ABSTRACT
Background
Children with intestinal failure (IF) receiving long-term parenteral nutrition (PN) have altered body composition (BC), but data on BC changes from start of PN onwards are lacking.
Objectives
We aimed to assess growth and BC in infants after neonatal intestinal surgery necessitating PN and at risk of IF, and to explore associations with clinical parameters.
Methods
A prospective cohort study in infants after intestinal surgery. IF was defined as PN dependency for >60 d. SD scores (SDS) for anthropometry were calculated until 6-mo corrected age. In a subgroup, fat mass (FM) and fat-free mass (FFM) were measured with air-displacement plethysmography at 2- and 6-mo corrected age. SDS for length-adjusted FM index and FFM index were calculated. Associations between cumulative amount of PN and BC parameters were analyzed with linear mixed-effect models.
Results
Ninety-five neonates were included (54% male, 35% born <32 wk) and 39 infants (41%) had IF. Studied infants had compromised anthropometric parameters during follow-up. At 6-mo corrected age, they remained smaller (median weight-for-age SDS –0.9 [IQR –1.5, 0.1], P < 0.001) than the normal population. In 57 infants, 93 BC measurements were performed. FM index SDS was lower than in healthy infants at 2- and 6-mo corrected age (–0.9 [–1.6, –0.3], P < 0.001 and –0.7 [–1.3, 0.1], P = 0.001, respectively), but FFM index SDS did not differ. A higher cumulative amount of PN predicted a higher FM index in female infants but lower FM index in male infants.
Conclusions
In this cohort of infants receiving PN after intestinal surgery, compromised anthropometrics, decreased FM, and adequate FFM were observed during the first 6 mo. Male and female infants seemed to respond differently to PN when it comes to FM index. Continuing growth monitoring after the age of 6 mo is strongly recommended, and further research should explore the benefit of incorporating ongoing BC monitoring during follow-up.
Keywords: intestinal failure, short bowel syndrome, parenteral nutrition, growth, body composition
Introduction
In children with intestinal failure (IF), the small intestine is either too short or dysfunctional despite adequate length, and therefore unable to absorb sufficient nutrients for growth and development (1). Conditions leading to IF include congenital anomalies of the small intestine such as intestinal atresia and gastroschisis, but also acquired neonatal diseases such as necrotizing enterocolitis (NEC) (2). In these neonates, parenteral nutrition (PN) is initiated shortly after intestinal surgery to prevent nutritional shortages (3). Currently, the effect of PN on growth is monitored using standard anthropometry such as weight and length trajectories. However, this approach does not provide information on the quality of growth, specifically on body composition (BC), in terms of fat mass (FM) and fat-free mass (FFM). Increased FM in infancy can lead to adiposity development, diabetes mellitus type 2, and cardiovascular disease later in life (4–11). The first 3 mo of life can already be seen as a critical window for adiposity development (4, 6, 9). FFM is important for muscle function and bone mass development and is associated with better cognitive function (12–14). Factors known to influence BC are, amongst others, prematurity, nutritional intake, and physical activity (15–17). Although previous studies have shown that older children on long-term PN have higher FM and lower FFM than healthy peers (18, 19), it remains unclear at what age alterations in BC begin. Studies on growth and BC in the first months after the start of PN are lacking. Early identification of those at risk of compromised BC may help to improve their long-term outcomes with timely interventions.
Our aims were to study 1) growth and BC in infants necessitating PN after neonatal intestinal surgery, up to 6-mo of corrected age, and 2) the associations between clinical and PN characteristics and infant growth and BC outcomes.
Methods
Study design and subjects
This observational prospective cohort study took place from March 2015 to March 2020 at the Erasmus MC Sophia Children's Hospital (Rotterdam, The Netherlands), which serves as a region-wide referral center for pediatric gastrointestinal surgery. It is difficult to predict which children will develop IF, defined by the North American Society for Pediatric Gastroenterology, Hepatology, and Nutrition (NASPGHAN) (20) as a duration of >60 d on PN. Therefore, we chose to include all neonates with conditions potentially leading to IF after intestinal surgery. Inclusion criteria were as follows: 1) need for PN, 2) after surgical conditions involving the small intestine such as gastroschisis or NEC with small bowel resection, 3) established in the neonatal period (<28 d after birth) or, if born preterm, established before term age. Infants meeting all 3 criteria were included.
The study was in line with the principles of the Declaration of Helsinki and was approved by the local Research Ethical Committee of the Erasmus Medical Center (MEC 2015–002, Dutch Trial Register NTR6080, https://www.trialregister.nl/trial/5892). Written informed consent was obtained from the participants’ caregivers.
Data collection and definitions
Demographic and clinical data, such as gestational age, sex, underlying disease, and intestinal surgery characteristics, were retrieved from the medical charts. Short bowel syndrome was defined as a resection of >70% of the small intestine, and/or a remaining length of the small intestine (measured from the ligament of Treitz onwards) of <50 cm in preterm infants or <75 cm in term born infants (21). The presence of cholestasis was defined as a serum conjugated bilirubin concentration of ≥40 µmol/L for ≥2 wk with the necessity of a clinical intervention (defined as reduction in lipid dose, discontinuation of lipids, switch to a fish-oil-based lipid emulsion [Omegaven®], or prescription of ursodeoxycholic acid), derived from the definition used by Belza et al. (22). An episode of a central-line associated blood stream infection was defined as the presence of a blood culture proven bacteremia (not being contamination) obtained from the catheter lumen (23). IF was defined as a duration of PN dependency of >60 d, calculated from the date of surgery onwards (20).
Data concerning enteral and parenteral nutrition and anthropometric parameters were assessed from the date of neonatal intestinal surgery up to 6 mo thereafter (see Supplementary Figure 1). The study visits were at 1, 2, 4, 8, 12, and 26 wk after surgery and occurred during hospitalization and outpatient clinic visits. BC measurements and anthropometry were performed around 2 mo and 6 mo of corrected age.
Measurements
Nutrition
PN and enteral nutrition (EN) were prescribed according to our local protocol which was based on the guidelines on pediatric parenteral nutrition from The European Society for Paediatric Gastroenterology Hepatology and Nutrition (ESPGHAN) and the European Society for Clinical Nutrition and Metabolism (ESPEN), supported by the European Society for Paediatric Research (ESPR) (3). A mixture of essential, semi-essential and nonessential amino acids was used for amino acid administration (Primene® 10% w/v). The type of parenteral lipid mostly used was a soybean oil emulsion (Intralipid®) until 2015, and a mixed soybean/medium-chain triglycerides/olive/fish oil lipid emulsion (SMOFlipid®) from 2016 onwards. In the case of persistent cholestasis (see above), a fish-oil-based lipid emulsion (Omegaven®) was used.
EN (oral or tube feeding), in the form of human milk or (preterm) formula (mostly polymeric infant formula or if indicated semi-elemental or monomeric formula), was initiated as soon as possible. The volume of EN was increased in the case of an acceptable vomiting frequency (≤3 times per day) and defecation frequency (<5 stools per day or ≤20–30 mL/kg/d stoma output). EN was fortified with either human milk fortifier or macronutrient additives in the case of impaired growth according to local protocol. PN administration was reduced accordingly if growth velocity was adequate and ceased at an enteral intake of ∼90–130 kcal/kg/d (depending on postnatal age and weight) (24). PN and EN prescribers (physicians and dietitians) were not aware of the BC results.
