Abstract
The National Antimicrobial Prescribing Survey (NAPS) is a web-based qualitative auditing platform that provides a standardized and validated tool to assist hospitals in assessing the appropriateness of antimicrobial prescribing practices. Since its release in 2013, the NAPS has been adopted by all hospital types within Australia, including public and private facilities, and supports them in meeting the national standards for accreditation. Hospitals can generate real-time reports to assist with local antimicrobial stewardship (AMS) activities and interventions. De-identified aggregate data from the NAPS are also submitted to the Antimicrobial Use and Resistance in Australia surveillance system, for national reporting purposes, and to strengthen national AMS strategies. With the successful implementation of the programme within Australia, the NAPS has now been adopted by countries with both well-resourced and resource-limited healthcare systems. We provide here a narrative review describing the experience of users utilizing the NAPS programme in Canada, Malaysia and Bhutan. We highlight the key barriers and facilitators to implementation and demonstrate that the NAPS methodology is feasible, generalizable and translatable to various settings and able to assist in initiatives to optimize the use of antimicrobials.
Introduction
National action plans to combat antimicrobial resistance (AMR) should ensure that the judicious use of antimicrobials is included as a key objective.1,2 One of the recommended activities is to encourage the adoption of antimicrobial stewardship (AMS) programmes, with the aim of enhancing patient healthcare outcomes while reducing the emergence and spread of AMR. Therefore WHO has prioritized identifying and measuring inappropriate antimicrobial prescribing practices.2
In 2011, the Australian Commission on Safety and Quality in Health Care (ACSQHC) published recommendations for implementing AMS programmes in all Australian hospitals.3 This was followed in 2013 by the introduction of detailed hospital accreditation criteria for AMS in the Australian National Safety and Quality Health Service Standards,4 which required the monitoring of antimicrobial prescribing appropriateness. Consequently, the need to determine the most appropriate tools for auditing antimicrobial use within Australian hospitals became apparent.
While the quantitative measurement of antimicrobial consumption will enable facilities to understand and compare the volume of antimicrobials used over time, these data alone cannot be used for a comprehensive analysis of prescribing practices at a patient or prescription level.5 Qualitative measurements of antimicrobial prescribing, including assessments of the concordance of prescribing with recommendations from evidence-based guidelines, which importantly, also take into account any documented, clinically justifiable reasons to vary from these guidelines (described as ‘appropriate’ prescribing), provide more useful information. These data can identify targets for quality improvement and assist in the coordination and evaluation of AMS initiatives. Where repeated patterns of variation emerge, this may also guide jurisdictional and national AMS strategies.3 However, if there is a lack of a standardized approach to assessing the quality of antimicrobial prescribing, peer comparison is often not possible, despite being a strong motivator for behaviour change.6,7
Most qualitative antimicrobial prescribing audits include an assessment of concordance with recommendations from evidence-based guidelines. However, there are many reasons why guideline concordance may not be feasible. Antimicrobial guidelines often do not cover the complex factors considered during antimicrobial prescribing. For example, many guidelines only provide empirical recommendations, or exclude particular patient groups such as children and immunocompromised patients. Appropriateness assessments allow consideration and evaluation of directed antimicrobial therapy, following culture and susceptibility results, complex patients, including those with multiple indications, and patients with allergy labels for the guideline-recommended antimicrobials. There have been several published tools designed to audit antimicrobial prescribing practices at a patient level, which compare aggregated hospital data internationally;8–10 however, these tools have not attempted to assess the appropriateness of the prescription and do not allow for detailed facility-level reports to support local AMS initiatives.
As part of a project supported by an Australian National Health and Medical Research Council grant, the National Centre for Antimicrobial Stewardship (NCAS) developed, and in 2011 and 2012, piloted, an antimicrobial prescribing point-prevalence survey.11 After the success of this pilot, an online auditing platform, the National Antimicrobial Prescribing Survey (NAPS), was developed.12 Released, in 2013, the Hospital NAPS is a standardized point-prevalence survey suitable for use in all hospitals to support real-time data collection and reporting, with dashboards and aggregation of data for benchmarking.13–19
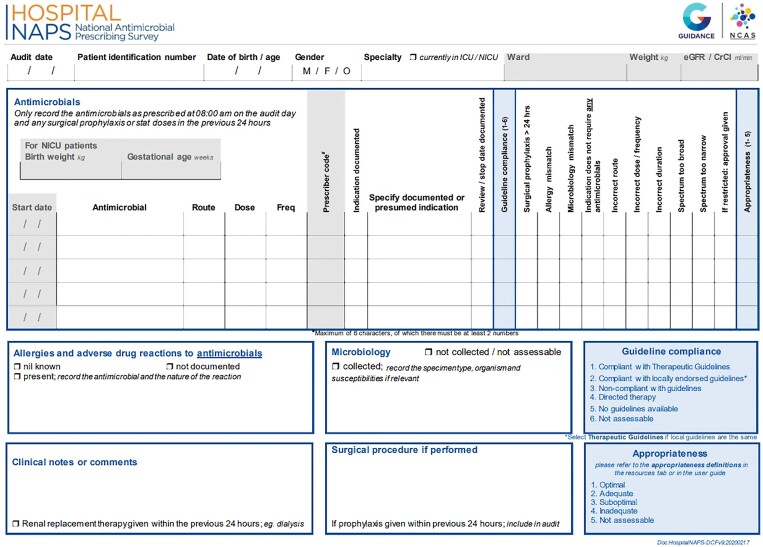
The survey tool was designed to be practical and generalizable and to facilitate the collection of qualitative antimicrobial prescribing data, such as the compliance with prescribing guidelines, the reasons for any non-compliance and an assessment of the appropriateness of the prescription. This requires a review of the patient’s relevant medical records, including medication charts, progress notes, surgical records, radiology, microbiology and other pathology results. Patient level data that are collected to assist with the assessment of prescribing appropriateness are presented in Figure 1, and include age, sex, weight, renal function, the indication for prescribing, allergy status, recent microbiology results and any other relevant clinical notes or comments. Each patient takes, on average, between 5 and 20 min to review and assess, depending on auditor experience and patient complexity, and the required information is entered through a dedicated data entry portal, which is available year round.
Figure 1.
Hospital NAPS data collection form.
