ABSTRACT
Background
Diet and physical activity (PA) are independent risk factors for obesity and chronic diseases including type 2 diabetes mellitus (T2DM) and metabolic syndrome (MetS). The temporal sequence of these exposures may be used to create patterns with relations to health status indicators.
Objectives
The objectives were to create clusters of joint temporal dietary and PA patterns (JTDPAPs) and to determine their association with health status indicators including BMI, waist circumference (WC), fasting plasma glucose, glycated hemoglobin, triglycerides, HDL cholesterol, total cholesterol, blood pressure, and disease status including obesity, T2DM, and MetS in US adults.
Methods
A 24-h dietary recall and random day of accelerometer data of 1836 participants from the cross-sectional NHANES 2003–2006 data were used to create JTDPAP clusters by constrained dynamic time warping, coupled with a kernel k-means clustering algorithm. Multivariate regression models determined associations between the 4 JTDPAP clusters and health and disease status indicators, controlling for potential confounders and adjusting for multiple comparisons.
Results
A JTDPAP cluster with proportionally equivalent energy consumed at 2 main eating occasions reaching ≤1600 and ≤2200 kcal from 11:00 to 13:00 and from 17:00 to 20:00, respectively, and the highest PA counts among 4 clusters from 08:00 to 20:00, was associated with significantly lower BMI (P < 0.0001), WC (P = 0.0001), total cholesterol (P = 0.02), and odds of obesity (OR: 0.2; 95% CI: 0.1, 0.5) than a JTDPAP cluster with proportionally equivalent energy consumed reaching ≤1600 and ≤1800 kcal from 11:00 to 14:00 and from 17:00 to 21:00, respectively, and high PA counts from 09:00 to 12:00.
Conclusions
The joint temporally patterned sequence of diet and PA can be used to cluster individuals with meaningful associations to BMI, WC, total cholesterol, and obesity. Temporal patterns hold promise for future development of lifestyle patterns that integrate additional temporal and contextual activities.
Keywords: temporal pattern, dietary patterns, physical activity patterns, obesity, BMI, waist circumference, adults, chrono-nutrition
Introduction
Diet (1) and physical activity (PA) (2) are modifiable risk factors when it comes to health. Both dietary and PA behaviors are expected to be interrelated, with potential synergistic effects on health status indicators (3–5). A systematic review to investigate the effect of the interaction of time, eating, and exercising on health showed that postmeal exercise may have a potential benefit on postprandial glycemic response compared with premeal exercise (5). This study demonstrated that the timing of diet and PA and their relation to each other in a day are related to health indicators and hold promise for the discovery of their joint patterns of activity and these patterns’ links to health.
Both dietary and PA behaviors occur in a temporal context or “temporal pattern” including daily rhythms that begin and end throughout a day (6). Previous work to derive clusters representing 4 distinct temporal dietary patterns in a representative US sample showed that participants with energy-equivalent and evenly distributed eating occasions throughout a day had higher diet quality (7), lower mean BMI and odds of obesity, and smaller waist circumference (WC) (8) than those with other temporal dietary patterns exhibiting 1 energy intake (EI) peak sometime in a day. Similarly, novel work integrating PA and corresponding timing throughout the day generated “temporal PA patterns.” Findings showed that those with the lowest PA counts (PACs) from 06:00 to 23:00 had significantly higher mean BMI and larger mean WC than those with higher PACs at either an early time (08:00–11:00) or later time (16:00–21:00) of the day (9).
Yet, the combination of the timing of both dietary EI and PA throughout a 24-h day simultaneously and investigation of their joint relation with health status indicators are not known. To fill this gap, this study describes the creation of “joint temporal dietary and PA patterns” (JTDPAPs), or the chronological succession of energy from dietary intake and varying PACs over time. Therefore, study objectives were to 1) create clusters of JTDPAPs using modern data-driven distance-based clustering algorithms, and 2) investigate the association between JTDPAP clusters and primary health status indicators including BMI, WC, and odds of being obese, as well as secondary health status indicators including fasting plasma glucose, glycated hemoglobin (HbA1c), triglycerides, HDL cholesterol, total cholesterol, blood pressure, and disease status including T2DM and MetS, among US adults. JTDPAPs are hypothesized to be linked with health status indicators and disease status among US adults aged 20–65 y using NHANES 2003–2006.
Methods
Participants and data set
NHANES is a cross-sectional survey that is carried out by the National Center for Health Statistics (NCHS) of the US CDC to assess the US noninstitutionalized civilian population's health and nutritional status. The participants of NHANES are selected using a complex, multistage sampling design. Selected participants undergo an in-person household interview, a physical health examination, and a phone follow-up interview. Participants’ sociodemographic information was collected during the in-person household interview, which included age, sex, race/ethnicity, and poverty to income ratio (PIR), using questionnaires. All participants consented to completing the survey approved by the NCHS Research Ethics Review Board (10).
Analytic sample
NHANES 2003–2006 was used owing to the availability of hip-worn PA accelerometer data. The analytic sample only included nonpregnant US adults ages 20–65 y with ≥1 reliable 24-h weekday dietary recall and 1 valid weekday accelerometer record. The temporal dietary and PA behaviors of pregnant women and participants outside of the age range are expected to entail unique life stage patterns and were excluded. Participants with missing sociodemographic, anthropometric, and health status indicator data were also excluded. Therefore, the study sample included 1836 participants (Supplemental Figure 1).
Dietary data assessment
The USDA Automated Multiple-Pass Method was used to collect 2 dietary recalls (11) detailing all the foods that participants reported consuming during a 24-h period, including information on the time of intake, amount, and type of each food, and detailed food descriptions (12). One valid dietary 24-h recall that met the minimum criteria in NHANES (12) with nonzero EI was used in this study. Previous research has found that weekdays and weekend days of dietary intake can differ (13). For this first attempt to generate JTDPAPs, weekday dietary recalls were chosen to maintain the largest possible sample; the first recall was used if it was for a weekday, otherwise, the second weekday recall was used. Each participant's EI for all reported foods and beverages was determined using the USDA Food and Nutrient Database for Dietary Studies (FNDDS) for 2003–2004 data (USDA FNDDS, version 2.0) and 2005–2006 data (USDA FNDDS, version 3.0). The duration time of the eating occasions was not available in NHANES, but 15 min/occasion was applied based on a previous study (14) so that energy reported at a time of eating was divided by 15 min to determine the energy per minute and applied to each minute within the 15-min eating occasion. Dietary data from the chosen valid weekday recall were also used to determine total EI over the entire day.
PA assessment
PA accelerometers were used to quantify the PA of each participant. The accelerometers recorded vertical accelerations as “counts per minute,” which represent the relative intensity of movements per minute (15), for 7 consecutive days after the health examination (16). Use of the accelerometer for >10 h/d was considered as a valid wear day (17). Previous research has determined that PA on weekdays or weekend days can differ (18). One random valid weekday was selected for each participant to ensure the selection probabilities for each weekday were equal (19). The random valid weekday of accelerometer data was also used to determine total PACs for the entire 24-h day. Because there is no gold standard to identify intensity levels and different cutoffs influenced estimates of PA intensity level, and specificity and sensitivity regarding health indicators (20, 21), the possible bias can be decreased by utilizing raw PACs, as in this study, rather than cutoffs or categorized intensity levels.
Anthropometric assessment and laboratory tests
Health status indicators were included to study their associations with JTDPAPs. Height, weight, and WC were measured (22), and BMI was calculated. Blood samples of participants were obtained based on standardized protocols (23, 24). After 8.5–24 h of fasting, triglycerides were measured enzymatically (25, 26) and a hexokinase method was used to measure fasting plasma glucose with a Roche/Hitachi 911 in years 2003–2004 or a Roche Cobas Mira in years 2005–2006 (27, 28). HDL cholesterol, total cholesterol, and HbA1c were measured without regard to fasting or nonfasting state (29). A direct immunoassay method was used to measure HDL cholesterol (30) and total cholesterol was measured enzymatically (31, 32). The equipment to measure HDL cholesterol and total cholesterol in years 2005–2006 was different, but the method and location were the same as those in 2003–2004 (30, 32). The blood lipid measurements were standardized for NHANES according to the CDC's lipid standardization program (33) and corrected using the Solomon Lab quality controls (30, 32). HPLC was used to measure HbA1c by a Primus CLC 330 and Primus CLC 385 (Primus Corporation) in years 2003–2004 and a Tosoh A1c 2.2 Plus Glycohemoglobin Analyzer (Tosoh Medics, Inc.) in years 2005–2006 (34, 35). A mercury sphygmomanometer was used to measure ≤4 systolic and diastolic blood pressures (36): if only 1 measurement was recorded, the value used in this study was the only measurement; if multiple measurements were recorded, the mean of the multiple measurements excluding the first measure was used.
Disease status classification
Disease status included obesity, T2DM, and MetS. Obesity was classified as BMI (in kg/m2) >30 (37). The number of participants with T2DM was determined by subtracting the number with type 1 diabetes from the total number of participants with diabetes. Diabetes was classified by participant self-reports of being told they had diabetes by a doctor or of taking medications that lowered glucose, or by fasting plasma glucose concentration ≥126 mg/dL or HbA1c ≥ 6.5% (38). Type 1 diabetes was classified when a participant reported being diagnosed with diabetes before being 30 y old and continuous insulin use since diagnosis (39). MetS was determined by ≥3 of 5 risk factors: 1) WC > 102 cm for men or >88 cm for women; 2) triglycerides >150 mg/dL; 3) HDL cholesterol < 40 mg/dL for men or <50 mg/dL for women; 4) hypertension (systolic blood pressure > 130/diastolic blood pressure > 85 mm Hg); or 5) impaired fasting plasma glucose >110 mg/dL (40).
Measures for covariates
Variables to classify survey year (2003–2004 and 2005–2006) and variables based on self-reported information to classify sex (male or female), race/ethnicity, age group (20–34, 35–49, and 50–65 y), and PIR were created and used to adjust the models. Furthermore, BMI, total PACs per day, and energy misreporting were also accounted for in the models. Race/ethnicity was reported and classified as Mexican American, other Hispanic, non-Hispanic white, non-Hispanic black, and other including multirace. PIR was the reported household income divided by the federal poverty guideline for household income, and classified as 0–0.99 (under poverty threshold), 1–1.99, 2–2.99, 3–3.99, 4–4.99, and ≥5 (41). BMI was classified as underweight (<18.5), normal weight (18.5–24.9), overweight (25.0–29.9), and obese (>30.0) (37). Energy misreporting was considered as a potential confounder modeled as total EI divided by estimated energy requirement (EER) (42–44), where EER was calculated using equations for adults based on age, sex, height, weight, and PA level according to the Institute of Medicine (45). According to previous studies, the vast majority (96.5%) of the US NHANES participant sample (and representative of the US population as a whole) did not meet the Physical Activity Guidelines for Americans and, similarly, the mean PACs of the 4 clusters indicated a light activity level (17, 46). Therefore, a low PA level was applied in the calculation to determine the EER (coefficient for men = 1.11 and women = 1.12) (44). For each participant whose data are reported in the NHANES data, the NCHS creates a weight based on the selection process. Weights were constructed when combining survey cycles 2003–2006 and used in the models, so the results are representative of the US civilian, noninstitutionalized population at the midpoint of the 4 y of the study (47, 48). The survey design of NHANES included stratification and clustering, which were both accounted for in the regression models according to NCHS guidelines to increase the precision of survey estimates (49).
