Abstract
To determine the presence of Bartonella henselae bacteremia in six cats, we compared isolation using blood culture with direct immunofluorescence on blood smears. Three cats that were positive by blood culture were also positive by direct immunofluorescence, and laser confocal microscopy confirmed the intraerythrocytic location of B. henselae.
Bartonella henselae is an agent of cat scratch disease and can be responsible for other human diseases such as bacillary angiomatosis, peliosis hepatitis, and endocarditis (12). The natural host of B. henselae is the domestic cat, and its vector is the cat flea. The organism can be isolated from the blood of 4 to 70% of apparently healthy cats depending on the cat population concerned and its geographic location (2, 5, 7). In infected cats, the target cells of B. henselae seem to be the erythrocytes (8, 14), although this has been refuted in a recent study (4). The detection of B. henselae in feline erythrocytes using immunological techniques has not been described, and the sensitivity of such methods is thus unknown. In our study we compared the use of blood cultures and a monoclonal antibody (MAb) against B. henselae in direct immunofluorescence tests to detect the organism in cat blood. Also, we used laser confocal microscopy to determine the position of B. henselae in blood smears that were positive by immunofluorescence.
The study was performed in June 2000 during an exercise to eradicate stray cats from a French military base. Six cats were captured using oral Dexeutanol (Instituto DEX, Seville, Spain) in fresh meat according to the manufacturer's instructions. Once sedated, the cats were anesthetized with Imalgène (Merial, Lyon, France), and 3 ml of blood was collected aseptically using intracardiac puncture. Cats were euthanized by intracardiac inoculation of Dolethal (Vétoquinol SA, Lure, France) according to the manufacturer's directions. Thin blood smears were made from the fresh blood and stored at room temperature until the direct immunofluorescence assays were carried out. The remaining fresh blood was stored at −20°C until it was thawed at room temperature and 1-ml aliquots were inoculated onto Columbia 5% sheep blood agar plates (BioMerieux, Marcy l'Etoile, France). The plates were placed in polyethylene bags to prevent desiccation and incubated at 37°C under 5% CO2 (Genbag CO2 system; BioMerieux). For 3 months the plates were examined weekly for evidence of bacterial growth. When Bartonella-like colonies were observed, they were characterized using the sequences of their 16S rRNA genes and the BLAST 2.0 program (National Center for Biotechnology Information) as described previously (6). The blood smears were examined for evidence of B. henselae infection using two specific MAbs prepared in our laboratory: the first (B3D4) was Bartonella genus specific (11) and the second (H2A10) (10) was B. henselae specific using standard immunofluorescence techniques (9). MAb H2A10 was of the immunoglobulin G2a (IgG2a) subclass and reacted with a 43-kDa epitope present only in B. henselae strains. This MAb does not cross-react with other Bartonella species, including B. quintana, B. elizabethae, B. clarridgeiae, B. bacilliformis, B. taylori, B. doshiae, and B. vinsonii, or with any of the bacteria already tested for MAb B3D4 (11).
Positive smears were examined under a laser scanning confocal microscope with an excitation wavelength of 488 nm and an emission wavelength of 617 nm to determine the location of the organisms in the erythrocytes. Sections of the erythrocytes were made in increments of 0.5 μm as previously described (13).
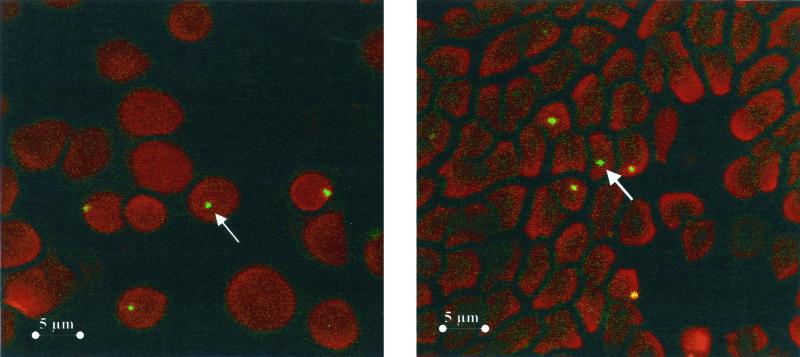
Bartonella organisms at 105 CFU/ml were isolated from the blood of three of six (50%) cats, and their 16S rRNA gene sequences were identical to that of B. henselae URLLY8 strain Marseille (3). The blood smears made from these cats had positive immunofluorescence when assayed either with MAb B3D4, specific for the Bartonella genus, or with MAb H2A10, specific to B. henselae strains (Fig. 1). Three to 8% (mean, 5%) of the erythrocytes were found to be infected, with most of these cells containing only one bacterium (average, 1.08 bacteria/erythrocyte). These results are consistent with previous reports that the prevalence of bacteremia in cats is 22 to 59% (5) and that the percentage of infected red blood cells varies from 1 to 20% (1, 2, 7). The average red cell count in the cat is 5 × 109/ml, and with the 5% level of infection we detected, there were around 2.5 × 108 infected erythrocytes per milliliter of blood. In our blood cultures, however, we detected only 105 CFU/ml, and we conclude that a large number of bacteria were killed during the processing of the blood and/or its storage at −20°C and thus that 1 in 2,500 bacteria was cultivable.
