Abstract
Study objective
Our goal is to review the outcomes of acute hypertensive/hypotensive episodes from articles published in the past 10 years that assessed the short- and long-term impact of acute hypertensive/hypotensive episodes in the perioperative setting.
Methods
We conducted a systematic peer review based upon PROSPERO and Cochrane Handbook protocols. The following study characteristics were collected: study type, author, year, population, sample size, their definition of acute hypertension, hypotension or other measures, and outcomes (probabilities, odds ratio, hazard ratio, and relative risk) and the p-values; and they were classified according to the type of surgery (cardiac and non-cardiac).
Results
A total of 3,680 articles were identified, and 66 articles fulfilled the criteria for data extraction. For the perioperative setting, the number of articles varies by outcome: 20 mortality, 16 renal outcomes, 6 stroke, 7 delirium and 34 other outcomes. Hypotension was reported to be associated with mortality (OR 1.02–20.826) as well as changes from the patient’s baseline blood pressure (BP) (OR 1.02–1.36); hypotension also had a role in the development of acute kidney injury (AKI) (OR 1.03–14.11). Postsurgical delirium was found in relation with BP lability (OR 1.018–1.038) and intra- and postsurgical hypotension (OR 1.05–1.22), and hypertension (OR 1.44–2.34). Increased OR (37.67) of intracranial hemorrhage was associated to postsurgical systolic BP >130 mmHg. There was a wide range of additional diverse outcomes related to hypo-, hypertension and BP lability.
Conclusions
The perioperative management of BP influences short- and long-term effects of surgical procedures in cardiac and non-cardiac interventions; these findings support the burden of BP fluctuations in this setting.
Introduction
Perioperative blood pressure (BP) variability, hypertension (HTN) and hypotension (HPT) have all been associated with hemodynamic instability and poor clinical outcomes [1]. Optimal pharmacologic control of BP requires intravenous (IV) agents that are easy to prepare and administer and that have rapid onset and offset of action that allows a predictable effect and easy dose-titration to properly fine-tune the BP of the patient, among other requirements [1].
Treatment choices for acute HTN depend on several factors in addition to BP measurement. These include evidence of end-organ damage (e.g., cerebral, cardiac, vascular, renal) presence of comorbidities (e.g., aortic dissection, acute myocardial infarction (AMI), bleeding) and ability to ingest and absorb oral medicines [2]. Examples of such clinical circumstances include perioperative HTN, in which rapid control of BP is essential to limit or prevent end-organ injury.
Both HTN and HPT in perioperative settings or acute HTN may result in a high economic burden for healthcare systems [3–5] due to perioperative complications requiring prolonged hospitalization.
Even though there are several international reference guidelines that account for the importance of management of perioperative BP [6–8], at present there are no universally accepted preoperative BP thresholds, as BP targets need to consider patient baseline BP, surgery type, and risk of short-term complications [9]. Furthermore, there is a clear gap in the knowledge of short- and long-term implications of acute hypo- and hypertensive perioperative episodes. Thus, a comprehensive, systematic review would have important and broad implications, particularly due to anecdotal evidence that suggests substantial underutilization of antihypertensive agents by clinical area.
The objective of this research is to systematically review the available evidence on critical outcomes potentially associated with hypertensive/hypotensive episodes (e.g., mortality, stroke, AMI, acute kidney injury (AKI), and others) in the perioperative setting for cardiac and non-cardiac surgeries, and to compare these findings to serve as a guide for clinical decision making, health policy and future research.
Methods
Study design
A systematic review of the literature was conducted in order to identify studies that analyzed the implications of HTN and HPT in the perioperative setting. The study design was formulated based upon PROSPERO and Cochrane Handbook protocols (Fig 1 and S1 Checklist and S1 File). The research protocol was registered in Open Science Framework (https://osf.io/vgjmu).
Fig 1. PICO(S) search strategy.
Inclusion and exclusion criteria
Studies were included based on the following criteria:
Original research articles from January 1, 2010, to December 31, 2020. The last ten calendar years to collect the latest evidence published.
Population 18 years of age or older.
Outcomes of acute hypertension/hypotension episodes in the perioperative settings.
Studies were excluded based on:
Key terms were not included in either the title or abstract (perioperative, intensive care, HTN, HPT, and BP).
Title or abstract included the following terms: pulmonary, intra-abdominal compartment syndrome, children, pediatric, infant, or pregnancy.
Non-English.
Duplicates.
Full text was inaccessible.
Other outcomes
Other types of studies not in the scope of the review
Database and search terms
Databases included in the search were PubMed, Google Scholar, and ScienceDirect. Our search terms included: perioperative, intensive care, HTN, HPT, and BP (S1 File).
Study selection and data extraction
In Round 1, the research team, which consisted of two researchers (MC and SP), reviewed each title and abstract to exclude articles that did not meet the inclusion criteria. An arbitration process then took place for any articles about which there was a disagreement. The research team had to come to a unanimous agreement. Those articles with acceptable titles and abstracts were acquired and reviewed again by both reviewers. Round 1 concluded after full-text arbitration.
In Round 2, the search included all articles that cited or were cited by the Round 1 articles based on a Web of Science search. The titles and abstracts of the citations underwent the same review process as Round 1. The citations with acceptable titles and abstracts were acquired and reviewed again by both reviewers. Round 2 concluded after full-text arbitration.
Data extraction was performed by two researchers and is summarized considering the following study characteristics: study type, author, year, population, sample size, their definition of acute HTN or HPT, and outcomes in terms of morbidity and mortality (probabilities, odds ratio (OR), hazard ratio (HR), and relative risk (RR)), as well as type of intervention, cardiac or non-cardiac surgery. When necessary, more information is provided.
Additionally, the quality of these studies was graded by using the Scottish Intercollegiate Guidelines (SIGN) [10].
Results
The literature search led to 3,680 articles being identified, of which, 1,054 were removed for being duplicates. We excluded 2,489 articles based on title and abstract review. We then assessed the full text of 137 articles. After this screening, we identified 66 articles for data extraction. This process is illustrated in the PRISMA diagram (Fig 2). Of these articles, 1 (1.5%) was graded as a moderate-high quality, 39 (59.1%) as moderate, and 26 (39.4%) as low quality (S1 Table).
Fig 2. PRISMA diagram.
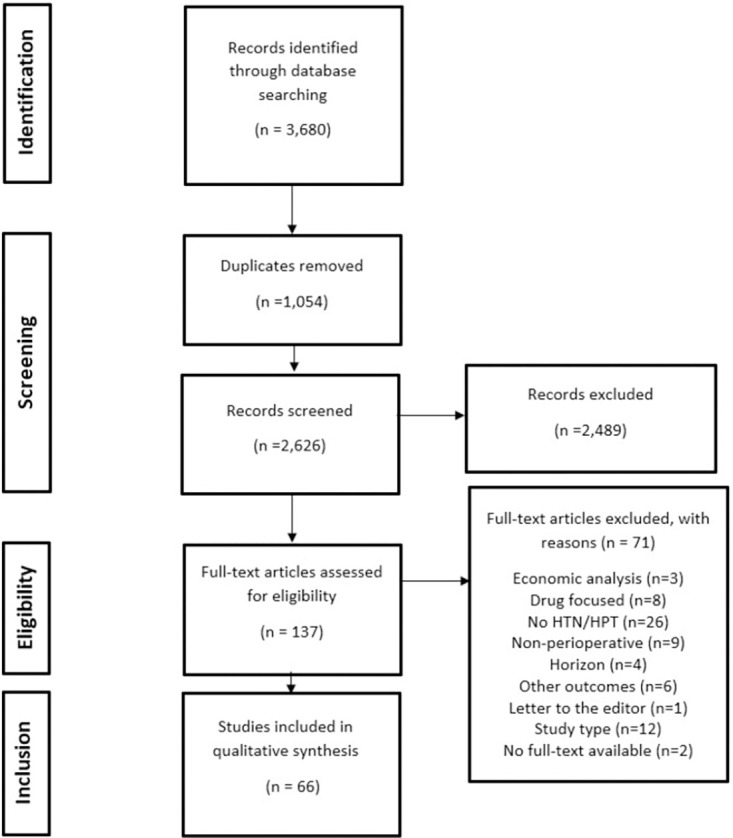
The study characteristics are shown in Table 1. Sample sizes ranged from 33 to 368,222 patients, with different population types and surgeries. Studies varied greatly in their definition of HTN/HPT. Article results were then grouped by cardiac or non-cardiac surgery, and outcome: mortality, stroke, kidney function, delirium, myocardial injury/AMI, and other outcomes associated with HPT, HTN, or other measures such as control of intraoperative mean arterial pressure (MAP) or variability within the surgery. The parameters were primarily OR, but we also found associations, HR, and RR. For the perioperative setting, the number of articles varies by outcome: 20 mortality, 16 renal outcomes, 6 stroke, 7 delirium, and 34 other outcomes. Since one study may include more than one outcome, the total sum of the reported outcomes may exceed the total number of studies.
Table 1. Study characteristics.
| Study title | Author | Year | Population | Sample Size | HPT/HTN |
|---|---|---|---|---|---|
| Association between preoperative pulse pressure and perioperative myocardial injury: an international observational cohort study of patients undergoing non-cardiac surgery [11] | Abbott et al. | 2017 | Non-cardiac surgery | 15,057 | HTN |
| A prospective international multicentre cohort study of intraoperative heart rate and systolic blood pressure and myocardial injury after noncardiac surgery: results of the VISION study [12] | Abbott et al. | 2018 | Non-cardiac surgery | 16,079 | HPT/HTN |
| Associations of intraoperative radial arterial systolic, diastolic, mean, and pulse pressures with myocardial and acute kidney injury after noncardiac surgery [13] | Ahuja et al. | 2020 | Non-cardiac surgery | 164,514 | HPT |
| Modifiable, postoperative risk factors for delayed discharge following total knee arthroplasty: the influence of hypotension and opioid use [14] | Anastasio et al. | 2020 | Total knee arthroplasty | 1,033 | HPT |
| Intraoperative systolic blood pressure variability predicts 30-day mortality in aortocoronary bypass surgery patients [15] | Aronson et al. | 2010 | Aortocoronary bypass graft surgery | 7,504 | HPT/HTN |
| Does perioperative systolic blood pressure variability predict mortality after cardiac surgery? an exploratory analysis of the ECLIPSE trials [16] | Aronson et al. | 2011 | Cardiac surgery | 1,512 | BP variability |
| Association between intraoperative low blood pressure and development of surgical site infection after colorectal surgery a retrospective cohort study [17] | Babazade et al. | 2016 | Colorectal surgery | 2,521 | HPT |
| High postoperative blood pressure after cardiac surgery is associated with acute kidney injury and death [18] | Balzer et al. | 2016 | Cardiac surgery | 5,225 | HTN |
| Hypotension during hip fracture surgery and postoperative morbidity [19] | Beecham et al. | 2020 | Hip fracture surgery | 52 | HPT |
| Intraoperative hypotension and perioperative ischemic stroke after general surgery a nested case-control study [20] | Bijker et al. | 2012 | Non-cardiac and non-neurosurgery | 48,241 | HPT |
| Can routine perioperative haemodynamic parameters predict postoperative morbidity after major surgery? [21] | Bonnet et al. | 2020 | Non-cardiac surgery | 50 | HPT |
| Association of intraoperative blood pressure instability with adverse outcomes after liver transplantation [22] | DeMaria et al. | 2013 | Orthotopic liver transplantation | 827 | HTN |
| Intraoperative hypotension is associated with adverse clinical outcomes after noncardiac surgery [23] | Gregory et al. | 2020 | Non-cardiac surgery | 368,222 | HPT |
| Intraoperative hypotension is associated with myocardial damage in noncardiac surgery: An observational study [24] | Hallqvist et al. | 2016 | Non-cardiac surgery | 300 | HPT |
| Intraoperative hypotension is associated with acute kidney injury in noncardiac surgery: An observational study[25] | Hallqvist et al. | 2018 | Non-cardiac surgery | 470 | HPT |
| Impact of intraoperative hypotension and blood pressure fluctuations on early postoperative delirium after non-cardiac surgery [26] | Hirsch et al. | 2015 | Non-cardiac surgery | 594 | HPT |
| Perioperative optimal blood pressure as determined by ultrasound tagged near infrared spectroscopy and its association with postoperative acute kidney injury in cardiac surgery patients [27] | Hori et al. | 2016a | Cardiac surgery | 110 | HPT |
| Blood pressure deviations from optimal mean arterial pressure during cardiac surgery measured with a novel monitor of cerebral blood flow and risk for perioperative delirium: a pilot study [28] | Hori et al. | 2016b | Cardiac surgery | 110 | HPT/HTN |
| The association between mild intraoperative hypotension and stroke in general surgery patients [29] | Hsieh et al. | 2016 | Non-cardiac, non-neurosurgery, and non-carotid surgery | 106,337 | HPT |
| Intraoperative hypotension is a risk factor for postoperative acute kidney injury after femoral neck fracture surgery: a retrospective study [30] | Jang et al. | 2019 | Femoral neck fracture surgery | 248 | HPT |
| Blood pressure coefficient of variation and its association with cardiac surgical outcomes [31] | Jinadasa et al. | 2018 | Cardiac surgery | 3,687 | HTN |
| Risk factors for emergence agitation in adults undergoing thoracoscopic lung surgery: a case-control study of 1,950 patients [32] | Kang et al. | 2020 | Thoracoscopic lung surgery | 1,950 | HPT/HTN |
| Intraoperative hypotension and flap loss in free tissue transfer surgery of the head and neck [33] | Kass et al. | 2018 | Head and neck surgery | 445 | HPT |
| Intraoperative hypotension is not associated with postoperative cognitive dysfunction in elderly patients undergoing general anesthesia for surgery: results of a randomized controlled pilot trial [34] | Langer et al. | 2019 | Non-cardiac surgery | 101 | HPT |
| Intraoperative arterial blood pressure lability is associated with improved 30-day survival [35] | Levin et al. | 2015 | Surgery | 52,919 | HTN |
| Perioperative risk factors associated with acute kidney injury in patients after brain tumor resection [36] | Li et al. | 2020a | Brain tumor resection | 460 | HPT |
| High variance of intraoperative blood pressure predicts early cerebral infarction after revascularization surgery in patients with Moyamoya disease [37] | Li et al. | 2020b | Revascularization surgery (Moyamoya disease) | 1,497 | HPT |
| Association of intraoperative hypotension with acute kidney injury after liver resection surgery: an observational cohort study [38] | Liao et al. | 2020 | Liver resection | 796 | HPT |
| Postoperative hypotension after noncardiac surgery and the association with myocardial injury [39] | Liem et al. | 2020 | Non-cardiac surgery | 1,710 | HPT |
| Perioperative cardiac complications in patients over 80 years of age with coronary artery disease undergoing noncardiac surgery: the incidence and risk factors [40] | Liu et al. | 2020 | Non-cardiac surgery | 547 | HPT |
| Association between perioperative hypotension and delirium in postoperative critically ill patients: a retrospective cohort analysis [41] | Maheshwari et al. | 2019 | Non-cardiac surgery | 1,083 | HPT |
| Intraoperative mean arterial pressure variability and 30-day mortality in patients having noncardiac surgery [42] | Mascha et al. | 2015 | Non-cardiac surgery | 104,401 | MAP Variability |
| Preoperative risk and the association between hypotension and postoperative acute kidney injury [43] | Mathis et al. | 2020 | Non-cardiac surgery | 138,021 | HPT |
| Prolonged heightened blood pressure following mechanical thrombectomy for acute stroke is associated with worse outcomes [44] | McCarthy et al. | 2020 | Mechanical thrombectomy | 212 | HTN |
| Association of postoperative blood pressure and bleeding after cardiac surgery [45] | McIlroy et al. | 2019 | Cardiac surgery | 793 | HTN |
| Relationship between intraoperative hypotension and acute kidney injury after living donor liver transplantation: a retrospective analysis [46] | Mizota et al. | 2017 | Liver transplantation | 231 | HPT |
| Association between intraoperative hypotension and hypertension and 30-day postoperative mortality in noncardiac surgery [47] | Monk et al. | 2015 | Non-cardiac surgery | 18,756 | HPT/HTN |
