Abstract
Background and Objective
Osteonecrosis of the femoral head (ONFH) is a devastating disease, and there is some evidence that extracorporeal shock wave therapy (ESWT) and intra-articular platelet-rich plasma (PRP) injection might alleviate pain and improve joint function in individuals with ONFH. The objective of this study was to compare the effectiveness and safety of PRP and ESWT in symptomatic ONFH patients.
Methods
A total of 60 patients aged 40–79 with unilateral ONFH at Association Research Circulation Osseous (ARCO) stages I, II, and III were randomly assigned to the PRP (N=30) or the ESWT group (N=30). Four treatment sessions were provided in both groups. Assessments were performed at baseline, and 1-, 3-, 6-, and 12-month. Primary outcomes were measured by the visual analogue scale (VAS), and pressure pain thresholds (PPTs). Secondary outcomes were assessed by Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC), Harris Hip Score (HHS), and magnetic resonance imaging (MRI). The linear mixed-model analysis was used to evaluate the differences between groups and within groups and the “group by time” interaction effects.
Results
There were significant differences between groups in terms of changes over time for VAS, PPTs, WOMAC, and HHS since 3-month and maintained up to 12-month (P<0.05, except for PPTs at 12-month). The simple main effects showed that the patients in PRP group had greater improvements in VAS (mean difference = −0.82, 95% CI [−1.39, −0.25], P=0.005), WOMAC (mean difference = −4.19, 95% CI [−7.00, −1.37], P=0.004), and HHS (mean difference = 5.28, 95% CI [1.94, 8.62], P=0.002). No related adverse events were reported.
Conclusion
This study supported the effectiveness and safety of both the PRP injection and ESWT in treating ONFH patients. For symptomatic patients with ONFH, intra-articular PRP injection appeared superior to ESWT in pain relief and functional improvement.
Keywords: necrosis of the femoral head, platelet-rich plasma, ultrasound-guided intra-articular injection, extracorporeal shock wave therapy
Introduction
Osteonecrosis of the femoral head (ONFH) may lead to progressive pain, hip joint dysfunction, and severely decreased life quality, affecting more and more patients worldwide. The aetiologies of ONFH are multifactorial and complicated, including both traumatic and non-traumatic (corticosteroid use, alcohol consumption, smoking, and genetic) causes.1–3 Histopathological changes of ONFH are usually characterized by osteocytes death and empty lacunae in the osseous matrix, which further develops into low bone mass, and subchondral trabecular collapse.4 Among the various diagnostic imaging examinations, MRI remains the most important diagnostic method with relatively high sensitivity and specificity, especially for patients with early-stage ONFH. The latest version of the Association Research Circulation Osseous (ARCO) classification system was revised in 2019, which has been proposed as one of the most popular classification systems for ONFH worldwide.5 In particular, not all avascular bone necrosis shares the same causes. For example, the possible correlation between the tyrosine kinase inhibitors, immunosuppressants, monoclonal antibodies, rapamycin inhibitors, and selective estrogen receptor modulators, and osteonecrosis of the jaws should be highlighted.6
Up to now, there are still no unified guidelines or recommendations for ONFH treatment, especially for patients accompanied by varying levels of pain symptoms. It is well accepted that patients with ARCO stage I and II were at early stages, with ARCO stage III and IV disease at late stages.7 For patients with early-stage ONFH, joint-preserving procedures are recommended, including both the non-surgical options (drugs, extracorporeal shock wave, and biologic treatment) and surgical options (core decompression, vascularized bone graft, and so on).8–10 Core decompression (CD) helps reduce the pressure in the femoral head, opens up the hardening zone, and further enhances the new bone regeneration.11 Total hip arthroplasty (THA) remains the most widespread and the only definitive procedure for end-stage ONFH patients.12 However, ONFH occurs predominantly in younger patients who are generally physically active and inclined to preserve the structural integrity of their joints. Thus, there is an urgent demand for developing safe and effective joint-preserving treatments.13 Extracorporeal shock wave therapy (ESWT) is a non-invasive and safe treatment proven effective in treating ONFH.14 It was proposed that ESWT helped to promote the revascularization and regeneration process of ONFH.15,16 Pilot clinical studies also confirmed the analgesic and therapeutic effects of ESWT in treating ONFH.
