ABSTRACT
Background
Endogenously formed advanced glycation end products (AGEs) may be important drivers of microvascular dysfunction and the microvascular complications of diabetes. AGEs are also formed in food products, especially during preparation methods involving dry heat.
Objectives
We aimed to assess cross-sectional associations between dietary AGE intake and generalized microvascular function in a population-based cohort.
Methods
In 3144 participants of the Maastricht Study (mean ± SD age: 60 ± 8 y, 51% men) the dietary AGEs Nε-(carboxymethyl)lysine (CML), Nε-(1-carboxyethyl)lysine (CEL), and Nδ-(5-hydro-5-methyl-4-imidazolon-2-yl)-ornithine (MG-H1) were estimated using the combination of our ultra-performance LC–tandem MS dietary AGE database and an FFQ. Microvascular function was determined in the retina as flicker light–induced arteriolar and venular dilation and as central retinal arteriolar and venular equivalents, in plasma as a z score of endothelial dysfunction biomarkers (soluble vascular adhesion molecule 1 and soluble intracellular adhesion molecule 1, soluble E-selectin, and von Willebrand factor), in skin as the heat-induced skin hyperemic response, and in urine as 24-h albuminuria. Associations were evaluated using multiple linear regression adjusting for demographic, cardiovascular, lifestyle, and dietary factors.
Results
Overall, intakes of CML, CEL, and MG-H1 were not associated with the microvascular outcomes. Although higher intake of CEL was associated with higher flicker light–induced venular dilation (β percentage change over baseline: 0.14; 95% CI: 0.02, 0.26) and lower plasma biomarker z score (β: −0.04 SD; 95% CI: −0.08, −0.00 SD), the effect sizes were small and their biological relevance can be questioned.
Conclusions
We did not show any strong association between habitual intake of dietary AGEs and generalized microvascular function. The contribution of dietary AGEs to generalized microvascular function should be further assessed in randomized controlled trials using specifically designed dietary interventions.
Keywords: dietary advanced glycation end products, microvascular function, population-based cohort, endothelial function, ultra-performance liquid chromatography tandem mass spectrometry
Introduction
Through a wide range of biological effects, advanced glycation end products (AGEs) may be important drivers of microvascular dysfunction (1, 2), which in turn is an important risk factor for microvascular diseases such as retinopathy, nephropathy, and neuropathy (3). Endogenous formation of AGEs, a heterogeneous group of sugar-modified proteins (4), is increased under conditions of chronic hyperglycemia and increased oxidative stress (5).
Aside from endogenous formation, AGEs are also formed during preparation of foods, especially in products such as heat-treated cereals and meats (6). We recently found in humans that dietary AGEs are associated with their concentrations in plasma, suggesting their absorption (7). Whether these dietary AGEs also contribute to microvascular function, and what mechanisms are involved, are currently unknown. Studies investigating the effects of dietary AGEs on microvascular function are scarce and mainly limited to plasma biomarkers of endothelial dysfunction. A meta-analysis of intervention trials showed that a low-AGE diet, compared with a high-AGE diet, reduces soluble vascular adhesion molecule 1 (sVCAM-1) concentrations (8). However, because microvascular function differs among tissues, measuring plasma biomarkers alone provides limited insight into general microvascular function (9). Whereas these plasma biomarkers reflect endothelial regulation of permeability, coagulation, fibrinolysis, and proliferation, microvascular function can be assessed by the endothelium-dependent vasodilation response to various stimuli, such as local heating of the skin and flicker light applied to the retina (9). One study investigating the effect of a low- or high-AGE diet for 6 wk found no differences in endothelial function (i.e., reactive hyperemia index) (10). However, most of these studies share common methodological limitations, namely relatively small sample sizes, short study durations, extreme differences in cooking methods to modulate dietary AGEs, and the usage of an immunohistochemistry-based database that contains only 1 dietary AGE, Nε-(carboxymethyl)lysine (CML). Several other AGEs are present in foods, including Nε-(1-carboxyethyl)lysine (CEL), and Nδ-(5-hydro-5-methyl-4-imidazolon-2-yl)-ornithine (MG-H1), which potentially have different biological effects. Because these AGEs are formed on different amino acids (CML and CEL from lysine, and MG-H1 from arginine), their intake may vary depending on an individual's dietary pattern. As such, the role of specific dietary AGEs in general microvascular function remains to be established.
In light of the foregoing, we investigated the association between habitual intake of the specific and well-characterized dietary AGEs CML, CEL, and MG-H1, and generalized microvascular function measured in the retina, skin, blood, and kidney in a large population-based cohort.
Methods
Study design and population
We used data from the Maastricht Study, an observational prospective population-based cohort study. The rationale and methodology have been described previously (11). In brief, the study focuses on the etiology, pathophysiology, complications, and comorbidities of type 2 diabetes mellitus (T2DM) and is characterized by an extensive phenotyping approach. Eligible for participation were all individuals aged between 40 and 75 y and living in the southern part of the Netherlands. Participants were recruited through mass media campaigns and from the municipal registries and the regional Diabetes Patient Registry via mailings. Recruitment was stratified according to known T2DM status, with an oversampling of individuals with T2DM, for reasons of efficiency. The present report includes cross-sectional data from the first 3451 participants, who completed the baseline survey between November 2010 and September 2013. The examinations of each participant were performed within a time window of 3 mo. The study has been approved by the institutional medical ethical committee (NL31329.068.10) and the Minister of Health, Welfare and Sports of the Netherlands (Permit 131088-105234-PG). All participants gave written informed consent. Primary outcomes of this study were measurements of microvascular function at several microvascular beds. In the retina, microvascular function was determined as flicker light–induced arteriolar and venular dilation and as central retinal arteriolar equivalent (CRAE) and central retinal venular equivalent (CRVE). In plasma, endothelial dysfunction was determined by endothelial dysfunction biomarkers [sVCAM-1 and soluble intracellular adhesion molecule 1 (sICAM-1), soluble E-selectin, and von Willebrand factor (vWf)]. In skin, this was determined as the heat-induced skin hyperemic response. Finally, in urine, this was determined as 24-h albuminuria.
Food intake and dietary AGEs
We assessed dietary intake by a validated 253-item FFQ (12). This FFQ contains 101 questions on consumption with a reference period of 1 y. The FFQ collected information on the intake of major food groups. All participants filled out the FFQ after their first visit to the study center.
Food intake was determined by the combination of frequency questions with quantity questions. For the frequency questions, 11 options were available ranging from “not used” to 7 d/wk. For the quantity questions, variable options were available based on 14 standard household servings, ranging from <1/d to >12/d. Average daily consumption of food items was then calculated by multiplying the frequency and amount. Energy and nutrient intakes were subsequently determined by transcribing food items into food codes embedded in the Dutch Food Composition Table 2011 (13). In addition, we determined the Dutch Healthy Diet (DHD) index based on these food intake data. The DHD index is a measure of diet quality because it assesses adherence to the Dutch dietary guidelines (14). A higher index has been associated with more nutrient-dense diets and lower risk of mortality (15, 16). In our statistical analyses we used a modified version of the DHD index that does not contain filtered coffee consumption because this information was not collected by the FFQ, and alcohol intake was not included because we already adjusted for alcohol intake as an individual variable.
Dietary AGE intake was determined by coupling the consumption of food items within the FFQ to our dietary AGE database (6). In this database, 3 major AGEs, CML, CEL, and MG-H1, were quantified in protein fractions of food products using highly specific ultra-performance liquid chromatography tandem mass spectrometry (UPLC-MS/MS). In total, this database includes >200 food products commonly consumed in a Western diet. For each participant, AGE intake was estimated as described previously (7). Some of the food products in the FFQ were not analyzed for AGE content. AGE contents of these specific products were estimated by matching them to other products that were comparable in macronutrient profile and preparation method. For example, for several fresh vegetables boiled in water, such as endive, beets, leek, and spinach, the same AGE content was used. By comparison, jarred peas and carrots were measured separately from fresh peas and carrots, because AGEs in jarred peas and carrots are higher because they contain added sugar and are heated to prolong shelf life (6). Only after completion of the FFQ were participants informed about their glucose metabolism status. As a result, estimation of dietary AGEs was not affected by a potential change in dietary habits upon dietary advice from health care workers in case participants were diagnosed with T2DM.
Assessment of microvascular function
All participants were asked to refrain from smoking and drinking caffeine-containing beverages for 3 h before the measurement. A light meal (breakfast or lunch), low in fat content, was allowed ≥90 min before the start of the measurements. For these microvascular measurements there is no international consensus on how to perform them in a standardized manner. Protocols for the Maastricht Study were designed based on our experience and best practice in other studies (9). All microvascular measurements have been extensively validated as described previously (17).