Collected PN characteristics included duration of PN in days, kcal per kg/d, and grams of carbohydrates, lipids, and amino acid infusion per kg/d. PN dependency was defined as percentage of energy intake provided by PN (%PN) = (daily energy intake in kcal provided by PN/total daily energy intake in kcal) × 100. To evaluate the effect of PN on BC, a cumulative amount of PN was calculated from the date of intestinal surgery up to 6-mo corrected age using AUC. This includes the duration of PN and PN dependency at different time points. The following formula was used: 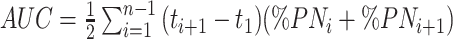 .
.
Anthropometrics
Weight, length, and head circumference were routinely measured according to our local protocol. Only during neonatal intensive care stay, length was not routinely measured in preterm infants. At the time of BC measurement, midupper arm circumference was measured to the nearest 0.1 cm with a tape measure (halfway between acromion of the shoulder and olecranon of the elbow of the left arm).
For preterm infants, gestational age, and sex-specific SD scores (SDS) were calculated for weight-for-age, length-for-age, weight-for-length, and head circumference-for-age ≤40 wk postmenstrual age, based on the Fenton growth charts (25, 26). For term born infants and preterm infants from 40 wk postmenstrual age onwards, weight-for-age, length-for-age, weight-for-length, head circumference-for-age, and midupper arm circumference-for-age SDS (adjusted for prematurity) were calculated with the WHO growth reference charts, available at https://www.who.int/childgrowth/software/en/ (27).
Body composition
BC was measured using the PEA POD® Infant Body Composition Tracking System (COSMED, Ltd) with air-displacement plethysmography (28, 29). In air-displacement plethysmography, based on whole-body densitometry, a 2-compartment model is derived consisting of FM and FFM (the latter including muscle, water, bone, and internal organs; also referred to as lean mass) (30). When an infant was receiving 24 h of PN administration or oxygen support, or was in isolation for infection prevention, BC measurement was not possible. BC measurements were performed by experienced personnel using a standardized protocol based on the manufacturer's instructions. Detailed description of the procedure is provided elsewhere (31). If applicable, a duplicate of the central venous catheter, feeding tube, enterostomy bag, or additional device was calibrated in the PEA POD® before the measurement according to the operator's manual. In the case of excessive movement or crying, resulting in invalid values of FM and FFM, the measurement was not included in the analysis.
To correct for the small body size of our study population, we calculated a length-adjusted FM index and FFM index, by dividing the FM or FFM by the square of length (19, 32). This helps the interpretation of associations and can indicate whether or not FM and FFM are in the normal range, proportional to length. Sex- and age-specific SDS for BC parameters were calculated using reference data from the United Kingdom (33). Individual SDS were assigned using the lambda-mu-sigma (LMS)method (34).
Statistical analysis
The primary outcome variable was FM; secondary outcome variables were FFM, weight, length, and head circumference. A sample size calculation was hampered by the lack of previous reports on children with PN and BC at 6-mo corrected age. Detecting a correlation ∼35% between the cumulative amount of PN and FM percentage would require 62 infants to reach a power of 80% using a 2-sided significance level of 0.05. Because of practical limitations of the PEA POD® and therefore the inability to measure every infant, we increased the sample size to 95 infants.
For the description of patient characteristics, categorical data were summarized as frequency counts (n) and percentages (%), and continuous data as median with IQR or range since many of the variables were not normally distributed. Duration of enterostomy, hospital stay, and PN administration until 26 wk after surgery were calculated with survival analysis, to take into account mortality and the fact that some infants were still receiving PN, having an enterostomy, or were admitted to the hospital at the end of the follow-up period.
One-sample Wilcoxon signed-rank tests (compared with zero) were used to determine whether anthropometric and BC parameters differed significantly from that of the normal population mean (0 SDS). Exploratory tests (chi-square tests, Mann–Whitney U tests, and 2-sample t-tests) were used to provide insight into differences between groups: 1) infants with versus without BC measurement, 2) male versus female infants, 3) infants born <32 wk (extremely and very preterm; the neonatal intensive care unit population) versus ≥32 weeks of gestation, 4) infants with >60 d of PN dependency (i.e. having IF) versus with shorter PN duration, 5) infants with versus without enterostomies, 6) infants with NEC versus non-NEC, and 7) infants with 1 versus multiple surgeries. For infants with 2 BC measurements, outcomes at 2- and 6-mo corrected age were compared with paired-sample t-tests. To explore the use of BMI as a measure of FM index, we used Hattori charts (35).
We analyzed the association between PN and longitudinal measurements of the FM index SDS and FFM index SDS with linear mixed-effects models. This type of model allows to account for the correlation among repeated measurements, for measurements taken at different time points per individual, and for the number of measurements within individuals. The fixed-effects part included the AUC of PN corrected for sex, the interaction of the AUC of PN with sex, gestational age in weeks, conditional weight-for-age SDS, receiving any human milk at the time of assessment, and the corrected age in weeks at the time of assessment (variables known from previous literature to be associated with BC outcomes) (36–39). Conditional weight-for-age SDS was calculated by regressing weight-for-age SDS at 2- or 6-mo corrected age on weight-for-age SDS at the date of intestinal surgery and then taking the standardized regression residual (to take into account any regression to the mean). Since the amount of human milk could not be quantified (in the case of breastfeeding), we chose to assess whether the infant was receiving any human milk at 2- and 6-mo corrected age. For the random-effects part, random intercepts were included. To select the optimal random-effects structure and to test whether the longitudinal evolutions were nonlinear, we used likelihood ratio tests based on the Akaike information criterion. We used t-tests to assess the predictive value of each covariate on the BC outcome variable. To evaluate the overall effect of the AUC of PN on BC outcomes, we compared the final model including the AUC of PN variable with a model without the AUC of PN using likelihood ratio tests. Model assumptions were checked by inspecting the residual plots. For illustrational purposes, effect plots were made for the effect of time and for the effect of AUC of PN for infants with median values of exploratory variables.
A P value <0.05 was considered statistically significant. Data analyses were performed using Statistical Package for the Social Sciences, Version 25.0 (IBM SPSS Statistics for Windows), R version 4.0.3 (R Foundation for Statistical Computing, http://www.R-project.org/), and GraphPad Prism version 8.00 for Windows (GraphPad Software, http://www.graphpad.com).
Results
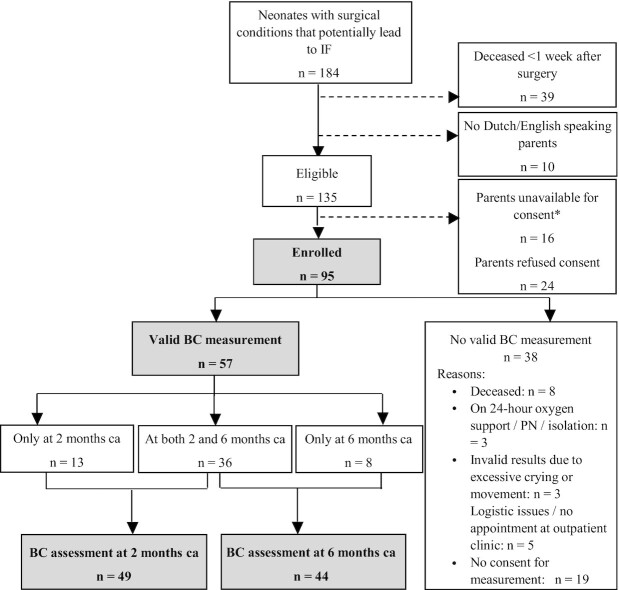
Of 135 eligible neonates, 95 were included in the study for assessment of nutrition and anthropometrics (see the flow chart in Figure 1). In 57 infants, 1 or 2 BC measurements could be performed. The 38 infants without BC measurement had a significantly lower gestational age but did not significantly differ from the group of infants with BC measurements in sex, presence of short bowel syndrome, underlying disease groups, PN duration, number of infants with home PN indication, or anthropometric parameter SDS (data not shown).