From the Australian Hospital NAPS 2020 results (Table 1), in terms of assessing compliance with guidelines, almost one-fifth of prescriptions (19.8%; ranging from 11.4% to 24.8%) are unable to be assessed, as they are for directed therapy, there are no prescribing guidelines available, or they are not assessable. In contrast, when assessing the appropriateness of the prescription, only 4.0% of prescriptions (ranging from 1.7% to 8.7%) are deemed not assessable. This demonstrates the importance in utilizing qualitative patient level data in the assessment of prescribing practices, to allow for a more comprehensive and accurate evaluation and in determining areas to concentrate AMS activities.
Table 1.
Hospital NAPS, 2020 key indicator results, by hospital peer group classification and funding type
| No. of participating facilities | No. of prescriptions | Compliance with evidence-based prescribing guidelines, %a | Appropriateness, % | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| compliant | non-compliant | directed therapy | no guideline available | not assessable | appropriate | inappropriate | not assessable | |||
| Hospital peer groupb | ||||||||||
| Principal referral | 30 | 9162 | 53.6 | 21.7 | 17.7 | 4.5 | 2.6 | 78.2 | 18.8 | 3.1 |
| Women’s and children’s | 6 | 310 | 76.1 | 11.2 | 6.1 | 5.6 | 1.0 | 84.9 | 13.4 | 1.7 |
| Children’s | 6 | 951 | 64.6 | 12.2 | 12.6 | 8.3 | 2.3 | 84.4 | 13.4 | 2.2 |
| Public acute group A | 58 | 6760 | 53.2 | 26.4 | 13.9 | 4.1 | 2.4 | 76.0 | 21.2 | 2.8 |
| Public acute group B | 29 | 1685 | 49.2 | 33.2 | 11.3 | 3.3 | 3.0 | 70.1 | 26.1 | 3.8 |
| Public acute group C | 68 | 2863 | 56.5 | 30.0 | 9.3 | 0.9 | 3.3 | 73.0 | 23.1 | 3.9 |
| Public acute group D | 54 | 978 | 51.6 | 37.0 | 8.1 | 0.8 | 2.5 | 67.6 | 28.9 | 3.5 |
| Private acute group A | 19 | 2581 | 49.9 | 33.6 | 10.6 | 2.1 | 3.8 | 66.3 | 29.0 | 4.8 |
| Private acute group B | 27 | 1715 | 52.0 | 28.3 | 10.3 | 3.1 | 6.3 | 66.1 | 25.2 | 8.7 |
| Private acute group C | 33 | 1354 | 44.8 | 39.4 | 8.4 | 2.0 | 5.3 | 59.5 | 34.6 | 5.9 |
| Private acute group D | 21 | 796 | 63.6 | 25.1 | 5.2 | 1.6 | 4.5 | 68.3 | 23.9 | 7.8 |
| Funding type | ||||||||||
| Public | 284 | 22 709 | 54.4 | 24.9 | 14.2 | 3.8 | 2.7 | 76.3 | 20.4 | 3.3 |
| Private | 122 | 6446 | 51.5 | 31.3 | 10.0 | 2.2 | 5.0 | 65.9 | 27.5 | 6.6 |
| Combined national result | 406 | 29 155 | 53.7 | 26.4 | 13.2 | 3.4 | 3.2 | 73.9 | 22.1 | 4.0 |
The Australian national antimicrobial prescribing guidelines are the ‘Therapeutic Guidelines’.51
Hospital peer groups are assigned by the Australian Institute of Health and Welfare.52 Principal referral—24 h emergency department, ICU, cardiac surgery, neurosurgery, infectious diseases, bone marrow transplant, organ transplant and burns units; Women’s and children’s—have both a children’s separations proportion over 50% and a women’s separations proportion over 25%; Children’s—proportion of separations with patients aged 0–14 over 80%; Public acute group A—24 h emergency department, ICU, coronary care unit, oncology unit; Public acute group B—24 h emergency department; Public acute group C—surgery, obstetric unit, emergency department; Public acute group D— hospitals that do not meet the service characteristics of the other public acute hospital groups; Private acute group A—24 h emergency department, ICU, special care nursery unit, coronary care unit, cardiac surgery unit, neurosurgery unit; Private acute group B—ICU, special care nursery unit, coronary care unit, cardiac surgery unit, neurosurgery unit; Private acute group C—acute psychiatry, surgery, rehabilitation; Private acute group D—hospitals that do not meet the service characteristics of the other private acute hospital groups.
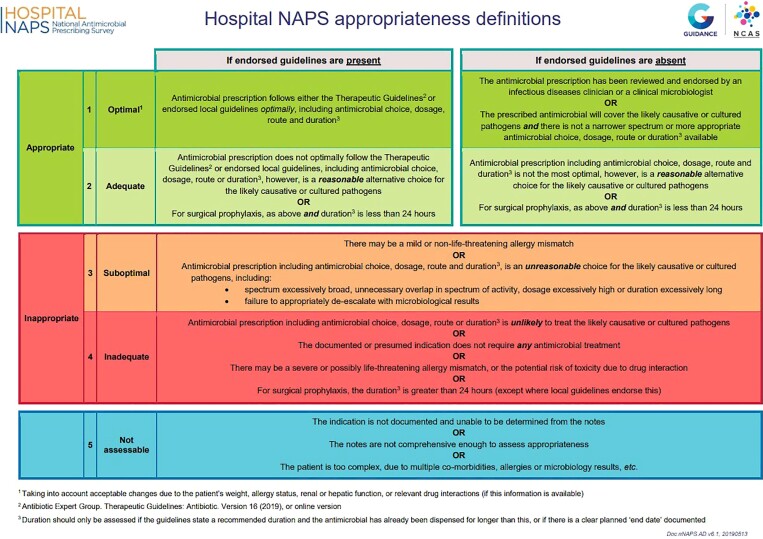
The Hospital NAPS uses standardized definitions, methodology and assessment algorithms to assist participating hospitals in determining the appropriateness of antimicrobial prescribing.11,13 Recognizing the fundamental importance of the indication for prescribing, a comprehensive and curated list of SNOMED CT-coded indications for antimicrobial use has also been developed and integrated into the programme. Collection of such standardized data, along with the appropriateness definitions guide (Figure 2) and year-round online and telephone support, allows for a more accurate assessment of the quality of prescribing and higher inter-rater reliability of the survey.20 More recently, internal validation rules and algorithms have been implemented for improved and more standardized data collection, evaluation and assessments, to further improve inter-rater reliability.
Figure 2.
Hospital NAPS appropriateness definitions.
