Abstract
Adverse childhood experiences (ACEs) put millions of children at risk for later health problems. As childhood represents a critical developmental period, it is important to understand how ACEs impact brain development in young children. In addition, children with attention-deficit/hyperactivity disorder (ADHD) are more likely than typically developing (TD) peers to experience ACEs. Therefore, the current study examined the impact of ACEs on early brain development, using a cumulative risk approach, in a large sample of children with and without ADHD. We examined 198 young children (Mage = 5.45, 82.3% Hispanic/Latino; 52.5% ADHD) across measures of brain volume, cortical thickness, neurite density index (NDI), and orientation dispersion index (ODI). Within NDI, there was a significant interaction between group and cumulative risk (ß = 0.18, p = .048), such that for children with ADHD, but not TD children, greater cumulate risk was associated with increased NDI in corpus callosum. No other interactions were detected. Additionally, when examining across groups, greater cumulative risk was associated with reduced ODI and volume in the cerebellum, although these findings did not survive a correction for multiple comparisons. Our results highlight the role early cumulative ACEs play in brain developmental across TD and children with ADHD.
Keywords: adverse childhood experiences (ACEs), attention-deficit/hyperactivity disorder (ADHD), cumulative risk, neurite density index (NDI), neuroimaging
A developmental psychopathology perspective advocates for 1) studying the full range of variation from normality to psychopathology, 2) understanding origins and mechanisms underlying psychopathology, and 3) use of multiple units and levels of analysis to study salient domains of functioning (Garber & Bradshaw, 2020; Miklosi, Mate, & Balazs, 2020). In the context of this conceptual approach, we examine the effects of cumulative adverse childhood experiences (ACEs) on structural brain development in typically developing (TD) and at-risk youth (i.e., children with attention-deficit/hyperactivity disorder; ADHD). Each year, ACEs put millions of children at risk for health problems (e.g., heart disease, obesity), psychological illness (e.g., alcoholism, depression, suicide), and even early death (Brown et al., 2009; Dube et al., 2002; McLaughlin et al., 2012). Typically, ACEs are explored in isolation, even though many of these risk factors co-occur and are cumulative (McLaughlin et al., 2010). Such co-occurring exogenous factors—low family income, parental psychopathology, stress—interact with endogenous characteristics of the child, such as their own psychopathology. Examining these factors within a cumulative risk model is thus most appropriate for understanding how ACEs affect brain development during early childhood, in which the brain is especially vulnerable to early experiences (Fox, Levitt, & Nelson, 2010). Despite this, most of the literature examining ACEs’ impact on brain development has been conducted with older, restricted samples that do not consider comorbid risk factors such as developmental disorders. This is especially problematic for common disorders appearing in early childhood, like ADHD, as such children are at increased risk for experiencing ACEs (Walker et al., 2020). Furthermore, the impact of ACEs on brain development may be exacerbated relative to TD children. Thus, the current study looks to fill these gaps by examining the impact of ACEs on early brain development, using a cumulative risk approach, in a large sample of young children with and without ADHD. In line with previous research, the current study will focus on seven ACEs: low family income and parental education (socioeconomic disadvantage), single-parent household status (family structure), and parental factors such as minority status, ADHD, stress, and emotion regulation (parental risk characteristics).
It is important to understand the impact of ACEs across a spectrum of presentations by studying the range of variation from normality to psychopathology. ACEs can lead to pervasive negative health outcomes that continue throughout adulthood (Mäntymaa et al., 2012; McLanahan, Tach, & Schneider, 2013). For example, children in single-parent homes are at an increased risk for decreased cognitive functioning and academic performance (Amato & Anthony, 2014; Brown, 2010), with increased risk for later obesity, mental health problems, antisocial behavior, and substance use (Duriancik & Goff, 2019; McLanahan et al., 2013). These risks are heightened in children with ADHD, as they are more likely to experience multiple ACEs such as socioeconomic disadvantage (Msall et al., 1998), low parental education (Law, Sideridis, Prock, & Sheridan, 2014; Machlin, McLaughlin, & Sheridan, 2020), parental divorce (Schermerhorn et al., 2012; Wymbs et al., 2008), high parental stress (Craig et al., 2016; Ronald, Pennell, & Whitehouse, 2011; Theule, Wiener, Tannock, & Jenkins, 2013), and parent psychopathology (Chronis et al., 2003; Vidair et al., 2011). Understanding the impact of cumulative ACEs across presentations (i.e., TD to ADHD) in early childhood can illuminate pathways of risk and resilience.
In addition to well-studied mental health outcomes, a number of studies have shown that ACEs are associated with neurobiological outcomes, specifically in grey matter brain regions and the white matter connectivity supporting these networks. Most studies have focused on grey matter volume and cortical thickness differences in the limbic system as a result of various ACEs. For example, ACEs have been associated with reductions in volume and thickness in the hippocampus, amygdala, anterior cingulate cortex, and orbitofrontal cortex (OFC), in addition to other cortical regions associated with limbic functions (Chad-Friedman et al., 2020; Duan, Hare, Staring, & Deligiannidis, 2019; Hanson, Chandra, Wolfe, & Pollak, 2011; Lawson, Duda, Avants, Wu, & Farah, 2013; Machlin et al., 2020; Marečková et al., 2019; Noble, Houston, Kan, & Sowell, 2012) (see Figure 1). Research in TD children has also shown reductions in volume of the cerebellum (Jackowski et al., 2008), and reduced cerebellar volume is a reliable finding in children with ADHD (Rubia, 2018). Indeed, the few studies examining ACEs in children with ADHD have also found that more ACEs were associated with reduced cerebellar volume, in addition to reductions in subcortical limbic regions (i.e., amygdala and hippocampus) (Machlin et al., 2020).
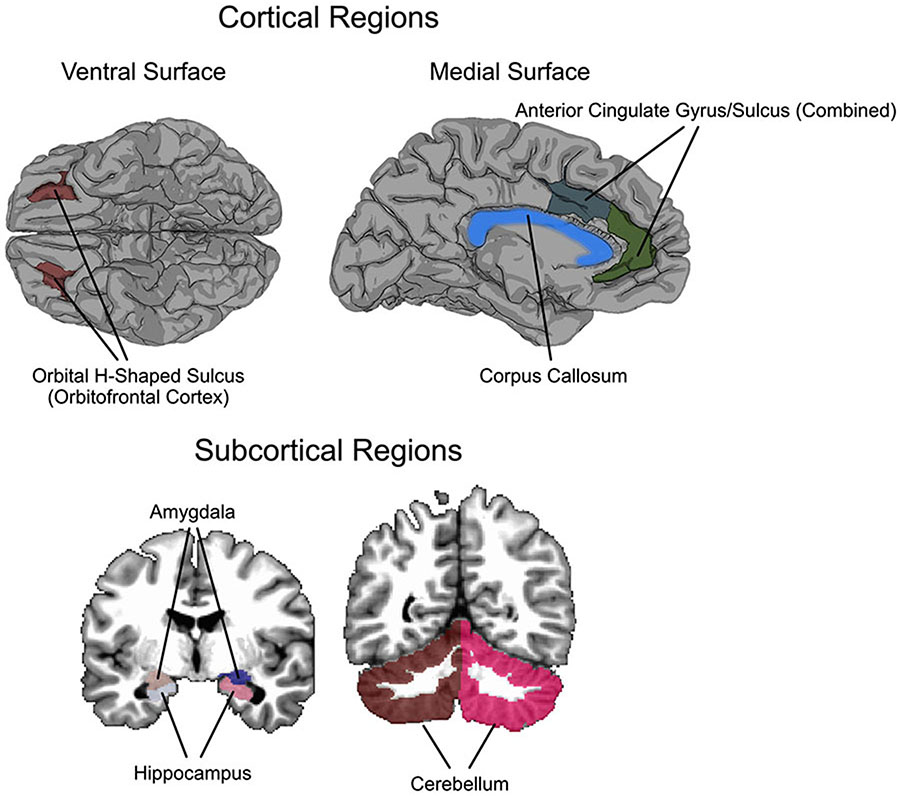
Figure 1. Cortical and Subcortical Regions of Interest.
Figure shows all regions of interest examined, including the orbital frontal cortex (OFC), anterior cingulate, corpus collosum (cortical regions), amygdala, hippocampus, and the cerebellum (subcortical regions).
