Abstract
Objectives
Cefiderocol is a siderophore cephalosporin active against MDR Gram-negatives including Stenotrophomonas maltophilia. Cefiderocol resistance remains uncommon and incompletely understood. We selected for cefiderocol-resistant S. maltophilia in vitro and characterized the genetic mechanisms and potential for cross-resistance to other antimicrobials.
Methods
We selected cefiderocol resistance in three clinical strains of S. maltophilia by serial passage in escalating concentrations of cefiderocol. Emergent cefiderocol-resistant isolates were subjected to repeat susceptibility testing against a panel of relevant antimicrobials. Isolates with confirmed MIC changes were whole genome sequenced.
Results
Each parent strain was initially susceptible to cefiderocol (MICs of 0.03125, 0.03125 and 0.125 mg/L), and one initially tested susceptible to ceftazidime/avibactam (MIC 4 mg/L). We recovered evolved isolates achieving cefiderocol resistance at MICs of 8–32 mg/L from each parental strain. Some cefiderocol resistant isolates reverted following one to four drug-free passages. Ceftazidime/avibactam MICs of passaged isolates repeatedly increased to ≥256 mg/L, and while other MICs were largely unchanged, trimethoprim/sulfamethoxazole MICs declined 4-fold in two strains. WGS revealed one evolved isolate carrying six coding mutations, while four were isogenic mutants of tonB, tolQ, smf-1 and the smeT promoter. Mutation of the smeT promoter downregulated the smeDEF efflux pump and reduced susceptibility to penicillins but increased susceptibility to several other classes including sulphonamides. Other mutations occurred in genes putatively involved in iron metabolism including smlt1148 and cirA.
Conclusions
S. maltophilia strains evolved cefiderocol resistance through different genetic pathways, but often involved iron transport. Future work is required to fully understand the role(s) of other genes in cefiderocol resistance.
Introduction
Cefiderocol is a siderophore cephalosporin with activity against Gram-negatives including many carbapenem-resistant strains, although most isolates included in studies have been Enterobacterales or P. aeruginosa.1,2 There is limited information about cefiderocol resistance among Stenotrophomonas maltophilia,1–5 a difficult-to-treat pathogen with many innate resistance mechanisms including chromosomally encoded class A serine and class B metallo-β-lactamases that render most β-lactams ineffective.6S. maltophilia is an important nosocomial pathogen among oncology patients that may also cause pneumonia in patients with obstructive lung disease or cystic fibrosis. The treatment of choice for S. maltophilia is trimethoprim/sulfamethoxazole, however, its utility is sometimes reduced by resistance or tolerability, especially among haematology and oncology patients.7 Due to its unique mechanism of action, cefiderocol is promising for the treatment of S. maltophilia. Since cefiderocol has recently been introduced and has a novel mechanism of action, clinical isolates that are resistant to cefiderocol are uncommon. Consequently, the full spectrum of mutations and mechanisms that could mediate cefiderocol resistance are incompletely understood, especially for S. maltophilia. Our study objective was to identify and describe possible mutational mechanisms of cefiderocol resistance in S. maltophilia using in vitro serial passage techniques and WGS.
Methods
Serial passage, susceptibility testing and phenotype stability
Using three independent, cefiderocol-susceptible S. maltophilia blood isolates obtained from the University of Washington Medical Center (W358, W359, and W364), we selected for cefiderocol resistance by serial passage in escalating concentrations of cefiderocol in cation-adjusted iron-depleted Mueller–Hinton broth beginning at 0.5× the MIC (CA-IDMHB; provided by Shionogi Inc. through International Health Management Associates). Once growth was visible (within 8 h to 2 days) a sample of the broth was diluted 1:1000 into fresh CA-IDMHB with twice the previous concentration of cefiderocol. Fifty microlitres was plated onto Mueller–Hinton agar and a Kirby–Bauer disc containing 30 mg of cefiderocol was added to screen for the emergence of isolates with reduced susceptibility. Emergent isolates with reduced zone sizes (<16 mm) on screening plates were subjected to repeat MIC testing in duplicate by broth microdilution (ATCC 27853 used as QC strain) to cefiderocol, ceftazidime, trimethoprim/sulfamethoxazole and minocycline, as well as ceftazidime/avibactam by MIC test strip (Liofilchem®). MICs were read after incubation at 37°C for 24 h. Passages continued for 21 days and cefiderocol concentrations ranged from 0.03 mg/L on Day 1 to as high as 32 mg/L by Day 21. Recovered isolates were passaged on drug-free medium for four passages with repeated MIC testing to assess stability of the resistance phenotype.