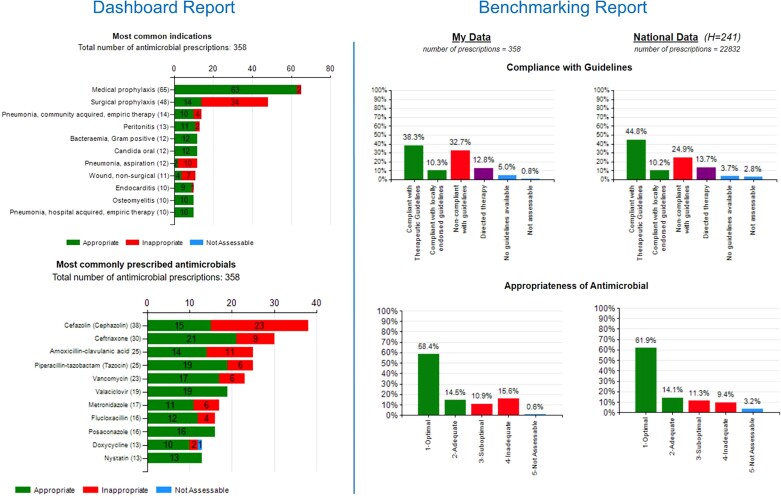
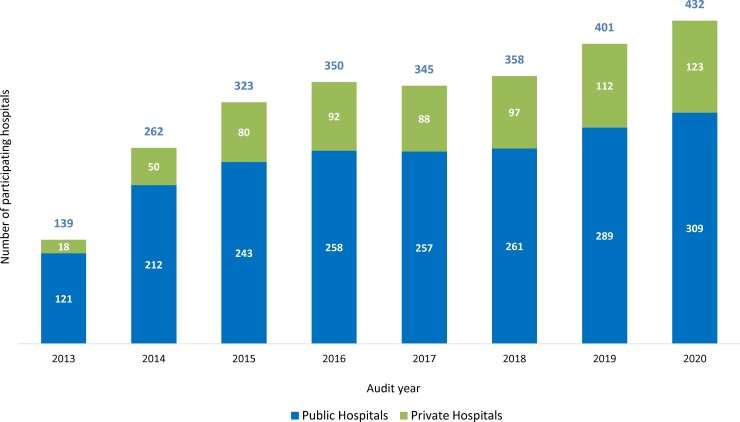
Hospitals are encouraged to complete the survey at least annually and online reports are immediately available and presented in a visually appealing and easy-to-understand format, highlighting key areas for improvement (Figure 3). Although voluntary, over 60% of all Australian public and private hospitals have participated in the survey (Figure 4).13–19 Following user feedback, and to meet the requirements of an ever-changing AMS landscape, there have been ongoing modifications including allowing flexible survey methodologies and many hospitals use the NAPS programme for more detailed, directed surveys of particular antimicrobials, indications or specialties. Due to the success of the Hospital NAPS, additional modules have been released, including Surgical NAPS,21–23 Quality Improvement NAPS12 and Aged Care NAPS.24–28
Figure 3.
Example of a Hospital NAPS dashboard and benchmarking report.
Figure 4.
Participation rate for public and private hospitals, by year of participation.
The uniformity of data collection enables benchmarking of results in real-time against other participating hospitals, allowing more targeted AMS initiatives at a local, regional or national level, based on high rates of prescribing inappropriateness, including by antimicrobial, indication, and specialty and according to facility type, patient case mix, size and location. Consequently, the NAPS programme was adopted as a core programme for the Antimicrobial Use and Resistance in Australia (AURA) reporting system,29–31 and has received funding support from the ACSQHC and the Australian Government’s Department of Health.
In addition to public reports,13–19,21–28 the nationally aggregated data from the NAPS have been analysed to identify and understand reasons for suboptimal prescribing in specific areas to help drive improvement initiatives. This includes determining the prevalence of antimicrobial allergy labels in inpatients, where the increased use of restricted antimicrobials in patients with cancer was discovered32 and allergy labels were associated with inappropriate use of broad-spectrum antimicrobials.33 Studies in the quality of antimicrobial use in paediatrics and neonates demonstrated risk factors for inappropriate antimicrobial prescribing were non-tertiary paediatric hospital admission and regional and remote hospital location,34 and substantial variation in dosing for antimicrobials prescribed for neonatal sepsis.35 Prescribing practices in haematology and oncology patients when compared with non-cancer acute inpatients revealed higher rates of appropriate prophylaxis when admitted under a haematology unit, although there were high rates of inappropriate carbapenem use in bone marrow transplant patients.36 Another key finding was that antimicrobial prescribing was more frequently inappropriate for some high-risk infections treated in rural and regional hospitals.37
Transferability of the NAPS programme
The Hospital NAPS has been successfully piloted in countries with well-resourced healthcare systems such as Canada, New Zealand and the UK, as well as countries with resource-limited healthcare settings, including Bhutan, Fiji, Malaysia, Papua New Guinea, Timor-Leste and Vietnam. We provide here a narrative review, including three countries and outlining their NAPS experiences.
Before the Hospital NAPS can be piloted internationally, several IT configurations are required, including creating a dedicated Hospital NAPS portal for each country to allow registration of the participating hospitals and auditors. Also, due to the various time zones encountered, and the time difference with Melbourne, Australia, the NAPS support team has developed in-application training videos and an eLearning module to support an in-country ‘train the trainer’ approach.
There have been minor modifications to each country’s NAPS database to reflect the local context and healthcare system. These include wording changes for the appropriateness assessment, the addition of antimicrobials available within the national or local antimicrobial formulary, additional routes of administration, frequencies and units, new classifications for auditors and facility types, and the modification and/or addition of indications for antimicrobial use.
Canada
Background
The Hospital NAPS was introduced to Canada by the Sinai Health-University Health Network Antimicrobial Stewardship Program (SH-UHN ASP) in 2018. They reviewed a variety of antimicrobial use (AMU) tools with the aim of addressing data gaps that had previously been identified in two AMS initiatives.38,39 These included a lack of data standardization, data on guideline compliance and definitions of appropriateness that could be applicable to any patient population. Furthermore, it was identified that timely access to AMU and appropriateness data was a challenge, and in some settings not currently possible.38,39 Following a demonstration to the SH-UHN ASP team and the Public Health Agency of Canada, it was agreed that the NAPS would not only meet their needs but remove barriers that had prevented the implementation of a national auditing programme.