Maturation of white matter in the brain is also susceptible to influence from early exposure to ACEs. This is not surprising, given the protracted developmental timeline of myelination of axons in early childhood through adolescence (Giedd et al., 1999). Several studies have shown reductions in volume or diffusion properties of the corpus callosum (Jackowski et al., 2008; McCarthy-Jones et al., 2018; Rinne-Albers et al., 2016). These changes persist into adulthood, suggesting prolonged negative impacts of early ACEs on brain development.
Information about gross grey matter and white matter changes are informative, but they do not provide information about subtle changes in local neural connections and structure. More recent methods, such as neurite orientation density and dispersion imaging (NODDI) have been developed to take advantage of the complex signal available in diffusion-weighted images (Zhang, Schneider, Wheeler-Kingshott, & Alexander, 2012). The neurite density metric (NDI) recovered from NODDI reconstruction can provide detailed information about how the cytoarchitecture of neurons changes in response to exposure to ACEs, specifically measuring the potential loss or maintenance of neurons. The advantage of this metric is that it can be used to investigate changes in both grey matter (primarily neurons) and white matter (primarily axons). Similarly, the orientation dispersion index (ODI) is sensitive to reduction or maintenance of the complexity of dendritic arborization. These indices can potentially provide information about changes in local neural organization in response to specific experiences, leading to a more comprehensive picture of the neural response to ACEs.
The Current Study
Although individual ACEs have been shown to impact later brain development (Chad-Friedman et al., 2020; Hair, Hanson, Wolfe, & Pollak, 2015), there is extremely limited research examining how cumulative risk factors impact brain development as early as preschool (Hawkey, Tillman, Luby, & Barch, 2018). While some studies have included only younger children (e.g., Luby et al., 2013), most include a large age range of children at different stages of brain development (e.g., children aged 3-21). Further, as children with ADHD are at an increased risk for experiencing these aforementioned ACEs, it is extremely important to understand if ACEs differentially impact brain development in children with ADHD. The current study looked to fill these gaps by examining how ACEs, utilizing a cumulative risk approach, are associated with brain development in young children with and without ADHD. Moreover, the current study tested if the impact of cumulative risk is exacerbated in children with ADHD compared to TD. The current study aimed to establish this comprehensive picture by examining volumetric, cortical thickness, NDI, and ODI differences in response to ACEs.
We hypothesized that cumulative risk would be negatively associated with children’s volume within the cerebellum, corpus collosum, the OFC, amygdala, hippocampus, and the anterior cingulate. We also hypothesized that cumulative risk would be negatively associated with cortical thickness in the OFC and cingulate. Given the limited studies on NDI and ODI within young children, we hypothesized that if cumulative risk interferes with synaptic formation, then a negative association with measures of NDI and a positive association with measures of ODI would be found. We expected to find these associations across TD children and those diagnosed with ADHD, although we expected that children with ADHD would have higher risk scores.
Methods and Materials
Participants and Recruitment
Children and their caregivers were recruited from local schools and mental health agencies via brochures, radio and newspaper ads, and open houses/parent workshops. All children were required to be enrolled in school during the previous year, have an estimated IQ of 70 or higher, and have no confirmed history of an autism spectrum disorder.
For the ADHD sample, ADHD diagnosis and comorbid disruptive behavior disorders were assessed through a combination of parent structured interview (Computerized-Diagnostic Interview Schedule for Children; C-DISC) (Shaffer, Fisher, Lucas, Dulcan, & Schwab-Stone, 2000), and parent and teacher ratings of symptoms and impairment (Disruptive Behavior Disorders Rating Scale, Impairment Rating Scale) (Fabiano et al., 2006; Pelham Jr, Gnagy, Greenslade, & Milich, 1992), as is recommended by standard practice (Pelham, Fabiano, & Massetti, 2005). Dual Ph.D. level clinician review was used to determine diagnosis and eligibility. For the TD sample, parents must have endorsed less than 4 ADHD symptoms (across either Inattention or Hyperactivity/Impulsivity according to the DSM-5), less than 4 Oppositional Defiant Disorder (ODD) symptoms, and indicated no clinically significant impairment (score below 3 on the impairment rating scale). The final sample included 198 young children (70.7% male; Mage = 5.45, SD = .89, 82.3% Hispanic/Latino) with an equivalent distribution of children diagnosed with ADHD (52.5%) and those characterized as TD (47.5%).
This study was approved by the university’s Institutional Review Board. All families participated in a one-time assessment, which included completion of the ADHD, ODD, and conduct disorder modules on the C-DISC and various questionnaires regarding their children’s behavioral, academic, and emotional functioning. Similar questionnaires were also obtained from children’s teachers. Children also completed a 25-minute MRI scan.
Risk Measures
Parental stress.
The Parenting Stress Index-Short Form (PSI-SF) (Abidin, 1995) is a 36 item self-report scale that measures stress in the parent-child relationship due to parent distress, difficult child behavior, and dysfunctional parent-child interaction. For the purposes of this study, the parental distress scale was used as a measure of parental stress (Cronbach’s a = .79).
Parental ADHD.
The ADHD Self-Report Scale (ASRS) (Kessler et al., 2005) is an 18-item self-report measure to assess manifestation of ADHD symptoms in people aged 18 years or older. The ASRS has previously demonstrated good internal consistency and concurrent validity (Adler et al., 2006). The total score was used in this study (Cronbach’s a = .89).
Parental emotion regulation.
The Difficulties in Emotion Regulation Scale Short Form (DERS-SF) (Kaufman et al., 2016; Victor & Klonsky, 2016) is an 18-item self-report measure that assesses the presence and frequency of symptoms of emotion dysregulation in adults. Responders are asked to rate the frequency at which they experience particular symptoms. The total score was used in this study with higher scores indicating more emotion dysregulation problems (Cronbach’s a = .80).
Cumulative risk index.
Consistent with prior work (Appleyard, Egeland, van Dulmen, & Alan Sroufe, 2005; Bagner & Graziano, 2013), we transformed seven variables into dichotomous variables, with a score of 1 indicating the presence of risk and 0 indicating no risk. The risk variables included 1) low family income; 2) parental education; 3) single-parent household status; 4) parental minority status; 5) parental ADHD; 6) parental stress, and 7) parental emotion regulation. Cumulative risk was calculated for each participant by summing the seven dichotomized variables (possible range in scores from 0 to 7), with higher scores indicating greater risk. See Table 1 for details on how risk scores were determined for each variable.
Table 1.
Descriptives and Cumulative Risk Factors
| Total Sample (N=198) |
ADHD only (n=104) |
TD Only (n=94) |
p | |
|---|---|---|---|---|
| Child Age | 5.45 (.89) | 5.47 (.91) | 5.43 (.87) | .742 |
| Child Sex (% male) | 70.7% | 74% | 67% | .279 |
| Child IQ | 99.74 (12.63) | 96.17 (12.92) | 103.68 (11.08) | < .001 |
| Child Ethnicity (% Latinx) | 82.3% | 81.7% | 83% | .712 |
| P/T DBD Inattention | 1.27 (1.06) | 2.25 (.60) | .39 (.41) | < .001 |
| P/T DBD Hyperactivity | 1.53 (1.03) | 2.37 (.56) | .59 (.47) | < .001 |
| P/T DBD ODD | .97 (.88) | 1.58 (.76) | .30 (.37) | < .001 |
| Risk Categories * | ||||
| Low Incomea | 36.9 % | 38.5 % | 35.1 % | .625 |
| Parental Educationb | 31.3% | 31.7% | 30.9% | .894 |
| Minority Statusc | 87.4 % | 88.5 % | 86.2 % | .628 |
| Single Parentd | 26.8 % | 25.0 % | 17.0 % | .003 |
| Parent Stresse | 16.2 % | 25.2 % | 6.4 % | < .001 |
| Parent ADHDf | 23.7 % | 33.7 % | 12.8% | .001 |
| Parent ERg | 19.7 % | 26.9 % | 11.7% | .007 |
| Cumulative Risk Scores * | ||||
| 0 | 4.0 % | 1.9 % | 6.4 % | |
| 1 | 28.3 | 22.1 % | 35.1 % | |
| 2 | 24.2 % | 20.2 % | 28.7 % | |
| 3 | 21.2 % | 26.0 % | 16.0 % | |
| 4 | 14.1 % | 16.3 % | 11.7% | |
| 5 | 4.5 % | 8.7 % | 0.0 % | |
| 6 | 2.5 % | 2.9 % | 2.1% | |
| 7 | 1.0% | 1.9% | 0.0% | |
| Total Risk Score | 2.41 (1.46) | 2.80 (1.52) | 2.00 (1.26) | < .001 |
= percent in risk group. Abbreviations: ADHD = attention-deficit/hyperactivity disorder, TD = typically developing, P/T = highest teacher or parent report, DBD = disruptive behaviors disorders rating scale, ODD = oppositional defiant disorder, ER = emotion regulation
Low income was dummy coded as above/below 150% of the poverty line.