WGS
WGS was performed on a selection of mutant isolates displaying elevated MICs of cefiderocol relative to their parental strains using the MiSeq platform with 300 cycle chemistries (Illumina, San Diego, CA, USA) to an average read depth of 86× (minimum depth of 22× and down-sampled to a maximum depth of 100×), as previously described.8 Genomes were analysed essentially as described by Roach et al.,8 except using ABySS 2.0.29 for de novo assembly, bwa-mem (v0.7.12)10 for alignment, and SAMtools (v1.1)11 for variant calling. Variant calling was performed using reference genomes identified as most closely matching the sequenced isolates. Mutations were manually verified using the Integrative Genomics Viewer,12 then annotated with sequence features using SnpEFF.13 Copy number variants were investigated using CNOGpro.14 Sequence data from this study are available from the NCBI Sequence Read Archive under accession PRJNA707063 (https://www.ncbi.nlm.nih.gov/bioproject/PRJNA707063/). Mutations in coding regions and susceptibility data are summarized in Table 1.
Table 1.
Changes in susceptibility (MICs, mg/L) to various antimicrobials among isolates recovered from cefiderocol serial passage experiments
| Strain | Time to isolate emergence (days) | FDC | CAZ/AVI | CAZ | MIN | TMP/SMX | FDC stability: no. of drug-free passes | Variants | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2 | 3 | 4 | gene | nucleic acid | amino acid | |||||||
| W357-Parent | — | 0.03125 | 4 | 128 | 4 | 0.5/9.5 | 0.03125 | 0.0625 | 0.03125 | — | — | — |
| W357-2-10 | 15 | 32 | ≥256 | 256 | 2 | 0.125/2.375 | 8 | 4.0–8.0 | 4.0–8.0 | smeT | c.−49_−48insTTGT | (promoter) |
| W358-Parent | — | 0.125 | 64 | ≥256 | 0.5 | 0.125/2.375 | 0.0625 | 0.0625 | 0.03125 | — | — | — |
| W358-1-25 | 21 | 32 | ≥256 | ≥256 | 0.5 | 0.25/4.75 | 16 | 16 | 8.0–16.0 | smlt1148 |
c.2413A > C |
p.Thr805Pro |
| tesB | c.−33T>C | (promoter) | ||||||||||
| panD | c.258_260delCAT | p.Ile87del | ||||||||||
| smlt3318 | c.383T>A | p.Leu128Gln | ||||||||||
| cysK1 | c.−59G>A | (promoter) | ||||||||||
| cirA | c.112_122dupTCCCGTTCCGC | p.Arg42fs | ||||||||||
| W358-2-2 | 12 | 8 | ≥256 | ≥256 | 0.5 | 0.125/2.375 | 1.0–2.0 | 1 | 1 | smf-1 | c.394A > G | p.Thr132Ala |
| W358-2-15 | 18 | 16 | ≥256 | ≥256 | 0.5 | 0.25/4.75 | 0.0625 | 0.0625 | 0.0625 | tolQ | c.647C > A | p.Pro216Gln |
| W364-Parent | — | 0.03125 | 48 | 64–128 | 1 | 1/19 | 0.03125 | 0.03125 | 0.03125 | — | — | — |
| W364-8 | 16 | 8 | ≥256 | 256 | 1 | 0.25/4.75 | 8 | 2.0–4.0 | 2 | tonB | c.376_384dupGCTCCGCCG | p.Ala126_Pro128dup |
FDC, cefiderocol; CAZ, ceftazidime; CAZ/AVI, ceftazidime/avibactam; MIN, minocycline; TMP/SMX, trimethoprim/sulfamethoxazole.
