Abstract
An array of negative psychological states – including depressive symptoms, perceived stress, rumination, and negative affect – have been linked to immune function and inflammatory responses. Herein we show evidence of gender-dependent associations between ex vivo lipopolysaccharide (LPS)-stimulated cytokine responses and such psychological states, in both cross-sectional and longitudinal analyses from three annual waves (N = 162 at baseline, 67.3% female). In cross-sectional analyses (at baseline), gender moderated the associations of depressive symptoms (previously reported), perceived stress (B = −0.043, 95%CI [−0.080, −0.015]), rumination (B = −0.500, [−1.015, −0.232]), negative affect (B = −0.020, [−0.020, −0.005]), and positive affect (B = 0.024, [0.008, 0.047]) with LPS-stimulated cytokine responses. In each analysis, negative psychological states were positively associated with LPS-stimulated cytokine responses among men but negatively among women (with associations for positive affect in the opposite direction). In longitudinal analyses (across three annual measurements), similar associations were seen for depressive symptoms (B = −0.024, [−0.059, −0.004]), perceived stress (B = −0.045, [−0.069, −0.024]), and rumination (B = −0.381, [−0.622, −0.120]). These results indicate that gender is a critical factor in associations between a broad array of negative psychological states and inflammatory responses and identify one pathway by which gender may influence psychosomatic health.
Keywords: inflammation, negative affect, perceived stress, rumination, depression, gender, cytokine
1. Introduction
Negative psychological states characterized by depressive symptoms or high self-reported levels of psychological stress have been associated with increased risk of disease and death [1–4]. This heightened risk may be partially explained by alterations in inflammation that accompany depressed or stressed states. For example, depressive symptoms and recent stress are each independently associated with heightened and prolonged inflammatory responses to immune challenge [5–10]. High inflammation, indexed by levels of inflammatory cytokines and C-reactive protein (CRP), is in turn implicated in the pathogenesis of many physical health conditions [11].
A large and growing literature has focused on gender1 differences in inflammatory responses to pathogens [12]. Our group recently showed that gender differences in immune functioning are evident in links between inflammation and depressive symptoms [13,14]. Specifically, higher depressive symptoms were associated with a larger ex vivo lipopolysaccharide (LPS)-stimulated inflammatory response in men, but with a lower response in women. A similar gender-dependent pattern between depressive symptoms and CRP levels was observed in a large sample of older adults [15]. Heightened levels of CRP and interleukin (IL)-6 have also been observed among men but not women with a current diagnosis of major depression compared to healthy controls [9,16]. Thus, there is emerging evidence that gender is an important determinant in the link between inflammatory responses and depressive symptomology [17].
Inflammation is theorized to represent a shared pathway linking an array of positive and negative psychological states with physical health [11,18]. As such, it is an open question if the aforementioned gender difference in the link between depressive symptoms and inflammatory responses is specific to depressive symptomology or if this association might be evident for other negative psychological states. This study explored that possibility, with a focus on perceived stress, rumination, and negative affect. Positive affect (a positive psychological state) was also assessed. Examining the extent to which gender-dependent, stimulated inflammatory responses generalize to a broader set of psychological constructs beyond depressive symptoms may provide new targets for understanding psychosomatic health for basic researchers and clinicians alike.
It is critical to better understand associations between perceived stress and gender-linked inflammatory responses because of the robust associations of stress with health [4]. The existing literature2 provides mixed evidence for associations between perceived stress and stimulated cytokine production. A positive relationship between perceived stress and ex vivo inflammatory responses to LPS has been reported in two studies [18,19], whereas two other studies demonstrated null relationships between perceived stress and 1) LPS-stimulated inflammatory responses [20] and 2) ex vivo influenza vaccine [21]. None of these prior studies examined or reported gender-specific associations between perceived stress and inflammatory responses. In other work, self-reported chronic interpersonal stress [22] and perceived stress [23] were each associated with heightened cytokine responses to ex vivo LPS stimulation in two studies of women. Because these latter two studies did not include men, these findings preclude an understanding of gender differences. Perceived stress and stressor exposure have also been linked to heightened basal cytokines and CRP [23], with some work further indicating that an allostatic load perspective is necessary to elucidate associations between stress and basal cytokine concentrations [24,25]. Overall, past research indicates that self-reported stress may be linked to stimulated inflammatory responses and circulating inflammation, but examination of gender is needed.
Trait rumination is the degree to which one tends to focus on unconstructive, repetitive thoughts [26]. Positive relationships have been reported between trait rumination and basal inflammation [27,28], and trait rumination has been associated with heightened inflammatory responses to psychosocial stress among women [29]. This prior work suggests there is an association between rumination tendencies and inflammation that may extend to ex vivo stimulated inflammatory responses. No work to our knowledge has examined rumination’s association with stimulated inflammatory responses or potential gender differences therein.
Levels of positive and negative affect and basal inflammation have been linked in multiple studies [14,30–32], but few studies have examined affect and stimulated inflammation. In past work, lower trait positive affect (but not negative affect) was associated with higher LPS-stimulated IL-10 for men but not women, and higher IL-6 responses for both men and women; no associations were evident between affect and IL-1β or tumor necrosis factor (TNF)-α [33]. One other study found a positive association between trait negative affect and LPS-stimulated IL-8 responses (not IL-1β, IL-6, or TNF-α) in a large sample of men and women [18].
Our prior work focused on gender-dependent associations between depressive symptoms and inflammation in cross-sectional data [13,14]. Here we extend these analyses in the same sample by examining, in a similar manner, an array of negative psychological states and their cross-sectional associations with inflammation. We further extend our prior work by examining longitudinal associations (including depressive symptoms) across three annual timepoints. In particular, we focus on whether stimulated inflammatory responses and a broader array of negative psychological states are coupled – i.e., whether changes in one measure are reflected in changes in the other measure across time – and whether the direction and magnitude of longitudinal coupling depends on gender. The goal for the present report was to examine the separate associations of inflammatory markers with depressive symptoms, perceived stress, rumination, and affect, both cross-sectionally and across three annual waves of data collection, and to assess gender differences. Gaining a better understanding of these associations will inform both clinical and mechanistic understandings of physical and mental health.
2. Material and Methods
2.1. Participants
The present work follows prior reports on this topic [13,14] from the “Effects of Stress on Cognitive Aging, Physiology and Emotion” project [34]. Participants were recruited using systematic probability sampling from the New York City Registered Voters List. Briefly, potential participants were sent recruitment letters explaining the study goals, after which eligible participants were enrolled via telephone. The parent study’s inclusion criteria consisted of ambulatory men and women aged 25–65 who resided in Co-Op City (the Bronx, New York), were fluent in English, and who were without visual impairment. As in Majd and colleagues’ work [13], the present report included the following additional criteria: No history of inflammatory-related illness (e.g., autoimmune disorders, diabetes, cancer, HIV, chronic infections, cardiovascular, kidney or liver disease), no history of psychiatric disorders other than depression, and not taking potent immunosuppressive drugs including oral corticosteroids. This sample was racially and ethnically diverse (64% African-American; 21% Hispanic) and consisted of 67% women.