Retinal arteriolar and venular dilation response
The retinal vascular response to flicker light was assessed with the Dynamic Vessel Analyzer (DVA, Imedos) as described previously (18). Briefly, pupils were dilated with 0.5% tropicamide and 2.5% phenylephrine ≥15 min before the start of the examination. For safety reasons, participants with an intraocular pressure >30 mm Hg were excluded from the measurements. For each participant, either the left or the right eye was selected depending on the time of day the measurement was performed and without reference to participant characteristics. A straight arteriolar or venular segment ∼1.5 mm in length located 0.5–2 disc diameter from the margin of the optic disc in the temporal section was examined. Vessel diameter was automatically and continuously measured for 150 s. A baseline recording of 50 s was followed by a 40-s flicker light exposure period (flicker frequency 12.5 Hz, bright-to-dark contrast ratio 25:1), followed by a 60-s recovery period. Baseline retinal vascular diameters and flicker light–induced retinal vascular dilation were automatically calculated with the integrated DVA software (version 4.51; Imedos). Baseline retinal arteriolar/venular diameter was calculated as the mean diameter of the 20–50 s recording and was expressed in measurement units (MU), where 1 MU is equal to 1 mm of the Gullstrand eye.
The flicker light–induced retinal vascular dilation was expressed as the percentage retinal vascular dilation over baseline and based on the mean dilation achieved at time points 10 and 40 s during the flicker stimulation period. This dilation response depends on a process called neurovascular coupling, which involves endothelial function (17).
Static retinal vessel calibers
All fundus photographs were taken with an auto-focus, auto-shot, and auto-tracking fundus camera (Model AFC-230; Nidek) in an optic disc–centered field of view of 45° in a darkened room, as described previously (19). Static retinal vessel analysis (1 image of the left or right eye was randomly chosen for each participant) was performed using the RHINO software (20, 21). Optic disc detection and arteriole/venule classification were corrected manually. Retinal vessel diameters were measured at 0.5–1.0 disc diameter away from the optic disc margin and were presented as CRAE and CRVE in MU. The scale factor is based on the optic disc diameter, which is assumed to be 1800 μm (22), i.e., 1 MU = 1 pixelsize × 1800 μm/pixelsize of optic disc diameter. CRAE and CRVE represent the equivalent single-vessel parent diameter for the 6 largest arterioles and largest venules in the region of interest, respectively. The calculations were based on the improved Knudtson–Parr–Hubbard formula (23). Fundus photographs of insufficient quality, e.g., obstructed by lashes or defocused, were evaluated and discussed with a second observer and excluded on mutual agreement. Narrowing of retinal arterioles and widening of retinal venules are regarded as early indicators of cardiovascular disease (24).
Plasma biomarkers of endothelial dysfunction
The plasma biomarkers of endothelial dysfunction sVCAM-1, sICAM-1, and soluble E-selectin were measured in EDTA plasma samples with commercially available 4-plex sandwich immunoassay kits [Meso Scale Discovery (MSD)], as described elsewhere (25). vWf was determined in citrated plasma with sandwich ELISA (Dako). Concentrations of vWf were expressed as percentages of the vWf detected in pooled citrated plasma of healthy volunteers. For the present study, the intra- and interassay CVs were 3.5% and 5.9% for sVCAM-1, 2.5% and 5.3% for sICAM-1, 6.4% and 6.0% for soluble E-selectin, and 3.2% and 5.4% for vWF, respectively.
Skin hyperemic response
Skin blood flow was measured as described previously by means of a laser-Doppler system (Periflux 5000; Perimed) equipped with a thermostatic laser-Doppler probe (PF457; Perimed) at the dorsal side of the wrist of the left hand (26). The laser-Doppler output was recorded for 25 min with a sample rate of 32 Hz, which gives semiquantitative assessment of skin blood flow expressed in arbitrary perfusion units. Skin blood flow was first recorded unheated for 2 min to serve as a baseline. After the 2 min of baseline, the temperature of the probe was rapidly and locally increased to 44°C and was then kept constant until the end of the measurement. The heat-induced skin hyperemic response was expressed as the percentage increase in average perfusion units during the 23-min heating phase over the mean baseline perfusion units. Skin perfusion during a period of local heating is thought to be mainly endothelium-dependent (27, 28) and this method is commonly used as a test of skin microvascular function (9).
Albuminuria
Two 24-h urine collections were used to assess urinary albumin excretion. Urinary albumin concentration was measured with a standard immunoturbidimetric assay by an automatic analyzer (owing to a change of supplier by the Beckman Synchron LX20, Beckman Coulter Inc.; and the Roche Cobas 6000, F. Hoffmann-La Roche) and multiplied by collection volume to obtain the 24-h urinary albumin excretion. A urinary albumin concentration below the detection limit of the assay (2 mg/L for the Beckman Synchron LX20 and 3 mg/L for the Roche Cobas 6000) was set at 1.5 mg/L before multiplying by collection volume. Only urine collections with a collection time between 20 h and 28 h were considered valid. If needed, urinary albumin excretion was extrapolated to a 24-h excretion. Albuminuria is seen as a risk marker for generalized endothelial dysfunction (29).
Glucose metabolism status
To determine glucose metabolism, all participants (except those who used insulin) underwent a standardized 7-point oral-glucose-tolerance test after an overnight fast. Blood samples were taken at baseline and at 15, 30, 45, 60, 90, and 120 min after ingestion of a 75-g glucose drink. For safety reasons, participants with a fasting glucose concentration >11.0 mmol/L, as determined by a finger prick, did not undergo the oral-glucose-tolerance test. For these individuals, fasting glucose concentration and information about glucose-lowering medication were used to determine glucose metabolism status. Glucose metabolism was classified according to the WHO 2006 criteria into normal glucose tolerance, impaired fasting glucose, impaired glucose tolerance, and T2DM (30). For this study, we defined having either impaired fasting glucose or impaired glucose tolerance as prediabetes.
Covariates
Smoking status and history of cardiovascular disease were assessed by a questionnaire. Smoking status was categorized into never, former, and current smoker. Waist circumference, total cholesterol, HDL cholesterol, LDL cholesterol, triglycerides, fasting plasma glucose, and glycosylated hemoglobin were determined as described elsewhere (11). Estimated glomerular filtration rate (eGFR) was computed with the Chronic Kidney Disease Epidemiology Collaboration formula, using serum creatinine and cystatin C (31). Information on the use of lipid-modifying, antihypertensive medication, and/or glucose-lowering medication, that is, generic names, doses, and frequencies, was collected during an in-person medication interview.
Statistical methods
Analyses were conducted using SPSS version 25 for Windows (IBM Corporation). Baseline characteristics are shown for the total sample, and stratified by a dietary AGE z score that represents an individual's overall AGE intake. Because we measured 3 AGEs in food items which differ in abundance, we first calculated z scores for CEL, CML, and MG-H1, which were then averaged into a single dietary AGE z score. A z score for the plasma biomarkers of endothelial dysfunction was calculated similarly. Differences in baseline characteristics between individuals stratified by the dietary AGE z score were tested by means of a one-way ANOVA or chi-square test, as appropriate. Likewise, participants who were excluded from the analyses because of missing covariates were compared with the included participants by the same statistical methods.
We performed multiple linear regression to investigate the association of each standardized dietary AGE and the combined dietary AGE z score with generalized microvascular function expressed as the retinal microvascular dilation response, static retinal vessel calibers, plasma biomarkers of endothelial function, skin microvascular function, and albuminuria (unstandardized). We fitted 2 regression models. In model 1, we adjusted for age (y), sex (male/female), and glucose metabolism status (normal glucose metabolism, prediabetes, T2DM, and other types of diabetes), the latter owing to oversampling of individuals with T2DM in the Maastricht Study. In model 2, we in addition adjusted for cardiovascular disease risk factors and dietary factors: waist circumference (cm), total:HDL cholesterol ratio, triglycerides (mmol/L), use of lipid-lowering medication (% yes), office systolic blood pressure, use of antihypertensive medication (% yes), smoking status (former, current, never), total energy intake (kcal/d), educational level (low/middle/high), alcohol intake (g/d), and the DHD index. For the association between dietary AGEs and urinary albumin excretion, model 2 of the regression model was in addition adjusted for estimated glomerular filtration rate. To check whether the assumption of linearity for linear regression was met, regression models using dietary AGEs as continuous and categorical exposures were compared with a likelihood ratio test. None of the models gave a significantly better fit of the data using dietary AGEs as categorical exposures (data not shown). Hence, dietary AGEs were entered as continuous exposures in all models. Because urinary albumin excretion was positively skewed, a normal distribution was obtained by ln transformation.
