Key Points
The Amperial assay was used to measure salivary SARS-CoV-2 S1 Abs.
IgG of 42 COVID-19 mRNA vaccinated individuals was longitudinally evaluated.
Twenty percent of subjects experienced a 90% drop of peak IgG levels over time.
Abstract
We used a noninvasive electrochemical quantitative assay for IgG Abs to SARS-CoV-2 S1 Ag in saliva to investigate the kinetics of Ab response in a community-based population that had received either the Pfizer or Moderna mRNA-based vaccine. Samples were received from a total of 97 individuals, including a subset of 42 individuals who collected samples twice weekly for 3 mo or longer. In all, >840 samples were collected and analyzed. In all individuals, salivary SARS-CoV-2 S1 IgG Ab levels rose sharply in the 2-wk period after their second vaccination, with peak Ab levels seen at 10–20 d after vaccination. We observed that 20%, 10%, and 2.4% of individuals providing serial samples had a 90%, 95%, and 99% drop, respectively, from peak levels during the duration of monitoring, and in two patients, Abs fell to prevaccination levels (5%). The use of noninvasive quantitative salivary Ab measurement can allow widespread, cost-effective monitoring of vaccine response.
Introduction
The pandemic caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has led to worldwide deaths and social and economic disruption. In the autumn of 2020, the Food and Drug Administration issued emergency use authorization for two mRNA-based vaccines manufactured by Pfizer/BioNTech (COMIRNATY; Pfizer) or Moderna/National Institute of Allergy and Infectious Diseases (Moderna). Both vaccines use mRNA sequences from the S1 domain of the SARS-CoV-2 spike protein (1–5) and require two doses given 21 or 28 d apart, respectively, to achieve 95% protection against SARS-CoV-2 infection (1–5). It is unclear whether all individuals developed Abs with 5% at risk for breakthrough infection or whether a modest fraction of individuals did not respond or develop Abs and remained at risk for infection.
Unlike the predicted statistics for the healthy general population, it is known that patients receiving immunosuppressive drugs and patients with cancer may not develop a robust Ab response to vaccine administration (6). It is possible that a fraction of individuals in the population may have an undetected immune deficiency that prevents them from responding appropriately to the standard vaccine regimen. Consequently, the Centers for Disease Control and Prevention (CDC) is currently recommending that booster immunizations be deployed beginning in the autumn of 2021 (7).
Several studies have demonstrated that circulating Ab levels decrease over time after either vaccination or infection (8–13). Breakthrough infections are being observed in fully vaccinated individuals. It is not known what level, if any, of circulating Abs is required to have immunoprotection against coronavirus disease 2019 (COVID-19) infection. Current publications report very little information regarding the kinetics of Ab levels in patients after vaccination, and these studies only report Ab levels at 5.5-wk and 90-d intervals after second vaccination, respectively (14, 15). Currently, research is underway to determine the necessity for booster immunization doses and the timing in protecting against SARS-CoV-2 infection, especially in light of the emergence of highly contagious variants such as the delta variant that may be less sensitive to the current vaccines.
It is clear that most, if not all, individuals receiving both doses of either the Pfizer or Moderna vaccine respond with a robust IgG response (1–5). However, what is lacking is frequent kinetic monitoring and long-term monitoring of Ab levels in a community-vaccinated population. Noninvasive monitoring using saliva allows frequent and long-term monitoring of vaccinated individuals and entire populations.
We have previously developed a saliva-based quantitative assay for IgG Abs to the S1 domain of spike protein in SARS-CoV-2 using a novel electrochemical platform formerly known as “EFIRM” (electric field–induced release and measurement) and now called “Amperial” (16). Previously, we used this assay to monitor patients who had recovered from COVID-19. This assay was >98% specific for individuals with prior COVID-19 infections and gave proportional results to serum assays performed at the same time for the same patient. Two other groups have similarly demonstrated the ability of saliva to be a surrogate for serum or plasma measurement of SARS-CoV-2 Abs (17, 18).
In this study we longitudinally evaluated individuals who had received an mRNA vaccination for SARS-CoV-2. The data demonstrate that quantifying Ab levels in saliva can be used to longitudinally follow individuals after vaccination and demonstrated that all individuals tested had a robust Ab response after two injections. However, a significant proportion of individuals experienced a decline in Ab levels of >90% in the 3–4-mo period after the completion of their vaccine regimen.