2.2. Protocol
Participants provided informed consent, then, in each wave, completed a paper survey at home and a laboratory visit at the Albert Einstein College of Medicine, followed by a 2-week burst of ecological momentary assessment (EMA; not reported here) in each of three waves3 (time between waves: Mean = 1.07 years, SD = 0.30). Following completion of each wave’s EMA, participants returned to the laboratory for a 12-hour fasting blood draw. Participants self-reported their gender as male or female (no participants reported non-binary gender) in the first wave. Data were collected between 2012 and 2017.
2.3. Self-report Measures
2.3.1. Depressive Symptoms.
The Patient Reported Outcome Measurement Information System – Depression (PROMIS Depression) short-form scale was used to measure depressive symptoms [35]. Eight items were presented regarding the frequency of depressive symptoms in the past seven days on a 1 (Never) to 5 (Always) scale (ICC = 0.79) 4. Raw scores were standardized to t-scores, such that the mean of the US population was fixed at a score of 50 (±10). Participants responded to questionnaires during the initial laboratory visit in each wave, which occurred approximately two weeks prior to the blood draw.
2.3.2. Perceived Stress Scale.
The Perceived Stress Scale [36] was used to index the frequency of participants’ subjective experiences of stressors in the past month (e.g., “How often have you been upset because of something that happened unexpectedly?”; ICC = 0.82). Participants responded to 14 items on a Likert scale of 1 (Never) to 5 (Very often).
2.3.3. Rumination and Reflection.
The Rumination and Reflection Questionnaire [26] was used to gauge the extent of participants’ tendency to focus on unconstructive, repetitive negative thoughts (rumination) and their focus motivated by curiosity and openness to experience (reflection). Twelve items each were averaged to index ruminative thinking (e.g., “I always seem to be “re-hashing” in my mind recent things I’ve said or done.”; ICC = 0.88) and reflective thinking (e.g., “I love exploring my “inner” self.”; ICC = 0.85) on a Likert scale of 1 (Strongly Disagree) to 5 (Strongly Agree).
2.3.4. Positive and Negative Affect.
Participants reported positive and negative affect experienced in the past month via the Brief Positive and Negative Affect Scale (PANAS) [37]. Positive and negative affect were scored as the sum of responses to a series of ten positive (i.e., alert, calm, relaxed, enthusiastic, excited, content, cheerful, satisfied, happy, and peaceful; ICC = 0.82) and ten negative adjectives (i.e., irritable, tense, bored, stressed, depressed, nervous, sad, sluggish, upset, and disappointed; ICC = 0.79) on a scale from 1 (Not at all) to 7 (Extremely).
2.4. Covariates
Age and BMI were included as covariates in all models in order to control for known associations between these variables and inflammation. Education (1 = at least some college; 0 = high school degree or less), household income (1 = $40K annual income or more; 0 = <$40K annual income)5, marital status (1 = married or remarried; 0 = divorced, separated, widowed, never married, or living with someone else but not married), and number of individuals living in the household were each explored as potential covariates given the possibility for gender differences in these variables. However, in each case, gender was not significantly associated with these variables in this sample (income: Χ2(1) = 0.047, p = 0.828; education: Χ2(1) = 0.124, p = 0.725; marital status: Χ2(1) = 0.285, p = 0.594; people living in household: BGENDER = −0.071, t(158) = −0.251, p = 0.802), so these variables were not included as covariates in subsequent analyses.
2.5. Bioassays
A certified phlebotomist drew 12-hour fasting blood between 7 AM and 11 AM at the Albert Einstein College of Medicine. Blood (5 mL) was collected in sodium heparin tubes to assess basal and stimulated cytokine levels and C-reactive protein (CRP).
To determine basal inflammation and CRP, whole blood was centrifuged at 1500g for 15 min at room temperature. The supernatant was aliquoted and stored at −80°C. To determine stimulated cytokine levels, 1 mL of whole blood was incubated with bacterial LPS (1 μg/mL, E. coli 055:B5, Sigma-100 mg) on a rotational shaker at 37°C in 5% CO2 for 2 h. Samples were then centrifuged at 1500g for 15 min at room temperature. The supernatant was aliquoted and stored at −80°C for future analysis. As described in Majd et al. (2018), basal and LPS-stimulated cytokines (IL-1β, IL-6, IL-8, IL-10, TNF-α) were quantified using multiplex magnetic bead arrays (Life Technologies, Grand Island NY). The minimum detection limit for these assays ranged from 0.02 to 2.77 pg/mL for each analyte and inter-assay CVs were 7.0 to 9.8%. CRP was quantified via enzyme-linked immunoassay (Cayman Chemical, Ann Arbor MI) with a detection limit of 46.9 pg/mL. Intra-assay CVs ranged from 1.9 to 7.0%; the inter-assay CV was 9.84%.
Confirmed values below the minimum detection limit were replaced with zeros. All cytokines were transformed via natural-log(x+1) to correct a positive skew in the distribution while maintaining a meaningful zero value. CRP was also positively skewed and was corrected with log-transformation for analysis [without the natural-log(x+1) transformation, as there were no samples below the minimum detection limit].
2.5.1. Composite Scores for Cytokines.
As described in prior work, individual cytokine levels were strongly correlated with one another, and a confirmatory factor analysis at baseline revealed support for a basal cytokine factor and for a stimulated cytokine factor (61% variance explained)[14]. We produced composite indices of cytokine levels by normalizing (z-score) each cytokine measurement based on Wave 1 means and standard deviations of each cytokine, then calculating the mean of these normalized values separately across the basal and stimulated cytokines. Prior work has used a similar composite approach to limit multiple comparisons [14,43].
2.6. Analyses
2.6.1. Cross-sectional.
We examined gender differences in the links between inflammation composites and the array of self-reported psychological states (other than depressive symptoms, which was examined previously; Majd et al., 2018) in the first wave of data collection (N = 162). Separate linear regression models were fit for each self-reported variable. A robustness check was performed for each of the separate interaction regression models that included the main effects of all the self-reported variables as covariates. Estimates are reported with bootstrap bias-corrected 95% confidence intervals (95%CI) using the adjusted bootstrap percentile (“Bca”) method.
2.6.2. Longitudinal.
We examined gender differences in the links between inflammation and self-reported psychological states across waves of data collection using a multilevel coupling approach [38]. This approach simply asks if changes in depressive symptoms (for example) are associated with changes in inflammation levels. That is, across three waves, when depressive symptoms decrease (or increase) more sharply within an individual, inflammation may also decrease (or increase) more sharply, such that the changes in the two variables are coupled over the course of the 3 annual waves. This coupling approach allows for the examination of interindividual differences (i.e., the effect of gender) in the association between the self-reported and inflammation variables while accounting for intraindividual variability in this association. A model to examine coupled links between depressive symptoms and inflammation was constructed as follows:
Level 1:
Level 2:
Full Model:
In this model, Inflammationij represents the level of inflammation (basal composite, stimulated composite, or CRP) for person i at time j; PROMISij represents depressive symptom levels for person i at time j. The coefficient g11 represents the estimate of the cross-level interaction between depressive levels and gender, which can be taken as evidence of gender differences in the association between depressive symptoms and inflammation. A separate model was fit for each self-reported negative variable (replacing PROMISij). For models that did not satisfactorily converge, u1i (the error term representing the participant-level random slope) was removed and the model re-fit (random intercepts were always included). A robustness check was performed for each coupling model by including the main effect of the average value of each of the self-reported variables as covariates in Level 2 of the multilevel model. Confidence intervals of estimates were bootstrap bias-corrected using the adjusted bootstrap percentile (“Bca”) method.