Creating JTDPAPs
Participants’ diet and PA data from the NHANES were extracted as 2-dimensional time series of length 1440 (24 h × 60 min), the entries of which are 2-dimensional vectors consisting of EIs and PACs at the timing (min) indicated by their indexes. For instance, participant q is represented by a 2D time series 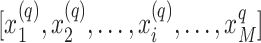 , where
, where ![]() is a 2D vector representing the EI and PAC at the ith minute of the day, and M = 1440 is the length of the time series. Distance-based clustering analysis was used to derive JTDPAPs; specifically, dynamic time warping (DTW) is a distance measure that was used to determine the pairwise distances between participants (50). Unlike the commonly used Euclidean distance which sums the squared differences of entries with the same indexes, DTW finds the optimal matching path such that the summed differences between matched entries are minimized under certain conditions (50). In addition, the Sakoe-Chiba band (51) was used to constrain the maximum temporal difference between matched entries to avoid pathological warping (e.g., matching activities in the morning to activities in the evening), and is denoted as constrained band DTW (CDTW).
is a 2D vector representing the EI and PAC at the ith minute of the day, and M = 1440 is the length of the time series. Distance-based clustering analysis was used to derive JTDPAPs; specifically, dynamic time warping (DTW) is a distance measure that was used to determine the pairwise distances between participants (50). Unlike the commonly used Euclidean distance which sums the squared differences of entries with the same indexes, DTW finds the optimal matching path such that the summed differences between matched entries are minimized under certain conditions (50). In addition, the Sakoe-Chiba band (51) was used to constrain the maximum temporal difference between matched entries to avoid pathological warping (e.g., matching activities in the morning to activities in the evening), and is denoted as constrained band DTW (CDTW).
The original CDTW was designed for 1-dimensional speech signals. To generalize CDTW to multidimensional time series such as the diet and PA time series, 2 commonly used methods (52), i.e., independent multivariate CDTW (CDTWI) and dependent multivariate CDTW (CDTWD), were adopted. CDTWI uses CDTW distances which were computed based on dietary data and PA data independently [i.e., to determine CDTWI for 1 pair of participants, 2 separate CDTW distances were computed:  and
and 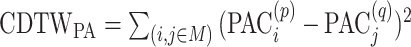 where M denotes the alignment (matching path) that is found by the DTW algorithm; it defines how entries/events (EI for diet) of participant p are matched to those in participant q.
where M denotes the alignment (matching path) that is found by the DTW algorithm; it defines how entries/events (EI for diet) of participant p are matched to those in participant q. 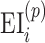 and
and 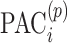 denote the EI and PACs in the ith minute of the day of participant p]; and the joint distance was determined from the equation:
denote the EI and PACs in the ith minute of the day of participant p]; and the joint distance was determined from the equation: 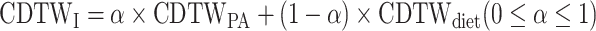 , where parameter α controls the emphasis on PA over diet. Note that CDTWI reduces to CDTWPA when α = 1 and to CDTWdiet when α = 0, and larger values of α indicate more emphasis on PA than dietary information in the clustering. In contrast, CDTWD computes the joint distance following a similar procedure as 1-dimensional CDTW. First, the difference between 2 entries, (EIi, PACi) and (EIj, PACj), was defined as
, where parameter α controls the emphasis on PA over diet. Note that CDTWI reduces to CDTWPA when α = 1 and to CDTWdiet when α = 0, and larger values of α indicate more emphasis on PA than dietary information in the clustering. In contrast, CDTWD computes the joint distance following a similar procedure as 1-dimensional CDTW. First, the difference between 2 entries, (EIi, PACi) and (EIj, PACj), was defined as 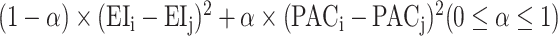 . Next, the same dynamic programming algorithm that finds the optimal matching path in CDTW was used here using the newly defined difference. The joint distance CDTWD was determined as the summed differences along the optimal matching path. The parameter α in CDTWD has similar effects on the derived JTDPAPs as in CDTWI. The discussion on multidimensional CDTW is kept necessarily brief in this article but more detail can be found in previous publications (50, 52).
. Next, the same dynamic programming algorithm that finds the optimal matching path in CDTW was used here using the newly defined difference. The joint distance CDTWD was determined as the summed differences along the optimal matching path. The parameter α in CDTWD has similar effects on the derived JTDPAPs as in CDTWI. The discussion on multidimensional CDTW is kept necessarily brief in this article but more detail can be found in previous publications (50, 52).
Both CDTWI and CDTWD were coupled with 3 popular distance-based clustering algorithms including kernel k-means (53), spectral clustering (54), and hierarchical agglomerative clustering (55) to partition the participants into 4 mutually exclusive clusters based on internal criteria including Silhouette Index and Dunn Index and external criteria including the number of significant inferential analysis results. In addition, the density balanced hierarchical cluster agglomeration (DBHCA), which exploits the definition of local reachability density (56), was used to develop a density-based, distance-aided cluster agglomeration method. In this method, pairwise CDTW with Sakoe-Chiba band and kernel k-means were coupled to derive 4 clusters based on dietary data and PA data independently (9, 57). The cross product of diet and PA clusters resulted in 16 initial clusters. Next, DBHCA agglomerateed the 16 initial joint clusters into 4 final clusters by repeatedly merging the 2 most similar clusters. Unlike previous hierarchical agglomerative methods (58–60), DBHCA follows a density-similarity-first strategy to avoid the chaining effect, and introduces a distance threshold to ensure diet and PA similarity between clusters. Python was used to perform all clustering.
Statistical analysis
The 3 aforementioned methods were evaluated using inferential analysis to determine the strength of the JTDPAP's relation with the health status indicators. For categorical disease status indicators for obesity (yes/no), T2DM (yes/no), and MetS (yes/no), the receiver operating characteristic curves were used to evaluate the models. The AUCs in all 3 categorical disease status models were >0.8. For continuous health status indicators including BMI, WC, fasting plasma glucose, HbA1c, triglycerides, HDL cholesterol, total cholesterol, and systolic and diastolic blood pressure, residual plots and outliers were checked, and triglycerides, HbA1c, total cholesterol, and fasting plasma glucose exhibited suspected outliers in the residuals. After rerunning the analysis without those points, the inferential analysis results did not change substantially. Further, the values that produced the extreme residuals were considered biologically possible and acceptable so they were retained. Ultimately, CDTWD with Sakoe-Chiba band = 240 and α = 0.0016 produced the strongest relations with health status indicators based on inferential analysis results that included the most significant differences between the 6 pairwise comparisons among all health status indicators, the highest model R2 values, or the lowest Akaike information criterion.
ANOVA and the Kruskal–Wallis test showed significant differences among the cluster means of HDL cholesterol and all other health status indicator models, respectively. Multiple linear regression models compared clusters of JTDPAPs on continuous health status indicators. Multiple logistic regression models compared clusters of JTDPAPs on categorical disease status indicators. Models using BMI, WC, and obesity as health status indicators were adjusted for survey year, age group, sex, race/ethnicity, PIR, total PACs per day, and energy misreporting, whereas models using other health and disease status indicators were in addition adjusted for BMI. The Tukey–Kramer adjustment was made for multiple comparisons. Adjusted P < 0.05 for comparisons among clusters was considered statistically significant. SAS version 9.4 (SAS Institute Inc) was used to complete the analysis.
Visualization
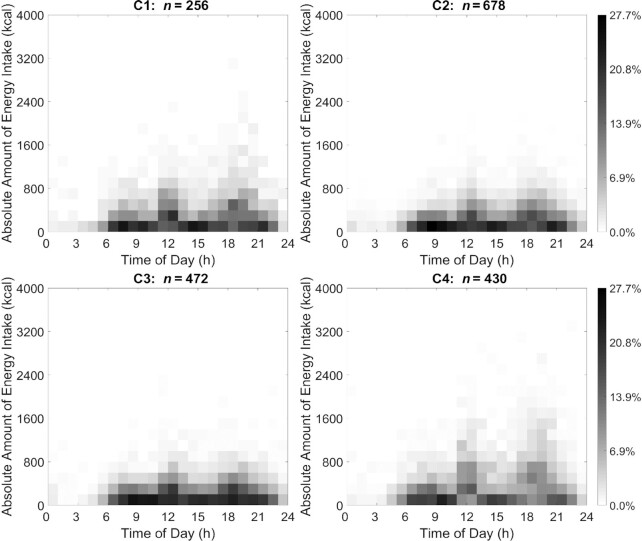
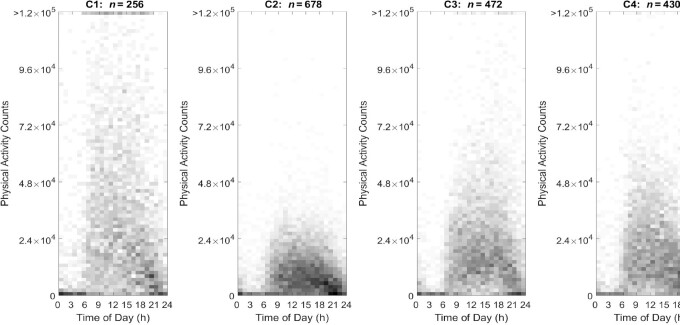
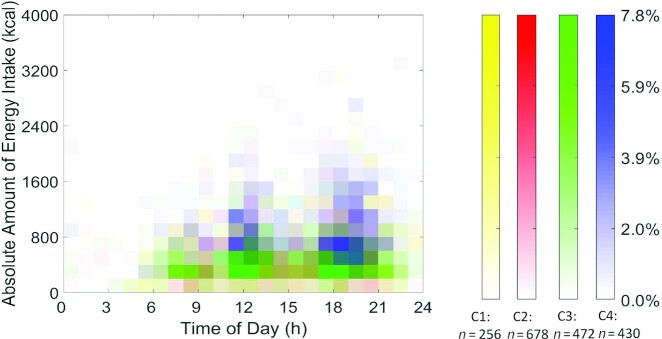
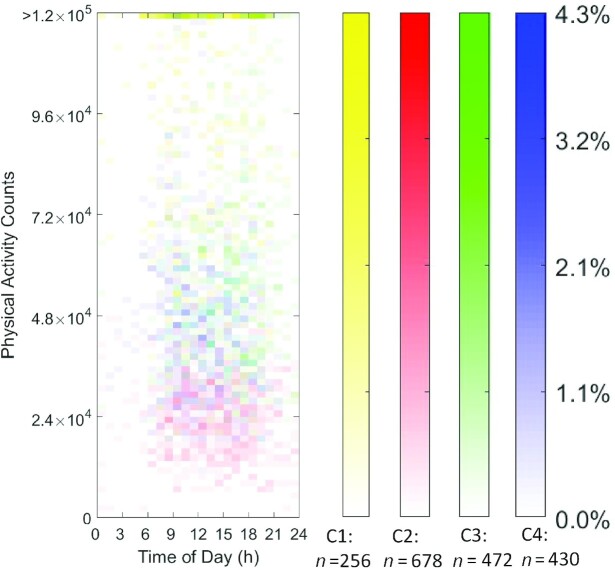
The visualization (Figures 1 and 2) used heat maps to reveal the distribution of nonzero eating occasions and nonzero PACs in 4 distinct JTDPAP clusters. The x axis indicated time ranging from 00:00 to 24:00, whereas the y axis was either absolute EI ranging from 0 kcal to 4000 kcal or PACs ranging from 0 to 1.2 × 105 (truncated) at a certain hour. Both EI and PAC were calculated from their minute records. The proportion of individuals in each cluster reporting eating occasions or PA was represented through shading and ranged from 0.0% to 27.7% or from 0.0% to 13.9% in the 4 JTDPAP clusters, respectively. The darker shading represented that a greater percentage of participants in the cluster reported the same amount of EI or PACs at that time. Figures 3 and 4 added color to show the distribution of the largest EIs (Figure 3) and PACs (Figure 4) throughout 24 h among the 4 clusters.