Cefiderocol MICs were repeated after passage from the freezer onto drug-free medium for up to four passes. Variants list the gene name, followed by the DNA variant and when relevant the amino acid changes.
RT-PCR
To assess the impact of the mutation in the smeT promoter observed in W357-2-10 we used RT-PCR to measure differences in the expression of the smeDEF operon regulator smeT, and separately, the operon itself using the first and last structural genes in that unit (smeD and smeF). RNA was extracted from mid-log phase cultures using RNeasy Bacterial Kit (Qiagen), and iScript Reverse Transcriptase was used for cDNA generation. Results were analysed using the double-delta Ct method, normalizing gene expression of sme gene transcripts against gyrA expression. Primer sequences for smeT,15smeD,16smeE17 and gyrA17 are published elsewhere.
Phenotype microarray
We investigated potential differences in candidate phenotypes between W357-2-10 and W357 using a phenotype microarray system (Biolog) due to the stability of the cefiderocol resistance phenotype and then unclear contribution of the smeT promotor mutation observed. Both strains were inoculated into panels PM9-PM13 and cellular respiration was monitored for 48 h as described previously.18 The parent and mutant respiration curves were integrated and compared then summarized in Table S1 and Figure S1 (available as Supplementary data at JAC-AMR Online).
Results
Isolates recovered from serial passage experiments and their MICs are listed in Table 1. We recovered isolates with reduced susceptibility to cefiderocol in all three genetic backgrounds. Ceftazidime/avibactam MICs increased from 4 mg/L, 48 mg/L and 64 mg/L to ≥256 mg/L in the evolved strains. Cross-resistance to other agents was limited, but trimethoprim/sulfamethoxazole MICs declined by 4-fold in two of the three genetic backgrounds (W357 and W364).
Whole genome analysis revealed isogenic mutations of tonB, smf-1, tolQ and the smeT promoter in 4/5 isolates, without other identifiable changes (Table 1). The remaining isolate carried six separate coding mutations.
The smeT-promoter mutant displayed the greatest increase in cefiderocol MIC from baseline (1024-fold), with MIC elevations of 128–256-fold above baseline persisting for ≥4 passages on drug-free medium. Mutants of smf-1, tolQ and tonB displayed smaller and less stable MIC elevations.
We also identified several other isolates from resistance screening plates that, despite elevated MICs, did not acquire identifiable mutations or changes in gene copy number, which has been suggested as a potential mechanism of heteroresistance. Such isolates’ resistance phenotypes were unstable and rapidly lost when subcultured on drug-free medium.
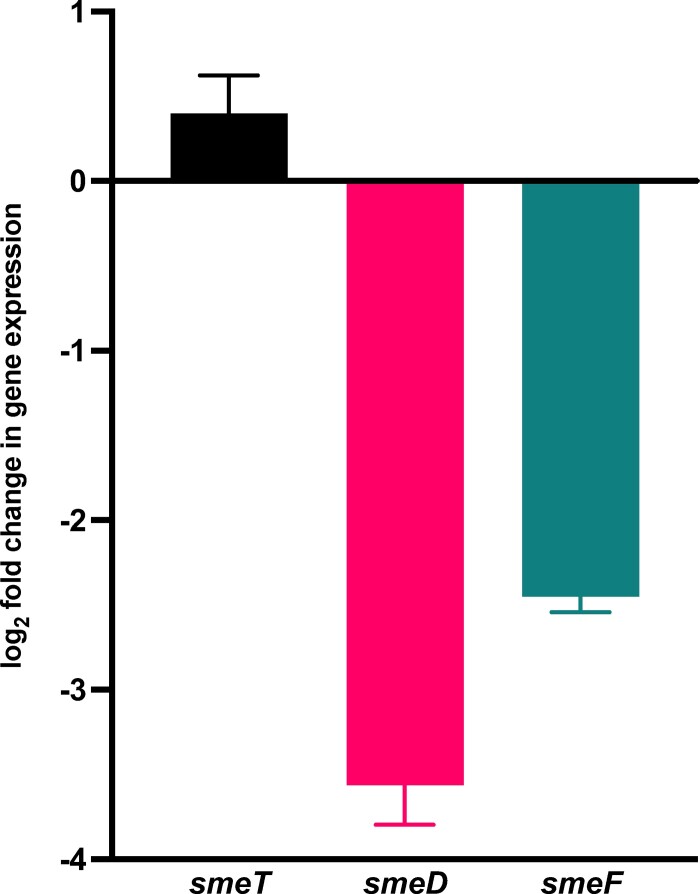
RT-PCR revealed that the smeT promoter mutation in W357-2-10 was associated with a >1.3-fold increase in smeT expression and a ∼10-fold reduction in smeDEF expression (Figure 1). Phenotype microarrays of W357 and W357-2-10 (Figure S1 and Table S1) indicate that, overall, W357-2-10 was more resistant to penicillins and vancomycin, while W357 was more resistant to a variety of intracellularly active agents (chloramphenicol, nalidixic acid, sulphonamides, tetracyclines) as well as polymyxin B and benzethonium chloride.