Characteristics of the pilot hospitals
Out of 1417 Canadian hospitals, the team chose a representative sample that included both urban and rural/remote hospitals of varying sizes across the country. Although 20 was the initial hospital sample size planned, widespread interest increased this to 38 participating hospitals. The hospital size range was 25 to 540 beds; 16 were teaching hospitals and 22 were non-teaching/community hospitals. The provinces represented were British Columbia, Alberta, Ontario, Quebec, New Brunswick, Prince Edward Island and Nova Scotia, which collectively constitute over 90% of Canada’s population.40
Implementation
To support the implementation, the SH-UHN ASP appointed a dedicated programme manager (Y.N.), who became both a super-user and the Canadian application administrator. The team customized the NAPS materials but utilized the Australian training videos. Prior to the launch, the SH-UHN ASP team tested the NAPS application platform to ensure consistency of use and adjudication around guidelines adherence using the NAPS framework. Support from the Australian NAPS support team included 2–4 h of demonstration and training sessions, establishing the Canadian database, and addition of provinces and sites to support benchmarking.
Funding source
The Canadian Hospital NAPS pilot programme was supported by the Public Health Agency of Canada, an unrestricted grant from BD Canada, and in-kind resources from the SH-UHN ASP.
Key findings
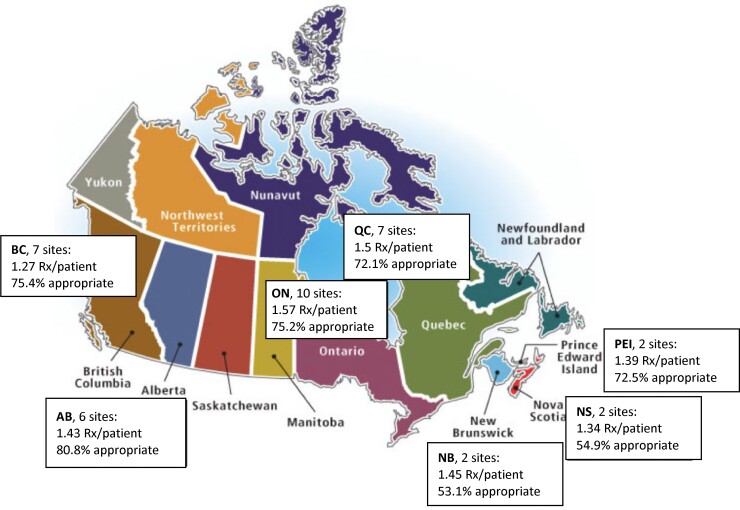
Among patients receiving at least one antimicrobial, those admitted to a teaching hospital received an average 1.48 prescriptions/patient, compared with 1.35 prescriptions/patient in non-teaching hospitals. The overall appropriateness of antimicrobial use was 73.7%, ranging from 53.1% to 80.8%. These figures are remarkably similar to the Australian surveys.19 Figure 5 summarizes the geographical variation in the quantity and quality of antimicrobial use.40
Figure 5.
Quantity and quality of antimicrobial prescribing by province among participating Canadian hospitals.
Implementation of NAPS in a specialized patient population
An AMS intervention in the haematology-oncology population using the Hospital NAPS is underway (led by M.S. and colleagues). Antimicrobials prescribed for patients in the acute leukaemia units were assessed against the institution’s high-risk febrile neutropenia protocol. The dashboard reports satisfy the requests from senior leadership for key performance indicators and an overview of prescribing patterns, while the detailed reports offer opportunities for patient-specific interventions between the AMS team and the primary leukaemia team. The NAPS is also being explored in the solid organ transplant population.41
Facilitators
The SH-UHN ASP had already adopted the concept of appropriateness assessment within their programme. The team had published a modified Delphi study on defining appropriateness of antimicrobial use in the critically ill,42 and documented their experiences with evaluating AMU in solid organ transplant recipients.43,44 The 24/7 availability of the platform provided flexibility to schedule audits when AMS teams had the human resources available. The programme was seen to have a remarkably high return on an AMS team’s time investment: the audits were relatively quick, and reports were available instantaneously. Such reports foster audit-and-feedback efforts, and the timeliness of the reports ensures AMS interventions are informed by data that are up to date and relevant for that hospital and context. Benchmarking and identifying trends nationally require using a tool that provides standardization of data collection but is still capable of meeting the specific needs of a diverse set of hospitals. As the data belong to the participating institution, access did not require reliance on the local IT department or permission from the NAPS support team or the NCAS.
The appropriateness assessment framework was intuitive. The NAPS had an established track record as a point-prevalence surveying tool and had been refined over the years based on feedback from clinicians who were the end-users. The NAPS supported the requirements that were identified by Canadian hospitals, enabling targeted or directed audits for quality improvement purposes, as well as repeat surveys that could be monitored using time-series run-charts. Dashboard reports and benchmarking at the patient care unit, service or hospital site level also allowed hospitals to compare themselves to their peer hospitals and provided an opportunity to have a deeper understanding of which AMU drivers may be institution specific as opposed to patient population specific. Furthermore, audits can be repeated on a smaller scale when fewer healthcare resources are available to conduct the audits. Finally, the auditing process and the ensuing results allowed the hospitals to meet the required organizational standards for hospital accreditation.
Barriers
In contrast to Australia, there are no national AMU guidelines in Canada. AMS programmes are organized by province, with provinces operating under a single health authority, several health authorities or none, and are managed at the hospital level. Accordingly, audits that use guidelines may rely on health authority guidelines, local guidelines or infectious diseases expertise where guidelines may not exist. This has implications for benchmarking guideline compliance specifically. In instances where a guideline did not exist, appropriateness may have been determined by a single infectious diseases expert’s advice, which is difficult to objectively verify. In Australia, there is a help desk to support remote appropriateness assessments. In Canada, the SH-UHN ASP provided similar support; this provided external validity and ensured the reliability of assessments.
Future planning
The Hospital NAPS initiative in Canada was implemented by a programme manager at a programmatic level as part of a national strategy, with the intention of sustaining the programme over time. The successful implementation of the pilot in Canada has led to an expansion of this AMS initiative nationally. From the 38 hospitals that originally participated in the pilot, the initiative has grown to include over 100 hospitals in Canada. Also, specialty clinical groups are advocating for the use of the Hospital NAPS so that they may leverage the benchmarking function specific to their patient population; for example, all the paediatric hospitals in Canada have registered to use the Hospital NAPS.