FIGURE 1.
EI heat maps representing 4 distinct JTDPAPs for US adults aged 20–65 y drawn from NHANES 2003–2006 (C1: n = 256; C2: n = 678; C3: n = 472; C4: n = 430). Distribution of 1836 participants’ EI in 4 JTDPAP clusters generated through the dependent constrained band dynamic time warping method is shown. The absolute EI ranging from 0 to 4000 kcal (y axis) ranging from 00:00 to 24:00 at the hourly level (x axis) for nonpregnant US adults aged 20–65 y as drawn from NHANES 2003–2006 is depicted. The proportion of participants in each of 4 JTDPAP clusters reporting EI is represented through shading ranging from 0.0% to 27.7% of participants. Darker shading represents a greater percentage of participants in the cluster reporting the same amount of EI at that time. C1, Cluster 1; C2, Cluster 2; C3, Cluster 3; C4, Cluster 4; EI, energy intake; JTDPAP, joint temporal dietary and physical activity pattern.
FIGURE 2.
PA heat maps representing 4 distinct JTDPAPs for US adults aged 20–65 y drawn from NHANES 2003–2006 (C1: n = 256; C2: n = 678; C3: n = 472; C4: n = 430). Distribution of PA of 4 JTDPAP clusters generated through the dependent constrained band dynamic time warping method is shown. PACs ranging from 0 cph to 1.2 × 105 cph (y axis, truncated) ranging from 00:00 to 24:00 (x axis) for US adults aged 20–65 y as drawn from NHANES 2003–2006 are depicted. The proportion of participants in each of 4 JTDPAP clusters reporting PA is represented through shading ranging from 0.0% to 13.9% of participants. Darker shading represents a greater percentage of participants in the cluster reporting the same number of PACs at that time. cph, counts per hour; C1, Cluster 1; C2, Cluster 2; C3, Cluster 3; C4, Cluster 4; JTDPAP, joint temporal dietary and physical activity pattern; PA, physical activity; PAC, physical activity count.
FIGURE 3.
The largest EI heat maps representing 4 distinct JTDPAPs for US adults aged 20–65 y drawn from NHANES 2003–2006. Heat maps (distribution) of the highest EI of 1836 participants in 4 JTDPAP clusters (C1: n = 256; C2: n = 678; C3: n = 472; C4: n = 430). The clusters were generated through the dependent constrained band dynamic time warping method for nonpregnant US adults aged 20–65 y as drawn from NHANES 2003–2006. The absolute EI (blocks in heat map) is characterized by amount (y axis: ranging from 0 to 4000 kcal) ranging from 00:00 to 24:00 at the hourly level (x axis). The shading of blocks (ranging from 0.0% to 7.8%) represents the proportion of participants in each cluster whose highest EI is characterized by the corresponding amount and timing. Darker shading represents a greater percentage of participants in the cluster reporting the same amount of EI at that time. C1, Cluster 1; C2, Cluster 2; C3, Cluster 3; C4, Cluster 4; EI, energy intake; JTDPAP, joint temporal dietary and physical activity pattern.
FIGURE 4.
The largest PA heat maps representing 4 distinct JTDPAPs for US adults aged 20–65 y drawn from NHANES 2003–2006. Heat maps (distribution) of the highest PA of participants in 4 JTDPAP clusters (C1: n = 256; C2: n = 678; C3: n = 472; C4: n = 430). The clusters were generated through the dependent constrained band dynamic time warping method for US adults aged 20–65 y as drawn from NHANES 2003–2006. The PA (blocks in heat map) is characterized by counts (y axis: ranging from 0 cph to 1.2 × 105 cph, truncated) ranging from 00:00 to 24:00 at the hourly level (x axis). The shading of blocks (ranging from 0.0% to 13.9%) represents the proportion of participants in each cluster whose highest PAC is characterized by the corresponding amount and timing. Darker shading represents a greater percentage of participants in the cluster reporting the same number of PACs at that time. cph, counts per hour; C1, Cluster 1; C2, Cluster 2; C3, Cluster 3; C4, Cluster 4; JTDPAP, joint temporal dietary and physical activity pattern; PA, physical activity; PAC, physical activity count.
Results
Table 1 shows the demographic characteristics of participants in the 4 JTDPAP clusters. Cluster 2 had the largest proportion (36.9%) of participants, whereas Cluster 1 had the smallest proportion (14.0%); Clusters 3 and 4 had similar proportions of participants (25.7% and 23.4%, respectively). There were significant differences in sex (P < 0.0001), age group (P < 0.0001), PIR (P = 0.007), and BMI (P < 0.0001) among clusters. Men made up the majority in Cluster 1 (73.4%) and Cluster 4 (70.3%), whereas women made up the majority in Cluster 2 (68.0%) and Cluster 3 (60.2%). In addition, the age group 20–34 y was more heavily represented in Cluster 1 (44.9%), the age group 50–65 y was more heavily represented in Cluster 2 (45.2%), and the age group 35–49 y was more heavily represented in Clusters 3 (40.1%) and 4 (41.4%). Further, participants with the lowest household PIR (0–0.99) accounted for the largest percentage (13.2%) and those with the highest household PIR (≥5.00) accounted for the smallest percentage (24.0%) in Cluster 2 compared with the 3 other clusters. Participants in Cluster 1 had a greater proportion (43.1%) of normal weight than the 3 other clusters; whereas Clusters 2 (41.8%) and 4 (39.4%) had greater proportions of participants with obesity than the 2 other clusters.
TABLE 1.
Characteristics of clusters representing joint temporal dietary and physical activity patterns of US adults aged 20–65 y as drawn from the NHANES, 2003–20061
| Characteristics | Total, n | Cluster 1 | Cluster 2 | Cluster 3 | Cluster 4 | P value2 |
|---|---|---|---|---|---|---|
| Total | 1836 | 256 (14.0) | 678 (36.9) | 472 (25.7) | 430 (23.4) | |
| Survey year | 0.19 | |||||
| 2003–2004 | 895 (49.3) | 131 (54.0) | 338 (51.2) | 222 (45.5) | 204 (48.0) | |
| 2005–2006 | 941 (50.7) | 125 (46.0) | 340 (48.8) | 250 (54.5) | 226 (52.0) | |
| Sex | <0.0001* | |||||
| Male | 933 (49.5) | 197 (73.4) | 226 (32.0) | 202 (39.8) | 308 (70.3) | |
| Female | 903 (50.5) | 59 (26.6) | 452 (68.0) | 270 (60.2) | 122 (29.7) | |
| Race/ethnicity | 0.09 | |||||
| Mexican American | 389 (8.4) | 54 (10.0) | 142 (7.6) | 107 (8.9) | 86 (8.1) | |
| Other Hispanic | 57 (3.2) | 11 (4.5) | 16 (2.6) | 18 (3.9) | 12 (2.6) | |
| Non-Hispanic white | 910 (71.5) | 127 (71.0) | 325 (69.3) | 229 (71.7) | 229 (74.5) | |
| Non-Hispanic black | 385 (10.5) | 56 (11.3) | 149 (11.4) | 96 (10.0) | 84 (9.4) | |
| Other | 95 (6.4) | 8 (3.2) | 46 (9.1) | 22 (5.5) | 19 (5.4) | |
| Age group, y | <0.0001* | |||||
| 20–34 | 560 (29.4) | 122 (44.9) | 138 (20.0) | 162 (32.5) | 138 (29.8) | |
| 35–49 | 623 (38.2) | 93 (37.2) | 193 (34.8) | 173 (40.1) | 164 (41.4) | |
| 50–65 | 653 (32.4) | 41 (18.0) | 347 (45.2) | 137 (27.3) | 128 (28.8) | |
| Household PIR | 0.007* | |||||
| 0–0.99 | 288 (9.9) | 39 (10.5) | 136 (13.2) | 57 (7.2) | 56 (7.8) | |
| 1.00–2.99 | 427 (18.0) | 62 (19.3) | 161 (20.4) | 116 (17.2) | 88 (14.6) | |
| 2.00–2.99 | 280 (15.9) | 40 (17.6) | 111 (16.0) | 73 (16.9) | 56 (13.4) | |
| 3.00–3.99 | 277 (17.1) | 39 (14.3) | 94 (17.3) | 79 (18.7) | 65 (16.9) | |
| 4.00–4.99 | 168 (11.1) | 24 (11.0) | 50 (9.1) | 39 (9.8) | 55 (15.3) | |
| ≥5.00 | 396 (28.1) | 52 (27.3) | 126 (24.0) | 108 (30.2) | 110 (31.9) | |
| BMI3 | <0.0001* | |||||
| Underweight | 24 (1.6) | 4 (2.2) | 10 (1.7) | 6 (1.3) | 4 (1.5) | |
| Normal weight | 519 (31.1) | 103 (43.1) | 164 (28.0) | 146 (34.4) | 106 (24.7) | |
| Overweight | 630 (33.5) | 94 (36.9) | 205 (28.4) | 181 (37.3) | 150 (34.5) | |
| Obese | 663 (33.8) | 55 (17.8) | 299 (41.8) | 139 (27.0) | 170 (39.4) |
Values are unweighted sample sizes and weighted percentages except for the total cluster size percentage. The percentages may not add up to 100% owing to rounding. *Significant difference: P < 0.05. PIR, poverty to income ratio.