Parental education was dummy coded as either parent having/not having a 4-year college degree.
Although race/ethnicity itself is not a risk factor, there is persistent evidence of racial/ethnic disparities in domains, such as health care, that may mitigate negative outcomes. Parental minority status is included as a proxy for such disparities, with a dummy code indicating Caucasian/Non-Hispanic or not.
Single parent was dummy coded as either single parent/not single parent household.
Parent report of clinically elevated distress on the Parenting Stress Index-Short was dummy coded as above/below 85th percentile.
Parent report of clinically elevated levels of ADHD on the ADHD Self-Report Scale was dummy coded as clinically elevated/not elevated.
Parent report of clinically elevated levels of emotion dysregulation on the Difficulties in Emotion Regulation Scale Short Form was dummy coded as clinically elevated/not elevated.
Image Acquisition & Processing
MRI acquisition & processing.
All imaging was performed using a research-dedicated 3-T Siemens MAGNETOM Prisma MRI scanner (V11C) with a 32-channel coil located on the University campus. Children first completed a mock scan. In the magnet children watched a child-friendly movie of their choice. Ear protection was used, and sound was presented through MRI compatible headphones.
We collected structural anatomical scans using a whole-head 3D T1-weighted acquisition inversion prepared RF-spoiled gradient echo protocol with prospective motion correction (Siemens vNAV; Tisdall et al., 2012). We collected 93 axial slices at 1 mm isotropic resolution. Each scan was reviewed by a licensed radiologist, and incidental findings were reported to the parent/guardian. We also collected multi-shell high-angular diffusion-weighted imaging (Harding, Galano, Martin, Huntington, & Schellenbach) data according to the Adolescent Brain and Cognitive Development (ABCD) protocol (Hagler et al., 2019). These scans were collected with a 1.7 mm isotropic voxel size, using multiband imaging echo planar imaging (EPI; acceleration factor = 3). The acquisition consisted of ninety-six diffusion directions, six b=0 frames, and four b-values (102 diffusion directions; 6 b=500 s/mm2, 15 b=1000 s/mm2, 15 b=2000 s/mm2, and 60 b=3000 s/mm2).
Diffusion-weighted imaging post-processing.
Initial post-processing was accomplished with DTIPrep v1.2.8 (Oguz et al., 2014), TORTOISE DIFFPREP v3.1.0 (Irfanoglu, Nayak, Jenkins, & Pierpaoli, 2017; Pierpaoli et al., 2010), AFNI (v 20.6.02), and FSL v6.0.1 topup (Andersson, Skare, & Ashburner, 2003; Smith et al., 2004). We also implemented a pre- and post-analysis quality check assessing signal-to-noise of each diffusion b-value (Roalf et al., 2016). Initial quality control was accomplished in DTIPrep to complete the following steps: 1) image/diffusion information check; 2) padding/cropping of data; 3) Rician noise removal; 4) slice-wise, interlace-wise, and gradient-wise intensity and motion checking. The number of acquisitions removed was used as a proxy for movement/bad data quality and was included as a covariate in subsequent regression analyses.
TORTOISE DIFFPREP was used to accomplish motion and eddy current correction, and registration to the T1-weighted structural scan, which was maintained in original subject space. An additional registration step established that the ROI mask (defined below) was appropriately registered to the diffusion image. This was accomplished in AFNI using a 12 degree of freedom affine registration of the T1 to the first b0 image of the DWI scan (AFNI fat_proc_map_to_dti using 3dAllineate). Registration was visually inspected at this phase and to assure alignment of the diffusion image to the T1-weighted image derived from the Freesurfer atlas.
Neurite orientation dispersion and density imaging (NODDI) metrics.
NODDI is an alternative diffusion model that can distinguish among three tissue-property contributions to the diffusion signal: intracellular, extracellular, and cerebrospinal fluid. The model is possible to implement with the multi-shell HARDI protocol (Zhang et al., 2012). With respect to the present study, the NODDI model allows estimation of the contributions of neurite morphology from the diffusion signal, and such estimates such as neurite density from the NODDI model have been verified with histology in animals (Sato et al., 2017) and pathological findings in humans (Sone et al., 2020). In the present study we focus on the NDI and ODI metrics, derived from the NODDI model, with higher values NDI correlated with higher density of neuronal tissue, and higher values of ODI indicating increased dendritic arborization and complexity (Shao et al., 2021). We computed the NDI and ODI metrics using the Microstructure Diffusion Toolbox (Harms, Fritz, Tobisch, Goebel, & Roebroeck, 2017; Harms & Roebroeck, 2018). The two diffusivities representing the diffusion coefficient of the isotropic compartment (diso) and the intrinsic diffusivity of the intra-neurite compartments (d∥) were fixed to diso = 3.00 x 10−3 mm2 s−1 (for free water in the brain at 37°C) and d∥ = 1.70 x 10−3 mm2 s−1, which are the standard values recommended in Zhang et al., (2012).
In addition to NDI and ODI, the NODDI model provides a compartment estimating the free-water isotropic diffusion component (ISO). This component can be used as a mask to mitigate partial volume effects, especially where brain tissue directly interfaces with cerebrospinal fluid (i.e., near the ventricles and in the extracortical space under the skull). We implemented a mask here such that voxels with an ISO volume fraction > .80 were removed from analysis, which masked the ventricles and extracortical space.
Construction of Cortical Surfaces and Semi-Automated Segmentation and Parcellation
For each participant, in order to provide a semi-automated segmentation of subcortical structures, a cortical parcellation, and an estimate of intracranial volume (Buckner et al., 2004), we constructed individual cortical surfaces for each subject from the T1-weighted volume using Freesurfer v6.0 (Dale, Fischl, & Sereno, 1999; Fischl, Sereno, & Dale, 1999). We then defined regions anatomically on individual cortical surfaces using the semi-automated Freesurfer parcellation procedure (Desikan et al., 2006; Fischl et al., 2004), which is itself based on the anatomical conventions of Duvernoy (1999).
We computed cortical thickness and subcortical volume as part of the standard FreeSurfer reconstruction pipeline (Fischl & Dale, 2000), as these have been shown to have high correspondence to histological measurements (Cardinale et al., 2014). The use of a program originally developed for studies on adults is a legitimate concern. However, Freesurfer has been used to successfully create brain surface representations for children (Tamnes et al., 2010), and even neonates (Pienaar, Fischl, Caviness, Makris, & Grant, 2008), and has been used in previous research on preschool children with ADHD (Jacobson et al., 2018). We employed a similar procedure as these prior studies.
Definition of Brain Regions
We focused on the regions reviewed in the Introduction, which comprise a distributed network of regions previously associated with ACEs in development, and identified several regions of interest (ROIs) which were based on the Destrieux parcellation from Freesurfer (Desikan et al., 2006; Fischl et al., 2004). These ROIs, detailed in Figure 1, were: 1) left and right amygdala; 2) left and right hippocampus; 3) left and right OFC; defined anatomically as the orbital H-shaped sulcus; 4) left and right anterior cingulate cortex, defined as the average of the anterior part of the cingulate gyrus and sulcus, and the middle-anterior part of the cingulate gyrus and sulcus; 5) cerebellum; and 6) corpus callosum. Data for volume were retrieved for all regions and data for cortical thickness were retrieved for cortical regions using Freesurfer v.6.0. The Freesurfer parcellation/segmentation was exported to the T1-weighted volume space in AFNI (@SUMA_Make_Spec_FS). Then NDI and ODI were retrieved for all regions defined in the T1-derived ROI mask (AFNI 3dROIstats), following visual verification of the registration of the Freesurfer parcellation/segmentation to the DWI scan in the volume space.
