Introduction
In recent years, Australia has vaccinated increasing numbers of its citizens against seasonal influenza via both government and private vaccine programs, largely between March and July. In 2020, this culminated in the distribution of 18 million doses of influenza vaccine.1
2021 has been the second year in which delivery of the seasonal influenza national immunization program (NIP) has been impacted by the COVID-19 pandemic. In 2020, the major challenge was implementing the program in the face of widespread non-pharmaceutical interventions (NPI) that were undertaken to limit the spread of COVID-19, which has been described previously.2
In 2021, challenges have arisen because the influenza NIP coincided with the Australian government’s ambition to administer ~50 million COVID-19 doses between February and October 2021.3
In this article we discuss the timeline of events linked to the 2021 season (Figure 1) and subsequent lessons learnt by public health experts and manufacturers from running concurrent programs with similar target populations, which relate to program planning and timing, the education, training and communication, and data collection. This may give insights for program managers in the Northern Hemisphere (NH) to plan for the arrival of this challenge during ongoing COVID-19 programs and the active discussion of booster shots for variants. We also consider what these lessons may mean for future influenza pandemics.
Figure 1.
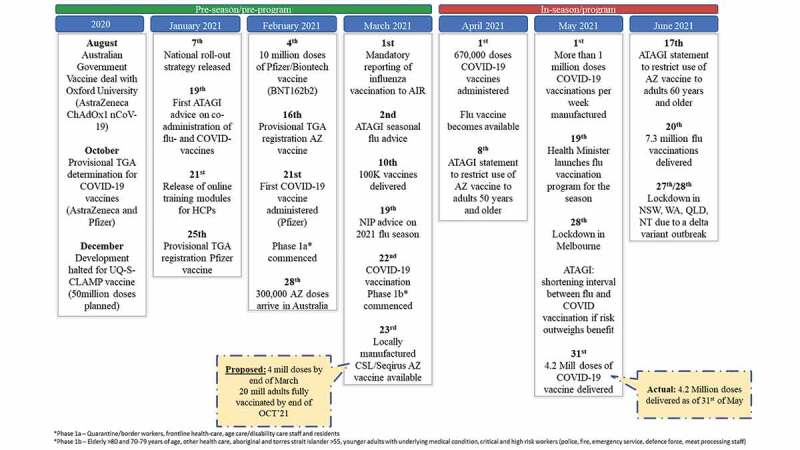
Timeline of events in the 2021 Southern Hemisphere flu season.
Background and timeline
Following the initial wave of COVID-19 infections that occurred across Australia between March and June 2020, international travel into and out of Australia was severely restricted and mandatory 14-day quarantine implemented.4 Since then, local interventions to restrict infection spread have been relaxed unless there are local outbreaks, however international travel remains restricted. Only one outbreak, in Victoria between July and November 2020, has resulted in prolonged NPI, the remaining outbreaks were smaller and addressed by implementing NPI or lockdowns up to 21 days in duration. NPI have also dramatically reduced influenza virus circulation, by June 30th, 2021, fewer than 400 cases were reported, compared to an average of over 40000 in the corresponding period in 2016–2019.5
These factors may have reduced the urgency for delivery of COVID-19 and flu vaccination. However, although timing is difficult to predict, it is likely both viruses will circulate widely again once international borders open fully. Therefore, vaccine programs against both viruses remain a public health priority; with COVID-19 currently given higher priority than flu.