Relatively, the platelet-rich plasma (PRP) intra-articular injection has not been fully discussed for ONFH management. While the use of PRP gel in association with core decompression as a surgical treatment has been much discussed and tried by various authors.17 Over the past few decades, PRP has emerged as a popular biologic treatment for many musculoskeletal diseases, including knee osteoarthritis, delayed or non-unions, osteonecrosis of the jaws, and tendon injuries.18–21 It is well-accepted that PRP has the potential to promote the healing process due to the autologous blood growth factors (GFs) released from α-granules within platelets.22,23 In 2017, Tao et al reported that the exosomes derived from human PRP via the tail vein injection could relieve apoptosis, promote angiogenesis and osteogenic differentiation in a rat model of ONFH.24 Recently, researchers have been paying more attention to the analgesic effects of PRP. Several clinical studies have demonstrated that PRP helped relieve pain symptoms in chronic diseases, including osteoarthritis and osteonecrosis, but the potential mechanisms are not fully understood. Even though the intra-articular PRP injection for hip osteoarthritis has revealed satisfactory treatment outcomes, little evidence on its effectiveness and safety in ONFH management existed.18,25 In 2020, our team reported that the intra-articular PRP injections improved pain symptoms and imaging findings of a late-stage ONFH (ARCO stage IV) patient.26 Despite the promising in vitro and in vivo results, it is still necessary to confirm the therapeutic effects of PRP through clinical randomized controlled trials.
The objective of this study was to compare the therapeutic effects of ultrasound-guided intra-articular PRP injections and extracorporeal shock wave therapy (ESWT) in symptomatic unilateral ONFH patients at ARCO I–III stages. Considering the various etiologies, the present study focused on the non-traumatic ONFH.
Materials and Methods
Study Design
This was a randomized, single-blind, parallel-group study with a 12-month follow-up. We aimed to evaluate the effectiveness and safety of intra-articular injection of PRP for treating ONFH compared to ESWT.
Participants and Study Procedure
The participants were recruited from the Department of Rehabilitation Medicine of Sun Yat-sen Memorial Hospital, Sun Yat-sen University from June 2019 to December 2019. This study was approved by the Medical Ethics Committee of Sun Yat-sen Memorial Hospital (2019-KY-086). The study protocol was registered at the Chinese Clinical Trial Registry (ID: ChiCTR1900023601).
Patients who met the following criteria were included: 1) between 40 and 80 years old, 2) diagnosed with non-traumatic ONFH for over 3 months; 3) ONFH identified by X-ray/MRI, accompanied with clinical symptoms and signs; 4) Visual Analogue Scale (VAS) ≥4; 5) ARCO stages I, II and III; 6) resistant to non-morphine or failed to respond to previous physical therapies. Exclusion criteria included 1) hip surgery history; 2) combined with infection, joint tumors, or tuberculosis; 3) blood coagulation disorders, or a history of drug abuse or oral anticoagulation; 4) abnormal psychological, cognitive disorders; 5) not suitable for injection because of local infection; 6) received the intra-articular injections in the past 3 months; 7) pregnancy.
At the baseline evaluation, all eligible participants’ demographic characteristics, medical history, and laboratory tests (blood biochemistry and blood cell analysis) were collected. Detailed physical and radiological examinations including the primary outcomes (Visual Analogue Scale and pressure pain thresholds) and secondary outcomes (Western Ontario and McMaster Universities Osteoarthritis Index, Harris Hip Score, and MRI) were evaluated before randomized allocation by a trained physician. The MRI scannings were used to assess the ARCO stages. All patients gave written informed consent to participate in the study.
