Abstract
Statins are among most commonly prescribed drugs for treatment of high blood cholesterol. Myotoxicity of statins in certain individuals is often a severe side effect leading to withdraw. Using C2C12 and H9c2 cells, both exhibiting characteristics of skeletal muscle cells, we addressed whether resveratrol (RSV) can prevent statin toxicity. Statins decreased cell viability in a dose and time-dependent manner. Among the five statins tested, atorvastatin, simvastatin, lovastatin, pravastatin and fluvastatin, simvastatin is the most toxic one. Simvastatin at 10 μM caused about 65% loss of metabolic activity as measured by MTT assays in C2C12 cells or H9c2 cells. Inhibition of metabolic activity correlates with an increase in caspase activity. RSV was found to protect H9c2 cells from simvastatin induced activation of caspase 3/7. However, such protection was not found in C2C12 cells. This cell type dependent effect of RSV adds to the complexity in muscle cell toxicity of statins.
Introduction
Cardiovascular disease is the leading cause of death worldwide. Large-scale clinical studies have shown that lowering blood cholesterol significantly reduces coronary disease-related deaths (1,2). Statins, the inhibitors of 3-hydroxy-3-methylglutaryl-coenzyme A (HMG-CoA) reductase, lower blood cholesterol by blocking mevalonate synthesis, the rate limiting step of cholesterol biosynthesis pathway in the liver. In addition to this canonical effect, recent in vitro and in vivo studies have found that statins exhibit non-canonical effects, including improving endothelial function, stabilizing atherosclerotic plaque and preventing thrombogenic events (3–5). Statins have also been found to exhibit anti-inflammatory and cardiac protective effect (6). For most people, statin drugs are safe and well tolerated. However, up to 30% of statin users reported intolerance often with muscular or joint discomfort, leading to noncompliance to the drug therapy. Statin associated muscle symptoms vary from benign myalgia or mild myopathy to myositis or fatal rhabdomyolysis (1,7,8). It has been observed that the symptoms of skeletal muscle myopathy are independent of the efficacy of statins in lowering cholesterol (9).
Mevalonate is also an intermediate for the synthesis of isoprenoids, such as farnesyl pyrophosphate and geranylgeranyl pyrophosphate. These isoprenoids are involved in protein prenylation and multiple cell functions, including posttranslational modifications and intracellular trafficking of signaling proteins (1,8,10). In cell culture models, mevalonate or geranylgeraniol has been found to prevent statin from inducing mesenchymal or muscle cell death (11,12). With experimental models of rat or human myotubes, apoptosis induced by statins was reversed by mevalonate or geranylgeraniol but not farnesol (13). Another downstream product of mevalonate metabolism is ubiquinone. Deficiency of ubiquinone has been postulated as an underline cause of myotoxicity. However, serum levels of ubiquinone do not predict myotoxicity, as patients experiencing the side effect of statins showed similar lower levels of ubiquinone as those did not present the side effect (14). Supplement of ubiquinone did not show clear reversal of muscle toxicity (15). With experimental models of muscle toxicity, ubiquinone failed to reverse apoptosis induced by statins. Drugs that can prevent myotoxicity without canceling the cholesterol lowering effect of statin have not been established.
Resveratrol (RSV) is an antioxidant polyphenol commonly found in the skin of grapes and has received extensive attention due to its preventive effects against aging and a variety of diseases including cardiovascular disease (16–18). In skeletal muscle, RSV has been shown to reduce oxidative stress levels in aging rats (19). Correlating with reduced oxidative stress, RSV enhances muscle contractility, skeletal muscle regeneration and recovery from disuse-induced atrophy (19). Others have reported that RSV improves mitochondrial function and muscle aerobic capacity (20). Prevention of muscle atrophy by a number of inducers including dexamethasone, TNF-alpha, denervation, chronic kidney disease or diabetes has been reported with RSV (21–25). Whether RSV can protect muscle cells from statin toxicity is not known.
Since a main factor of muscle toxicity of statins is induction of cell death and there are individual variations in developing muscle toxicity, we investigated whether or not RSV protects against statin toxicity using two different cell lines, C2C12 and H9c2 cells. C2C12 cells are murine myoblasts derived from satellite cells (26), whereas H9c2 cells were cloned from embryonic BD1X rat heart tissues but exhibit characteristics of skeletal muscle (27). These cell lines provide a useful tool for initial identification of compounds capable of protecting against statin toxicity.
Materials and Methods
Chemicals
All statins and RSV were purchased from Toronto Research Chemicals Inc. (Toronto, Canada). Atorvastatin was dissolved in methanol, while simvastatin, lovastatin, fluvastatin, and pravastatin were prepared in ethanol. Simvastatin was converted into the active form by dissolving 8 mg in 0.2 mL ethanol and adding 0.3 mL of 0.1N NaOH for heating at 50 °C for 2 hours before adjusted pH to 7.2 with 1N HCl. The active form was stored in −20 °C before use after adjusting the final volume to 1 mL using distilled H2O. To activate lovastatin, 25 mg of lovastatin was dissolved in 0.625 mL ethanol, added with 0.938 mL of 0.1 N NaOH, then heated at 50 °C for 2 hours, before adjusting pH to 7.2 with 1N HCl. After adjusting final volume to 6 ml with distilled H2O, the sample was aliquoted and stored at −20 °C. All stock solutions were stored at −20 °C and were diluted to 100x concentration in phosphate-buffered saline (PBS) prior to adding to cells for the final concentration of 1x in culture. 3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide (MTT) was purchased from Sigma-Aldrich (St. Louis. MO). Ac-DEVD-AMC was obtained from Cayman Chemical Company (Ann Arbor. MI).