The potential for concurrent COVID-19 and flu vaccination programs first arose in August 2020 when the Australian government entered an early supply and manufacturing agreement with Astra Zeneca (AZ) for their vaccine candidate ChAdOx1 nCoV-19. 3.8 million doses were to be supplied from overseas and 50 million doses were to be manufactured onshore by CSL.6,7 Subsequently, 20 million doses of the Pfizer vaccine candidate (BNT162b2) were purchased on advice from the Scientific Industry Technical Advisory Group.8 In October 2020 these products received provisional TGA determinations that allowed an accelerated pathway leading to approval in early 2021.9,10–12
The national roll-out strategy, released in early January 2021, provided a roadmap to fully vaccinate Australia by October 2021. The three phases in the plan prioritized population groups according to risk of exposure to infection, risk of severe disease, and societal impact starting with phase 1a which included older age groups, disability care residents and workers, and frontline healthcare and quarantine workers. Phase 2 captured the balance of adults before vaccination of adolescents under 18 years in Phase 3. The roadmap also gave insight into potential vaccination sites, proposing standalone vaccination hubs equipped for the Pfizer vaccine’s storage.13
In late January 2021, the Australian Technical Advisory Group for Immunization (ATAGI) recommended 14-days separation between COVID-19 vaccination and other vaccinations, including flu, under most circumstances meaning COVID-19 and flu vaccines should not be administered at the same visit. This decision was due to the lack of COVID-19 vaccine co-administration data and to allow effective safety monitoring of COVID-19 vaccines given alone.14
On February 21st, 2021, the COVID-19 vaccination program commenced with the administration of Pfizer vaccine to an 84-year-old female.15 Initial vaccine uptake was slow, largely due to limitations in Pfizer vaccine supply and the restricted locations capable of storing this vaccine. Meanwhile, healthcare professionals participating in the COVID-19 program were assigned comprehensive online training modules covering dosing, administration, and co-administration recommendations.16
The first AZ vaccine doses arrived in Australia in late February; vaccinations commenced shortly afterward.6 The initial overseas supply was limited, constraining vaccination rates even though GP practices were also included as vaccination sites.17 As a result, by April 1st 670,000 COVID-19 vaccines had been administered; representing only 17% of the initial 4 million dose target set for the end of March.18
At the end of March AZ vaccine doses manufactured locally at CSL and Seqirus’ Australian sites became available.19 These doses were released at a rate of ~1 million doses per week, allowing increased supply to immunizers and thereby the potential to increase vaccination rates.3,20
However, as doses administered increased, reports of thrombosis with thrombocytopenia, a rare but potentially serious side effect, emerged overseas and in Australia. Continuing evaluation of the benefit-to-risk profile led ATAGI to first restrict use of AZ vaccine to adults 50 years and above and later to adults 60 years and older.21,22 These recommendation changes attracted widespread media attention that may have impacted on the public’s confidence and willingness to receive the AZ vaccine, exacerbated by availability of an alternative vaccine and limited urgency due to low COVID-19 spread.23
Whilst timelines and recommendations for the COVID-19 vaccine program have regularly evolved, the doses for the seasonal influenza NIP and annual private market vaccination were approved and delivered within normal timeframes in March and April. Consistent with this, ATAGI advice on high-risk groups funded for influenza vaccines was released in March, however, a strong focus on COVID-19 vaccines led to the health minister officially launching the 2021 flu NIP two months later on May 19, 2021.24 By June 20th, 7.31 million flu vaccinations had been delivered Australia wide according to Australian Immunization Register (AIR) records, in contrast this figure was achieved by 27 May in 2020.1,25 Changes to AIR reporting make comparison to previous years challenging, however, FluTracking project data reveals that flu vaccine coverage is likely down as only 68.1% of participants in the survey week ending 20 June 2021 (n = 66,230 respondents) had been vaccinated for flu, compared to 80.4% for week ending 21 June 2020 (n = 72,900) and 74% in the week ending 23 June 2019 (n = 40,159).26–28 Notably, 2021 rates are below even 2019 levels for all cohorts (<5 years: 43.7% vs 72.5%, 5–17 years: 31.1% vs 47.8%, 18–64 years: 64.7% vs 73.6%, 65+ years 86.1% vs 88.6% and working with patients: 74.6% vs 85.6%) indicating factors beyond delay due to co-administration exclusion are likely at fault.26,28
Lessons learned
Target populations
During the COVID-19 impacted 2020 influenza vaccine program the Australian government required all individuals working or living in aged care/nursing/long-term care or assisted living facilities to receive a flu vaccine by May 1, 2020.1 The Australian Government did not renew this for 2021, however, all state governments except Victoria kept similar requirements in place.29–36 Subsequently, demand for flu vaccine in these target populations continued beyond the 2020 flu program into the early stages of the 2021 flu vaccine season. The lesson learnt is these programs need to be coordinated with availability of seasonal flu stock, particularly where COVID-19 may change when the annual flu vaccine program launches.