FIGURE 1.
Flow chart of inclusion in study. Studied infants are shown in bold/gray (N = 95 for anthropometrics and nutritional intake, N = 57 for BC). *Parents were not asked to participate due to a complex social situation in which there was no available legal guardian to give consent or due to quick transfer to another hospital. BC, body composition; ca, (for prematurity) corrected age; IF, intestinal failure; PN, parenteral nutrition.
Patient characteristics
Patient characteristics are presented in Table 1. Thirty-three (35%) neonates were born ≤32 weeks of gestation and 51 (54%) were male. At a median age of 2 d, neonates were operated on for gastroschisis (n = 34), NEC (n = 26), intestinal atresia (n = 19), or other pathology (n = 16). Short bowel syndrome was present in 9 infants. From the 22 infants that developed cholestasis, 8 were switched to a fish-oil-based lipid emulsion. In 16 infants, cholestasis resolved (2 persistent cholestasis, 4 missing data). Eight infants deceased during follow-up; all were born before 32 weeks of gestation and the most common cause of death was a complicated course of sepsis.
TABLE 1.
Patient characteristics1
| Total cohort | PN dependency ≤60 d | PN dependency >60 d | ||
|---|---|---|---|---|
| N = 95 | N = 56 | N = 39 | ||
| n (%) or median [IQR] | n (%) or median [IQR] | n (%) or median [IQR] | ||
| Birth Related | ||||
| Sex, male | 51 (54) | 31 (55) | 20 (51) | |
| Gestational age, wk | 35.6 [27.6, 37.3] | 36.9 [29.6, 37.4] | 33.7 [25.9, 36.1] | |
| Very preterm (gestational age <32 wk) | 33 (35) | 15 (27) | 18 (46) | |
| Caesarean delivery | 39 (41) | 19 (30) | 22 (56) | |
| Maternal age at delivery, y | 29 [24, 32] | 29 [25, 33] | 29 [24, 31] | |
| Maternal age <20 y | 6 (6) | 4 (7) | 2 (5) | |
| Apgar score at 5 min | n = 91 | 9 [8, 10] | 9 [8, 10] | 8 [7, 9] |
| Birth weight, g | 2250 [1000, 2800] | 2555 [1298, 2946] | 1870 [800, 2415] | |
| Birth weight SDS | –0.2 [–0.9, 0.6] | 0.0 [–0.7, 0.5] | –0.2 [–1.4, 0.6] | |
| Small for gestational age (<10th percentile) | 17 (18) | 7 (13) | 10 (26) | |
| Large for gestational age (>90th percentile) | 7 (7) | 2 (4) | 5 (13) | |
| Head circumference at birth, cm | n = 62 | 29.7 [23.5, 32.5] | 32.0 [24.0, 33.2] | 25.2 [22.0, 30.0] |
| Head circumference SDS | –0.5 [–1.0, 0.2] | –0.2 [–0.7, 0.2] | –0.7 [–1.0, 0.1] | |
| Intestinal Failure Related | ||||
| Postnatal age at intestinal surgery, d | 2 [0, 12] | 1 [0, 10] | 3 [0, 18] | |
| Underlying disease | ||||
| Gastroschisis | 34 (36) | 22 (39) | 12 (31) | |
| Necrotizing enterocolitis | 26 (27) | 14 (25) | 12 (31) | |
| Intestinal atresia | 19 (20) | 14 (25) | 5 (13) | |
| Other2 | 16 (17) | 6 (11) | 10 (25) | |
| Remaining small intestinal length | n = 29 | 70.0 [46.0, 102.5] | 87.0 [71.3, 123.8] | 50.0 [35.0, 90.0] |
| Short bowel syndrome3 | 9 (9) | 0 (0) | 9 (23) | |
| Ileocecal valve not in situ | 13 (14) | 5 (9) | 8 (21) | |
| Enterostomy | 41 (43) | 17 (30) | 24 (62) | |
| Duration of enterostomy,4 wk | 9.6 [7.7, 13.0] | 8.9 [6.1, 10.4] | 10.4 [8.4, 13.6] | |
| Number of surgeries | ||||
| 1 | 31 (33) | 29 (52) | 3 (8) | |
| Multiple | 64 (67) | 27 (48) | 36 (92) | |
| Number of CLABSIs5 | ||||
| None | 47 (49) | 39 (70) | 7 (18) | |
| 1 | 26 (27) | 13 (23) | 14 (36) | |
| Multiple | 22 (23) | 4 (7) | 18 (46) | |
| Cholestasis6 | 22 (23) | 6 (11) | 16 (41) | |
| Duration of first hospital stay,4 wk | 8.7 [4.9, 16.3] | 6.1 [3.4, 8.1] | 16.4 [13.1, 21.4] | |
| Total PN duration,4 wk | 7.9 [4.1, 15.1] | 5.4 [2.7, 7.1] | 16.3 [11.7, 26.0] | |
| PN duration before intestinal surgery, wk | n = 36 | 1.6 [0.9, 2.4] | 1.4 [1.0, 2.2] | 1.9 [0.9, 2.9] |
| PN duration from date of surgery onwards,4 wk | 7.0 [3.7, 13.0] | 4.1 [2.6, 6.3] | 15.4 [11.1, 24.1] |
CLABSI, central line-associated blood stream infection; PN, parenteral nutrition; SDS, SD score.
Other underlying diseases include volvulus (n = 4), perforation of the small bowel (n = 5), strangulation ileus (n = 2), ileal stenosis (n = 1), milk curd ileus (n = 1), and meconium peritonitis (n = 3).
Defined as resection of ≥70% of small intestine, and/or remaining small bowel length of <50 cm in preterm neonates or <75 cm in term neonates.
Calculated with the Kaplan–Meier method until 26 wk of follow-up.
CLABSI: blood culture proven, treated with antibiotics, until 26 wk of follow-up.
Cholestasis: serum direct bilirubin of >40 µmol/L (≈>2 mg/dL) for >14 consecutive days with need for intervention (decrease/stop/change in type of lipids and/or ursodeoxycholic acid), until 26 wk of follow-up.
Nutrition
PN was already initiated before intestinal surgery in 36 infants. Median PN duration after surgery was 7.0 wk. At ∼4 wk after surgery, infants received a median of 50% from energy intake by EN. The courses of energy intake provided by PN and EN from the date of surgery onwards for all infants together and only for infants receiving PN at each specific time point are shown in Supplementary Figure 2. In Supplementary Figure 3, a survival curve for PN weaning is presented. Ten weeks after intestinal surgery, ∼50% of infants with NEC had completely weaned off PN, compared with 75% of infants with gastroschisis and intestinal atresia. However, for total duration of PN dependency, no statistically significant difference between the underlying disease groups was found. Thirty-nine infants met the criteria for IF by receiving PN for >60 d, of whom 12 were discharged with home PN during or after the 26 wk follow-up period. A comparison of patient characteristics of the group with >60 d PN dependency versus ≤60 d PN dependency is presented in Table 1.
The distribution and macronutrient contribution of PN and the type of EN during follow-up is described in Supplementary Table 1. In the first 8 wk of follow-up, the majority of infants received human milk if they were receiving any EN.