Cell Culture
C2C12 murine myoblasts were a generous gift from Dr. Donna Zhang’s Lab. H9c2 rat myoblasts were obtained from the American Type Culture Collection (Manassas, VA). Cells were maintained by weekly subculture in 100 mm dishes (Genesee Scientific, San Diego, CA) in Dulbecco’s Modified Eagle’s Medium (DMEM, GIBCO, Grand Island. NY) supplemented with heat-inactivated 10% fetal bovine serum (FBS, Omega Scientific, Tarzana, CA), containing 25 mM glucose, 1 mM sodium pyruvate, 2 mM L-glutamine and 100 unit/ml penicillin/streptomycin. Upon reaching 70–80% confluency, cells were subcultured for stock, seeded into 100 mm dishes for Western blot experiments, or into 6-well or 24-well plates for cytotoxicity assays. Cells were treated with various doses of statins at 24 hours after plating when the density reached 80% confluency. Resveratrol was added to cells 1 hour before statin treatment.
MTT Assay
Cells were seeded at a density of 3×104 per well in a 24-well plate containing 1 mL culture medium. After 24 hours of seeding, statins were added for 24 hour treatment and at the end of the treatment, MTT was added to the culture media to a final concentration of 0.16 mg/mL. Following 30 min incubation in a tissue culture incubator at 37°C with 5% CO2, purple crystals of reduced formazan were observed inside cells under a phase contrast microscope. The culture medium was removed and the formazan crystals were dissolved in isopropanol containing 4 mM HCL and 0.1% NP-40. Cell viability was calculated by comparing the absorbance at 570 nm subtracted from the background reading at 690 nm of treated cells to that of untreated cells, which were considered as 100% viable.
Morphology and Cell Number Counting
Cells were grown on 6-well plates or 100 mm dishes. Following incubation with simvastatin with or without RSV, the morphology of cells was recorded under a phase contrast microscope (EVOS FL, Life Technologies) with 4x sitting. The percentage of viable cells was determined by counting cells from randomly selected 3 fields from each sample.
Annexin V Staining
Cells were seeded onto cover glasses placed in a 24 well plate at a density of 3×104 cells/mL/well. At 20–24 hours after seeding, cells were incubated with simvastatin with or without RSV for 16–20 h. After washing with PBS, 20 μL of Annexin V-Fluos (Roche Life Science) was added in 100 μL of Annexin V binding buffer to each well for 15 min incubation at room temperature in the dark. Cell samples were washed briefly with PBS before fixation in 2% formalin for 10 min at room temperature. After washing with PBS, the cover glass was mounted on a microscope slide for examination and recording images under Olympus BX53 fluorescent microscope with attached digital camera.
Caspase Activity Assay
At 16 hr after statin treatment, detached cells from 6-well plates were collected by centrifugation at 3000 RPM for 3 minutes and washed once with PBS before combining with the adherent cells from the same well. Cells were scraped off the well in 60 μL of lysis buffer (0.5% Nonidet P-40, 0.5 mM EDTA, 150 mM NaCl, and 50 mM Tris pH 7.5). Caspase 3/7 activity was measured using a fluorogenic substrate Acetyl Asp-Glu-Val-Asp-7-amino-4-methylcoumarin (Ac-DEVD-AMC). Cell lysates (50 μL/well) were incubated at 37°C for 1 h with a master mix containing 10 μM of synthetic peptide substrate Ac-DEVD-AMC in a total volume of 200 μL in a 96-well plate. Caspase-3/7 activity was expressed as fluorescent intensity unit detected by a 96-well fluorescence plate reader (BioTek Synergy 2, Winnski, VT) with an excitation wavelength of 365 nm and an emission wavelength of 450 nm.
Western Blot Analysis
Cells were washed twice with ice cold PBS for protein extraction using RIPA buffer [(50 mM Tris-HCL pH 7.4, 1% NP-40, 0.1% SDS, 150 mM NaCl, 2 mM EDTA, 50 mM NaF with freshly added protease inhibitor cocktails tablets (Fisher Scientific, Pittsburgh, PA)]. After removing insoluble cell debris by centrifugation at 12,000xg for 10 min, protein concentration in the supernatant was determined by the bicinchoninic acid (BCA) assay (Pierce, Rockford, IL). An equal amount of proteins from different samples were boiled in SDS buffer and loaded for separation based on molecular weight by electrophoresis using 12% polyacrylamide gel. Separated proteins were transferred onto a PVDF membrane (Bio-Rad, Hercules CA) by electrophoresis at 100 Volts and 4 °C for 1 hour, or at 30 Volts and 4 °C for overnight. After blocking the membrane for non-specific protein binding using 5% nonfat milk in Tris-buffered saline containing 0.05% Tween 20 (TBST) at room temperature (RT) for 1 hour, PVDF membranes were incubated overnight at 4°C with primary antibodies (1:1,000, Rabbit polyclonal, Cell Signaling Technology, Beverly, MA) against caspase-3 or caspase-7. Vinculin was probed as an internal loading control using a polyclonal antibody (1:1,000, H300, Santa Cruz Biotechnology Inc, CA). The membranes were washed in TBST and incubated with appropriate secondary antibodies (Santa Cruz Biotechnology Inc, CA) conjugated with horseradish peroxidase for 1.5 h at room temperature. Enhanced chemiluminescence (ECL) was used to detect bound antibodies by ChemiDoc XRS+ imaging system with Image Lab software (Bio-Rad, Hercules, CA).
Statistical Analysis
All cytotoxicity experiments were performed in triplicates (treatment) and repeated at least three times. Significant differences were determined by Analysis of Variance (ANOVA) with p<0.05 or student’s t-test with p<0.01.
Results
Cytotoxicity of Statins
Four commonly prescribed statins, Atorvastatin, Simvastatin, Lovastatin, and Pravastatin were tested for cytotoxicity, plus Fluvastatin, which has a weak activity in reducing blood cholesterol. MTT reduction is one of the most commonly used methods for measuring metabolic viability. The yellow tetrazolium of MTT is reduced by NAD (P) H oxidoreductase in the mitochondria to purple colored formazan, allowing high throughput spectrophotometric quantification. Decreases in MTT conversion reflect the degree of metabolic inactivation of the cells.