Materials and Methods
SARS-CoV-2 salivary assay equipment
The Amperial platform uses a proprietary 96-well microtiter plate containing gold electrodes at the bottom of each well and an electrochemical reader system (EZLife Bio Inc, Los Angeles, CA). The detailed description of the Amperial COVID-19 Ab assay and the assay performance and validation have been described previously (16). We selected the S1 Ag as the capture Ab because both the Pfizer and Moderna vaccines use mRNA coding for the S1 Ag. In addition, we have found that Abs to S1 Ag are more specific than Abs for receptor-binding domain (RBD) in uninfected and unimmunized populations (16).
Summary of Amperial quantitative saliva assay for anti-S1 IgG to SARS-CoV-2
Initially, plates are prepared by the addition, in alternate rows, of a native pyrrole solution and a pyrrole solution containing recombinant S1 to the bottom of the 96-well gold electrode plate. The pyrrole solution is then polymerized by applying a square wave current using the Amperial reader.
Saliva samples are then diluted 1:10, and 30 µl of solution is added to each well. Each patient sample is added to both a native pyrrole well and a well containing S1 Ag. After incubation, signal generation is performed by successive incubations and wash cycles with biotinylated goat anti-human IgG Fc and Poly80 HRP.
In the final step, Ultra-TMB is added to the wells, and the plate is immediately inserted into the Amperial reader, where simultaneous measurement of electrochemical current for all 96 wells is performed two discrete times for 60 s. All patient values are normalized by subtracting the signal of the polymer-only wells with the Ag-polymer–coated wells. A 3-point standard curve is established for each plate by the addition of standards to each plate, allowing conversion from measured current to pg/ml.
An assay for IgA anti-S1 Ab was performed identically to the IgG assay with the substitution of goat anti-human IgA for the goat anti-human IgG Fc.
Human subjects
The research protocol and consents were approved by the Western Internal Review Board (Study 1302611; expiration date: March 19, 2022). Individuals under the age of 18 y, receiving immunosuppressive drugs, with prior COVID-19 infections, or having received cancer chemotherapy were excluded from the study.
Volunteers who had previously received a Pfizer (BioNTech), Moderna, or Johnson and Johnson vaccine for SARS-CoV-2 were consented. Subjects were issued a questionnaire to collect information about vaccination dates and vaccine type, along with questions to eliminate subjects who met exclusionary criteria.
The study had both a longitudinal and a cross-sectional study arm. For the longitudinal study, a cohort receiving either the Pfizer (n = 15) or Moderna (n = 27) mRNA vaccine was monitored with a first-morning, twice-weekly collection. Collection first thing in the morning was used as a means to reduce potential confounding effects of diurnal variations, food or beverage ingestion, and smoking. Collections continued for up to 8 mo after vaccination for some individuals. We analyzed saliva at a single time point for another 31 and 24 individuals receiving the Pfizer and Moderna vaccines, respectively. This allowed us to make several conclusions regarding the kinetics of COVID-19 vaccine response in community-vaccinated populations. In all, >840 samples were collected and analyzed.
Sample processing device and protocol
Saliva samples were collected using the OraSure oral fluid collection device (OraSure Technologies, Bethlehem, PA), which consists of an absorbent pad on the end of a long wand and a collection tube containing preservative solution. Subjects insert the absorbent pad into their mouth between their cheek and gum for a minimum of 2 min to absorb adequate saliva fluids. The absorbent pad is then immersed into a collection tube, and the wand is broken at a scored breakpoint to allow the device to be securely capped. Individuals participating in longitudinal studies placed the capped collectors in a resealable zipper storage bag and then into their home freezer until shipping them to the laboratory at ambient temperature. Individuals providing single samples kept the samples at room temperature until shipment to the laboratory.
Upon receipt at a central laboratory, the tube is uncapped, the pad gently pressed on the inside of the tube to squeeze out saliva, and the saliva transferred into a labeled microcentrifuge tube for testing. Samples are stored at −80°C for long-term storage. Previous evaluations of the collection system (16) have demonstrated the system can store the samples at room temperature for >10 d without significant degradation in Ab levels.
The collection protocol recommended by the manufacturer specifies centrifugation to remove the fluid from the collector. We have demonstrated that squeezing the pad against the side of the tube yields similar results to centrifugation, so we eliminated the centrifugation step in our protocol.
Variant Ag (delta)
To determine whether the Abs of vaccinated or convalescent patients were reactive to the delta variant, we used SARS-CoV-2 variant S1 Ag B.1.6.617.2 (40591-V08H23; SinoBiological, Wayne, PA), a recombinant Ag that included T194, G142D, E156G, 157-158 deletions and the L452R, T478K, D614G, and 681R mutations. Identical amounts of this variant Ag are immobilized in the gel, and the standard assay is performed.