| Postoperative systolic blood pressure as a risk factor for haematoma following thyroid surgery [48] | Morton and Vandal. | 2015 | Thyroid surgery | 621 | HTN |
| Defining an intraoperative hypotension threshold in association with de novo renal replacement therapy after cardiac surgery [49] | Ngu et al. | 2020 | Cardiac surgery | 6,523 | HPT |
| Blood pressure excursions below the cerebral autoregulation threshold during cardiac surgery are associated with acute kidney injury [50] | Ono et al. | 2013 | Cardiac surgery | 348 | MAP below limit of autoregulation |
| Duration and magnitude of blood pressure below cerebral autoregulation threshold during cardiopulmonary bypass is associated with major morbidity and operative mortality [51] | Ono et al. | 2014 | Cardiac surgery | 450 | MAP below limit of autoregulation |
| Elevated blood pressure after craniotomy: A prospective observational study [52] | Perez et al. | 2020 | Craniotomy | 282 | HTN |
| Intraoperative blood pressure changes as a risk factor for anastomotic leakage in colorectal surgery [53] | Post et al. | 2012 | Colorectal surgery | 285 | HTN |
| Impact of intraoperative hypotension during cardiopulmonary bypass on acute kidney injury after coronary artery bypass grafting [54] | Rettig et al. | 2017 | Cardiac surgery | 1,891 | HPT |
| The impact of preoperative risk on the association between hypotension and mortality after cardiac surgery: an observational study [55] | Ristovic et al. | 2020 | Cardiac surgery | 6,627 | HPT |
| Relationship between perioperative hypotension and perioperative cardiovascular events in patients with coronary artery disease undergoing major noncardiac surgery [56] | Roshanov et al. | 2019 | Non-cardiac surgery | 955 | HPT |
| Intra-operative hypotension is a risk factor for post-operative silent brain ischaemia in patients with pre-operative hypertension undergoing carotid endarterectomy [57] | Rots et al. | 2020 | Carotid endarterectomy | 55 | HPT/HTN |
| Relationship between Intraoperative Hypotension, defined by either reduction from baseline or absolute thresholds, and acute kidney and myocardial injury after noncardiac surgery [58] | Salmasi et al. | 2017 | Non-cardiac surgery | 57,315 | HPT |
| Period-dependent associations between hypotension during and for four days after noncardiac surgery and a composite of myocardial infarction and death [59] | Sessler et al. | 2018 | Non-cardiac surgery | 9,765 | HPT |
| Perioperative hypotension and discharge outcomes in non-critically injured trauma patients, a single centre retrospective cohort study [60] | Sheffy et al. | 2017 | Trauma surgery | 1,744 | HPT |
| Intraoperative hypotension, new onset atrial fibrillation, and adverse outcome after carotid endarterectomy [61] | Sposato et al. | 2011 | Carotid endarterectomy | 186 | HPT |
| Association of intraoperative hypotension with acute kidney injury after elective noncardiac surgery [62] | Sun et al. | 2015 | Non-cardiac surgery | 5,127 | HPT |
| Association of intraoperative hypotension with acute kidney injury after noncardiac surgery in patients younger than 60 years old [63] | Tang et al. | 2019 | Non-cardiac surgery | 4,952 | HPT |
| Impact of intraoperative hypotension on hospital stay in major abdominal surgery [64] | Tassoudis et al. | 2011 | Abdominal surgery | 100 | HPT |
| Association between postoperative mean arterial blood pressure and myocardial injury after noncardiac surgery [65] | van Lier et al. | 2018 | Non-cardiac surgery | 2,211 | HPT |
| Association between intraoperative hypotension and myocardial injury after vascular surgery [66] | van Waes. | 2016 | Non-cardiac surgery | 890 | HPT |
| Relationship between intraoperative mean arterial pressure and clinical outcomes after noncardiac surgery: toward an empirical definition of hypotension [67] | Walsh et al. | 2013 | Non-cardiac surgery | 33,330 | HPT |
| Association between intraoperative blood pressure and postoperative delirium in elderly hip fracture patients [68] | Wang et al. | 2015 | Hip fracture surgery | 103 | HTN |
| Intraoperative hypotension and delirium after on-pump cardiac surgery [69] | Wesselink et al. | 2015 | Cardiac surgery | 743 | HPT |
| Concurrence of intraoperative hypotension, low minimum alveolar concentration, and low bispectral index is associated with postoperative death [70] | Willingham et al. | 2015 | Any surgery | 13,198 | HPT |
| Intraoperative blood pressure variability predicts postoperative mortality in non-cardiac surgery-a prospective observational cohort study [71] | Wiorek and Krzych. | 2019 | Non-cardiac surgery | 835 | BP Variability |
| Optimal blood pressure decreases acute kidney injury after gastrointestinal surgery in elderly hypertensive patients: A randomized study Optimal blood pressure reduces acute kidney injury [72] | Wu et al. | 2017 | Gastrointestinal surgery | 678 | Control of MAP |
| Postoperative hypotension and surgical site infections after colorectal surgery: a retrospective cohort study [73] | Yilmaz et al. | 2018 | Colorectal surgery | 5,896 | HPT |
| Association of perioperative blood pressure with long-term survival in rectal cancer patients [74] | Yu et al. | 2016 | Colorectal surgery | 358 | HTN |
| Greater intraprocedural systolic blood pressure and blood pressure variability are associated with contrast-induced neurotoxicity after neurointerventional procedures [75] | Zevallos et al. | 2020 | Neurointerventions | 33 | BP Variability |
| Perioperative blood pressure control in carotid artery stenosis patients with carotid angioplasty stenting: a retrospective analysis of 173 cases [76] | Zheng et al. | 2020 | Carotid angioplasty stenting | 173 | HTN |
Abbreviations: BP: blood pressure, HPT: hypotension, HTN: hypertension, MAP: mean arterial pressure.
Mortality
Twenty articles reporting mortality outcomes in relation to perioperative HPT, HTN, and other measures were retrieved from the search.
Hypotension as a risk factor
Ten articles had results for mortality associated with HPT (Table 2). There were two articles focused on cardiac surgery and what these authors demonstrated was that values such as the duration of min excursion of BP <95 mmHg were associated with increased odds (1.03) for 30-day mortality [15], and that MAP below 55 mmHg for more than 10 minutes postsurgery or between 55–64 mmHg intraoperative or postsurgery are also associated with increased odds of death [55]. What was mainly seen in non-cardiac surgeries was that perioperative HPT entailed a higher risk of mortality, with increased odds of 30-day mortality of 1.81 when systolic blood pressure (SBP) was below 100 mmHg, as reported by Abbott et al. [12] Other authors also reported increased risk for 30-day mortality with diverse OR such as 1.79 for patients with intraoperative MAP <55 mmHg for more than 20 minutes [67] or 20.826 for intraoperative MAP below 40 mmHg for more than 5 minutes when comparing this patient group with patients with intraoperative BP levels between 60–109 mmHg [47]. The incidence of mortality was reported to be statistically significant for those patients with MAP below 67 mmHg versus above 67.3 mmHg (p<0.001) [65]. Some authors also reported an association between the duration of HPT (measured both as the MAP or SBP) and a higher risk of mortality [47, 67]. In addition, an association between the threshold and the increased odds for 30-day and 90-day mortality has been recently demonstrated: the lower the MAP the higher the odds, as reported by Gregory et al. [23].
Table 2. Mortality associated with perioperative hypotension.
| Author | Year | Sample size | Definition of hypotension | Result* | P-value | Duration |
|---|---|---|---|---|---|---|
| Aronson et al.# [15] | 2010 | 7,504 | AUC <95 (mmHg x min) | 1.02 (1.002–1.041) | 0.03 | 30-day |
| Excursion <95 [AUC for 20% (mmHg x min)] | 0.95 (0.916–0.994) | 0.02 | 30-day | |||
| Minutes <95 mmHg per excursion | 1.03 (1.008–1.042) | 0.003 | 30-day | |||
| Mean excursion nadir <95 mmHg | 1.05 (1.019–1.084) | 0.002 | 30-day | |||
| Ristovic et al.# [55] | 2020 | 6,627 | MAP 55–64 mmHg 10 min during cardiopulmonary bypass | 1.10 (1.00–1.21) | 0.049 | |
| MAP <55 mmHg 10 min post cardiopulmonary bypass | 1.30 (1.13–1.49) | 0.002 | ||||
| MAP 55–64 mmHg 10 min post cardiopulmonary bypass | 1.18 (1.07–1.30) | 0.001 | ||||
| Abbott et al. [12] | 2018 | 16,079 | SBP <100 mmHg | 1.81 (1.39–2.37) | <0.01 | 30-day |
| Gregory et al. [23] | 2020 | 368,222 | Absolute maximum decrease (for each 5 mmHg under IOH MAP thresholds) 75 mmHg | 1.16 (1.14–1.17) | 30-day | |
| Absolute maximum decrease (for each 5 mmHg under IOH MAP thresholds) 65 mmHg | 1.21 (1.18–1.23) | 30-day | ||||
| Absolute maximum decrease (for each 5 mmHg under IOH MAP thresholds) 55 mmHg | 1.30 (1.26–1.35) | 30-day | ||||
| Absolute maximum decrease (for each 5 mmHg under IOH MAP thresholds) 75 mmHg | 1.13 (1.12–1.14) | 90-day | ||||
| Absolute maximum decrease (for each 5 mmHg under IOH MAP thresholds) 65 mmHg | 1.17 (1.15–1.20) | 90-day | ||||
| Absolute maximum decrease (for each 5 mmHg under IOH MAP thresholds) 55 mmHg | 1.26 (1.22–1.30) | 90-day | ||||
| Levin et al. [35] | 2015 | 52,919 | MAP <50 mmHg 5 min; Derivation cohort (ASA IV or V) | 1.19 (1.09–1.29) | <0.001 | 30-day |
| MAP <50 mmHg 5 min; Validation cohort (ASA IV or V) | 1.15 (1.04–1.26) | 0.01 | 30-day | |||
| Monk et al. [47] | 2015 | 18,756 | SBP <70 mmHg for >5 min (Reference 90–159 mmHg) | 2.898 (1.719–4.886) | <0.0001 | 30-day |
| MAP 40–49 mmHg for >5 min (Reference 60–109 mmHg) | 2.433 (1.285–4.608) | <0.0001 | 30-day | |||
| MAP <40 mmHg for >5 min (Reference 60–109 mmHg) | 20.826 (8.884–48.822) | <0.0001 | 30-day | |||
| DBP <30 mmHg for >5 min | 3.181 (1.826–5.540) | <0.001 | 30-day | |||
| MBP decrease >50% from baseline for >5 min | 2.721 (1.489–4.974) | 0.005 | 30-day | |||
| Sessler et al. [59] | 2018 | 9,765 | Intra 10-min increase in HPT | 1.12 (1.05–1.20) | <0.01 | 30-day |
| Post 10-min increase in HPT | 1.03 (1.01–1.06) | <0.01 | 30-day | |||
| van Lier et al. [65] | 2018 | 2,211 | MAP 31.0–67.0 mmHg | 7% Incidence | <0.001 | 30-day |
| MAP 67.3–76.3 mmHg | 2.90% Incidence | |||||
| MAP 76.7–86.0 mmHg | 1.90% Incidence | |||||
| MAP 86.7–122.3 mmHg | 2.70% Incidence | |||||
| Walsh et al. [67] | 2013 | 33,330 | MAP <55 mmHg for 1–5 min | 1.16 (0.91–1.46) | 30-day | |
| MAP <55 mmHg for 6–10 min | 1.16 (0.84–1.60) | 30-day | ||||
| MAP <55 mmHg for 11–20 min | 1.26 (0.89–1.80) | 30-day | ||||
| MAP <55 mmHg for >20 min | 1.79 (1.21–2.65) | 30-day | ||||
| Willingham et al. [70] | 2015 | 13,198 | Low MAP 15 min | 1.11 (1.08–1.14) HR | <0.001 | 30-day |
| Low MAP 15 min | 1.10 (1.08–1.12) HR | <0.001 | 90-day |
Abbreviations: ASA: American Society of Anesthesiologists; AUC: area under the curve, DBP: diastolic blood pressure; CI: confidence interval, IOH: intraoperative hypotension, HPT: hypotension, HR: hazard ratio, MAP: mean arterial pressure, MBP: mean blood pressure, min: minutes, OR: odds ratio, SBP: systolic blood pressure.
*Generally, OR (95% CI), unless otherwise stated.
# These articles refer to cardiac surgery.
Hypertension as a risk factor
Ten articles studied mortality in patients with perioperative HTN (Table 3). Four articles investigated the outcomes of cardiac surgery and all but one found statistically significant associations, for example for every 0.10 increase in the coefficient of variation of the SBP an increase in the odds of death of 150% was reported by Jinadasa et al. [31], and postoperative HTN (SBP >130 mmHg) was found to be related to higher in-hospital mortality rates [18]. Other authors reported increased odds of 1.03 per minutes above 135 mmHg per BP excursion [15]. One of the articles reported non-statistical significance for the influence of HTN on mortality; the study analyzed the increase of 1 mmHg in SBP [45]. Regarding the non-cardiac surgeries, while some of the studies found an association of HTN and reduced odds of mortality [12, 35], a recent article found increased odds of long-term mortality associated with the maximum SBP in days 2 (OR 1.28) and 3 (OR 1.30) after surgery [44]. One study associated preoperative SBP above 120 mmHg with increased mortality HR in cancer patients [74]. Zheng et al. showed that the incidence of in-hospital death was higher in those patients with BP >130 mmHg 24h after the intervention (16.70%, p-value 0.001) [76].
Table 3. Mortality associated with perioperative hypertension.
| Author | Year | Sample size | Definition of hypertension | Result* | P-value | Duration |
|---|---|---|---|---|---|---|
| Aronson et al.# [15] | 2010 | 7,504 | AUC >135 (mmHg x min) | 1.02 (0.991–1.055) | 0.16 | 30-day |
| Excursions >135 [AUC for 20% (mmHg x min)] | 0.99 (0.929–1.058) | 0.79 | 30-day | |||
| Min >135 mmHg per excursion | 1.03 (1.001–1.055) | 0.04 | 30-day | |||
| Mean excursion peak >135 mmHg | 1.01 (0.989–1.016) | 0.14 | 30-day | |||
| Balzer et al.# [18] | 2016 | 5,225 | >130 mmHg postoperative | 0.001 | 30-day | |
| Jinadasa et al.# [31] | 2018 | 3,687 | SBP coefficient of variation | 2.50 (1.60–3.92) | <0.01 | 30-day |
| Pre bypass coefficient of variation | 1.75 (1.17–2.62) | 0.01 | 30-day | |||
| Post bypass coefficient of variation | 1.40 (0.97–2.04) | 0.08 | 30-day | |||
| MAP coefficient of variation | 1.16 (0.98–1.37) | 0.09 | 30-day | |||
| Pre bypass coefficient of variation | 1.08 (0.95–1.23) | 0.24 | 30-day | |||
| Bypass coefficient of variation | 1.07 (0.87–1.31) | 0.53 | 30-day | |||
| Post bypass coefficient of variation | 1.06 (0.85–1.32) | 0.63 | 30-day | |||
| McIlroy et al.# [45] | 2019 | 793 | SBP 1 mmHg increase | 0.98 (0.94–1.02) | 0.35 | Hospital mortality |
| MAP 1 mmHg increase | 0.91 (0.84–0.99) | 0.03 | Hospital mortality | |||
| Abbott et al. [12] | 2018 | 16,079 | SBP >160 mmHg | 0.76 (0.58–0.99) | 0.04 | 30-day |
| Levin et al. [35] | 2015 | 52,919 | Baseline MAP (per 10 mmHg increase); Derivation cohort | 0.89 (0.84–0.95) | <0.001 | 30-day |
| Baseline MAP (per 10 mmHg increase); Validation cohort | 0.91 (0.84–0.99) | 0.03 | 30-day | |||
| MAP >120 mmHg, per 5 min increase; Derivation cohort | 0.91 (0.84–0.99) | 0.03 | 30-day | |||
| MAP >120 mmHg, per 5 min increase; Validation cohort | 1.00 (0.93–1.08) | 0.98 | 30-day | |||
| McCarthy et al. [44] | 2020 | 212 | Maximum SBP day 1 (increases of 10 mmHg) | - | ||
| Maximum SBP day 2 (increases of 10 mmHg) | 1.28 (1.04–1.58) | 0.021 | Last follow-up (median 89.5 days) | |||
| Maximum SBP day 3 (increases of 10 mmHg) | 1.30 (1.06–1.63) | 0.015 | Last follow-up (median 89.5 days) | |||
| Peak SBP change from day 1 to 2 | - | |||||
| Peak SBP change from day 2 to 3 | - | |||||
| Monk et al. [47] | 2015 | 18,756 | SBP >180 mmHg for >5 min (Reference 90–159 mmHg) | 0.904 (0.545–1.500) | 1 | 30-day |
| MAP >130mmHg for >5 min | 0.947 (0.624–1.435) | 1 | 30-day | |||
| DBP >120 mmHg for >5 min | 1.357 (0.714–2.580) | 1 | 30-day | |||
| Yu et al. [74] | 2016 | 358 | Preoperative SBP ≥120 mmHg | 1.97 (1.08–3.60) HR | 0.028 | Disease-free survival |
| Preoperative SBP ≥120 mmHg | 2.85 (1.00–8.25) HR | 0.05 | Cancer-free survival | |||
| Zheng et al. [76] | 2020 | 173 | 24h post-CAS BP <120 mmHg | 0% Incidence | 0.001 | In-hospital death |
| 24h post-CAS BP 120–130 mmHg | 0% Incidence | In-hospital death | ||||
| 24h post-CAS BP >130 mmHg | 16.70% Incidence | In-hospital death |
Abbreviations: AUC: area under the curve, CAS: carotid angioplasty stenting, CI: confidence interval, DBP: diastolic blood pressure, HR: hazard ratio, MAP: mean arterial pressure, min: minutes, OR: odds ratio, SBP: systolic blood pressure.