We also performed several sensitivity analyses. Firstly, we introduced further adjustment for covariates reflecting microangiopathy in the regression models: history of cardiovascular disease, eGFR, retinopathy, and albuminuria (except for analyses in which albuminuria was the outcome). These covariates may introduce overcorrection, because they also reflect microvascular function. Secondly, we introduced further adjustment for physical activity in the regression models [as assessed by accelerometer data (ActivPAL), explained in the Supplemental Methods]. Because accelerometer data were not available in a relatively large number of participants, performing a complete case analysis with this covariate might have resulted in selection bias. Thirdly, we explored possible confounding by antihypertensive medication through further specification into renin–angiotensin–aldosterone system inhibitors and other types of antihypertensive medication. Fourthly, we used ambulant 24-h blood pressure measurements instead of office blood pressure measurements obtained during the vascular measurements (explained in the Supplemental Methods). Fifthly, we used BMI instead of waist circumference as a measure of obesity. In addition, we performed interaction analyses for age, sex, BMI, glucose metabolism status (as dummy variables), and eGFR (thus also adding eGFR as a covariate in the fully adjusted model) by adding interaction terms in our model. The interaction analyses for T2DM and BMI (with substitution of BMI for waist circumference in the regression model) were performed to investigate potential underreporting of individuals with T2DM, overweight, or obesity.
β Coefficients are reported with their 95% CIs and represent the effect on microvascular outcomes per 1-SD higher dietary AGE intake. Unless stated otherwise, β coefficients represent the fully adjusted model (model 2). P values < 0.05 were considered statistically significant.
Results
Population characteristics
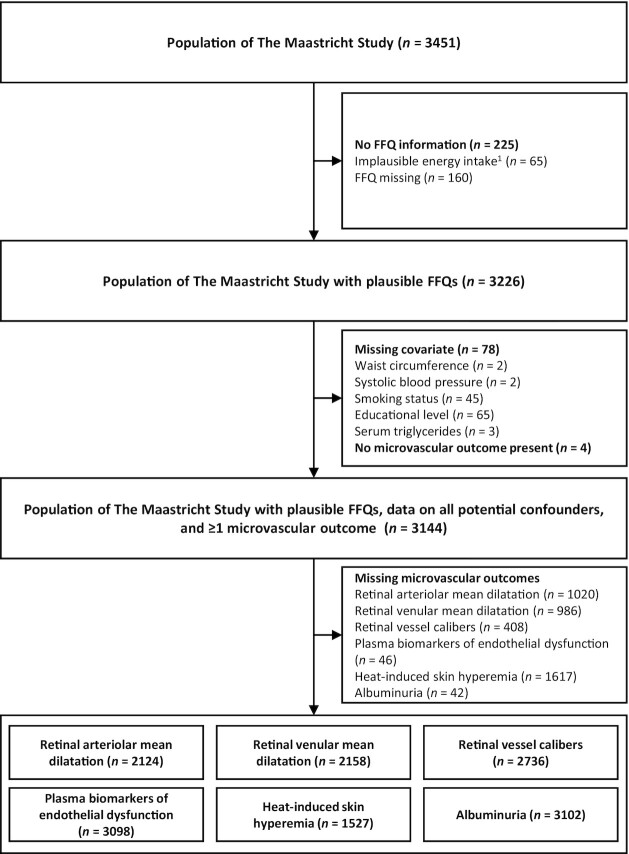
The selection of participants with available data on dietary AGEs, potential confounders, and estimates of microvascular function is shown in Figure 1, and their characteristics are shown in Table 1. Mean energy intake was 2182 kcal/d, intake of dietary CML was 3.3 mg/d, of CEL 3.0 mg/d, and of MG-H1 24.3 mg/d, which is comparable with other Dutch cohorts (Supplemental Table 1). Participants in the highest dietary AGE quartile were more likely to be younger men, more physically active, consumed more energy and coffee, and had a slightly less healthy diet (P < 0.05). These participants had slightly higher systolic and diastolic blood pressure, total:HDL cholesterol ratio, and albuminuria (P < 0.05). None of the other microvascular outcomes were statistically different among quartiles of dietary AGE intake. Although the microvascular outcomes could not be assessed in all participants, the selected populations were largely comparable (Supplemental Table 2). In general, participants excluded from analyses because of missing variables had a slightly worse cardiovascular disease risk profile (Supplemental Tables 3–8).
FIGURE 1.
Flowchart of participant selection for the different microvascular outcomes. Please note that missing variables do not necessarily add up because they are not mutually exclusive, e.g., an individual may have missing information on both smoking status and educational level. 1Implausible energy intake: <500 kcal or >3500 kcal for women, <800 or >4000 kcal for men.
TABLE 1.
Characteristics of those with information on all potential confounders and ≥1 microvascular outcome, stratified by overall dietary AGE intake1
| Total sample (n = 3144) | Dietary AGE quartiles (z score for all dietary AGEs) | ||||
|---|---|---|---|---|---|
| Characteristics | Q1 (n = 786) | Q2 (n = 786) | Q3 (n = 786) | Q4 (n = 786) | |
| Demographics | |||||
| Age, y | 59.9 ± 8.2 | 60.7 ± 8.1 | 60.0 ± 8.4 | 59.6 ± 8.1 | 59.2 ± 8.1 |
| Males | 51.0 | 35.7 | 48.7 | 52.8 | 66.5 |
| Educational level: low/medium/high | 33.5/28.2/38.3 | 38.0/26.1/35.9 | 32.3/27.9/39.9 | 34.0/28.6/37.4 | 29.9/30.2/39.9 |
| Glucose metabolism status: NGM/prediabetes/T2DM/other | 56.6/14.7/27.7/1.0 | 55.3/13.6/30.1/1.0 | 55.9/13.9/29.5/0.8 | 58.0/15.8/25.1/1.1 | 57.1/15.5/26.1/1.3 |
| Lifestyle | |||||
| Smoking: never/former/current | 35.0/52.2/12.8 | 32.9/52.7/14.4 | 36.0/49.9/14.1 | 35.5/52.5/12.0 | 35.8/53.7/10.6 |
| Waist circumference, cm | |||||
| Males | 101.5 ± 12.0 | 103.0 ± 11.8 | 101.6 ± 12.1 | 101.6 ± 11.5 | 100.6 ± 12.5 |
| Females | 89.8 ± 12.8 | 90.1 ± 13.5 | 88.8 ± 11.5 | 89.7 ± 12.5 | 91.2 ± 13.8 |
| BMI, kg/m2 | 27.0 ± 4.5 | 27.1 ± 4.7 | 26.8 ± 4.3 | 27.0 ± 4.4 | 27.2 ± 4.7 |
| Physical activity,2 min/d | 119.3 ± 42.0 | 115.3 ± 43.4 | 116.5 ± 38.7 | 118.5 ± 39.6 | 127.0 ± 45.3 |
| Biological | |||||
| Office systolic BP, mm Hg | 135.0 ± 18.2 | 134.7 ± 18.9 | 133.9 ± 18.2 | 135.5 ± 18.4 | 135.9 ± 17.4 |
| Office diastolic BP, mm Hg | 76.1 ± 9.9 | 75.4 ± 9.9 | 75.3 ± 9.8 | 76.8 ± 9.8 | 77.0 ± 10.0 |
| Antihypertensive medication, yes | 40.3 | 44.3 | 40.2 | 38.9 | 37.9 |
| Total:HDL cholesterol ratio | 3.7 ± 1.2 | 3.6 ± 1.2 | 3.6 ± 1.2 | 3.7 ± 1.1 | 3.8 ± 1.2 |
| Triglycerides, mmol/L | 1.4 ± 0.9 | 1.4 ± 0.8 | 1.4 ± 0.9 | 1.4 ± 0.8 | 1.4 ± 0.9 |
| Lipid-modifying medication, yes | 36.5 | 38.6 | 38.0 | 34.5 | 35.0 |
| Glucose-lowering medication, yes | |||||
| Insulin | 6.8 | 8.0 | 6.9 | 6.5 | 6.0 |
| Oral | 20.2 | 21.2 | 21.8 | 18.8 | 19.1 |