Results
Kinetic studies
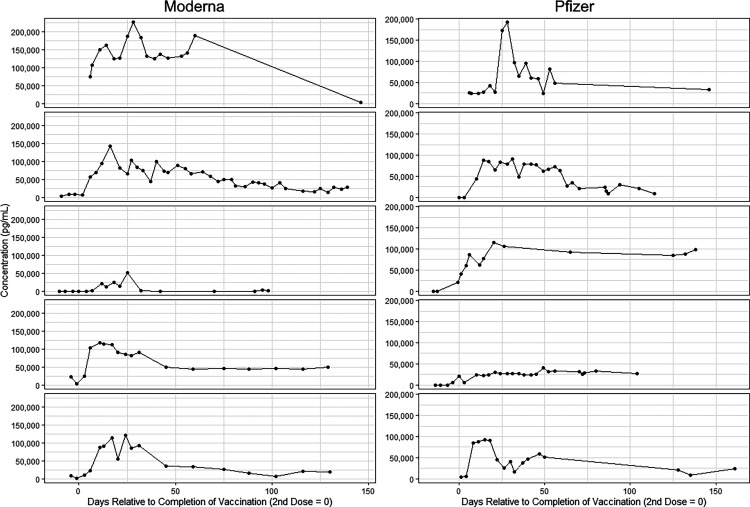
We analyzed 42 vaccinated patients (27 Moderna, 15 Pfizer) who provided twice-weekly samples for a period of several weeks. The general patterns for all patients were comparable. (Fig. 1 demonstrates eight representative samples from this longitudinal study. The curves are oriented with time 0 being the date of the second injection. The general patterns are similar for individuals receiving both vaccines with a spike in SARS-CoV-2 S1 IgG Ab production 1–2 wk after the second injection followed by a steady decrease in Ab levels. There were differences in the robustness of response, however, with most volunteers having robust responses with 100–200 ng/ml of IgG to SARS-CoV-2 S1 as a peak response followed by a gradual decrease in levels over time.
FIGURE 1.
Representative individual kinetic experiments with Pfizer and Moderna vaccines, with graphs centered on time 0 as the day of the second vaccination.
As can be seen in (Fig 1, however, two individuals responded with a maximum level of 50 ng/ml. Individual 3 in the Pfizer group had a peak response of ∼50 ng/ml and then stabilized at ∼25 ng/ml. Individual 3 in the Moderna group had a short-duration peak of 50 ng/ml followed by a return to baseline 30 d after the second vaccination. All but two patients experienced a gradual but steady decline in SARS-CoV-2 IgG S1 Ab levels. These decreasing levels may eventually correlate with the need for booster vaccinations.
Clinical trial data revealed ∼50% protection for individuals 2 wk after receiving their first immunization with either the Modern or Pfizer vaccine (1–5). We wondered whether this could be a function of Ab response in vaccinated patients. Of the 42 subjects who were serially monitored, 36 supplied samples before the second dose. In the subjects whose samples were collected before the second dose, 88% of the Moderna subjects had detectable SARS-CoV-2 IgG S1 IgG Abs before the second dose, and 50% of the Pfizer subjects had detectable SARS-CoV-2 IgG S1 IgG Abs before the second dose (see Table I).
Table I.
Summary of subjects with measurable Abs before completion of second dose
| Moderna | Pfizer | Combined | |
|---|---|---|---|
| Individuals with data collected before second dose | 26 | 10 | 36 |
| Abs produced before second dose | 23 (88%) | 5 (50%) | 28 (77%) |
Summary statistics for kinetic studies
Table II shows the summary statistics for the volunteers participating in the kinetic studies. Table III summarizes basic demographics regarding the sex and age of the kinetic study participants. The average time to maximum SARS-CoV-2 S1 IgG Ab level was 22 d after the second dose for Moderna and 30 d after the second dose for Pfizer. The maximum levels were nearly identical for the two vaccines, with Moderna-vaccinated individuals having average peak levels of 127 ng/ml and 130 ng/ml, respectively, for Moderna-vaccinated and Pfizer-vaccinated individuals. These levels are similar to those we observed in convalescent hospitalized patients with COVID-19 and fivefold higher than more mildly symptomatic individuals (16).
Table II.
Summary statistics for kinetic studies on vaccinated volunteers
| Moderna | Pfizer | Combined | |
|---|---|---|---|
| Total subjects for longitudinal study | 27 | 15 | 42 |
| Average time after second dose to maximum Ab level | 22 d | 28 d | 24 d |
| Average maximum Ab level | 127 ng/ml | 127 ng/ml | 127 ng/ml |
Table III.