*Generally, OR (95% CI), unless otherwise stated.
#These articles refer to cardiac surgery.
Blood pressure variability
Five articles studied intraoperative BP variability (Table 4) and what was mostly demonstrated was that changes from the baseline of each patient were associated with higher mortality outcomes. Within cardiac surgeries, Aronson et al. [16] reported 30-day mortality related to the extent of SBP excursions intraoperatively (outside the range 75–135 mmHg), and pre- and postoperatively (outside the range 85–145 mmHg); another study found that MAP below the limit of autoregulation was also associated with increased odds of mortality in patients undergoing coronary artery bypass grafting and/or valve surgery [51]. The same results were reported for patients undergoing non-cardiac surgery by Mascha et al. [42], who found that cumulative time of MAP less than 80, 70, 60, 55, and 50 mmHg were associated with higher odds of 30-day mortality. Small changes, 1% of variability in both DBP and SBP were also related to postoperative mortality [71]. Finally, the control of MAP during surgery between the limits 65–110 mmHg was reported to reduce the risk of mortality [72].
Table 4. Mortality associated with perioperative variability in blood pressure (and other measures of blood pressure).
| Author | Year | Sample size | Definition of measure | Result* | P-value | Duration | Measure |
|---|---|---|---|---|---|---|---|
| Aronson et al.# [16] | 2011 | 1,512 | SBP, 65–135 mmHg intraoperative 75–145 mmHg pre and post | 1.142 (0.989–1.319) | 0.0707 | 30-day | SBP Variability |
| SBP, 75–135 mmHg intraoperative 85–145 mmHg pre and post | 1.16 (1.039–1.295) | 0.0082 | 30-day | SBP Variability | |||
| SBP, 85–135 mmHg intraoperative 95–145 mmHg pre and post | 1.18 (1.076–1.295) | 0.0005 | 30-day | SBP Variability | |||
| SBP, 95–135 mmHg intraoperative 105–145 mmHg pre and post | 1.153 (1.074–1.238) | <0.01 | 30-day | SBP Variability | |||
| SBP, 105–135 mmHg intraoperative 115–145 mmHg pre and post | 1.105 (1.053–1.160) | <0.01 | 30-day | SBP Variability | |||
| Ono et al.# [51] | 2014 | 450 | AUC MAP<LLA (mmHg x min/h) | 1.36 (1.08–1.71) | 0.008 | Morbidity or mortality | MAP below limit of autoregulation |
| Mascha et al. [42] | 2015 | 104,401 | Average real variability-MAP 25th percentile | 1.14 (1.03–1.25) | 0.01 | 30-day | MAP Variability |
| Average real variability-MAP median | 1.0 (reference) | 30-day | MAP Variability | ||||
| Average real variability-MAP 75th percentile | 0.94 (0.88–0.99) | 0.018 | 30-day | MAP Variability | |||
| 10-min sustained minimum MAP (mmHg) minimum MAP <70 | 0.76 (0.72–0.80) | <0.01 | 30-day | MAP Variability | |||
| 10-min sustained minimum MAP (mmHg) minimum MAP ≥70 | 1.02 (0.95–1.10) | 0.59 | 30-day | MAP Variability | |||
| Cumulative min of MAP <50 mmHg (10 min) | 1.23 (1.15–1.30) | <0.001 | 30-day | MAP Variability | |||
| Cumulative min of MAP <55 mmHg (10 min) | 1.13 (1.09–1.17) | <0.001 | 30-day | MAP Variability | |||
| Cumulative min of MAP <60 mmHg (10 min) | 1.09 (1.07–1.11) | <0.001 | 30-day | MAP Variability | |||
| Cumulative min of MAP <70 mmHg (10 min) | 1.04 (1.03–1.05) | <0.001 | 30-day | MAP Variability | |||
| Cumulative min of MAP <80 mmHg (10 min) | 1.02 (1.01–1.03) | <0.001 | 30-day | MAP Variability | |||
| Wiorek and Krzych. [71] | 2019 | 835 | SBP variability per 1% | 1.10 (1.00–1.21) | 0.05 | 30-day | SBP Variability |
| Mean BP variability per 1% | 1.10 (0.99–1.23) | 0.06 | 30-day | SBP Variability | |||
| DBP variability per 1% | 1.10 (1.01–1.21) | 0.03 | 30-day | SBP Variability | |||
| Wu et al. [72] | 2017 | 678 | MAP 65 to 79 mmHg | 3% Incidence | 0.671 | 28-day mortality incidence | Control of MAP |
| MAP 80 to 95 mmHg | 2.9% Incidence | Control of MAP | |||||
| MAP 96 to 110 mmHg | 3.8% Incidence | Control of MAP |
Abbreviations: AUC MAP<LLA: area under the curve of mean arterial pressure less than the lower limit of autoregulation during the cardiopulmonary bypass, BP: blood pressure, CI: confidence interval, DBP: diastolic blood pressure, MAP: mean arterial pressure, min: minutes, OR: odds ratio, SBP: systolic blood pressure.
*Generally, OR (95% CI), unless otherwise stated.
# These articles refer to cardiac surgery.
Renal outcomes
Sixteen articles investigated the influence of perioperative HPT and HTN in renal outcomes such as AKI or renal failure.
Hypotension as a risk factor
Thirteen articles reported an association between perioperative HPT and increased risk of developing AKI or other adverse renal outcomes (Table 5). There were two works investigating these outcomes in cardiac surgery and it was seen that an area under the curve (AUC) below the optimal BP as well as MAP below 65 mmHg were associated with increased odds of developing AKI and requiring de novo renal replacement therapy, respectively. For de novo renal replacement therapy higher odds were reported with lower values of MAP [27, 49]. In non-cardiac surgeries an increase of 1.51 odds was associated with a 10% decrease in MAP [46], while other authors demonstrated a cumulative effect of HPT over time, for example Salmasi et al. [58], who found increased OR for longer times in the same MAP ranges. The same results were observed by Sun et al. [62] for patients with MAP <55 mmHg (11–20 minutes, OR 2.34; >20 minutes, OR 3.53). Tang et al. [63] reported OR of 3.25 and 14.11 for patients with MAP <55 mmHg for 11–20 minutes and >20 minutes, respectively. Walsh et al. [67] described higher OR with the longest time exposed to MAP <55 mmHg. Mathis et al. found statistically significant increased odds for developing AKI when stratifying patients by preoperative risk; odds were increased for high preoperative risk patients with absolute MAP 60–64 mmHg, 55–59 mmHg, 50–54 mmHg, and <50 mmHg, as well as for patients with the highest preoperative risk in the ranges of MAP 55–59 mmHg, 50–54 mmHg, and <50 mmHg; and medium risk patient with MAP below 50 mmHg. These authors reported statistically significant increased odds for low, medium, and high preoperative risk patients when there was a relative decrease in MAP higher than 40% of their baseline MAP [43]. The absolute maximum decrease in intraoperative MAP below 75 mmHg had a statistically significant influence on the risk of needing continuous renal replacement therapy or dialysis, with the risk increasing with each 5 mmHg decrease as reported by Gregory et al. [23].
Table 5. Acute kidney injury and other renal outcomes associated with perioperative hypotension.
| Author | Year | Sample size | Definition of hypotension | Results* | P-value | Injury |
|---|---|---|---|---|---|---|
| Hori et al.# [27] | 2016a | 110 | AUC <optimal BP (MAP with the lowest CFx) | 1.03 (1.01–1.05) | 0.017 | AKI |
| Ngu et al.# [49] | 2020 | 6,523 | MAP (mmHg) per 10 min post cardiopulmonary bypass, <55 mmHg | 1.13 (1.05–1.23) | 0.002 | De novo renal replacement therapy |
| MAP (mmHg) per 10 min post cardiopulmonary bypass, 55–64 mmHg | 1.12 (1.06–1.18) | 0.0001 | De novo renal replacement therapy | |||
| MAP (mmHg) per 10 min post cardiopulmonary bypass, <65 mmHg | 1.01 (0.96–1.07) | 0.651 | De novo renal replacement therapy | |||
| Ahuja et al. [13] | 2020 | 164,514 | AUC under SBP <90 mmHg, Q1 1–21 mmHg x min | 0.99 (0.80–1.24) | 0.991 | AKI |
| AUC under SBP <90 mmHg, Q2 22–66 mmHg x min | 1.14 (0.92–1.41) | 0.15 | AKI | |||
| AUC under SBP <90 mmHg, Q3 67–166 mmHg x min | 1.09 (0.87–1.35) | 0.345 | AKI | |||
| AUC under SBP <90 mmHg, Q4 >166 mmHg x min | 1.41 (1.14–1.75) | >0.001 | AKI | |||
| AUC under MAP <65 mmHg, Q1 1–25 mmHg x min | 1.07 (0.84–1.36) | 0.474 | AKI | |||
| AUC under MAP <65 mmHg, Q2 26–78 mmHg x min | 1.19 (0.94–1.50) | 0.069 | AKI | |||
| AUC under MAP <65 mmHg, Q3 79–198 mmHg x min | 1.06 (0.83–1.35) | 0.562 | AKI | |||
| AUC under MAP <65 mmHg, Q4 >198 mmHg x min | 1.43 (1.12–1.82) | <0.001 | AKI | |||
| AUC under DBP <50 mmHg, Q1 1–28 mmHg x min | 1.07 (0.84–1.36) | 0.477 | AKI | |||
| AUC under DBP <50 mmHg, Q2 29–99 mmHg x min | 1.07 (0.84–1.36) | 0.51 | AKI | |||
| AUC under DBP <50 mmHg, Q3 100–289 mmHg x min | 1.07 (0.83–1.35) | 0.562 | AKI | |||
| AUC under DBP <50 mmHg, Q4 >289 mmHg x min | 1.14 (0.89–1.47) | 0.191 | AKI | |||
| Gregory et al. [23] | 2020 | 368,222 | Absolute maximum decrease (for each 5 mmHg under IOH MAP thresholds) 75 mmHg | 1.10 (1.07–1.14) | Continuous renal replacement therapy/Dialysis | |
| Absolute maximum decrease (for each 5 mmHg under IOH MAP thresholds) 65 mmHg | 1.12 (1.07–1.17) | Continuous renal replacement therapy/Dialysis | ||||
| Absolute maximum decrease (for each 5 mmHg under IOH MAP thresholds) 55 mmHg | 1.12 (1.03–1.21) | Continuous renal replacement therapy/Dialysis | ||||
| Hallqvist et al. [25] | 2018 | 470 | The percentage decrease in SBP relative to baseline for more than 5 min (>40 to ≤50%) vs. ≤40% | 1.61 (0.99–2.62) | 0.054 | AKI |
| The percentage decrease in SBP relative to baseline for more than 5 min (>50%) vs. ≤40% | 2.27 (1.20–4.30) | 0.013 | AKI | |||
| The percentage decrease in SBP relative to baseline for more than 5 min (>40 to ≤50%) vs. ≤40% | 1.48 (0.90–2.44) | 0.11 | AKI | |||
| The percentage decrease in SBP relative to baseline for more than 5 min (>50%) vs. ≤40%b | 2.02 (1.05–3.89) | 0.034 | AKI | |||
| Jang et al.[30] | 2019 | 248 | SBP <80 mmHg or a mean BP <55–60 mmHg more than 5 min | 5.14 (1.54–20.35) | 0.012 | AKI |
| Liao et al. [38] | 2020 | 796 | Lowest absolute MAP <65 mmHg >10 min during surgery | 2.565 (1.271–5.177) | 0.009 | AKI |
| Mathis et al. [43] | 2020 | 138,021 | Absolute MAP 60–64 mmHg, low preoperative risk | 0.96 (0.73–1.27) | AKI | |
| Absolute MAP 60–64 mmHg, medium preoperative risk | 1.12 (0.93–1.36) | AKI | ||||
| Absolute MAP 60–64 mmHg, high preoperative risk | 1.18 (1.03–1.36) | <0.05 | AKI | |||
| Absolute MAP 60–64 mmHg, highest preoperative risk | 1.10 (0.99–1.22) | AKI | ||||
| Absolute MAP 55–59 mmHg, low preoperative risk | 0.90 (0.64–1.27) | AKI | ||||
| Absolute MAP 55–59 mmHg, medium preoperative risk | 1.18 (0.94–1.47) | AKI | ||||
| Absolute MAP 55–59 mmHg, high preoperative risk | 1.22 (1.04–1.43) | <0.05 | AKI | |||
| Absolute MAP 55–59 mmHg, highest preoperative risk | 1.39 (1.25–1.54) | <0.05 | AKI | |||
| Absolute MAP 50–54 mmHg, low preoperative risk | 1.44 (0.92–2.25) | AKI | ||||
| Absolute MAP 50–54 mmHg, medium preoperative risk | 1.23 (0.88–1.74) | AKI | ||||
| Absolute MAP 50–54 mmHg, high preoperative risk | 1.38 (1.08–1.76) | <0.05 | AKI | |||
| Absolute MAP 50–54 mmHg, highest preoperative risk | 1.57 (1.36–1.83) | <0.05 | AKI | |||
| Absolute MAP <50 mmHg, low preoperative risk | 1.32 (0.69–2.51) | AKI | ||||
| Absolute MAP <50 mmHg, medium preoperative risk | 1.77 (1.20–2.61) | <0.05 | AKI | |||
| Absolute MAP <50 mmHg, high preoperative risk | 1.64 (1.25–2.14) | <0.05 | AKI | |||
| Absolute MAP <50 mmHg, highest preoperative risk | 2.12 (1.79–2.50) | <0.05 | AKI | |||
| MAP 20–30% below baseline, low preoperative risk | 1.05 (0.79–1.39) | AKI | ||||
| MAP 20–30% below baseline, medium preoperative risk | 0.91 (0.74–1.11) | AKI | ||||
| MAP 20–30% below baseline, high preoperative risk | 0.93 (0.80–1.08) | AKI | ||||
| MAP 20–30% below baseline, highest preoperative risk | 0.86 (0.78–0.95) | AKI | ||||
| MAP 30–40% below baseline, low preoperative risk | 1.03 (0.77–1.38) | AKI | ||||
| MAP 30–40% below baseline, medium preoperative risk | 0.99 (0.81–1.21) | AKI | ||||
| MAP 30–40% below baseline, high preoperative risk | 1.03 (0.89–1.20) | AKI | ||||
| MAP 30–40% below baseline, highest preoperative risk | 0.91 (0.82–1.00) | AKI | ||||
| MAP >40% below baseline, low preoperative risk | 1.48 (1.09–2.00) | <0.05 | AKI | |||
| MAP >40% below baseline, medium preoperative risk | 1.40 (1.14–1.73) | <0.05 | AKI | |||
| MAP >40% below baseline, high preoperative risk | 1.24 (1.06–1.45) | <0.05 | AKI | |||
| MAP >40% below baseline, highest preoperative risk | 1.09 (0.98–1.22) | AKI | ||||
| Mizota et al. [46] | 2017 | 231 | Nadir MAP (per 10 mmHg decrease) | 2.11 (1.32–3.47) | 0.002 | AKI |
| Relative decrease in MAP (per 10% decrease) | 1.51 (1.11–2.09) | 0.01 | AKI | |||
| MAP 40–49 mmHg 1–9 min | 1.64 (0.49–5.43) | 0.419 | AKI | |||
| MAP 40–49 mmHg >10 min | 2.11 (0.61–7.22) | 0.236 | AKI | |||
| MAP <40 mmHg 1–9 min | 3.80 (1.17–12.30) | 0.026 | AKI | |||
| MAP <40 mmHg for >10 min | 5.06 (1.26–20.40) | 0.022 | AKI | |||
| Salmasi et al. [58] | 2017 | 57,315 | Time under MAP <65 mmHg 13–28 min | 1.20 (1.02–1.40) | 0.0049 | AKI |
| Time under MAP <65 mmHg >28 min | 1.35 (1.14–1.58) | <0.001 | AKI | |||
| Time in the lowest MAP categories, 50–55 mmHg 1 min | 1.25 (1.02–1.54) | 0.0061 | AKI | |||
| Time in the lowest MAP categories, 50–55 mmHg 2–4 min | 1.29 (1.04–1.61) | 0.0029 | AKI | |||
| Time in the lowest MAP categories, 50–55 mmHg >4 min | 1.43 (1.06–1.92) | 0.0031 | AKI | |||
| Time in the lowest MAP categories, <50 mmHg 2–4 min | 1.23 (1.00–1.50) | 0.011 | AKI | |||
| Time in the lowest MAP categories, <50 mmHg >4 min | 1.43 (1.15–1.78) | <0.0001 | AKI | |||
| Sun et al. [62] | 2015 | 5,127 | MAP <55 mmHg 1–5 min | 1.35 (0.98–1.86) | AKI | |
| MAP <55 mmHg 6–10 min | 1.45(0.94–2.22) | AKI | ||||
| MAP <55 mmHg 11–20 min | 2.34 (1.35–4.05) | AKI | ||||
| MAP <55 mmHg >20 min | 3.53 (1.51–8.25) | AKI | ||||
| MAP <60 mmHg 1–5 min | 1.10 (0.70–1.74) | AKI | ||||
| MAP<60 mmHg 6–10 min | 1.08(0.65–1.78) | AKI | ||||
| MAP <60 mmHg 11–20 min | 1.84(1.11–3.06) | AKI | ||||
| MAP <60 mmHg >20 min | 1.70(0.93–3.10) | AKI | ||||
| MAP <65 mmHg 1–5 min | 1.28 (0.57–2.87) | AKI | ||||
| MAP <65 mmHg 6–10 min | 1.56(0.69–3.50) | AKI | ||||
| MAP <65 mmHg 11–20 min | 1.57(0.70–3.53) | AKI | ||||
| MAP <65 mmHg >20 min | 2.25(0.99–5.07) | AKI | ||||
| Tang et al. [63] | 2019 | 4,952 | MAP <55 mmHg 1–5 min | 1.01 (0.51–2) | AKI | |
| MAP 55–59 mmHg 1–5 min | 0.75 (0.35–1.62) | AKI | ||||
| MAP 60–64 mmHg 1–5 min | 0.53 (0.23–1.24) | AKI | ||||
| MAP <55 mmHg 6–10 min | 0.96 (0.31–2.96) | AKI | ||||
| MAP 55–59 mmHg 6–10 min | 1.14 (0.37–3.51) | AKI | ||||
| MAP 60–64 mmHg 6–10 min | 0.23 (0.06–0.92) | <0.05 | AKI | |||
| MAP <55 mmHg 11–20 min | 3.25 (1.04–10.14) | <0.05 | AKI | |||
| MAP 55–59 mmHg 11–20 min | 2.40 (0.85–6.75) | AKI | ||||
| MAP 60–64 mmHg 11–20 min | 0.40 (0.11–1.47) | AKI | ||||
| MAP <55 mmHg >20 min | 14.11 (5.02–39.69) | <0.001 | AKI | |||
| MAP 55–59 mmHg >20 min | 7.46 (3.14–17.72) | <0.001 | AKI | |||
| MAP 60–64 mmHg >20 min | 2.78 (1.18–6.51) | <0.05 | AKI | |||
| Walsh et al. [67] | 2013 | 33,330 | MAP <55 mmHg 1–5 min | 1.18 (1.06–1.31) | AKI | |
| MAP <55 mmHg 6–10 min | 1.19 (1.03–1.39 | AKI | ||||
| MAP <55 mmHg 11–20 min | 1.32 (1.11–1.56) | AKI | ||||
| MAP <55 mmHg >20 min | 1.51 (1.24–1.84) | AKI |
Abbreviations: AUC: area under the curve, AKI: acute kidney injury, CFx: correlation flow index, CI: confidence interval, DBP: diastolic blood pressure, IOH: intraoperative hypotension, MAP: mean arterial pressure, min: minutes, OR: odds ratio, Q: quartile, SBP: systolic blood pressure.