Rao–Scott F adjusted χ2 test is a goodness-of-fit, 1-sided test. Analyses were adjusted for aspects of survey design including stratification, clustering, and weight.
BMI: categories were classified based on the WHO (95).
Characteristics of JTDPAPs
Characteristics of JTDPAPs are summarized in Table 2 based on the cluster visualizations in Figures 1–4. In respect to dietary intake, the absolute EI in Cluster 4 was significantly higher than in the 3 other clusters. The absolute EIs in Clusters 2 and 3 were both less but more concentrated between 0 and 600 kcal around noon and in the evening than in Clusters 1 and 4, which were more concentrated between 0 and 800 kcal with a more dispersed distribution. Two main EI peaks from all 4 clusters occurred around 12:00 and 18:00. Clusters 1 and 4 displayed more EI around noon and in the evening, whereas Clusters 2 and 3 exhibited more energy-equivalent eating occasions with no distinct EI peaks.
TABLE 2.
Qualitative description of separate energy and PA pattern clusters representing joint temporal dietary and physical activity patterns of US adults aged 20–65 y as drawn from the NHANES, 2003–20061
| Cluster 1 | Cluster 2 | Cluster 3 | Cluster 4 | |
|---|---|---|---|---|
| n (%) | 256 (14.0) | 678 (36.9) | 472 (25.7) | 430 (23.4) |
| Characteristics of EI patterns | Mean ± SEM EI is 2558 ± 69 kcal/d. Peaks in EI were at 2 main occasions reaching ≤1600 and ≤2200 kcal at 11:00–13:00 and 17:00–20:00 where 59.35% and 75.8% of the cluster consumed 0–600 kcal, respectively | Mean ± SEM EI is 1808 ± 28 kcal/d. Proportionally equivalent peaks in EI were at 2 main occasions reaching ≤1000 kcal at 11:00–14:00 and 17:00–20:00 where 79.5% and 81.3% of the cluster consumed 0–600 kcal, respectively | Mean ± SEM EI is 1817 ± 34 kcal/d. Proportionally equivalent peaks in EI were at 2 main occasions reaching ≤1000 kcal at 11:00–04:00 and 17:00–20:00 where 74.2% and 78.6% of the cluster consumed 0–600 kcal, respectively | Mean ± SEM EI is 3017 ± 46 kcal/d. Peaks in EI were at 2 main occasions reaching ≤1600 and ≤1800 kcal at 11:00–14:00 and 17:00–21:00 where 46.5% and 31.9% of the cluster consumed 200–800 kcal, respectively |
| Characteristics of PA patterns | Mean PA (SEM) is 5.7 × 105 (1.2 × 104) counts/d. Highest PACs reached >1.2 × 105 cph and 92.2% of the cluster engaged in PACs between 1.8 × 104 and 3.6 × 104 cph between 08:00 and 20:00 | Mean PA (SEM) is 1.7 × 105 (2.4 × 103) counts/d. Lowest PACs reached ≤3.8 × 104 cph and 98.1% of the cluster engaged in PACs between 0.3 × 104 and 1.2 × 104 cph between 09:00 and 22:00 | Mean PA (SEM) is 3.4 × 105 (4.7 × 103) counts/d. High PACs reached ≤8.8 × 104 cph and 75.4% of the cluster engaged in PACs between 1.2 × 104 and 2.4 × 104 cph between 16:00 and 20:00 | Mean PA (SEM) is 3.0 × 105 (5.0 × 103) counts/d. High PACs reached ≤6.2 × 104 cph and 58.6% of the cluster engaged in PACs between 1.2 × 104 and 2.4 × 104 cph between 09:00 and 12:00 |
1cph, counts per hour; EI, energy intake; PA, physical activity; PAC, physical activity count.
With respect to PA, Cluster 1 demonstrated the highest PACs, Cluster 2 demonstrated the lowest PACs, and Clusters 3 and 4 demonstrated similar PACs in the middle among the 4 clusters. The intensity of activity in Cluster 3 tended to increase toward later hours from 16:00 to 20:00; however, Cluster 4 intensity tended to be greater toward the earlier hours from 09:00 to 12:00 and decrease toward later hours after 13:00. This suggested that Cluster 3 had more of an evening PA pattern, whereas Cluster 4 had more of an early morning PA pattern.
Associations of JTDPAPs with obesity
The primary result of this study is that JTDPAPs are associated with obesity-related health indicators. Participants in Cluster 1 had significantly lower mean BMI and smaller WC than those in Clusters 2 and 4 as well as those in Cluster 3 than those in Clusters 2 and 4 in the unadjusted model (P < 0.05) (Supplemental Tables 1 and 2). Participants in Cluster 4 had significantly higher mean BMI and larger WC than those in Clusters 1 (P < 0.0001, P = 0.0001, respectively), 2 (P = 0.01, P = 0.05, respectively), and 3 (P < 0.0001, P < 0.0001, respectively), and participants in Cluster 3 also had significantly lower mean BMI and smaller WC than those in Cluster 2 (P = 0.001, P = 0.003, respectively) in the adjusted models (Tables 3 and 4). The greatest significant differences in mean BMI and mean WC were present between Clusters 3 and 4 (β: −3.7 ± 0.5 and β: −8.3 ± 1.3 cm, respectively) in the adjusted model (Tables 3 and 4).
TABLE 3.
Adjusted regression model results for mean BMI with clusters representing joint temporal dietary and physical activity patterns of US adults aged 20–65 y as drawn from the NHANES, 2003–20061
| Adjusted models2 | n, % | BMI,3 kg/m2 | β4 ± SE compared with Cluster 2 | 95% CI | Adjusted P value | β4 ± SE compared with Cluster 3 | 95% CI | Adjusted P value | β4 ± SE compared with Cluster 4 | 95% CI | Adjusted P value |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Cluster 1 | 256 (14.0) | 26.8 ± 0.3 | −1.3 ± 0.6 | −2.9, 0.4 | 0.17 | 0.5 ± 0.5 | −0.8, 1.9 | 0.71 | −3.1 ± 0.5 | −4.6, −1.7* | <0.0001 |
| Cluster 2 | 678 (36.9) | 30.0 ± 0.3 | 1.8 ± 0.4 | 0.7, 3.0* | 0.001 | −1.8 ± 0.5 | −3.3, −0.4* | 0.01 | |||
| Cluster 3 | 472 (25.7) | 27.8 ± 0.3 | −3.7 ± 0.5 | −5.1, −2.2* | <0.0001 | ||||||
| Cluster 4 | 430 (23.4) | 29.5 ± 0.3 |
There were significant differences in mean BMI between Clusters 1 and 2, Clusters 1 and 4, Clusters 2 and 3, and Clusters 3 and 4 in the unadjusted model at P < 0.05 (see Supplemental Table 1). *Significant difference: adjusted P < 0.05.
Multiple linear regression models were used and were adjusted for survey year, age group, sex, race/ethnicity, poverty to income ratio, energy misreporting, and total physical activity counts per day.
Values are mean ± SEM.
β represents the difference of mean BMI between 2 compared clusters. Least square means were used to calculate the differences in mean BMI.
TABLE 4.
Adjusted regression model results for mean WC (cm) with clusters representing joint temporal dietary and physical activity patterns of US adults aged 20–65 y as drawn from the NHANES, 2003–20061
| Adjusted models2 | n, % | WC,3 cm | β4 ± SE compared with Cluster 2 | 95% CI | Adjusted P value | β4 ± SE compared with Cluster 3 | 95% CI | Adjusted P value | β4 ± SE compared with Cluster 4 | 95% CI | Adjusted P value |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Cluster 1 | 256 (14.0) | 93.4 ± 0.9 | −3.5 ± 1.9 | −8.6, 1.7 | 0.28 | 1.0 ± 1.5 | −3.0, 5.0 | 0.91 | −7.3 ± 1.4 | −11.2, −3.4* | 0.0001 |
| Cluster 2 | 678 (36.9) | 100.7 ± 0.6 | 4.5 ± 1.2 | 1.3, 7.6* | 0.003 | −3.8 ± 1.4 | −7.6, −0.1* | 0.05 | |||
| Cluster 3 | 472 (25.7) | 94.8 ± 0.6 | −8.3 ± 1.3 | −11.9, −4.6* | <0.0001 | ||||||
| Cluster 4 | 430 (23.4) | 100.4 ± 0.8 |
There were significant differences in mean WC between Clusters 1 and 2, Clusters 1 and 4, Clusters 2 and 3, and Clusters 3 and 4 in the unadjusted model at P < 0.05 (see Supplemental Table 2). *Significant difference: adjusted P < 0.05. WC, waist circumference.
Multiple linear regression models were used and were adjusted for survey year, age group, sex, race/ethnicity, poverty to income ratio, energy misreporting, and total physical activity counts per day.
Values are mean ± SEM.
β represents the difference of mean WC between 2 compared clusters. Least square means were used to calculate the differences in mean WC.
There were significant differences among all comparisons of 2 clusters except for Clusters 2 and 4 in the odds of being obese to normal weight in the unadjusted model (P < 0.05) (Supplemental Table 3). The significant differences remained between Cluster 4 and Clusters 1 (P < 0.0001), 2 (P = 0.004), and 3 (P < 0.0001) in the adjusted models (Table 5). The greatest significant difference in odds of being obese was present between Clusters 1 and 4 in the adjusted model (OR: 0.2; 95% CI: 0.1, 0.5).
TABLE 5.