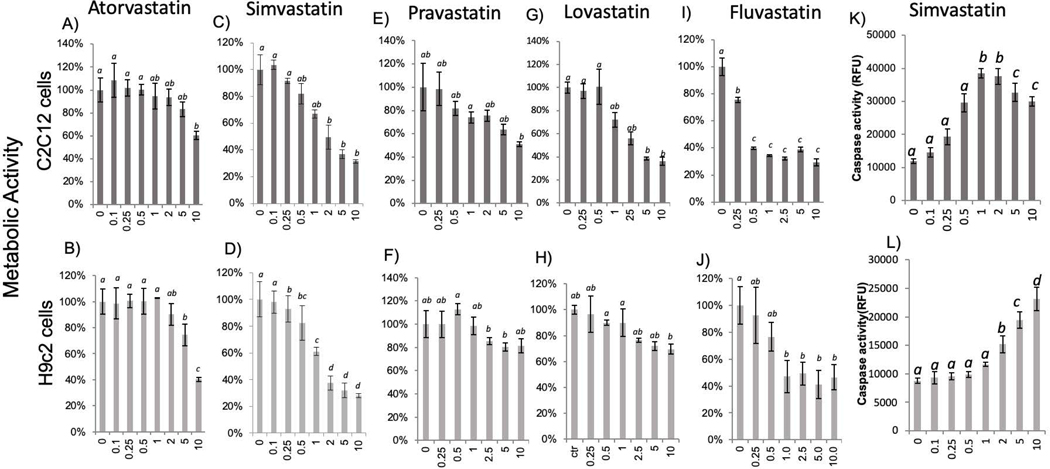
Dose-dependent response studies were performed to define the most versus the least toxic statins in two muscle cell lines using the dose range of 0.1–10 μM. As shown in figure 1, all statins induced a dose-dependent inhibition of metabolic viability. Atorvastatin and pravastatin are least toxic in either cell line, which exhibited similar trend of dose responses (Fig 1). Although metabolic viability significantly decreased with lovastatin and fluvastatin, Simvastatin appears to be most toxic with an LC50 of 2.6 μM and 1.9 μM in C2C12 and H9c2 respectively. The LC50 value was calculated based the viability as measured by MTT assay as a percentage over control untreated plotted on the Y axis and the concentration of the statins in micromolar plotted on the X axis. A linear or logarithmic equation depending on the dose response curve obtained was used to calculate the LC50.
Fig 1. Statin Dose Dependent Inhibition of Metabolic Activity and Activation of Caspase.
C2C12 cells (top panel) or H9C2 cells (bottom panel) were seeded in 24-well plates and were incubated with statins at indicated dose upon 80% confluency for 24 hours before measurements of metabolic activity using MTT assay (A-J) or for 16 hours for measurements of caspase activity using DEVD-AMC as a substrate (K, L). The results are from one experiment representative of three and are expressed as means ± standard deviation (SD) from triplicates. Statistical significance (p≤0.05) was determined by ANNOVA and indicated by an alphabetic letter. The means labeled with “a” is significantly different from that labeled with “b”, “c” or “d”, where as “ab” indicates no significant difference from “a” or “b”.
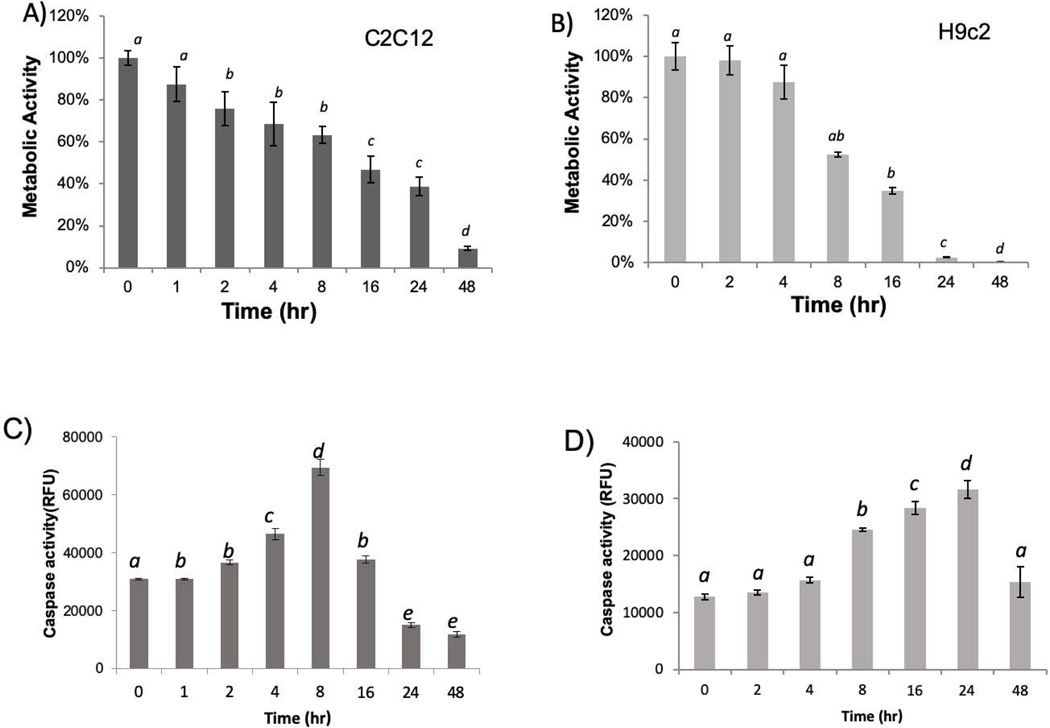
Since simvastatin is the most potent drug in inducing cytotoxicity among all the five statins, simvastatin was used for studying time-dependence of metabolic inhibition. Both C2C12 and H9c2 cells showed time dependent loss of viability as measured by MTT assay from 1 to 48 hours, and C2C12 cells maintained about 40% metabolic activity at 24 hours and required 48 hours for near complete elimination of metabolic viability (Fig 2A). In contrast, H9c2 cells lost most if not all metabolic activity by 24 hours (Fig 2B). Therefore, although both cells exhibited a similar trend in time dependent loss of metabolic viability, H9c2 cells showed a steeper decline after 8 hours.