Figure 1.
Log2 fold change in expression of smeTDEF operon elements in mutant W357-2-10 relative to parent strain W357. Expression of sme elements was determined by RT-PCR and normalized to gyrA expression and is shown relative to the gene expression in the parental strain. Error bars indicate standard deviation of measurements.
Discussion
In this study we determined that cefiderocol resistance evolved in S. maltophilia in vitro can emerge via a variety of genetic pathways and with variable degrees of phenotypic stability. Our results suggest that reduced susceptibility could be inducible in some passaged isolates, without any detectible genetic changes, but the basis and significance of these elusive and unstable phenotypes is unclear. While we did not observe mutations in genes associated with canonical β-lactam resistance, such as those that encode penicillin-binding proteins or β-lactamases, we did observe some concurrent increases in MICs of ceftazidime and ceftazidime/avibactam among our resistant isolates. The passaged isolate with the highest resistance carried six separate coding mutations, but most harboured isogenic mutations of single genes (Table 1).
The smeT promoter mutant (W357-2-10) exhibited the greatest and most stable resistance phenotype of all the single-gene mutants identified in the study. This mutant exhibited cross-resistance to ceftazidime/avibactam. Phenotype microarray data also showed that W357-2-10 had reduced susceptibility to oxacillin, nafcillin and cloxacillin, but other β-lactams on the panels (amoxicillin, ampicillin, azlocillin, carbenicillin, cefazolin, ceftriaxone, cefuroxime, cephalothin and penicillin) had similar activity at the concentrations tested, which are proprietary (Biolog). Although none of these agents is generally presumed to be meaningfully active against S. maltophilia, such differences suggest some degree of β-lactam cross-resistance. SmeT is a repressor of the S. maltophilia TetR family MDR efflux pump smeDEF.19,20smeDEF overexpression, typically related to smeT mutation, has been associated with resistance to a variety of antimicrobials including tetracyclines, trimethoprim/sulfamethoxazole and fluoroquinolones.21 In contrast, we observed a mutation in the smeT promoter region accompanying reductions in trimethoprim/sulfamethoxazole MICs. Furthermore, the candidate phenotypes from the microarray data showed that the strain with the smeT promoter mutation was potentially more susceptible to demeclocycline, doxycycline, nalidixic acid and four sulphonamides. Collectively, these susceptibility profiles suggest that a reduction in SmeDEF activity, which was confirmed by our RT-PCR showing upregulated smeT and decreased smeDEF expression, contributes to cefiderocol resistance. It is presently unclear how reduced smeDEF expression contributes to this phenotype, but the effect may be indirect since previous work suggests β-lactams are poor substrates for SmeDEF.