Odds of obesity relative to normal weight status and covariate-adjusted regression model results for clusters representing joint temporal dietary and physical activity patterns of US adults aged 20–65 y as drawn from the NHANES, 2003–20061
| Adjusted models2 | n, % | OR3 compared with Cluster 2 | 95% CI | Adjusted P value | OR3 compared with Cluster 3 | 95% CI | Adjusted P value | OR3 compared with Cluster 4 | 95% CI | Adjusted P value |
|---|---|---|---|---|---|---|---|---|---|---|
| Cluster 1 | 256 (14.0) | 0.6 | 0.3, 1.1 | 0.17 | 0.9 | 0.5, 1.6 | 0.96 | 0.2 | 0.1, 0.5* | <0.0001 |
| Cluster 2 | 678 (36.9) | 1.5 | 0.9, 2.5 | 0.15 | 0.4 | 0.2, 0.8* | 0.004 | |||
| Cluster 3 | 472 (25.7) | 0.3 | 0.1, 0.5* | <0.0001 | ||||||
| Cluster 4 | 430 (23.4) |
There were significant differences in OR of obesity to normal weight among all clusters except Clusters 2 and 4 in the unadjusted model at P < 0.05 (see Supplemental Table 3). *Significant difference: adjusted P < 0.05.
Multiple logistic regression models were used and were adjusted for survey year, age group, sex, race/ethnicity, poverty to income ratio, energy misreporting, and total physical activity counts per day.
OR of obesity to normal weight between 2 compared clusters. Obesity was classified as BMI ≥ 30 kg/m2 (95).
Association between JTDPAPs and other health status indicators
The secondary result of this study is that JTDPAPs are associated with other health indicators. Supplemental Tables 4–12 present the association of JTDPAPs with other health status indicators, T2DM, and MetS in the unadjusted model. There were 3 significant differences in triglycerides between Clusters 1 and 2 or 3, and Clusters 2 and 4, and 3 significant differences in total cholesterol between Cluster 1 and the 3 other clusters, in the adjusted models (Tables 6 and 7). There were no significant differences in the adjusted models for all other examined health status indicators, T2DM, and MetS (Supplemental Tables 13–19).
TABLE 6.
Adjusted regression model results for mean triglycerides with clusters representing joint temporal dietary and physical activity patterns of US adults aged 20–65 y as drawn from the NHANES, 2003–20061
| Adjusted models2 | n, % | Triglycerides,3 mg/dL | β4 ± SE compared with Cluster 2 | 95% CI | Adjusted P value | β4 ± SE compared with Cluster 3 | 95% CI | Adjusted P value | β4 ± SE compared with Cluster 4 | 95% CI | Adjusted P value |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Cluster 1 | 256 (14.0) | 121.3 ± 6.0 | −40.6 ± 10.0 | −67.8, −13.4* | 0.002 | −28.7 ± 9.1 | −53.6, −3.8* | 0.02 | −13.5 ± 9.9 | −40.3, 13.3 | 0.53 |
| Cluster 2 | 678 (36.9) | 155.5 ± 6.3 | 11.9 ± 7.6 | −8.7, 32.5 | 0.41 | 27.1 ± 8.3 | 4.6, 49.6* | 0.01 | |||
| Cluster 3 | 472 (25.7) | 136.8 ± 5.7 | 15.2 ± 10.3 | −12.7, 43.1 | 0.46 | ||||||
| Cluster 4 | 430 (23.4) | 149.2 ± 6.8 |
There were significant differences in mean triglycerides between Clusters 1 and 2, Clusters 1 and 4, and Clusters 2 and 3 in the unadjusted model at P < 0.05 (see Supplemental Table 4). *Significant difference: adjusted P < 0.05.
Multiple linear regression models were used and were adjusted for survey year, age group, sex, race/ethnicity, poverty to income ratio, BMI, energy misreporting, and total physical activity counts per day.
Values are mean ± SEM.
β represents the difference of mean triglycerides between 2 compared clusters. Least square means were used to calculate the differences in mean triglycerides.
TABLE 7.
Adjusted regression model results for mean total cholesterol with clusters representing joint temporal dietary and physical activity patterns of US adults aged 20–65 y as drawn from the NHANES, 2003–20061
| Adjusted models2 | n, % | Total cholesterol,3 mg/dL | β4 ± SE compared with Cluster 2 | 95% CI | Adjusted P value | β4 ± SE compared with Cluster 3 | 95% CI | Adjusted P value | β4 ± SE compared with Cluster 4 | 95% CI | Adjusted P value |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Cluster 1 | 256 (14.0) | 188.8 ± 2.3 | −16.0 ± 5.1 | −30.0, −2.0* | 0.02 | −11.6 ± 3.8 | −21.9, −1.3* | 0.02 | −14.9 ± 4.8 | −27.8, −1.9* | 0.02 |
| Cluster 2 | 678 (36.9) | 201.5 ± 1.9 | 4.4 ± 2.6 | −2.7, 11.5 | 0.36 | 1.1 ± 2.8 | −6.6, 8.9 | 0.98 | |||
| Cluster 3 | 472 (25.7) | 197.9 ± 1.7 | −3.3 ± 3.5 | −12.7, 6.2 | 0.79 | ||||||
| Cluster 4 | 430 (23.4) | 202.5 ± 1.9 |
There were significant differences in mean total cholesterol between Clusters 1 and 2, Clusters 1 and 3, and Clusters 1 and 4 in the unadjusted model at P < 0.05 (see Supplemental Table 5). *Significant difference: adjusted P < 0.05.
Multiple linear regression models were used and were adjusted for survey year, age group, sex, race/ethnicity, poverty to income ratio, BMI, energy misreporting, and total physical activity counts per day.
Values are mean ± SEM.
β represents the difference of mean total cholesterol between 2 compared clusters. Least square means were used to calculate the differences in mean total cholesterol.
Discussion
In this study, primarily, clusters representing JTDPAPs among US adults ages 20–65 y were created using a novel methodology. Even though previous studies investigated the potential role of timing of eating and PA for health (7–9, 61–65), to our knowledge, this is the first study to create JTDPAPs that integrated the amount/counts, frequency, and timing of dietary intake and PA throughout 24 h. JTDPAPs are associated with BMI, WC, triglycerides, total cholesterol, and odds of being obese among investigated health or disease status indicators. The circadian system has an important effect on the regulation of metabolism, physiology, and behavior and may help to explain why the timing of diet and PA exposures is important to health. Biological rhythms are coordinated in the circadian system through the involvement of clock genes (66–68). Diet and PA are behavioral factors that can have an impact on the circadian system. If these external behaviors are misaligned with the original circadian system, such as in eating late at night, metabolism may be impaired and could lead to dysfunction (69–74).
The significant mean differences in BMI, WC, triglycerides, but not total cholesterol, among JTDPAP clusters were both statistically significant and clinically meaningful (75–79), showing a potential for future clinical application. JTDPAPs could be an important health exposure that holds promise for early detection of lifestyle factors to promote health and prevent disease.
Participants in Cluster 1 had significantly lower BMI, total cholesterol concentration, odds of obesity, and smaller WC than those in Cluster 4. A pattern with 3 evenly spaced eating occasions with higher EI during 2 main eating occasions from 11:00 to 13:00 and 17:00 to 20:00 and higher PACs during the day, may bring more benefits to health than a pattern with higher overall EI and an energy peak from 17:00 to 21:00 and lower PACs throughout a day. These primary findings are in line with the previous finding that suggested lower EI and higher PA level are positively associated with a lower prevalence of obesity-related health outcomes (8, 9, 15, 80–83). Moreover, being in Cluster 1 was also associated with significantly lower total cholesterol than the 3 other clusters, a result that is also consistent with previous PA studies showing higher PA level is positively associated with a lower concentration of total cholesterol (84–86). Furthermore, Cluster 1 participants demonstrated peaks of PACs before corresponding eating occasions, which may indicate participants have a high level of PA before eating. Cluster 1 predominantly included men and the age group 20–34 y, which is consistent with previous studies showing young adults have higher PA intensities than older adults (87).
Cluster 2 had a significantly higher mean BMI and larger mean WC than Cluster 3. Because Clusters 2 and 3 had similar dietary patterns during the day, this result suggests that PA may have a main effect on modulating BMI and WC as supported by previous studies (15, 80–83). Cluster 2 with the lowest PACs among the 4 clusters had the highest number of participants (36.9%), corroborating evidence that US adults engage in high amounts of sedentary behavior (88). In addition, Cluster 2 predominantly included women, ages 50–65 y with low PIR and odds of obesity, which aligns with previous findings of lower PA and EI with advanced age (89, 90) and among women (82), especially those with low income (91, 92).
Results of no significant differences among JTDPAP clusters in terms of the other short-term health status indicators and disease statuses examined in this study were unexpected. A systematic review demonstrated that participants who exercised postmeal at any time of day had lower postprandial glycemia than those who exercised premeal (5). Further, a cross-sectional study showed that a temporal dietary pattern with “later lunch” (13:00–14:00) was associated with hypertension compared with that with “conventional lunch” (12:00–13:00) in women (62). Potential reasons for no significant differences in these health status indicators of this study may be due to the similar timing of dietary intake among the 4 clusters yet varying amounts of EI or individual variability in short-term serum biomarkers being larger than among long-term indicators like BMI and WC. If not, this finding may imply that JTDPAPs may be more closely related to long-term health status indicators like BMI and WC rather than short-term indicators such as blood pressure.
One of the strengths of the study was the comprehensive method to integrate temporal dietary and PA exposures together over a 24-h day. Using this method, the clusters were not generated from a predefined standard but data-driven methods based on the true nature of the behaviors. Even though this is the first attempt to integrate multiple behaviors, additional multidimensionality of dietary intake and PA such as diet quality and activity type, along with other factors such as comorbidity status, medication use, and other lifestyle behaviors such as smoking, should be incorporated in future studies. But because of the cross-sectional study design, this study cannot be used to infer causation. In addition, the patterns generated integrate both EI and PA over time, but the influences of factors of behavior and timing are not distinguishable because the goal was to determine if their integration had a significant relation with health. Another limitation is that the dietary and PA data are from 1 weekday dietary recall and 1 weekday accelerometer record, respectively, likely representing different days because of NHANES protocol, yet their combination assumes the data represent participants’ regular patterns. NHANES includes ≤2 dietary recalls and 7 d of PA accelerometer data, so the sample size would be further reduced if a second recall or another day of PA was also an inclusion criterion, even though two 24-h dietary recalls or multiple PA accelerometer data may better identify participants’ usual temporal eating pattern and temporal PA pattern. In addition, because little is known about temporal dietary and PA behavior over multiple days, future studies should explore the integration of the amount/intensity and temporal sequence of dietary intake and PA over multiple days. A single 24-h dietary recall may be considered to be representative to estimate the general dietary pattern if days of the week of dietary recalls are evenly selected (93), and 1 valid random day of PA data is also considered to be sufficient at the population level (19). The dietary data in this study did not evenly distribute across the week, thus, weekend compared with weekday patterns should be further investigated and the conclusion of this study should be cautiously generalized because only weekday data were used. Accelerometers are not waterproof and only record uniaxial movement, so they did not record or accurately record all types of activity such as swimming or elliptical training (16), but main sources of PA for most people like walking were accounted for. Moreover, because total cholesterol may not be measured at fasting state, it may result in higher concentration than fasting total cholesterol (94), but compared with the fasting lipid profile, the diagnostic accuracy of the nonfasting lipid profile was significantly higher for the assessment of lipoprotein coronary risk (94). Finally, the study sample is only 8.97% of the original population in NHANES 2003–2006 because many participants in the original sample were outside of the age range 20–65 y and did not have valid fasting health status indicator information. Owing to the relatively small sample size, the unique JTDPAPs that may exist in the population, such as night shift patterns, may not be observed because these smaller patterns were combined with other patterns preventing observation of their unique temporal characteristics.