This approach differs from other longitudinal approaches (e.g., growth-curve modeling) as coupling does not require either variable to steadily increase or decrease; the coupling approach merely determines if changes in the two variables are consistently associated across time [38]. As with other multilevel approaches, coupling is able to use all available data, despite loss of participants across waves. For the present protocol, coupling is arguably more appropriate than a growth-curve model because there was not strong a priori expectation of a specific trajectory of inflammation or negative psychological states across 3 annual waves among the age range evident in this sample of adults.
2.6.3. Follow-up Analyses.
Two follow-up analyses were conducted on both sets of cross-sectional and longitudinal models. First, we examined self-reported, probable depression as a covariate. Participants were asked “Have you ever been diagnosed with or received treatment for emotional or psychiatric problems?” Participants that responded with any version of the term “depression” were coded as having had probable depression (although it is not known whether these responses represent major depressive disorder).
Second, we conducted follow-up analyses focused on participants’ menopausal status. Women self-reported their menopausal status in the first wave of data collection. We re-ran all analyses with the gender variable replaced with a two-level, Helmert contrast-coded variable that compared men versus women in the first level (controlling for differences between pre- and post-menopausal women) and that compared pre- versus post-menopausal women (controlling for differences between men and women) in the second level.
3. Results
3.1. Preliminary Results
Of the 162 participants at baseline who met this report’s criteria, 95 in the second wave and 71 in the third wave had both self-report and inflammation data available (see Table 1 for descriptive statistics). Health status in this sample was diverse as indexed by relatively high CRP concentrations (e.g., CRP in Wave 1, mean = 5.55 mg/L, SD = 8.14) compared to the clinical cut-off for CRP as high risk for cardiovascular disease (> 3.0 mg/L).
Table 1.
Mean (SD) of study variables.
| Full Sample | Men | Women | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Wave 1 | Wave 2 | Wave 3 | Wave 1 | Wave 2 | Wave 3 | Wave 1 | Wave 2 | Wave 3 | |
|
| |||||||||
| Sample Size | 162 | 95 | 71 | 53 | 32 | 22 | 109 | 63 | 49 |
| Age | 44.4 (11.2) | 44.2 (11.0) | 45.0 (11.1) | 44.7 (11.1) | 44.4 (10.3) | 45.8 (10.6) | 44.3 (11.2) | 44.1 (11.4) | 44.6 (11.3) |
| Basal Cytokine Composite (standardized units) | −0.05 (0.704) | −0.186 (0.419) | 0.025 (0.581) | 0.131 (1.032) | −0.137 (0.562) | 0.107 (0.664) | −0.138 (0.45) | −0.209 (0.328) | −0.014 (0.54) |
| Stimulated Cytokine Composite (standardized units) | 0.005 (0.743) | 0.03 (0.979) | −0.154 (1.116) | 0.104 (0.625) | 0.269 (0.826) | 0.061 (1.026) | −0.043 (0.792) | −0.084 (1.03) | −0.251 (1.15) |
| CRP (mg/L) | 5.55 (8.14) | 6.66 (11.68) | 7.29 (13.19) | 5.42 (9.58) | 4.77 (11.17) | 5.19 (10.59) | 5.62 (7.38) | 7.54 (11.88) | 8.27 (14.24) |
| Depressive Symptoms | 52.5 (9.2) | 52.5 (9) | 51.3 (9.2) | 53.4 (8.9) | 51.9 (9) | 51.1 (9.6) | 52.1 (9.4) | 52.8 (9) | 51.5 (9) |
| Perceived Stress | 25.8 (7.4) | 24.6 (7.3) | 24.3 (7.6) | 26.5 (7) | 24.6 (6.1) | 23.6 (6.2) | 25.5 (7.7) | 24.6 (7.9) | 24.6 (8.1) |
| Rumination | 3.08 (0.82) | 3.11 (0.88) | 2.96 (0.66) | 3.14 (0.75) | 3.15 (0.85) | 2.97 (0.65) | 3.05 (0.85) | 3.09 (0.9) | 2.95 (0.67) |
| Reflection | 3.4 (0.7) | 3.42 (0.67) | 3.36 (0.6) | 3.51 (0.75) | 3.41 (0.7) | 3.34 (0.58) | 3.35 (0.68) | 3.42 (0.65) | 3.37 (0.61) |
| Negative Affect | 36.2 (12.8) | 36.4 (12.9) | 34.3 (13.1) | 35 (13.4) | 34.5 (13) | 32.5 (13.6) | 36.8 (12.5) | 37.3 (12.9) | 35.1 (13) |
| Positive Affect | 44.8 (12.1) | 44.8 (11.3) | 45.6 (11.1) | 44.9 (11.4) | 45.5 (11.6) | 46.6 (11.3) | 44.7 (12.4) | 44.4 (11.3) | 45.1 (11.1) |
3.2. Cross-Sectional Analyses at Baseline
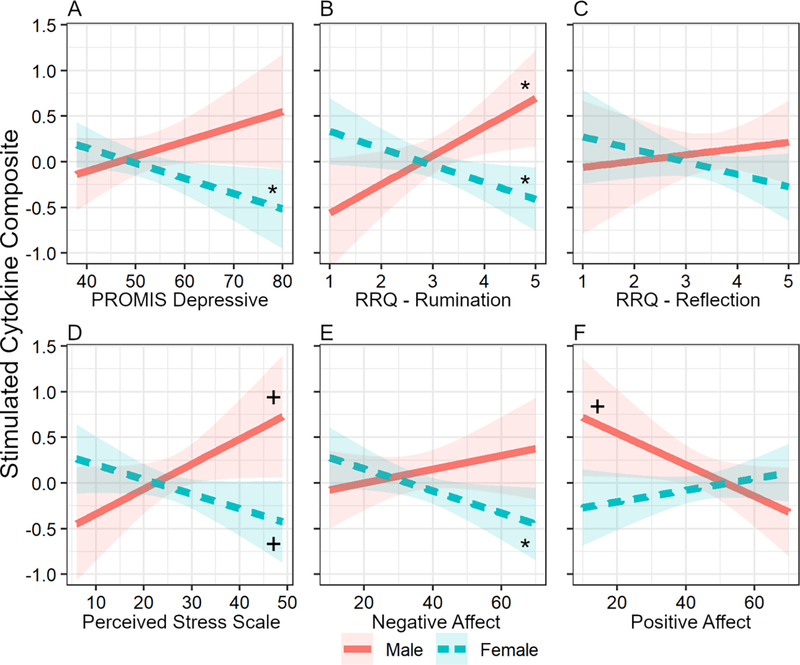
When examined cross-sectionally at baseline (wave 1), gender differences were evident in the association between self-report variables and stimulated cytokine levels. Much like depressive symptoms (previously reported in Majd et al., 2018), perceived stress (B = −0.043, 95%CI[−0.080, −0.015], t(156) = −2.53, p = 0.013), a tendency toward ruminative thinking (but not reflective thinking) (B = −0.500, [−1.015, −0.232], t(155) = −3.18, p = 0.002), and negative affect (B = −0.020, [−0.020, −0.005], t(156) = −2.07, p = 0.040) were positively associated with stimulated cytokines in men and negatively associated with stimulated cytokines in women (Table 2; Figure 1; see also Table S1). An inverse of this association was observed for positive affect: Men with lower positive affect exhibited higher stimulated cytokine levels whereas women with lower positive affect displayed lower stimulated cytokine levels (B = 0.024, [0.008, 0.047], t(156) = 2.24, p = 0.027). No such associations were seen for basal cytokines or CRP.