Malaysia
Background
The driver for the collaboration with the NCAS and use of the Hospital NAPS was that increased rates of multidrug-resistant (MDR) organisms and increasing broad-spectrum antimicrobial use were being observed. The Malaysian healthcare system is funded by the government, and AMS policies, programmes and guidelines have been available in Malaysia since 2014;45 however, there is no standardized surveillance system to monitor antibiotic prescribing practices in Malaysia. Furthermore, the Malaysian Action Plan on Antimicrobial Resistance (MyAP-AMR)46 and Malaysian Society for Quality in Health (MSQH)47 have outlined the need to implement an audit programme using a standardized tool that enables the assessment of the quality of prescribing and appropriateness of use, and to monitor improvement. The Malaysian health service accreditation system47 requires the establishment of a hospital infection and antibiotic control committee but there is no clear indication that appropriate antibiotic prescribing is a specific criterion. AMS activities are mainly in tertiary public hospitals.
Characteristics of pilot hospitals
Two university hospitals in Kuala Lumpur were selected: the University Malaysia Medical Centre (UMMC), which is the largest and oldest hospital with 1649 beds, nine infectious diseases physicians and two infectious diseases pharmacists, and the University Kebangsaan Medical Centre (UKMC), with 900 beds, two infectious diseases physicians and two infectious diseases pharmacists. Each hospital has an established AMS programme. UMMC has had antimicrobial guidelines since 2014, and these are reviewed every 2 years.48
Implementation
Endorsement was received from the Hospital Infection and Antibiotic Control Committee (HIACC) and the Medical Advisory Council (MAC), and local ethics approval was obtained in both hospitals.
A prospective hospital-wide audit was conducted in both UMMC and UKMC between 22 and 30 April 2019. The Hospital NAPS auditing programme was coordinated by PhD candidates and audits were conducted by infectious diseases physicians and pharmacists. An initial 1 h training session was provided with ongoing access to the online training materials. Assistance with difficult assessments was provided by the NAPS support team as required. Results of the audits were disseminated to management (presented at the HIACC and MAC meetings) and relevant prescribers through symposia, workshops and meetings with each department or unit. The project was designed to be completed in three phases: (i) a baseline hospital point-prevalence survey; (ii) development of a targeted AMS bundle based on a point-prevalence survey in each hospital; and (iii) evaluation of the impact of the implementation of the AMS bundle.
Funding source
Pfizer Independent Grants for Learning & Change (IGLC) provided project funding and The Joint Commission (USA) provided administrative oversight for the Malaysia NAPS pilot.
Key findings
Concordance of prescribing with guidelines and appropriateness of antimicrobial prescribing were similar between the two hospitals, at approximately 60%.49 Based on the results, key areas to target AMS interventions were the medical department in UMMC and surgical department in UKMC.
Special situations
In UMMC, a targeted point-prevalence survey was performed in November 2019 across 12 medical wards. A total of 434 prescriptions were assessed. Overall, the compliance with prescribing guidelines was 48.1%, and 58.2% of prescriptions were assessed as appropriate (the difference between the two being prescriptions that varied from guidelines for documented clinically justifiable reasons).49 The most common reasons for inappropriate antimicrobial use were the unnecessary use of broad-spectrum antimicrobials and the lack of a documented indication for antimicrobial use. A focus group was undertaken, using the nominal group technique. From this, the medical teams identified clinicians’ needs, including education and the introduction of interventions within the electronic medical record system (a pop-up reminder after 48 h, links to prescribing guidelines and antimicrobial history listed in case notes).
The UKMC point-prevalence survey was performed in January 2020 across 13 surgical wards; 318 procedures and 146 surgical prophylaxis prescriptions were assessed. Overall, compliance of antimicrobial prescribing with recommended guidelines was 56.0%, and 60.5% of antimicrobial prescriptions were assessed as appropriate. A frequent source of inappropriate use was surgical antibiotic prophylaxis.49 In response to these findings, the surgical teams requested a surgical prophylaxis prescribing care bundle, consisting of extra resources, a prescribing checklist as well as regular audits with prescriber feedback. The MAC also approved an automatic stop order for surgical prophylaxis after 24 h.
Facilitators
AMS policies and programmes have been available in Malaysia since 2014,45 and there were established multidisciplinary AMS committees in the two study hospitals. The NAPS was viewed as an internationally recognized tool that utilized a standardized assessment matrix for compliance and appropriateness. In fact, there were no modifications required other than some minor changes to indications and drug names in the database. This allowed clinicians to feel more reassured when feedback was provided, and there was greater acceptance of the validity of the data, which led to changes in hospital policies and procedures.
Barriers
AMS programmes are in their infancy in Malaysia and many clinicians are unaware of what it involves, which can lead to a lack of acceptance of recommendations by prescribers. There is also a prescribing hierarchy present, with senior doctors more inclined to prescribe based on experience rather than in accordance with guidelines. There is a lack of clinical resources and staff, including infectious diseases-trained clinical pharmacists, to carry out more active AMS initiatives.
Future planning
The investigator team report that the validated and standardized reports were trusted by the clinical staff. Local AMS champions have been appointed, and a basic interactive online AMS module has been developed. The results of the NAPS have also led to the antibiotic guidelines being updated. As outlined in the study design, further NAPS auditing will be undertaken to evaluate the impact of the implementation of the AMS bundles.
Bhutan
Background
The implementation of the Hospital NAPS in Bhutan was championed by a physician (P.C.) based at the Jigme Dorji Wangchuck National Referral Hospital, Thimphu, who has an interest in infectious diseases and AMS, and is a Fleming Fund fellow for antimicrobial usage in Bhutan. The Fleming Fund is a UK aid programme that supports low- and middle-income countries (LMICs) to generate, share and use data to improve antimicrobial use, and encourages investment in detection, monitoring and prevention of AMR. As part of this fellowship scheme, P.C. was tasked with developing an understanding of how antimicrobials are used within Bhutan. This was to complement the Royal Government of Bhutan’s National Action Plan on Antimicrobial Resistance (2018–22),50 whose objective 3 is to institute a surveillance and monitoring system for antimicrobial resistance and antimicrobial use.
Implementation
Other than establishing the administrative components in the NAPS database for Bhutan, there were no other modifications required. Online training of the local NAPS team by the Australian support team was provided (1 h), and oversight of an initial set of prescriptions to ensure that the appropriateness assessment was being correctly applied (2 h).
Funding source
The NAPS programme was implemented in Bhutan as part of the antimicrobial prescribing quality auditing requirements of a Fleming Fund fellowship programme.