Vaccination planning and timing
Lower uptake of flu vaccine in SH21 compared to SH20 and SH19 suggests that the COVID-19 vaccine program has, at least in part, had an impact on flu vaccine uptake. However, lessons relating to the planning, timing and location of vaccination could help to improve coordination and integration between these programs and increase flu vaccine uptake.
To achieve the objectives of both vaccine programs it was important to plan the sequence for how each COVID-19 vaccine eligible group could receive their flu vaccine as well. Therefore, ensuring these programs were organized by healthcare professionals with substantial immunization experience, such as state government flu teams, could have ensured more channels for flu vaccine were included. Without this, different systems for booking and management were being used, creating a barrier to scheduling patient’s flu vaccine appointment when they received the COVID-19 vaccine.
Similarly, the additional sites at which COVID-19 vaccines were administered presented challenges for co-ordinating recall of individual patients for the flu vaccine around their COVID-19 vaccine. Anecdotally, some GP clinics saw good recall of older adults for flu vaccination, however, younger groups proved more challenging. Therefore, it is important to appreciate how concerted efforts to recall flu vaccine eligible patients around their COVID-19 eligibility could improve flu vaccine coverage. For example, patients ≤18 years old could be recalled for their flu vaccine well ahead of phase 3. Additionally, the impact of increased working from home on workplace vaccine program coverage, and whether hours of operation of vaccine clinics enable workers to get vaccinated should also be addressed.
Flu vaccine uptake could also have been improved through prompt distribution of flu vaccines to all programs following release by the TGA, coupled with immediately announcing their availability. This would allow individuals to be vaccinated before they became eligible for the COVID-19 vaccine and would also have been favorable at GPs as it allows opportunistic vaccination of patients presenting after the practices weekly COVID-19 vaccine allocation was exhausted (initially 50 doses per week for most practices), or vaccination of COVID-19 hesitant patients accepting of flu.37
The impact of the 14-day co- and near-administration exclusion window around COVID-19 vaccination also needs to be considered. This recommendation created the potential for a 5–6-week period during which flu vaccination would not be recommended; excluding a sizable portion of the typical flu vaccine period (April and May) for those receiving COVID-19 vaccine in phase 1. Practically, the decision to recommend a 12-week interval for AZ vaccine, based on the real-world evidence for effectiveness, created a 7–8-week window between doses in which flu vaccine could be administered. GP clinics, who could access both COVID-19 and flu vaccines, have taken advantage of this to schedule patients flu vaccine 2–8 weeks from their first COVID-19 dose, thus completing all three vaccinations in 12-weeks. The change in recommendation to 7-days expanded this window and introduced a 1-week opening in the Pfizer vaccine schedule.38 Hence, COVID-19 vaccine programs must minimize exclusion periods, and ideally consider routine co-administration as co-administration data starts to emerge, to maximize opportunities for flu vaccination.39
The important role of communication
Clear, consistent, simple, and timely messages to both vaccinators and the public are vital for ensuring high-rates of COVID-19 and flu vaccine uptake, particularly for communicating to those at greater risk of co-infection. Therefore, variation on messages needs to be avoided, as was seen from the confusion caused by health professional training modules incorrectly stating a 7-day co-administration window initially, which also required many health workers to complete additional training to clarify.
Contributing to the lower flu vaccine uptake in 2021 may be the later announcement of the NIP flu program compared to 2020 and messaging heavily focused on COVID-19 vaccine alone. In contrast, New Zealand produced a strong communications campaign for older adults, who were not eligible for COVID-19 vaccination at the time, to get their flu vaccine before COVID-19 achieving over 60% uptake within approximately 4 weeks.40 The lesson therefore is timely and repeated messaging, co-ordinated by public health authorities ahead of flu vaccine availability, is critical for instilling a sense of urgency and importance among the public regarding receiving both vaccines.
