Abstract
Somatic antigen of Ascaris lumbricoides was purified to homogeneity (molecular mass, 34 kDa) by ammonium sulfate fractionation and successive chromatographic procedures, namely, gel permeation, ion exchange, and high-performance gel permeation liquid chromatographies. The antigen showed strong binding with immunoglobulin G (IgG) in Ascaris-infested patients and was cross-reactive with IgE and IgG in patients infested with other nematodes. It reacted specifically with IgG4 (P < 0.001) in 63 Ascaris-infested patients, which represented 65% of the total IgG response, though cross-reactivity with IgG1, IgG2, and IgG3 subclasses was observed, indicating the unique specificity of this test system and its potential utility in the serodiagnosis of ascariasis.
Ascaris lumbricoides infects about one-fourth of the world's population (21) and is considered to be one of the causes of various other ailments, namely, intestinal obstruction, acute pancreatitis, acute appendicitis (12), and malnutrition in children (9). Despite the high prevalence of Ascaris infection, no good method for diagnosing ascariasis in the context of an epidemiological investigation has yet been devised except for parasitological screening for the presence of eggs in stool. However, this method poses logistical and social problems. The use of an alternative method, such as serodiagnosis, is limited by the extensive cross-reactivity between the antigenic epitopes of different nematodes infective to humans. Ascariasis is associated with elevated immunoglobulin E (IgE) and IgG responses in humans and animals (11, 24). Serological tests based on IgG detection may overestimate the prevalence of infection, due to the persistence of antibodies for a long time after the deworming of patients. Although a novel, specific, and sensitive technique for the serodiagnosis of ascariasis that involves the assessment of Ascaris excretory-secretory (ES) antigen-specific IgG4 has been developed (3), procurement of ES antigen from living worms is limited due to the small number and death of the worms after deworming.
As a substance released from living worms, ES antigen possesses a significant antibody response; however, the source of the ES antigen is unknown. The possibility of its derivation from the worm's somatic cell component could not be ruled out. If ES antigen responsible for the IgG4 response in the infected host persists in the worm's somatic cell component(s), this could be a chief alternative source of IgG4-specific antigen for the diagnosis of ascariasis, due to its easy availability from the whole worm rather than from the ES antigen.
The present study describes the purification of the A. lumbricoides somatic antigen and its reactivity with serum IgE and IgG, especially with subclasses of IgG by enzyme-linked immunosorbent assay (ELISA), which may be useful markers for diagnosis of Ascaris infection in an epidemiological study.
Sixty-three patients (29 males and 34 females, 8 to 65 years of age) from urban and rural areas of West Bengal, India, infested with Ascaris were treated with albendazole (400-mg single dose), and during the first 72 h of their posttreatment period, stool samples were collected for three consecutive days from each subject and examined under a microscope. The number of worms expelled (range, 1 to 50) was counted to provide an estimate of the worm burden for each patient. Sera separated from pretreatment peripheral blood were stored in aliquots at −50°C for analysis. For a comparison of the parasitological screening with the serological evidence of ascariasis, stool samples from 126 dyspeptic patients were collected for three consecutive days and examined for the presence of eggs and/or larvae of helminths as before. Ten subjects (six males and four females, 20 to 50 years of age) with no known history of worm infection and with an absence of intestinal nematodes, confirmed by stool examination, served as controls. Groups of 10 subjects (five males and five females, 5 to 40 years of age) infested with hookworm, Strongyloides stercoralis, and Trichuris trichura, as confirmed by stool tests, were studied. Sera from control subjects and helminth-infested patients were stored at −50°C, and those with mixed infections (i.e., those infested with more than one type of nematode) were excluded from this study.
A. lumbricoides worms were collected from stool from each patient, washed thoroughly with saline, and dissected longitudinally. The body wall of each worm was again washed, homogenized in Tris-buffered saline (TBS; 50 mM, pH 8.0), centrifuged (10,000 rpm; Sorvall RC5B refrigerated centrifuge) for 1 h at 4°C, and concentrated (PM10 membrane). After protein estimation (16), the solution was stored in aliquots at −50°C.