Characteristics of pilot hospitals
To achieve the fellowship workplan activity, the Hospital NAPS was utilized to audit three main referral hospitals and one district hospital. These hospitals were selected as they were the sentinel sites for surveillance under the Fleming Fund country grant; they were the largest regional hospitals with microbiology facilities, with bed numbers ranging from 60 to 150.
Key findings
The automated reports generated by the Hospital NAPS allowed, for the first time, an understanding of how antimicrobials were used in Bhutan, including through identification of the indications for use and of indications and antimicrobials associated with inappropriate prescribing practices. From the three hospitals audited, 166 patients’ charts were reviewed, and 73 patients were identified as receiving antimicrobials and included in the data collection. From these, the average prevalence of antimicrobial use was 44.0%, the compliance with locally endorsed guidelines was 42.6%, and 54.6% of prescriptions were deemed appropriate. The team is yet to present the data to stakeholders and have not yet finished the survey at one site due to the COVID-19 pandemic and current unavailability of personnel.
Facilitators
This Fleming Fund grant was administered by the Peter Doherty Institute for Infection and Immunity (University of Melbourne and Royal Melbourne Hospital) in Melbourne, Australia, which provided immediate access to the NAPS support team and hence the opportunity to pilot the tool in Bhutanese hospitals with supervision and mentorship from AMS experts (K.B., K.T., R.J.).
The key factors that have contributed to the successful pilot include: the survey data are locally owned; the audit methodology is flexible; the survey is suitable for all hospital types; the web-based platform is not dependent on local IT infrastructure and resourcing; the data fields captured are practical and meaningful for AMS programmes; the assessment matrix for appropriateness did not require national or even local guidelines; and the pre-formatted and automated reports were very popular in the absence of local statistical support.
Barriers
There was no major barrier except the emergence of the COVID-19 pandemic, which delayed the training and reduced the clinical resources available for the NAPS programme.
Future planning
There is a plan to repeat surveys when resources are available and provide feedback to the prescribers and the Ministry of Health.
Conclusions
The volume of antimicrobial use and appropriateness of prescribing are essential metrics to guide and support national and local AMS programmes. We have demonstrated that national surveillance of antimicrobial prescribing appropriateness can deliver aggregated and meaningful ‘data for action’ to support quality improvement initiatives. Australia has an internationally recognized track record in the development of AMS programmes in hospitals, which has been supported through the incorporation of AMS in health service quality and safety standards. The NAPS has facilitated the collection of actionable data, and enabled local, jurisdictional and national health authorities to promote evidence-based efforts aimed at improving antimicrobial use in healthcare.
Internationally, it is well recognized that national and jurisdictional health authorities need to act urgently to mitigate the impacts of AMR, but they need support to implement and build on effective strategies. The successful implementation and piloting of the NAPS programme internationally provides support for such programmes in both advanced and LMIC healthcare settings. The success and sustainability of the NAPS in Australia is partly attributable to its incorporation into the national AMR strategy (and the national hospital accreditation standards specifically), but also an iterative approach that has continuously addressed the contexts, workflow patterns and needs of AMS programmes.
Acknowledgements
We would like to thank the many health system leadership and hospital team members who supported and participated in the local NAPS programme in each country, as well as the NAPS team and associated programme leaders, clinicians and researchers in Australia, Canada, Malaysia and Bhutan.
Funding
The Australian NAPS programme is supported by the Australian Commission on Safety and Quality in Health Care and the Australian Government’s Department of Health, the Guidance Group, Royal Melbourne Hospital and the National Centre for Antimicrobial Stewardship, Centre of Research Excellence (National Health and Medical Research Council grant APP1079625). The Canadian NAPS pilot programme was supported by the Public Health Agency of Canada, an unrestricted grant from BD Canada (AGR2017-04251JH), and in-kind resources from the Sinai Health-University Health Network Antimicrobial Stewardship Program. Pfizer Independent Grants for Learning & Change (IGLC) provided project funding (40867041) and The Joint Commission (USA) provided administrative oversight for the Malaysian NAPS pilot. The NAPS project in Bhutan received funding support from the UK Fleming Fund programme on AMR.
Transparency declarations
None to declare.
References
- 1. WHO . WHO Global Strategy for Containment of Antimicrobial Resistance. 2001. https://www.who.int/drugresistance/WHO_Global_Strategy_English.pdf.
- 2. WHO . Global Action Plan on Antimicrobial Resistance. 2015. https://www.who.int/publications/i/item/9789241509763.
- 3. Australian Commission on Safety and Quality in Health Care . Antimicrobial Stewardship in Australian Hospitals. 2011. https://www.safetyandquality.gov.au/sites/default/files/migrated/Antimicrobial_stewardship_prelim_execsummary.pdf.
- 4. Australian Commission on Safety and Quality in Health Care . National Safety and Quality Health Service Standards. 2013. https://www.safetyandquality.gov.au/standards/nsqhs-standards.
- 5. Thursky KA. The implementation challenges of undertaking national antimicrobial usage surveillance. Clin Infect Dis 2021; 73: 223–5. [DOI] [PubMed] [Google Scholar]
- 6. Meeker D, Linder JA, Fox CRet al. Effect of behavioral interventions on inappropriate antibiotic prescribing among primary care practices: a randomized clinical trial. JAMA 2016; 315: 562–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Borek AJ, Wanat M, Atkins Let al. Optimising antimicrobial stewardship interventions in English primary care: a behavioural analysis of qualitative and intervention studies. BMJ Open 2020; 10: e039284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. University of Antwerp Global Point Prevalence Survey of Antimicrobial Consumption and Resistance (Global-PPS). 2021. https://www.global-pps.com.
- 9. WHO . Point-Prevalence Survey on Antibiotic Use in Hospitals. 2021. https://www.who.int/initiatives/glass/glass-modules-8. [Google Scholar]
- 10. ECDC . ESAC-Net Reporting Protocol 2021. 2021. https://www.ecdc.europa.eu/en/publications-data/esac-net-reporting-protocol-2021.
- 11. James R, Upjohn L, Cotta Met al. Measuring antimicrobial prescribing quality in Australian hospitals: development and evaluation of a national antimicrobial prescribing survey tool. J Antimicrob Chemother 2015; 70: 1912–8. [DOI] [PubMed] [Google Scholar]
- 12. National Centre for Antimicrobial Stewardship . National Antimicrobial Prescribing Survey. 2021. https://www.naps.org.au/.