In conclusion, JTDPAPs are significantly associated with BMI, WC, triglycerides, total cholesterol, and obesity. A JTDPAP cluster with 2 energy-equivalent main eating occasions and higher PACs may be associated with more favorable health indicators including lower BMI, total cholesterol concentration, odds of obesity, and smaller WC in US adults than other JTDPAPs. The integration of weekday EI, PACs, and the timing of those exposures is possible in generating JTDPAPs that are associated with health status indicators and could contribute to early detection of lifestyle behavioral patterns prone to obesity.
Supplementary Material
Acknowledgments
The authors’ responsibilities were as follows—LL and JG: analyzed the data; LL: wrote the paper; HAE-M, EJD, SBG, AB, EAR, EH, JG, and MMA: critically reviewed and edited the manuscript; HAE-M: had primary responsibility for the final content; and all authors: designed the research and read and approved the final manuscript. The authors report no conflicts of interest.
Notes
Supported by National Cancer Institute of the NIH award R21CA224764 (to HAE-M) and Purdue University (to HAE-M).
Supplemental Figure 1 and Supplemental Tables 1–19 are available from the “Supplementary data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/ajcn/.
Abbreviations used: CDTW, constrained band dynamic time warping; CDTWD, dependent multivariate constrained band dynamic time warping; CDTWI, independent multivariate constrained band dynamic time warping; DBHCA, density balanced hierarchical cluster agglomeration; DTW, dynamic time warping; EER, estimated energy requirement; EI, energy intake; FNDDS, Food and Nutrient Database for Dietary Studies; HbA1c, glycated hemoglobin; JTDPAP, joint temporal dietary and physical activity pattern; MetS, metabolic syndrome; NCHS, National Center for Health Statistics; PA, physical activity; PAC, physical activity count; PIR, poverty to income ratio; T2DM, type 2 diabetes mellitus; WC, waist circumference.
Contributor Information
Luotao Lin, Department of Nutrition Science, Purdue University, West Lafayette, IN, USA.
Jiaqi Guo, School of Electrical and Computer Engineering, Purdue University, West Lafayette, IN, USA.
Marah M Aqeel, Department of Nutrition Science, Purdue University, West Lafayette, IN, USA.
Saul B Gelfand, School of Electrical and Computer Engineering, Purdue University, West Lafayette, IN, USA.
Edward J Delp, School of Electrical and Computer Engineering, Purdue University, West Lafayette, IN, USA.
Anindya Bhadra, Department of Statistics, Purdue University, West Lafayette, IN, USA.
Elizabeth A Richards, School of Nursing, Purdue University, West Lafayette, IN, USA.
Erin Hennessy, Friedman School of Nutrition Science and Policy, Tufts University, Boston, MA, USA.
Heather A Eicher-Miller, Department of Nutrition Science, Purdue University, West Lafayette, IN, USA.
Data Availability
Data described in the article and code book are publicly and freely available without restriction at https://www.cdc.gov/nchs/nhanes/index.htm. Analytic code will be made available upon request pending application and approval.
References
- 1. Dietary Guidelines Advisory Committee. Scientific Report of the 2015 Dietary Guidelines Advisory Committee: advisory report to the Secretary of Health and Human Services and the Secretary of Agriculture. Washington (DC): USDA Agricultural Research Service; 2015. [Google Scholar]
- 2. 2018 Physical Activity Guidelines Advisory Committee. 2018 Physical Activity Guidelines Advisory Committee scientific report. Washington (DC): US Department of Health and Human Services; 2018. [Google Scholar]
- 3. Busch V, Van Stel HF, Schrijvers AJ, de Leeuw JR. Clustering of health-related behaviors, health outcomes and demographics in Dutch adolescents: a cross-sectional study. BMC Public Health. 2013;13(1):1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Elliot CA, Hamlin MJ. Combined diet and physical activity is better than diet or physical activity alone at improving health outcomes for patients in New Zealand's primary care intervention. BMC Public Health. 2018;18(1):230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Aqeel MM, Forster A, Richards EA, Hennessy E, McGowan B, Bhadra A, Guo J, Gelfand SB, Delp EJ, Eicher-Miller HA. The effect of timing of exercise and eating on postprandial response in adults: a systematic review. Nutrients. 2020;12(1):221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Zhang Y, Liu L. Understanding temporal pattern of human activities using Temporal Areas of Interest. Appl Geogr. 2018;94:95–106. [Google Scholar]
- 7. Eicher-Miller HA, Khanna N, Boushey CJ, Gelfand SB, Delp EJ. Temporal dietary patterns derived among the adult participants of the National Health and Nutrition Examination Survey 1999-2004 are associated with diet quality. J Acad Nutr Diet. 2016;116(2):283–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Aqeel MM, Guo J, Lin L, Gelfand SB, Delp EJ, Bhadra A, Richards EA, Hennessy E, Eicher-Miller HA. Temporal dietary patterns are associated with obesity in US adults. J Nutr. 2020;150(12):3259–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Aqeel M, Guo J, Lin L, Gelfand S, Delp E, Bhadra A, Richards EA, Hennessy E, Eicher-Miller HA. Temporal physical activity patterns are associated with obesity in U.S. adults. Prev Med. 2021;148:106538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. National Center for Health Statistics (NCHS). NCHS Research Ethics Review Board (ERB) approval. [Internet]. Hyattsville, MD: NCHS; 2017; [cited 13 April, 2021]. Available from: http://www.cdc.gov/nchs/nhanes/irba98.htm. [Google Scholar]
- 11. Agricultural Research Service, USDA. AMPM - USDA Automated Multiple-Pass Method. [Internet]. Beltsville, MD: Food Surveys Research Group; 2021; [cited 13 April, 2021]. Available from: https://www.ars.usda.gov/northeast-area/beltsville-md-bhnrc/beltsville-human-nutrition-research-center/food-surveys-research-group/docs/ampm-usda-automated-multiple-pass-method/#what. [Google Scholar]
- 12. National Center for Health Statistics (NCHS). NHANES dietary data. [Internet]. Hyattsville, MD: NCHS; 2021; [cited 13 April, 2021]. Available from: https://wwwn.cdc.gov/nchs/nhanes/Search/DataPage.aspx?Component=Dietary. [Google Scholar]
- 13. An R. Weekend-weekday differences in diet among U.S. adults, 2003–2012. Ann Epidemiol. 2016;26(1):57–65. [DOI] [PubMed] [Google Scholar]
- 14. Gibney MJ, Wolever TMS. Periodicity of eating and human health: present perspective and future directions. Br J Nutr. 1997;77(S1):S3–5. [DOI] [PubMed] [Google Scholar]
- 15. Luke A, Dugas LR, Durazo-Arvizu RA, Cao G, Cooper RS. Assessing physical activity and its relationship to cardiovascular risk factors: NHANES 2003-2006. BMC Public Health. 2011;11(1):387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. National Center for Health Statistics (NCHS). National Health and Nutrition Examination Survey 2005-2006 data documentation, codebook, and frequencies: Physical Activity Monitor (PAXRAW_D). [Internet]. Hyattsville, MD: NCHS; 2008; [cited 13 April, 2021]. Available from: https://wwwn.cdc.gov/Nchs/Nhanes/2005-2006/PAXRAW_D.htm. [Google Scholar]
- 17. Troiano RP, Berrigan D, Dodd KW, Mâsse LC, Tilert T, McDowell M. Physical activity in the United States measured by accelerometer. Med Sci Sports Exerc. 2008;40(1):181–8. [DOI] [PubMed] [Google Scholar]
- 18. Sigmundová D, Sigmund E, Badura P, Vokáčová J, Trhlíková L, Bucksch J. Weekday-weekend patterns of physical activity and screen time in parents and their pre-schoolers. BMC Public Health. 2016;16(1):898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Wolff-Hughes DL, McClain JJ, Dodd KW, Berrigan D, Troiano RP. Number of accelerometer monitoring days needed for stable group-level estimates of activity. Physiol Meas. 2016;37(9):1447–55. [DOI] [PubMed] [Google Scholar]
- 20. Loprinzi PD, Lee H, Cardinal BJ, Crespo CJ, Andersen RE, Smit E. The relationship of actigraph accelerometer cut-points for estimating physical activity with selected health outcomes: results from NHANES 2003–06. Res Q Exerc Sport. 2012;83(3):422–30. [DOI] [PubMed] [Google Scholar]
- 21. Bornstein DB, Beets MW, Byun W, Welk G, Bottai M, Dowda M, Pate R. Equating accelerometer estimates of moderate-to-vigorous physical activity: in search of the Rosetta Stone. J Sci Med Sport. 2011;14(5):404–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. McDowell MA, Fryar CD, Ogden CL, Flegal KM. Anthropometric reference data for children and adults: United States, 2003–2006. Natl Health Stat Report. 2008;(10):1–48. [PubMed] [Google Scholar]
- 23. National Center for Health Statistics (NCHS). Laboratory Procedures Manual 2003-2004. [Internet]. Hyattsville, MD: NCHS; 2004; [cited 13 April, 2021]. Available from: https://wwwn.cdc.gov/nchs/data/nhanes/2003-2004/manuals/lab.pdf. [Google Scholar]
- 24. National Center for Health Statistics (NCHS). Laboratory Procedures Manual 2005-2006[Internet]. Hyattsville, MD: NCHS; 2005; [cited 13 April, 2021]. Available from: https://wwwn.cdc.gov/nchs/data/nhanes/2005-2006/manuals/lab.pdf. [Google Scholar]
- 25. National Center for Health Statistics (NCHS). National Health and Nutrition Examination Survey 2003-2004 data documentation, codebook, and frequencies: cholesterol-LDL & triglycerides (L13AM_C). [Internet]. Hyattsville, MD: NCHS; 2008; [cited 13 April, 2021]. Available from: https://wwwn.cdc.gov/Nchs/Nhanes/2003-2004/L13AM_C.htm. [Google Scholar]
