ABSTRACT
Although routine vaccinations in children decreased during the coronavirus disease 2019 (COVID-19) pandemic, pneumococcal vaccine coverage in older adults has remained unknown. This study was performed to investigate the impact of the COVID-19 pandemic on pneumococcal vaccination in Japan. The numbers of 23-valent pneumococcal polysaccharide vaccines (PPV23) shipped was obtained from an office memorandum released by the Ministry of Health, Labor and Welfare. The results showed that the COVID-19 pandemic increased the need for pneumococcal vaccination, causing shipping restrictions of pneumococcal vaccines. Regular vaccination is still important because there may be a shortage of vaccines during a pandemic.
KEYWORDS: COVID-19, pandemic, pneumococcal vaccination
Dear Editor:
Routine vaccinations, essential tools for individuals and communities to avoid the spread of vaccine-preventable diseases, decreased among children during the coronavirus disease 2019 (COVID-19) pandemic.1 Of the routine vaccinations, influenza and pneumococcal vaccines are especially important for elderly persons to reduce pneumonia mortality. Although the influenza vaccine policy during the COVID-19 pandemic has already been discussed elsewhere,2 there is little research reporting pneumococcal vaccine use. Pneumococcal vaccination, which is routinely recommended for older adults, might also have been affected by the COVID-19 pandemic. The aim was to investigate the impact of the COVID-19 pandemic on pneumococcal vaccination in Japan.
We obtained the numbers of 23-valent pneumococcal polysaccharide vaccines (PPV23) shipped from an office memorandum released by the Ministry of Health, Labor and Welfare.3 The office memorandum showed the number of pneumococcal vaccines shipped every three months. We compared the number of pneumococcal vaccines shipped every three months in 2020 with that in 2019.
The number of pneumococcal vaccines shipped in 2019 was 352,000 from April to June, 271,000 from July to September, and 473,000 from October to December (Figure 1). In 2020, a total of 497,000 pneumococcal vaccines were shipped from January to March. After April, the number of pneumococcal vaccines shipped every three months was higher than in the previous year. A total of 475,000 pneumococcal vaccines were shipped from April to June. The shipment of vaccines from July to September was 594,000, which was almost double that in 2019. Due to the rapid increase in shipments of pneumococcal vaccines, pharmaceutical companies began to restrict shipments in October 2020.4 The number of pneumococcal vaccines shipped after October 2020 was not available because it was not yet released.
Figure 1.
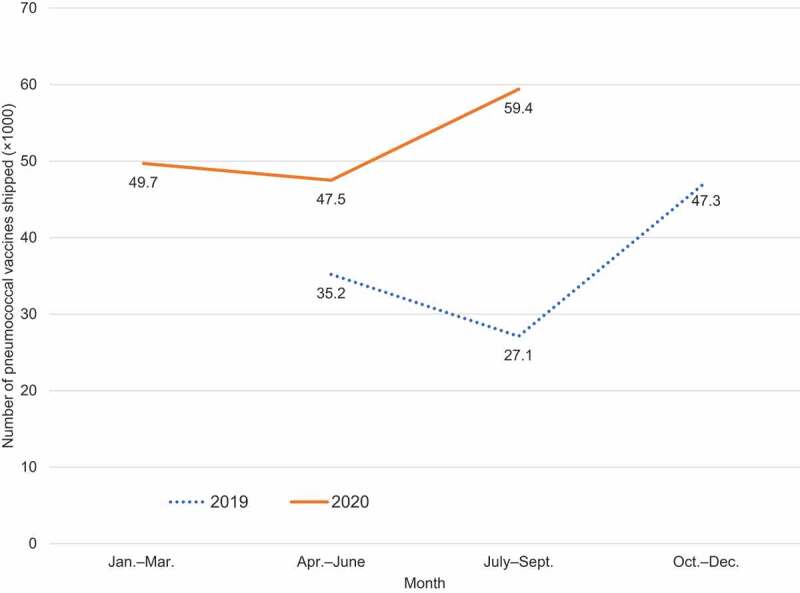
The number of pneumococcal vaccines shipped every three months in 2019 and in 2020.
It was found that the COVID-19 pandemic increased the need for pneumococcal vaccination in Japan, consequently leading to shipping restrictions. An increase in vaccination shipping might have occurred because of fear of the pneumonia epidemic in winter. Alternatively, some people might have been unable to get vaccinated in the spring. The declaration of a state of emergency was issued from April 4 to May 25 in Japan, which would have affected people’s behavior during this period. The limitation of our report is that the number of vaccines shipped may not represent the actual vaccination rates. In conclusion, the COVID-19 pandemic impacted pneumococcal vaccination in Japan. Regular vaccination is still important because vaccination shipping appears to be affected by the prevalence of infectious diseases, especially in the case of a pandemic that may cause a shortage of vaccines.
Funding Statement
This work did not receive grants from funding agencies in the public, commercial, or not-for-profit sectors.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
References
- 1.Santoli JM, Lindley MC, DeSilva MB, Kharbanda EO, Daley MF, Galloway L, Gee J, Glover M, Herring B, Kang Y, et al. Effects of the COVID-19 pandemic on routine pediatric vaccine ordering and administration - United States, 2020. MMWR Morb Mortal Wkly Rep. 2020;69:591–93. doi: 10.15585/mmwr.mm6919e2. [DOI] [PubMed] [Google Scholar]
- 2.Richmond H, Rees N, McHale S, Rak A, Anderson J.. Seasonal influenza vaccination during a pandemic. Hum Vaccin Immunother. 2020;16:2219–21. doi: 10.1080/21645515.2020.1793713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ministry of Health, Labour and Welfare, Japan . Supply forecast for pneumococcal vaccine. Health Service Bureau; [accessed 2021 Jun 12]. https://www.mhlw.go.jp/content/000700140.pdf.
- 4.Japanese Society of Chemotherapy . Notice of shipping restrictions of pneumococcal vaccine. MSD K.K. (Tokyo, Japan) [accessed 2021 Jun 14]. http://www.chemotherapy.or.jp/notice/349.pdf.