Anthropometrics
Median birth weight SDS was –0.2 (IQR –0.9, 0.6). Figure 2 shows the compromised course of growth from surgery onwards for male and female infants separately. At 2-mo corrected age, almost one-third of the infants had a weight-for-age below –2 SDS. At 6-mo corrected age this number decreased to 9%. Infants remained smaller (median weight-for-age SDS –0.9 [IQR –1.5, 0.1], P < 0.001) and shorter (median length-for-age SDS –0.4 [IQR –1.3, 0.4], P = 0.003) than the normal population at 6-mo corrected age.
FIGURE 2.
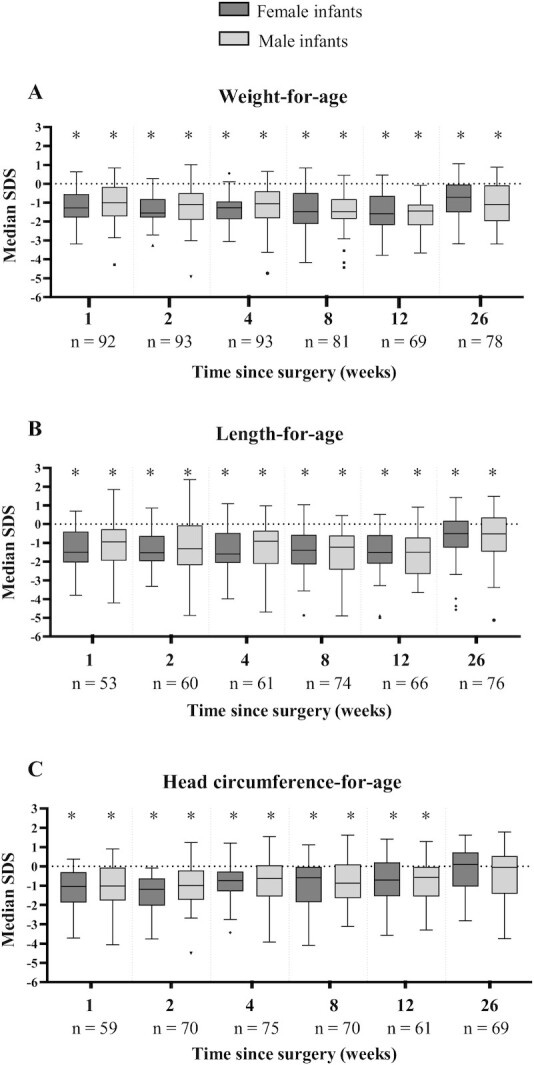
Anthropometric parameters from the date of neonatal intestinal surgery onwards for male infants and female infants separately. Box plots represent median SDS with IQR and whiskers of min-max range (with outliers as dots). SDS were calculated with WHO growth reference charts for term infants and with Fenton growth curves for preterm infants ≤40 wk of postmenstrual age and WHO growth reference charts thereafter. *Significantly different from zero (normal population mean) (analyzed with 1-sample Wilcoxon test). SDS, SD score.
Body composition
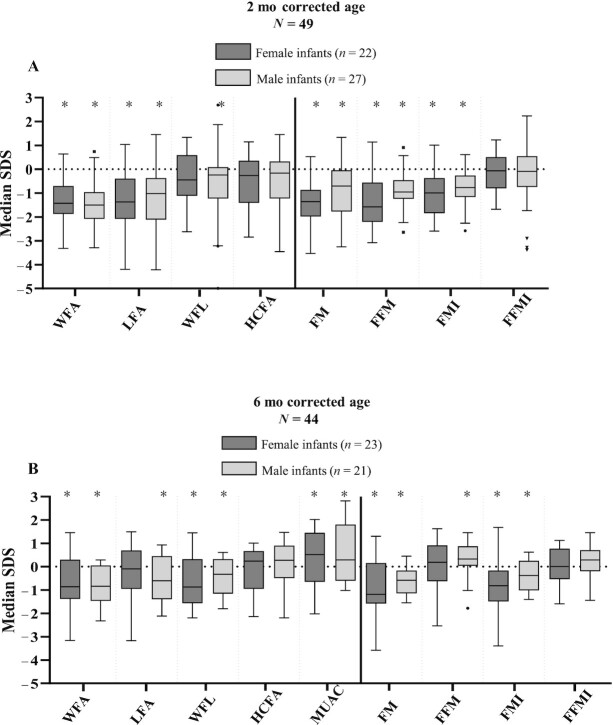
In 57 (60%) infants, 93 air-displacement plethysmography measurements were performed; 49 at 2-mo and 44 at 6-mo corrected age. Median FM percentage was 16.5% (IQR 13.1, 20.7) and 21.4% (IQR 18.1, 25.5) at 2- and 6-mo corrected age, respectively. In Figure 3, median SDS for anthropometric and BC parameters in this subgroup are presented for male and female infants separately. Studied infants had a significantly lower median FM index SDS than the reference population at both 2- and 6-mo corrected age (–0.9 [IQR –1.6, –0.3] and –0.7 [IQR –1.3, 0.1], P < 0.001 and P = 0.001, respectively), but median FFM index SDS did not significantly differ from the reference population. No significant differences in BC outcomes were found between infants born <32 weeks and ≥32 weeks of gestation, between the sexes, between infants with enterostomies and those without, between infants with NEC and non-NEC, or between infants with 1 and multiple surgeries. At 2-mo corrected age, 6 (12%) infants had an FM index below –2 SDS, and at 6-mo corrected age this number was 3 (7%). At 2-mo corrected age, 9 (18%) infants had an FM below –2 SDS, whereas at 6-mo corrected age this number decreased to 4 (9%).
FIGURE 3.
Anthropometric and body composition parameters expressed in SDS in selected group of infants with body composition measurement at 2-mo corrected age (A) and 6-mo corrected age (B) for male infants and female infants separately. Box plots represent median SDS with IQR and whiskers of min-max range (with outliers as dots). SDS were calculated with WHO growth reference charts for anthropometric parameters, and with UK reference values for body composition parameters (33). *Significantly different from zero (normal population mean) (analyzed with 1-sample Wilcoxon test). Missing data: n = 3 for HCFA at 2-mo corrected age, n = 2 for HCFA, and n = 3 for MUAC at 6-mo corrected age. FFM, fat-free mass; FFMI, fat-free mass index; FM, fat mass; FMI, fat mass index; HCFA, head circumference-for-age; LFA, length-for-age; MUAC, midupper arm circumference; SDS, SD score; WFA, weight-for-age; WFL, weight-for-length.
In the 36 infants who had air-displacement plethysmography measurements at both 2- and 6-mo corrected age, there was a significant increase in SDS during the follow-up period for weight-for-age (+0.7 SDS [95% CI: 0.4, 1.1]), length-for-age (+0.8 SDS [95% CI: 0.5, 1.1]), head circumference-for-age (+0.5 SDS [95% CI: 0.3, 0.8]), FFM (+1.3 SDS [95% CI: 1.0, 1.6]), and FFM index (+0.4 SDS [95% CI: 0.1, 0.8]). This was not the case for weight-for-length SDS, FM SDS, and FM index SDS. Supplementary Figure 4A and 4B represent Hattori charts and show that a given BMI can correspond with a wide range of FM index values.
The group of infants receiving PN for >60 d did not significantly differ in anthropometric or BC parameters at 2- and 6-mo corrected age from the infants with shorter PN duration.