*OR (95%CI),
# These articles refer to cardiac surgery.
Hypertension as a risk factor
Jinadasa et al. [31] carried out an investigation in 3,687 patients undergoing cardiac surgery about HTN through the coefficient of variation (CV) of both SBP and MAP in cardiac surgical outcomes. In this study, the CV of SBP was found to be a predictor of renal failure [104% increased odds; OR 2.04 (95%CI 1.33–3.14), p-value: 0.001], as well as the CV during the prebypass [OR 1.59 (95%CI 1.07–2.37), p-value: 0.02] and postbypass phases [OR 1.56 (95%CI 1.09–2.22), p-value:0.010]. No other measure was found to be associated with the prediction of renal failure.
Control of MAP
A MAP below the lower limit of autoregulation monitored during cardiopulmonary bypass as calculated by a continuous, moving Pearson’s correlation coefficient between MAP and processed near-infrared spectroscopy signals to generate the variable cerebral oximetry index, was reported to increase the relative risk of AKI in cardiac surgical patients [relative risk 1.02 (95%CI 1.01–1.03), p-value<0.0001] in a study carried out by Ono et al. that included 348 patients [50]. And the intraoperative control of MAP in the range 80–95 mmHg could reduce postoperative AKI in elderly hypertensive patients (n = 678) after major abdominal surgery according to Wu et al. being the incidence (SD) of 31% (13.5%) in patients with a MAP between 65–79 mmHg; 13% (6.3%) in patients with MAP between 80–95 mmHg, and 27% (12.9%) in patients with MAP between 96–110 mmHg, p-value = 0.033 [72].
Stroke
Six of the retrieved articles investigated the relationship between HPT, HTN, and control of MAP during surgery and stroke.
Hypotension as a risk factor
Four articles that studied the relationship between intraoperative HPT and stroke were identified (Table 6) and none of their results found a statistically significant difference in the influence of perioperative HPT on stroke. The approaches included absolute SBP below 100, 90, 80, and 70 mmHg; relative decreases of 10, 20, 30, and 40% from baseline; and MAP below 70, 65, and 60 mmHg [20, 29].
Table 6. Stroke associated with intraoperative hypotension.
| Author | Year | Sample size | Definition of hypotension | Results* | P-value |
|---|---|---|---|---|---|
| Bijker et al. [20] | 2012 | 48,241 | Absolute SBP <100 mmHg | 1.005 (0.993–1.016) | 0.205 |
| Absolute SBP <90 mmHg | 1.006 (0.991–1.022) | 0.182 | |||
| Absolute SBP <80 mmHg | 1.007 (0.981–1.034) | 0.368 | |||
| Absolute SBP <70 mmHg | 1.002 (0.952–1.051) | 0.918 | |||
| SBP percentage decrease from baseline >10% | 1.010 (0.997–1.023) | 0.01 | |||
| SBP percentage decrease from baseline >20% | 1.010 (0.999–1.022) | 0.003 | |||
| SBP percentage decrease from baseline >30% | 1.010 (0.999–1.022) | 0.003 | |||
| SBP percentage decrease from baseline >40% | 1.011 (0.996–1.025) | 0.02 | |||
| Absolute mean BP <70 mmHg | 1.003 (0.993–1.014)α | 0.296 | |||
| Absolute mean BP <60 mmHg | 1.003 (0.988–1.014)α | 0.468 | |||
| Absolute mean BP <50 mmHg | 1.004 (0.962–1.046)α | 0.755 | |||
| Absolute mean BP <40 mmHg | 1.013 (0.939–1.088)α | 0.563 | |||
| Mean BP decrease from baseline >10% | 1.004 (0.991–1.016)α | 0.365 | |||
| Mean BP decrease from baseline >20% | 1.008 (0.996–1.021)α | 0.028 | |||
| Mean BP decrease from baseline >30% | 1.013 (1.000–1.025)α | <0.001 | |||
| Mean BP decrease from baseline >40% | 1.015 (0.999–1.031)α | 0.003 | |||
| Gregory et al. [23] | 2020 | 368,222 | Absolute maximum decrease (for each 5 mmHg under IOH MAP thresholds) 75 mmHg | 1.00 (0.97–1.02) | |
| Absolute maximum decrease (for each 5 mmHg under IOH MAP thresholds) 65 mmHg | 1.00 (0.96–1.04) | ||||
| Absolute maximum decrease (for each 5 mmHg under IOH MAP thresholds) 55 mmHg | 1.02 (0.95–1.09) | ||||
| Hsieh et al. [29] | 2016 | 106,337 | MAP <70 mmHg | 0.49 (0.18–1.38) | |
| MAP <70 mmHg | 1.07 (0.76–1.53)β | ||||
| MAP <65 mmHg | 0.68 (0.27–1.71) | ||||
| MAP <65 mmHg | 1.16 (0.76–1.77)β | ||||
| MAP <60 mmHg | 0.72 (0.24–2.17) | ||||
| MAP <60 mmHg | 1.26 (0.73–2.19)β | ||||
| Li et al. [37] | 2020b | 1,497 | Average real variance-SBP (mmHg/min) | 3.18 (1.32–10.30)α | 0.002 |
| Average real variance-DBP (mmHg/min) | 4.04 (1.04–16.82)α | 0.006 | |||
| Average real variance-MAP (mmHg/min) | 4.02 (1.22–17.46)α | 0.004 | |||
| Maximum drop-DBP (mmHg) | 1.08 (1.02–1.15)α | 0.001 | |||
| Maximum drop-MAP (mmHg) | 1.06 (1.00–1.12)α | 0.006 | |||
| Minimal SBP (mmHg) | 1.03 (0.99–1.08)α | 0.048 | |||
| Time weighted average-SBP (mmHg) | 1.06 (1.02–1.11)α | <0.001 | |||
| Time weighted average-MAP (mmHg) | 1.06 (1.00–1.12)α | 0.008 | |||
| Relative SBP-intraoperative HPT duration, <60% baseline | 1.02 (1.00–1.04)α | 0.037 | |||
| Average real variance-SBP (mmHg/min) in massive infarction | 5.83 (0.77–52.91)α | 0.025 | |||
| Average real variance-DBP (mmHg/min) in massive infarction | 12.90 (0.69–259.94)α | 0.021 | |||
| Average real variance-MAP (mmHg/min) in massive infarction | 20.22 (1.08–578.16)α | 0.011 | |||
| Maximum drop-DBP (mmHg) | 1.10 (1.01–1.22)α | 0.006 | |||
| Maximum drop-MAP (mmHg) | 1.11 (1.01–1.22)α | 0.005 |
Abbreviations: BP: blood pressure, CI: confidence interval, DBP: diastolic blood pressure, HPT: hypotension, IOH: intraoperative hypotension, MAP: mean arterial pressure, min: minutes, OR: odds ratio, SBP: systolic blood pressure.
*Generally, OR (95% CI), unless otherwise stated;
α, OR adjusted (99.9% CI);
β, OR (Ratio of Geometric Mean).
Hypertension as a risk factor
Zheng et al. [76] investigated the influence of baseline and 24h after intervention SBP on the incidence of stroke in 173 patients undergoing carotid angioplasty stenting (CAS). Baseline SBP was not found to be statistically associated with stroke incidence being the incidence 0% in those patients with baseline SBP <120 mmHg, 7.90% for SBP 120–130 mmHg, and 4.10% for SBP >130 mmHg; p-value: 0.41. Post-intervention SBP increases incidence, increasing proportionally with higher levels of SBP, but no statistical significance was found; 3.50% incidence in patients with 24h post-CAS SBP <120 mmHg, 7.50% for 24h post-CAS SBP 120–130 mmHg, and 11.10% in 24h post-CAS SBP >130 mmHg; p-value = 0.174.
Control of MAP
In the study performed by Wu et al. in 678 patients, there was no statistically significant difference in stroke after gastrointestinal surgery in elderly patients whose ranges of MAP were 65–79 mmHg (0.43% incidence), 80–95 mmHg (0.48% incidence), and 96–110 mmHg (0.47%); p-value = 0.341 [72].
Delirium
There were seven articles reporting results for the association of perioperative HPT, HTN and postsurgical delirium.
Hypotension as a risk factor
Studies investigating the effect of perioperative HPT on delirium (n = 5) (Table 7) found that what might be related to the development of postsurgical delirium is BP fluctuation or variance, the so-called lability, and no absolute or relative values of HPT themselves both in cardiac and non-cardiac surgical patients [26]. In those articles studying cardiac surgeries, influence of HPT over delirium was demonstrated [28, 69]. Additionally, other authors studying non-cardiac interventions, reported increased HR for delirium in those patients with intraoperative and postoperative HPT (during intensive care unit (ICU) stay) [41] and increased OR of delirium for decreases of intraoperative MAP in ranges below 75 mmHg [23]. The study by Hirsch et al. [26] reported increased OR for the prediction of the number of days of delirium, both with MAP (OR 1.038, 95% CI 1.010–1.067, p-value = 0.008) and SBP (OR 1.018 95% CI 1.005–1.030, p-value = 0.004) variance as measured by mmHg2 per 10 units decrease. Another study found no statistically significant results for the association of HPT with postoperative delirium in elderly patients undergoing non-cardiac surgery [34].
Table 7. Delirium associated with perioperative hypotension.
| Author | Year | Sample size | Definition of hypotension | Results* | P-value |
|---|---|---|---|---|---|
| Hori et al.# [28] | 2016b | 110 | AUC <optimal MAP (mmHg x h) overall | Incidence | 0.7 |
| AUC <optimal MAP (mmHg x h) postoperative day 1 | Incidence | 0.5 | |||
| AUC <optimal MAP (mmHg x h) postoperative day 2 | Incidence | 0.61 | |||
| AUC <optimal MAP (mmHg x h) postoperative day 3 | Incidence | 0.82 | |||
| Wesselink et al.# [69] | 2015 | 743 | MAP <60 mmHg | 1.04 (0.99–1.10)α | 0.04 |
| MAP <50 mmHg | 1.22 (1.02–1.66)α | 0.04 | |||
| MAP decrease >30% relative to baseline | 1.00 (0.99–1.01)α | 0.45 | |||
| MAP decrease >40% relative to baseline | 1.00 (0.99–1.02) α | 0.55 | |||
| Gregory et al. [23] | 2020 | 368,222 | Absolute maximum decrease (for each 5 mmHg under IOH MAP thresholds) 75 mmHg | 1.04 (1.01–1.07) | |
| Absolute maximum decrease (for each 5 mmHg under IOH MAP thresholds) 65 mmHg | 1.05 (1.01–1.10) | ||||
| Absolute maximum decrease (for each 5 mmHg under IOH MAP thresholds) 55 mmHg | 1.07 (1.00–1.15) | ||||
| Langer et al. [34] | 2019 | 101 | MAP ≥90% time below of preoperative values | 0.19 | |
| Maheshwari et al. [41] | 2019 | 1,083 | Intraoperative period: TWA of MAP <65 mmHg | 1.11 (1.03–1.20) HR | 0.009 |
| ICU stay: Lowest MAP on each day (10 mmHg decrease in lowest MAP) | 1.12 (1.04–1.20) HR | 0.003 | |||
| MAP <50 mmHg | 1.14 (0.98–1.53)α | 0.09 |
Abbreviations: AUC: area under the curve, CI: confidence interval, HR: hazard ratio, ICU: intensive care unit, IOH: intraoperative hypotension; MAP: mean arterial pressure, OR: odds ratio, SBP: systolic blood pressure, TWA: time-weighted average.
*Generally, OR (95% CI), unless otherwise stated in the Outcome type column.
# These articles refer to cardiac surgery.
α, OR adjusted (99% CI).
Hypertension as a risk factor
Two different studies demonstrated the implication of intraoperative HTN for postsurgical delirium. (Table 8) Hori et al. [28] found a statistically significant association of the AUC above the optimal MAP (as measured in mmHg x h) during cardiac surgery and the development of delirium on postoperative day 2, and the other reported increased odds (2.34) for delirium when suffering an increase of 10 mmHg in mean surgery mean arterial pressure (msMAP) when msMAP was equal or above 80 mmHg during trauma surgery [68].