Table 2.
Cross-sectional analysis (baseline only) linking negative self-reported psychological states to gender-specific stimulated cytokine levels
| Full Sample | Simple slopes | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Men |
Women |
||||||||
| B a | CI | p | B b | CI | p | B b | CI | p | |
|
| |||||||||
| Depressive symptoms c | −0.033 | [−0.071, −0.010] | .017 | 0.016 | [−0.006, 0.039] | .154 | −0.017 | [−0.031, −0.002] | .027 |
| Perceived Stress | −0.043 | [−0.085, −0.015] | .013 | 0.027 | [−0.001, 0.056] | .061 | −0.016 | [−0.034, 0.002] | .084 |
| Rumination | −0.500 | [−1.001, −0.209] | .002 | 0.314 | [0.052, 0.576] | .020 | −0.186 | [−0.348, −0.023] | .026 |
| Reflection | −0.206 | [−0.471, 0.124] | .249 | 0.069 | [−0.207, 0.344] | .625 | −0.137 | [−0.345, 0.070] | .197 |
| Negative Affect | −0.020 | [−0.037, −0.005] | .040 | 0.008 | [−0.007, 0.022] | .322 | −0.012 | [−0.023, −0.001] | .035 |
| Positive Affect | 0.024 | [0.008, 0.048] | .027 | −0.017 | [−0.035, 0.0002] | .055 | 0.006 | [−0.005, 0.018] | .269 |
Notes:
Represents the regression coefficient for the interaction between gender and a given negative self-reported psychological state. See supplemental materials for full model results.
Represent the simple-slope for linking self-reported negative psychological states to stimulated cytokine levels for men and women
The cross-sectional, gender-dependent association of depressive symptoms and individual cytokines (not a composite cytokine score) was reported previously for this sample in Majd et al., 2018
Figure 1.
The association of negative psychological states with LPS-stimulated cytokine response depends on gender at baseline. Gender × negative psychological state interaction was significant unless otherwise stated. A. PROMIS depressive symptom scale (previously reported in Majd et al., 2018). B. RRQ- Rumination scale. C. RRQ – Reflection scale (the coefficient for this interaction with gender was non-significant). D. Perceived Stress Scale. E. PANAS – Negative Affect. F. PANAS – Positive Affect. Ribbons around lines represent 95% confidence intervals. Simple slopes: *p < .05; +p ≤ .08.
3.2.1. Robustness check.
Including the main effects of each of the self-reported variables as covariates did not affect the magnitude or interpretation of any of the models performed (Table S3).
3.2.2. Follow-up analyses.
The covariate for self-reported depression did not alter results or interpretation of the cross-sectional analyses (Table S4A). In addition, no differences were evident between pre- and post-menopausal women and the interaction terms for menopause status and negative psychological states did not associate with stimulated cytokine levels. The inclusion of menopause status did not change the results or interpretation of the cross-sectional psychological state × gender analyses (Table S5A).
3.3. Longitudinal Analyses
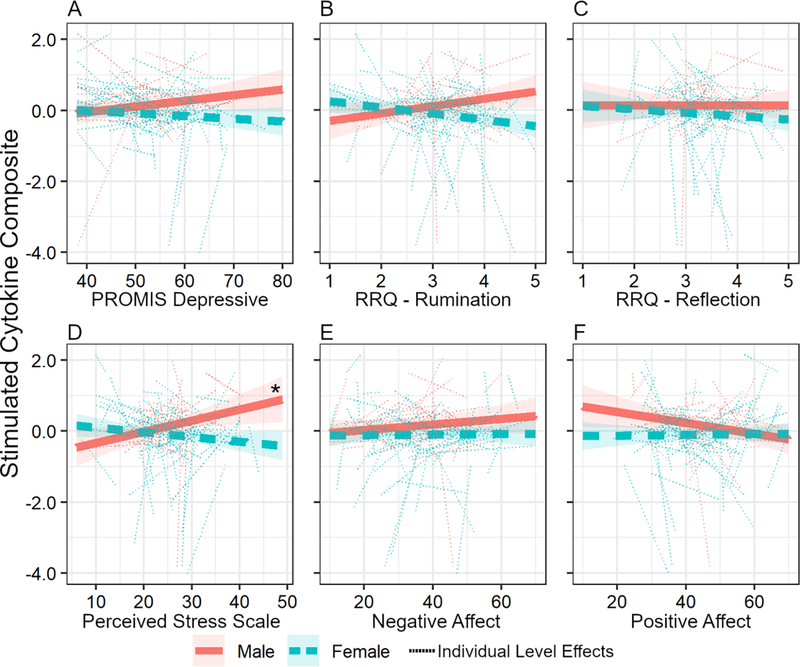
Within each longitudinal model, random intercepts and slopes were highly correlated, which necessitated removal of the random error term for individual slopes (results are reported with the individual level error terms removed; see supplement for comparison of models with and without these error terms). We first examined the coupling of depressive symptoms with inflammation across the three waves. The pattern that we reported cross-sectionally [13] remained evident in the longitudinal coupling analyses, although the effect was somewhat weaker, with a bias-corrected confidence interval that does not include zero and marginal statistical significance when including age and BMI as covariates (B = −0.024, [−0.059, −0.004], t(84.6) = −1.78, p = 0.051; Figure 2; Table S2). Specifically, across the three waves of data collection, change in depressive symptoms was positively coupled with changes in stimulated cytokine levels in men and negatively coupled with changes in stimulated cytokine levels in women (Table 3).
Figure 2.
Longitudinal coupling of negative psychological states with LPS-stimulated cytokine response depends on gender. The solid red and dashed blue lines represent the fixed effects for male and females; the thin dotted lines represent the individual level coupling effects (but note that the models did not support this complexity, and individual slopes were ultimately removed). The gender × negative psychological state interaction was significant unless otherwise stated. A. PROMIS depressive symptom scale B. RRQ-Rumination scale. C. RRQ – Reflection scale (the coefficient for this interaction with gender was non-significant). D. Perceived Stress Scale. E. PANAS – Negative Affect (the coefficient for this interaction with gender was non-significant). F. PANAS – Positive Affect (the coefficient for this interaction with gender was non-significant). The * indicates the 95%CI of the estimated simple slope did not contain zero.
Table 3.
Longitudinal coupling analysis for gender-specific associations of negative self-reported psychological states and stimulated cytokine levels.
| Full Sample | Simple Slopesa | ||||||
|---|---|---|---|---|---|---|---|
|
Male
|
Female
|
||||||
| B b | CI | p | B c | CI | B c | CI | |
|
| |||||||
| Depressive symptoms | −0.024 | [−0.058, −0.004] | .051 | 0.016 | [−0.003, 0.035] | −0.007 | [−0.021, 0.006] |
| Perceived Stress | −0.045 | [−0.075, −0.026] | .004 | 0.031 | [0.005, 0.057] | −0.013 | [−0.029, 0.003] |
| Rumination | −0.375 | [−0.626, −0.122] | .007 | 0.207 | [−0.023, 0.437] | −0.174 | [−0.325, −0.024] |
| Reflectior | −0.097 | [−0.393, 0.184] | .551 | 0.002 | [−0.254, 0.258] | −0.095 | [−0.282, 0.091] |
| Negative Affect | −0.007 | [−0.021, 0.007] | .408 | 0.008 | [−0.006, 0.022] | 0.001 | [−0.009, 0.011] |
| Positive Affect | 0.016 | [0.000, 0.034] | .088 | −0.015 | [−0.031, 0.0004] | 0.001 | [−0.009, 0.012] |
Notes:
P values cannot be reliably calculated for simple slopes produced within the longitudinal coupling analyses. We encourage readers to consider the size and direction of the confidence intervals instead.