- 26. National Center for Health Statistics (NCHS). National Health and Nutrition Examination Survey 2005-2006 data documentation, codebook, and frequencies: cholesterol - LDL, triglyceride & apoliprotein (ApoB) (TRIGLY_D). [Internet]. Hyattsville, MD: NCHS; 2008; [cited 13 April, 2021]. Available from: https://wwwn.cdc.gov/Nchs/Nhanes/2005-2006/TRIGLY_D.htm. [Google Scholar]
- 27. National Center for Health Statistics (NCHS). National Health and Nutrition Examination Survey 2003-2004 data documentation, codebook, and frequencies: plasma fasting glucose, serum C-peptide & insulin (L10AM_C). [Internet]. Hyattsville, MD: NCHS; 2016; [cited 13 April, 2021]. Available from: https://wwwn.cdc.gov/Nchs/Nhanes/2003-2004/L10AM_C.htm. [Google Scholar]
- 28. National Center for Health Statistics (NCHS). National Health and Nutrition Examination Survey 2005-2006 data documentation, codebook, and frequencies: plasma fasting glucose & insulin (GLU_D). [Internet]. Hyattsville, MD: NCHS; 2016; [cited 13 April, 2021]. Available from: https://wwwn.cdc.gov/Nchs/Nhanes/2005-2006/GLU_D.htm. [Google Scholar]
- 29. Cohen JD, Cziraky MJ, Cai Q, Wallace A, Wasser T, Crouse JR, Jacobson TA. 30-year trends in serum lipids among United States adults: results from the National Health and Nutrition Examination Surveys II, III, and 1999–2006. Am J Cardiol. 2010;106(7):969–75. [DOI] [PubMed] [Google Scholar]
- 30. National Center for Health Statistics (NCHS). National Health and Nutrition Examination Survey 2005-2006 data documentation, codebook, and frequencies: cholesterol - HDL (HDL_D). [Internet]. Hyattsville, MD: NCHS; 2010; [cited 13 April, 2021]. Available from: https://wwwn.cdc.gov/Nchs/Nhanes/2005-2006/HDL_D.htm. [Google Scholar]
- 31. National Center for Health Statistics (NCHS). National Health and Nutrition Examination Survey 2005-2006 data documentation, codebook, and frequencies: cholesterol - total (TCHOL_D). [Internet]. Hyattsville, MD: NCHS; 2010; [cited 13 April, 2021]. Available from: https://wwwn.cdc.gov/Nchs/Nhanes/2005-2006/TCHOL_D.htm. [Google Scholar]
- 32. National Center for Health Statistics (NCHS). National Health and Nutrition Examination Survey 2003-2004 data documentation, codebook, and frequencies: cholesterol - total & HDL (l13_c). [Internet]. Hyattsville, MD: NCHS; 2010; [cited 13 April, 2021]. Available from: https://wwwn.cdc.gov/Nchs/Nhanes/2003-2004/L13_C.htm. [Google Scholar]
- 33. Myers GL, Cooper GR, Winn CL, Smith SJ. The Centers for Disease Control-National Heart, Lung and Blood Institute Lipid Standardization Program. An approach to accurate and precise lipid measurements. Clin Lab Med. 1989;9(1):105–36. [PubMed] [Google Scholar]
- 34. National Center for Health Statistics (NCHS). National Health and Nutrition Examination Survey 2003-2004 data documentation, codebook, and frequencies: glycohemoglobin (L10_C). [Internet]. Hyattsville, MD: NCHS; 2012; [cited 13 April, 2021]. Available from: https://wwwn.cdc.gov/Nchs/Nhanes/2003-2004/L10_C.htm. [Google Scholar]
- 35. National Center for Health Statistics (NCHS). NHANES 2005-2006 data documentation, codebook, and frequencies: glycohemoglobin (GHB_D). [Internet]. Hyattsville, MD: NCHS; 2012; [cited 13 April, 2021]. Available from: https://wwwn.cdc.gov/Nchs/Nhanes/2005-2006/GHB_D.htm. [Google Scholar]
- 36. Mellen PB, Gao SK, Vitolins MZ, Goff DC. Deteriorating dietary habits among adults with hypertension: DASH dietary accordance, NHANES 1988-1994 and 1999-2004. Arch Intern Med. 2008;168(3):308–14. [DOI] [PubMed] [Google Scholar]
- 37. World Health Organization. Body Mass Index - BMI. [Internet]. Geneva, Switzerland: WHO; 2021; [cited 13 April, 2021]. Available from: http://www.euro.who.int/en/health-topics/disease-prevention/nutrition/a-healthy-lifestyle/body-mass-index-bmi. [Google Scholar]
- 38. American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2014;37(Supplement_1):S81–90. [DOI] [PubMed] [Google Scholar]
- 39. Nelson KM, Reiber G, Boyko EJ. Diet and exercise among adults with type 2 diabetes: findings from the third National Health and Nutrition Examination Survey (NHANES III). Diabetes Care. 2002;25(10):1722–8. [DOI] [PubMed] [Google Scholar]
- 40. Alexander CM, Landsman PB, Teutsch SM, Haffner SM. NCEP-defined metabolic syndrome, diabetes, and prevalence of coronary heart disease among NHANES III participants age 50 years and older. Diabetes. 2003;52(5):1210–14. [DOI] [PubMed] [Google Scholar]
- 41. United States Census Bureau. Poverty thresholds. [Internet]. Suitland, MD: US Census Bureau; 2020; [cited 13 April, 2021]. Available from: https://www.census.gov/topics/income-poverty/poverty/guidance/poverty-measures.html. [Google Scholar]
- 42. Murakami K, Livingstone MBE. Associations between meal and snack frequency and overweight and abdominal obesity in US children and adolescents from National Health and Nutrition Examination Survey (NHANES) 2003–2012. Br J Nutr. 2016;115(10):1819–29. [DOI] [PubMed] [Google Scholar]
- 43. Wang JB, Patterson RE, Ang A, Emond JA, Shetty N, Arab L. Timing of energy intake during the day is associated with the risk of obesity in adults. J Hum Nutr Diet. 2014;27:255–62. [DOI] [PubMed] [Google Scholar]
- 44. Leech RM, Worsley A, Timperio A, McNaughton SA. The role of energy intake and energy misreporting in the associations between eating patterns and adiposity. Eur J Clin Nutr. 2018;72(1):142–7. [DOI] [PubMed] [Google Scholar]
- 45. Food and Nutrition Board, Institute of Medicine. Dietary Reference Intakes for energy, carbohydrate, fiber, fat, fatty acids, cholesterol, protein, and amino acids. Washington (DC): National Academy Press; 2005. [DOI] [PubMed] [Google Scholar]
- 46. Evenson KR, Wen F, Metzger JS, Herring AH. Physical activity and sedentary behavior patterns using accelerometry from a national sample of United States adults. Int J Behav Nutr Phys Act. 2015;12(1):20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. National Center for Health Statistics (NCHS). NHANES tutorials - module 3 - weighting. [Internet]. Hyattsville, MD: NCHS; 2021; [cited 13 April, 2021]. Available from: https://wwwn.cdc.gov/nchs/nhanes/tutorials/module3.aspx. [Google Scholar]
- 48. National Center for Health Statistics (NCHS). National Health and Nutrition Examination Survey 2003-2004 data documentation, codebook, and frequencies: demographic variables & sample weights (DEMO_C). [Internet]. Hyattsville, MD: NCHS; 2009; [cited 13 April, 2021]. Available from: https://wwwn.cdc.gov/Nchs/Nhanes/2003-2004/DEMO_C.htm. [Google Scholar]
- 49. National Center for Health Statistics (NCHS). NHANES tutorials - module 2 - sample design. [Internet]. Hyattsville, MD: NCHS; 2021; [cited 13 April, 2021]. Available from: https://wwwn.cdc.gov/nchs/nhanes/tutorials/module2.aspx. [Google Scholar]
- 50. Sakoe H, Chiba S. Dynamic programming algorithm optimization for spoken word recognition. IEEE Trans Acoust Speech Signal Process. 1978;26(1):43–9. [Google Scholar]
- 51. Geler Z, Kurbalija V, Radovanović M, Ivanović M. Impact of the Sakoe-Chiba band on the DTW time series distance measure for kNN classification. In: Buchmann R, Kifor CV, Yu J, editors. International Conference on Knowledge Science, Engineering and Management; 16–18 October, 2014; Sibiu, Romania. Cham, Switzerland: Springer; 2014. p. 105–14. [Google Scholar]
- 52. Shokoohi-Yekta M, Hu B, Jin H, Wang J, Keogh E. Generalizing DTW to the multi-dimensional case requires an adaptive approach. Data Min Knowl Discov. 2017;31(1):1–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Dhillon IS, Guan Y, Kulis B. Kernel k-means: spectral clustering and normalized cuts. In: Proceedings of the tenth ACM SIGKDD international conference on Knowledge discovery and data mining; 22–25 August, 2004; Seattle, WA. New York: ACM; 2004. p. 551–6. [Google Scholar]
- 54. Ng AY, Jordan MI, Weiss Y. On spectral clustering: analysis and an algorithm. In: Dietterich TG, Becker S, Ghahramani Z, editors. NIPS’01: proceedings of the 14th International Conference on neural information processing systems: natural and synthetic; 3–8 December, 2001; Vancouver, Canada. Cambridge, MA: MIT Press; 2002. p. 849–56. [Google Scholar]
- 55. Rokach L, Maimon O. Clustering methods. In: Maimon O, Rokach L, editors. Data mining and knowledge discovery handbook. Boston, MA: Springer; 2005. p. 321–52. [Google Scholar]
- 56. Breunig MM, Kriegel H-P, Ng RT, Sander J. LOF: identifying density-based local outliers. In: Proceedings of the 2000 ACM SIGMOD international conference on Management of data; 15–18 May, 2000; Dallas, TX. New York: ACM; 2000. p. 93–104. [Google Scholar]
- 57. Khanna N, Eicher-Miller HA, Boushey CJ, Gelfand SB, Delp EJ. Temporal dietary patterns using kernel k-means clustering. In: 2011 IEEE International Symposium on Multimedia; 5–7 December, 2011; Dana Point, CA. Piscataway, NJ: IEEE; 2011. p. 375–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Zhu Y, Ting KM, Carman MJ. Density-ratio based clustering for discovering clusters with varying densities. Pattern Recognit. 2016;60:983–97. [Google Scholar]
- 59. Ester M, Kriegel H-P, Sander J, Xu X. A density-based algorithm for discovering clusters in large spatial databases with noise. In: Simoudis E, Han J, Fayyad U, editors. KDD’96: Proceedings of the Second International Conference on Knowledge Discovery and Data Mining; 2–4 August, 1996; Portland, OR. Palo Alto, CA: AAAI Press; 1996. p. 226–31. [Google Scholar]