It was unsurprising to recover a mutation of iron transporter tonB given the known role of TonB-dependent receptors in active transport of siderophore drug conjugates.22 Ceftazidime resistance has also been linked to TonB function, so while the relevant strain was initially resistant to ceftazidime, tonB mutations could theoretically mediate cross-resistance between siderophore conjugates and traditional β-lactams.23
It is unclear how smf-1, which affects fimbriae and surface adhesion, or transmembrane transporter tolQ, affect cefiderocol susceptibility, and these mechanisms warrant further investigation. Of the six mutated genes observed in W358-1-25 two have a plausible link to cefiderocol resistance: cirA, which is a TonB-dependent receptor family protein previously implicated in cefiderocol resistance,24 and smlt1148, a putative iron transport protein. Other mutations involve amino acid and fatty acid metabolism in ways that may contribute to metabolic resistance phenotypes.25
Conclusions
Our study suggests that disparate mutations, many in genes relating to iron transport, occur with the emergence of cefiderocol resistance in S. maltophilia in vitro. We also report a counterintuitive relationship between the smeDEF transporter downregulation and cefiderocol resistance, which warrants further study. Future work should also focus on whether cefiderocol exposure, especially clinically meaningful kinetic exposures, can select for changes in MDR efflux pumps that could affect other classes of antimicrobials.
Supplementary Material
Acknowledgements
We thank Dr Lori Bourassa for helping to screen and select the original clinical isolates, and Dr Colin Manoil and Samuel Lee for helping with the Biolog Phenotype Microarray experiments.
Funding
This study was supported by an investigator-initiated research grant from Shionogi Inc. B.J.W. is also supported in part by National Institute of Allergy and Infectious Diseases of the National Institutes of Health under the award number R01AI136979. S.J.S. is also supported in part by SINGH19R0 from the Cystic Fibrosis Foundation.
Transparency declarations
B.J.W. and A.B. report research grants from Shionogi Inc. All other authors: none to declare.
Author contributions
B.J.W., A.B. and S.J.S. conceived of and designed the study. N.K.A. and B.J.W. performed the serial passage, resistance screening and susceptibility testing. K.P., A.W., E.A.H. and S.J.S. performed the WGS and analysis, and the RT-PCR. B.J.W. wrote the first draft, and all authors reviewed the data, prepared the manuscript and approved the final version.
Supplementary data
Table S1 and Figure S1 are available as Supplementary data at JAC-AMR Online.
References
- 1. Hackel MA, Tsuji M, Yamano Yet al. . In vitro activity of the siderophore cephalosporin, cefiderocol, against a recent collection of clinically relevant Gram-negative bacilli from North America and Europe, including carbapenem-nonsusceptible isolates (SIDERO-WT-2014 study). Antimicrob Agents Chemother 2017; 61: e00093-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hackel MA, Tsuji M, Yamano Yet al. . In vitro activity of the siderophore cephalosporin, cefiderocol, against carbapenem-nonsusceptible and multidrug-resistant isolates of Gram-negative bacilli collected worldwide in 2014 to 2016. Antimicrob Agents Chemother 2018; 62: e01968-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Nakamura R, Oota M, Matsumoto Set al. . In vitro activity and in vivo efficacy of cefiderocol against Stenotrophomonas maltophilia. Antimicrob Agents Chemother 2021; 65: e01436-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Biagi M, Vialichka A, Jurkovic Met al. . Activity of cefiderocol alone and in combination with levofloxacin, minocycline, polymyxin B, or trimethoprim-sulfamethoxazole against multidrug-resistant Stenotrophomonas maltophilia. Antimicrob Agents Chemother 2020; 64: e00559-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Gill CM, Abdelraouf K, Oota Met al. . Discrepancy in sustained efficacy and resistance emergence under human-simulated exposure of cefiderocol against Stenotrophomonas maltophilia between in vitro chemostat and in vivo murine infection models. J Antimicrob Chemother 2021; 76: 2615–21. [DOI] [PubMed] [Google Scholar]