These communications from government and health professionals should also be co-ordinated to ensure a consistent, repeated, message. The media also have an important role to play through ensuring the consistency of messages is maintained through informative articles that reflect the weight of public health experts’ consensus. Failure to achieve this may undermine public confidence in specific vaccines or vaccination, as seen from the reduced confidence in AZ vaccine following evolving messages.41
Data collection and co-ordination
The distinct sites for COVID-19 vaccine administration meant patients vaccination status needed to be co-ordinated across multiple delivery channels to ensure exclusion periods were adhered to and that records of vaccination were retained. Therefore, the Australian Government introduced legislation that made reporting to the AIR mandatory, practice software was also updated to transmit data to the AIR.42 Collectively, this created a reasonably accurate central database of patient’s vaccination status. However, it is important to ensure all providers can access the database as residential aged care facilities did not have access and therefore could not advise on the vaccination status of residents during transfers or for future mop-up programs.43,44
In addition to providers, individual patients must also be able to access their vaccination data to demonstrate their vaccination status, such as is required when entering an aged-care facility. Digital certificates housed within digital wallets on patient’s smart devices have been touted as a solution to this challenge, which should be supported by widespread education to ensure everyone knows how to use these tools.45 In the interim, it is valuable to provide patients with a written record and education on how to access their electronic medical record, which is currently where that information is housed.
Monitoring the safety of new COVID-19 vaccines has been a focus of the vaccination program. To this end, efforts by AusVax Safety, a collaboration led by the National Center for Immunization Research and Surveillance, to communicate real-time updates on vaccine side effects to immunizers via electronic messaging based on their surveillance has been well received by immunizers who found it useful in communication with patients.46 However, co-administration exclusion periods mean safety data collection from real-world sources has been severely limited. Hence, vaccine programs need to ensure prospective co-administration studies are being established.
Planning for future flu seasons incl. NH 2021/22
At the current rates of global progress, COVID-19 vaccination programs will continue to be delivered alongside influenza vaccine programs for at least the next few seasons, ideally as co-administered vaccines once enough data emerges. There is also a possibility that COVID-19 vaccine booster doses may need to be delivered seasonally for the longer term, depending on the emergence of vaccine escape mutants and the duration of protection of COVID-19 vaccines. Hence, future vaccine programs will need to be designed to achieve high uptake of both vaccinations with consideration to the lessons from previous seasons (Box 1), eligibility for both vaccines, delivery networks (e.g., public vs. private), flu and COVID-19 epidemiology and the residual protection offered by previous vaccination.
Box 1.
Summary of lessons
To maximize uptake of both vaccines:
|
All involved professionals need access to vaccine records for program co-ordination, and individual patients need access to their record to demonstrate their vaccine status.
With respect to epidemiology, NH countries such as Canada, Germany, UK, and US, are entering this challenge for the NH21/22 flu season with fluctuating COVID-19 case numbers and low influenza circulation, as distinct from Australia which had negligible case number for both diseases. These countries are also at different stages of their vaccine roll-outs, with 30.9%, 37%, 46.3% and 48.8% of Canadian, German, US and UK residents fully vaccinated respectively on 30 June 2021.18 Hence, they may prioritize their programs differently to the SH approach.
What about the next (Influenza-) pandemic?
Emergence of new viruses and viral variants with pandemic potential, including influenza and corona viruses, mean the next pandemic is a matter of ‘if,’ not ‘when.’ The development and subsequent roll-out of COVID-19 vaccines offer many learnings for future pandemics with key focus areas being research policy, manufacturing, communications, and collaboration.
The rapid COVID-19 vaccine development programs were built on a large body of basic research into coronaviruses initiated following the SARS-CoV-1 epidemic. Also critical has been the unprecedented data sharing and academic-industry collaboration, including sharing of technology platforms and knowledge. Fostering such collaborations and supporting infectious diseases research should become a pillar of pre-pandemic preparedness to ensure rapid vaccine development in the next pandemic.
