Abstract
The nucleocapsid (N) protein of subgroup C (United States-specific) avian pneumovirus (APV/US) was expressed in Escherichia coli, and antibodies to the recombinant N protein were shown to specifically recognize the ≈47-kDa N protein of APV/US by Western immunoblot analysis. The recombinant APV/US N protein was used in a sandwich-capture enzyme-linked immunosorbent assay (ELISA), and the resulting assay was found to be more sensitive and specific than the routine indirect ELISA for the detection of APV/US antibodies in turkey sera.
Avian pneumovirus (APV) is an emerging respiratory viral pathogen in turkeys, causing severe economic loss to the turkey industry in the United States (2, 5, 7, 8). Studies in our laboratories and by others have shown that APV isolates from the United States differ genetically and antigenically from the European A and B subtypes of APV and have therefore been designated subgroup C APV (APV/US) (3, 9, 10, 12, 13). In general, APV infections are diagnosed by serology, reverse transcription-PCR, and virus isolation assays (5, 14). Routine serologic diagnosis of APV infection is performed by an enzyme-linked immunosorbent assay (ELISA) that uses crude lysates of APV-infected Vero cells as the antigen (2). Discrepancies in the results of crude cell lysate-based ELISAs have been reported previously and depend on the viral strain and the method of antigen preparation (4).
The recombinant matrix (M) protein of subgroup C APV was recently used in an indirect ELISA that was found to be very sensitive test for the detection of anti-APV antibodies (6). However, this assay is not suitable for specific detection of subgroup C (APV/US) infection (unpublished data). We have recently sequenced the nucleocapsid (N) protein gene of APV subgroup C isolates and have found that the degree of N-protein amino acid sequence identity is 99.7% among APV subgroup C isolates but that the APV subgroup C N protein has only 69% amino acid sequence identity with the N proteins of European APV subgroups A and B (3). Additionally, since the N protein is among the most highly expressed of all pneumovirus proteins and is also immunogenic (1, 11, 16), we postulated that it would serve as a superior diagnostic antigen for the specific detection of APV/US infections. Consistent with this hypothesis, ELISAs based on the recombinant N protein have previously been developed for bovine and human respiratory syncytial viruses, both of which are mammalian pneumoviruses related to APV (1, 11). In the study described here, we expressed the N protein in Escherichia coli, and we report here on the utility of the recombinant N protein in a diagnostic ELISA (N-ELISA) for detection of APV antibodies.
APV N-protein gene cloning and expression.
The N-protein gene of the Colorado isolate (APV/CO) (10) of APV subgroup C (positions 14 to 1198; GenBank accession number AF176590) (3) was amplified by PCR with primers N1 (5′-ATGTCTCTTCAGGGGATTCAGCTTAG) and N2 (5′ -TTACTCATAATCATTCTGGCCTTC), and the gel-purified PCR product was cloned into the pCRT7/NT-TOPO vector (Invitrogen, Carlsbad, Calif.). The construct was transformed into E. coli BL21(DE3)/pLysS cells (Invitrogen), and the expression of recombinant N protein was induced by the addition of 0.5 mM isopropyl-β-d-thiogalactopyranoside. Four hours following induction, the cells were lysed by sonication and the recombinant N protein was purified. The cloned N-protein gene was expressed as a soluble protein with an N-terminal 6× His tag downstream of a T7 promoter, yielding a fusion protein of 429 amino acids. The expression of recombinant N protein was monitored by Western blot analysis with express detector nickel-horseradish peroxidase conjugate (Kirkegaard & Perry Laboratories, Gaithersburg, Md.). Maximum expression was observed at 4 h postinduction under native lysis conditions, and the N protein was affinity purified with nickel-nitrilotriacetic acid (Ni-NTA) agarose (Qiagen, Valencia, Calif.). To raise hyperimmune serum, the recombinant N protein was further purified by resolution in a 10% polyacrylamide gel by discontinuous sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) (15), followed by staining of the gel with Coomassie blue, excision of the ≈47-kDa band from the gel, and homogenization of the resulting material in phosphate-buffered saline (PBS; pH 7.2).
Preparation of antiserum to recombinant APV N protein.
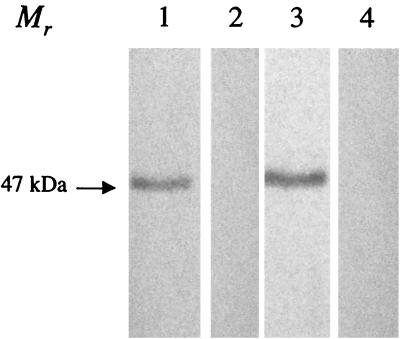
Two New Zealand White rabbits were each given three subcutaneous injections of approximately 100 μg of gel-purified recombinant N protein with Freund's complete adjuvant on day 0 and with incomplete adjuvant on days 14 and 28. A final intravenous injection was given on day 35, and the rabbits were bled at 72 h postinjection. Antiserum was tested by Western immunoblotting with purified APV protein and recombinant N protein as described previously (6). Antiserum to the recombinant N protein specifically detected a single ≈47-kDa protein in both partially purified APV/CO and purified N-protein preparations by Western immunoblot analysis (Fig. 1).