Fig 2. Time Dependent Inhibition of Metabolic Activity and Activation of Caspase.
C2C12 cells or H9C2 cells were seeded in 24-well plates. When cells reached 80% confluency, simvastatin was added to cells to 10 μM in a reversed time scale, so that the metabolic activity can be measured by MTT assay at the same time (A, B), and caspase activity can be measured at the same time using DEVD-AMC as the substrate (C, D). The results are from one experiment representative of three and are expressed as means ± standard deviation (SD) from triplicates. Statistical significance is indicated as described in Fig 1.
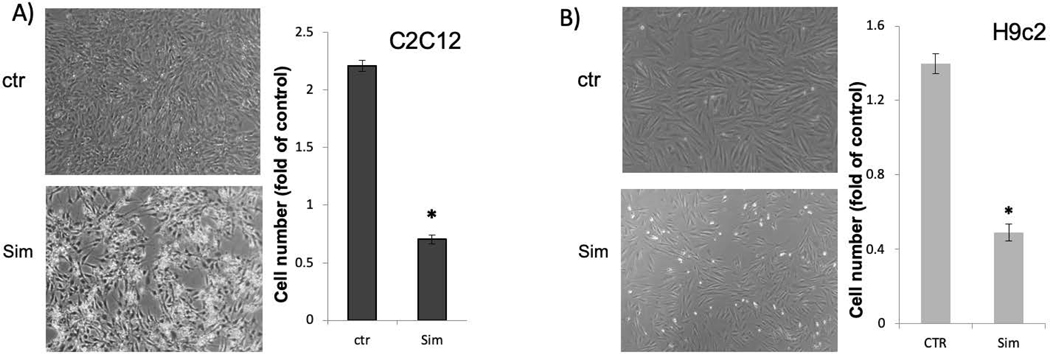
Morphologic observation and cell number counting of C2C12 cells and H9c2 cells suggest that statins induced cytostasis as well as cell death (Fig 3). C2C12 cells showed a faster proliferative rate, with the number of cells doubled over 24 hours. Treatment of simvastatin for 24 hours caused about 25% loss of C2C12 cells (Fig 3). H9c2 cells is slower in proliferation, showed about 50% loss of cell number (Fig 3). In addition to inhibition of cell number increases, detached cells in statin treated groups showing shrinkage, typical morphology of apoptotic cells (Fig 3).
Fig 3. Loss of Cell Number due to Statin Treatment.
C2C12 cells or H9c2 cells were seeded in 6-well plates. When cells reached 60–80% confluency, simvastatin was added to cells to 10 μM. Cell morphology was from untreated control versus 10 mM simvastatin treated cells at 24 hours after under an inverted microscope with 4x lens. Cell numbers of control or treated group over that at 24 hour before the treatment were recorded for counting and expressed as the percentage. 100% means no change in cell number, whereas percentage above 100% indicates cell proliferation, and less than 100% indicates loss of cells. The results are from one experiment representative of three and are expressed as means ± standard deviation (SD) from triplicates. An asterisk indicates significant difference (P<0.01) by student’s t-test.
Using caspase −3/7 activity assay, we measured apoptosis quantitatively. A higher baseline caspase activity in C2C12 cells reflects a higher number of cells compared to H9c2 cells at the time of measurements. Nevertheless, a dose and time dependent increase of caspase activity was observed in C2C12 cells and H9c2 cells with simvastatin treatment (Fig 1K&L, 2C&D). The maximal fold of caspase activity increase is similar between C2C12 cells and H9c2 cells within each experiment of comparing the effect of simvastatin. Caspase 3/7 activity increased with the increasing dose of simvastatin from 0.1 to 1 μM and reached the highest with 1 μM in C2C12 cells and declined with higher doses (Fig 1K). The decline of caspase activity with 5 to 10 μM simvastatin may indicate a mixed mode of cell death, with high doses are expected to cause necrosis in addition to apoptosis (28). H9c2 cells showed somewhat resistance until 1 μM of simvastatin and dose dependent increases of caspase activity in 2 to 10 μM dose range (Fig 1L). The time course studies revealed that caspase activation reached the highest at 8 hours and declined after 16 hours in C2C12 (Fig 2C). The peak of caspase activation occurred at 24 hours and decreased at 48 hours in H9c2 cells (Fig 2D). The decline of caspase after 16 hours for C2C12 cells or 24 hours for H9c2 cells indicates that the process of apoptosis occurred and finished within the period of time, and discontinued afterwards.