Associations between clinical parameters and body composition
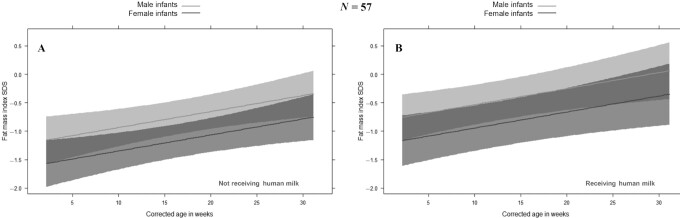
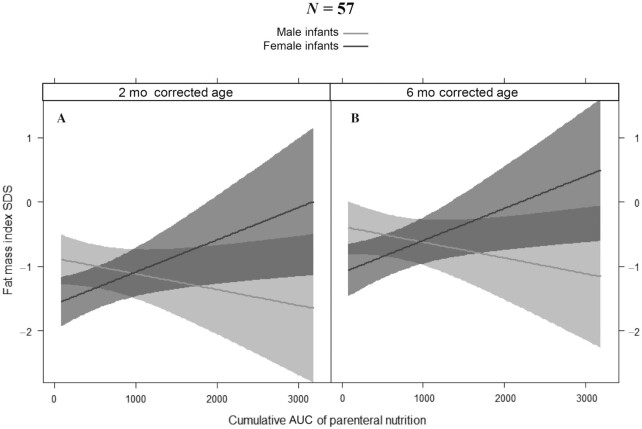
To further explore the role of PN in predicting BC, we studied associations in a mixed-effects model of repeated measurements. Figure 4 depicts the effect plots of FM index SDS for male and female infants over time, corrected for AUC of PN, gestational age, and receiving any human milk (Figure 4B) or not (Figure 4A). Male sex (P = 0.019) was independently associated with a higher FM index SDS. Also, the cumulative amount of PN (P = 0.029) was associated with FM index SDS, but the direction of the association was different for male infants compared with female infants (meaning that there was an interaction between sex and AUC of PN, P = 0.011): in female infants, FM index SDS increased with increasing AUC of PN, but in male infants, FM index decreased with increasing AUC of PN. This effect is shown in Figure 5, which represents the effect plot of the AUC of PN on FM index SDS for male and female infants at 2- and 6-mo corrected age, adjusted for gestational age and receiving any human milk. When repeating the model with conditional weight-for-age included, the association of FM index SDS with AUC of PN was still present. Aside from patterns of BC changes, exploration of several other characteristics between male and female infants at birth, and 2- and 6-mo corrected age, revealed no differences in terms of birth weight SDS, number of infants small/large for gestational age, number of infants born preterm (<37 wk gestational age) or very preterm (<32 wk gestational age), gestational age, underlying disease (gastroschisis versus intestinal atresia versus NEC versus other), number of infants with IF (PN dependency >60 d), or in anthropometric and BC parameter SDS at 2- and 6-mo corrected age.
FIGURE 4.
Effect plot of the fat mass index SDS for male and female infants over time. Linear mixed-effects model with effect plots of predictions (lines) and 95% CIs (bands) for fat mass index SDS. A: When taking the median gestational age and AUC of PN while receiving no human milk at the time of measurement, the fat mass index SDS increases with increasing age for both male and female infants, but for male infants, the fat mass index SDS is higher than for female infants. B: When taking the median gestational age and AUC of PN while receiving human milk at the time of measurement, the fat mass index SDS increases with increasing age for both male and female infants, but for male infants, the fat mass index SDS is higher than for female infants. PN, parenteral nutrition; SDS, SD score.
FIGURE 5.
Effect plot of the fat mass index SDS for male and female infants at 2-mo corrected age (A) and at 6-mo corrected age (B) with increasing AUC of parenteral nutrition. Linear mixed-effects model with effect plots of predictions (lines) and 95% CIs (bands) for fat mass index SDS. When gestational age and receiving any human milk remain the same, the fat mass index SDS increases with increasing AUC of parenteral nutrition in female infants but decreases in male infants. SDS, SD score.
Receiving human milk was not significantly associated with a higher FFM index SDS over time (P = 0.069), neither was the overall effect of the AUC of PN on FFM index SDS (P = 0.578).
Discussion
In this unique prospective study, we showed that among infants who received PN for a median duration of 7 wk after intestinal surgery, growth parameters and FM index were compromised in the first 6 mo of life, but FFM index was adequate. The cumulative amount of PN they received was positively associated with the FM index in female infants, but negatively associated with the FM index in male infants. Our findings are reassuring and suggest that patients in this early phase of disease are not overfed while on PN.
We found compromised weight-for-age and length-for-age during the complete follow-up period, consistent with the results of studies assessing growth from birth up to 2 y of age in patients who underwent neonatal gastrointestinal surgery (40–43). A possible explanation for poor growth despite EN and PN prescription according to international guidelines is that energy requirements can be even higher especially during periods of recovery when catch-up growth needs to be achieved (44). Fortunately, an increase in weight-, length-, and head circumference-for-age SDS was seen by the end of follow-up at 26 wk after intestinal surgery, but SDS were still lower than the normal population. Possibly, postsurgical neonates require more strict monitoring of nutritional intake and anthropometric parameters combined with individualized and sometimes more aggressive feeding regimens. Another explanation for poor growth may be the concern that infants with cholestasis/intestinal-failure-associated liver disease receive inadequate calories because of a reduction in the amount of intravenous lipids by changing to fish-oil-based lipid emulsions at a lower dose. However, in a previous study in infants with IF and cholestasis, infants receiving lower dosed fish-oil-based lipid emulsions showed comparable growth to infants receiving soybean emulsions (45). We know from previous studies that in older children with IF, height-for-age may decrease after ceasing PN, which emphasizes the need for continued growth monitoring of these patients (46).
Our finding of decreased FM and adequate FFM is not consistent with the increased FM and decreased FFM seen in older children receiving long-term PN (18, 19). This difference in BC outcome might be explained by different study populations, as we only included surgical patients whereas children in these previous studies (18, 19) included children with not only surgical conditions but also enteropathies and motility disorders. Persistent intestinal inflammation observed in some of these children may also exacerbate FM accumulation (18). More importantly, the children in previous studies were much older (18, 19). Decreased physical activity has been associated with increased FM and decreased FFM (16, 17), and is prevalent in children with IF (47). Lack of physical activity becomes more apparent with increasing age: we speculate that this contributes to increasing differences in BC outcomes between infants and older children. Our results of low FM are consistent with findings of a study comparing BC in infants after neonatal gastrointestinal surgery with healthy peers (40).
Almost half of the infants of the present study were PN dependent for >60 d and therefore met criteria for IF (20). Infants with NEC received PN for a longer period of time than infants with gastroschisis and intestinal atresia, consistent with previous studies (41, 43, 48, 49). The group of infants with >60 d of PN did not differ in anthropometric or BC parameters, compared with the group with shorter PN duration. However, in our exploratory analysis when taking into account multiple factors, PN did play a role in predicting the FM index. Surprisingly, the FM index of male infants decreased but that of female infants increased with increasing cumulative amount of PN. An explanation for this difference may be found in the sex difference in composition of human milk. Maternal human milk has been reported to naturally supply more lipids and energy to male infants than to female infants (50). In a recent review, the authors describe that in studies with predominantly human milk, no growth differences between male and female infants are seen. Conversely, there are sex differences in growth in studies with PN administration (providing the same nutrients to male and female infants, not adjusted to sex) (51). Possibly, decreases in FM index in male infants receiving predominantly PN could be due to needing more lipids and energy than provided. Moreover, in general, it is known that females at various ages, from infancy to adulthood, use energy at a slower rate than males (52, 53) and are more efficient in conserving energy and storing it as fat (33, 54). Although this phenomenon has not been studied in infants with IF, it could be a possible explanation for the increased FM index with increasing amount of PN in female infants. Yet, our results should be interpreted with caution because of the small number of infants.