The extract was precipitated with ammonium sulfate to give products of 30, 70, and finally 100% saturation; these were centrifuged as before, dissolved separately in TBS, dialyzed against the same buffer, and tested for antigenicity by ELISA using sera from Ascaris-infested patients. The most immunogenic fraction (30 to 70%, 1 ml, 20 mg of protein) was resolved into four fractions on a Sephacryl S-300 column (1.6 by 90 cm) with TBS as the eluent; these fractions were concentrated and tested for their binding activities with specific IgE and IgG. Fraction III (0.5 ml, 0.5 mg of protein/ml), which showed high binding activity with specific IgE and IgG, was further passed through a Resource-Q anion exchanger (6.4 by 30 mm) in a fast protein liquid chromatography (FPLC) system with a continuous gradient set up with TBS. The eluted fractions were concentrated and assayed for antigenic activity as before. The more active fraction was further purified by high-performance gel permeation liquid chromatography (HPGPLC) on a protein PAC 300 SW column (7.5 by 75 mm) with 10 mM phosphate-buffered saline (pH 6.8) as the eluent.
Homogeneity of the purified somatic antigen (pSAg) was tested by 7.5% alkaline polyacrylamide gel electrophoresis (4). The molecular size of pSAg was determined by sodium dodecyl sulfate (SDS)–7.5% polyacrylamide gel electrophoresis (14) with the following protein markers: bovine serum albumin (66 kDa), ovalbumin (45 kDa), glyceraldehyde-3-phosphate dehydrogenase (36 kDa), carbonic anhydrase (29 kDa), trypsinogen (24 kDa), trypsin inhibitor (20 kDa), lactalbumin (14 kDa), and aprotinin (6.5 kDa).
For ELISA, each fraction of SAg (10 μg/well) in carbonate buffer (pH 9.6), coated on a 96-well assay plate (Flow Laboratories) and blocked with phosphate-buffered saline–Tween 20 (0.05%) containing bovine serum albumin (1%), was incubated with sera from patients (100 μl), which were diluted to 1:64. Biotinylated goat anti-human IgE, IgG, IgG1, IgG2, IgG3, and IgG4 (Sigma), diluted to 1:1,000, were added, following antibiotin-coupled peroxidase. Color developed by o-phenylenediamine and H2O2 was measured at 492 nm in an ELISA reader to quantify results. For the ELISA inhibition assay, sera from patients, diluted to 1:100, were incubated with SAg and its fractions prior to ELISA. Antigenic proteins needed for inhibition were calculated from a graph of percentage inhibition determined from ELISA reference values.
A nonparametric Mann-Whitney U test was used for testing the significance of differences in worm load between sexes, and Spearman's rank correlation coefficient was used to assess the relation between age, isotype levels, and worm load.
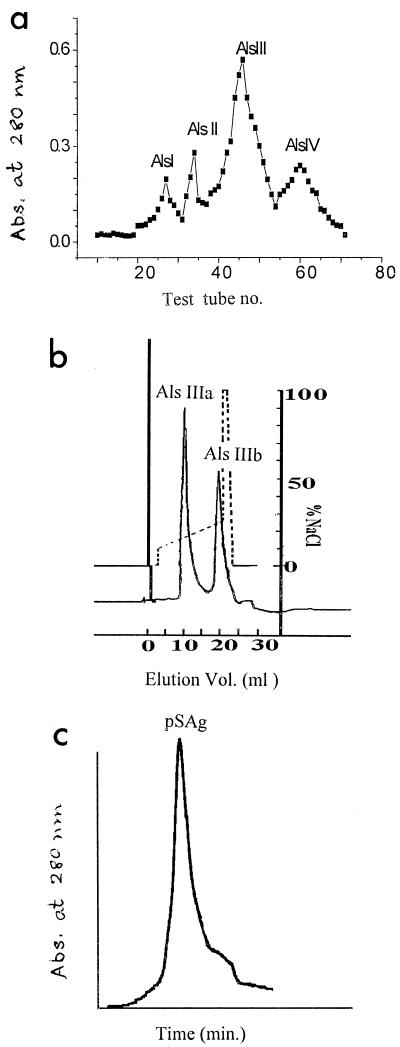
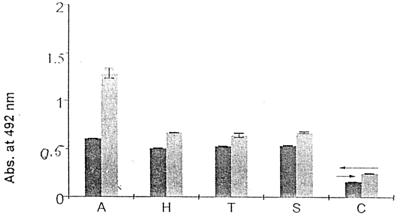
The ammonium sulfate fraction of A. lumbricoides somatic extract was separated into four fractions by Sephacryl S-300 column chromatography (Fig. 1a). Als III, being the most immunogenic fraction, was separated into two fractions (Fig. 1b), of which Als IIIb, being the more immunogenic of the two fractions as tested by an ELISA inhibition study, resulted in pSAg by HPGPLC (Fig. 1c). pSAg-specific IgG and IgE were present in the sera of other nematode-infected patients (Fig. 2), suggesting the nonspecificity of this test system; however, specific IgG in Ascaris-infested patients was significantly elevated (P < 0.05). pSAg was homogenous, having a molecular size of ≈34 kDa (data not shown).