Resveratrol Inhibits Apoptosis in H9c2 Cells
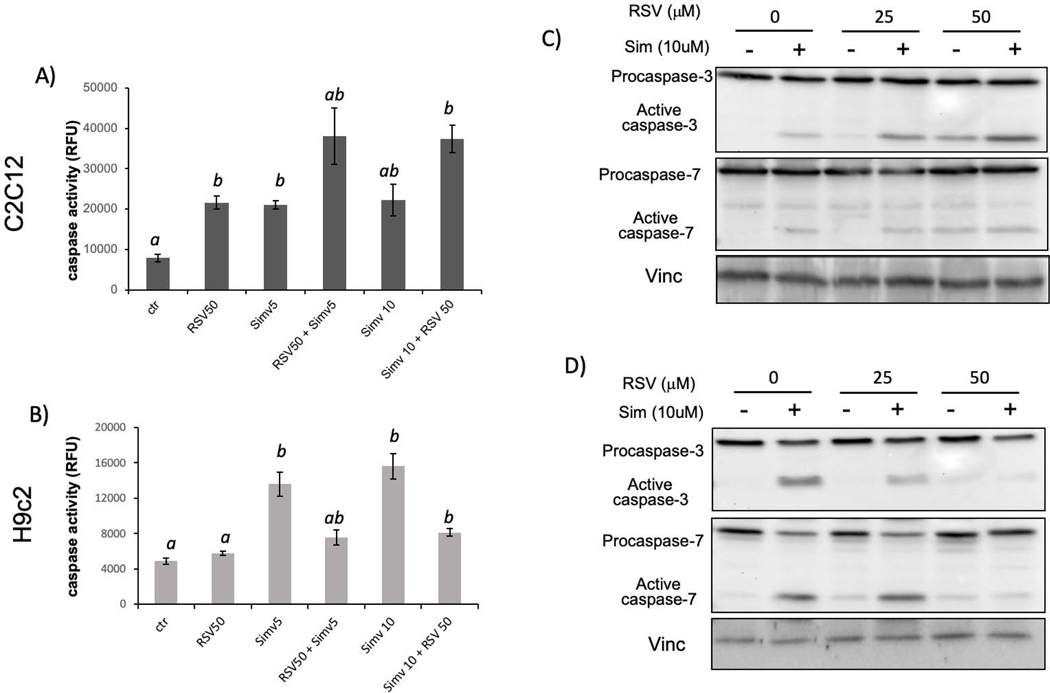
Caspase activity assay provides a quantitative measure of apoptosis and was used to determine the effect of RSV on statin induced cytotoxicity. C2C12 or H9c2 cells were pretreated with RSV (50 μM) for 1 h before simvastatin (5 and 10 μM) treatment. Cells were incubated for 16 h for caspase activity assay. Interestingly, RSV inhibited caspase activation in H9c2 cells but not in C2C12 cells (Fig 4A&B). In C2C12 cells, RSV induced activation of caspase 3/7, which appeared to be additive with simvastatin (Fig 4A).
Fig 4. Resveratrol Inhibits Simvastatin from Activating Caspase-3 and Caspase-7.
C2C12 cells or H9C2 cells were seeded in 24-well plates or 100 mm dishes. When cells reached 80% confluency, resveratrol was added to cells 1 hour prior to simvastatin treatment. Cells were harvested at 16 hours after for measurements of caspase activity using DEVD-AMC as a substrate or for Western blot to measure procaspase-3, procaspase-7 and active forms. The results are from one experiment representative of three and are expressed as means ± standard deviation (SD) from triplicates (A, B). Statistical significance is indicated as described in Fig 1.
To confirm caspase activation with simvastatin and the inhibition by RSV in H9c2 cells, we measured caspase 3 and caspase 7 cleavage by Western blot. Caspase 3 protein was probed using an anti-caspase 3 antibody that recognizes the full length 32 KDa form and 17 KDa cleaved form of caspase 3. Similarly, anti-caspase 7 antibody recognized the full length 37 KDa and 20 KDa cleaved form of caspase 7. Vinculin was used as a loading control. In C2C12 cells, there is cleavage of caspase 3 and caspase 7 due to simvastatin treatment, with or without RSV pretreatment (Fig 4C). In contrast, in H9c2 cells, the cleavage of caspase 3 or caspase 7 due to simvastatin treatment was inhibited by 50 μM RSV pretreatment (Fig 4D). Therefore, the caspase cleavage results are consistent with that of caspase activity assay, showing that RSV mediated protection against Simvastatin toxicity is specific for H9c2 cells.
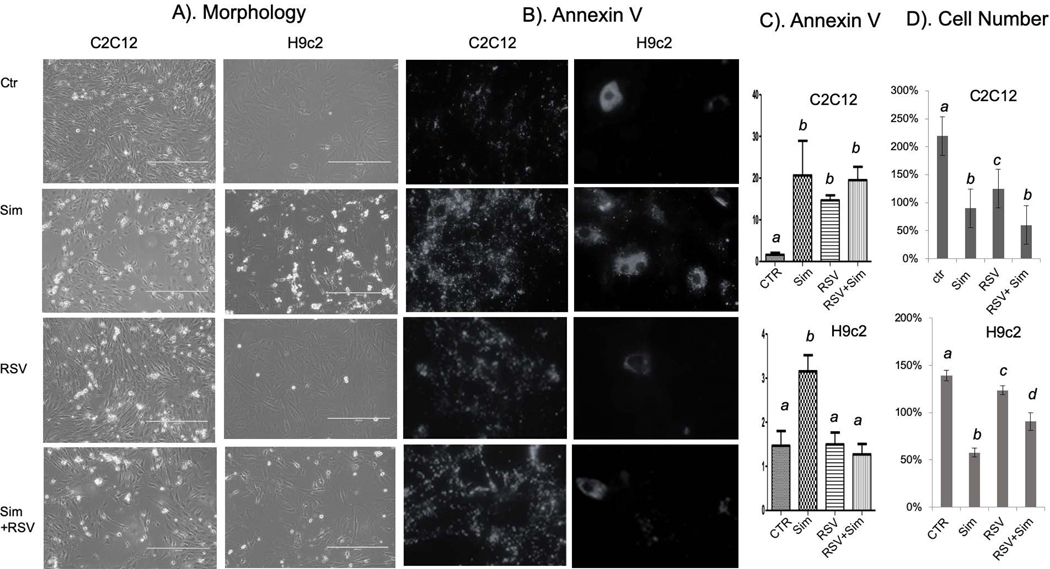
Cell morphology was recorded using light microscopy to further demonstrate the effect of RSV on apoptosis induced by simvastatin (Fig 5A). Treatment with 10 μM simvastatin resulted in condensed, rounding up and floating either C2C12 or H9c2cells. RSV treatment reversed cell morphology to normal in H9c2 cells with fewer apoptotic cells but failed to do so in C2C12 cells (Fig 5A).
Fig 5. Morphological Evidence and Annexin V Staining Support that Resveratrol Prevents Simvastatin from Inducing Apoptosis.