| eGFR,3 mL · min−1 · 1.73 m−2 | 88.0 ± 15.1 | 86.8 ± 15.4 | 88.3 ± 14.5 | 88.1 ± 14.6 | 88.6 ± 15.1 |
| Retinopathy,3 yes | 1.6 | 1.0 | 2.0 | 1.5 | 2.1 |
| Medical history of cardiovascular disease,3 yes | 16.9 | 17.2 | 18.1 | 17.0 | 15.3 |
| Dietary intake | |||||
| Energy intake, kcal/d | 2182 ± 606 | 1620 ± 377 | 1988 ± 348 | 2302 ± 400 | 2819 ± 523 |
| Alcohol, g/d | 8.5 [1.4–18.8] | 7.2 [0.5–18.6] | 8.4 [1.5–17.6] | 8.6 [1.7–17.3] | 9.4 [2.5–20.5] |
| Coffee, g/d | 468.3 ± 296.9 | 418.9 ± 259.0 | 453.3 ± 274.9 | 476.8 ± 300.0 | 524.1 ± 338.5 |
| Tea, g/d | 139.8 [13.0–325.0] | 132.4 [7.5–325.0] | 139.8 [17.5–355.0] | 125.0 [19.4–325.0] | 162.5 [19.4–346.1] |
| Dutch Healthy Diet index4 | 76.0 ± 14.2 | 78.2 ± 14.2 | 77.0 ± 13.8 | 75.4 ± 14.2 | 73.4 ± 14.3 |
| Dietary CML, mg/d | 3.3 ± 1.1 | 2.0 ± 0.4 | 2.8 ± 0.3 | 3.5 ± 0.4 | 4.7 ± 0.9 |
| Dietary CEL, mg/d | 3.0 ± 1.2 | 1.8 ± 0.4 | 2.5 ± 0.3 | 3.1 ± 0.4 | 4.5 ± 1.4 |
| Dietary MG-H1, mg/d | 24.3 ± 8.8 | 15.5 ± 3.1 | 21.0 ± 2.5 | 25.7 ± 3.0 | 35.1 ± 8.8 |
| Retinal microvascular measurements5 | |||||
| Flicker light–induced arteriolar dilation response, % | 3.0 ± 2.8 | 3.0 ± 2.8 | 2.9 ± 2.8 | 3.1 ± 2.8 | 3.2 ± 2.8 |
| Flicker light–induced venular dilation response, % | 3.9 ± 2.2 | 3.9 ± 2.3 | 3.7 ± 2.1 | 3.8 ± 2.2 | 4.0 ± 2.2 |
| CRAE, µm | 142.3 ± 20.3 | 142.8 ± 20.8 | 142.9 ± 20.5 | 142.4 ± 19.7 | 141.2 ± 20.1 |
| CRVE, µm | 214.6 ± 31.4 | 214.3 ± 32.8 | 214.2 ± 30.8 | 215.9 ± 30.5 | 213.7 ± 31.4 |
| Plasma biomarkers of endothelial dysfunction6 | |||||
| sICAM-1, ng/mL | 354.2 ± 99.9 | 362.2 ± 112.9 | 352.9 ± 93.5 | 351.6 ± 90.4 | 350.2 ± 101.0 |
| sVCAM-1, ng/mL | 428.2 ± 101.3 | 429.8 ± 111.8 | 426.0 ± 101.4 | 426.2 ± 90.8 | 430.9 ± 101.3 |
| E-selectin, ng/mL | 118.3 ± 65.2 | 117.9 ± 66.7 | 117.0 ± 71.1 | 119.1 ± 59.9 | 118.3 ± 65.2 |
| von Willebrand factor, % | 132.6 ± 48.4 | 134.3 ± 51.1 | 131.9 ± 47.1 | 132.0 ± 48.4 | 132.4 ± 47.0 |
| Biomarker z score, SD | 0.0 ± 1.0 | 0.0 ± 1.1 | −0.0 ± 1.0 | −0.0 ± 0.9 | −0.0 ± 1.0 |
| Skin microvascular measurements7 | |||||
| Baseline skin blood flow, PU | 11.1 ± 6.5 | 11.3 ± 7.2 | 10.9 ± 6.1 | 11.1 ± 6.5 | 11.2 ± 6.0 |
| Skin hyperemic response, % | 1124.4 ± 774.1 | 1155.8 ± 788.7 | 1140.6 ± 759.0 | 1129.8 ± 793.0 | 1069.9 ± 752.8 |
| Kidney microvascular measurements | |||||
| Albuminuria,3 mg/24 h | 6.7 [4.0–11.9] | 6.9 [4.2–12.8] | 6.5 [3.8–11.8] | 6.4 [3.9–10.7] | 7.1 [4.3–12.3] |
Values are means ± SDs, medians [IQRs], or percentages. AGE, advanced glycation end product; BP, blood pressure; CEL, Nε-(1-carboxyethyl)lysine; CML, Nε-(carboxymethyl)lysine; CRAE, central retinal arteriolar equivalent; CRVE, central retinal venular equivalent; eGFR, estimated glomerular filtration rate; MG-H1, Nδ-(5-hydro-5-methyl-4-imidazolon-2-yl)-ornithine; NGM, normal glucose metabolism; other, type 1 diabetes or surgery/medicine-induced; PU, perfusion units; Q, quartile; sICAM-1, soluble intracellular adhesion molecule-1; sVCAM-1, soluble vascular adhesion molecule-1; T2DM, type 2 diabetes mellitus.
Physical activity data available in n = 2434.
Markers of microangiopathy, n = 3048.
Modified version of the Dutch Healthy Diet index that does not include alcohol intake.
Static retinal imaging, n = 2736; dynamic retinal imaging, n = 2124 for arteriolar dilation and n = 2158 for venular dilation.
Plasma biomarkers of endothelial dysfunction, n = 3098.
Skin microvascular measurements, n = 1527.
Dietary AGEs and retinal microcirculation
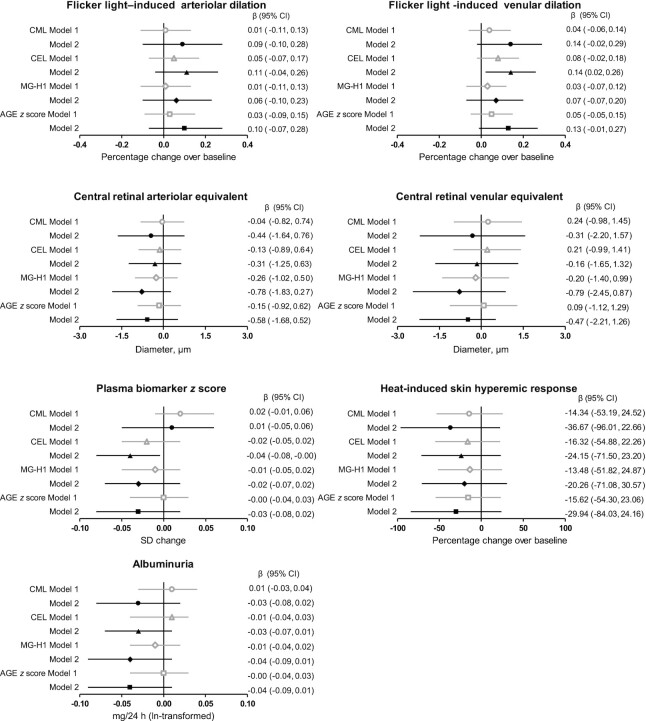
Baseline retinal vessel calibers before flicker light were not different between quartiles of dietary AGE intake (data not shown). In the fully adjusted model (model 2), dietary CML, CEL, MG-H1, and the combined dietary AGE z score were not associated with flicker light–induced retinal arteriolar dilation, with β (percentage change over baseline) of 0.09 (95% CI: −0.10, 0.28), 0.11 (95% CI: −0.04, 0.26), 0.06 (95% CI: −0.10, 0.23), and 0.10 (95% CI: −0.07, 0.28), respectively (Figure 2). Greater intake of CEL, but not CML, MG-H1, or the combined dietary AGE z score, was associated with greater flicker light–induced retinal venular dilation: β: 0.14 (95% CI: 0.02, 0.26), β: 0.14 (95% CI: −0.02, 0.29), β: 0.07 (95% CI: −0.07, 0.20), and β: 0.13 (95% CI: −0.01, 0.27), respectively (Figure 2).
FIGURE 2.
Multivariate-adjusted associations of dietary AGEs (in SD/d) and microvascular measurements tested with multiple linear regression analysis. βs (95% CIs) indicate the difference in microvascular outcome per 1-SD change in dietary AGE intake. Model 1: adjusted for participant characteristics: age, sex, and glucose metabolism status. Model 2: in addition adjusted for cardiovascular disease risk factors and lifestyle factors: waist circumference, total:HDL cholesterol ratio, triglycerides, smoking habits, use of lipid-lowering medication, office systolic blood pressure, use of antihypertensive medication, caloric intake, educational level, alcohol intake, and the Dutch Healthy Diet index. For albuminuria, model 2 was in addition adjusted for estimated glomerular filtration rate. Sample sizes (n): flicker light–induced arteriolar dilation: 2124; flicker light–induced venular dilation: 2158; central retinal arteriolar and venular equivalents: 2736; plasma biomarker z score: 3098; heat-induced skin hyperemia: 1527; and albuminuria: 3102. AGE, advanced glycation end product; CEL, Nε-(1-carboxyethyl)lysine; CML, Nε-(carboxymethyl)lysine; MG-H1, Nδ-(5-hydro-5-methyl-4-imidazolon-2-yl)-ornithine.