FIG. 1.
(a) Gel permeation chromatography of the 30 to 70% ammonium sulfate fraction of A. lumbricoides SAg on a Sephacryl S-300 column. (b) Separation of Als III on a Resource-Q anion exchanger by FPLC. (c) Purification of Als IIIb by HPGPLC on a protein PAC 300 SW column. Abs., absorbance.
FIG. 2.
pSAg-specific IgE and IgG response in patients with different nematode infections. ←, control cut-off value for IgG; →, control cut-off value for IgE. Dark gray bars, specific IgE response; light gray bars, specific IgG response. A, A. lumbricoides; H, hookworm; T, T. trichura; S, S. stercoralis; C, control. Abs., absorbance.
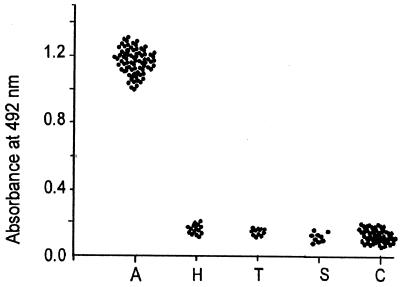
The cross-reactivities in regard to IgE and IgG turned our attention towards IgG subclass response in the study population, which showed that pSAg predominantly reacted with IgG4 in the sera of 63 Ascaris-infested patients, comprising 65% of the total IgG response; no such reactivity was observed with hookworm-, Trichuris-, and Strongyloides-infested patients (P < 0.001) (Fig. 3). Cross-reactivity was observed with pSAg-specific IgG1 (20% of hookworm, 10% each of T. trichura and S. stercoralis), IgG2 (60% of hookworm, 80% of T. trichura, and 60% of S. stercoralis), and IgG3 (20% of hookworm, 10% of T. trichura, and 30% of S. stercoralis).
FIG. 3.
pSAg-specific IgG4 response by ELISA in sera of different groups of individuals: Ascaris-infested (n = 63), hookworm-infested (n = 10), T. trichura-infested (n = 10), S. stercoralis-infested (n = 10), and control (n = 10) subjects.
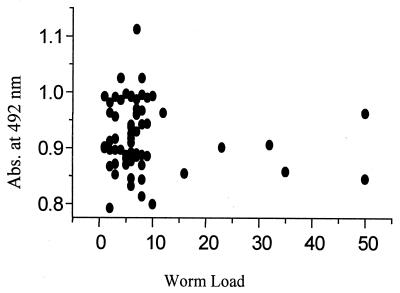
A comparison of the results of stool examination and of serological evidence of ascariasis in 126 individuals (Table 1) shows that these results are compatible and that such a comparison can be regarded a sensitive assay. Figure 4 shows the relationship between absorbance levels of IgG4 response and worm loads of patients.
TABLE 1.
Comparison of stool examination and serological evidence of ascariasis
| Subjects | Ascaris/IgG4a
|
|||
|---|---|---|---|---|
| +/+ | +/− | −/+ | −/− | |
| Men (n = 33) | 23/23 | 0/0 | 0/0 | 10/10 |
| Women (n = 28) | 16/16 | 0/0 | 0/0 | 12/12 |
| Boys (n = 34) | 24/24 | 0/0 | 0/0 | 10/10 |
| Girls (n = 31) | 20/20 | 0/0 | 0/0 | 11/11 |
| Total (n = 126) | 83/83 | 0/0 | 0/0 | 43/43 |
Values are the number of samples with the indicated results.