Our study has several strengths. To our knowledge, this study is the first to prospectively assess longitudinal BC outcomes in infants after neonatal intestinal surgery. This allowed us to evaluate FM and FFM changes over time. We reached a high participation rate with 95 out of 119 (80%) of the approached parents consenting to participate in the study, making it a representative sample of the population. Using air-displacement plethysmography for the assessment of BC has important advantages over other BC techniques as it is relatively quick, has no exposure to radiation, no motion artifacts, and the possibility to correct for the central venous catheter and other additional devices (19, 33). Moreover, we have confirmed that it is important to measure BC in addition to standard anthropometrics, since BMI was not a good substitute for FM and FFM index in infants, as was seen before in older children (19).
A limitation of this study was that, unfortunately, it was not possible to include all eligible patients because of quick transfer to another hospital or unavailability of a legal guardian to obtain consent. We excluded patients from non-Dutch or non-English speaking parents; in the future, we should consider translating informed consent folders. Also, we were not able to perform an air-displacement plethysmography assessment in every participating infant, mainly due to logistical reasons. However, the infants included with no BC measurement did not significantly differ in sex, underlying disease, presence of short bowel syndrome, duration of PN dependency, or anthropometrics SDS from the infants with BC measurements, limiting possible selection bias on BC outcomes. Another limitation is the need to use different reference data sources for calculating anthropometric SDS before 40 wk of gestational age and after (Fenton and WHO, respectively), which could introduce a systematic error. When inspecting the raw data, this did not seem to be the case. Finally, actual enteral energy intake could not be calculated because we could not quantify the intake of breastfeeding throughout the study period.
BC abnormalities in early childhood are associated with adverse long-term health effects. In the first months of life we did not observe worrisome alterations in BC in infants after neonatal intestinal surgery. FFM increased faster than the reference population and towards normal, which looks like a successful catch-up in FFM, whereas FM did not increase faster than the reference population. This is reassuring, since gaining FFM is beneficial and gaining FM too quickly is not. However, we should be careful that catch-up growth does not proceed too quickly after 6-mo corrected age, because rapid weight gain, especially FM gain, in early infancy predisposes to midchildhood overweight and obesity (55). The question arises when the turning point will be, if there is one. Future studies should focus on growth and BC outcomes after 6-mo corrected age, to find out if continuation of monitoring is needed to detect deteriorations later in life. Our finding that sex and the amount of PN interact in their effect on FM also needs further investigation to find out if PN prescription should possibly be sex specific.
In conclusion, we observed compromised weight and length parameters, and low rather than excess fat accumulation in infants requiring PN after neonatal intestinal surgery during the first 6 mo. However, a higher amount of PN was associated with a higher FM index in female infants. Whether abnormalities in growth and BC persist during childhood necessitating ongoing monitoring after the age of 6 mo is yet to be explored by further research.
Supplementary Material
Acknowledgments
We are grateful to Roos Robert, Bo van Schijndel, and Leslie Strengers for performing several air-displacement plethysmography measurements and collecting data. We thank the Erasmus MC Sophia Children's Hospital, Department of Pediatrics, Division of Endocrinology, for the opportunity to use the PEA POD®.
The authors’ responsibilities were as follows—EGN, JFO, RMHW, EHHMR, BAEdK, and JMH: designed the research; LEV, EGN, WLMK, JFO, MJV, JAR, JCKW, MSF, BAEdK, and JMH: conducted the research; LEV and DR: performed statistical analysis; LEV: drafted the manuscript; LEV, EGN, JCKW, MSF, BAEdK, and JMH: revised the manuscript; LEV and JMH: had primary responsibility for content of the manuscript; all authors: critically reviewed the manuscript for important intellectual content and read and approved the final manuscript. The authors report no conflicts of interest.
Notes
LEV's salary was supported by funding from the Stichting Vrienden van het Sophia (Erasmus MC Sophia Children's Hospital, “Sporten voor Sophia” event). The funder had no role in the design, conduct, or data-analysis of the study, or drafting of the manuscript.
Supplemental Figures 1–4 and Supplemental Table 1 are available from the “Supplementary data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/ajcn/.
LEV and EGN share first authorship.
Abbreviations used: BC, body composition; EN, enteral nutrition; FFM, fat-free mass; FM, fat mass; IF, intestinal failure; NEC, necrotizing enterocolitis; PN, parenteral nutrition; SDS, SD score.
Contributor Information
Lotte E Vlug, Department of Pediatrics, Division of Gastroenterology, Erasmus MC University Medical Center Sophia Children's Hospital, Rotterdam, The Netherlands.
Esther G Neelis, Department of Pediatrics, Division of Gastroenterology, Erasmus MC University Medical Center Sophia Children's Hospital, Rotterdam, The Netherlands.
Jonathan C K Wells, Childhood Nutrition Research Centre, University College London Great Ormond Street Institute of Child Health, London, United Kingdom; Population, Policy, and Practice Programme, University College London Great Ormond Street Institute of Child Health, London, United Kingdom.
Mary S Fewtrell, Childhood Nutrition Research Centre, University College London Great Ormond Street Institute of Child Health, London, United Kingdom; Population, Policy, and Practice Programme, University College London Great Ormond Street Institute of Child Health, London, United Kingdom.
Wendy L M Kastelijn, Department of Internal Medicine, Division of Dietetics, Erasmus MC University Medical Center Sophia Children's Hospital, Rotterdam, The Netherlands.
Joanne F Olieman, Department of Internal Medicine, Division of Dietetics, Erasmus MC University Medical Center Sophia Children's Hospital, Rotterdam, The Netherlands.
Marijn J Vermeulen, Department of Neonatology, Erasmus MC University Medical Center Sophia Children's Hospital, Rotterdam, The Netherlands.
Jorine A Roelants, Department of Neonatology, Erasmus MC University Medical Center Sophia Children's Hospital, Rotterdam, The Netherlands.
Dimitris Rizopoulos, Department of Biostatistics, Erasmus MC University Medical Center, Rotterdam, The Netherlands.
René M H Wijnen, Department of Pediatric Surgery, Erasmus MC University Medical Center Sophia Children's Hospital, Rotterdam, The Netherlands.
Edmond H H M Rings, Department of Pediatrics, Division of Gastroenterology, Erasmus MC University Medical Center Sophia Children's Hospital, Rotterdam, The Netherlands; Department of Pediatrics, Division of Gastroenterology, Leiden University Medical Center Willem Alexander Children's Hospital, Leiden, The Netherlands.
Barbara A E de Koning, Department of Pediatrics, Division of Gastroenterology, Erasmus MC University Medical Center Sophia Children's Hospital, Rotterdam, The Netherlands.
Jessie M Hulst, Department of Pediatrics, Division of Gastroenterology, Erasmus MC University Medical Center Sophia Children's Hospital, Rotterdam, The Netherlands; Division of Pediatric Gastroenterology, Hepatology and Nutrition, The Hospital for Sick Children, Toronto, Canada.
Data Availability
Data described in the manuscript, code book, and analytic code will be made available upon request to the corresponding author.