Meanwhile, delays and uncertainties in deliveries of overseas vaccine supplies highlights why scalable, onshore production is essential. To this end, the Australian government has entered an $800 million partnership with Seqirus to secure local cell-based influenza vaccine manufacturing capacity; an important investment that reflects how cell-based vaccines can be more rapidly scaled in response to an influenza pandemic than existing egg-based capacity.47 Similarly, state, and federal governments are establishing partnerships for the development of local mRNA vaccine capacity.48–50
Delivery of annual vaccination programs for seasonal influenza will again be a consideration during future pandemics and during the rollout of pandemic vaccine programs. In 2020, following a strong influenza campaign the uptake of influenza vaccine was unprecedented, however in 2021, when focus was on the COVID-19 vaccine the influenza program was less successful. This shows that it will be critical for government and other stakeholders to remember the communication and program planning lessons from this pandemic (Box 1 and Ref 2) to ensure high vaccine uptake in a timely fashion.
Conclusions
Opportunities to maximize influenza vaccine uptake were lost in Australia in 2021 due to a lack of an integrated program. A centralized co-ordinated campaign informed by policy recommendations and the timing, and availability of both influenza vaccines and COVID-19 vaccines is required and should be continuously updated and shared. This would enable a focus on influenza vaccine, either before the availability of COVID vaccines, or between COVID doses.
Acknowledgments
We appreciate the excellent medical writing and editorial assistance provided by Anja Giese of Otway Pharmaceutical Development and Consulting. Funding for this editorial assistance was provided by Seqirus Pty Ltd, Melbourne, Australia.
Funding Statement
This work was supported by the Seqirus Pty Ltd, Melbourne, Australia.
Disclosure of potential conflicts of interest
PGVB has received financial support for education delivery, research, or advisory board attendance from Seqirus and Pfizer. A.N has received financial support for education delivery, or advisory board attendance from Seqirus, Sanofi, GSK, MSD and Pfizer. C.R.M reports grants from Sanofi and other support from Seqirus, AstraZeneca Australia, and Janssen, outside of the submitted work. A.T.K, C.C., and J.A. are employees of Seqirus Pty Ltd, Melbourne, Australia.
References
- 1.Ministers Department of Health . Flu vaccination more important than ever during the month of April; 2020. April 1. [Online]. [accessed 2021 June 21]. https://www.health.gov.au/ministers/the-hon-greg-hunt-mp/media/flu-vaccination-more-important-than-ever-during-the-month-of-april.
- 2.Richmond H, Rees N, McHale S, Rak A, Anderson J.. Seasonal influenza vaccination during a pandemic. Hum Vaccin Immunother. 2020. Sep 1;6(9):2219–21. doi: 10.1080/21645515.2020.1793713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Press Conference Australian Parliament House . Transcript; 2021. January 25. [Online]. [accessed 2021 June 21]. https://www.pm.gov.au/media/press-conference-australian-parliament-house-act-25-01-21.
- 4.Prime Minister of Australia . Media release. Border restrictions; 2020. March 19. [Online]. [accessed 2021 June 30]. https://www.pm.gov.au/media/border-restrictions.
- 5.Australia Government Depeartment of Health . National notifiable diseases surveillance system, 1991-2021 [Online]. [accessed 2021 June 30]. http://www9.health.gov.au/cda/source/cda-index.cfm.
- 6.Prime Minister of Australia . Joint media release. 300,000 AstraZeneca vaccine doses arrive in Australia; 2021. February 28. [Online]. [accessed 2021 June 21]. https://www.pm.gov.au/media/300000-astrazeneca-vaccine-doses-arrive-australia.
- 7.Prime Minister of Australia . Media release. New deal secures potential COVID-19 vaccine for every Australian; 2020. August 19. [Online]. [accessed 2021 July 12]. https://www.pm.gov.au/media/new-deal-secures-potential-covid-19-vaccine-every-australian.
- 8.Prime Minister of Australia . Joint media release. Greater access - additional 10 million Pfizer-Biontech vaccines; 2021. February 4. [Online]. [accessed 2021 June 21]. https://www.pm.gov.au/media/greater-access-additional-10-million-pfizer-biontech-vaccines.
- 9.Australian Government Department of Health. Therapeutic Goods Administration (TGA). TGA grantssecond provisional determination for a COVID-19 vaccine; 2020 October 14. [Online]. [accessed 2021 June 21]. https://www.tga.gov.au/tga-grants-second-provisional-determination-covid-19-vaccine.