Quality Control of Magnetic Resonance Imaging Scans
Movement artifacts in T1-weighted MRI scans are common, especially in pediatric populations in this age range, and especially in children with ADHD. Fortunately, Freesurfer is robust to movement-related artifacts, as, except in extreme cases, the program is able to accurately identify intensity differences between white matter and grey matter inherent in the T1-weighted image. In some cases, however, manual intervention is necessary. In this manual intervention, each individual MRI scan is inspected, and in cases where the program does not adequately identify the appropriate regional boundaries, manual edits are employed. We also visually rated each T1-weighted image on a seven-point scale ranging from “Poor = 1” to “Excellent = 4”, with allowances for half-points (e.g., 3.5). Scans for both groups were generally rated “Very Good” to “Excellent”, with an average of 3.56 (SD = 0.59) for the ADHD group, and 3.44 (SD = 0.68) for the TD group. There were no significant group differences for the quality of the scans, t(195) = −1.39, p = 0.17).
Data Analyses
All analyses were conducted using SPSS Version 26. Data were first inspected for missingness, with no missing data present for any variables of interest. We then examined whether there were differences in cumulative risk categories between ADHD and TD groups.
Next, multiple regression analyses were conducted to examine how cumulative risk (the predictor) was associated with brain measures (the outcome). Thus, we examined volume, NDI, and ODI of the cerebellum, corpus collosum, OFC, amygdala, hippocampus, and the anterior cingulate. For cortical regions (i.e., OFC and anterior cingulate) we also examined cortical thickness. These regions were chosen based on previous literature linking early risk factors to brain development, as we reviewed in the Introduction. For all regressions, the following covariates of non-interest were included: child age, child sex, child IQ, average cortical thickness (for cortical ROIs), intracranial volume (for brain volume measures), average brain NDI (for NDI measures), and average brain ODI (for ODI measures). Intracranial volume was defined using the procedure from Buckner et al., (2004).
The first set of regressions also included diagnostic status as a moderating variable on cumulative risk (i.e., group [ADHD vs. TD] by cumulative risk interaction). This assesses whether the impact of ACEs on brain development is exacerbated in children with ADHD relative to TD children. In a second set of regressions, we removed the categorical ADHD diagnosis and examined, as covariates, more continuous measures of inattention, hyperactivity, and oppositional defiant behaviors from the Disruptive Behaviors Disorders (DBD) rating scale. For the DBD, the highest score from either the parent or the teacher was used.
Correction for Multiple Comparisons
We focused on a small number of brain regions based on our review of the literature, but the number of comparisons necessitates statistical correction to control for Type I error. We employed the False Discovery Rate (FDR) correction (Benjamini & Hochberg, 1995) at two different nominal levels (q = .05 and .10), which defines the proportion of errors committed by falsely rejecting null hypotheses. Family was defined within each brain measure. Thus, there were ten comparisons each for volume, NDI and ODI, and four comparisons for cortical thickness. We interpret results in the context of these FDR proportions, and in the context of effect sizes considered against the associated 95% CIs.
Results
Descriptive and demographic variables are presented in Table 1. As expected, there were significant group differences in inattention, hyperactivity, and oppositional defiant behaviors. In addition, there were significant differences in several risk categories, including single parent status, parental stress, parental ADHD, parental emotion dysregulation, and total cumulative risk scores.
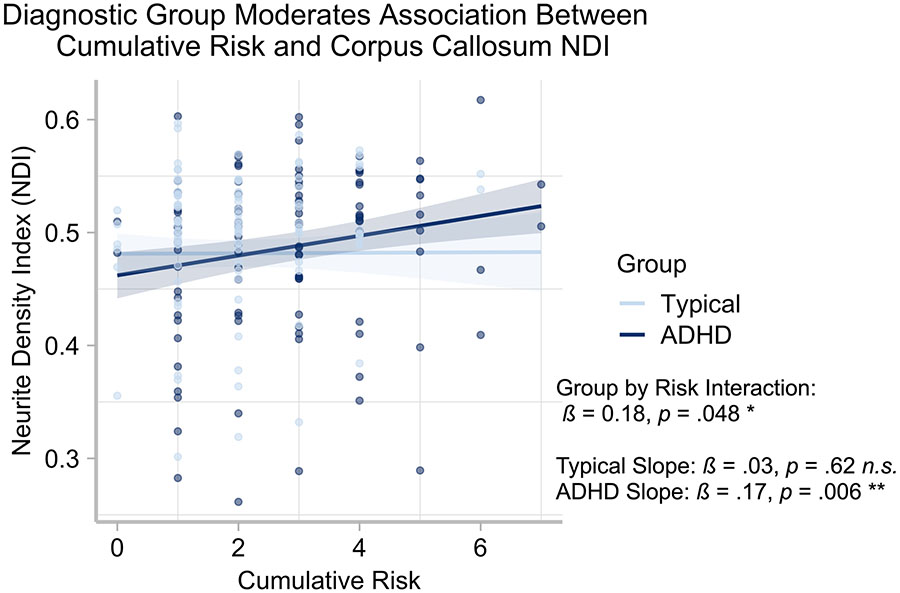
Confirming group differences, we examined whether group status moderated the association between cumulative risk and brain outcomes. There was a significant interaction between group and cumulative risk when predicting the corpus callosum NDI (ß = 0.18, B = 0.009, t(189) = 1.99, p = .048, [B CI 95% 0.0001, 0.016]; see Figure 2). Probing of the interaction revealed there was no association for TD children (ß = 0.03, B= .001, t(88) = 0.49, p = .62, [B CI 95% −.005, .007]). However, for children with ADHD, greater cumulative risk scores were associated with increased NDI (ß = 0.17, B= .008, t(97) = 2.81, p = .006, [B CI 95% .002, .013]; see Figure 2). There were no significant interactions across other areas of interest (p’s > .05).
Figure 2. Diagnostic Group Moderates Association between corpus callosum NDI and Cumulative Risk.
Figure show the significant interaction of group (i.e., ADHD and typically developing children; TD) and cumulative risk. Analyses controlled for child age, child sex, child IQ, and mean white matter neurite density index (NDI).
Next, we examined the main effect of cumulative risk across the diagnostic groups, adding the continuous measures of inattention, hyperactivity, and ODD as covariates. Table 2 shows these results. Within NDI, higher cumulative risk scores were significantly associated with increased NDI in the corpus callosum. However, this main effect is best interpreted in the context of the significant group by risk interaction (noted above) showing that the effect holds for children with ADHD, but not TD children.
Table 2.