Represents the cross-level interaction of the fixed effects of gender and negative self-reported psychological states on stimulated cytokine levels.
Represents the simple slope self-reported negative psychological states to stimulated cytokine levels for men and women.
We next examined the broader set of self-report variables using this longitudinal coupling approach. Perceived stress (B = −0.045, [−0.069, −0.024], t(256.7) = −2.89, p = 0.004) and rumination (B = −0.381, [−0.622, −0.120], t(218.8) = −2.70, p = 0.007; but not reflection) were positively coupled with stimulated cytokines in men and negatively coupled with stimulated cytokines in women across three waves of data collection (Table 3; Table S2). Positive and negative affect were not found to be longitudinally coupled to stimulated cytokines.
3.3.1. Robustness checks.
Inclusion of individual-level mean values for each of the self-reported variables as covariates did not affect the magnitude or interpretation of interactions of gender with perceived stress or rumination; the interaction of gender × depressive symptoms was marginal in this model (Table S3).
3.3.2. Follow-up analyses.
The inclusion of the self-reported depression covariate did not change the results or interpretation of the longitudinal analyses (Table S4B). In addition, no differences were evident between pre- and post-menopausal women and the interaction of menopause status and negative psychological states did not associate with stimulated cytokines. The depressive symptom × gender interaction was marginal when controlling for menopause status (and the bootstrap bias-corrected confidence interval included zero); the inclusion of menopause status otherwise did not change the results or interpretation of the psychological state × gender analyses (Table S5B).
3.4. Other biomarkers of inflammation
Analyses with basal cytokines and CRP did not demonstrate significant gender-dependent associations with any of the negative psychological states.
4. Discussion
The present results add to our understanding of gender differences in the link between depressive symptoms and systemic inflammatory responses by demonstrating that these patterns generalize to an array of negative psychological states cross-sectionally and over three annual measurements. In men, higher self-reported levels for an array of negative psychological states [i.e., higher depressive symptoms [13], perceived stress, rumination, negative affect, and lower positive affect] were related to higher ex vivo stimulated inflammatory responses. Conversely, in women, these associations between negative psychological states and stimulated cytokine levels were negatively correlated. Similar gender differences were apparent in the longitudinal coupling of stimulated inflammatory responses with depressive symptoms, perceived stress, and rumination across three annual measurements. These longitudinal results suggest that psychological states and inflammation are entwined and persistent over time: Changes in depressive symptoms, rumination, and stress correlated with changes in stimulated cytokine responses across three annual assessments.
Overall, these findings emphasize the importance of considering gender as a factor, rather than as a confounder, in studies of psychosomatic health. To wit, if gender had only been considered as a covariate in the present study, associations between negative psychological states and stimulated inflammation would not have been evident. Our results suggest that gender is important for understanding links between inflammation and reactions to may have translational implications for psychosomatic health. For example, heightened inflammatory responses are a crucial factor leading to men’s increased morbidity and mortality due to conditions like sepsis [12] and the novel coronavirus [39] and blunted inflammatory responses have been linked to poorer wound healing and weaker responses to vaccine among women [40,41]. This focus on gender comes amidst burgeoning evidence of the critical importance of considering sex and gender in health research [42]. The present results indicate that an array of negative psychological states – depressive symptoms, perceived stress, rumination, heightened negative affect, and reduced positive affect – may influence gender-dependent inflammatory responses and health outcomes.
The variables investigated in this report have similar features in that they are self-reported psychological states with negative valence (or that lack positive valence) occurring in the past few months. Yet, these variables are also distinct concepts that differ in salience and severity. Prior work has tended to approach these questions from within a given discipline’s silo – for example, by focusing on depressive symptoms per se as the singular psychological component related to immune alterations despite prior theorizing that inflammation represents a shared pathway linking psychological states and physical health [11,18]. Other psychological states that share these features or other unknown features may show similar gender-dependent associations with stimulated cytokine levels, such as bereavement [43] or anger and hostility [44,45]. Role conflict – a negative psychological state based on the degree to which the demands of one’s social roles (e.g., being a parent, spouse, and friend) pull an individual in different directions – has also been associated with heightened stimulated inflammatory responses for men, but not women [46]. In sum, a variety of psychological states – including psychological states that may be culturally specific or that may depend on social-contextual or idiosyncratic factors – may impact health in a gender-dependent manner via similar inflammatory routes. A broader approach that investigates wider sets of psychological concepts may provide new avenues for understanding gender differences in mental and physical health outcomes.
The present results point to a specific, shared pathway linking psychological states and inflammation: The gender-dependent associations between negative psychological states and inflammation were specific to LPS-stimulated inflammatory responses and were not evident for basal inflammation (either cytokine or CRP levels), a pattern evident in prior reports from this same sample [13,14]. Several factors may contribute to this finding and parsing these factors may be helpful for delineating mechanisms. For example, compared to cytokines, CRP is a less specific inflammatory biomarker that generally fluctuates on a slower timeline; it may be elevated for a number of reasons, including due to metabolic irregularities [47,48]. CRP levels in this sample were relatively high compared to other studies, perhaps indicative of metabolic-related inflammation that may obscure gender-dependent associations seen in prior work (15; for additional discussion, see 31). Regarding basal cytokines, these exist at relatively low concentrations in the absence of acute infection or other immunological challenge; stimulated cytokine concentrations occur at reliably measurable levels, are more variable, and represent a measure of inflammation distinct from basal cytokines [49]. This natural constriction of range in basal cytokine concentrations may reduce the ability to detect associations with psychological states without larger samples.
Alternatively, there may be aspects of stimulated inflammatory responses (versus other inflammatory biomarkers) that are specifically dependent on gender. For example, men have been observed to respond with heightened inflammation to microbial agents (e.g., bacterial infection, which LPS mimics) compared to women who demonstrate more robust adaptive immune responses (i.e., protective humoral and cellular mediated responses) [12,50]. A variety of explanations for these gender-specific immune responses have been examined including biological factors, such as genetic differences linked to the X chromosome [51], modulation of immune activity by sex hormones [52], and psychosocial factors such as behavioral gender differences that, in turn, relate to antigen exposure [53,54]. Basic research using a rodent model of sepsis has also determined that sexual dimorphisms exist in the pro-inflammatory pathways involved in responding to microbial challenge, including Toll-like receptor (TLR)-4 and nuclear factor kappa B (NF-κB) mediated routes [55]. Hence, the extent to which the observed associations are specific to gender dimorphisms in these pathways could be investigated more directly by testing sensitivity and expression of TLR-4 and NF-κB pro-inflammatory pathways [55,56]. Future work should continue to investigate a broad array of inflammatory measures – for example, CRP, basal and stimulated cytokines, and more specific elements of pro- and anti-inflammatory mechanistic pathways – to better understand psychosomatic associations with basal inflammation and inflammatory responses.