FIG. 1.
Digital sections of feline red blood cells infected with B. henselae as viewed by laser scanning confocal microscopy. Sections were taken in 0.5-μm increments from top to bottom. B. henselae organisms were revealed with a specific MAb. Arrows indicate intracellular B. henselae.
By laser scanning confocal microscopy B. henselae was seen within the erythrocytes, particularly near the cell membrane but also in the center of the cell (Fig. 1). These findings using immunological techniques confirm the intraerythrocytic location of B. henselae in naturally infected cats. Also, our findings show that direct fluorescence with a specific MAb is a sensitive, rapid, and simple technique which may replace blood cultures for detecting Bartonella infections in cats.
Acknowledgments
We thank Patrick Kelly for review of the manuscript and Robert Pistoresi for technical assistance.
REFERENCES
- 1.Bergmans A M C, Schellekens J F P, Van Embden J D A, Schouls L M. Predominance of two Bartonella henselae variants among cat scratch disease patients in the Netherlands. J Clin Microbiol. 1996;34:254–260. doi: 10.1128/jcm.34.2.254-260.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chomel B B, Abbott R C, Kasten R W, Floyd-Hawkins K A, Kass P H, Glaser C A, Pedersen N C, Koehler J E. Bartonella henselae prevalence in domestic cats in California: risk factors and association between bacteremia and antibody titers. J Clin Microbiol. 1995;33:2445–2450. doi: 10.1128/jcm.33.9.2445-2450.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Drancourt M, Birtles R J, Chaumentin G, Vandenesch F, Etienne J, Raoult D. New serotype of Bartonella henselae in endocarditis and cat-scratch disease. Lancet. 1996;347:441–443. doi: 10.1016/s0140-6736(96)90012-4. [DOI] [PubMed] [Google Scholar]
- 4.Guptill L, Wu C C, Glickman L, Turek J, Slater L, HogenEsch H. Extracellular Bartonella henselae and artifactual intraerythrocytic pseudoinclusions in experimentally infected cats. Vet Microbiol. 2000;76:283–290. doi: 10.1016/s0378-1135(00)00240-6. [DOI] [PubMed] [Google Scholar]
- 5.Heller R, Artois M, Xemar V, De Briel D, Gehin H, Jaulhac B, Monteil H, Piemont Y. Prevalence of Bartonella henselae and Bartonella clarridgeiae in stray cats. J Clin Microbiol. 1997;35:1327–1331. doi: 10.1128/jcm.35.6.1327-1331.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Joblet C, Roux V, Drancourt M, Gouvernet J, Raoult D. Identification of Bartonella (Rochalimaea) species among fastidious gram-negative bacteria on the basis of the partial sequence of the citrate synthase gene. J Clin Microbiol. 1995;33:1879–1883. doi: 10.1128/jcm.33.7.1879-1883.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Koehler J E, Glaser C A, Tappero J W. Rochalimaea henselae infection: a new zoonosis with the domestic cat as a reservoir. JAMA. 1994;271:531–535. doi: 10.1001/jama.271.7.531. [DOI] [PubMed] [Google Scholar]
- 8.Kordick D L, Breitschwerdt E B. Intraerythrocytic presence of Bartonella henselae. J Clin Microbiol. 1995;33:1655–1656. doi: 10.1128/jcm.33.6.1655-1656.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.La Scola B, Raoult D. Culture of Bartonella quintana and Bartonella henselae from human samples: a 5-year experience (1993 to 1998) J Clin Microbiol. 1999;37:1899–1905. doi: 10.1128/jcm.37.6.1899-1905.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liang Z. Rôle des anticorps monoclonaux dans la classification et l'identification des bactéries intracellularies. Ph. D. thesis. Marseille, France: Université de la Méditerranée; 2001. [Google Scholar]
- 11.Liang, Z., B. La Scola, and D. Raoult. Production of Bartonella genus-specific monoclonal antibodies. Clin. Diagn. Lab. Immun., in press. [DOI] [PMC free article] [PubMed]
- 12.Maurin M, Birtles R J, Raoult D. Current knowledge of Bartonella species. Eur J Clin Microbiol Infect Dis. 1997;16:487–506. doi: 10.1007/BF01708232. [DOI] [PubMed] [Google Scholar]
- 13.Meconi S, Jacomo V, Boquet P, Raoult D, Mege J L, Capo C. Coxiella burnetii induces reorganization of the actin cytoskeleton in human monocytes. Infect Immun. 1998;66:5527–5533. doi: 10.1128/iai.66.11.5527-5533.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mehock J R, Greene C E, Gherardini F C, Hahn T W, Krause D C. Bartonella henselae invasion of feline erythrocytes in vitro. Infect Immun. 1998;66:3462–3466. doi: 10.1128/iai.66.7.3462-3466.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]