C2C12 cells or H9C2 cells were seeded in 24-well plates containing coverglass or 6-well plates. When cells reached 60–80% confluency, resveratrol was added to cells 1 hour prior to simvastatin treatment. Morphology was recorded under an inverted microscope with 4xlens. Cells on coverglasses were stained with Annexin V for recording images under a fluorescence microscope and Annexin V positive cells were scored under a view of 20x lens. At lease 4 views were scored for each treatment group for counting Annexin V positive cells (C). Cell number was counted prior to drug treatment and at 24 hours after drug treatment. Cell number was calculated as percentage over the number prior to drug treatment (D). The results are from one experiment representative of three (A, B), means ± standard error (SE) from counting at least 4 views per group (C) or means + standard deviation (SD) from triplicates of one representative experiment. Statistical significance is indicated as described in Fig 1.
Annexin V staining was performed to confirm H9c2 cell specific inhibition of apoptosis by RSV. Annexin V is a 36 KDa protein that has a high affinity for phosphatidylserine, which normally locates in the inner side of the plasma membrane, but migrates to the outer surface of apoptotic cells where it readily binds to Annexin V. Positive Annexin V staining increased in C2C12 cells due to simvastatin treatment with or without RSV pretreatment (Fig 5B&C). However, RSV pretreatment blocked simvastatin from inducing Annexin binding in H9c2 cells (Fig 5B&C).
Apoptosis results in a loss of cell number in adherent culture. To determine cell number loss, cells were seeded on 6- well plates at 3×104cells/mL and allowed to attach overnight. The next day, 3 random pictures were taken from each well. At this time point (T0), the number of cells is set to 100%. Cells were then incubated in the absence or presence of RSV (50 μM) for 1 hour prior to incubation with 10 μM simvastatin for 16 h-20 h. At 16 h later (T16), cell number was recorded under a phase contrast microscope. Three random visual fields from triplicate samples were chosen and the number of adherent cells was counted using ImageJ software. The number is expressed as a % of T0 control (Fig 5D). The number of untreated cells increased to 220% and 140% in C2C12 and H9c2 cells respectively due to continuous cell proliferation. However, with simvastatin treatment, the number declined to about 80% or 50% in C2C12 or H9c2 cells respectively. RSV alone induced loss of cell number in C2C12 cells and did not reverse simvastatin induced loss of cell number (Fig 5D). In contrast, RSV did not impair cell growth dramatically in H9c2 cells, but inhibited simvastatin from inducing loss of cell number (Fig 5D). These data added together supporting RSV protects H9c2 cells but not C2C12 cells from undergoing apoptosis.
The inhibitory effect of RSV on simvastatin induced apoptosis promoted us to determine whether RSV can block simvastatin from inhibiting metabolic viability. We conducted MTT assay in C2c12 and H9c2 cells treated with simvastatin with or without RSV pretreatment. The metabolic viability rate was reduced with simvastatin treatment at 5 or 10 μM dose in both cell lines, which was not affected by RSV pretreatment (data not shown).
Discussion
Our data indicate that statins induce cytotoxicity in H9c2 and C2C12 in a time and dose-dependent manner. We found that simvastatin is the most potent inducer of cytotoxicity, consistent with the findings from clinical observations. Such feature of simvastatin may be related to its lipophilicity, implying its ability to cross cell membrane more readily than the hydrophilic statins, e.g. pravastatin. Interestingly, RSV inhibited apoptosis only in H9c2 as shown in caspase-3/7 activity, caspase 3/7 cleavage, Annexin staining and cell morphology, but not C2C12 cells. Therefore, although C2C12 and H9c2 cells share the relevance with their characteristics of skeletal muscle cells and their response to statins, they respond to RSV differently.
RSV has been reported to inhibit apoptosis of cardiomyocytes by experimental ischemic injury (29). This is consistent with our observation of RSV inhibiting apoptosis of H9c2 cells, since this cell line was originally derived from rat myocardial tissue (27). Although the mechanism of apoptosis inhibition remains to be elucidated, RSV has been shown to decrease oxidant generation and increase antioxidant defense systems (18,30–32). However, we did not observe a protective effect against statin toxicity by N-acetylcysteine, tert-butylhydroquinone, butylated hydroxyaniosole and phenylenediamine, the antioxidants have often been used for cell culture experiments (data not shown). The cell type dependent protection of RSV also points to a mechanism outside antioxidant.
The benefit of combining RSV with statin use has been tested in experimental animals related to the reported functions of RSV as an antioxidant, angiogenesis, anti-platelet, or anti-inflammatory agent. RSV improves the efficacy of statins by reducing oxidized LDL, LDL, total cholesterol, or triglycerides (33). In a clinical trial with 75 enrollments of high risk cardiovascular patients on statin treatment, 1 year supplement of RSV-rich grape product resulted in significant improvement of fibrinolytic status and decreases of inflammatory biomarkers (34). With hypercholesterolemia rats, the combination of statin plus RSV is cardiac protective against ischemic injury (29). Current pharmacologic treatment of acute myocardial ischemia in the clinic include statins for cardiac protection (35–39). Combining RSV with statins is expected to achieve further reduction in cardiac injury. Animal experiments have also showed a role of RSV for inhibition of heart failure in the models of pressure overload, myocarditis and cardiomyopathy due to doxorubicin chemotherapy (40). These lines of evidence support the concept of combining RSV with statins for improved outcome in treatment of dyslipidemia, coronary disease and heart failure.
Statin toxicity studies in humans have revealed genetic elements in influencing individual’s susceptibility. The severity of side effects appears to be affected by heritable factors as well as epigenetic factors. Genome wide association studies revealed a particular SNP in the solute carrier anion transporter family 1B1 (SLCO1B1) gene alters one amino acid in its encoded protein, a hepatic organic anion transporter OATP1B1, in patients with simvastatin-induced myopathy. This SNP contributes to an increased plasma concentration of statins (41,42). Other genetic factors affecting statin toxicity include UDP glucuronosyltransferase, which metabolizes the lactone form of statins. Statin intolerance in humans have also been linked to ryanodine receptors, leukocyte immunoglobulin-like receptor, and serotonin receptor genes HTR3B and HTR7 (1). While cell culture experiments do not replicate the intolerance aspect of adverse drug reaction, the difference in genetic background of each cell line may influence the difference in cytotoxicity response.