- 13. National Centre for Antimicrobial Stewardship and Australian Commission on Safety and Quality in Health Care . Antimicrobial Prescribing Practice in Australia: Results of the 2013 National Antimicrobial Prescribing Survey. 2014. https://www.safetyandquality.gov.au/our-work/antimicrobial-resistance/antimicrobial-use-and-resistance-australia-surveillance-system/aura-surveillance-system-data-sources/appropriateness-antimicrobial-use#hospital-naps.
- 14. National Centre for Antimicrobial Stewardship and Australian Commission on Safety and Quality in Health Care . Antimicrobial Prescribing Practice in Australia: Results of the 2014 National Antimicrobial Prescribing Survey. 2015. https://www.safetyandquality.gov.au/our-work/antimicrobial-resistance/antimicrobial-use-and-resistance-australia-surveillance-system/aura-surveillance-system-data-sources/appropriateness-antimicrobial-use#hospital-naps.
- 15. National Centre for Antimicrobial Stewardship and Australian Commission on Safety and Quality in Health Care . Antimicrobial Prescribing Practice in Australia: Results of the 2015 National Antimicrobial Prescribing Survey. 2016. https://www.safetyandquality.gov.au/our-work/antimicrobial-resistance/antimicrobial-use-and-resistance-australia-surveillance-system/aura-surveillance-system-data-sources/appropriateness-antimicrobial-use#hospital-naps.
- 16. National Centre for Antimicrobial Stewardship and Australian Commission on Safety and Quality in Health Care . Antimicrobial Prescribing Practice in Australia: Results of the 2016 National Antimicrobial Prescribing Survey. 2018. https://www.safetyandquality.gov.au/our-work/antimicrobial-resistance/antimicrobial-use-and-resistance-australia-surveillance-system/aura-surveillance-system-data-sources/appropriateness-antimicrobial-use#hospital-naps.
- 17. National Centre for Antimicrobial Stewardship and Australian Commission on Safety and Quality in Health Care . Antimicrobial Prescribing Practice in Australia: Results of the 2017 National Antimicrobial Prescribing Survey. 2018. https://www.safetyandquality.gov.au/our-work/antimicrobial-resistance/antimicrobial-use-and-resistance-australia-surveillance-system/aura-surveillance-system-data-sources/appropriateness-antimicrobial-use#hospital-naps.
- 18. National Centre for Antimicrobial Stewardship and Australian Commission on Safety and Quality in Health Care . Antimicrobial Prescribing Practice in Australian hospitals: Results of the 2018 Hospital National Antimicrobial Prescribing Survey. 2020. https://www.safetyandquality.gov.au/our-work/antimicrobial-resistance/antimicrobial-use-and-resistance-australia-surveillance-system/aura-surveillance-system-data-sources/appropriateness-antimicrobial-use#hospital-naps.
- 19. National Centre for Antimicrobial Stewardship and Australian Commission on Safety and Quality in Health Care . Antimicrobial Prescribing Practice in Australian hospitals: Results of the 2019 Hospital National Antimicrobial Prescribing Survey. 2021. https://www.safetyandquality.gov.au/our-work/antimicrobial-resistance/antimicrobial-use-and-resistance-australia-surveillance-system/aura-surveillance-system-data-sources/appropriateness-antimicrobial-use#hospital-naps.
- 20. Cotta MO, Spelman T, Chen Cet al. Evaluating antimicrobial therapy: how reliable are remote assessors? Infect Dis Health 2016; 21: 3–10. [Google Scholar]
- 21. National Centre for Antimicrobial Stewardship and Australian Commission on Safety and Quality in Health Care . Surgical National Antimicrobial Prescribing Survey in Australia: Results of the 2016 Pilot. 2017. https://www.safetyandquality.gov.au/our-work/antimicrobial-resistance/antimicrobial-use-and-resistance-australia-surveillance-system/aura-surveillance-system-data-sources/appropriateness-antimicrobial-use#surgical-naps.
- 22. National Centre for Antimicrobial Stewardship . Surgical Prophylaxis Prescribing in Australian Hospitals: Results of the 2017 and 2018 Surgical National Antimicrobial Prescribing Surveys. 2019. https://www.ncas-australia.org/ncas-publications.
- 23. National Centre for Antimicrobial Stewardship and Australian Commission on Safety and Quality in Health Care . Surgical Prophylaxis Prescribing in Australian Hospitals: Results of the 2019 Surgical National Antimicrobial Prescribing Survey. 2020. https://www.safetyandquality.gov.au/our-work/antimicrobial-resistance/antimicrobial-use-and-resistance-australia-surveillance-system/aura-surveillance-system-data-sources/appropriateness-antimicrobial-use#surgical-naps.
- 24. National Centre for Antimicrobial Stewardship and Australian Commission on Safety and Quality in Health Care . Antimicrobial Prescribing and Infections in Australian Residential Aged Care Facilities: Results of the 2015 Aged Care National Antimicrobial Prescribing Survey Pilot. 2016. https://www.safetyandquality.gov.au/our-work/antimicrobial-resistance/antimicrobial-use-and-resistance-australia-surveillance-system/antimicrobial-prescribing-australian-residential-aged-care.
- 25. National Centre for Antimicrobial Stewardship and Australian Commission on Safety and Quality in Health Care . 2016 Aged Care National Antimicrobial Prescribing Survey Report. 2017. https://www.safetyandquality.gov.au/our-work/antimicrobial-resistance/antimicrobial-use-and-resistance-australia-surveillance-system/antimicrobial-prescribing-australian-residential-aged-care.
- 26. National Centre for Antimicrobial Stewardship and Australian Commission on Safety and Quality in Health Care . 2017 Aged Care National Antimicrobial Prescribing Survey Report. 2018. https://www.safetyandquality.gov.au/our-work/antimicrobial-resistance/antimicrobial-use-and-resistance-australia-surveillance-system/antimicrobial-prescribing-australian-residential-aged-care.
- 27. National Centre for Antimicrobial Stewardship and Australian Commission on Safety and Quality in Health Care . 2018 Aged Care National Antimicrobial Prescribing Survey Report. 2019. https://www.safetyandquality.gov.au/our-work/antimicrobial-resistance/antimicrobial-use-and-resistance-australia-surveillance-system/antimicrobial-prescribing-australian-residential-aged-care.