Associations between cumulative risks and brain morphometric measures
| B (SE) | β | t-value | p | 95% CI for B | |
|---|---|---|---|---|---|
| Neurite Density Index (NDI) | |||||
| Corpus Collosum | .006 (.002) | .08 | 2.67 | .009 ++ | .002, .010 |
| Cerebellum | −.008 (.005) | −.08 | −1.64 | .10 | −.020, .002 |
| Left Hemisphere | |||||
| OFC | .0008 (.002) | −.02 | 0.36 | .72 | −.004, .005 |
| Amygdala | .002 (.002) | .03 | 0.77 | .44 | −.002, .006 |
| Hippocampus | .0001 (.002) | .003 | 0.06 | .95 | −.004, .004 |
| Cingulate | −.0003 (.002) | −.01 | −0.14 | .89 | −.004, .004 |
| Right Hemisphere | |||||
| OFC | −.002 (.002) | −.05 | −1.11 | .27 | −.007, .002 |
| Amygdala | .004 (.002) | .07 | 1.85 | .07 | −.0002, .008 |
| Hippocampus | −.002 (.002) | −.03 | −0.77 | .44 | −.005, .002 |
| Cingulate | .0003 (.002) | .01 | 0.15 | .88 | −.004, .004 |
| Orientation Dispersion Index (ODI) | |||||
| Corpus Collosum | −.0002 (.004) | −.003 | −0.06 | .95 | −.008, .008 |
| Cerebellum | −.01 (.005) | −.10 | −2.02 | .045 | −.021, −.0003 |
| Left Hemisphere | |||||
| OFC | .001 (.003) | .02 | 0.37 | .71 | -.005, .008 |
| Amygdala | −.0007 (.002) | −.02 | −0.32 | .75 | −.005, .004 |
| Hippocampus | −.0005 (.002) | −.01 | −0.21 | .83 | −.005, .004 |
| Cingulate | −.001 (.003) | −.03 | −0.47 | .64 | −.007, .004 |
| Right Hemisphere | |||||
| OFC | −.001 (.003) | −.03 | −1.55 | .12 | −.001, .010 |
| Amygdala | .0005 (.002) | .01 | 0.22 | .83 | −.004, .005 |
| Hippocampus | −.001 (.003) | .02 | 0.47 | .64 | −.004, .006 |
| Cingulate | −.002 (.003) | −.04 | −0.86 | .39 | −.007, .003 |
| Volume | |||||
| Corpus Collosum | 5.09 (3.81) | .09 | 1.33 | .18 | −2.43, 12.61 |
| Cerebellum | −410.57 (185.06) | −.11 | −2.22 | .028 | −775.63, −45.52 |
| Left Hemisphere | |||||
| OFC | .000 (.000) | .02 | 0.21 | .83 | −.001, .001 |
| Amygdala | 2.34 (6.92) | .02 | 0.34 | .74 | −11.31, 15.98 |
| Hippocampus | −6.64 (14.52) | −.03 | −0.46 | .65 | −35.33, 22.03 |
| Cingulate | .000 (.000) | .03 | 0.42 | .67 | −.001, .001 |
| Right Hemisphere | |||||
| OFC | .000 (.000) | .02 | 0.31 | .75 | −.001, .001 |
| Amygdala | 4.04 (8.02) | .03 | 0.50 | .62 | −11.78, 19.87 |
| Hippocampus | −25.19 (14.88) | −.10 | −1.69 | .09 | −54.54, 4.16 |
| Cingulate | .000 (.000) | −.02 | −0.26 | .79 | −.001, .001 |
| Cortical Thickness | |||||
| Left Hemisphere | |||||
| OFC | .008 (.008) | .08 | 1.00 | .32 | −.008, .024 |
| Cingulate | .008 (.005) | .10 | 1.50 | .14 | −.003, .019 |
| Right Hemisphere | |||||
| OFC | .007 (.008) | .07 | 0.91 | .36 | −.008, .022 |
| Cingulate | .004 (.006) | .05 | 0.77 | .45 | −.006, .013 |
Bold indicates that the p value is less than the nominal alpha of .05. p-values marked with + indicate that these effects survived a False Discovery Rate (FDR) correction for multiple comparisons at q = .05. Those with ++ indicate survival at q = .10. Abbreviations: OFC = orbital frontal cortex. All regressions controlled for child symptoms of inattention, hyperactivity, oppositional defiant disorder, child age, child sex, and child IQ. Volume regressions controlled for total cranial volume, thickness regression controls for total average thickness, and NDI and ODI regressions controlled for mean white matter NDI or ODI, respectively.
Examining the ODI measure, cumulative risk was significantly associated with reduced ODI of the cerebellum. However, this result did not survive FDR correction at q = .05 or .10. No other statistically significant effects for ODI were identified. Examining volume, we found that cumulative risk was negatively associated with cerebellar volume, although again this did not survive the multiple comparison correction. For the thickness measure, no statistically significant effects were identified in any regions that were examined.
Discussion
In this study, we demonstrated that greater cumulative ACEs were associated with increased NDI in the corpus collosum across all children. However, an interaction emerged indicating that for the TD children, there was no significant association between cumulative risk and neurite density. In contrast, for children diagnosed with ADHD, increased risk was associated with increased NDI. The differential association between cumulative ACEs and microstructural indices of neurite density in corpus callosum underscores the potential negative consequences to brain development in this region, especially in children who are at increased risk for cumulative ACEs (Jackowski et al., 2008; McCarthy-Jones et al., 2018; Rinne-Albers et al., 2016). Furthermore, this interaction reinforces the notion that endogenous characteristics of the child (i.e., existing psychopathology) interact with environmental factors to affect brain development in early childhood. Taken together, our results highlight the role early cumulative ACEs play in brain developmental across TD and children with ADHD.
Adverse Childhood Experiences Affect Axonal Density in Corpus Callosum
The strongest effect of ACEs on brain development in our preschool sample was detected using the more sensitive measure of brain morphology, namely in the novel measure of neurite density derived from the NODDI diffusion model. Thus, we did not detect strong effects for more common metrics of volume and cortical thickness, even though these effects have been reported in the previous literature (Chad-Friedman et al., 2020; Duan et al., 2019; Lawson et al., 2013; Machlin et al., 2020; Marečková et al., 2019; Noble et al., 2012). Further, while one effect for ODI (in cerebellum) was nominally significant, it did not survive FDR correction even at the more liberal q = .10 level. The finding for cerebellar volume also does not survive this FDR correction. Thus, we focus our initial discussion on the NDI metric as it pertains to corpus callosum microstructure.
Interpretation of the NDI metric as it pertains to white or grey matter microstructure must proceed with caution. The diffusion signal in grey matter is derived from a combination of several tissue components, including axons, dendrites, and cell bodies of both neurons and glia. The NODDI model helps to segregate these contributions to some degree, and indeed in grey matter, the NDI metric from the NODDI model has been verified in several histologic studies to be sensitive to the density of neurons, such that reduced NDI is associated with the loss or reduction of neurons in cases of lesion or tumor (Shao et al., 2021) or degenerative disease (Kamagata et al., 2016). There is also some modest sensitivity to density differences in cytoarchitectonically diverse tissue samples (Crombe et al., 2018).
In white matter, signal contributions are derived mainly from axons and glia. Developmental studies of neurite density, measured by the NODDI model, show increases in NDI in the white matter from ages 7 to 63 years (Chang et al., 2015) and in grey matter from ages 0 to 14-years (Zhao et al., 2021). However, our main finding is with respect to NDI in the corpus callosum, which is a dense collection of interhemispheric fibers, and thus the NODDI measure in this region is most sensitive to axonal density, not neural or glial cell body density or dendritic density. Fortunately, two studies have linked NDI in the corpus callosum to histological differences in axonal density in developmental and adult samples. Indeed, NODDI of the corpus callosum closely aligns with the known longitudinal distribution of fiber density in the corpus callosum, such that the NDI metric decreases with a high degree of correlation as fiber density increases (Garic, Yeh, Graziano, & Dick, 2021; Genc, Malpas, Ball, Silk, & Seal, 2018). These studies found that this association applies to children in the age range we study here. We can thus speculate that the maintenance of callosal fibers following exposure to ACEs, indicated by the positive association with NDI and cumulative risk, may reflect a disruption of callosal axonal pruning, a process that takes place in typical development in response to experience (LaMantia & Rakic, 1990). Atypical axonal pruning in the corpus callosum is linked with a number of psychopathologies (Raine et al., 2003) and seems to mainly affect excitatory rather than inhibitory interhemispheric connections (Saugstad, 1994). Functionally, this may translate to altered network connectivity across the two hemispheres, such that typical processes of establishment of functional laterality over development are disrupted (Everts et al., 2009). Such disruption may impact the neural processes implementing several cognitive and affective functions, including the onset of mental health disorders associated with early risk exposure (McLaughlin et al., 2012). Notably, this disruption seems to be specific to children with ADHD who are repeatedly exposed to stressful situations, as the association with ACEs and corpus callosum NDI only applied to the ADHD group (Figure 2). One can speculate that children with ADHD already differ to some degree in terms of their trajectories of brain development relative to TD children (Rubia, 2007), and that the additional burden of repeated ACEs exacerbates these differences. However, the directionality of this proposed causal pathway is speculative given the quasi-experimental nature of the study design. That said, it is an intriguing possibility that could be explored in future work.
Adverse Childhood Experiences May Influence Cerebellar Development
Two findings related to ODI and volume of the cerebellum were nominally significant, but did not survive FDR correction. Thus, our brief Discussion below should be considered in that context. Here, we found that greater cumulative risk scores were associated with reduced ODI and volume of the cerebellum. This is an interesting result when considered in the context of cerebellar development and function. First, with respect to cerebellar development, the cerebellum is unique with respect to the rest of the brain because, unlike regions of the cortex and other subcortical areas, neural proliferation in the cerebellum proceeds beyond birth, and refinement of cerebellar neuronal maps is heavily experience-dependent (Sotelo, 2004). Thus, the cerebellum may be especially sensitive to cumulative ACEs, as developmental processes related to neural proliferation may be affected both pre- and postnatally. Second, with respect to cerebellar function, the cerebellum has been implicated in a number of cognitive, affective, and sensorimotor processes, and it is densely connected to cortical regions supporting function in these domains. For example, lesions of the posterior lobe of the cerebellum result in a well-described cerebellar cognitive affective syndrome, which manifests as deficits in executive function, visual spatial processing, linguistic processing, and emotion regulation (Schmahmann, 2019). The cerebellum is part of a comprehensive cortico-subcortical network supporting these functions and given its potential susceptibility to experiential influences during development, it may contribute significantly to negative outcomes following exposure to ACEs in both children with and without ADHD.