- 10.Australian Government Department of Health . Therapeutic Goods Administration (TGA). TGA grants provisional determination for COVID-19 vaccine; 2020. October 9. [Online]. [accessed 2021 June 21]. https://www.tga.gov.au/tga-grants-provisional-determination-covid-19-vaccine
- 11.Australian Government Department of Health . Therapeutic Goods Administration (TGA). TGA provisionally approves Pfizer COVID-19 vaccine; 2021. January 25. [Online]. [accessed 2021 June 30]. https://www.tga.gov.au/media-release/tga-provisionally-approves-pfizer-covid-19-vaccine.
- 12.Australian Government Department of Health . Therapeutic Goods Administration (TGA). TGA provisionally approves AstraZeneca’s COVID-19 vaccine; 2021. February 16. [Online]. [accessed 2021 June 30]. https://www.tga.gov.au/media-release/tga-provisionally-approves-astrazenecas-covid-19-vaccine.
- 13.Australian Government Department of Health . COVID-19 vaccination. Australia’s COVID-19 vaccine national roll-out strategy; 2021. January 7. [Online]. [accessed 2021 June 30]. https://www.health.gov.au/resources/publications/covid-19-vaccination-australias-covid-19-vaccine-national-roll-out-strategy.
- 14.Australian Technical Advisory Group on Immunisation (ATAGI) . Advice on relative timing of administering influenza and COVID-19 vaccines in 2021; 2021. January 19. [Online]. [accessed 2021 June 21]. https://www.health.gov.au/sites/default/files/documents/2021/01/covid-19-vaccination-atagi-advice-on-influenza-and-covid-19-vaccines.pdf.
- 15.Australian Government Department of Health . Media release. First COVID-19 vaccinations; 2021. February 21. [Online]. [accessed 2021 June 21]. https://www.pm.gov.au/media/first-covid-19-vaccinations.
- 16.Ministers Department of Health . Mobilising Australia’s COVID-19 vaccine workforce; 2021. January 21. [Online]. [accessed 2021 June 30]. https://www.health.gov.au/ministers/the-hon-greg-hunt-mp/media/mobilising-australias-covid-19-vaccine-workforce.
- 17.ABC news – Italy . EU refuse AstraZeneca request to ship 250,000 doses of vaccine to Australia; 2021. March 5. [Online]. [accessed 2021 June 21]. https://www.abc.net.au/news/2021-03-05/italy-eu-block-250000-astrazeneca-doses-to-australia/13218348.
- 18.Our world in data - COVID-19 doses administered, [Online]. [accessed 2021 July 8]. https://ourworldindata.org/coronavirus#coronavirus-country-profiles.
- 19.Australian Government Department of Health . Therapeutic Goods Administration (TGA). Melbourne-made COVID-19 vaccine now available; 2021. March 23. [Online]. [Accessed 2021 June 30]. https://www.tga.gov.au/media-release/melbourne-made-covid-19-vaccine-now-available.
- 20.CSL . Update on CSL COVID-19 vaccine manufacturing numbers; 2021. May 1. [Online]. [accessed 2021 June 30]. https://www.csl.com/news/2021/20210501-update-on-csl-covid-19-vaccine-manufacturing-numbers.
- 21.Australian Government Department of Health . Australian Technical Advisory Group on Immunisation (ATAGI). Statement on AstraZeneca vaccine in response to new vaccine safety concerns; 2021. April 8. [Online]. [accessed 2021 June 21]. https://www.health.gov.au/news/atagi-statement-on-astrazeneca-vaccine-in-response-to-new-vaccine-safety-concerns.
- 22.Australian Government Department of Health . Australian Therapeutic Advisory Group on Immunisation (ATAGI). Statement on revised recommendations on the use of COVID-19 vaccine AstraZeneca; 2021. June 17. [Online]. [accessed 2021 June 30]. https://www.health.gov.au/news/atagi-statement-on-revised-recommendations-on-the-use-of-covid-19-vaccine-astrazeneca-17-june-2021.