Summary demographics of longitudinally monitored cohort
| Variable | Data |
|---|---|
| Age, y | |
| <20 | 0 |
| 20–29 | 0 |
| 30–39 | 2 |
| 40–49 | 5 |
| 50–59 | 1 |
| 60–69 | 20 |
| 70–79 | 12 |
| ≥80 | 2 |
| Sex | |
| Male | 20 |
| Female | 22 |
| Vaccine | |
| Pfizer | 15 |
| Moderna | 27 |
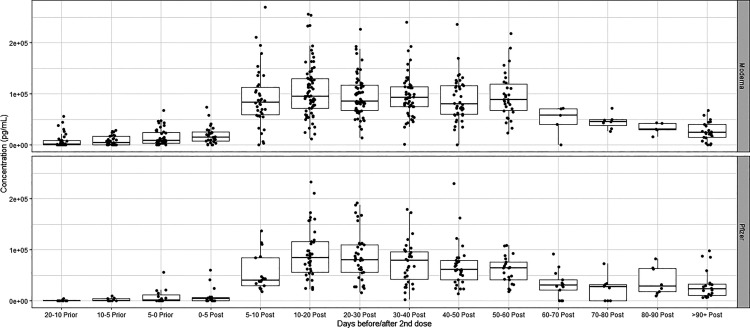
In addition to the 42 volunteers participating in the kinetic studies, we had an additional 53 individuals who submitted single samples for this study. (Fig. 2 is a summary of all the data representing 840 individual time points, including the multiple time points for the 42 volunteers submitting multiple samples and the 53 volunteers submitting a single sample. The data are represented with the date of the second immunization set as time 0.
FIGURE 2.
Samples collected from volunteer subjects (n = 99) at different time intervals for Pfizer (n = 47) and Moderna (n = 52) vaccines were tested and binned to different time intervals relative to completion of the second dose of mRNA vaccine.
(Fig. 2 is a summary of these data showing a box plot of weekly SARS-CoV-2 S1 IgG Ab levels for individuals both before and after second vaccination. The trend is clear. Robust IgG Ab levels to SARS-CoV-2 S1 are present for the initial 60 d after the second vaccination. Subsequently, levels begin falling gradually but consistently. The data demonstrate a steady decrease in Ab levels with increasing time after vaccination.
We initially tested vaccinated subjects for IgA subtype of SARS-CoV-2 S1 Abs. We were unable to detect any IgA SARS-CoV-2 S1 Abs in the saliva of vaccinated patients (see Supplemental Fig. 1).
Kinetic studies and demographics
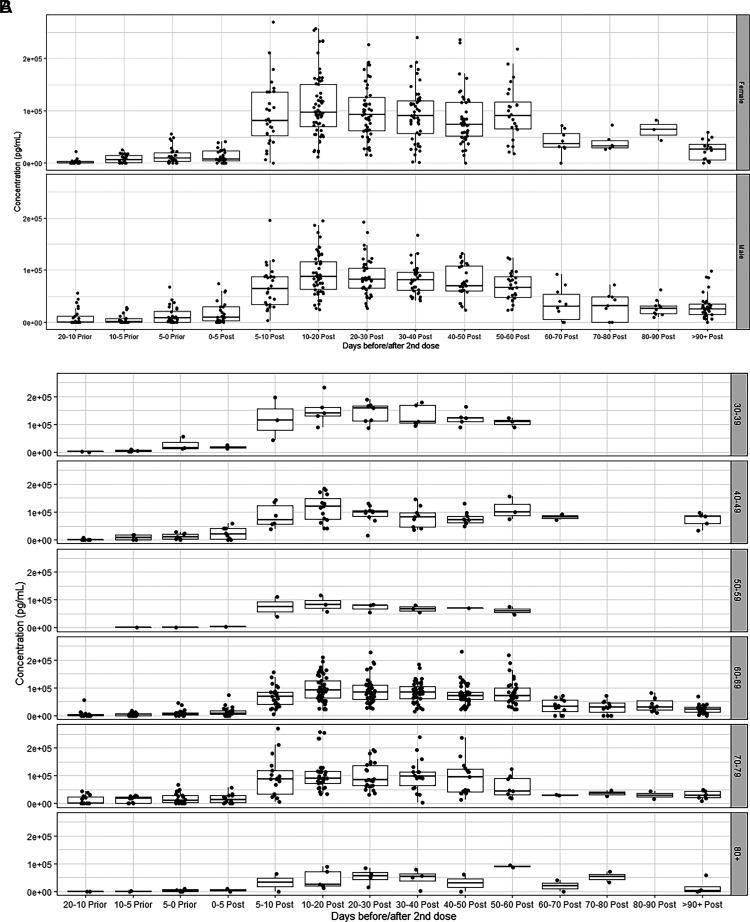
We investigated whether age or sex had any effect on vaccine kinetics. (Fig. 3A represents IgG SARS-CoV-3 S1 Abs with respect to sex, and (Fig. 3B represents Abs with respect to age in 10-y bins. There are clearly no sex differences in vaccine response. There is no apparent decrease in S1 IgG Ab response with age until 80 y. Although our study only has two patients in that age range, it is clear that their peak values are less than those of younger individuals. However, all other age groups had similar responses.