FIG. 1.
Western immunoblot analysis of recombinant N protein and partially purified APV with hyperimmune antiserum to N protein raised in rabbits. Recombinant N protein (lanes 1 and 2) and partially purified APV proteins (lanes 3 and 4) were separated by SDS-PAGE, transferred to a nitrocellulose membrane, and incubated with a 1:20,000 dilution of N-protein-specific rabbit hyperimmune serum or normal rabbit serum, followed by incubation with a 1:5,000 dilution of anti-rabbit IgG horseradish peroxidase. Immunoreactive bands were visualized with the tetramethylbenzidine substrate system. While hyperimmune N-protein-specific antisera detected a single band with an Mr of ≈47,000 in lanes containing N and APV proteins (lanes 1 and 3, respectively), no reaction was observed with N or APV proteins by using preimmune rabbit serum (lanes 2 and 4, respectively).
Development of recombinant N-protein-based capture-sandwich ELISA.
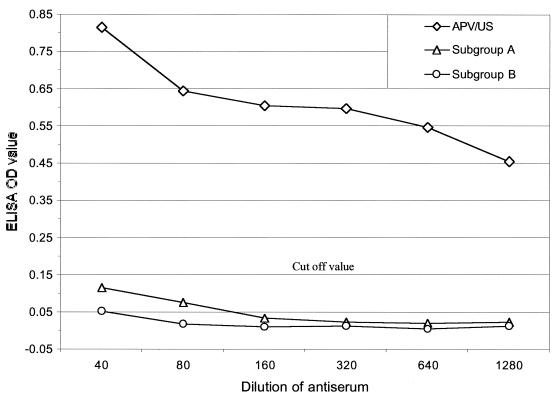
We next evaluated the utility of the recombinant N protein for APV antibody detection by ELISA. Alternate rows of ELISA plates (Immulon 1B; Dynatech, Chantilly, Va.) were coated with 100 μl of rabbit N-protein-specific antiserum and preimmune rabbit serum (positive and negative rows, respectively) diluted 1:1,500 in 0.05 M sodium carbonate buffer (pH 9.6) by incubation at 37°C for 2 h. Nonspecific binding sites were blocked by incubation with 4% fetal horse serum (100 μl) in PBS containing 0.05% Tween 20 (PBS-T) overnight at 4°C. Ni-NTA column-purified N protein (50 μl, ≈100 ng/well) appropriately diluted in ELISA dilution and blocking reagent (Kirkegaard & Perry Laboratories) was added as antigen, and the mixture was incubated for 1 h at room temperature. The plates were then washed five times with PBS-T, serial twofold dilutions of turkey sera a (50 μl) in ELISA dilution and blocking reagent were added to duplicate wells of positive and negative rows, and the plates were incubated for 1 h at room temperature. After washing of the plates five times, 50 μl of anti-turkey immunoglobulin G-horseradish peroxidase conjugate (1:1,500) diluted in ELISA dilution and blocking reagent was added to each well, and the plates were incubated for 1 h at room temperature. Following two additional washings, the substrate chromogen o-phenylenediamine (0.04%) in citrate phosphate buffer (pH 5.0) containing 0.04% H2O2 was added (100 μl) to each well, the plates were incubated for 10 min, and the reaction was stopped by the addition of 25 μl of 2.5 M H2SO4. The results of the assay were expressed as the difference between the mean absorbance (A490/A405) of each sample in wells coated with protein N-positive serum and protein N-negative serum. Hyperimmune sera against APV subgroup A (UK 14/1), subgroup B (Hungarian 657/4; obtained from National Veterinary Services Laboratories, Ames, Iowa), and subgroup C (APV/US/MN1a; prepared in our laboratory) raised in turkeys were tested by this ELISA. Antiserum to the APV/US isolate reacted strongly in the N-ELISA when it was tested at dilutions that started at 1:40 and that went through 1:1,280. However, antisera to subgroups A and B failed to react in the N-ELISA at any of these dilutions, indicating that this assay is specific for APV/US isolates (Fig. 2). A single test dilution of 1:40 was selected for subsequent assays of turkey sera. The cutoff value (0.15) for a positive test was determined as the mean absorbance plus 3 standard deviations for a panel of turkey sera (n = 24) that were known to be free of APV infection. The specificity of the assay was determined by evaluation of serum samples from experimental turkeys (n = 55) that were free of APV infection. All samples from this group of APV-negative turkeys were negative by the N-ELISA, indicating that this assay is highly specific. The sensitivity of the assay was determined by evaluating serum specimens (n = 81) from turkeys that were experimentally infected with subgroup C APV (APV/US/MN1a) and collected 4 weeks postinfection. All 81 samples were positive by the N-ELISA, and hence, the diagnostic sensitivity of this assay was 100%.