Abbreviations:
- PBS
Phosphate Buffered saline
- RSV
Resveratrol
- MTT
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
- HMG-CoA
3-hydroxy-3- methylglutaryl-coenzyme A
References
- 1.Ward NC, Watts GF, Eckel RH. Response by Ward et al to Letter Regarding Article, “Statin Toxicity: Mechanistic Insights and Clinical Implications”. Circ Res 2019;124(12):e121–e122. [DOI] [PubMed] [Google Scholar]
- 2.Yebyo HG, Aschmann HE, Kaufmann M, Puhan MA. Comparative effectiveness and safety of statins as a class and of specific statins for primary prevention of cardiovascular disease: A systematic review, meta-analysis, and network meta-analysis of randomized trials with 94,283 participants. Am Heart J 2019;210:18–28. [DOI] [PubMed] [Google Scholar]
- 3.Zhang ZJ, Cheng Q, Jiang GX, Marroquin OC. Statins in prevention of repeat revascularization after percutaneous coronary intervention--a meta-analysis of randomized clinical trials. Pharmacol Res 2010;61(4):316–20. [DOI] [PubMed] [Google Scholar]
- 4.Sakellarios AI, Fotiadis DI. The pleiotropic effect of statins on the atherosclerotic plaque and coronary heart disease. Trends Cardiovasc Med 2019. [DOI] [PubMed] [Google Scholar]
- 5.Almeida SO, Budoff M. Effect of statins on atherosclerotic plaque. Trends Cardiovasc Med 2019. [DOI] [PubMed] [Google Scholar]
- 6.Tousoulis D, Psarros C, Demosthenous M, Patel R, Antoniades C, Stefanadis C. Innate and adaptive inflammation as a therapeutic target in vascular disease: the emerging role of statins. J Am Coll Cardiol 2014;63(23):2491–2502. [DOI] [PubMed] [Google Scholar]
- 7.Zhou Z, Curtis AJ, Breslin M, Nelson M. Letter by Zhou et al Regarding Article, “Statin Toxicity: Mechanistic Insights and Clinical Implications”. Circ Res 2019;124(12):e120. [DOI] [PubMed] [Google Scholar]
- 8.Norata GD, Tibolla G, Catapano AL. Statins and skeletal muscles toxicity: from clinical trials to everyday practice. Pharmacol Res 2014;88:107–13. [DOI] [PubMed] [Google Scholar]
- 9.Skottheim IB, Gedde-Dahl A, Hejazifar S, Hoel K, Asberg A. Statin induced myotoxicity: the lactone forms are more potent than the acid forms in human skeletal muscle cells in vitro. Eur J Pharm Sci 2008;33(4–5):317–25. [DOI] [PubMed] [Google Scholar]
- 10.Liao JK. Isoprenoids as mediators of the biological effects of statins. J Clin Invest 2002;110(3):285–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jaskiewicz A, Pajak B, Litwiniuk A, Urbanska K, Orzechowski A. Geranylgeraniol Prevents Statin-Dependent Myotoxicity in C2C12 Muscle Cells through RAP1 GTPase Prenylation and Cytoprotective Autophagy. Oxid Med Cell Longev 2018;2018:6463807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li Y, Muller AL, Ngo MA, Sran K, Bellan D, Arora RC, Kirshenbaum LA, Freed DH. Statins impair survival of primary human mesenchymal progenitor cells via mevalonate depletion, NF-kappaB signaling, and Bnip3. J Cardiovasc Transl Res 2015;8(2):96–105. [DOI] [PubMed] [Google Scholar]
- 13.Johnson TE, Zhang X, Bleicher KB, Dysart G, Loughlin AF, Schaefer WH, Umbenhauer DR. Statins induce apoptosis in rat and human myotube cultures by inhibiting protein geranylgeranylation but not ubiquinone. Toxicol Appl Pharmacol 2004;200(3):237–50. [DOI] [PubMed] [Google Scholar]
- 14.Skilving I, Acimovic J, Rane A, Ovesjo ML, Bjorkhem-Bergman L. Statin-induced Myopathy and Ubiquinone Levels in Serum - Results from a Prospective, Observational Study. Basic Clin Pharmacol Toxicol 2015;117(2):133–6. [DOI] [PubMed] [Google Scholar]
- 15.Schaars CF, Stalenhoef AF. Effects of ubiquinone (coenzyme Q10) on myopathy in statin users. Curr Opin Lipidol 2008;19(6):553–7. [DOI] [PubMed] [Google Scholar]
- 16.Bonkowski MS, Sinclair DA. Slowing ageing by design: the rise of NAD(+) and sirtuin-activating compounds. Nat Rev Mol Cell Biol 2016;17(11):679–690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gliemann L, Nyberg M, Hellsten Y. Effects of exercise training and resveratrol on vascular health in aging. Free Radic Biol Med 2016;98:165–176. [DOI] [PubMed] [Google Scholar]
- 18.Xia N, Daiber A, Forstermann U, Li H. Antioxidant effects of resveratrol in the cardiovascular system. Br J Pharmacol 2017;174(12):1633–1646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bennett BT, Mohamed JS, Alway SE. Effects of resveratrol on the recovery of muscle mass following disuse in the plantaris muscle of aged rats. PLoS One 2013;8(12):e83518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.de Oliveira MR, Nabavi SF, Manayi A, Daglia M, Hajheydari Z, Nabavi SM. Resveratrol and the mitochondria: From triggering the intrinsic apoptotic pathway to inducing mitochondrial biogenesis, a mechanistic view. Biochim Biophys Acta 2016;1860(4):727–45. [DOI] [PubMed] [Google Scholar]