FIGURE 3.
(A) Longitudinally collected samples (n = 42), by sex. (B) Longitudinally collected samples, by age range.
Summary of subjects with significant drops from peak value
Table IV is a summary of volunteers who experienced drops of >90%, >95%, and >99% from their peak values, grouped by vaccine type. Although a higher percentage of Pfizer-vaccinated patients experienced a decrease of 90% or more (33% versus 15%) and 95% or more (13% versus 8%), no Pfizer volunteers experienced a 99% drop, whereas one Moderna-vaccinated patient experienced a 99% drop in Ab levels (see Table IV). The numbers are not sufficient to form any conclusions regarding any potential differences between the two vaccines, but they do show that, with time, IgG Abs to SARS-CoV-2 S1 levels drop to 90% of their peak level in 20% of community-vaccinated individuals.
Table IV.
Individuals with large drops in Ab levels
| Moderna | Pfizer | Combined | |
|---|---|---|---|
| Subjects with ≥90% drop | 15% (4 of 27) | 33% (5 of 15) | 19% (8 of 42) |
| Subjects with ≥95% drop | 8% (2 of 27) | 13% (2 of 15) | 10% (4 of 42) |
| Subjects with ≥99% drop | 4% (1 of 27) | 0% (0 of 15) | 2.4% (1 of 42) |
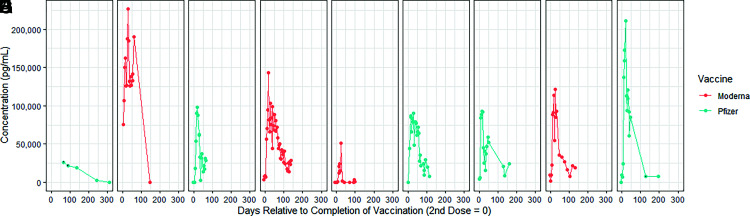
(Fig. 4 contains the kinetic plots of the nine vaccinated individuals who experienced >90% drops in SARS-CoV-2 S1 IgG Ab levels after vaccination. For subjects who experienced significant drops and had no other samples collected within a 2-wk time frame, duplicates were made to confirm the drop (Fig. 4A, 4B, and 4I). It is apparent that there is no correlation with the original peak value with prediction of an eventual 90% drop in Ab level. Two individuals had peak levels >200 ng/ml, indicating a robust initial response. There were two volunteers who had initial peak values of only 50 ng/ml whose Abs also dropped to low levels. Two individuals (5%) had their Abs drop to undetectable levels, one a Pfizer patient and one a Moderna patient. The age distributions of the nine individuals are as follows: one (50%) subject >80 y old, two (16%) subjects between 70 and 79 y old, and six (30%) subjects between 60 and 69 y old. The sex distribution of the nine individuals consisted of five females and four males. Of note is that no individual younger than age 60 y experienced a >90% drop in Ab level during the study.
FIGURE 4.
(A–I) Plots of individuals measured with >90% drop from peak. Panels (A)–(I) represent nine individual volunteers.
Case study: prednisone effects
A 69-y-old male was given a 3-wk course of prednisone (50 mg/d for 14 d followed by 1 wk of tapering) for a nasal polyp ∼2 mo after his second dose of vaccine. The kinetics of his Ab production is shown in (Fig. 5 with the time period that the prednisone was being administered highlighted in gray. As can be seen, Ab levels began falling with the onset of treatment to baseline levels and remained suppressed for several weeks after the taper. However, SARS-CoV-2 S1 IgG Ab levels eventually did rebound and then began to slowly decline thereafter.
FIGURE 5.