FIG. 2.
Sandwich N-ELISA showing reactivity with different subgroups of avian pneumovirus. Twofold serial dilutions of antiserum against APV subgroups A, B, and C were tested by the N-ELISA as described in the text. The cutoff value of the absorbance at 490 nm for a positive result was 0.15.
Comparison of N-ELISA with routine APV-ELISA.
One hundred eighty-three serum samples from turkeys suspected of being infected with APV and submitted to the Minnesota Veterinary Diagnostic Laboratory in the year 2000 were tested by both routine APV-specific ELISA (APV-ELISA) and capture N-ELISA. The routine indirect ELISA, which used APV/CO-infected Vero cells as the antigen for coating, was performed as described previously (2). While 143 samples gave identical results by both assays (85 positive and 58 negative), the capture N-ELISA detected APV antibodies in 38 more samples than the APV-ELISA (Table 1). Of these 38 samples, 37 were from turkey flocks that had experienced clear clinical signs of APV disease, and routine APV-ELISA failed to detect APV antibodies in these samples. These results suggest that the capture N-ELISA is more sensitive than the routine ELISA for the detection of antibodies to APV in turkey sera. The two samples that were positive by the routine APV-ELISA but negative by the capture N-ELISA (Table 1) were from a turkey flock for which all other samples tested (8 of 10) were negative by the routine ELISA. Consistent with this observation, Western immunoblot analysis with recombinant N protein and purified APV proteins also failed to detect anti-APV antibodies in these sera (Fig. 3). These results also suggest that the capture N-ELISA is more specific than the routine indirect assay for the detection of APV antibodies in turkey sera.
TABLE 1.
Comparison of whole-virus APV-ELISA and capture N-ELISA for detection of APV antibodies in turkey sera
| Capture N-ELISA result | No. of samples with the following whole-virus indirect APV-ELISA result:
|
||
|---|---|---|---|
| Positive | Negative | Total | |
| Positive | 85 | 38 | 123 |
| Negative | 2 | 58 | 60 |
| Total | 87 | 96 | 183 |
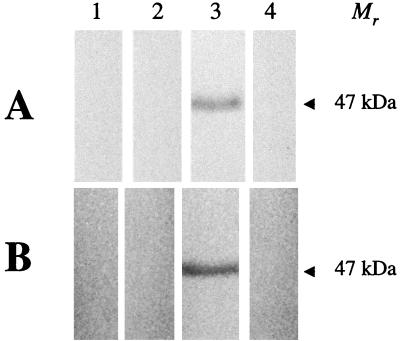
FIG. 3.
Western immunoblot analysis of turkey sera suspected of being infected with APV by using recombinant N protein (A) and partially purified APV proteins (B). Nitrocellulose membrane strips were reacted with sera from two turkeys that were APV antibody positive by routine ELISA but negative with the capture N-ELISA system (lanes 1 and 2, respectively) and with known APV-positive and -negative sera (lanes 3 and 4, respectively). The results demonstrate that the two samples positive by the routine ELISA do not show any evidence of APV-specific antibodies and thus have false-positive results.
Concluding comments.
The specific and sensitive diagnosis of APV-infected turkey flocks continues to be a challenge due to the absence of sensitive and specific serologic diagnostic tests. Since the N protein gene is among the most highly expressed genes in pneumoviruses, we evaluated the utility of the N protein as a sensitive diagnostic antigen. The results of our studies suggest that a recombinant APV N protein produced in E. coli can successfully be used as a diagnostic antigen in a capture ELISA for the specific and sensitive diagnosis of APV infection in turkeys. The recombinant N-protein-based assay was also found to be more sensitive and specific than the whole viral antigen-based ELISA that is in use (2). Studies are under way to assess whether this N-protein-based immunoassay may detect infection earlier than previously described serologic assays, since in a related virus (bovine respiratory syncytial virus), antibodies to the N protein appear early and predominate throughout infection (16).
Acknowledgments
This study was supported in part by grants from the Minnesota Agricultural Experiment Station.
We thank Arshud Dar and Ling Ling Li for numerous suggestions and technical assistance. We are grateful to B. Panigrahy, National Veterinary Services Laboratories, for providing antisera to APV subgroups A and B.