FIG. 4.
Relationship between absorbance levels of IgG4 response and A. lumbricoides worm load. Abs., absorbance.
The Mann-Whitney U test showed no significant difference (P > 0.05) between males (n = 29) and females (n = 34) regarding the worm load. Spearman's rank correlation coefficient analyses also revealed no significant association between isotypes, age, and worm load between sexes (P > 0.05).
Adult A. lumbricoides worms produce pSAg-specific IgE and IgG in infested humans. We observed significant cross-reactivity of A. lumbricoides ES antigen, fraction Als IIIb, with IgE and IgG of different nematode-infected patients (3), which was not specific for serodiagnosis. The present study shows that, in ascariasis, distribution of specific antibodies occurs among the IgG subclasses and IgG4 response is highly elevated. IgG4 response was studied in lymphatic filarial infections (13), onchocerciasis (5, 17), hookworm infection (19), strongyloidiasis (22), and ascariasis (3), and this subclass appears to be a marker of active infection that is useful for serodiagnosis. In our Ascaris-infested study population, IgG4 level was found to be independent of the worm load. IgG4 antibody is prominent in total antigen-specific IgG response when antigenic exposure is chronic (1), and this finding is supported by results of several clinical studies showing high IgG or IgG4 levels in subjects with various atopic disorders (2, 6–8, 20, 23, 25) and chronic schistosomiasis (10), as well as those with chronic filariasis (18). The fact that this immunoglobulin cannot be induced in humans by the epitope phosphorylcholine, which is shared by all helminthic parasites (15), and consequently that the possibilities of false-positive results are omitted, enables the assay system to be more predictive about Ascaris invasion.
Recombinant proteins, though they have been used for diagnostic purposes, still show limitations in clinical tests and are high in cost and not readily available in less developed regions, where the disease is an important public health issue. In this regard, somatic antigen, which contains epitope against specific IgG4 and is widely available, may be considered an alternative diagnostic method of nominal expense and no cross-reactivity. This increased specificity of an IgG4-based assay may be used for serodiagnosis of ascariasis in large populations.
REFERENCES
- 1.Aalberse R C, Van Der Gaag R, Van Leeuwen J. Serologic aspects of IgG4 antibodies. I. Prolonged immunization results in an IgG4-restricted response. J Immunol. 1983;130:722–726. [PubMed] [Google Scholar]
- 2.Bruynzee P L B, Berrens L. IgE and IgG antibodies in specific human allergies. Int Arch Allergy Appl Immunol. 1979;58:344–350. doi: 10.1159/000232211. [DOI] [PubMed] [Google Scholar]
- 3.Chatterjee B P, Santra A, Roy Karmakar P, Guha Majumder D N. Evaluation of IgG4 response in ascariasis by ELISA for serodiagnosis. Trop Med Int Health. 1996;1:633–639. doi: 10.1111/j.1365-3156.1996.tb00088.x. [DOI] [PubMed] [Google Scholar]
- 4.Davis B J. Disc-electrophoresis-II, method and application to human serum proteins. Ann N Y Acad Sci. 1964;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- 5.Egwang T G, Nguiri C, Kombila M, Duong T H, Lenoble D R. Elevated antifilarial IgG4 antibody levels in microfilaremic and microfilaridermic Gabonese adults and children. Am J Trop Med Hyg. 1993;49:135–142. doi: 10.4269/ajtmh.1993.49.135. [DOI] [PubMed] [Google Scholar]
- 6.Gwynn C M, Smith J M, Leon G L, Stanworth D R. Role of IgG4 subclass in childhood allergy. Lancet. 1978;i:910–911. doi: 10.1016/s0140-6736(78)90685-2. [DOI] [PubMed] [Google Scholar]