- 23.The Guardian . Essential poll: fewer than 50% of over-50s willing to get AstraZeneca vaccine; 2021. April 27. [Online]. [accessed 2021 June 30]. https://www.theguardian.com/australia-news/2021/apr/27/essential-poll-fewer-than-50-of-over-50s-willing-to-get-pfizer-or-astrazeneca-vaccines.
- 24.Ministers Department of Health . National flu vaccination program officially launches; 2021. May 20. [Online]. [accessed 2021 June 21]. https://www.health.gov.au/ministers/the-hon-greg-hunt-mp/media/national-flu-vaccination-program-officially-launches.
- 25.Australian Government Department of Health . Influenza (flu) immunisation data; 2021. June 20. [Online]. [accessed 2021 June 30]. https://www.health.gov.au/sites/default/files/documents/2021/06/influenza-flu-immunisation-data-influenza-flu-immunisation-data-20-june-2021.pdf.
- 26.Flu Tracking Tracking COVID-19 . Flu tracking reports weekly interim report, week ending 23 June 2019; 2019. June 23. [Online]. [accessed 2021 June 30]. https://info.flutracking.net/reports-2/australia-reports/.
- 27.Flu Tracking Tracking COVID-19 . Flu tracking reports weekly interim report, week ending 21 June 2020; 2020. June 21. [Online]. [accessed 2021 June 30]. https://info.flutracking.net/reports-2/australia-reports/.
- 28.Flu Tracking Tracking COVID-19 . Flu tracking reports weekly interim report, week ending 20 June 2021; 2021. June 20. [Online]. [accessed 2021 June 30]. https://info.flutracking.net/reports-2/australia-reports/.
- 29.Queensland Government . Queensland health. Age care direction (No.28); 2021. April 28. [Online]. [accessed 2021 July 10]. https://www.health.qld.gov.au/system-governance/legislation/cho-public-health-directions-under-expanded-public-health-act-powers/revoked/aged-care-28.
- 30.South Australia Emergency Management Direction 2021 . 2021. March 10. [Online]. [accessed 2021 July 10]. https://legislation.Sa.gov.au/web/information/CV19/EMA-CEASED/Emergency%20Management%20(Residential%20Aged%20Care%20Facilities%20No%2033)(COVID-19)%20Direction%202021_11.3.2021_CEASED.pdf.
- 31.Public Health Act 2016 (WA) . Visitors to residential aged care facilities directions (NO 7); 2021. February 23. [Online]. [accessed 2021 July 10]. https://www.wa.gov.au/sites/default/files/2021-02/240221-Visitors-to-Residential-Aged-Care-Facilities-Directions-No7.pdf.
- 32.ACT Government . Public health (Residential Aged Care Facilities) emergency direction 2021 (No 2); 2021. February 4. [Online]. [accessed 2021 July 10]. https://www.legislation.act.gov.au/View/ni/2021-56/20210207-75852/PDF/2021-56.PDF.
- 33.Northern Territory of Australia Public and Environment Health Act 2011 . COVID-19 directions (No 40) 2020. Directions for aged care facilities; 2020. June 24. [Online]. [accessed 2021 July 11]. https://coronavirus.nt.gov.au/__data/assets/pdf_file/0006/898953/cho-directions-no40-directions-aged-care-facilities.pdf.
- 34.Public health (COVID-19 gathering restrictions) order 2021; 2021. March 29. [Online]. [accessed 2021 July 10]. https://legislation.Nsw.gov.au/file/Public%20Health%20(COVID-19%20Gathering%20Restrictions)%20Order%202021_210503.pdf.
- 35.NSW Government Health . Advice to residental aged care facilities; 2021. July 10. [Online]. [accessed 2021 July 10]. https://www.health.nsw.gov.au/Infectious/covid-19/Pages/racf-latest-advice.aspx.
- 36.Vic.GOV.AU Coronavirus . Visiting care facilities; 2021. July 8. [Online]. [accessed 2021 July 10]. https://www.coronavirus.vic.gov.au/visiting-care-facilities.