Table 8. Delirium associated with perioperative hypertension.
| Author | Year | Sample size | Definition of HT | Results* | P-value | Time |
|---|---|---|---|---|---|---|
| Hori et al.# [28] | 2016b | 110 | AUC >optimal MAP (mmHg x h) overall | Incidence | 0.4 | Overall |
| AUC >optimal MAP (mmHg x h) postoperative day 1 | Incidence | 0.12 | POD 1 | |||
| AUC >optimal MAP (mmHg x h) postoperative day 2 | Incidence | 0.031 | POD 2 | |||
| AUC >optimal MAP (mmHg x h) postoperative day 3 | Incidence | 0.59 | POD 3 | |||
| Wang et al. [68] | 2015 | 103 | Per 10 mmHg increase of msMAP if msMAP was <80 mmHg | 0.21 (0.05–0.86) | 0.03 | |
| Per 10 mmHg increase of msMAP if msMAP was ≥80 mmHg | 2.34 (1.11–4.94) | 0.03 | ||||
| MAP value at baseline | 1.44 (0.92–2.25) | 0.11 |
Abbreviations: AUC: area under the curve, CI: confidence interval, HT: hypertension, ICU: intensive care unit, msMAP: average MAP during surgery, MAP: mean arterial pressure, OR: odds ratio, POD: postoperative day, SBP: systolic blood pressure.
*Generally, OR (95% CI), unless otherwise stated,
# This article refers to cardiac surgery.
Cardiovascular and cerebrovascular outcomes
There were 18 articles reporting results for the association of HPT and HTN with cardiovascular and cerebrovascular adverse outcomes such as AMI, myocardial injury after non-cardiac surgery (MINS), and others.
Hypotension as a risk factor
HPT association with cardiovascular and cerebrovascular outcomes was investigated in 15 articles (Table 9). One of them reported results from patients undergoing cardiac surgery. The work by Sposato et al. [61] described the new onset of atrial fibrillation associated with intraoperative SBP below 80 mmHg for 15 minutes or more with increased odds of 9.6 in cardiac surgery. The rest of the articles referred to non-cardiac surgery; some of them studied HPT in relation to various cardiac outcomes and found a statistically significant association between SBP <100 mmHg and myocardial injury, increasing with an OR of 1.21 [12], this relation of HPT was also seen when the decrease in SBP is more than 50% relative to baseline values for more than 5 minutes, with OR of 4.4 for myocardial damage [24]. Other studies such as that of Roshanov et al. [56] reported that not just the intraoperative HPT is relevant for the development of cardiovascular events (SBP <90 mmHg for more than 10 minutes, OR 2.43) but also postoperative HPT (SBP <90 mmHg for more than 10 minutes, OR 2.17); there was also evidence showing that in addition to the postoperative MAP, the time under different thresholds also has an influence on the development of AMI [39]. Additionally it has been published that time under MAP also has an influence on myocardial outcomes, as demonstrated by Salmasi et al. [58], odds for MINS (AMI after non-cardiac surgery) MAP <65 mmHg during 13–28 minutes were 1.34, while odds for MAP <65 mmHg during more than 28 minutes were 1.60; influence was also seen for MAP <50 mmHg, in this category just one minutes below 50 mmHg was found to be statistically significant associated with the development of MINS. As well as Salmasi et al., van Lier et al. [65] saw that there was a statistically significant relationship between the levels of HPT and the incidence of AMI, with the relationship being inversely proportional. Walsh et al. [67] also found that a longer time below <55 mmHg had an impact on the greater odds for both myocardial injury and cardiac complication. Gregory et al. reported statistically significant increased odds for developing MACCE (composite measure of all-cause mortality, AMI, or acute ischemic stroke) by any measure of MAP under any threshold considered, they also found increased odds for AMI [23].
Table 9. Cardiovascular and cerebrovascular outcomes associated with perioperative hypotension.
| Author | Year | Sample size | Definition of hypotension | Result* | P-value | Event |
|---|---|---|---|---|---|---|
| Sposato et al.# [61] | 2011 | 186 | Intraoperative SBP <80 mmHg ≥15 min | 9.6 (1.9–47.4) | 0.006 | New onset atrial fibrillation |
| Abbott et al. [12] | 2018 | 16,079 | SBP <100 mmHg | 1.21 (1.05–1.39) | 0.01 | Myocardial injury |
| SBP <100 mmHg | 1.21 (0.98–1.49) | 0.07 | AMI | |||
| Ahuja et al. [13] | 2020 | 164,514 | AUC under SBP <90 mmHg, Q1 1–21 mmHg x min | 0.85 (0.65–1.10)γ | 0.107 | MINS |
| AUC under SBP <90 mmHg, Q2 22–66 mmHg x min | 1.00 (0.78–1.29) γ | 0.974 | MINS | |||
| AUC under SBP <90 mmHg, Q3 67–166 mmHg x min | 1.16 (0.91–1.49) γ | 0.122 | MINS | |||
| AUC under SBP <90 mmHg, Q4 >166 mmHg x min | 1.65 (1.30–2.09) γ | >0.001 | MINS | |||
| AUC under MAP <65 mmHg, Q1 1–25 mmHg x min | 1.03 (0.78–1.38) γ | 0.766 | MINS | |||
| AUC under MAP <65 mmHg, Q2 26–78 mmHg x min | 1.09 (0.82–1.46) γ | 0.438 | MINS | |||
| AUC under MAP <65 mmHg, Q3 79–198 mmHg x min | 1.37 (1.04–1.81) γ | 0.004 | MINS | |||
| AUC under MAP <65 mmHg, Q4 >198 mmHg x min | 1.51 (1.14–2.00) γ | <0.001 | MINS | |||
| AUC under DBP <50 mmHg, Q1 1–28 mmHg x min | 1.01 (0.75–1.35) γ | 0.956 | MINS | |||
| AUC under DBP <50 mmHg, Q2 29–99 mmHg x min | 1.15 (0.86–1.54) γ | 0.223 | MINS | |||
| AUC under DBP <50 mmHg, Q3 100–289 mmHg x min | 1.20 (0.90–1.60) γ | 0.117 | MINS | |||
| AUC under DBP <50 mmHg, Q4> 289 mmHg x min | 1.23 (0.91–1.65) | 0.081 | MINS | |||
| Gregory et al. [23] | 2020 | 368,222 | Absolute maximum decrease (for each 5 mmHg under IOH MAP thresholds) 75 mmHg | 1.12 (1.11–1.14) | <0.001 | MACCE |
| Absolute maximum decrease (for each 5 mmHg under IOH MAP thresholds) 65 mmHg | 1.17 (1.15–1.19) | <0.001 | MACCE | |||
| Absolute maximum decrease (for each 5 mmHg under IOH MAP thresholds) 55 mmHg | 1.26 (1.22–1.29) | <0.001 | MACCE | |||
| TWA-MAP (75 mmHg) | 1.03 (1.03–1.04) | <0.001 | MACCE | |||
| TWA-MAP (65 mmHg) | 1.07 (1.06–1.08) | <0.001 | MACCE | |||
| TWA-MAP (55 mmHg) | 1.14 (1.12–1.16) | <0.001 | MACCE | |||
| Absolute cumulative time (75 mmHg) | 1.02 (1.02–1.03) | <0.001 | MACCE | |||
| Absolute cumulative time (65 mmHg) | 1.03 (1.03–1.04) | <0.001 | MACCE | |||
| Absolute cumulative time (55 mmHg) | 1.05 (1.04–1.06) | <0.001 | MACCE | |||
| Absolute maximum decrease (for each 5 mmHg under IOH MAP thresholds) 75 mmHg | 1.07 (1.04–1.10) | AMI | ||||
| Absolute maximum decrease (for each 5 mmHg under IOH MAP thresholds) 65 mmHg | 1.10 (1.05–1.14) | AMI | ||||
| Absolute maximum decrease (for each 5 mmHg under IOH MAP thresholds) 55 mmHg | 1.12 (1.05–1.21) | AMI | ||||
| Hallqvist et al. [24] | 2016 | 300 | Decrease in SBP more than 50% relative to baseline for more than 5 min | 4.4 (1.8–11.1) | Myocardial damage | |
| Kass et al. [33] | 2018 | 445 | Baseline MAP, per 10 mmHg increase | 0.96 (0.75–1.257) | 0.782 | Flap loss |
| MAP <60 mmHg for >20 episodes, per episode increase | 1.22 (1.11–1.35) | <0.01 | Flap loss | |||
| Liem et al. [39] | 2020 | 1,710 | Postoperative MAP <60 mmHg 0-1h | 1.54 (1.05–2.24) | 0.027 | Myocardial injury |
| Postoperative MAP <60 mmHg 1-2h | 2.62 (1.39–4.80) | 0.002 | Myocardial injury | |||
| Postoperative MAP <60 mmHg 2-4h | 3.26 (1.57–6.48) | 0.001 | Myocardial injury | |||
| Postoperative MAP <65 mmHg >4h | 2.98 (1.78–4.98) | <0.001 | Myocardial injury | |||
| Postoperative MAP <70 mmHg >4h | 2.18 (1.37–3.51) | 0.001 | Myocardial injury | |||
| Postoperative MAP <75 mmHg >4h | 2.03 (1.19–3.60) | 0.012 | Myocardial injury | |||
| Liu et al. [40] | 2020 | 547 | Intraoperative HPT (SBP <90 mmHg or 70% baseline or DBP <60 mmHg lasting at least 10 min) | 25.50% Incidence | 0.003 | Perioperative cardiac complications |
| Roshanov et al. [56] | 2019 | 955 | Any SBP <90 mmHg more than 10 min | 3.17 (1.99–5.06) HR | <0.01 | Cardiovascular events |
| Intraoperative SBP <90 mmHg more than 10 min | 2.43 (1.50–3.95) HR | <0.01 | Cardiovascular events | |||
| Postoperative SBP <90 mmHg more than 10 min | 2.17 (1.35–3.49) HR | 0.001 | Cardiovascular events | |||
| Rots et al. [57] | 2020 | 55 | SBP intraoperative—SBP preoperative (mean differences) | 0.024 | Silent brain ischaemia | |
| Salmasi et al. [58] | 2017 | 57,315 | Time under MAP <65 mmHg 13–28 min | 1.34 (1.06–1.60) | 0.0015 | MINS |
| Time under MAP <65 mmHg >28 min | 1.60 (1.28–2.01) | <0.001 | MINS | |||
| Time under MAP <50 mmHg 1 min | 1.49 (1.13–1.96) | <0.001 | MINS | |||
| Time under MAP <50 mmHg 2–4 min | 1.63 (1.26–2.10) | <0.001 | MINS | |||
| Time MAP <50 mmHg >4 min | 1.97 (1.50–2.59) | <0.001 | MINS | |||
| Sessler et al. [59] | 2018 | 9,765 | Intraoperative 10-min increase in HPT | 1.03 (0.97–1.10) | 0.162 | AMI |
| Remaining day of surgery 10min increase in HPT | 1.03 (1.00–1.05) | 0.002 | AMI | |||
| van Lier et al. [65] | 2018 | 2,211 | MAP 31.0–67.0 mmHg | 4.80% Incidence | p = 0.005; OR 0.991 (0.983–0.999) | AMI |
| MAP 67.3–76.3 mmHg | 2.90% Incidence | AMI | ||||
| MAP 76.7–86.0 mmHg | 1.40% Incidence | AMI | ||||
| MAP 86.7–122.3 mmHg | Incidence | AMI | ||||
| van Waes et al. [66] | 2016 | 890 | MAP <50 mmHg 2–5 min | 1.3 (0.8–2.2)δ | 0.21 | Myocardial injury |
| MAP <50 mmHg 6–10 min | 2.0 (1.1–3.6)δ | 0.003 | Myocardial injury | |||
| MAP <50 mmHg 11–20 min | 1.0 (0.4–2.2)δ | 0.89 | Myocardial injury | |||
| MAP <50 mmHg 21–30 min | 2.0 (0.8–5.1)δ | 0.08 | Myocardial injury | |||
| MAP <50 mmHg >30 min | 1.5 (0.4–6.7)δ | 0.47 | Myocardial injury | |||
| MAP <60 mmHg 2–5 min | 1.1 (0.7–1.7)δ | 0.52 | Myocardial injury | |||
| MAP <60 mmHg 6–10 min | 0.9 (0.5–1.6)δ | 0.58 | Myocardial injury | |||
| MAP <60 mmHg 11–20 min | 1.5 (1.0–2.3)δ | 0.02 | Myocardial injury | |||
| MAP <60 mmHg 21–30 min | 1.5 (1.0–2.5)δ | 0.02 | Myocardial injury | |||
| MAP <60 mmHg >30 min | 1.7 (1.1–2.6)δ | 0.004 | Myocardial injury | |||
| Walsh et al. [67] | 2013 | 33,330 | MAP <55 mmHg 1–5 min | 1.30 (1.06–1.58) | Myocardial injury | |
| MAP <55 mmHg 6–10 min | 1.47 (1.13–1.93) | Myocardial injury | ||||
| MAP <55 mmHg 11–20 min | 1.79 (1.33–2.39) | Myocardial injury | ||||
| MAP <55 mmHg > 20 min | 1.82 (1.31–2.55) | Myocardial injury | ||||
| MAP <55 mmHg 1–5 min | 1.35 (1.15–1.58) | Cardiac complication | ||||
| MAP <55 mmHg 6–10 min | 1.46 (1.17–1.83) | Cardiac complication | ||||
| MAP <55 mmHg 11–20 min | 1.50 (1.16–1.94) | Cardiac complication | ||||
| MAP <55 mmHg >20 min | 1.95 (1.46–2.60) | Cardiac complication |
Abbreviations: AMI: acute myocardial infarction, DBP: diastolic blood pressure, HPT: hypotension, HR: hazard ratio, IOH: intraoperative hypotension, MACCE: composite measure of all-cause mortality, AMI, or acute ischemic stroke, MAP: mean arterial pressure, min: minutes, MINS: myocardial infarction after non-cardiac surgery, OR: odds ratio, SBP: systolic blood pressure, TWA: time-weighted average.
*Generally, OR (95% CI), unless otherwise stated in the Outcome type column.
# This article refers to cardiac surgery.
γ, OR adjusted (98.75% CI);
δ, RR (98.8% CI).
Hypertension as a risk factor
Studies investigating the relationship between HTN and cardiac or cerebrovascular outcomes in non-cardiac surgery were also retrieved from our search (n = 5) (Table 10). Abbott et al. [12] described a statistically significant association between SBP above 160 mmHg and myocardial injury and AMI, which had been found to not be associated in a previous study [11]. Outcomes such as silent brain ischemia were found to be statistically significant related to a 20% increase of baseline SBP during carotid endarterectomy [57]. Further evidence regarding the influence of postoperative HTN has been described by Zheng et al. [76], who reported increased incidence of intracranial hemorrhage and unfavorable discharge rising with higher ranges of postoperative SBP; these authors report increased OR of intracranial hemorrhage when SBP postsurgery is above 130 mmHg [OR 37.67 (6.79–209.01), p<0.0001].
Table 10. Cardiac and cerebrovascular outcomes associated with perioperative hypertension.
| Author | Year | Sample size | Definition of hypertension | Result* | P-value | Event |
|---|---|---|---|---|---|---|
| Abbott et al. [11] | 2017 | 15,057 | SBP <120 mmHg | 1.07 (0.91–1.24) | 0.43 | Myocardial injury |
| SBP 120–131 mmHg | 1.19 (0.97–1.44) | 0.09 | Myocardial injury | |||
| SBP 132–143 mmHg | 0.95 (0.83–1.10) | 0.52 | Myocardial injury | |||
| SBP 144–159 mmHg | 0.93 (0.80–1.07) | 0.29 | Myocardial injury | |||
| SBP ≥160 mmHg | 0.90 (0.75–1.07) | 0.24 | Myocardial injury | |||
| Abbott et al. [12] | 2018 | 16,079 | SBP >160 mmHg | 1.16 (1.01–1.34) | 0.04 | Myocardial injury |
| SBP >160 mmHg | 1.34 (1.09–1.64) | 0.01 | AMI | |||
| Pérez et al. [52] | 2020 | 282 | Post craniotomy severe HTN (SBP ≥170 mmHg) | 0% Incidence | Intracerebral haemorrhage | |
| Post craniotomy severe HTN (SBP ≥170 mmHg) | 2.70% Incidence | Ischemic stroke | ||||
| Rots et al. [57] | 2020 | 55 | SBP intraoperative increase ≥20% of SBP preoperative (duration of relative HTN) | 0.048 | Silent brain ischaemia | |
| Zheng et al. [76] | 2020 | 173 | Baseline SBP <120 mmHg | 4.20% Incidence | 0.803 | Intracranial haemorrhage |
| Baseline SBP 120–130 mmHg | 7.90% Incidence | Intracranial haemorrhage | ||||
| Baseline SBP >130 mmHg | 9.60% Incidence | Intracranial haemorrhage | ||||
| 24h post-CAS SBP <120 mmHg | 2.60% Incidence | <0.0001 | Intracranial haemorrhage | |||
| 24h post-CAS SBP 120–130 mmHg | 5.00% Incidence | Intracranial haemorrhage | ||||
| 24h post-CAS SBP >130 mmHg | 50% Incidence | Intracranial haemorrhage | ||||
| 24h post-CAS SBP >130 mmHg | 37.67 (6.79–209.01) | <0.0001 | Intracranial haemorrhage |
Abbreviations: AMI: acute myocardial infarction, CAS: carotid angioplasty stenting, HTN: hypertension, SBP: systolic blood pressure.