Three aspects of this study limit the generalizability of the results and warrant further investigation. First, participants were a subsample of a diverse population who were not selected based on a particular health condition. It is therefore unclear the extent to which these patterns may be evident in populations with prevalent health conditions, including mental health conditions such as major depressive disorder. Second, the focus on basal and stimulated cytokine biomarkers and CRP is somewhat limited, especially given evidence that women tend to respond to pathogens with greater humoral and cell-mediated immune responses than men (e.g., high immunoglobulin-M concentrations, more robust antibody production) [12]. Examining other biomarkers of innate and adaptive immune functioning in response to other stimuli [e.g., polyinosinic-polycytidylic acid (poly I:C), which mimics viral infection, or viral vaccine challenges [21]] may reveal additional associations between negative psychological states and immune functioning that differ by gender (cf. 57). Third, the longitudinal analyses for negative and positive affect did not replicate the cross-sectional results and an explanation for this pattern of results is not presently available6. Weaker associations between affect and inflammation may have been obscured by lower sample sizes in later waves or perhaps changes in affect levels were simply unrelated to changes in LPS-stimulated inflammatory responses. Future research with larger samples might employ intensive measurement over longer time periods with broader indices of immune functioning to further delineate associations between negative psychological states, gender, and physical health.
4.1. Conclusions
Ultimately, the present work indicates that gender is a critical factor for understanding psychosomatic health. Here we demonstrate that gender differences in the association between depressive symptoms and stimulated inflammatory responses 1) generalize to an array of negative psychological states (with the reverse pattern evident for positive affect), and 2) are evident across three annual assessments. These findings suggest that alterations in inflammatory responses to immune challenge are a commonality shared by a variety of psychological states; subsequent health implications need to be more fully explored. Continued investigation of gender in this context is needed to improve our understanding of how, when, and for whom physical health conditions are linked with depressive symptoms, stress, or other negative psychological states. Such work is essential to gain a full understanding of gender as a critical factor in the intertwining of mental and physical health.
Supplementary Material
Highlights.
Inflammation was linked with depressive symptoms, rumination, stress, affect.
LPS-stimulated inflammation positively related to array of negative states in men.
For women, array of negative states linked with lower inflammatory responses.
Effects evident in cross-sectional and longitudinal coupling analyses.
Acknowledgements
This research was supported in part by National Institute of Health (NIH) grants R01 AG039409 (Sliwinski, PI), R01 AG042595 (Graham-Engeland and Engeland, mPIs), and T32 AG049676 (Knight, via Pennsylvania State University).
Footnotes
Declaration of Interests: None.
Here we use “gender” to refer to the totality of biological and psychosocial differences between human men and women [61].
There is a small literature focused on changes in LPS-stimulated inflammatory responses due to acute experimental stressors. For example, acute stress reduced men’s but not women’s LPS-stimulated cytokine responses in two laboratory studies, with some nuance for which cytokines were affected [58, 59]. However, self-reported perceived stress, which we examine here, is a distinct concept from acute, laboratory-induced stress.
A fourth wave was completed for a much smaller number of participants but is not included here.
As an index of measure stability of each measure with the present sample, we report the intraclass correlation coefficient for the “average of k fixed raters,” or ICC(3,k) in the parlance of Shrout and Fleiss [62], using the psych package (Version 2.0.8; [60]) in R.
Income was split at the $40K income level to best reflect federal income limits for poverty in the United States, which was about $25K by the end of the study. Splitting the data at a lower categorical response in the income questionnaire would have included individuals who were below the poverty line in the higher income group. The median value from this survey question was 4 (mean = 3.93; mode = 3).
The longitudinal analysis with depressive symptoms was also revealed to be less robust than analyses with other psychological states, with marginal significance, in follow-up analyses focused on menopause status. As this effect was slightly weaker than other effects in the main analyses (i.e., with marginal significance but a bootstrap bias-corrected confidence interval that did not include zero), we do not interpret the marginal results to be specific to the inclusion of menopause status per se.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Zheng D, Macera CA, Croft JB, Giles WH, Davis D, Scott WK, Major depression and all-cause mortality among white adults in the United States, Ann. Epidemiol. 7 (1997) 213–218. 10.1016/S1047-2797(97)00014-8. [DOI] [PubMed] [Google Scholar]
- [2].Vasunilashorn S, Glei DA, Weinstein M, Goldman N, Perceived stress and mortality in a Taiwanese older adult population, Stress. 16 (2013) 600–606. 10.3109/10253890.2013.823943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Nielsen NR, Kristensen TS, Schnohr P, Gronbaek M, Perceived stress and cause-specific mortality among men and women: Results from a prospective cohort study, Am. J. Epidemiol. 168 (2008) 481–491. 10.1093/aje/kwn157. [DOI] [PubMed] [Google Scholar]
- [4].O’Connor DB, Thayer JF, Vedhara K, Stress and Health: A Review of Psychobiological Processes, Annu. Rev. Psychol. 72 (2021) 663–688. 10.1146/annurev-psych-062520-122331. [DOI] [PubMed] [Google Scholar]
- [5].Cyranowski JM, Marsland AL, Bromberger JT, Whiteside TL, Chang Y, Matthews KA, Depressive symptoms and production of proinflammatory cytokines by peripheral blood mononuclear cells stimulated in vitro, Brain. Behav. Immun. 21 (2007) 229–237. 10.1016/j.bbi.2006.07.005. [DOI] [PubMed] [Google Scholar]
- [6].Huang C-J, Stewart JK, Franco RL, Evans RK, Lee ZP, Cruz TD, Webb HE, Acevedo EO, LPS-stimulated tumor necrosis factor-alpha and interleukin-6 mRNA and cytokine responses following acute psychological stress, Psychoneuroendocrinology. 36 (2011) 1553–1561. 10.1016/j.psyneuen.2011.04.009. [DOI] [PubMed] [Google Scholar]
- [7].Kiecolt-Glaser JK, McGuire L, Robles TF, Glaser R, Emotions, morbidity, and mortality: New perspectives from psychoneuroimmunology, Annu. Rev. Psychol. 53 (2002) 83–107. 10.1146/annurev.psych.53.100901.135217. [DOI] [PubMed] [Google Scholar]