In the fully adjusted model, dietary CML, CEL, MG-H1, and the combined dietary AGE z score were not associated with CRAE (β: −0.44 µm; 95% CI: −1.64, 0.76 µm; β: −0.31 µm; 95% CI: −1.25, 0.63 µm; β: −0.78 µm; 95% CI: −1.83, 0.27 µm; and β: −0.58 µm; 95% CI: −1.68, 0.52 µm, respectively) or CRVE (β: −0.31 µm; 95% CI: −2.20, 1.57 µm; β: −0.16 µm; 95% CI: −1.65, 1.32 µm; β: −0.79 µm; 95% CI: −2.45, 0.87 µm; and β: −0.47 µm; 95% CI: −2.21, 1.26 µm, respectively) (Figure 2).
Dietary AGEs and plasma biomarkers of endothelial dysfunction
With the individual biomarkers combined into a single z score, greater intake of CEL, but not CML, MG-H1, or the combined dietary AGE z score, was associated with lower plasma endothelial dysfunction biomarkers z score in the fully adjusted model, with β of −0.04 SD (95% CI: −0.08, −0.00 SD), 0.01 SD (95% CI: −0.05, 0.06 SD), −0.02 SD (95% CI: −0.07, 0.02 SD), and −0.03 SD (95% CI: −0.08, 0.02 SD), respectively (Figure 2). Dietary CML, CEL, MG-H1, and the combined dietary AGE z score were not associated with any of the individual plasma biomarkers (Supplemental Table 9).
Dietary AGEs, the skin microvascular hyperemic response, and albuminuria
In the fully adjusted model, dietary AGEs CML, CEL, MG-H1, and the combined dietary AGE z score were not associated with the heat-induced skin hyperemic response, with β (percentage change over baseline) of −36.67 (95% CI: −96.01, 22.66), −24.15 (95% CI: −71.50, 23.20), −20.26 (95% CI: −71.08, 30.57), and −29.94 (95% CI: −84.03, 24.16), respectively, or 24-h albumin excretion, with β (mg/24 h, ln-transformed) of −0.03 (95% CI: −0.08, 0.02), −0.03 (95% CI: −0.07, 0.01), −0.04 (95% CI: −0.09, 0.01), and −0.04 (95% CI: −0.09, 0.01), respectively (Figure 2).
Sensitivity analyses
In general, further adjustment of model 2 for either physical activity or markers of microangiopathy did not significantly alter the observed effect sizes of the aforementioned associations. However, with further adjustment for physical activity (analyses in which fewer participants were available), statistical significance for the associations of CEL intake with the endothelial function biomarker z score (n = 2405) and flicker light–induced venular dilation (n = 1817) were already lost in the crude model (Supplemental Tables 10–13). All other sensitivity analyses did not materially change the results (data not shown).
Interaction analyses
In general, the observed associations between dietary AGEs and microvascular function were not modified by age, sex, BMI, glucose metabolism status, or eGFR (P-interaction > 0.05). Although the associations of dietary CML and MG-H1 with CRVE were significantly modified by presence of T2DM (P-interaction = 0.04), the associations of dietary CML and MG-H1 with CRVE in this subgroup was not statistically significantly different compared with associations among people with normal glucose metabolism or prediabetes (Supplemental Table 14). Contrarily, greater MG-H1 intake was associated with lower CRVE (β: −3.39 µm; 95% CI: −6.62, −0.15 µm) in participants with prediabetes (Supplemental Table 14).
Discussion
In this cross-sectional study, we are the first, to our knowledge, to investigate the association between 3 dietary AGEs, measured in selected food items using UPLC-MS/MS, and a broad array of microvascular measurements in a population-based cohort. We observed no consistent associations between habitual intake of the dietary AGEs CML, CEL, and MG-H1 and several microvascular measurements. Although there was a statistically significant association between higher intake of CEL, higher flicker light–induced venular dilation, and lower z score of combined plasma endothelial dysfunction biomarkers, this did not apply to the individual plasma biomarkers and the effect sizes were small.
Increasing evidence suggests that AGEs of dietary origin contribute to the body AGE pool. It is thought that dietary AGEs, consumed as whole proteins, undergo digestion in the gastrointestinal tract and subsequently enter the circulation in their free form (7). In the circulation, these free AGEs are in direct contact with the vascular endothelium and could potentially modulate microvascular function (9). AGEs are involved in endothelial function, at least partly, through binding to the receptor for AGEs (RAGE) expressed by endothelial cells, which subsequently leads to generation of reactive oxygen species and activation of NF-κB (32). However, this mechanism does not apply to free AGEs of dietary origin, because only protein-bound AGEs seem to have affinity for RAGE (33). In line with this, it is still uncertain whether dietary AGEs have any (harmful) consequences. Although meta-analyses suggest that a diet high in AGEs is linked to insulin resistance (34), increased inflammatory markers (35), and atherogenic dyslipidemia (8), they also conclude that high-quality trials are lacking (8, 35) and several individual studies found no such effects (10, 36–38). In addition, individual AGEs can potentially elicit different biological effects. Although we present the combined dietary AGE z score for easier interpretation of overall dietary AGE intake, it is important to consider that this may lead to loss of information. For example, biological effects of specific dietary AGEs could partly be mediated by interactions with the gut microbiome. Although some strains of Escherichia coli were shown to degrade CML into several metabolites with unknown biological effects (39), such effects have not yet been described for CEL and MG-H1.
We are the first, to our knowledge, to investigate associations between dietary AGEs and the retinal microcirculation in humans. We found no associations between dietary AGEs, central retinal arteriolar and venular diameters, and flicker light–induced arteriolar dilation, but surprisingly there was a small but statistically significant association between higher CEL intake and higher flicker light–induced venular dilation. Lower flicker light–induced retinal venular dilation was recently shown to be an independent predictor of all-cause mortality in end-stage renal disease patients (40). Factors that contribute to this response are inflammation, endothelial function, and NO bioavailability (41). As previously mentioned, studies investigating the effect of dietary AGEs on inflammation have yielded conflicting results (10, 35–38). We observed no robust associations between dietary AGEs and the central retinal venular diameter, which is also linked to systemic inflammation (42), or any of the other endothelium-dependent microvascular outcomes. Furthermore, statistical significance for the association between dietary CEL and flicker light–induced venular dilation was not consistent in sensitivity analyses with fewer participants. In light of the foregoing, its biological relevance can be questioned.
We showed a statistically significant but small association of a higher intake of CEL, but not CML, MG-H1, or the combined dietary AGE z score, with lower combined plasma endothelial dysfunction biomarkers z score. We found no associations between dietary AGEs and the individual plasma biomarkers. These findings are partly in disagreement with previous studies, although results so far have been inconsistent. Whereas some authors reported a decrease in sVCAM-1 after a low-AGE diet and an increase after a high-AGE diet (43–45), others found no difference (10). There are several possible explanations for the discrepancies between these studies and the present study. Next to differences in study design (cross-sectional compared with a controlled dietary intervention), our analyses were performed in a larger sample, and we investigated 3 dietary AGEs as assessed by UPLC-MS/MS, as opposed to only CML assessed by immunohistochemistry. In addition, we included not only sVCAM-1 but also sICAM-1, soluble E-selectin, and vWf. However, again, the observed effect size is small and thus its biological relevance may be questioned.
We found no association between intake of dietary AGEs and the heat-induced skin hyperemic response. This is in agreement with a previous study, where no associations were found between dietary AGEs, assessed by a 3-d food record, and either postocclusive or heat-induced skin hyperemia in 51 patients with chronic kidney disease and 51 controls (46). In contrast, Negrean et al. (47) showed an acute impairment in postocclusive skin hyperemia in 20 patients with T2DM after a fried/broiled high-AGE meal compared with a steamed/boiled low-AGE meal. However, owing to the extreme differences in preparation methods, the carcinogenic compound acrylamide or reduced micronutrient bioavailability may also have contributed to the observed differences (48). In line with this, the authors were not able to reproduce this impairment with administration of a specifically designed low- or high-AGE drink (49).
Finally, we found no association between dietary AGEs and albuminuria. Although there is little research to compare with, our findings are in agreement with findings by Harcourt et al. (50), who observed no difference in albuminuria in 11 healthy but overweight individuals after a 2-wk high-AGE diet. Albuminuria is an estimate not only of renal microvascular dysfunction (29) but also of several other microvascular pathologies (51, 52). As such, it may act as a marker of generalized endothelial dysfunction (53), and the null finding in the present study strengthens the observed lack of associations between dietary AGEs and our other microvascular outcomes.