Case study plot of a Pfizer-vaccinated individual who was administered prednisone after his vaccination. Gray shaded area indicates the period of time when prednisone was taken.
Evaluation of delta variant Ag to salivary Abs
The delta variant of SARS-CoV-2 has become the predominant variant in the United States. We therefore investigated whether Abs present in convalescent and vaccinated patients are capable of recognizing the S1 Ag of the delta variant. We designed an Amperial assay, substituting monoclonal S1 delta variant Ag for the wild-type S1 Ag (see Materials and Methods). The standard curves for this assay compared with the wild-type Ag assay are shown in (Fig. 6A and demonstrate very similar assay characteristics. There is some indication of slightly reduced binding efficiency for anti-S1 IgG mAbs to the delta variant versus the wild-type S1 Ag, but these differences are not enough to alter testing results.
FIGURE 6.
(A) Standard curves for both the wild-type and delta variant S1 Ags. (B) Comparison of wild-type anti–SARS-CoV-2 IgG S1 and B.1.6.617.2 variant S1 SARS-CoV-2 Ag.
Next, we investigated whether Abs present in convalescent individuals and vaccinated individuals could recognize and bind to the delta variant S1 Ag. A total of three pre-2019 samples were used as controls along with one immunodeficient organ transplant patient run for reference. Three samples from convalescent patients with detectable Ab levels were used. These patients were infected before the delta variant emerged. In addition, three Pfizer-vaccinated and four Moderna-vaccinated individuals with detectable Abs were analyzed. All samples were assayed in parallel using both the wild-type assay and the delta Amperial assay.
These data are shown in (Fig. 6B. For all cases of vaccinated and convalescent subjects, there was no significant reduction of apparent Ab concentration in saliva to the delta variant versus the wild type.
Discussion
This study demonstrates that although all individuals vaccinated with Pfizer or Moderna vaccine develop a robust anti–SARS-CoV-2 S1 IgG Ab response, the response wanes over time. Approximately 20% of vaccinated individuals experience a drop-off >90% 90 d after vaccination. In two (5%) serially monitored patients, Ab levels became undetectable. The ability to monitor vaccine response noninvasively can be an important way to identify individuals who may require additional injections without straining strained health care resources.
Although some variability is seen among individuals in terms of fluctuating levels, it is easy to determine trends over time using serial saliva monitoring. Previous studies have determined that serum and saliva levels are highly correlated (16–18). However, one cannot predict the absolute serum level by measuring the salivary level. It appears that each individual has his or her own gradient between saliva and serum. However, as the data in the article demonstrate, that gradient remains relatively constant over time, allowing longitudinal monitoring to be performed.
Community immunity from widespread vaccination is a key component in preventing COVID-19 infections and in curbing the pandemic. Several questions remain unanswered. Our data can help provide the answers to some of these questions, discussed in the next paragraphs.
Does everyone respond to vaccination with a robust immune response? In this study, all vaccinated individuals did respond, although some with much lower SARS-CoV-2 S1 IgG Ab levels than the average. Ab monitoring after vaccination could identify the individuals who did not react to vaccination with a robust Ab response and allow these individuals to have an immunologic evaluation or an additional injection or a different vaccine type.
Will booster vaccination be necessary? Recent data regarding breakthrough infections and CDC recommendations are for immunocompromised individuals, and patients receiving immunosuppressive therapy should receive a third dose of the vaccine, regardless of timing. Health care workers and high-risk individuals are now eligible to receive boosters. Our data support this approach in that most individuals experience a continuous drop in SARS-CoV-2 S1 IgG Ab levels with time, and 5% of individuals’ Abs dropped to undetectable levels. However, no individual under the age of 60 has experienced a 90% drop in S1 IgG Ab levels. Although it is not clear what level of Abs, if any, is necessary to prevent COVID-19 infection, individuals with baseline levels of Abs may be at higher risk of acquiring a breakthrough infection.
Will a fourth vaccination be needed? Future kinetic studies will be necessary to determine if SARS-CoV-2 IgG Ab levels will remain stable after a third vaccination. Noninvasive monitoring using saliva home collection provides a low-cost, effective way to perform population monitoring of vaccine levels after a third vaccination.
Will the current vaccines protect against the delta variant? Our data show that SARS-CoV-2 S1 IgG Abs produced in convalescent patients and mRNA-vaccinated subjects do recognize the delta variant. In addition, convalescent individuals infected before the emergence of the delta variant also developed Abs that recognize the delta variant. Although this cannot ensure an equal protection level against serious infection, it is reassuring.