References
- 1. Fleming CR, Remington M. Intestinal failure. In: Hill GL. editor, Nutrition and the Surgical Patient. Edinburgh: Churchill Livingstone; 1981. p. 219–35. [Google Scholar]
- 2. Goulet O, Ruemmele F. Causes and management of intestinal failure in children. Gastroenterology. 2006;130(2):S16–28. [DOI] [PubMed] [Google Scholar]
- 3. Koletzko B, Goulet O, Hunt J, Krohn K, Shamir R, Parenteral Nutrition Guidelines Working Group, European Society for Clinical Nutrition and Metabolism, European Society of Paediatric Gastroenterology, Hepatology and Nutrition (ESPGHAN), European Society of Paediatric Research (ESPR) . 1. Guidelines on Paediatric Parenteral Nutrition of the European Society of Paediatric Gastroenterology, Hepatology and Nutrition (ESPGHAN) and the European Society for Clinical Nutrition and Metabolism (ESPEN), supported by the European Society of Paediatric Research (ESPR). J Pediatr Gastroenterol Nutr. 2005;41:Suppl 2:S1–87. [DOI] [PubMed] [Google Scholar]
- 4. Breij LM, Kerkhof GF, Hokken-Koelega AC. Accelerated infant weight gain and risk for nonalcoholic fatty liver disease in early adulthood. The Journal of Clinical Endocrinology & Metabolism. 2014;99(4):1189–95. [DOI] [PubMed] [Google Scholar]
- 5. Chung ST, Onuzuruike AU, Magge SN. Cardiometabolic risk in obese children. Ann N Y Acad Sci. 2018;1411(1):166–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Singhal A, Lucas A. Early origins of cardiovascular disease: is there a unifying hypothesis?. Lancet North Am Ed. 2004;363(9421):1642–5. [DOI] [PubMed] [Google Scholar]
- 7. Kelishadi R, Haghdoost AA, Jamshidi F, Aliramezany M, Moosazadeh M. Low birthweight or rapid catch-up growth: which is more associated with cardiovascular disease and its risk factors in later life? A systematic review and cryptanalysis. Paediatrics and International Child Health. 2015;35(2):110–23. [DOI] [PubMed] [Google Scholar]
- 8. Santos S, Gaillard R, Oliveira A, Barros H, Hofman A, Franco OH, Jaddoe VW. Subcutaneous fat mass in infancy and cardiovascular risk factors at school-age: the generation r study. Obesity. 2016;24(2):424–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Leunissen RW, Stijnen T, Hokken-Koelega AC. Influence of birth size on body composition in early adulthood: the programming factors for growth and metabolism (PROGRAM)-study. Clin Endocrinol (Oxf). 2009;70(2):245–51. [DOI] [PubMed] [Google Scholar]
- 10. Wells JCK. Using body composition assessment to evaluate the double burden of malnutrition. Ann Nutr Metab. 2019;75(2):103–8. [DOI] [PubMed] [Google Scholar]
- 11. Scheurer JM, Zhang L, Plummer EA, Hultgren SA, Demerath EW, Ramel SE. Body composition changes from infancy to 4 years and associations with early childhood cognition in preterm and full-term children. Neonatology. 2018;114(2):169–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Jeddi M, Dabbaghmanesh MH, Ranjbar Omrani G, Ayatollahi SM, Bagheri Z, Bakhshayeshkaram M. Relative importance of lean and fat mass on bone mineral density in Iranian children and adolescents. International Journal of Endocrinology and Metabolism. 2015;13(3):e25542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Rokoff LB, Rifas-Shiman SL, Switkowski KM, Young JG, Rosen CJ, Oken E, Fleisch AF. Body composition and bone mineral density in childhood. Bone. 2019;121:9–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Pfister KM, Zhang L, Miller NC, Ingolfsland EC, Demerath EW, Ramel SE. Early body composition changes are associated with neurodevelopmental and metabolic outcomes at 4 years of age in very preterm infants. Pediatr Res. 2018;84(5):713–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Strydom K, Van Niekerk E, Dhansay MA. Factors affecting body composition in preterm infants: assessment techniques and nutritional interventions. Pediatrics & Neonatology. 2019;60(2):121–8. [DOI] [PubMed] [Google Scholar]
- 16. Correa-Rodriguez M, Rueda-Medina B, Gonzalez-Jimenez E, Schmidt-RioValle J. Associations between body composition, nutrition, and physical activity in young adults. Am J Hum Biol. 2017;29(1):e22903. [DOI] [PubMed] [Google Scholar]
- 17. Bosch L, Wells JCK, Lum S, Reid AM. Associations of extracurricular physical activity patterns and body composition components in a multi-ethnic population of UK children (the size and lung function in children study): a multilevel modelling analysis. BMC Public Health. 2019;19(1):573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Pichler J, Chomtho S, Fewtrell M, Macdonald S, Hill S. Body composition in paediatric intestinal failure patients receiving long-term parenteral nutrition. Arch Dis Child. 2014;99(2):147–53. [DOI] [PubMed] [Google Scholar]
- 19. Neelis E, Kouwenhoven S, Olieman J, Tabbers M, Jonkers C, Wells J, Fewtrell M, Wijnen R, Rings E, de Koning B et al. Body composition using air displacement plethysmography in children with intestinal failure receiving long-term home parenteral nutrition. JPEN J Parenter Enteral Nutr. 2020;; 44(2):318–26. [DOI] [PubMed] [Google Scholar]
- 20. Merritt RJ, Cohran V, Raphael BP, Sentongo T, Volpert D, Warner BW, Goday PS, Nutrition Committee of the North American Society for Pediatric Gastroenterology H, Nutrition . Intestinal rehabilitation programs in the management of pediatric intestinal failure and short bowel syndrome. Journal of Pediatric Gastroenterology & Nutrition. 2017;65(5):588–96. [DOI] [PubMed] [Google Scholar]
- 21. Olieman JF, Tibboel D, Penning C. Growth and nutritional aspects of infantile short bowel syndrome for the past 2 decades. J Pediatr Surg. 2008;43(11):2061–9. [DOI] [PubMed] [Google Scholar]
- 22. Belza C, Fitzgerald K, de Silva N, Avitzur Y, Wales PW. Early predictors of enteral autonomy in pediatric intestinal failure resulting from short bowel syndrome: development of a disease severity scoring tool. Journal of Parenteral and Enteral Nutrition. 2019;43(8):961–9. [DOI] [PubMed] [Google Scholar]
- 23. Wendel D, Mezoff EA, Raghu VK, Kinberg S, Soden J, Avitzur Y, Rudolph JA, Gniadek M, Cohran VC, Venick RS et al. Management of central venous access in children with intestinal failure: a position paper from the NASPGHAN intestinal rehabilitation special interest group. Journal of Pediatric Gastroenterology & Nutrition. 2021;72(3):474–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Marino L, Meyer R, Kruizenga HM, Wierdsma NJ. Disease specific classifications and requirements. Dietetic pocket guide paediatrics. Amsterdam, VU University Press, 2019; 209–22. [Google Scholar]
- 25. Fenton TR, Kim JH. A systematic review and meta-analysis to revise the Fenton growth chart for preterm infants. BMC Pediatrics. 2013;13(1):59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Cormack BE, Embleton ND, van Goudoever JB, Hay WW Jr, Bloomfield FH. Comparing apples with apples: it is time for standardized reporting of neonatal nutrition and growth studies. Pediatr Res. 2016;79(6):810–20. [DOI] [PubMed] [Google Scholar]
- 27. WHO Multicentre Growth Reference Study Group . WHO child growth standards based on length/height, weight and age. Acta Paediatr Suppl. 2006;450:76–85. [DOI] [PubMed] [Google Scholar]