*OR (95% CI).
Other outcomes
In total, 16 articles were retrieved reporting other types of outcomes associated with perioperative HPT or HTN.
Hypotension as a risk factor
Nine of them investigated perioperative HPT and other relevant outcomes such as myocardial injury or surgical site infection (Table 11). Other studies such as that of Roshanov et al. [56] reported hypotensive events in postoperative days 0 and 1 was also found to be associated with increased length of hospital stay (OR 1.305, p = 0.0239) [14, 21]; and sepsis/systemic inflammatory response syndrome [23]. Other outcomes that were investigated were: surgical site infection, that was found to be not statistically significant in relation to MAP <55 mmHg and SBP <80 mmHg, but odds of 1.08 were found in association with minimum postoperative MAP, per 5 mmHg decrease by Yilmaz et al. [73]; or flap loss that was associated with MAP <60 mmHg for more than 20 episodes and per each episode the odds were 1.22 [33]. Increased risk of poor discharge outcomes (defined as death, discharge to a nursing facility, or discharge to hospice care) were reported by Sheffy et al. [60] for those patients with perioperative SBP <90 mmHg or <110 mmHg undergoing trauma surgery. Tassoudis et al. [64] described increased odds for hospital stay, complications, and duration of complications related to intraoperative MAP below 60 mmHg or MAP below 70 mmHg and MAP decrease above 30% from baseline values. Emergence agitation was associated with SBP <90 mmHg intraoperatively (OR 1.636, p = 0.025) in patients undergoing thoracoscopic lung surgery [32].
Table 11. Diverse outcomes associated with perioperative hypotension.
| Author | Year | Sample size | Definition of hypotension | Result* | P-value | Event |
|---|---|---|---|---|---|---|
| Anastasio et al. [14] | 2020 | 1,033 | Hypotensive events (SBP <90 mmHg or DBP <60 mmHg for any single reading) on POD 0/1 | 1.305 (1.036–1.645) | 0.0239 | LoS >3 days |
| Babazade et al. [17] | 2016 | 2,521 | MAP <55 mmHg | 0.97 (0.81–1.17) | 0.71 | Surgical site infection |
| SBP <80 mmHg | 0.96 (0.84–1.11) | 0.54 | Surgical site infection | |||
| Cumulative min of MAP <55 mmHg | 0.97 (0.91–1.04) HR | 0.36 | Time to discharge alive | |||
| Cumulative min of SBP <80 mmHg | 0.97 (0.93–1.01) HR | 0.13 | Time to discharge alive | |||
| Beecham et al. [19] | 2020 | 52 | 1-min increase cumulative time of SAP <80% pre-induction | 1.020 (1.008–1.035) | 0.003 | Clavien-Dindo (progression to a higher level) |
| Bonnet et al. [21] | 2020 | 50 | SBP <100 mmHg (duration) | 0.38 | Comprehensive Complications Index | |
| MAP <75 mmHg (duration) | 0.03 | Comprehensive Complications Index | ||||
| MAP <55 mmHg (duration) | 0.11 | Comprehensive Complications Index | ||||
| SBP <100 mmHg (duration) | 0.35 | LoS | ||||
| MAP <75 mmHg (duration) | 0.03 | LoS | ||||
| MAP <55 mmHg (duration) | 0.53 | LoS | ||||
| Gregory et al. [23] | 2020 | 368,222 | Absolute maximum decrease (for each 5 mmHg under IOH MAP thresholds) 75 mmHg | 1.15 (1.12–1.18) | Sepsis/Systemic inflammatory response syndrome | |
| Absolute maximum decrease (for each 5 mmHg under IOH MAP thresholds) 65 mmHg | 1.21 (1.16–1.26) | Sepsis/Systemic inflammatory response syndrome | ||||
| Absolute maximum decrease (for each 5 mmHg under IOH MAP thresholds) 55 mmHg | 1.28 (1.20–1.37) | Sepsis/Systemic inflammatory response syndrome | ||||
| Kang et al. [32] | 2020 | 1,950 | Intraoperative hypotension (SBP < 90 mmHg assessed more than 5 min apart) | 1.636 (1.064–2.514) | 0.025 | Emergence agitation |
| Sheffy et al. [60] | 2017 | 1,744 | Perioperative SBP <90 mmHg | 1.55 (1.04–2.31) RR | <0.05 | Poor discharge outcome |
| Perioperative SBP <110 mmHg | 1.87 (1.17–2.98) RR | <0.01 | Poor discharge outcome | |||
| Tassoudis et al. [64] | 2011 | 100 | Intraoperative MAP <60 mmHg or MAP <70 mmHg and MAP decrease >30% compared to baseline value | 4.56 (1.85–10.96) | 0.001 | LoS |
| Intraoperative MAP <60 mmHg or MAP <70 mmHg and MAP decrease >30% compared to baseline value | 5.10 (1.95–13.35) | 0.001 | Complications | |||
| Intraoperative MAP <60 mmHg or MAP <70 mmHg and MAP decrease >30% compared to baseline value | 4.54 (1.88–10.92) | 0.001 | Duration of complications | |||
| Yilmaz et al. [73] | 2018 | 5,896 | Postoperative TWA-MAP Quintile (mmHg) [OR for a 5-mmHg decrease in TWA-MAP or minimum MAP] | 1.03 (0.99–1.08) | 0.16 | Surgical site infection |
| Minimum postoperative MAP (per 5 mmHg decrease) | 1.08 (1.03–1.12) | 0.001 | Surgical site infection |
Abbreviations: DBP: diastolic blood pressure, HR: hazard ratio, IOH: intraoperative hypotension, LoS: length of hospital stay, MAP: mean arterial pressure, min: minutes, POD: postoperative day, RR: risk ratio, SAP: systolic arterial pressure, SBP: systolic blood pressure, TWA: time-weighted average.
*Generally, OR (95%CI), unless otherwise stated in the Outcome type column.
Hypertension as a risk factor
There were eight studies investigating the relationship between HTN and various outcomes that were retrieved from our search (Table 12). Other outcomes have been associated with HTN as well: hematoma [48], emergence agitation [32], and anastomotic leakage [53]. Postoperative HTN was reported to have a negative impact on functional independence of patients undergoing mechanical thrombectomy, especially for those patients with maximum SBP in postoperative day (POD) 3 [44]. There were two articles on cardiac surgical patients and one of the studies found statistically significant increased odds for the length of hospital stay due to HTN [18].
Table 12. Diverse outcomes associated with perioperative hypertension.
| Author | Year | Sample size | Definition of hypertension | Result* | P-value | Event |
|---|---|---|---|---|---|---|
| Balzer et al.# [18] | 2016 | 5,225 | SBP >130 mmHg | 0.119 | LoS in ICU | |
| SBP >130 mmHg | 0.024 | LoS in hospital | ||||
| SBP >130 mmHg | 0.842 | Time of ventilation | ||||
| SBP >130 mmHg | 0.666 | Drainage output 24h after surgery | ||||
| McIlroy et al.# [45] | 2019 | 793 | SBP >145 mmHg | 0.58 (0.08–4.42) | 0.6 | Unplanned return to operating room for bleeding on postoperative day 0 or 1 |
| MAP >95 mmHg | 1.32 (0.30–5.81) | 0.71 | Unplanned return to operating room for bleeding on postoperative day 0 or 1 | |||
| Kang et al. [32] | 2020 | 1,950 | Intraoperative HTN (SBP ≥160 mmHg assessed more than 5 min apart) | 1.608 (1.056–2.448) | 0.027 | Emergence agitation |
| McCarthy et al. [44] | 2020 | 212 | Maximum SBP day 1 (increases of 10 mmHg) | 0.85 (0.73–0.98) | 0.031 | Functional independence |
| Maximum SBP day 2 (increases of 10 mmHg) | 0.90 (0.84–0.95) | 0.042 | Functional independence | |||
| Maximum SBP day 3 (increases of 10 mmHg) | 0.59 (0.47–0.74) | <0.0001 | Functional independence | |||
| Peak SBP change from day 1 to 2 | - | - | Functional independence | |||
| Peak SBP change from day 2 to 3 | 0.97 (0.95–0.99) | 0.006 | Functional independence | |||
| Morton and Vandal. [48] | 2015 | 621 | Every 10 points rise of highest SBP recordings | 1.39 (1.09–1.8) | Hematoma | |
| Pérez et al. [52] | 2020 | 282 | Post craniotomy severe HTN (SBP ≥170 mmHg) | 10.80% Incidence | 0.017 | Renal failure |
| Post et al. [53] | 2012 | 285 | SBP ≥150 mmHg | Incidence | 0.78 | Anastomotic leakage |
| DBP ≥90 mmHg | Incidence | 0.008 | Anastomotic leakage | |||
| Zheng et al. [76] | 2020 | 173 | 24h post-CAS SBP <120 mmHg | 1.70% Incidence | 0.002 | Unfavourable discharge |
| 24h post-CAS SBP 120–130 mmHg | 0% Incidence | Unfavourable discharge | ||||
| 24h post-CAS SBP >130 mmHg | 22.20% Incidence | Unfavourable discharge |
Abbreviations: CAS: carotid angioplasty stenting, DBP: diastolic blood pressure, HTN: hypertension, ICU: intensive care unit, LoS: length of stay, MAP: mean arterial pressure, min: minutes, SBP: systolic blood pressure.
# These articles refer to cardiac surgery.
*Generally, OR (95%CI), unless otherwise stated.
Blood pressure variation and control of mean arterial pressure
In non-cardiac surgeries, variation in BP (Table 13) has been reported to be correlated with adverse outcomes, such as primary graft non-function, ICU ventilator for more than 3 days, sepsis, prothrombin (PT) >16, poor early graft function and re-transplant, however, no data was available for these outcomes [22]. Additionally, an absolute fractional change of 10% in MAP was described as being protective against primary graft non-function [22]. Moreover, a work studying the influence of control of intraoperative MAP showed that the control of MAP between the range 80–95 mmHg had a protective action against hospital-acquired pneumonia, admission to the ICU and length of stay in ICU [72]. Lastly, the study by Zevallos et al. [75] analyzed the mean differences between intraprocedural SBP, DBP, and MAP, comparing cases and controls of patients developing contrast-induced neurotoxicity after neurointerventional procedures, and found statistically significant differences in all of them and concluded that HTN might have a role in increasing blood-brain barrier permeability, allowing the leak of the contrast.
Table 13. Diverse outcomes associated with perioperative control of mean arterial pressure and blood pressure variation.
| Author | Year | Sample size | Definition of measure | Result | P-value | Event |
|---|---|---|---|---|---|---|
| DeMaria et al. [22] | 2013 | 827 | Absolute fractional change in MAP <10% | Protective against adverse outcome (data not shown) | Primary graft non-function | |
| MAP <50 mmHg | Correlated with adverse event (data not shown) | Primary graft non-function | ||||
| MAP <50 mmHg | Correlated with adverse event (data not shown) | ICU ventilator >3 days | ||||
| MAP <50 mmHg | Correlated with adverse event (data not shown) | Sepsis | ||||
| MAP <50 mmHg | Correlated with adverse event (data not shown) | PT >16 | ||||
| MAP >120 mmHg | Correlated with adverse event (data not shown) | Poor early graft function | ||||
| MAP >120 mmHg | Correlated with adverse event (data not shown) | Re-transplant | ||||
| Wu et al. [72] | 2017 | 678 | MAP 65 to 79 mmHg | 3.90% Incidence | 0.542 | Surgical site infection |
| MAP 80 to 95 mmHg | 3.40% Incidence | Surgical site infection | ||||
| MAP 96 to 110 mmHg | 3.40% Incidence | Surgical site infection | ||||
| MAP 65 to 79 mmHg | 11.30% Incidence | 0.014 | Hospital acquired pneumonia | |||
| MAP 80 to 95 mmHg | 6.70% Incidence | Hospital acquired pneumonia | ||||
| MAP 96 to 110 mmHg | 10.40% Incidence | Hospital acquired pneumonia | ||||
| MAP 65 to 79 mmHg | 8.40% Incidence | 0.015 | Admission to ICU | |||
| MAP 80 to 95 mmHg | 4.40% Incidence | Admission to ICU | ||||
| MAP 96 to 110 mmHg | 7.60% Incidence | Admission to ICU | ||||
| MAP 65 to 79 mmHg | 2 (1–7) Median (IQR) | 0.025 | Stay in ICU | |||
| MAP 80 to 95 mmHg | 1 (1–3) Median (IQR) | Stay in ICU | ||||
| MAP 96 to 110 mmHg | 2 (1–6) Median IQR) | Stay in ICU | ||||
| Zevallos et al. [75] | 2020 | 33 | SBP cases—SBP controls | 23.41 Average intraprocedural values | 0.005 | Contrast-induced neurotoxicity |
| DBP cases—DBP controls | 10.67 Average intraprocedural values | 0.008 | Contrast-induced neurotoxicity | |||
| MAP cases—MAP controls | 13.79 Average intraprocedural values | 0.008 | Contrast-induced neurotoxicity | |||
Abbreviations: DBP: diastolic blood pressure, CI: confidence interval, ICU: intensive care unit, IQR: interquartile range, MAP: mean arterial pressure, OR: odds ratio, PT: prothrombin, SBP: systolic blood pressure.
Discussion
The main finding of this work is the existence of evidence supporting the burden of BP excursions in patients undergoing cardiac and non-cardiac surgeries that entail higher risks for negative outcomes after surgery. This review includes a wide range of surgical procedures and addresses several surgical outcomes that could be related to organ damage. There is also evidence that perioperative BP management is important for graft survival, flap loss, and length of hospital stay [18, 22, 33].
What can be highlighted from the results is that it is not just acute HPT or acute HTN that play a role in the development of adverse post-surgical outcomes but also the variation or lability in the values of BP from the patients’ baseline. It is important to characterize each patient’s baseline BP values and assess the intrapatient variability; this approach might have more clinically relevant results than establishing standard thresholds for all the patients [71, 77]. A few studies also incorporated time spent outside of the determined acceptable BP range as a critical factor [3, 46], as adverse outcomes seem to be related to the time the patient spends above or below the optimal ranges: the longer the time, the higher the risk [46, 55, 58, 62, 63, 67]. Regarding long-term outcomes, it seems that no differential long-term effects are expected in the absence of short-term effects, however, further evidence would be needed in order to confirm this hypothesis due to the difficulty of tracing long-term consequences back to the surgery. Nonetheless, a need for the intensification of periopertive medicine has been reported due to the emergence of long-term consequences of postoperative complications; there is an opportunity for the improvement of follow-up, rehabilitation programs, and the fine-tuning of medical management [78].
What has also been seen is that within cardiac surgery all outcomes are significantly associated with BP excursions. Even though this might be expected, there is also evidence for the relationship of HPT and HTN with negative outcomes such as AKI, stroke, discharge to a nursing facility or hospice care, or even death, in non-critically ill trauma surgeries [60] and other type of surgeries.
We highlight the associated risks, especially for severe HPT and HTN [12, 46, 74]. AMI and myocardial injury were also significant events that have been researched extensively, as HPT and HTN have been associated with the risk of both [11, 12, 24, 59, 66, 67]. Both HPT and HTN have also both been described as influencing the risk of stroke in patients undergoing non-cardiac surgeries.