- 60. Campello R, Moulavi D, Sander J. Density-based clustering based on hierarchical density estimates. In: Pei J, Tseng VS, Cao L, Motoda H, Xu G, editors.Advances in Knowledge Discovery and Data Mining: 17th Pacific-Asia Conference. Berlin and Heidelberg, Germany: Springer; 2013. p. 160–72. [Google Scholar]
- 61. Leech RM, Timperio A, Livingstone KM, Worsley A, McNaughton SA. Temporal eating patterns: associations with nutrient intakes, diet quality, and measures of adiposity. Am J Clin Nutr. 2017;106(4):1121–30. [DOI] [PubMed] [Google Scholar]
- 62. Leech RM, Timperio A, Worsley A, McNaughton SA. Eating patterns of Australian adults: associations with blood pressure and hypertension prevalence. Eur J Nutr. 2019;58(5):1899–909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Chastin SFM, Palarea-Albaladejo J, Dontje ML, Skelton DA. Combined effects of time spent in physical activity, sedentary behaviors and sleep on obesity and cardio-metabolic health markers: a novel compositional data analysis approach. PLoS One. 2015;10(10):e0139984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Niemelä M, Kangas M, Farrahi V, Kiviniemi A, Leinonen A-M, Ahola R, Puukka K, Auvinen J, Korpelainen R, Jämsä T. Intensity and temporal patterns of physical activity and cardiovascular disease risk in midlife. Prev Med. 2019;124:33–41. [DOI] [PubMed] [Google Scholar]
- 65. Zerón-Rugerio MF, Díez-Noguera A, Izquierdo-Pulido M, Cambras T. Higher eating frequency is associated with lower adiposity and robust circadian rhythms: a cross-sectional study. Am J Clin Nutr. 2021;113(1):17–27. [DOI] [PubMed] [Google Scholar]
- 66. Jamshed H, Beyl RA, Della Manna DL, Yang ES, Ravussin E, Peterson CM. Early time-restricted feeding improves 24-hour glucose levels and affects markers of the circadian clock, aging, and autophagy in humans. Nutrients. 2019;11(6):1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Garaulet M, Gómez-Abellán P, Alburquerque-Béjar JJ, Lee Y-C, Ordovás JM, Scheer FA. Timing of food intake predicts weight loss effectiveness. Int J Obes. 2013;37(4):604–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Garaulet M, Vera B, Bonnet-Rubio G, Gómez-Abellán P, Lee Y-C, Ordovás JM. Lunch eating predicts weight-loss effectiveness in carriers of the common allele at PERILIPIN1: the ONTIME (Obesity, Nutrigenetics, Timing, Mediterranean) study. Am J Clin Nutr. 2016;104(4):1160–6. [DOI] [PubMed] [Google Scholar]
- 69. Poggiogalle E, Jamshed H, Peterson CM. Circadian regulation of glucose, lipid, and energy metabolism in humans. Metabolism. 2018;84:11–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Morris CJ, Yang JN, Garcia JI, Myers S, Bozzi I, Wang W, Buxton OM, Shea SA, Scheer FA. Endogenous circadian system and circadian misalignment impact glucose tolerance via separate mechanisms in humans. Proc Natl Acad Sci U S A. 2015;112(17):E2225–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Bo S, Fadda M, Castiglione A, Ciccone G, De Francesco A, Fedele D, Guggino A, Caprino MP, Ferrara S, Boggio MV et al. Is the timing of caloric intake associated with variation in diet-induced thermogenesis and in the metabolic pattern? A randomized cross-over study. Int J Obes. 2015;39(12):1689–95. [DOI] [PubMed] [Google Scholar]
- 72. Van Cauter E, Shapiro ET, Tillil H, Polonsky KS. Circadian modulation of glucose and insulin responses to meals: relationship to cortisol rhythm. Am J Physiol. 1992;262(4 Pt 1):E467–75. [DOI] [PubMed] [Google Scholar]
- 73. Biston P, Van Cauter E, Ofek G, Linkowski P, Polonsky KS, Degaute JP. Diurnal variations in cardiovascular function and glucose regulation in normotensive humans. Hypertension. 1996;28(5):863–71. [DOI] [PubMed] [Google Scholar]
- 74. Dashti HS, Gómez-Abellán P, Qian J, Esteban A, Morales E, Scheer FA, Garaulet M. Late eating is associated with cardiometabolic risk traits, obesogenic behaviors, and impaired weight loss. Am J Clin Nutr. 2021;113(1):154–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Bodegard J, Sundström J, Svennblad B, Östgren CJ, Nilsson PM, Johansson G. Changes in body mass index following newly diagnosed type 2 diabetes and risk of cardiovascular mortality: a cohort study of 8486 primary-care patients. Diabetes Metab. 2013;39(4):306–13. [DOI] [PubMed] [Google Scholar]
- 76. Mulligan AA, Lentjes MAH, Luben RN, Wareham NJ, Khaw K-T. Changes in waist circumference and risk of all-cause and CVD mortality: results from the European Prospective Investigation into Cancer in Norfolk (EPIC-Norfolk) cohort study. BMC Cardiovasc Disord. 2019;19(1):238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Cerhan JR, Moore SC, Jacobs EJ, Kitahara CM, Rosenberg PS, Adami H-O, Ebbert JO, English DR, Gapstur SM, Giles GG et al. A pooled analysis of waist circumference and mortality in 650,000 adults. Mayo Clin Proc. 2014;89(3):335–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Tirosh A, Rudich A, Shochat T, Tekes-Manova D, Israeli E, Henkin Y, Kochba I, Shai I. Changes in triglyceride levels and risk for coronary heart disease in young men. Ann Intern Med. 2007;147(6):377–85. [DOI] [PubMed] [Google Scholar]
- 79. Jeong S-M, Choi S, Kim K, Kim S-M, Lee G, Son JS, Yun JM, Park SM. Association of change in total cholesterol level with mortality: a population-based study. PLoS One. 2018;13(4):e0196030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Arsenault BJ, Rana JS, Lemieux I, Després J-P, Kastelein JJP, Boekholdt SM, Wareham NJ, Khaw K-T. Physical inactivity, abdominal obesity and risk of coronary heart disease in apparently healthy men and women. Int J Obes. 2010;34(2):340–7. [DOI] [PubMed] [Google Scholar]
- 81. Diaz KM, Howard VJ, Hutto B, Colabianchi N, Vena JE, Blair SN, Hooker SP. Patterns of sedentary behavior in US middle-age and older adults: the REGARDS study. Med Sci Sports Exerc. 2016;48(3):430–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Carlson SA, Densmore D, Fulton JE, Yore MM, Kohl HW. Differences in physical activity prevalence and trends from 3 U.S. surveillance systems: NHIS, NHANES, and BRFSS. J Phys Act Health. 2009;6(s1):S18–27. [DOI] [PubMed] [Google Scholar]
- 83. Dyck DV, Cerin E, De Bourdeaudhuij I, Hinckson E, Reis RS, Davey R, Sarmiento OL, Mitas J, Troelsen J, MacFarlane D et al. International study of objectively measured physical activity and sedentary time with body mass index and obesity: IPEN adult study. Int J Obes. 2015;39(2):199–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Marzel A, Kouyos RD, Reinschmidt S, Balzer K, Garon F, Spitaleri M, Matthes N, Suter P, Weber R, Staehelin C et al. Dietary patterns and physical activity correlate with total cholesterol independently of lipid-lowering drugs and antiretroviral therapy in aging people living with human immunodeficiency virus. Open Forum Infect Dis. 2018;5(4):ofy067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Mora S, Lee I-M, Buring JE, Ridker PM. Association of physical activity and body mass index with novel and traditional cardiovascular biomarkers in women. JAMA. 2006;295(12):1412–19. [DOI] [PubMed] [Google Scholar]
- 86. Skoumas J, Pitsavos C, Panagiotakos DB, Chrysohoou C, Zeimbekis A, Papaioannou I, Toutouza M, Toutouzas P, Stefanadis C. Physical activity, high density lipoprotein cholesterol and other lipids levels, in men and women from the ATTICA study. Lipids Health Dis. 2003;2(1):1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Bassett DR, Wyatt HR, Thompson H, Peters JC, Hill JO. Pedometer-measured physical activity and health behaviors in United States adults. Med Sci Sports Exerc. 2010;42(10):1819–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Yang L, Cao C, Kantor ED, Nguyen LH, Zheng X, Park Y, Giovannucci EL, Matthews CE, Colditz GA, Cao Y. Trends in sedentary behavior among the US population, 2001-2016. JAMA. 2019;321(16):1587–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Healy GN, Matthews CE, Dunstan DW, Winkler EAH, Owen N. Sedentary time and cardio-metabolic biomarkers in US adults: NHANES 2003–06. Eur Heart J. 2011;32(5):590–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Wakimoto P, Block G. Dietary intake, dietary patterns, and changes with age: an epidemiological perspective. J Gerontol A Biol Sci Med Sci. 2001;56(Suppl 2):65–80. [DOI] [PubMed] [Google Scholar]
- 91. Townsend MS, Peerson J, Love B, Achterberg C, Murphy SP. Food insecurity is positively related to overweight in women. J Nutr. 2001;131(6):1738–45. [DOI] [PubMed] [Google Scholar]
- 92. Basiotis PP, Lino M. Insight 26: July 2002: food insufficiency and prevalence of overweight among adult women. Fam Econ Nutr Rev. 2003;15(2):55–7. [Google Scholar]
- 93. Ahluwalia N, Dwyer J, Terry A, Moshfegh A, Johnson C. Update on NHANES dietary data: focus on collection, release, analytical considerations, and uses to inform public policy. Adv Nutr. 2016;7(1):121–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Fatima S, Ijaz A, Sharif TB, Khan DA, Siddique A. Accuracy of non-fasting lipid profile for the assessment of lipoprotein coronary risk. J Coll Physicians Surg Pak. 2016;26(12):954–7. [PubMed] [Google Scholar]
- 95. World Health Organization. Obesity and overweight. [Internet]. Geneva, Switzerland: WHO; 2021; [cited 14 April, 2021]. Available from: https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data described in the article and code book are publicly and freely available without restriction at https://www.cdc.gov/nchs/nhanes/index.htm. Analytic code will be made available upon request pending application and approval.