The enrolled patients were randomly assigned at 1:1 ratio into the PRP group (N=30) or ESWT group (N=30). The randomization was conducted using the Website tool (https://www.random.org/). No other analgesic drugs or physical therapies were administered to the patients in both groups during the treatment and 12-month follow-up period. The baseline and follow-up outcome assessors and data administrators were blinded to the allocation, but the patients and treatment providers were not blinded because of the nature of this trial design.
Interventions
Extracorporeal Shock Wave Treatment
The ESWT was applied using Gymna Shockmaster 500 and the femoral artery was confirmed with ultrasound Doppler beforehand to avoid potential injuries. Patients were treated with the affected hip in mild adduction and internal rotation. The ESWT procedures were performed without anesthesia and the clean coupling gel was used at the interface to decrease the loss of energy between the probe and skin. Before treatment, the physician reviewed the MRI scannings to ensure that the impulses were focused around the necrotic tissue of the femoral head. Four selected spots were located on the hardened layer around the lesion based on patients’ MRI scannings, and a dose of 1000 impulses at an energy flux density (EFD) of 0.50 mJ/mm2 was applied at each point in one single session, for about 15 minutes. Patients received consecutive four weekly sessions of treatment in the entire cycle. After each session, patients were instructed to walk with crutches (partial weight-bearing). The ESWT procedure was replicable and performed by the same operator. The study nurse recorded adverse reactions, including persistent unbearable pain and swelling.
Ultrasound-Guided Intra-Articular Platelet-Rich Plasma Injection
The PRP preparation procedures were performed under sterile conditions. A total of 18 mL of whole-blood sample was drawn from the antecubital vein into 10-mL syringes containing 1 mL 3.8% (w/v) sodium citrate. The autologous PRP was prepared in a two-step centrifugation process and the harvest and transfer methods were the same as our previously described protocol.26 At room temperature (23°C), the blood samples were centrifuged for 10 min at 1600 revolutions per minute (r.p.m.). The supernatant buffy coat and plasma were transferred into the other tubes. Then, the samples were centrifuged at 3200 r.p.m for another 10 min and the lower layer plasma was obtained as concentrated PRP. A small sample of PRP (0.5mL) was usually sent to the laboratory for quantitative analysis of platelet and leukocyte counts.
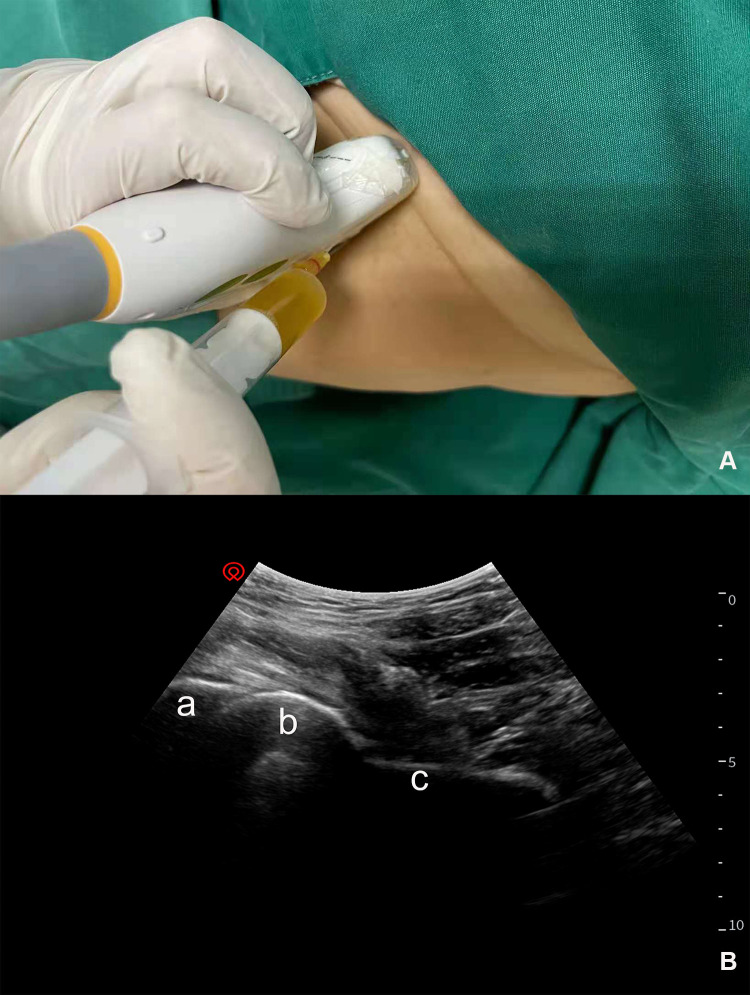
Similar to the injection protocol introduced in our previous study, the PRP injections were performed with patients in the supine position.26 All the injections were performed using SONIMAGE HS1 PLUS. Preliminary sonographic examinations including the ultrasound Doppler of the affected hip were performed to identify the vascular and nerve structures. Before injection, the intra-articular fluid would be evacuated if presented. We usually chose 20–22G needles for injections according to patients’ individualized body sizes. With the low-frequency convex array (C5-2, 2–5MHz) probe aligned along the long axis of the femoral neck, the needle was advanced into the anterior joint recess at the femoral head-neck