It is important to consider whether a potentially low consumption of dietary AGEs may have influenced the ability to detect an association. However, AGE intake in the current cohort was largely comparable with those of other Dutch cohorts in which associations between dietary AGEs and several outcomes have been reported (7, 54, 55). Furthermore, the difference in intake between opposing quartiles of our cohort was approximately double that of the difference between the low- and high-AGE diets of a well-executed crossover randomized controlled trial (RCT) in humans that observed a change in insulin sensitivity (56). As such, dietary AGE intake in our cohort should have been sufficient to detect an association with an outcome.
The current study has several strengths. Primarily, we investigated associations between dietary AGEs and measurements from several microvascular beds in a large study sample. Combined with the extensive phenotyping and population-based approach of the Maastricht Study, this enabled us to draw conclusions on generalized microvascular function while controlling for several possible confounders. In addition, we used our UPLC-MS/MS-based dietary AGE database (6) and a validated FFQ to capture the majority of sources of AGEs. The FFQ covers >96% of total energy intake and a large list of nutrients (12), and has been updated with several high-AGE products that are commonly consumed in our cohort (blood sausage, toasted bread, beef stew). Lastly, whereas biological effects of dietary AGEs are often investigated under extreme circumstances (heated and nonheated food products), our FFQ-based approach measured habitual intake.
The current study also has several limitations. Several uncertainties may negatively affect the accuracy of estimated dietary AGE intake. The use of FFQs may introduce recall bias (57) and our FFQ and AGE database do not include detailed information about food preparation for all foods. Although this only applied to a minority of food products within the AGE database, food preparation, referring mainly to cooking techniques and heating duration, largely determines the quantity of AGEs in food (58). However, this will apply to all studies that use this approach, and our estimates of dietary AGE intake were in line with these other cohorts (54, 59, 60). Nonetheless, combining our dietary AGE database and an FFQ is currently the most accurate method to determine dietary AGE intake in larger cohorts. Despite our cross-sectional analyses being adjusted for a comprehensive set of a priori–defined covariates, we cannot completely rule out residual confounding. In addition, although we generally did not observe effect modification by age, sex, BMI, glucose metabolism status, or kidney function, our research question should be formally tested in other populations.
In summary, we did not find any strong association between habitual intake of dietary AGEs and generalized microvascular function. Nonetheless, our findings would benefit most from confirmation by longitudinal studies and RCTs in which participants receive specifically designed dietary interventions in order to minimize residual confounding by certain food products or the food preparation methods.
Supplementary Material
Acknowledgments
The authors’ responsibilities were as follows—AMAL: was responsible for data analysis, for data interpretation, and wrote the manuscript; AJHMH, SJMPE, and CGS: edited the manuscript; AJHMH, SJMPE, AAK, MTS, TTJMB, CABW, RMAH, BdG, MvG, CDAS, and CGS: participated in the data collection, conception, and design of the Maastricht Study; and all authors: read and approved the final manuscript. The authors report no conflicts of interest.
Notes
Supported by the European Regional Development Fund via OP-Zuid, the Province of Limburg, Dutch Ministry of Economic Affairs grant 31O.041, Stichting De Weijerhorst (Maastricht, Netherlands), the Pearl String Initiative Diabetes (Amsterdam, Netherlands), the Cardiovascular Center (Maastricht, Netherlands), CARIM School for Cardiovascular Diseases (Maastricht, Netherlands), CAPHRI Care and Public Health Research Institute (Maastricht, Netherlands), NUTRIM School for Nutrition and Translational Research in Metabolism (Maastricht, Netherlands), Stichting Annadal (Maastricht, Netherlands), Health Foundation Limburg (Maastricht, Netherlands), and by unrestricted grants from Janssen-Cilag BV (Tilburg, Netherlands), Novo Nordisk Farma BV (Alphen aan den Rijn, Netherlands), and Sanofi-Aventis Netherlands BV (Gouda, Netherlands). CGS has received funding from ZonMw and Diabetesfonds.
The funders had no role in the design of the current study, nor the writing, analysis, and interpretation process.
Supplemental Methods and Supplemental Tables 1–14 are available from the “Supplementary data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/ajcn/.
Abbreviations used: AGE, advanced glycation end product; CEL, Nε-(1-carboxyethyl)lysine; CML, Nε-(carboxymethyl)lysine; CRAE, central retinal arteriolar equivalent; CRVE, central retinal venular equivalent; DHD, Dutch Healthy Diet; DVA, Dynamic Vessel Analyzer; eGFR, estimated glomerular filtration rate; MG-H1, Nδ-(5-hydro-5-methyl-4-imidazolon-2-yl)-ornithine; MU, measurement units; RAGE, receptor for advanced glycation end products; RCT, randomized controlled trial; sICAM-1, soluble intracellular adhesion molecule 1; sVCAM-1, soluble vascular adhesion molecule 1; T2DM, type 2 diabetes mellitus; UPLC-MS/MS, ultra-performance liquid chromatography tandem mass spectrometry; vWf, von Willebrand factor.
Contributor Information
Armand M A Linkens, Department of Internal Medicine, Maastricht University Medical Center, Maastricht, Netherlands; CARIM School for Cardiovascular Diseases, Maastricht University, Maastricht, Netherlands.
Alfons J H M Houben, Department of Internal Medicine, Maastricht University Medical Center, Maastricht, Netherlands; CARIM School for Cardiovascular Diseases, Maastricht University, Maastricht, Netherlands.
Abraham A Kroon, Department of Internal Medicine, Maastricht University Medical Center, Maastricht, Netherlands; CARIM School for Cardiovascular Diseases, Maastricht University, Maastricht, Netherlands.
Miranda T Schram, Department of Internal Medicine, Maastricht University Medical Center, Maastricht, Netherlands; CARIM School for Cardiovascular Diseases, Maastricht University, Maastricht, Netherlands.
Tos T J M Berendschot, University Eye Clinic Maastricht, Maastricht University Medical Center, Maastricht, Netherlands.
Carroll A B Webers, University Eye Clinic Maastricht, Maastricht University Medical Center, Maastricht, Netherlands.
Marleen van Greevenbroek, Department of Internal Medicine, Maastricht University Medical Center, Maastricht, Netherlands; CARIM School for Cardiovascular Diseases, Maastricht University, Maastricht, Netherlands.
Ronald M A Henry, Department of Internal Medicine, Maastricht University Medical Center, Maastricht, Netherlands; CARIM School for Cardiovascular Diseases, Maastricht University, Maastricht, Netherlands; Heart and Vascular Center, Maastricht University Medical Center, Maastricht, Netherlands.
Bastiaan de Galan, Department of Internal Medicine, Maastricht University Medical Center, Maastricht, Netherlands; CARIM School for Cardiovascular Diseases, Maastricht University, Maastricht, Netherlands.
Coen D A Stehouwer, Department of Internal Medicine, Maastricht University Medical Center, Maastricht, Netherlands; CARIM School for Cardiovascular Diseases, Maastricht University, Maastricht, Netherlands.
Simone J M P Eussen, CARIM School for Cardiovascular Diseases, Maastricht University, Maastricht, Netherlands; Department of Epidemiology, Maastricht University, Maastricht, Netherlands; CAPHRI School for Care and Public Health Research Unit, Maastricht University, Maastricht, Netherlands.
Casper G Schalkwijk, Department of Internal Medicine, Maastricht University Medical Center, Maastricht, Netherlands; CARIM School for Cardiovascular Diseases, Maastricht University, Maastricht, Netherlands.
Data Availability
The data that underlie the findings of this study are available from the corresponding author and The Maastricht Study Management Team (research.dms@mumc.nl) upon reasonable request.