- [8].Glaser R, Kiecolt-Glaser JK, Stress-induced immune dysfunction: implications for health, Nat. Rev. Immunol. 5 (2005) 243–251. 10.1038/nri1571. [DOI] [PubMed] [Google Scholar]
- [9].Vogelzangs N, de Jonge P, Smit JH, Bahn S, Penninx BW, Cytokine production capacity in depression and anxiety, Transl. Psychiatry. 6 (2016) e825–e825. 10.1038/tp.2016.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Lee SM, Te S, Breen EC, Olmstead R, Irwin MR, Cho JH, Circulating versus lipopolysaccharide-induced inflammatory markers as correlates of subthreshold depressive symptoms in older adults, World J. Biol. Psychiatry. 21 (2020) 634–641. 10.1080/15622975.2019.1671608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Miller GE, Chen E, Cole SW, Health psychology: Developing biologically plausible models linking the social world and physical health, Annu. Rev. Psychol. 60 (2009) 501–524. 10.1146/annurev.psych.60.110707.163551. [DOI] [PubMed] [Google Scholar]
- [12].Marriott I, Huet-Hudson YM, Sexual dimorphism in innate immune responses to infectious organisms, Immunol. Res. 34 (2006) 177–192. 10.1385/IR:34:3:177. [DOI] [PubMed] [Google Scholar]
- [13].Majd M, Graham-Engeland JE, Smyth JM, Sliwinski MJ, Lipton RB, Katz MJ, Engeland CG, Distinct inflammatory response patterns are evident among men and women with higher depressive symptoms, Physiol. Behav. (2018). 10.1016/j.physbeh.2017.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Knight EL, Majd M, Graham-Engeland JE, Smyth JM, Sliwinski MJ, Engeland CG, Gender differences in the link between depressive symptoms and ex vivo inflammatory responses are associated with markers of endotoxemia, Brain, Behav. Immun. - Heal. 2 (2020) 100013. 10.1016/j.bbih.2019.100013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Niles AN, Smirnova M, Lin J, O’Donovan A, Gender differences in longitudinal relationships between depression and anxiety symptoms and inflammation in the health and retirement study, Psychoneuroendocrinology. 95 (2018) 149–157. 10.1016/j.psyneuen.2018.05.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Ford DE, Erlinger TP, Depression and C-Reactive Protein in US Adults, Arch. Intern. Med. 164 (2004) 1010. 10.1001/archinte.164.9.1010. [DOI] [PubMed] [Google Scholar]
- [17].Majd M, Saunders EFH, Engeland CG, Inflammation and the dimensions of depression: A review, Front. Neuroendocrinol. 56 (2020) 100800. 10.1016/j.yfrne.2019.100800. [DOI] [PubMed] [Google Scholar]
- [18].Marsland AL, Sathanoori R, Muldoon MF, Manuck SB, Stimulated production of interleukin-8 covaries with psychosocial risk factors for inflammatory disease among middle-aged community volunteers, Brain. Behav. Immun. 21 (2007) 218–228. 10.1016/j.bbi.2006.07.006. [DOI] [PubMed] [Google Scholar]
- [19].Bellingrath S, Rohleder N, Kudielka BM, Effort–reward-imbalance in healthy teachers is associated with higher LPS-stimulated production and lower glucocorticoid sensitivity of interleukin-6 in vitro, Biol. Psychol. 92 (2013) 403–409. 10.1016/j.biopsycho.2012.12.003. [DOI] [PubMed] [Google Scholar]
- [20].Koh KB, Lee Y-J, Beyn KM, Chu SH, Kim DM, Seo WY, Effects of high and low stress on proinflammatory and antiinflammatory cytokines, Psychophysiology. 49 (2012) 1290–1297. 10.1111/j.1469-8986.2012.01409.x. [DOI] [PubMed] [Google Scholar]
- [21].Sribanditmongkol V, Neal JL, Patrick TE, Szalacha LA, McCarthy DO, Effect of Perceived Stress on Cytokine Production in Healthy College Students, West. J. Nurs. Res. 37 (2015) 481–493. 10.1177/0193945914545658. [DOI] [PubMed] [Google Scholar]
- [22].Miller GE, Rohleder N, Cole SW, Chronic Interpersonal Stress Predicts Activation of Pro- and Anti-Inflammatory Signaling Pathways 6 Months Later, Psychosom. Med. 71 (2009) 57–62. 10.1097/PSY.0b013e318190d7de. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Martínez de Toda I, Miguélez L, Siboni L, Vida C, De la Fuente M, High perceived stress in women is linked to oxidation, inflammation and immunosenescence, Biogerontology. 20 (2019) 823–835. 10.1007/s10522-019-09829-y. [DOI] [PubMed] [Google Scholar]
- [24].Knight EL, Jiang Y, Rodriguez-Stanley J, Almeida DM, Engeland CG, Zilioli S, Perceived stress is linked to heightened biomarkers of inflammation via diurnal cortisol in a national sample of adults, Brain. Behav. Immun. 93 (2021) 206–213. 10.1016/j.bbi.2021.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Goldman N, Glei DA, Seplaki C, Liu I-W, Weinstein M, Perceived stress and physiological dysregulation in older adults, Stress. 8 (2005) 95–105. 10.1080/10253890500141905. [DOI] [PubMed] [Google Scholar]
- [26].Trapnell PD, Campbell JD, Private self-consciousness and the five-factor model of personality: Distinguishing rumination from reflection, J. Pers. Soc. Psychol. (1999). 10.1037/0022-3514.76.2.284. [DOI] [PubMed] [Google Scholar]
- [27].Moriarity DP, Ng T, Curley EE, Anne McArthur B, Ellman LM, Coe CL, Abramson LY, Alloy LB, Reward Sensitivity, Cognitive Response Style, and Inflammatory Response to an Acute Stressor in Adolescents, J. Youth Adolesc. 49 (2020) 2149–2159. 10.1007/s10964-020-01216-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Moriarity DP, Ng T, Titone MK, Chat IKY, Nusslock R, Miller GE, Alloy LB, Reward Responsiveness and Ruminative Styles Interact to Predict Inflammation and Mood Symptomatology, Behav. Ther. (2020). 10.1016/j.beth.2019.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Zoccola PM, Figueroa WS, Rabideau EM, Woody A, Benencia F, Differential effects of poststressor rumination and distraction on cortisol and C-reactive protein., Heal. Psychol. 33 (2014) 1606–1609. 10.1037/hea0000019. [DOI] [PubMed] [Google Scholar]
- [30].Jones DR, Graham-Engeland JE, Positive affect and peripheral inflammatory markers among adults: A narrative review, Psychoneuroendocrinology. (2021). 10.1016/j.psyneuen.2020.104892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Graham-Engeland JE, Sin NL, Smyth JM, Jones DR, Knight EL, Sliwinski MJ, Almeida DM, Katz MJ, Lipton RB, Engeland CG, Negative and positive affect as predictors of inflammation: Timing matters, Brain. Behav. Immun. (2018). 10.1016/j.bbi.2018.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Steptoe A, O’Donnell K, Badrick E, Kumari M, Marmot M, Neuroendocrine and inflammatory factors associated with positive affect in healthy men and women: The Whitehall II study, Am. J. Epidemiol. (2008). 10.1093/aje/kwm252. [DOI] [PubMed] [Google Scholar]