Could any individuals in low-risk groups benefit from booster vaccination? Our data suggest that ∼20% of individuals experience a fall >90% of SARS-CoV-2 S1 IgG Ab levels 3 mo after completion of their vaccination protocol. These individuals might benefit from early booster shots to prevent breakthrough infections. If an individual with a low SARS-CoV-2 S1 IgG Ab level is identified by saliva testing, further evaluation can be performed using serum titers to confirm the initial observation.
We should stress that it has not been determined what level of circulating SARS-CoV-2 S1 IgG Abs, if any, is necessary to prevent COVID infection. Furthermore, this study focused primarily on SARS-CoV-2 S1 IgG Abs, and there are other SARS-CoV-2 Ab tests (such as for the RBD component of the spike protein) or isotypes (IgM, IgA) that may possess different dynamics. The data in this article must be interpreted in light of these study limitations. An Amperial quantitative assay for RBD IgG has already been developed, and studies are underway to compare the RBD and S1 response to vaccination.
Nevertheless, the ability to noninvasively and cost-efficiently quantify COVID-19 Ab levels could be an important tool in investigating the relationship between circulating Abs and immunity. We have observed that i.m. vaccination does not induce a sustained salivary IgA response. This is not surprising in that the salivary glands are not directly exposed to Ag after an i.m. injection. Further studies may be helpful in further elucidating this point.
The data presented in this study regarding the salivary monitoring of SARS-CoV-2 S1 IgG are congruent with recommendations given by the CDC and established literature regarding SARS-CoV-2 Abs in vaccinated populations. Although there still remains a need for a more comprehensive evaluation of the relationship between salivary SARS-CoV-2 Abs and those present in the blood, our work demonstrates that our noninvasive quantitative saliva assay could be valuable for evaluating a community-vaccinated population and to further investigate the relationship between circulating Abs and COVID-19 immunity.
Supplementary Material
Acknowledgments
We thank the volunteers who participated in the study.
This work was supported by National Center for Advancing Translational Sciences Grant U18 TR003778 and National Heart, Lung, and Blood Institute Grant U54 HL119893 (to D.T.W.W.).
The online version of this article contains supplemental material.
- CDC
- Centers for Disease Control and Prevention
- COVID-19
- coronavirus disease 2019
- RBD
- receptor-binding domain
- SARS-CoV-2
- severe acute respiratory syndrome coronavirus 2
Disclosures
D.T.W.W. is a consultant for Colgate-Palmolive, Mars Wrigley, and GlaxoSmithKline. D.T.W.W. also has equity in Liquid Diagnostics LLC and RNAmeTRIX. C.M.S., M.K.T., and R.A.B. are shareholders in Liquid Diagnostics LLC. M.K.T. is a paid consultant for Liquid Diagnostics LLC. M.K.T. is a shareholder for EZLife Bio Inc. R.A.B. is a consultant for Amgen and Bristol Myers Squibb. The other author has no financial conflicts of interest.
References
- 1. Jackson L. A., Anderson E. J., Rouphael N. G., Roberts P. C., Makhene M., Coler R. N., McCullough M. P., Chappell J. D., Denison M. R., Stevens L. J., et al. mRNA-1273 Study Group . 2020. An mRNA vaccine against SARS-CoV-2 — preliminary report. N. Engl. J. Med. 383: 1920–1931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Barrett J. R., Belij-Rammerstorfer S., Dold C., Ewer K. J., Folegatti P. M., Gilbride C., Halkerston R., Hill J., Jenkin D., Stockdale L., et al. Oxford COVID Vaccine Trial Group . 2021. Phase 1/2 trial of SARS-CoV-2 vaccine ChAdOx1 nCoV-19 with a booster dose induces multifunctional antibody responses. Nat. Med. 27: 279–288. [DOI] [PubMed] [Google Scholar]
- 3. Sahin U., Muik A., Derhovanessian E., Vogler I., Kranz L. M., Vormehr M., Baum A., Pascal K., Quandt J., Maurus D., et al. 2020. COVID-19 vaccine BNT162b1 elicits human antibody and TH1 T cell responses. [Published erratum appears in 2021 Nature 590: E17.] Nature 586: 594–599. [DOI] [PubMed] [Google Scholar]