References
- 1. Bierhaus A, Hofmann MA, Ziegler R, Nawroth PP. AGEs and their interaction with AGE-receptors in vascular disease and diabetes mellitus. I. The AGE concept. Cardiovasc Res. 1998;37(3):586–600. [DOI] [PubMed] [Google Scholar]
- 2. Singh VP, Bali A, Singh N, Jaggi AS. Advanced glycation end products and diabetic complications. Korean J Physiol Pharmacol. 2014;18(1):1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Stehouwer CDA. Microvascular dysfunction and hyperglycemia: a vicious cycle with widespread consequences. Diabetes. 2018;67(9):1729–41. [DOI] [PubMed] [Google Scholar]
- 4. Farmer DG, Kennedy S. RAGE, vascular tone and vascular disease. Pharmacol Ther. 2009;124(2):185–94. [DOI] [PubMed] [Google Scholar]
- 5. Brownlee M. Advanced protein glycosylation in diabetes and aging. Annu Rev Med. 1995;46(1):223–34. [DOI] [PubMed] [Google Scholar]
- 6. Scheijen J, Clevers E, Engelen L, Dagnelie PC, Brouns F, Stehouwer CDA, Schalkwijk CG. Analysis of advanced glycation endproducts in selected food items by ultra-performance liquid chromatography tandem mass spectrometry: presentation of a dietary AGE database. Food Chem. 2016;190:1145–50. [DOI] [PubMed] [Google Scholar]
- 7. Scheijen J, Hanssen NMJ, van Greevenbroek MM, Van der Kallen CJ, Feskens EJM, Stehouwer CDA, Schalkwijk CG. Dietary intake of advanced glycation endproducts is associated with higher levels of advanced glycation endproducts in plasma and urine: the CODAM study. Clin Nutr. 2018;37(3):919–25. [DOI] [PubMed] [Google Scholar]
- 8. Baye E, Kiriakova V, Uribarri J, Moran LJ, de Courten B. Consumption of diets with low advanced glycation end products improves cardiometabolic parameters: meta-analysis of randomised controlled trials. Sci Rep. 2017;7:2266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Houben A, Martens RJH, Stehouwer CDA. Assessing microvascular function in humans from a chronic disease perspective. J Am Soc Nephrol. 2017;28(12):3461–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Semba RD, Gebauer SK, Baer DJ, Sun K, Turner R, Silber HA, Talegawkar S, Ferrucci L, Novotny JA. Dietary intake of advanced glycation end products did not affect endothelial function and inflammation in healthy adults in a randomized controlled trial. J Nutr. 2014;144(7):1037–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Schram MT, Sep SJ, van der Kallen CJ, Dagnelie PC, Koster A, Schaper N, Henry RM, Stehouwer CD. The Maastricht Study: an extensive phenotyping study on determinants of type 2 diabetes, its complications and its comorbidities. Eur J Epidemiol. 2014;29(6):439–51. [DOI] [PubMed] [Google Scholar]
- 12. van Dongen MCJM, Wijckmans-Duysens NEG, den Biggelaar LJCJ, Ocké MC, Meijboom S, Brants HAM, de Vries JHM, Feskens EJM, Bueno-de-Mesquita HB, Geelen A et al. The Maastricht FFQ: development and validation of a comprehensive food frequency questionnaire for the Maastricht study. Nutrition. 2019;62:39–46. [DOI] [PubMed] [Google Scholar]
- 13. Rijksinstituut voor Volksgezondheid en Milieu . Dutch food composition table (NEVO). [Internet]. Bilthoven (Netherlands): RIVM; 2011. [Cited 2019 Feb 6]. Available from: https://www.rivm.nl/nederlands-voedingsstoffenbestand/toegang-nevo-gegevens/nevo-online/zoeken-bekijken-en-vergelijken. [Google Scholar]
- 14. Looman M, Feskens EJM, de Rijk M, Meijboom S, Biesbroek S, Temme EHM, de Vries J, Geelen A. Development and evaluation of the Dutch Healthy Diet index 2015. Public Health Nutr. 2017;20(13):2289–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. van Lee L, Feskens EJ, Hooft van Huysduynen EJ, de Vries JH, van ’t Veer P, Geelen A. The Dutch Healthy Diet Index as assessed by 24 h recalls and FFQ: associations with biomarkers from a cross-sectional study. J Nutr Sci. 2013;2:e40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. van Lee L, Geelen A, Kiefte-de Jong JC, Witteman JCM, Hofman A, Vonk N, Jankovic N, Hooft van Huysduynen EJC, de Vries JHM, van ’t Veer P et al. Adherence to the Dutch dietary guidelines is inversely associated with 20-year mortality in a large prospective cohort study. Eur J Clin Nutr. 2016;70(2):262–8. [DOI] [PubMed] [Google Scholar]
- 17. Li W, Schram MT, Sörensen BM, van Agtmaal MJM, Berendschot T, Webers CAB, Jansen JFA, Backes WH, Gronenschild EHBM, Schalkwijk CG et al. Microvascular phenotyping in the Maastricht Study: design and main findings, 2010–2018. Am J Epidemiol. 2020;189(9):873–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Sörensen BM, Houben AJHM, Berendschot TTJM, Schouten JSAG, Kroon AA, van der Kallen CJH, Henry RMA, Koster A, Sep SJS, Dagnelie PC et al. Prediabetes and type 2 diabetes are associated with generalized microvascular dysfunction: the Maastricht Study. Circulation. 2016;134(18):1339–52. [DOI] [PubMed] [Google Scholar]
- 19. Li W, Schram MT, Berendschot TTJM, Webers CAB, Kroon AA, van der Kallen CJH, Henry RMA, Schaper NC, Huang F, Dashtbozorg B et al. Type 2 diabetes and HbA1C are independently associated with wider retinal arterioles: the Maastricht study. Diabetologia. 2020;63(7):1408–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. ter Haar Romeny BM, Bekkers EJ, Zhang J, Abbasi-Sureshjani S, Huang F, Duits R, Dashtbozorg B, Berendschot TTJM, Smit-Ockeloen I, Eppenhof KAJ et al. Brain-inspired algorithms for retinal image analysis. Mach Vis Appl. 2016;27(8):1117–35. [Google Scholar]
- 21. Bekkers E, Duits R, Berendschot T, ter Haar Romeny B. A multi-orientation analysis approach to retinal vessel tracking. J Math Imaging Vision. 2014;49(3):583–610. [Google Scholar]
- 22. Jonas JB, Gusek GC, Naumann GO. Optic disc, cup and neuroretinal rim size, configuration and correlations in normal eyes. Invest Ophthalmol Vis Sci. 1988;29(7):1151–8. [PubMed] [Google Scholar]
- 23. Knudtson MD, Lee KE, Hubbard LD, Wong TY, Klein R, Klein BEK. Revised formulas for summarizing retinal vessel diameters. Curr Eye Res. 2003;27(3):143–9. [DOI] [PubMed] [Google Scholar]
- 24. Seidelmann SB, Claggett B, Bravo PE, Gupta A, Farhad H, Klein BE, Klein R, Di Carli M, Solomon SD. Retinal vessel calibers in predicting long-term cardiovascular outcomes: the Atherosclerosis Risk in Communities Study. Circulation. 2016;134(18):1328–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Geraets AFJ, van Agtmaal MJM, Stehouwer CDA, Sörensen BM, Berendschot TTJM, Webers CAB, Schaper NC, Henry RMA, van der Kallen CJH, Eussen SJPM et al. Association of markers of microvascular dysfunction with prevalent and incident depressive symptoms: the Maastricht Study. Hypertension. 2020;76(2):342–9. [DOI] [PubMed] [Google Scholar]
- 26. Muris DMJ, Houben AJHM, Kroon AA, Henry RMA, van der Kallen CJH, Sep SJS, Koster A, Dagnelie PC, Schram MT, Stehouwer CDA. Age, waist circumference, and blood pressure are associated with skin microvascular flow motion: the Maastricht Study. J Hypertens. 2014;32(12):2439–49.; discussion 2449. [DOI] [PubMed] [Google Scholar]
- 27. Brunt VE, Minson CT. Cutaneous thermal hyperemia: more than skin deep. J Appl Physiol (1985). 2011;111(1):5–7. [DOI] [PubMed] [Google Scholar]
- 28. Brunt VE, Minson CT. KCa channels and epoxyeicosatrienoic acids: major contributors to thermal hyperaemia in human skin. J Physiol. 2012;590(15):3523–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Stehouwer CD, Smulders YM. Microalbuminuria and risk for cardiovascular disease: analysis of potential mechanisms. J Am Soc Nephrol. 2006;17(8):2106–11. [DOI] [PubMed] [Google Scholar]
- 30. World Health Organization . Definition and diagnosis of diabetes mellitus and intermediate hyperglycemia: report of a WHO/IDF consultation. Geneva (Switzerland): WHO; 2006. [Google Scholar]
- 31. Inker LA, Schmid CH, Tighiouart H, Eckfeldt JH, Feldman HI, Greene T, Kusek JW, Manzi J, Van Lente F, Zhang YL et al. Estimating glomerular filtration rate from serum creatinine and cystatin C. N Engl J Med. 2012;367(1):20–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Basta G, Lazzerini G, Massaro M, Simoncini T, Tanganelli P, Fu C, Kislinger T, Stern DM, Schmidt AM, De Caterina R. Advanced glycation end products activate endothelium through signal-transduction receptor RAGE: a mechanism for amplification of inflammatory responses. Circulation. 2002;105(7):816–22. [DOI] [PubMed] [Google Scholar]
