroy PC 3rd, Wong JB, O'Brien MJ. et al. A risk prediction index for advanced colorectal neoplasia at screening colonoscopy. Am J Gastroenterol 2015;110:1062–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Erben V, Carr PR, Holleczek B. et al. Strong associations of a healthy lifestyle with all stages of colorectal carcinogenesis: results from a large cohort of participants of screening colonoscopy. Int J Cancer 2019;144:2135–43. [DOI] [PubMed] [Google Scholar]
- 19. Imperiale TF, Monahan PO, Stump TE. et al. Derivation and validation of a scoring system to stratify risk for advanced colorectal neoplasia in asymptomatic adults: a cross-sectional study. Ann Intern Med 2015;163:339–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Tao S, Hoffmeister M, Brenner H.. Development and validation of a scoring system to identify individuals at high risk for advanced colorectal neoplasms who should undergo colonoscopy screening. Clin Gastroenterol Hepatol 2014;12:478–85. [DOI] [PubMed] [Google Scholar]
- 21. Ning Y, Wang L, Giovannucci EL.. A quantitative analysis of body mass index and colorectal cancer: findings from 56 observational studies. Obes Rev 2010;11:19–30. [DOI] [PubMed] [Google Scholar]
- 22. Abar L, Vieira AR, Aune D. et al. Height and body fatness and colorectal cancer risk: an update of the WCRF-AICR systematic review of published prospective studies. Eur J Nutr 2018;57:1701–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Botteri E, Iodice S, Bagnardi V. et al. Smoking and colorectal cancer: a meta-analysis. JAMA 2008;300:2765–78. [DOI] [PubMed] [Google Scholar]
- 24. McNabb S, Harrison TA, Albanes D. et al. Meta-analysis of 16 studies of the association of alcohol with colorectal cancer. Int J Cancer 2020;146:861–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Sekiguchi M, Kakugawa Y, Matsumoto M. et al. A scoring model for predicting advanced colorectal neoplasia in a screened population of asymptomatic Japanese individuals. J Gastroenterol 2018;53:1109–19. [DOI] [PubMed] [Google Scholar]
- 26. Park YM, Kim HS, Park JJ. et al. A simple scoring model for advanced colorectal neoplasm in asymptomatic subjects aged 40-49 years. BMC Gastroenterol 2017;17:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Cai QC, Yu ED, Xiao Y. et al. Derivation and validation of a prediction rule for estimating advanced colorectal neoplasm risk in average-risk Chinese. Am J Epidemiol 2012;175:584–93. [DOI] [PubMed] [Google Scholar]
- 28. Vieira AR, Abar L, Chan DSM. et al. Foods and beverages and colorectal cancer risk: a systematic review and meta-analysis of cohort studies, an update of the evidence of the WCRF-AICR Continuous Update Project. Ann Oncol 2017;28:1788–802. [DOI] [PubMed] [Google Scholar]
- 29. Balavarca Y, Weigl K, Thomsen H. et al. Performance of individual and joint risk stratification by an environmental risk score and a genetic risk score in a colorectal cancer screening setting. Int J Cancer 2020;146:627–34. [DOI] [PubMed] [Google Scholar]
- 30. Jeon J, Du M, Schoen RE. et al. ; Colorectal Transdisciplinary Study and Genetics and Epidemiology of Colorectal Cancer Consortium. Determining risk of colorectal cancer and starting age of screening based on lifestyle, environmental, and genetic factors. Gastroenterology 2018;154:2152–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Carr PR, Weigl K, Edelmann D. et al. Estimation of absolute risk of colorectal cancer based on healthy lifestyle, genetic risk, and colonoscopy status in a population-based study. Gastroenterology 2020;159:129–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Yang HJ, Choi S, Park SK. et al. Derivation and validation of a risk scoring model to predict advanced colorectal neoplasm in adults of all ages. J Gastroenterol Hepatol 2017;32:1328–35. [DOI] [PubMed] [Google Scholar]