- 28. Ellis KJ, Yao M, Shypailo RJ, Urlando A, Wong WW, Heird WC. Body-composition assessment in infancy: air-displacement plethysmography compared with a reference 4-compartment model. Am J Clin Nutr. 2007;85(1):90–5. [DOI] [PubMed] [Google Scholar]
- 29. Mazahery H, von Hurst PR, McKinlay CJD, Cormack BE, Conlon CA. Air displacement plethysmography (pea pod) in full-term and pre-term infants: a comprehensive review of accuracy, reproducibility, and practical challenges. Maternal Health, Neonatology and Perinatology. 2018;4(1):12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ma G, Yao M, Liu Y, Lin A, Zou H, Urlando A, Wong WW, Nommsen-Rivers L, Dewey KG. Validation of a new pediatric air-displacement plethysmograph for assessing body composition in infants. Am J Clin Nutr. 2004;79(4):653–60. [DOI] [PubMed] [Google Scholar]
- 31. Sainz RD, Urlando A. Evaluation of a new pediatric air-displacement plethysmograph for body-composition assessment by means of chemical analysis of bovine tissue phantoms. Am J Clin Nutr. 2003;77(2):364–70. [DOI] [PubMed] [Google Scholar]
- 32. VanItallie TB, Yang MU, Heymsfield SB, Funk RC, Boileau RA. Height-normalized indices of the body's fat-free mass and fat mass: potentially useful indicators of nutritional status. Am J Clin Nutr. 1990;52(6):953–9. [DOI] [PubMed] [Google Scholar]
- 33. Wells JCK, Davies PSW, Fewtrell MS, Cole TJ. Body composition reference charts for UK infants and children aged 6 weeks to 5 years based on measurement of total body water by isotope dilution. Eur J Clin Nutr. 2020;74(1):141–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Cole TJ, Green PJ. Smoothing reference centile curves: the LMS method and penalized likelihood. Stat Med. 1992;11(10):1305–19. [DOI] [PubMed] [Google Scholar]
- 35. Wells JC. A Hattori chart analysis of body mass index in infants and children. Int J Obes. 2000;24(3):325–9. [DOI] [PubMed] [Google Scholar]
- 36. Meyers JM, Greecher CP, Shaffer ML, Shenberger JS. Potential influence of total parenteral nutrition on body composition at discharge in preterm infants. J Matern Fetal Neonatal Med. 2013;26(15):1548–53. [DOI] [PubMed] [Google Scholar]
- 37. Breij LM, Abrahamse-Berkeveld M, Acton D, De Lucia Rolfe E, Ong KK, Hokken-Koelega ACS. Impact of early infant growth, duration of breastfeeding and maternal factors on total body fat mass and visceral fat at 3 and 6 months of age. Ann Nutr Metab. 2017;71(3–4):203–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Fields DA, Krishnan S, Wisniewski AB. Sex differences in body composition early in life. Gender Medicine. 2009;6(2):369–75. [DOI] [PubMed] [Google Scholar]
- 39. Wells JC, Dumith SC, Ekelund U, Reichert FF, Menezes AM, Victora CG, Hallal PC. Associations of intrauterine and postnatal weight and length gains with adolescent body composition: prospective birth cohort study from Brazil. J Adolesc Health. 2012;51(6 Suppl):S58–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. De Cunto A, Paviotti G, Travan L, Bua J, Cont G, Demarini S. Impact of surgery for neonatal gastrointestinal diseases on weight and fat mass. J Pediatr. 2015;167(3):568–71. [DOI] [PubMed] [Google Scholar]
- 41. Varma S, Bartlett EL, Nam L, Shores DR. Use of breast milk and other feeding practices following gastrointestinal surgery in infants. Journal of Pediatric Gastroenterology & Nutrition. 2019;68(2):264–71. [DOI] [PubMed] [Google Scholar]
- 42. McLaughlin CM, Channabasappa N, Pace J, Nguyen H, Piper HG. Growth trajectory in children with short bowel syndrome during the first 2 years of life. Journal of Pediatric Gastroenterology & Nutrition. 2018;66(3):484–8. [DOI] [PubMed] [Google Scholar]
- 43. Hall NJ, Drewett M, Burge DM, Eaton S. Growth pattern of infants with gastroschisis in the neonatal period. Clinical Nutrition ESPEN. 2019;32:82–7. [DOI] [PubMed] [Google Scholar]
- 44. Pierro A, Eaton S. Metabolism and nutrition in the surgical neonate. Semin Pediatr Surg. 2008;17(4):276–84. [DOI] [PubMed] [Google Scholar]
- 45. Raphael BP, Mitchell PD, Gura KM, Potemkin AK, Squires RH, Puder M, Duggan CP. Growth in infants and children with intestinal failure-associated liver disease treated with intravenous fish oil. Journal of Pediatric Gastroenterology & Nutrition. 2020;70(2):261–8. [DOI] [PubMed] [Google Scholar]
- 46. Neelis E, Olieman J, Rizopoulos D, Wijnen R, Rings E, de Koning B, Hulst J. Growth, body composition, and micronutrient abnormalities during and after weaning off home parenteral nutrition. Journal of Pediatric Gastroenterology & Nutrition. 2018;67(5):e95–e100. [DOI] [PubMed] [Google Scholar]
- 47. So S, Patterson C, Evans C, Wales PW. Motor proficiency and generalized self-efficacy toward physical activity in children with intestinal failure. Journal of Pediatric Gastroenterology & Nutrition. 2019;68(1):7–12. [DOI] [PubMed] [Google Scholar]
- 48. Allin BSR, Long AM, Gupta A, Lakhoo K, Knight M, British Association of Paediatric Surgeons Congenital Anomalies Surveillance System Necrotising Enterocolitis C . One-year outcomes following surgery for necrotising enterocolitis: a UK-wide cohort study. Archives of Disease in Childhood - Fetal and Neonatal Edition. 2018;103(5):F46–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Hong CR, Zurakowski D, Fullerton BS, Ariagno K, Jaksic T, Mehta NM. Nutrition delivery and growth outcomes in infants with gastroschisis. Journal of Parenteral and Enteral Nutrition. 2018;42(5):913–9. [DOI] [PubMed] [Google Scholar]
- 50. Thakkar SK, Giuffrida F, Cristina CH, De Castro CA, Mukherjee R, Tran LA, Steenhout P, Lee le Y, Destaillats F. Dynamics of human milk nutrient composition of women from Singapore with a special focus on lipids. Am J Hum Biol. 2013;25(6):770–9. [DOI] [PubMed] [Google Scholar]
- 51. Tottman AC, Oliver CJ, Alsweiler JM, Cormack BE. Do preterm girls need different nutrition to preterm boys? Sex-specific nutrition for the preterm infant. Pediatr Res. 2021;89:313–17. [DOI] [PubMed] [Google Scholar]
- 52. Wells JC, Davies PS. The effect of diet and sex on sleeping metabolic rate in 12-week-old infants. Eur J Clin Nutr. 1995;49(5):329–35. [PubMed] [Google Scholar]
- 53. Butte NF, Wong WW, Hopkinson JM, Heinz CJ, Mehta NR, Smith EO. Energy requirements derived from total energy expenditure and energy deposition during the first 2 y of life. Am J Clin Nutr. 2000;72(6):1558–69. [DOI] [PubMed] [Google Scholar]
- 54. Wu BN, O'Sullivan AJ. Sex differences in energy metabolism need to be considered with lifestyle modifications in humans. Journal of Nutrition and Metabolism. 2011;2011:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Zheng M, Lamb KE, Grimes C, Laws R, Bolton K, Ong KK, Campbell K. Rapid weight gain during infancy and subsequent adiposity: a systematic review and meta-analysis of evidence. Obes Rev. 2018;19(3):321–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data described in the manuscript, code book, and analytic code will be made available upon request to the corresponding author.