- 37.Ministers Department of Health . GPs to get increased supplies of COVID-19 vaccines; 2021. May 5. [Online]. [accessed 2021 June 21]. https://www.health.gov.au/ministers/the-hon-greg-hunt-mp/media/gps-to-get-increased-supplies-of-covid-19-vaccines.
- 38.Australian Government Department of Health . Australian Technical Advisory Group on Immunisation (ATAGI). COVID 19 vaccination. ATAGI clinical guidance on COVID-19 vaccine in Australia in 2021; 2021. June 17. [Online]. [accessed 2021 June 21]. https://www.health.gov.au/sites/default/files/documents/2021/06/covid-19-vaccination-atagi-clinical-guidance-on-covid-19-vaccine-in-australia-in-2021_1.pdf.
- 39.Toback S, Galiza E, Cosgrove C, Galloway J, Goodman AL, Swift PA, Rajaram S, Graves-Jones A, Edelman J, Burns F, et al. Safety, immunogenicity, and efficacy of a COVID-19 vaccine (NVX-CoV2373) co-administered with seasonal influenza vaccines. medRxiv (Preprint Available Online, Submitted to the Lancet). 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Stuff, National News New Zealand . Health; 2021. May 17. [Online]. [accessed 2021 June 30]. https://www.stuff.co.nz/national/health/300309713/flu-jab-rolls-out-to-new-zealanders-under-65-after-monthlong-delay-in-schedule.
- 41.Royal Australian College of General Physicians (RACGP) . RACGP urges support for vaccine counselling; 2021. June 17. [Online]. [accessed 2021 June 30]. https://www.racgp.org.au/gp-news/media-releases/2021-media-releases/june-2021/racgp-urges-support-for-vaccine-counselling.
- 42.Ministers Department of Health . Building a stronger Australian immunisation register; 2021. February 5. [Online]. [accessed 2021 June 21]. https://www.health.gov.au/ministers/the-hon-greg-hunt-mp/media/building-a-stronger-australian-immunisation-register.
- 43.Australian Government Services Australia . Australian Immunication Register (AIR). Application to register as a vaccination provider form (IM004); 2020. February 20. [Online]. [accessed 2021 July 10]. https://www.servicesaustralia.gov.au/organisations/health-professionals/forms/im004.
- 44.Australian Government . COVID-19 vaccination; 2021. April 14. [Online]. [accessed 2021 July 10]. https://www.health.gov.au/sites/default/files/documents/2021/04/covid-19-vaccination-clinical-governance-requirements-for-covid-19-vaccination-in-residential-aged-care-covid-19-vaccination-clinical-governance-requirements-for-covid-19-vaccination-clinics-.
- 45.Prime Minister of Australia . National cabinet statement; 2021. June 4. [Online]. [accessed 2021 June 30]. https://www.pm.gov.au/media/national-cabinet-statement-040621.
- 46.AusVaxSafety . An National Centre for Immunisation Research and Surveillance (NCIRS) led collaboration. COVID-19 vaccine safety surveillance; 2021. [Online]. [accessed 2021 June 21]. https://www.ausvaxsafety.org.au/our-work/covid-19-vaccine-safety-surveillance.
- 47.Seqirus . Seqirus will build world-class, next generation $800m influenza vaccine manufacturing facility in Australia; 2020. November 16. [Online]. [accessed 2021 July 12]. https://www.seqirus.com/news/seqirus-will-build-world-class-vaccine-manufacturing-facility.
- 48.Premier of Victoria . Victoria ready to lead on new vaccination manufacturing; 2021. April 21. [Online]. [accessed 2021 June 30]. https://www.premier.vic.gov.au/victoria-ready-lead-new-vaccine-manufacturing.
- 49.NSW Government . NSW looks to lead the way with mRNA vaccines; 2021. May 4. [Online]. [accessed 2021 July 11]. https://www.nsw.gov.au/media-releases/nsw-looks-to-lead-way-mrna-vaccines.
- 50.The Hon Christian Porter MP . Australia to develop onshore mRNA manufacturing; 2021. May 21. [Online]. [accessed 2021 July 11]. https://www.minister.industry.gov.au/ministers/porter/media-releases/australia-develop-onshore-mrna-manufacturing.