- 4. Wang Z., Schmidt F., Weisblum Y., Muecksch F., Barnes C. O., Finkin S., Schaefer-Babajew D., Cipolla M., Gaebler C., Lieberman J. A., et al. 2021. mRNA vaccine-elicited antibodies to SARS-CoV-2 and circulating variants. Nature 592: 616–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ella R., Vadrevu K. M., Jogdand H., Prasad S., Reddy S., Sarangi V., Ganneru B., Sapkal G., Yadav P., Abraham P., et al. 2021. Safety and immunogenicity of an inactivated SARS-CoV-2 vaccine, BBV152: a double-blind, randomised, phase 1 trial. Lancet Infect. Dis. 21: 637–646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Monin L., Laing A. G., Muñoz-Ruiz M., McKenzie D. R., Del Molino Del Barrio I., Alaguthurai T., Domingo-Vila C., Hayday T. S., Graham C., Seow J., et al. 2021. Safety and immunogenicity of one versus two doses of the COVID-19 vaccine BNT162b2 for patients with cancer: interim analysis of a prospective observational study. Lancet Oncol. 22: 765–778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Centers for Disease Control and Prevention. COVID-19 booster shots. Available at: https://www.cdc.gov/coronavirus/2019-ncov/vaccines/booster-shot.html. Accessed: August 31, 2021. [PubMed]
- 8. Xiang T., Liang B., Fang Y., Lu S., Li S., Wang H., Li H., Yang X., Shen S., Zhu B., et al. 2021. Declining levels of neutralizing antibodies against SARS-CoV-2 in convalescent COVID-19 patients one year post symptom onset. Front. Immunol. 12: 708523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Robbiani D. F., Gaebler C., Muecksch F., Lorenzi J. C. C., Wang Z., Cho A., Agudelo M., Barnes C. O., Gazumyan A., Finkin S., et al. 2020. Convergent antibody responses to SARS-CoV-2 in convalescent individuals. Nature 584: 437–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ni L., Ye F., Cheng M.-L., Feng Y., Deng Y.-Q., Zhao H., Wei P., Ge J., Gou M., Li X., et al. 2020. Detection of SARS-CoV-2-specific humoral and cellular immunity in COVID-19 convalescent individuals. Immunity 52: 971–977.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Rodda L. B., Netland J., Shehata L., Pruner K. B., Morawski P. A., Thouvenel C. D., Takehara K. K., Eggenberger J., Hemann E. A., Waterman H. R., et al. 2021. Functional SARS-CoV-2-specific immune memory persists after mild COVID-19. Cell 184: 169–183.e17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Zhao J., Yuan Q., Wang H., Liu W., Liao X., Su Y., Wang X., Yuan J., Li T., Li J., et al. 2020. Antibody responses to SARS-CoV-2 in patients with novel coronavirus disease 2019. Clin. Infect. Dis. 71: 2027–2034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Li K., Huang B., Wu M., Zhong A., Li L., Cai Y., Wang Z., Wu L., Zhu M., Li J., et al. 2020. Dynamic changes in anti-SARS-CoV-2 antibodies during SARS-CoV-2 infection and recovery from COVID-19. Nat. Commun. 11: 6044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Eliakim-Raz N., Massarweh A., Stemmer A., Stemmer S. M.. 2021. Durability of response to SARS-CoV-2 BNT162b2 vaccination in patients on active anticancer treatment. JAMA Oncol. 7: 1716–1718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Widge A. T., Rouphael N. G., Jackson L. A., Anderson E. J., Roberts P. C., Makhene M., Chappell J. D., Denison M. R., Stevens L. J., Pruijssers A. J., et al. mRNA-1273 Study Group . 2021. Durability of responses after SARS-CoV-2 mRNA-1273 vaccination. N. Engl. J. Med. 384: 80–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Chiang S. H., Tu M., Cheng J., Wei F., Li F., Chia D., Garner O., Chandrasekaran S., Bender R., Strom C. M., Wong D. T. W.. 2021. Development and validation of a quantitative, non-invasive, highly sensitive and specific, electrochemical assay for anti-SARS-CoV-2 IgG antibodies in saliva. PLoS One 16: e0251342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Pisanic N., Randad P. R., Kruczynski K., Manabe Y. C., Thomas D. L., Pekosz A., Klein S. L., Betenbaugh M. J., Clarke W. A., Laeyendecker O., et al. 2020. COVID-19 serology at population scale: SARS-CoV-2-specific antibody responses in saliva. J. Clin. Microbiol. 59: e02204-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Isho B., Abe K. T., Zuo M., Jamal A. J., Rathod B., Wang J. H., Li Z., Chao G., Rojas O. L., Bang Y. M., et al. 2020. Persistence of serum and saliva antibody responses to SARS-CoV-2 spike antigens in COVID-19 patients. Sci. Immunol. 5: eabe5511. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.