More research needs to be done in order to determine the best strategies to deal with these morbidities in addition to developing new therapeutic strategies to better utilize current medications. Furthermore, a recent meta-analysis assessing the effect of vasoactive drugs (dobutamine, ephedrine, norepinephrine, nitroglycerine, theodrenaline/cafedrine, dopamine, dopexamine, colloids, phenylephrine, inotropes, nitrates, adrenaline) in the perioperative setting has concluded that these drugs might reduce postoperative complications and length of hospital stay in adults having major abdominal surgery [79]. It is clear that the opportunity loss of these agents controlling BP excursions in perioperative settings could be lower or partially compensated by the minimization of health resources that would be required to manage related complications. In this regard a few articles have provided some preliminary insights. Aronson et al. analyzed the impact of IV antihypertensive treatment for the management of perioperative BP during cardiac surgery and found that there was an association with shorter time to extubation and shorter ICU stays [80]. For some authors, anesthetic drugs and opioids can be used to control HTN [81–83]. However, their use can imply an increased risk of HPT and for this reason IV BP lowering drugs ideally should be used to control acute HTN episodes [59, 67, 84]. From a pharmacodynamic perspective, it would be reasonable to opt for a strategy that uses a specific type of therapy for each desired pharmacological effect for comprehensive intraprocedural patient management, to optimize their perioperative clinical evolution. Therefore, an opioid (e.g., remifentanil) would be the best option for managing acute pain, while keeping the level of anesthesia achieved separated from pain relief with the use of a hypnotic agent (e.g., propofol) and resorting to an IV antihypertensive that allows rapid, controlled, and predictable reductions in BP within a desired range. This evidence supports the view that new anti-hypertensive and vasoactive agents with favorable pharmacodynamic and pharmacokinetic properties might have a role in many clinical areas and thus, it would be pertinent to further investigate their role to guarantee the fine-tuning of BP and an individualized therapy for patients.
For the management of acute HPT there are also many therapeutic alternatives as described in a recent publication [85]. Fluid administration is a non-pharmacological approach intended to increase stroke volume and cardiac output but other options are also available within the pharmacological armamentarium, such as vasopressors and positive inotropic agents including ephedrine, phenylephrine; additional options are vasopressin and terlipressin, norepinephrine, epinephrine, and other less used agents, angiotensin II, vitamin C, hydroxicobalamin, dopamine, dobutamine, or milrinone. Recommended pharmacological treatments in case of acute HPT in the operating room are epinephrine or phenylephrine in case of mild HPT, and epinephrine in case of significant or refractory HPT [86].
Another study by Aronson et al. examined BP variability and demonstrated that by controlling BP during cardiac surgery, patients spent less time on mechanical ventilation and in the hospital, therefore avoiding the associated adverse outcomes and economic costs [5]. Getsios et al. took an in-depth look at clevidipine and found that it provided higher cost savings than other IV antihypertensives [4]. Keuffel et al. used Monte Carlo simulations to determine the association between intraoperative HPT and hospital expenditures, and concluded that limiting HPT during non-cardiac surgery will have an impact on hospital cost reductions, which may be highly relevant for resource allocation decisions [3]. This literature research also provides a basis for further research and potential economic models of reducing complications related to HPT and HTN.
Limitations
Although the methodology applied in this research meets international guidelines in the field, a few limitations should be noted. Firstly, in the absence of international guidelines on perioperative HPT and HTN definition (BP thresholds), articles vary greatly in how they define HTN and HPT. This could lead to issues when trying to compare results across studies and prevents performing a meta-analysis in order to assess the effect based on the published evidence due to the heterogeneity among studies. Also, there may be a publication bias that impedes the dissemination of negative findings.
Secondly, these sixty-six articles contain a wealth of evidence on the burden of acute HPT and HTN; however, the evidence remains uneven and difficult to synthesize due to differences in statistical presentation and target population.
Finally, any assessment of the burden is also complicated by the fact that the frequency of the long-term outcomes of acute HPT and HTN may be consequences of their short-term effects (e.g., stroke, AKI, hemorrhage, atrial fibrillation, and others.).
Conclusion
This review presents the available evidence on the burden of acute HPT and HTN across perioperative settings, and what seems to be clear is that HPT, HTN, and even fluctuations in BP from the patient’s baseline, entail a high burden for patients undergoing diverse types of surgeries. This suggests the potential benefit from improved management of BP in terms of short- and long-term effects of surgical procedures in patient outcomes.
Supporting information
(DOC)
(TXT)
(DOCX)
Data Availability
All relevant data are within the paper and its Supporting information files.
Funding Statement
Funding was provided by Ferrer, Barcelona, Spain. The funder provided support in the form of salaries for authors[IL], but did not have any additional role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript. The specific roles of these authors are articulated in the ‘author contributions’ section.
References
- 1.Varon J, Marik PE. Perioperative hypertension management. Vascular Health and Risk Management. Vasc Health Risk Manag; 2008. pp. 615–627. doi: 10.2147/vhrm.s2471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cheung AT. Exploring an optimum intra/postoperative management strategy for acute hypertension in the cardiac surgery patient. Journal of Cardiac Surgery. J Card Surg; 2006. doi: 10.1111/j.1540-8191.2006.00214.x [DOI] [PubMed] [Google Scholar]
- 3.Keuffel EL, Rizzo J, Stevens M, Gunnarsson C, Maheshwari K. Hospital costs associated with intraoperative hypotension among non-cardiac surgical patients in the US: a simulation model. J Med Econ. 2019;22: 645–651. doi: 10.1080/13696998.2019.1591147 [DOI] [PubMed] [Google Scholar]
- 4.Getsios D, Wang Y, Stolar M, Williams G, Ishak KJ, Hu M, et al. Improved perioperative blood pressure control leads to reduced hospital costs. Expert Opin Pharmacother. 2013;14: 1285–1293. doi: 10.1517/14656566.2013.798646 [DOI] [PubMed] [Google Scholar]
- 5.Aronson S, Levy JH, Lumb PD, Fontes M, Wang Y, Crothers TA, et al. Impact of perioperative blood pressure variability on health resource utilization after cardiac surgery: An analysis of the ECLIPSE trials. J Cardiothorac Vasc Anesth. 2014;28: 579–585. doi: 10.1053/j.jvca.2014.01.004 [DOI] [PubMed] [Google Scholar]
- 6.Kristensen SD, Knuuti J, Saraste A, Anker S, Bøtker HE, De Hert S, et al. 2014 ESC/ESA Guidelines on non-cardiac surgery. Eur J Anaesthesiol. 2014;31: 517–573. doi: 10.1097/EJA.0000000000000150 [DOI] [PubMed] [Google Scholar]
- 7.Grupo de trabajo. Vía Clínica de Recuperación Intensificada en Cirugía Abdominal (RICA). 2018.
- 8.Hartle A, McCormack T, Carlisle J, Anderson S, Pichel A, Beckett N, et al. The measurement of adult blood pressure and management of hypertension before elective surgery. Anaesthesia. 2016;71: 326–337. doi: 10.1111/anae.13348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Meng L, Yu W, Wang T, Zhang L, Heerdt PM, Gelb AW. Blood pressure targets in perioperative care provisional considerations based on a comprehensive literature review. Hypertension. Lippincott Williams and Wilkins; 2018. pp. 806–817. doi: 10.1161/HYPERTENSIONAHA.118.11688 [DOI] [PubMed] [Google Scholar]
- 10.Scottish Intercollegiate Guidelines Network. SIGN 50 A guideline developer’s handbook. Edinburgh; 2011. https://www.sign.ac.uk/assets/sign50_2011.pdf
- 11.Abbott TEF, Pearse RM, Archbold RA, Wragg A, Kam E, Ahmad T, et al. Association between preoperative pulse pressure and perioperative myocardial injury: An international observational cohort study of patients undergoing non-cardiac surgery. Br J Anaesth. 2017;119: 78–86. doi: 10.1093/bja/aex165 [DOI] [PubMed] [Google Scholar]
- 12.Abbott TEF, Pearse RM, Archbold RA, Ahmad T, Niebrzegowska E, Wragg A, et al. A Prospective International Multicentre Cohort Study of Intraoperative Heart Rate and Systolic Blood Pressure and Myocardial Injury After Noncardiac Surgery. Anesth Analg. 2018;126: 1936–1945. doi: 10.1213/ANE.0000000000002560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ahuja S, Mascha EJ, Yang D, Maheshwari K, Cohen B, Khanna AK, et al. Associations of intraoperative radial arterial systolic, diastolic, mean, and pulse pressures with myocardial and acute kidney injury after noncardiac surgery a retrospective cohort analysis. Anesthesiology. 2020;132: 291–306. doi: 10.1097/ALN.0000000000003048 [DOI] [PubMed] [Google Scholar]
- 14.Anastasio AT, Farley KX, Boden SD, Bradbury TL, Premkumar A, Gottschalk MB. Modifiable, Postoperative Risk Factors for Delayed Discharge Following Total Knee Arthroplasty: The Influence of Hypotension and Opioid Use. J Arthroplasty. 2020;35: 82–88. doi: 10.1016/j.arth.2019.07.047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Aronson S, Stafford-Smith M, Phillips-Bute B, Shaw A, Gaca J, Newman M. Intraoperative systolic blood pressure variability predicts 30-day mortality in aortocoronary bypass surgery patients. Anesthesiology. 2010;113: 305–312. doi: 10.1097/ALN.0b013e3181e07ee9 [DOI] [PubMed] [Google Scholar]
- 16.Aronson S, Dyke CM, Levy JH, Cheung AT, Lumb PD, Avery EG, et al. Does perioperative systolic blood pressure variability predict mortality after cardiac surgery? An exploratory analysis of the ECLIPSE trials. Anesth Analg. 2011;113: 19–30. doi: 10.1213/ANE.0b013e31820f9231 [DOI] [PubMed] [Google Scholar]
- 17.Babazade R, Yilmaz HO, Zimmerman NM, Stocchi L, Gorgun E, Kessler H, et al. Association Between Intraoperative Low Blood Pressure and Development of Surgical Site Infection After Colorectal Surgery. Ann Surg. 2016;264: 1058–1064. doi: 10.1097/SLA.0000000000001607 [DOI] [PubMed] [Google Scholar]
- 18.Balzer F, Aronson S, Campagna JA, Ding L, Treskatsch S, Spies C, et al. High Postoperative Blood Pressure After Cardiac Surgery Is Associated With Acute Kidney Injury and Death. J Cardiothorac Vasc Anesth. 2016;30: 1562–1570. doi: 10.1053/j.jvca.2016.05.040 [DOI] [PubMed] [Google Scholar]
- 19.Beecham G, Cusack R, Vencken S, Crilly G, Buggy DJ. Hypotension during hip fracture surgery and postoperative morbidity. Ir J Med Sci. 2020;189: 1087–1096. doi: 10.1007/s11845-020-02175-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bijker JB, Persoon S, Peelen LM, Moons KGM, Kalkman CJ, Kappelle LJ, et al. Intraoperative hypotension and perioperative ischemic stroke after general surgery, a nested case-control study. Anesthesiology. 2012;116: 658–664. doi: 10.1097/ALN.0b013e3182472320 [DOI] [PubMed] [Google Scholar]
- 21.Bonnet J-F, Buggy E, Cusack B, Sherwin A, Wall T, Fitzgibbon M, et al. Can routine perioperative haemodynamic parameters predict postoperative morbidity after major surgery? Perioper Med. 2020;9: 9. doi: 10.1186/s13741-020-0139-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.DeMaria S, Nürnberg J, Lin H, Contreras-Saldivar A, Levin M, Flax K, et al. Association of intraoperative blood pressure instability with adverse outcomes after liver transplantation—Minerva Anestesiologica 2013 June;79(6):604–16—Minerva Medica—Journals. Minerva Anestesiol. 2013;79: 604–616. Available: https://www.minervamedica.it/en/journals/minerva-anestesiologica/article.php?cod=R02Y2013N06A0604 [PubMed] [Google Scholar]
- 23.Gregory A, Stapelfeldt WH, Khanna AK, Smischney NJ, Boero IJ, Chen Q, et al. Intraoperative hypotension is associated with adverse clinical outcomes after noncardiac surgery. Anesth Analg. 2021;132: 1654–1665. doi: 10.1213/ANE.0000000000005250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hallqvist L, Mårtensson J, Granath F, Sahlén A, Bell M. Intraoperative hypotension is associated with myocardial damage in noncardiac surgery: An observational study. Eur J Anaesthesiol. 2016;33: 450–456. doi: 10.1097/EJA.0000000000000429 [DOI] [PubMed] [Google Scholar]
- 25.Hallqvist L, Granath F, Huldt E, Bell M. Intraoperative hypotension is associated with acute kidney injury in noncardiac surgery. Eur J Anaesthesiol. 2018;35: 273–279. doi: 10.1097/EJA.0000000000000735 [DOI] [PubMed] [Google Scholar]
- 26.Hirsch J, DePalma G, Tsai TT, Sands LP, Leung JM. Impact of intraoperative hypotension and blood pressure fluctuations on early postoperative delirium after non-cardiac surgery. Br J Anaesth. 2015;115: 418–426. doi: 10.1093/bja/aeu458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hori D, Hogue C, Adachi H, Max L, Price J, Sciortino C, et al. Perioperative optimal blood pressure as determined by ultrasound tagged near infrared spectroscopy and its association with postoperative acute kidney injury in cardiac surgery patients. Interact Cardiovasc Thorac Surg. 2016;22: 445–451. doi: 10.1093/icvts/ivv371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hori D, Max L, Laflam A, Brown C, Neufeld KJ, Adachi H, et al. Blood Pressure Deviations from Optimal Mean Arterial Pressure during Cardiac Surgery Measured with a Novel Monitor of Cerebral Blood Flow and Risk for Perioperative Delirium: A Pilot Study. J Cardiothorac Vasc Anesth. 2016;30: 606–612. doi: 10.1053/j.jvca.2016.01.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hsieh JK, Dalton JE, Yang D, Farag ES, Sessler DI, Kurz AM. The Association between Mild Intraoperative Hypotension and Stroke in General Surgery Patients. Anesth Analg. 2016;123: 933–939. doi: 10.1213/ANE.0000000000001526 [DOI] [PubMed] [Google Scholar]
- 30.Jang WY, Jung JK, Lee DK, Han SB. Intraoperative hypotension is a risk factor for postoperative acute kidney injury after femoral neck fracture surgery: A retrospective study. BMC Musculoskelet Disord. 2019;20. doi: 10.1186/s12891-019-2496-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jinadasa SP, Mueller A, Prasad V, Subramaniam K, Heldt T, Novack V, et al. Blood pressure coefficient of variation and its association with cardiac surgical outcomes. Anesth Analg. 2018;127: 832–839. doi: 10.1213/ANE.0000000000003362 [DOI] [PubMed] [Google Scholar]
- 32.Kang X, Lin K, Tang H, Tang X, Bao F, Gan S, et al. Risk Factors for Emergence Agitation in Adults Undergoing Thoracoscopic Lung Surgery: A Case-Control Study of 1,950 Patients. J Cardiothorac Vasc Anesth. 2020;34: 2403–2409. doi: 10.1053/j.jvca.2020.02.046 [DOI] [PubMed] [Google Scholar]
- 33.Kass JL, Lakha S, Levin MA, Joseph T, Lin HM, Genden EM, et al. Intraoperative hypotension and flap loss in free tissue transfer surgery of the head and neck. Head Neck. 2018;40: 2334–2339. doi: 10.1002/hed.25190 [DOI] [PubMed] [Google Scholar]