REFERENCES
- 1.Buraphacheep W, Britt W J, Sullender W M. Detection of antibodies to respiratory syncytial virus attachment and nucleocapsid proteins with recombinant baculovirus-expressed antigens. J Clin Microbiol. 1997;35:354–357. doi: 10.1128/jcm.35.2.354-357.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chiang S J, Dar A, Goyal S M, Nagaraja K V, Halvorson D A, Kapur V. Seroprevalence of avian pneumovirus in Minnesota turkeys. J Vet Diagn Investig. 2000;12:381–384. doi: 10.1177/104063870001200214. [DOI] [PubMed] [Google Scholar]
- 3.Dar, A. M., S. Munir, S. M. Goyal, M. S. Abrahamsen, and V. Kapur. Sequence analysis of the nucleocapsid and phosphoprotein genes of avian pneumovirus circulating in the US. Virus Res., in press. [DOI] [PubMed]
- 4.Eterradossi N, Toquin D, Guittet M, Bennejean G. Discrepancies in turkey rhinotracheitis ELISA results using different antigens. Vet Rec. 1992;131:563–564. [PubMed] [Google Scholar]
- 5.Goyal S M, Chiang S J, Dar A M, Nagaraja K V, Shaw D P, Halvorson D A, Kapur V. Isolation of avian pneumovirus from an outbreak of respiratory illness in Minnesota turkeys. J Vet Diagn Investig. 2000;12:166–168. doi: 10.1177/104063870001200214. [DOI] [PubMed] [Google Scholar]
- 6.Gulati B R, Cameron K T, Seal B S, Goyal S M, Halvorson D A, Njenga M K. Development of a highly sensitive and specific enzyme-linked immunosorbent assay based on recombinant matrix protein for detection of avian pneumovirus antibodies. J Clin Microbiol. 2000;38:4010–4014. doi: 10.1128/jcm.38.11.4010-4014.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jirjis F F, Noll S L, Halvorson D A, Nagaraja K V, Townsend E L, Sheikh A M, Shaw D P. Avian pneumovirus infection in Minnesota turkeys: experimental reproduction of the disease. Avian Dis. 2000;44:222–226. [PubMed] [Google Scholar]
- 8.Kleven S H. Proceedings of the U.S. Animal Health Association 101st Annual Meeting. U.S. Richmond, Va: Animal Health Association; 1997. Report of the Committee on Transmissible Diseases of Poultry and Other Avian Species; pp. 473–491. [Google Scholar]
- 9.Li J, Ling R, Randhawa J S, Shaw K, Davis P J, Juhasz K, Pringle C R, Easton A J, Cavanagh D. Sequence of the nucleocapsid protein gene of subgroup A and B avian pneumoviruses. Virus Res. 1996;41:185–191. doi: 10.1016/0168-1702(96)01288-9. [DOI] [PubMed] [Google Scholar]
- 10.Panigrahy B, Senne D A, Pedersen J C, Gidlewski T, Edson R K. Experimental and serologic observations on avian pneumovirus (APV/turkey/Colorado/97) infection in turkeys. Avian Dis. 2000;44:17–22. [PubMed] [Google Scholar]
- 11.Samal S K, Pastey M K, McPhillips T, Carmel D K, Mohanty S B. Reliable confirmation of antibodies to bovine respiratory syncytial virus (BRSV) by enzyme-linked immunosorbent assay using BRSV nucleocapsid protein expressed in insect cells. J Clin Microbiol. 1993;31:3147–3152. doi: 10.1128/jcm.31.12.3147-3152.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Seal B S. Matrix protein gene nucleotide and predicted amino acid sequence demonstrate that the first U.S. avian pneumovirus isolate is distinct from European strains. Virus Res. 1998;58:45–52. doi: 10.1016/s0168-1702(98)00098-7. [DOI] [PubMed] [Google Scholar]
- 13.Seal B S, Sellers H S, Meinersmann R J. Fusion protein predicted amino acid sequence of the first U.S. avian pneumovirus isolate and lack of heterogeneity among other U.S. isolates. Virus Res. 2000;66:139–147. doi: 10.1016/s0168-1702(99)00133-1. [DOI] [PubMed] [Google Scholar]
- 14.Shin H J, Rajashekara G, Jirjis F F, Shaw D P, Goyal S M, Halvorson D A, Nagaraja K V. Specific detection of avian pneumovirus (APV) U.S. isolates by RT-PCR. Arch Virol. 2000;145:1239–1246. doi: 10.1007/s007050070123. [DOI] [PubMed] [Google Scholar]
- 15.Towbin H, Staehelin T, Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci USA. 1979;76:4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Westenbrink F, Kimman T G, Brinkhof J M. Analysis of the antibody response to bovine respiratory syncytial virus proteins in calves. J Gen Virol. 1989;70:591–601. doi: 10.1099/0022-1317-70-3-591. [DOI] [PubMed] [Google Scholar]