- 7.Gwynn C M, Smith G, Leon L, Stanworth D R. IgE and IgG4 subclass in atopic families. Clin Allergy. 1979;9:119–123. doi: 10.1111/j.1365-2222.1979.tb01530.x. [DOI] [PubMed] [Google Scholar]
- 8.Gwynn C M, Almosawi T, Stanworth D R. Clinical association with serum allergen specific IgG4 antibodies. Clin Allergy. 1982;12:459–464. doi: 10.1111/j.1365-2222.1982.tb01644.x. [DOI] [PubMed] [Google Scholar]
- 9.Hlaing T. Ascariasis and childhood malnutrition. Parasitology. 1993;107:S125–S136. doi: 10.1017/s0031182000075557. [DOI] [PubMed] [Google Scholar]
- 10.Iskander R, Das P K, Aalberse R C. IgG4 antibodies in Egyptian patients with schistosomiasis. Int Arch Allergy Appl Immunol. 1981;66:200–207. doi: 10.1159/000232819. [DOI] [PubMed] [Google Scholar]
- 11.Jarrett E E E, Miller H R P. Production and activities of IgE in helminth infection. Prog Allergy. 1982;32:178–233. [PubMed] [Google Scholar]
- 12.Khroo M S. Ascariasis. Gastroenterol Clin N Am. 1996;25:553–577. doi: 10.1016/s0889-8553(05)70263-6. [DOI] [PubMed] [Google Scholar]
- 13.Kwan-Lim G E, Forsyth K P, Maizels R M. Filarial specific IgG4 response correlates with active Wuchereria bancrofti infection. J Immunol. 1990;145:4298–4305. [PubMed] [Google Scholar]
- 14.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 15.Lal R B, Ottesen E A. Enhanced diagnostic specificity in human filariasis by IgG4 antibody assessment. J Infect Dis. 1988;158:1034–1037. doi: 10.1093/infdis/158.5.1034. [DOI] [PubMed] [Google Scholar]
- 16.Lowry O H, Rosebrough N H, Farr A L, Randall R J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 17.Lucius R, Kern A, Seeber F, Pogonka T, Willenbucher J, Taylor H R, Pinder M, Ghalib A W, Schulz-Key H, Soboslay P. Specific and sensitive IgG4 immunodiagnosis of onchocerciasis with a recombinant 33 kD Onchocerca volvulus protein (Ov 33) Trop Med Parasitol. 1992;43:139–145. [PubMed] [Google Scholar]
- 18.Ottesen E A, Skvaril E, Tripathy S P, Poindexter R W, Hussain R. Prominence of IgG4 in the IgG antibody response to human filariasis. J Immunol. 1985;134:2707–2712. [PubMed] [Google Scholar]
- 19.Palmer D L, Bradley M, Bundy D A. IgG4 responses to antigens of adult Necator americanus. Potential for use in large scale epidemiological studies. Bull W H O. 1996;74:381–386. [PMC free article] [PubMed] [Google Scholar]
- 20.Parish W E. The clinical relevance of heat stable short term sensitizing anaphylactic IgG antibodies (IgGs-Ts) and of relative activities of IgG4 and IgG2. Br J Dermatol. 1981;105:223–231. doi: 10.1111/j.1365-2133.1981.tb01210.x. [DOI] [PubMed] [Google Scholar]
- 21.Pawlowski Z S, Davis A. Morbidity and mortality in ascariasis. In: Crompton D W T, Nesheim M C, Pawlowski Z S, editors. Ascariasis and its prevention and control. London, England: Taylor and Francis; 1989. pp. 71–86. [Google Scholar]
- 22.Ramachandran S, Thompson R W, Gam A A, Neva F A. Recombinant cDNA clones for immunodiagnosis of strongyloidiasis. J Infect Dis. 1998;177:196–203. doi: 10.1086/513817. [DOI] [PubMed] [Google Scholar]
- 23.Shakib F, McLaughlan P, Stanworth D R, Smith E, Fairburn E. Elevated serum IgE and IgG4 in patients with atopic dermatitis. Br J Dermatol. 1977;97:59–63. doi: 10.1111/j.1365-2133.1977.tb15428.x. [DOI] [PubMed] [Google Scholar]
- 24.Stromberg B E. IgE and IgG1 antibody production by a soluble product of Ascaris suum in the guinea pig. Immunology. 1979;38:489–496. [PMC free article] [PubMed] [Google Scholar]
- 25.Van Toorenenbergen A W, Aalberse R C. Allergen-specific IgE and IgG4 antibody levels in sera and the capacity of these sera to sensitize the basophil leucocytes in vitro. Clin Allergy. 1982;12:451–458. doi: 10.1111/j.1365-2222.1982.tb01643.x. [DOI] [PubMed] [Google Scholar]