Limitations
While the current study represents the first step in understanding how cumulative risk impacts brain development in young children with and without ADHD, it is not without limitations. First, the current study is cross-sectional, which substantially limits our ability to make causal claims. Longitudinal investigation of the cumulative impact over development is necessary to better understand the neurobiological sequelae of ACEs throughout development. However, this snapshot of the preschool period does provide an opportunity to understand resilience to ACEs as children develop and may especially be relevant for understanding what factors predict later resilience. The current study also focuses on children diagnosed with ADHD as the clinical group of interest, which may limit generalizability to other clinical disorders that emerge in childhood. However, as children with ADHD are notably at higher risk for experiencing ACEs, a finding that was replicated in the present study, the current study extends our understanding of this common childhood disorder. Finally, an additional methodological limitation was the use of the standard recommended values for the diffusion coefficients of diso and the intrinsic diffusivity of the intra-neurite compartments d∥, which were fixed to diso = 3.00 x 10−3 mm2 s−1 (for free water in the brain at 37°C) and d∥ = 1.70 x 10−3 mm2 s−1. Studies have shown that these simplifying model assumptions for parallel diffusivity are reasonable for white matter in adults, but may be sub-optimal for grey matter, or for infants earlier in development (Fukutomi et al., 2018; Guerrero et al., 2019). Such optimal parallel diffusivity values may vary across the brain, which may lead to better fitting NODDI models in some regions as opposed to others. This is a limitation when both grey matter and white matter regions are considered in the same analysis.
Conclusion
Taken together, the impact of cumulative ACEs on microstructural indices of cellularity across TD and children with ADHD underscores the potential negative consequences of early ACEs on brain. Future work should investigate if early intervention of malleable risk factors (e.g., parent stress, parent ADHD) will prevent and/or reverse the negative impact of ACEs on brain development and alter subsequent psychosocial functioning.
Acknowledgments:
ASD and PAG were supported by grants from NIMH (R01MH112588) and NIDDK (R01DK119814). MMH was supported by a grant from NIDA (T32DA04344).
Footnotes
Disclosures: MMH, ASD, and PAG report no biomedical financial interests or potential conflicts of interest.
The data that support the findings of this study are available on request from the corresponding author [ASD].
References
- Abidin R (1995). Manual for the parenting stress index. Odessa, FL: Psychological Assessment Resources. [Google Scholar]
- Adler LA, Spencer T, Faraone SV, Kessler RC, Howes MJ, Biederman J, & Secnik K (2006). Validity of pilot Adult ADHD Self-Report Scale (ASRS) to rate adult ADHD symptoms. Annals of Clinical Psychiatry, 18(3), 145–148. [DOI] [PubMed] [Google Scholar]
- Amato PR, & Anthony CJ (2014). Estimating the effects of parental divorce and death with fixed effects models. Journal of Marriage and Family, 76(2), 370–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson JL, Skare S, & Ashburner J (2003). How to correct susceptibility distortions in spin-echo echo-planar images: application to diffusion tensor imaging. Neuroimage, 20(2), 870–888. [DOI] [PubMed] [Google Scholar]
- Appleyard K, Egeland B, van Dulmen MH, & Alan Sroufe L (2005). When more is not better: The role of cumulative risk in child behavior outcomes. Journal of Child Psychology and Psychiatry, 46(3), 235–245. [DOI] [PubMed] [Google Scholar]
- Bagner DM, & Graziano PA (2013). Barriers to success in parent training for young children with developmental delay: The role of cumulative risk. Behavior modification, 37(3), 356–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamini Y, & Hochberg Y (1995). Controlling the false discovery rate: a practical and powerful approach to multiple testing. Journal of the royal statistical society. Series B (Methodological), 289–300. [Google Scholar]
- Brown DW, Anda RF, Tiemeier H, Felitti VJ, Edwards VJ, Croft JB, & Giles WH (2009). Adverse childhood experiences and the risk of premature mortality. American journal of preventive medicine, 37(5), 389–396. [DOI] [PubMed] [Google Scholar]
- Brown SL (2010). Marriage and child well-being: Research and policy perspectives. Journal of Marriage and Family, 72(5), 1059–1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckner RL, Head D, Parker J, Fotenos AF, Marcus D, Morris JC, & Snyder AZ (2004). A unified approach for morphometric and functional data analysis in young, old, and demented adults using automated atlas-based head size normalization: reliability and validation against manual measurement of total intracranial volume. Neuroimage, 23(2), 724–738. doi: 10.1016/j.neuroimage.2004.06.018 [DOI] [PubMed] [Google Scholar]
- Cardinale F, Chinnici G, Bramerio M, Mai R, Sartori I, Cossu M, … Caborni C (2014). Validation of FreeSurfer-estimated brain cortical thickness: comparison with histologic measurements. Neuroinformatics, 12(4), 535–542. [DOI] [PubMed] [Google Scholar]
- Chad-Friedman E, Botdorf M, Riggins T, & Dougherty LR (2020). Early childhood cumulative risk is associated with decreased global brain measures, cortical thickness, and cognitive functioning in school-age children. Developmental Psychobiology. [DOI] [PubMed] [Google Scholar]
- Chang YS, Owen JP, Pojman NJ, Thieu T, Bukshpun P, Wakahiro ML, … Sherr EH (2015). White matter changes of neurite density and fiber orientation dispersion during human brain maturation. PloS one, 10(6), e0123656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chronis AM, Lahey BB, Pelham WE Jr, Kipp HL, Baumann BL, & Lee SS (2003). Psychopathology and substance abuse in parents of young children with attention-deficit/hyperactivity disorder. Journal of the American Academy of Child & Adolescent Psychiatry, 42(12), 1424–1432. [DOI] [PubMed] [Google Scholar]
- Craig F, Operto FF, De Giacomo A, Margari L, Frolli A, Conson M, … Margari F (2016). Parenting stress among parents of children with neurodevelopmental disorders. Psychiatry research, 242, 121–129. [DOI] [PubMed] [Google Scholar]
- Crombe A, Planche V, Raffard G, Bourel J, Dubourdieu N, Panatier A, … Hiba B (2018). Deciphering the microstructure of hippocampal subfields with in vivo DTI and NODDI: applications to experimental multiple sclerosis. Neuroimage, 172, 357–368. [DOI] [PubMed] [Google Scholar]
- Dale AM, Fischl B, & Sereno MI (1999). Cortical surface-based analysis. I. Segmentation and surface reconstruction. Neuroimage, 9(2), 179–194. doi: 10.1006/nimg.1998.0395 [DOI] [PubMed] [Google Scholar]
- Desikan RS, Segonne F, Fischl B, Quinn BT, Dickerson BC, Blacker D, … Killiany RJ (2006). An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage, 31(3), 968–980. doi: 10.1016/j.neuroimage.2006.01.021 [DOI] [PubMed] [Google Scholar]
- Duan C, Hare MM, Staring M, & Deligiannidis KM (2019). Examining the relationship between perinatal depression and neurodevelopment in infants and children through structural and functional neuroimaging research. International Review of Psychiatry, 31(3), 264–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dube SR, Anda RF, Felitti VJ, Edwards VJ, & Croft JB (2002). Adverse childhood experiences and personal alcohol abuse as an adult. Addictive behaviors, 27(5), 713–725. [DOI] [PubMed] [Google Scholar]
- Duriancik DM, & Goff CR (2019). Children of single-parent households are at a higher risk of obesity: A systematic review. Journal of Child Health Care, 23(3), 358–369. [DOI] [PubMed] [Google Scholar]
- Duvernoy HM, Bourgouin P, Cabanis EA, Cattin F, Guyot J, Iba-Zizen MT, … vannson JL (1999). The human brain: Surface, three-dimensional sectional anatomy with MRI, and blood supply. New York: Springer-Wien. [Google Scholar]
- Everts R, Lidzba K, Wilke M, Kiefer C, Mordasini M, Schroth G, … Steinlin M (2009). Strengthening of laterality of verbal and visuospatial functions during childhood and adolescence. Human brain mapping, 30(2), 473–483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabiano GA, Pelham J, William E, Waschbusch DA, Gnagy EM, Lahey BB, Chronis AM, … Burrows-MacLean L (2006). A practical measure of impairment: Psychometric properties of the impairment rating scale in samples of children with attention deficit hyperactivity disorder and two school-based samples. Journal of Clinical Child and Adolescent Psychology, 35(3), 369–385. [DOI] [PubMed] [Google Scholar]