- [33].Prather AA, Marsland AL, Muldoon MF, Manuck SB, Positive affective style covaries with stimulated IL-6 and IL-10 production in a middle-aged community sample, Brain. Behav. Immun. (2007). 10.1016/j.bbi.2007.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Scott SB, Graham-Engeland JE, Engeland CG, Smyth JM, Almeida DM, Katz MJ, Lipton RB, Mogle JA, Munoz E, Ram N, Sliwinski MJ, The effects of stress on cognitive aging, physiology, and emotion (ESCAPE) project, BMC Psychiatry. 15 (2015) 146. 10.1186/s12888-015-0497-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Cella D, Yount S, Rothrock N, Gershon R, Cook K, Reeve B, Ader D, Fries JF, Bruce B, Rose M, The Patient-Reported Outcomes Measurement Information System (PROMIS), Med. Care. 45 (2007) S3–S11. 10.1097/01.mlr.0000258615.42478.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Cohen S, Kamarck T, Mermelstein R, A global measure of perceived stress., J. Health Soc. Behav. 24 (1983) 385–396. 10.2307/2136404. [DOI] [PubMed] [Google Scholar]
- [37].Watson D, Clark LA, Tellegen A, Development and validation of brief measures of positive and negative affect: The PANAS scales., J. Pers. Soc. Psychol. (1988). 10.1037//0022-3514.54.6.1063. [DOI] [PubMed] [Google Scholar]
- [38].Marceau K, Ruttle PL, Shirtcliff EA, Hastings PD, Klimes-Dougan B, Zahn-Waxler C, Within-person coupling of changes in cortisol, testosterone, and DHEA across the day in adolescents, Dev. Psychobiol. 57 (2015) 654–669. 10.1002/dev.21173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Gargaglioni LH, Marques DA, Let’s talk about sex in the context of COVID-19, J. Appl. Physiol. 128 (2020) 1533–1538. 10.1152/japplphysiol.00335.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Kiecolt-Glaser JK, Glaser R, Gravenstein S, Malarkey WB, Sheridan J, Glaser R, Chronic stress and age-related increases in the proinflammatory cytokine IL-6, Proc. Natl. Acad. Sci. U. S. A. (1996). 10.1073/pnas.93.7.3043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Kiecolt-Glaser JK, Marucha PT, Mercado AM, Malarkey WB, Glaser R, Slowing of wound healing by psychological stress, Lancet. 346 (1995) 1194–1196. 10.1016/S0140-6736(95)92899-5. [DOI] [PubMed] [Google Scholar]
- [42].Mauvais-Jarvis F, Bairey Merz N, Barnes PJ, Brinton RD, Carrero JJ, DeMeo DL, De Vries GJ, Epperson CN, Govindan R, Klein SL, Lonardo A, Maki PM, McCullough LD, Regitz-Zagrosek V, Regensteiner JG, Rubin JB, Sandberg K, Suzuki A, Sex and gender: modifiers of health, disease, and medicine, Lancet. (2020). 10.1016/S0140-6736(20)31561-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Fagundes CP, Murdock KW, LeRoy A, Baameur F, Thayer JF, Heijnen C, Spousal bereavement is associated with more pronounced ex vivo cytokine production and lower heart rate variability: Mechanisms underlying cardiovascular risk?, Psychoneuroendocrinology. (2018). 10.1016/j.psyneuen.2018.04.010. [DOI] [PubMed] [Google Scholar]
- [44].Suarez EC, Lewis JG, Krishnan RR, Young KH, Enhanced expression of cytokines and chemokines by blood monocytes to in vitro lipopolysaccharide stimulation are associated with hostility and severity of depressive symptoms in healthy women, Psychoneuroendocrinology. 29 (2004) 1119–1128. 10.1016/j.psyneuen.2004.01.002. [DOI] [PubMed] [Google Scholar]
- [45].Suarez EC, Lewis JG, Kuhn C, The relation of aggression, hostility, and anger to lipopolysaccharide-stimulated tumor necrosis factor (TNF)-α by blood monocytes from normal men, Brain. Behav. Immun. 16 (2002) 675–684. 10.1016/S0889-1591(02)00019-3. [DOI] [PubMed] [Google Scholar]
- [46].Schreier HMC, Hoffer LC, Chen E, Social role conflict predicts stimulated cytokine production among men, not women, Brain. Behav. Immun. 58 (2016) 272–279. 10.1016/j.bbi.2016.07.156. [DOI] [PubMed] [Google Scholar]
- [47].Black PH, Stress and the inflammatory response: A review of neurogenic inflammation, Brain. Behav. Immun. 16 (2002) 622–653. 10.1016/S0889-1591(02)00021-1. [DOI] [PubMed] [Google Scholar]
- [48].Marsland AL, Walsh C, Lockwood K, John-Henderson NA, The effects of acute psychological stress on circulating and stimulated inflammatory markers: A systematic review and meta-analysis, Brain. Behav. Immun. 64 (2017) 208–219. 10.1016/j.bbi.2017.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Davis KM, Engeland CG, Murdock KW, Ex vivo LPS-stimulated cytokine production is associated with cortisol curves in response to acute psychosocial stress, Psychoneuroendocrinology. 121 (2020) 104863. 10.1016/j.psyneuen.2020.104863. [DOI] [PubMed] [Google Scholar]
- [50].Oertelt-Prigione S, The influence of sex and gender on the immune response, Autoimmun. Rev. 11 (2012) A479–A485. 10.1016/j.autrev.2011.11.022. [DOI] [PubMed] [Google Scholar]
- [51].Libert C, Dejager L, Pinheiro I, The X chromosome in immune functions: when a chromosome makes the difference, Nat. Rev. Immunol. 10 (2010) 594–604. 10.1038/nri2815. [DOI] [PubMed] [Google Scholar]
- [52].Da Silva JAP, Sex hormones and glucocorticoids: Interactions with the immune system, in: Ann. N. Y. Acad. Sci, 1999. 10.1111/j.1749-6632.1999.tb07628.x. [DOI] [PubMed] [Google Scholar]
- [53].Vázquez-Martínez ER, García-Gómez E, Camacho-Arroyo I, González-Pedrajo B, Sexual dimorphism in bacterial infections, Biol. Sex Differ. 9 (2018) 27. 10.1186/s13293-018-0187-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Khan M, A plausible explanation for male dominance in typhoid ileal perforation, Clin. Exp. Gastroenterol. (2012) 213. 10.2147/CEG.S36569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Marriott I, Bost KL, Huet-Hudson YM, Sexual dimorphism in expression of receptors for bacterial lipopolysaccharides in murine macrophages: A possible mechanism for gender-based differences in endotoxic shock susceptibility, J. Reprod. Immunol. 71 (2006) 12–27. 10.1016/j.jri.2006.01.004. [DOI] [PubMed] [Google Scholar]
- [56].Cho JH-J, Irwin MR, Eisenberger NI, Lamkin DM, Cole SW, Transcriptomic predictors of inflammation-induced depressed mood, Neuropsychopharmacology. 44 (2019) 923–929. 10.1038/s41386-019-0316-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Suarez EC, Sex differences in the relation of depressive symptoms, hostility, and anger expression to indices of glucose metabolism in nondiabetic adults, Heal. Psychol. (2006). 10.1037/0278-6133.25.4.484. [DOI] [PubMed] [Google Scholar]
- [58].Prather AA, Carroll JE, Fury JM, McDade KK, Ross D, Marsland AL, Gender differences in stimulated cytokine production following acute psychological stress, Brain. Behav. Immun. (2009). 10.1016/j.bbi.2008.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Rohleder N, Schommer NC, Hellhammer DH, Engel R, Kirschbaum C, Sex differences in glucocorticoid sensitivity of proinflammatory cytokine production after psychosocial stress, Psychosom. Med. 63 (2001) 966–972. 10.1097/00006842-200111000-00016. [DOI] [PubMed] [Google Scholar]
- [60].Revelle W, psych: Procedures for Personality and Psychological Research, (2020). https://cran.r-project.org/package=psych.
- [61].Darnall BD, Suarez EC, Sex and gender in psychoneuroimmunology research: Past, present and future, Brain. Behav. Immun. 23 (2009) 595–604. 10.1016/j.bbi.2009.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Shrout PE, Fleiss JL, Intraclass correlations: Uses in assessing rater reliability, Psychol. Bull. (1979). 10.1037/0033-2909.86.2.420. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.