- 21.Alamdari N, Aversa Z, Castillero E, Gurav A, Petkova V, Tizio S, Hasselgren PO. Resveratrol prevents dexamethasone-induced expression of the muscle atrophy-related ubiquitin ligases atrogin-1 and MuRF1 in cultured myotubes through a SIRT1-dependent mechanism. Biochem Biophys Res Commun 2012;417(1):528–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang DT, Yin Y, Yang YJ, Lv PJ, Shi Y, Lu L, Wei LB. Resveratrol prevents TNF-alpha-induced muscle atrophy via regulation of Akt/mTOR/FoxO1 signaling in C2C12 myotubes. Int Immunopharmacol 2014;19(2):206–13. [DOI] [PubMed] [Google Scholar]
- 23.Asami Y, Aizawa M, Kinoshita M, Ishikawa J, Sakuma K. Resveratrol attenuates denervation-induced muscle atrophy due to the blockade of atrogin-1 and p62 accumulation. Int J Med Sci 2018;15(6):628–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sun LJ, Sun YN, Chen SJ, Liu S, Jiang GR. Resveratrol attenuates skeletal muscle atrophy induced by chronic kidney disease via MuRF1 signaling pathway. Biochem Biophys Res Commun 2017;487(1):83–89. [DOI] [PubMed] [Google Scholar]
- 25.Wang D, Sun H, Song G, Yang Y, Zou X, Han P, Li S. Resveratrol Improves Muscle Atrophy by Modulating Mitochondrial Quality Control in STZ-Induced Diabetic Mice. Mol Nutr Food Res 2018;62(9):e1700941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Blau HM, Pavlath GK, Hardeman EC, Chiu CP, Silberstein L, Webster SG, Miller SC, Webster C. Plasticity of the differentiated state. Science 1985;230(4727):758–66. [DOI] [PubMed] [Google Scholar]
- 27.Kimes BW, Brandt BL. Properties of a clonal muscle cell line from rat heart. Experimental Cell Research 1976;98(2):367–81. [DOI] [PubMed] [Google Scholar]
- 28.Chen Q, Tu V, Wu Y, Bahl J. Hydrogen Peroxide Dose Dependent Induction of Cell Death or Hypertrophy in Cardiomyocytes. Arch Biochem Biophys 2000;373(1):242–248. [DOI] [PubMed] [Google Scholar]
- 29.Penumathsa SV, Thirunavukkarasu M, Koneru S, Juhasz B, Zhan L, Pant R, Menon VP, Otani H, Maulik N. Statin and resveratrol in combination induces cardioprotection against myocardial infarction in hypercholesterolemic rat. J Mol Cell Cardiol 2007;42(3):508–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Carrizzo A, Forte M, Damato A, Trimarco V, Salzano F, Bartolo M, Maciag A, Puca AA, Vecchione C. Antioxidant effects of resveratrol in cardiovascular, cerebral and metabolic diseases. Food Chem Toxicol 2013;61:215–26. [DOI] [PubMed] [Google Scholar]
- 31.de la Lastra CA, Villegas I. Resveratrol as an antioxidant and pro-oxidant agent: mechanisms and clinical implications. Biochem Soc Trans 2007;35(Pt 5):1156–60. [DOI] [PubMed] [Google Scholar]
- 32.Kovacic P, Somanathan R. Multifaceted approach to resveratrol bioactivity: Focus on antioxidant action, cell signaling and safety. Oxid Med Cell Longev 2010;3(2):86–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Houston M. The role of nutraceutical supplements in the treatment of dyslipidemia. J Clin Hypertens (Greenwich) 2012;14(2):121–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tome-Carneiro J, Gonzalvez M, Larrosa M, Yanez-Gascon MJ, Garcia-Almagro FJ, Ruiz-Ros JA, Garcia-Conesa MT, Tomas-Barberan FA, Espin JC. One-year consumption of a grape nutraceutical containing resveratrol improves the inflammatory and fibrinolytic status of patients in primary prevention of cardiovascular disease. Am J Cardiol 2012;110(3):356–63. [DOI] [PubMed] [Google Scholar]
- 35.Merla R, Daher IN, Ye Y, Uretsky BF, Birnbaum Y. Pretreatment with statins may reduce cardiovascular morbidity and mortality after elective surgery and percutaneous coronary intervention: clinical evidence and possible underlying mechanisms. Am Heart J 2007;154(2):391–402. [DOI] [PubMed] [Google Scholar]
- 36.Papageorgiou N, Zacharia E, Briasoulis A, Androulakis E, Tousoulis D. Statins and myocardial infarction: Type, dose, and administration time: Does it matter? Trends Cardiovasc Med 2016;26(5):433–41. [DOI] [PubMed] [Google Scholar]
- 37.Lampropoulos K, Megalou A, Bazoukis G, Tse G, Manolis A. Pre-loading therapy with statins in patients with angina and acute coronary syndromes undergoing PCI. J Interv Cardiol 2017;30(6):507–513. [DOI] [PubMed] [Google Scholar]
- 38.Ludman A, Venugopal V, Yellon DM, Hausenloy DJ. Statins and cardioprotection--more than just lipid lowering? Pharmacol Ther 2009;122(1):30–43. [DOI] [PubMed] [Google Scholar]
- 39.Arnett DK, Blumenthal RS, Albert MA, Buroker AB, Goldberger ZD, Hahn EJ, Himmelfarb CD, Khera A, Lloyd-Jones D, McEvoy JW and others. 2019 ACC/AHA Guideline on the Primary Prevention of Cardiovascular Disease: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation 2019;140(11):e596–e646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sung MM, Dyck JR. Therapeutic potential of resveratrol in heart failure. Ann N Y Acad Sci 2015;1348(1):32–45. [DOI] [PubMed] [Google Scholar]
- 41.Group SC, Link E, Parish S, Armitage J, Bowman L, Heath S, Matsuda F, Gut I, Lathrop M, Collins R. SLCO1B1 variants and statin-induced myopathy--a genomewide study. N Engl J Med 2008;359(8):789–99. [DOI] [PubMed] [Google Scholar]
- 42.Vladutiu GD, Isackson PJ. SLCO1B1 variants and statin-induced myopathy. N Engl J Med 2009;360(3):304. [DOI] [PubMed] [Google Scholar]