- Fischl B, & Dale AM (2000). Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proceedings of the National Academy of Sciences, 97(20), 11050–11055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B, Sereno MI, & Dale AM (1999). Cortical surface-based analysis: II: inflation, flattening, and a surface-based coordinate system. Neuroimage, 9(2), 195–207. [DOI] [PubMed] [Google Scholar]
- Fischl B, Van Der Kouwe A, Destrieux C, Halgren E, Ségonne F, Salat DH, … Kennedy D (2004). Automatically parcellating the human cerebral cortex. Cerebral Cortex, 14(1), 11–22. [DOI] [PubMed] [Google Scholar]
- Fox SE, Levitt P, & Nelson CA III (2010). How the timing and quality of early experiences influence the development of brain architecture. Child development, 81(1), 28–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukutomi H, Glasser MF, Zhang H, Autio JA, Coalson TS, Okada T, … Hayashi T (2018). Neurite imaging reveals microstructural variations in human cerebral cortical gray matter. Neuroimage, 182, 488–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garber J, & Bradshaw CP (2020). Developmental Psychopathology and the Research Domain Criteria: Friend or Foe? Journal of Clinical Child & Adolescent Psychology, 49(3), 341–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garic D, Yeh F-C, Graziano P, & Dick AS (2021). In vivo restricted diffusion imaging (RDI) is sensitive to differences in axonal density in typical children and adults. Brain Structure and Function, 226(8), 2689–2705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genc S, Malpas CB, Ball G, Silk TJ, & Seal ML (2018). Age, sex, and puberty related development of the corpus callosum: a multi-technique diffusion MRI study. Brain Structure and Function, 223(6), 2753–2765. [DOI] [PubMed] [Google Scholar]
- Giedd JN, Blumenthal J, Jeffries NO, Castellanos FX, Liu H, Zijdenbos A, … Rapoport JL (1999). Brain development during childhood and adolescence: a longitudinal MRI study. Nature neuroscience, 2(10), 861–863. [DOI] [PubMed] [Google Scholar]
- Guerrero JM, Adluru N, Bendlin BB, Goldsmith HH, Schaefer SM, Davidson RJ, … Alexander AL (2019). Optimizing the intrinsic parallel diffusivity in NODDI: an extensive empirical evaluation. PloS one, 14(9), e0217118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagler DJ Jr, Hatton S, Cornejo MD, Makowski C, Fair DA, Dick AS, … Harms MP (2019). Image processing and analysis methods for the Adolescent Brain Cognitive Development Study. Neuroimage, 202, 116091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hair NL, Hanson JL, Wolfe BL, & Pollak SD (2015). Association of child poverty, brain development, and academic achievement. JAMA pediatrics, 169(9), 822–829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson JL, Chandra A, Wolfe BL, & Pollak SD (2011). Association between income and the hippocampus. PloS one, 6(5), e18712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harding K, Galano J, Martin J, Huntington L, & Schellenbach CJ (2007). Healthy Families America® Effectiveness: A comprehensive review of outcomes. Journal of Prevention & Intervention in the Community, 34(1-2), 149–179. [DOI] [PubMed] [Google Scholar]
- Harms RL, Fritz F, Tobisch A, Goebel R, & Roebroeck A (2017). Robust and fast nonlinear optimization of diffusion MRI microstructure models. Neuroimage, 155, 82–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harms RL, & Roebroeck A (2018). Robust and fast markov chain monte carlo sampling of diffusion mri microstructure models. Frontiers in neuroinformatics, 12, 97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkey EJ, Tillman R, Luby JL, & Barch DM (2018). Preschool Executive Function Predicts Childhood Resting-State Functional Connectivity and Attention-Deficit/Hyperactivity Disorder and Depression. Biological Psychiatry: Cognitive Neuroscience and Neuroimaging, 3(11), 927–936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irfanoglu MO, Nayak A, Jenkins J, & Pierpaoli C (2017). TORTOISE v3: Improvements and new features of the NIH diffusion MRI processing pipeline. Paper presented at the ISMRM Annual Meeting. Hawaii, USA. [Google Scholar]
- Jackowski AP, Douglas-Palumberi H, Jackowski M, Win L, Schultz RT, Staib LW, … Kaufman J (2008). Corpus callosum in maltreated children with posttraumatic stress disorder: a diffusion tensor imaging study. Psychiatry Research: Neuroimaging, 162(3), 256–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson LA, Crocetti D, Dirlikov B, Slifer K, Denckla MB, Mostofsky SH, & Mahone EM (2018). Anomalous brain development is evident in preschoolers with attention-deficit/hyperactivity disorder. Journal of the International Neuropsychological Society, 24(6), 531–539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamagata K, Hatano T, Okuzumi A, Motoi Y, Abe O, Shimoji K, … Kumamaru KK (2016). Neurite orientation dispersion and density imaging in the substantia nigra in idiopathic Parkinson disease. European Radiology, 26(8), 2567–2577. [DOI] [PubMed] [Google Scholar]
- Kaufman EA, Xia M, Fosco G, Yaptangco M, Skidmore CR, & Crowell SE (2016). The Difficulties in Emotion Regulation Scale Short Form (DERS-SF): Validation and replication in adolescent and adult samples. Journal of psychopathology and behavioral assessment, 38(3), 443–455. [Google Scholar]
- Kessler RC, Adler L, Ames M, Demler O, Faraone S, Hiripi E, … Spencer T (2005). The World Health Organization Adult ADHD Self-Report Scale (ASRS): a short screening scale for use in the general population. Psychological medicine, 35(2), 245. [DOI] [PubMed] [Google Scholar]
- LaMantia A, & Rakic P (1990). Axon overproduction and elimination in the corpus callosum of the developing rhesus monkey. Journal of Neuroscience, 10(7), 2156–2175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Law EC, Sideridis GD, Prock LA, & Sheridan MA (2014). Attention-deficit/hyperactivity disorder in young children: predictors of diagnostic stability. Pediatrics, 133(4), 659–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawson GM, Duda JT, Avants BB, Wu J, & Farah MJ (2013). Associations between children's socioeconomic status and prefrontal cortical thickness. Developmental science, 16(5), 641–652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luby J, Belden A, Botteron K, Marrus N, Harms MP, Babb C, … Barch D (2013). The effects of poverty on childhood brain development: the mediating effect of caregiving and stressful life events. JAMA pediatrics, 167(12), 1135–1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machlin L, McLaughlin KA, & Sheridan MA (2020). Brain structure mediates the association between socioeconomic status and attention-deficit/hyperactivity disorder. Developmental science, 23(1), e12844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mäntymaa M, Puura K, Luoma I, Latva R, Salmelin RK, & Tamminen T (2012). Predicting internalizing and externalizing problems at five years by child and parental factors in infancy and toddlerhood. Child Psychiatry & Human Development, 43(2), 153–170. [DOI] [PubMed] [Google Scholar]
- Marečková K, Klasnja A, Bencurova P, Andrýsková L, Brázdil M, & Paus T (2019). Prenatal stress, mood, and gray matter volume in young adulthood. Cerebral Cortex, 29(3), 1244–1250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy-Jones S, Oestreich LK, Lyall AE, Kikinis Z, Newell DT, Savadjiev P, … Whitford TJ (2018). Childhood adversity associated with white matter alteration in the corpus callosum, corona radiata, and uncinate fasciculus of psychiatrically healthy adults. Brain imaging and behavior, 12(2), 449–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLanahan S, Tach L, & Schneider D (2013). The causal effects of father absence. Annual review of sociology, 39, 399–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin KA, Green JG, Gruber MJ, Sampson NA, Zaslavsky AM, & Kessler RC (2010). Childhood adversities and adult psychopathology in the National Comorbidity Survey Replication (NCS-R) III: associations with functional impairment related to DSM-IV disorders. Psychological medicine, 40(5), 847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin KA, Green JG, Gruber MJ, Sampson NA, Zaslavsky AM, & Kessler RC (2012). Childhood adversities and first onset of psychiatric disorders in a national sample of US adolescents. Archives of General Psychiatry, 69(11), 1151–1160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miklosi M, Mate O, & Balazs T (2020). Developmental Psychopathology Perspective of Attention Defi cit/Hyperactivity Disorder (ADHD). Neuropsychopharmacologia Hungarica: a Magyar Pszichofarmakologiai Egyesulet Lapja= Official Journal of the Hungarian Association of Psychopharmacology, 22(3), 112–120. [PubMed] [Google Scholar]