- 34.Langer T, Santini A, Zadek F, Chiodi M, Pugni P, Cordolcini V, et al. Intraoperative hypotension is not associated with postoperative cognitive dysfunction in elderly patients undergoing general anesthesia for surgery: results of a randomized controlled pilot trial. J Clin Anesth. 2019;52: 111–118. doi: 10.1016/j.jclinane.2018.09.021 [DOI] [PubMed] [Google Scholar]
- 35.Levin MA, Fischer GW, Lin HM, Mccormick PJ, Krol M, Reich DL. Intraoperative arterial blood pressure lability is associated with improved 30 day survival. Br J Anaesth. 2015;115: 716–726. doi: 10.1093/bja/aev293 [DOI] [PubMed] [Google Scholar]
- 36.Li J, Zhao Y, Yan X, Li R, Zhang X, Zeng M, et al. Perioperative risk factors associated with acute kidney injury in patients after brain tumor resection. J Neurosurg Anesth. 2020;In press. doi: 10.1097/ANA.0000000000000716 [DOI] [PubMed] [Google Scholar]
- 37.Li J, Zhao Y, Zhao M, Cao P, Liu X, Ren H, et al. High variance of intraoperative blood pressure predicts early cerebral infarction after revascularization surgery in patients with Moyamoya disease. Neurosurg Rev. 2020;43: 759–769. doi: 10.1007/s10143-019-01118-z [DOI] [PubMed] [Google Scholar]
- 38.Liao P, Zhao S, Lyu L, Yi X, Ji X, Sun J, et al. Association of intraoperative hypotension with acute kidney injury after liver resection surgery: an observational cohort study. BMC Nephrol. 2020;21: 456. doi: 10.1186/s12882-020-02109-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Liem VGB, Hoeks SE, Mol KHJM, Potters JW, Grüne F, Stolker RJ, et al. Postoperative Hypotension after Noncardiac Surgery and the Association with Myocardial Injury. Anesthesiology. 2020;133: 510–522. doi: 10.1097/ALN.0000000000003368 [DOI] [PubMed] [Google Scholar]
- 40.Liu Z, Xu G, Xu L, Zhang Y, Huang Y. Perioperative Cardiac Complications in Patients Over 80 Years of Age with Coronary Artery Disease Undergoing Noncardiac Surgery: The Incidence and Risk Factors. Clin Interv Aging. 2020;Volume 15: 1181–1191. doi: 10.2147/CIA.S252160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Maheshwari K, Ahuja S, Khanna AK, Mao G, Perez-Protto S, Farag E, et al. Association between perioperative hypotension and delirium in postoperative critically ill patients: A retrospective cohort analysis. Anesth Analg. 2020;130: 636–643. doi: 10.1213/ANE.0000000000004517 [DOI] [PubMed] [Google Scholar]
- 42.Mascha EJ, Yang D, Weiss S, Sessler DI. Intraoperative Mean Arterial Pressure Variability and 30-day Mortality in Patients Having Noncardiac Surgery. Anesthesiology. 2015;123: 79–91. doi: 10.1097/ALN.0000000000000686 [DOI] [PubMed] [Google Scholar]
- 43.Mathis MR, Naik BI, Freundlich RE, Shanks AM, Heung M, Kim M, et al. Preoperative risk and the association between hypotension and postoperative acute kidney injury. Anesthesiology. 2020;132: 461–475. doi: 10.1097/ALN.0000000000003063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.McCarthy DJ, Ayodele M, Luther E, Sheinberg D, Bryant JP, Elwardany O, et al. Prolonged Heightened Blood Pressure Following Mechanical Thrombectomy for Acute Stroke is Associated with Worse Outcomes. Neurocrit Care. 2020;32: 198–205. doi: 10.1007/s12028-019-00803-7 [DOI] [PubMed] [Google Scholar]
- 45.McIlroy D, Murphy D, Kasza J, Bhatia D, Marasco S. Association of postoperative blood pressure and bleeding after cardiac surgery. J Thorac Cardiovasc Surg. 2019;158: 1370–1379.e6. doi: 10.1016/j.jtcvs.2019.01.063 [DOI] [PubMed] [Google Scholar]
- 46.Mizota T, Hamada M, Matsukawa S, Seo H, Tanaka T, Segawa H. Relationship Between Intraoperative Hypotension and Acute Kidney Injury After Living Donor Liver Transplantation: A Retrospective Analysis. J Cardiothorac Vasc Anesth. 2017;31: 582–589. doi: 10.1053/j.jvca.2016.12.002 [DOI] [PubMed] [Google Scholar]
- 47.Monk TG, Bronsert MR, Henderson WG, Mangione MP, Sum-Ping STJ, Bentt DR, et al. Association between intraoperative hypotension and hypertension and 30-day postoperative mortality in noncardiac surgery. Anesthesiology. 2015;123: 307–319. doi: 10.1097/ALN.0000000000000756 [DOI] [PubMed] [Google Scholar]
- 48.Morton RP, Vandal AC. Postoperative Systolic Blood Pressure as a risk factor for haematoma following thyroid surgery. Clin Otolaryngol. 2015;40: 462–467. doi: 10.1111/coa.12407 [DOI] [PubMed] [Google Scholar]
- 49.Ngu JMC, Jabagi H, Chung AM, Boodhwani M, Ruel M, Bourke M, et al. Defining an Intraoperative Hypotension Threshold in Association with de Novo Renal Replacement Therapy after Cardiac Surgery. Anesthesiology. 2020;132: 1447–1457. doi: 10.1097/ALN.0000000000003254 [DOI] [PubMed] [Google Scholar]
- 50.Ono M, Arnaoutakis GJ, Fine DM, Brady K, Easley RB, Zheng Y, et al. Blood Pressure Excursions Below the Cerebral Autoregulation Threshold During Cardiac Surgery are Associated With Acute Kidney Injury*. Crit Care Med. 2013;41: 464–471. doi: 10.1097/CCM.0b013e31826ab3a1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ono M, Brady K, Easley RB, Brown C, Kraut M, Gottesman RF, et al. Duration and magnitude of blood pressure below cerebral autoregulation threshold during cardiopulmonary bypass is associated with major morbidity and operative mortality. J Thorac Cardiovasc Surg. 2014;147: 483–489. doi: 10.1016/j.jtcvs.2013.07.069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Perez CA, Stutzman S, Jansen T, Perera A, Jannusch S, Atem F, et al. Elevated blood pressure after craniotomy: A prospective observational study. J Crit Care. 2020;60: 235–240. doi: 10.1016/j.jcrc.2020.08.013 [DOI] [PubMed] [Google Scholar]
- 53.Post IL, Verheijen PM, Pronk A, Siccama I, Houweling PL. Intraoperative blood pressure changes as a risk factor for anastomotic leakage in colorectal surgery. Int J Colorectal Dis. 2012;27: 765–772. doi: 10.1007/s00384-011-1381-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rettig TCD, Peelen LM, Geuzebroek GSC, van Klei WA, Boer C, van der Veer JW, et al. Impact of Intraoperative Hypotension During Cardiopulmonary Bypass on Acute Kidney Injury After Coronary Artery Bypass Grafting. J Cardiothorac Vasc Anesth. 2017;31: 522–528. doi: 10.1053/j.jvca.2016.07.040 [DOI] [PubMed] [Google Scholar]
- 55.Ristovic V, de Roock S, Mesana TG, van Diepen S, Sun LY. The Impact of Preoperative Risk on the Association between Hypotension and Mortality after Cardiac Surgery: An Observational Study. J Clin Med. 2020;9: 2057. doi: 10.3390/jcm9072057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Roshanov PS, Sheth T, Duceppe E, Tandon V, Bessissow A, Chan MTV, et al. Relationship between Perioperative Hypotension and Perioperative Cardiovascular Events in Patients with Coronary Artery Disease Undergoing Major Noncardiac Surgery. Anesthesiology. 2019;130: 756–766. doi: 10.1097/ALN.0000000000002654 [DOI] [PubMed] [Google Scholar]
- 57.Rots ML, Fassaert LMM, Kappelle LJ, de Groot MCH, Haitjema S, Bonati LH, et al. Intra-Operative Hypotension is a Risk Factor for Post-operative Silent Brain Ischaemia in Patients With Pre-operative Hypertension Undergoing Carotid Endarterectomy. Eur J Vasc Endovasc Surg. 2020;59: 526–534. doi: 10.1016/j.ejvs.2020.01.007 [DOI] [PubMed] [Google Scholar]
- 58.Salmasi V, Maheshwari K, Yang D, Mascha EJ, Singh A, Sessler DI, et al. Relationship between Intraoperative Hypotension, Defined by Either Reduction from Baseline or Absolute Thresholds, and Acute Kidney and Myocardial Injury after Noncardiac Surgery. Anesthesiology. 2017;126: 47–65. doi: 10.1097/ALN.0000000000001432 [DOI] [PubMed] [Google Scholar]
- 59.Sessler DI, Meyhoff CS, Zimmerman NM, Mao G, Leslie K, Vásquez SM, et al. Period-dependent Associations between Hypotension during and for Four Days after Noncardiac Surgery and a Composite of Myocardial Infarction and Death: A Substudy of the POISE-2 Trial. Anesthesiology. 2018;128: 317–327. doi: 10.1097/ALN.0000000000001985 [DOI] [PubMed] [Google Scholar]
- 60.Sheffy N, Bentov I, Mills B, Nair BG, Rooke GA, Vavilala MS. Perioperative hypotension and discharge outcomes in non-critically injured trauma patients, a single centre retrospective cohort study. Injury. 2017;48: 1956–1963. doi: 10.1016/j.injury.2017.06.023 [DOI] [PubMed] [Google Scholar]
- 61.Sposato LA, Suárez A, Jáuregui A, Riccio PM, Altounian M, Andreoli MG, et al. Intraoperative hypotension, new onset atrial fibrillation, and adverse outcome after carotid endarterectomy. J Neurol Sci. 2011;309: 5–8. doi: 10.1016/j.jns.2011.07.052 [DOI] [PubMed] [Google Scholar]
- 62.Sun LY, Wijeysundera DN, Tait GA, Beattie WS. Association of intraoperative hypotension with acute kidney injury after elective noncardiac surgery. Anesthesiology. 2015;123: 515–523. doi: 10.1097/ALN.0000000000000765 [DOI] [PubMed] [Google Scholar]
- 63.Tang Y, Zhu C, Liu J, Wang A, Duan K, Li B, et al. Association of Intraoperative Hypotension with Acute Kidney Injury after Noncardiac Surgery in Patients Younger than 60 Years Old. Kidney Blood Press Res. 2019;44: 211–221. doi: 10.1159/000498990 [DOI] [PubMed] [Google Scholar]
- 64.Tassoudis V, Vretzakis G, Petsiti A, Stamatiou G, Bouzia K, Melekos M, et al. Impact of intraoperative hypotension on hospital stay in major abdominal surgery. J Anesth. 2011;25: 492–499. doi: 10.1007/s00540-011-1152-1 [DOI] [PubMed] [Google Scholar]
- 65.van Lier F, Wesdorp FHIM, Liem VGB, Potters JW, Grüne F, Boersma H, et al. Association between postoperative mean arterial blood pressure and myocardial injury after noncardiac surgery. Br J Anaesth. 2018;120: 77–83. doi: 10.1016/j.bja.2017.11.002 [DOI] [PubMed] [Google Scholar]
- 66.Van Waes JAR, Van Klei WA, Wijeysundera DN, Van Wolfswinkel L, Lindsay TF, Beattie WS. Association between intraoperative hypotension and myocardial injury after vascular surgery. Anesthesiology. 2016;124: 35–44. doi: 10.1097/ALN.0000000000000922 [DOI] [PubMed] [Google Scholar]
- 67.Walsh M, Devereaux PJ, Garg AX, Kurz A, Turan A, Rodseth RN, et al. Relationship between intraoperative mean arterial pressure and clinical outcomes after noncardiac surgery: Toward an empirical definition of hypotension. Anesthesiology. 2013;119: 507–515. doi: 10.1097/ALN.0b013e3182a10e26 [DOI] [PubMed] [Google Scholar]
- 68.Wang NY, Hirao A, Sieber F. Association between intraoperative blood pressure and postoperative delirium in elderly hip fracture patients. PLoS One. 2015;10. doi: 10.1371/journal.pone.0123892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wesselink EM, Kappen TH, Van Klei WA, Dieleman JM, Van Dijk D, Slooter AJC. Intraoperative hypotension and delirium after on-pump cardiac surgery. Br J Anaesth. 2015;115: 427–433. doi: 10.1093/bja/aev256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Willingham MD, Karren E, Shanks AM, O’Connor MF, Jacobsohn E, Kheterpal S, et al. Concurrence of intraoperative hypotension, low minimum alveolar concentration, and low bispectral index is associated with postoperative death. Anesthesiology. 2015;123: 775–785. doi: 10.1097/ALN.0000000000000822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wiórek A, Krzych ŁJ. Intraoperative blood pressure variability predicts postoperative mortality in non-cardiac surgery—A prospective observational cohort study. Int J Environ Res Public Health. 2019;16. doi: 10.3390/ijerph16224380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wu X, Jiang Z, Ying J, Han Y, Chen Z. Optimal blood pressure decreases acute kidney injury after gastrointestinal surgery in elderly hypertensive patients: A randomized study: Optimal blood pressure reduces acute kidney injury. J Clin Anesth. 2017;43: 77–83. doi: 10.1016/j.jclinane.2017.09.004 [DOI] [PubMed] [Google Scholar]
- 73.Yilmaz HO, Babazade R, Leung S, Zimmerman NM, Makarova N, Saasouh W, et al. Postoperative hypotension and surgical site infections after colorectal surgery: A retrospective cohort study. Anesth Analg. 2018;127: 1129–1136. doi: 10.1213/ANE.0000000000003666 [DOI] [PubMed] [Google Scholar]
- 74.Yu H-C, Luo Y-X, Peng H, Wang X-L, Yang Z-H, Huang M-J, et al. Association of perioperative blood pressure with long-term survival in rectal cancer patients. Chin J Cancer. 2016;35: 38. doi: 10.1186/s40880-016-0100-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zevallos CB, Dai B, Dandapat S, Quispe-Orozco D, Holcombe A, Ansari S, et al. Greater intraprocedural systolic blood pressure and blood pressure variability are associated with contrast-induced neurotoxicity after neurointerventional procedures. J Neurol Sci. 2021;420: 117209. doi: 10.1016/j.jns.2020.117209 [DOI] [PubMed] [Google Scholar]
- 76.Zheng L, Li J, Liu H, Guo H, Zhao L, Bai H, et al. Perioperative Blood Pressure Control in Carotid Artery Stenosis Patients With Carotid Angioplasty Stenting: A Retrospective Analysis of 173 Cases. Front Neurol. 2020;11: 567623. doi: 10.3389/fneur.2020.567623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Xue FS, Li RP, Liu GP. Association of intraoperative arterial blood pressure lability with postoperative adverse outcome: no one size fits all. Br J Anaesth. 2016;117: 258–69. doi: 10.1093/bja/aew199 [DOI] [PubMed] [Google Scholar]
- 78.Toner A, Hamilton M. The long-term effects of postoperative complications. Curr Opin Crit Care. 2013;19: 364–368. doi: 10.1097/MCC.0b013e3283632f77 [DOI] [PubMed] [Google Scholar]
- 79.Deng C, Bellomo R, Myles P. Systematic review and meta-analysis of the perioperative use of vasoactive drugs on postoperative outcomes after major abdominal surgery. British Journal of Anaesthesia. Elsevier Ltd; 2020. pp. 513–524. doi: 10.1016/j.bja.2020.01.021 [DOI] [PubMed] [Google Scholar]
- 80.Aronson S, Dasta JF, Levy JH, Lumb PD, Fontes M, Wang Y, et al. A cost analysis of the impact of a new intravenous antihypertensive in managing perioperative blood pressure during cardiac surgery. Hosp Pract (1995). 2014;42: 26–32. doi: 10.3810/hp.2014.08.1115 [DOI] [PubMed] [Google Scholar]
- 81.Lonjaret L, Lairez O, Geeraerts T, Minville V. Optimal perioperative management of arterial blood pressure. Integr Blood Press Control. 2014;7: 49. doi: 10.2147/IBPC.S45292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Paix AD, Williamson JA, Runciman WB. Crisis management during anaesthesia: difficult intubation. Qual Saf Health Care. 2005;14: e5–e5. doi: 10.1136/qshc.2002.004135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lien SF, Bisognano JD. Perioperative hypertension: Defining at-risk patients and their management. Curr Hypertens Rep. 2012;14: 432–441. doi: 10.1007/s11906-012-0287-2 [DOI] [PubMed] [Google Scholar]
- 84.Monk TG, Saini V, Weldon BC, Sigl JC. Anesthetic management and one-year mortality after noncardiac surgery. Anesth Analg. 2005;100: 4–10. doi: 10.1213/01.ANE.0000147519.82841.5E [DOI] [PubMed] [Google Scholar]
- 85.London MJ. Hemodynamic management during anesthesia in adults. In: UpToDate [Internet]. 2020 [cited 16 Apr 2021]. https://www.uptodate.com/contents/hemodynamic-management-during-anesthesia-in-adults
- 86.Seifert PC, Wadlund DL. Crisis Management of Hypotension in the OR. AORN J. 2015;102: 64–73. doi: 10.1016/j.aorn.2015.05.002 [DOI] [PubMed] [Google Scholar]
- 87.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLoS Med. 2009;6: e1000097. doi: 10.1371/journal.pmed.1000097 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOC)
(TXT)
(DOCX)
Data Availability Statement
All relevant data are within the paper and its Supporting information files.