- 6. Sanchez MB. Antibiotic resistance in the opportunistic pathogen Stenotrophomonas maltophilia. Front Microbiol 2015; 6: 658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Fuchs M, Scheid C, Schulz Aet al. . Trimethoprim/sulfamethoxazole prophylaxis impairs function of mobilised autologous peripheral blood stem cells. Bone Marrow Transplant 2000; 26: 815–6. [DOI] [PubMed] [Google Scholar]
- 8. Roach DJ, Burton JN, Lee Cet al. . A year of infection in the intensive care unit: prospective whole genome sequencing of bacterial clinical isolates reveals cryptic transmissions and novel microbiota. PLoS Genet 2015; 11: e1005413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Jackman SD, Vandervalk BP, Mohamadi Het al. . ABySS 2.0: resource-efficient assembly of large genomes using a Bloom filter. Genome Res 2017; 27: 768–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Li H, Durbin R. Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics 2009; 25: 1754–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Li H, Handsaker B, Wysoker Aet al. . The sequence alignment/map format and SAMtools. Bioinformatics 2009; 25: 2078–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Robinson JT, Thorvaldsdóttir H, Wenger AMet al. . Variant review with the integrative genomics viewer. Cancer Res 2017; 77: e31–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Cingolani P, Platts A, Wang LLet al. . A program for annotating and predicting the effects of single nucleotide polymorphisms, SnpEff: SNPs in the genome of Drosophila melanogaster strain w1118; iso-2; iso-3. Fly (Austin) 2012; 6: 80–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Brynildsrud O, Snipen L-G, Bohlin J. CNOGpro: detection and quantification of CNVs in prokaryotic whole-genome sequencing data. Bioinformatics 2015; 31: 1708–15. [DOI] [PubMed] [Google Scholar]
- 15. Wang G, Xu N, Yang Let al. . Community acquired Stenotrophomonas maltophilia discitis: diagnosis aided by shotgun metagenomic sequencing. Int J Infect Dis 2019; 81: 1–3. [DOI] [PubMed] [Google Scholar]
- 16. Zhang L, Li X-Z, Poole K. SmeDEF multidrug efflux pump contributes to intrinsic multidrug resistance in Stenotrophomonas maltophilia. Antimicrob Agents Chemother 2001; 45: 3497–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Gould VC. SmeDEF-mediated antimicrobial drug resistance in Stenotrophomonas maltophilia clinical isolates having defined phylogenetic relationships. J Antimicrob Chemother 2006; 57: 1070–6. [DOI] [PubMed] [Google Scholar]
- 18. Martin SE, Melander RJ, Brackett CMet al. . Small molecule potentiation of Gram-positive selective antibiotics against Acinetobacter baumannii. ACS Infect Dis 2019; 5: 1223–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hernández A, Maté MJ, Sánchez-Díaz PCet al. . Structural and functional analysis of SmeT, the repressor of the Stenotrophomonas maltophilia multidrug efflux pump SmeDEF. J Biol Chem 2009; 284: 14428–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Sánchez P, Alonso A, Martinez JL. Cloning and characterization of SmeT, a repressor of the Stenotrophomonas maltophilia multidrug efflux pump SmeDEF. Antimicrob Agents Chemother 2002; 46: 3386–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Sánchez MB, Martínez JL. The efflux pump SmeDEF contributes to trimethoprim-sulfamethoxazole resistance in Stenotrophomonas maltophilia. Antimicrob Agents Chemother 2015; 59: 4347–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Luscher A, Moynié L, Auguste PSet al. . TonB-dependent receptor repertoire of Pseudomonas aeruginosa for uptake of siderophore-drug conjugates. Antimicrob Agents Chemother 2018; 62: e00097-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Calvopiña K, Dulyayangkul P, Heesom KJet al. . TonB-dependent uptake of β-lactam antibiotics in the opportunistic human pathogen Stenotrophomonas maltophilia. Mol Microbiol 2020; 113: 492–503. [DOI] [PubMed] [Google Scholar]
- 24. Ito A, Sato T, Ota Met al. . In vitro antibacterial properties of cefiderocol, a novel siderophore cephalosporin, against Gram-negative bacteria. Antimicrob Agents Chemother 2017; 62: e01454-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lopatkin AJ, Bening SC, Manson ALet al. . Clinically relevant mutations in core metabolic genes confer antibiotic resistance. Science 2021; 371: eaba0862. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.