- 28. National Centre for Antimicrobial Stewardship and Australian Commission on Safety and Quality in Health Care . 2019 Aged Care National Antimicrobial Prescribing Survey Report. 2020. https://www.safetyandquality.gov.au/our-work/antimicrobial-resistance/antimicrobial-use-and-resistance-australia-surveillance-system/antimicrobial-prescribing-australian-residential-aged-care.
- 29. Australian Commission on Safety and Quality in Health Care . AURA 2017 Second Australian Report on Antimicrobial Use and Resistance in Human Health. 2017. https://www.safetyandquality.gov.au/publications-and-resources/resource-library/aura-2017-second-australian-report-antimicrobial-use-and-resistance-human-health.
- 30. Australian Commission on Safety and Quality in Health Care . AURA 2019: Third Australian Report on Antimicrobial Use and Resistance in Human Health. 2019. https://www.safetyandquality.gov.au/our-work/antimicrobial-resistance/antimicrobial-use-and-resistance-australia-surveillance-system/aura-2019.
- 31. Australian Commission on Safety and Quality in Health Care . AURA 2021: Fourth Australian Report on Antimicrobial Use and Resistance in Human Health. 2021. https://www.safetyandquality.gov.au/our-work/antimicrobial-resistance/antimicrobial-use-and-resistance-australia-surveillance-system/aura-2021.
- 32. Trubiano JA, Leung VK, Chu MYet al. The impact of antimicrobial allergy labels on antimicrobial usage in cancer patients. Antimicrob Resist Infect Control 2015; 4: 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Trubiano JA, Chen C, Cheng ACet al. Antimicrobial allergy ‘labels’ drive inappropriate antimicrobial prescribing: lessons for stewardship. J Antimicrob Chemother 2016; 71: 1715–22. [DOI] [PubMed] [Google Scholar]
- 34. McMullan BJ, Hall L, James Ret al. Antibiotic appropriateness and guideline adherence in hospitalized children: results of a nationwide study. J Antimicrob Chemother 2020; 75: 738–46. [DOI] [PubMed] [Google Scholar]
- 35. McMullan B, Cooper C, Spotswood Net al. Antibiotic prescribing in neonatal sepsis: an Australian nationwide survey. BMJ Paediatrics Open 2020; 4: e000643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Douglas AP, Hall L, James RSet al. Quality of inpatient antimicrobial use in hematology and oncology patients. Infect Control Hosp Epidemiol 2021; 42: 1235–44. [DOI] [PubMed] [Google Scholar]
- 37. Bishop JL, Schulz TR, Kong DCMet al. Similarities and differences in antimicrobial prescribing between major city hospitals and regional and remote hospitals in Australia. Int J Antimicrob Agents 2019; 53: 171–6. [DOI] [PubMed] [Google Scholar]
- 38. Morris A, Nakamachi Y. CAHO Adopting Research to Improve Care (ARTIC) Program - Antimicrobial Stewardship Program (ASP) in Intensive Care Units (ICU). Final Report for the Council of Academic Hospitals of Ontario. Council of Academic Hospitals of Ontario, 2014. [Google Scholar]
- 39. Nakamachi Y, Senthinathan A, Morris A. Adopting Research to Improve Care (ARTIC) Community Hospital ICU Local Leadership (CHILL) Antimicrobial Stewardship Program (ASP). Final Report for the Council of Academic Hospitals of Ontario. Council of Academic Hospitals of Ontario, 2016. [Google Scholar]
- 40. Nakamachi Y, So M, Morris A. Canadian National Antimicrobial Prescribing Survey (NAPS) Pilot. Final Report for the Public Health Agency of Canada. Public Health Agency of Canada, 2019. [Google Scholar]
- 41. So M, Hand J, Forrest Get al. White paper on antimicrobial stewardship in solid organ transplant recipients. Am J Transplant 2022; 1: 96–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Dresser LD, Bell CM, Steinberg Met al. Use of a structured panel process to define antimicrobial prescribing appropriateness in critical care. J Antimicrob Chemother 2018; 73: 246–9. [DOI] [PubMed] [Google Scholar]
- 43. So M, Yang DY, Bell Cet al. Solid organ transplant patients: are there opportunities for antimicrobial stewardship? Clin Transplant 2016; 30: 659–68. [DOI] [PubMed] [Google Scholar]
- 44. So M, Morris AM, Nelson Set al. Antimicrobial stewardship by academic detailing improves antimicrobial prescribing in solid organ transplant patients. Eur J Clin Microbiol Infect Dis 2019; 38: 1915–23. [DOI] [PubMed] [Google Scholar]
- 45. Ministry of Health Malaysia . Protocol on Antimicrobial Stewardship Program in Healthcare Facilities. 2014. http://www.aesculapseguridaddelpaciente.org.mx/docs/antimicrobianos/Protocol_on_Antimicrobial_Stewardship.pdf.
- 46. Ministry of Health and Ministry of Agriculture and Agro-Based Industry Malaysia . Malaysian Action Plan on Antimicrobial Resistance (MyAP-AMR) 2017-2021. 2017. https://www.moh.gov.my/moh/resources/Penerbitan/Garis%20Panduan/Garis%20panduan%20Umum%20(Awam)/National_Action_Plan_-_FINAL_29_june.pdf. [Google Scholar]
- 47. Malaysian Society for Quality in Health. MSQH Standards . 2017. http://www.msqh.com.my/web/index.php/en/accreditation-programme/hospital-accreditation-programme/5th-edition/5th-edition-hospital-accreditation-standards.
- 48. University Malaya Medical Centre . UMMC On-line Antibiotic Guideline. 2021. https://farmasi.ummc.edu.my/ummc-on-line-antibiotic-guideline. [Google Scholar]
- 49. Jamaluddin NAH, Periyasamy P, Lau CLet al. Point prevalence survey of antimicrobial use in a Malaysian tertiary care university hospital. Antibiotics 2021; 10: 531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Royal Government of Bhutan . National Action Plan on Antimicrobial Resistance [2018-2022]. 2017. https://www.moh.gov.bt/wp-content/uploads/ict-files/2021/04/NATIONAL-ACTION-PLAN-ON-AMR.pdf. [Google Scholar]
- 51. Therapeutic Guidelines Ltd . Therapeutic Guidelines: Antibiotic. 2021. https://www.tg.org.au/.
- 52. Australian Institute of Health and Welfare . Australian Hospital Peer Groups. 2015. https://www.aihw.gov.au/reports/hospitals/australian-hospital-peer-groups/summary.