- 33. Penfold SA, Coughlan MT, Patel SK, Srivastava PM, Sourris KC, Steer D, Webster D E, Thomas MC, MacIsaac RJ, Jerums G et al. Circulating high-molecular-weight RAGE ligands activate pathways implicated in the development of diabetic nephropathy. Kidney Int. 2010;78(3):287–95. [DOI] [PubMed] [Google Scholar]
- 34. Sohouli MH, Fatahi S, Sharifi-Zahabi E, Santos HO, Tripathi N, Lari A, Pourrajab B, Kord-Varkaneh H, Găman MA, Shidfar F. The impact of low advanced glycation end products diet on metabolic risk factors: a systematic review and meta-analysis of randomized controlled trials. Adv Nutr. 2021;12(3):766–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Clarke RE, Dordevic AL, Tan SM, Ryan L, Coughlan MT. Dietary advanced glycation end products and risk factors for chronic disease: a systematic review of randomised controlled trials. Nutrients. 2016;8(3):125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Baye E, de Courten MP, Walker K, Ranasinha S, Earnest A, Forbes JM, de Courten B. Effect of dietary advanced glycation end products on inflammation and cardiovascular risks in healthy overweight adults: a randomised crossover trial. Sci Rep. 2017;7(1):4123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Luévano-Contreras C, Garay-Sevilla ME, Wrobel K, Malacara JM, Wrobel K. Dietary advanced glycation end products restriction diminishes inflammation markers and oxidative stress in patients with type 2 diabetes mellitus. J Clin Biochem Nutr. 2013;52(1):22–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Uribarri J, Cai W, Pyzik R, Goodman S, Chen X, Zhu L, Ramdas M, Striker GE, Vlassara H. Suppression of native defense mechanisms, SIRT1 and PPARγ, by dietary glycoxidants precedes disease in adult humans; relevance to lifestyle-engendered chronic diseases. Amino Acids. 2014;46(2):301–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Hellwig M, Auerbach C, Müller N, Samuel P, Kammann S, Beer F, Gunzer F, Henle T. Metabolization of the advanced glycation end product N-ɛ-carboxymethyllysine (CML) by different probiotic E. coli strains. J Agric Food Chem. 2019;67(7):1963–72. [DOI] [PubMed] [Google Scholar]
- 40. Gunthner R, Hanssen H, Hauser C, Angermann S, Lorenz G, Kemmner S, Matschkal J, Braunisch MC, Kuechle C, Renders L et al. Impaired retinal vessel dilation predicts mortality in end-stage renal disease. Circ Res. 2019;124(12):1796–807. [DOI] [PubMed] [Google Scholar]
- 41. Newman EA. Functional hyperemia and mechanisms of neurovascular coupling in the retinal vasculature. J Cereb Blood Flow Metab. 2013;33(11):1685–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Wong TY, Islam FMA, Klein R, Klein BEK, Cotch MF, Castro C, Sharrett AR, Shahar E. Retinal vascular caliber, cardiovascular risk factors, and inflammation: the Multi-Ethnic Study of Atherosclerosis (MESA). Invest Ophthalmol Vis Sci. 2006;47(6):2341–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Vlassara H, Cai W, Goodman S, Pyzik R, Yong A, Chen X, Zhu L, Neade T, Beeri M, Silverman JM et al. Protection against loss of innate defenses in adulthood by low advanced glycation end products (AGE) intake: role of the antiinflammatory AGE receptor-1. J Clin Endocrinol Metab. 2009;94(11):4483–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Vlassara H, Cai W, Crandall J, Goldberg T, Oberstein R, Dardaine V, Peppa M, Rayfield EJ. Inflammatory mediators are induced by dietary glycotoxins, a major risk factor for diabetic angiopathy. Proc Natl Acad Sci U S A. 2002;99(24):15596–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Vlassara H, Cai W, Tripp E, Pyzik R, Yee K, Goldberg L, Tansman L, Chen X, Mani V, Fayad ZA et al. Oral AGE restriction ameliorates insulin resistance in obese individuals with the metabolic syndrome: a randomised controlled trial. Diabetologia. 2016;59(10):2181–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Linden E, Cai W, He JC, Xue C, Li Z, Winston J, Vlassara H, Uribarri J. Endothelial dysfunction in patients with chronic kidney disease results from advanced glycation end products (AGE)-mediated inhibition of endothelial nitric oxide synthase through RAGE activation. Clin J Am Soc Nephrol. 2008;3(3):691–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Negrean M, Stirban A, Stratmann B, Gawlowski T, Horstmann T, Götting C, Kleesiek K, Mueller-Roesel M, Koschinsky T, Uribarri J et al. Effects of low- and high-advanced glycation endproduct meals on macro- and microvascular endothelial function and oxidative stress in patients with type 2 diabetes mellitus. Am J Clin Nutr. 2007;85(5):1236–43. [DOI] [PubMed] [Google Scholar]
- 48. Nowotny K, Schröter D, Schreiner M, Grune T. Dietary advanced glycation end products and their relevance for human health. Ageing Res Rev. 2018;47:55–66. [DOI] [PubMed] [Google Scholar]
- 49. Stirban A, Kotsi P, Franke K, Strijowski U, Cai W, Götting C, Tschoepe D. Acute macrovascular dysfunction in patients with type 2 diabetes induced by ingestion of advanced glycated β-lactoglobulins. Diabetes Care. 2013;36(5):1278–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Harcourt BE, Sourris KC, Coughlan MT, Walker KZ, Dougherty SL, Andrikopoulos S, Morley AL, Thallas-Bonke V, Chand V, Penfold SA et al. Targeted reduction of advanced glycation improves renal function in obesity. Kidney Int. 2011;80(2):190–8. [DOI] [PubMed] [Google Scholar]
- 51. Spijkerman AM, Gall MA, Tarnow L, Twisk JW, Lauritzen E, Lund-Andersen H, Emeis J, Parving HH, Stehouwer CD. Endothelial dysfunction and low-grade inflammation and the progression of retinopathy in type 2 diabetes. Diabet Med. 2007;24(9):969–76. [DOI] [PubMed] [Google Scholar]
- 52. Ingelsson E, Sundström J, Lind L, Risérus U, Larsson A, Basu S, Ärnlöv J. Low-grade albuminuria and the incidence of heart failure in a community-based cohort of elderly men. Eur Heart J. 2007;28(14):1739–45. [DOI] [PubMed] [Google Scholar]
- 53. Martens RJH, Houben AJHM, Kooman JP, Berendschot TTJM, Dagnelie PC, van der Kallen CJH, Kroon AA, Leunissen KML, van der Sande FM, Schaper NC et al. Microvascular endothelial dysfunction is associated with albuminuria: the Maastricht Study. J Hypertens. 2018;36(5):1178–87. [DOI] [PubMed] [Google Scholar]
- 54. Waqas K, Chen J, van der Eerden BCJ, Ikram MA, Uitterlinden AG, Voortman T, Zillikens MC. Dietary advanced glycation end-products (dAGEs) intake and bone health: a cross-sectional analysis in the Rotterdam Study. Nutrients. 2020;12(8):2377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Chen J, Waqas K, Tan RC, Voortman T, Ikram MA, Nijsten TEC, de Groot LCPGM, Uitterlinden AG, Zillikens MC. The association between dietary and skin advanced glycation end products: the Rotterdam Study. Am J Clin Nutr. 2020;112(1):129–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. de Courten B, de Courten MPJ, Soldatos G, Dougherty SL, Straznicky N, Schlaich M, Sourris KC, Chand V, Scheijen JLJM, Kingwell BA et al. Diet low in advanced glycation end products increases insulin sensitivity in healthy overweight individuals: a double-blind, randomized, crossover trial. Am J Clin Nutr. 2016;103(6):1426–33. [DOI] [PubMed] [Google Scholar]
- 57. Naska A, Lagiou A, Lagiou P. Dietary assessment methods in epidemiological research: current state of the art and future prospects. F1000Res. 2017;6:926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Poulsen MW, Hedegaard RV, Andersen JM, de Courten B, Bugel S, Nielsen J, Skibsted LH, Dragsted LO. Advanced glycation endproducts in food and their effects on health. Food Chem Toxicol. 2013;60:10–37. [DOI] [PubMed] [Google Scholar]
- 59. Cordova R, Knaze V, Viallon V, Rust P, Schalkwijk CG, Weiderpass E, Wagner K-H, Mayen-Chacon A-L, Aglago EK, Dahm CC et al. Dietary intake of advanced glycation end products (AGEs) and changes in body weight in European adults. Eur J Nutr. 2020;59(7):2893–904. [DOI] [PubMed] [Google Scholar]
- 60. Nagata C, Wada K, Yamakawa M, Nakashima Y, Koda S, Uji T, Oba S. Dietary intake of Nɛ-carboxymethyl-lysine, a major advanced glycation end product, is not associated with increased risk of mortality in Japanese adults in the Takayama study. J Nutr. 2020;150(10):2799–805. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that underlie the findings of this study are available from the corresponding author and The Maastricht Study Management Team (research.dms@mumc.nl) upon reasonable request.