- Msall ME, Bier J-A, LaGasse L, Tremont M, & Lester B (1998). The vulnerable preschool child: the impact of biomedical and social risks on neurodevelopmental function. Paper presented at the Seminars in pediatric neurology. [DOI] [PubMed] [Google Scholar]
- Noble KG, Houston SM, Kan E, & Sowell ER (2012). Neural correlates of socioeconomic status in the developing human brain. Developmental science, 15(4), 516–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oguz I, Farzinfar M, Matsui J, Budin F, Liu Z, Gerig G, … Styner MA (2014). DTIPrep: quality control of diffusion-weighted images. Frontiers in neuroinformatics, 8, 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelham J, William E, Fabiano GA, & Massetti GM (2005). Evidence-based assessment of attention deficit hyperactivity disorder in children and adolescents. Journal of Clinical Child and Adolescent Psychology, 34(3), 449–476. [DOI] [PubMed] [Google Scholar]
- Pelham WE Jr, Gnagy EM, Greenslade KE, & Milich R (1992). Teacher ratings of DSM-III-R symptoms for the disruptive behavior disorders. Journal of the American Academy of Child & Adolescent Psychiatry, 31(2), 210–218. [DOI] [PubMed] [Google Scholar]
- Pienaar R, Fischl B, Caviness V, Makris N, & Grant PE (2008). A methodology for analyzing curvature in the developing brain from preterm to adult. International journal of imaging systems technology, 18(1), 42–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierpaoli C, Walker L, Irfanoglu M, Barnett A, Basser P, Chang L, … Sarlls J (2010). TORTOISE: an integrated software package for processing of diffusion MRI data. Paper presented at the ISMRM 18th annual meeting. [Google Scholar]
- Raine A, Lencz T, Taylor K, Hellige JB, Bihrle S, Lacasse L, … Colletti P (2003). Corpus callosum abnormalities in psychopathic antisocial individuals. Archives of General Psychiatry, 60(11), 1134–1142. [DOI] [PubMed] [Google Scholar]
- Rinne-Albers MA, Van Der Werff SJ, van Hoof M-J, van Lang ND, Lamers-Winkelman F, Rombouts SA, … van der Wee NJ (2016). Abnormalities of white matter integrity in the corpus callosum of adolescents with PTSD after childhood sexual abuse: a DTI study. European child & adolescent psychiatry, 25(8), 869–878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roalf DR, Quarmley M, Elliott MA, Satterthwaite TD, Vandekar SN, Ruparel K, … Hopson R (2016). The impact of quality assurance assessment on diffusion tensor imaging outcomes in a large-scale population-based cohort. Neuroimage, 125, 903–919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronald A, Pennell CE, & Whitehouse AJ (2011). Prenatal maternal stress associated with ADHD and autistic traits in early childhood. Frontiers in psychology, 1, 223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubia K (2007). Neuro-anatomic evidence for the maturational delay hypothesis of ADHD. Proceedings of the National Academy of Sciences, 104(50), 19663–19664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubia K (2018). Cognitive neuroscience of attention deficit hyperactivity disorder (ADHD) and its clinical translation. Frontiers in human neuroscience, 12, 100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato K, Kerever A, Kamagata K, Tsuruta K, Irie R, Tagawa K, … Aoki I (2017). Understanding microstructure of the brain by comparison of neurite orientation dispersion and density imaging (NODDI) with transparent mouse brain. Acta radiologica open, 6(4), 2058460117703816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saugstad LF (1994). The maturational theory of brain development and cerebral excitability in the multifactorially inherited manic-depressive psychosis and schizophrenia. International Journal of Psychophysiology, 18(3), 189–203. [DOI] [PubMed] [Google Scholar]
- Schermerhorn AC, D'Onofrio BM, Slutske WS, Emery RE, Turkheimer E, Harden KP, … Martin NG (2012). Offspring ADHD as a risk factor for parental marital problems: Controls for genetic and environmental confounds. Twin Research and Human Genetics, 15(6), 700–713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmahmann JD (2019). The cerebellum and cognition. Neuroscience letters, 688, 62–75. [DOI] [PubMed] [Google Scholar]
- Shaffer D, Fisher P, Lucas CP, Dulcan MK, & Schwab-Stone ME (2000). NIMH Diagnostic Interview Schedule for Children Version IV (NIMH DISC-IV): description, differences from previous versions, and reliability of some common diagnoses. Journal of the American Academy of Child & Adolescent Psychiatry, 39(1), 28–38. [DOI] [PubMed] [Google Scholar]
- Shao X, Zhang X, Xu W, Zhang Z, Zhang J, Guo H, … Zhang W (2021). Neurite orientation dispersion and density imaging parameters may help for the evaluation of epileptogenic tubers in tuberous sclerosis complex patients. European Radiology, 1–10. [DOI] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TE, Johansen-Berg H, … Flitney DE (2004). Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage, 23, S208–S219. [DOI] [PubMed] [Google Scholar]
- Sone D, Shigemoto Y, Ogawa M, Maikusa N, Okita K, Takano H, … Matsuda H (2020). Association between neurite metrics and tau/inflammatory pathology in Alzheimer's disease. Alzheimer's & Dementia: Diagnosis, Assessment & Disease Monitoring, 12(1), e12125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sotelo C (2004). Cellular and genetic regulation of the development of the cerebellar system. Progress in neurobiology, 72(5), 295–339. [DOI] [PubMed] [Google Scholar]
- Tamnes CK, Ostby Y, Fjell AM, Westlye LT, Due-Tonnessen P, & Walhovd KB (2010). Brain maturation in adolescence and young adulthood: regional age-related changes in cortical thickness and white matter volume and microstructure. Cereb Cortex, 20(3), 534–548. doi: 10.1093/cercor/bhp118 [DOI] [PubMed] [Google Scholar]
- Theule J, Wiener J, Tannock R, & Jenkins JM (2013). Parenting stress in families of children with ADHD: A meta-analysis. Journal of Emotional and Behavioral Disorders, 21(1), 3–17. [Google Scholar]
- Tisdall MD, Hess AT, Reuter M, Meintjes EM, Fischl B, & van der Kouwe AJ (2012). Volumetric navigators for prospective motion correction and selective reacquisition in neuroanatomical MRI. Magnetic resonance in medicine, 68(2), 389–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Victor SE, & Klonsky ED (2016). Validation of a brief version of the difficulties in emotion regulation scale (DERS-18) in five samples. Journal of psychopathology and behavioral assessment, 38(4), 582–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vidair HB, Reyes JA, Shen S, Parrilla-Escobar MA, Heleniak CM, Hollin IL, … Rynn MA (2011). Screening parents during child evaluations: exploring parent and child psychopathology in the same clinic. Journal of the American Academy of Child & Adolescent Psychiatry, 50(5), 441–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker CS, Walker BH, Brown DC, Buttross S, & Sarver DE (2020). Defining the role of exposure to ACEs in ADHD: examination in a national sample of US children. Child abuse & neglect, 112, 104884. [DOI] [PubMed] [Google Scholar]
- Wymbs BT, Pelham WE Jr, Molina BS, Gnagy EM, Wilson TK, & Greenhouse JB (2008). Rate and predictors of divorce among parents of youths with ADHD. Journal of consulting and clinical psychology, 76(5), 735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Schneider T, Wheeler-Kingshott CA, & Alexander DC (2012). NODDI: practical in vivo neurite orientation dispersion and density imaging of the human brain. Neuroimage, 61(4), 1000–1016. [DOI] [PubMed] [Google Scholar]
- Zhao X, Shi J, Dai F, Wei L, Zhang B, Yu X, … Wang H (2021). Brain Development From Newborn to Adolescence: Evaluation by Neurite Orientation Dispersion and Density Imaging. Frontiers in human neuroscience, 15, 122. [DOI] [PMC free article] [PubMed] [Google Scholar]