junction. PRP diffusion could be visualized under sonographic guidance. The tolerable hip joint motion of the affected side was assisted by a physician assistant to ensure the PRP distribution on the ball-and-socket synovial joint. All patients in the PRP group received four weekly injections at a one-week interval (see Figure 1). All adverse reactions and complications, including persistent joint swelling, infections, allergies, and severe posttreatment pain were recorded by the study nurse in written form.
Figure 1.
Ultrasound-guided PRP injection for ONFH. The low-frequency convex array probe was aligned with the long axis of the affected femoral head (A) and the needle was advanced using anterior longitudinal approach (B). (a) acetabulum; (b) femoral head; (c) femoral neck.
Assessment Outcomes
The primary outcomes included the VAS and PPTs, and the secondary outcomes included the WOMAC, HHS, and MRI scannings. The clinical outcome assessments were scheduled at baseline before randomization and at 1, 3, 6, and 12 months post-treatment. In particular, the MRI was evaluated at baseline and 12-month follow-up. The assessors were blinded to the type of treatment administered.
Visual Analogue Scale (VAS)
Visual Analogue Scale (VAS) provides a simple way to subjectively evaluate pain intensity, which has been widely used in clinical practice and among researchers. Participants were instructed to mark a point along the 10-point Likert scale (0 to 10 cm, indicating no pain to most severe pain). According to a published study, the mean reduction in VAS of 1.0 cm represents the minimal clinical important difference (MCID) of pain alleviation.27
Pressure Pain Thresholds (PPTs)
Fischer et al recommended that a specially designed digital pressure algometer could be used to evaluate the pressure pain thresholds (PPTs). PPT is usually defined as the least amount of pressure to provoke pain, and the lower value usually indicates hyperalgesia and peripheral sensitization. Thus, it is recommended that the PPT could be used as an important objective indication of pain.28 Based on a previously published study, the PPTs of hips were measured at 5cm distally and 2cm anteriorly of the greater trochanter.29 Before the tests, the patients were instructed to differentiate the pain and sensations of pressure, and they were asked to give a sign as soon as they felt the pain. An experienced assessor performed the assessment with the algometer (Model PTH AF2; Pain Diagnostics and Thermography, Great Neck, NY) by placing the 1 cm2 rubber tip to the affected hip at a rate of 1 kg/sec. All the measurements were performed by a single assessor blinded to allocation. We performed three repetitive measures at an interval of 60 seconds, and the average values were determined as the final results.
Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC)
The WOMAC was developed in the 1980s and designed as an evaluation tool for knee and hip osteoarthritis. In the past several decades, the WOMAC has been widely used to evaluate clinically patient‐relevant changes and therapeutic effects of specific interventions in hip joint disorders. The complete WOMAC scale covers three dimensions: pain, stiffness, and physical function. The total score is 96, and the sub-total scores are 20, 8, and 68 for the above three domains. The Likert version of WOMAC was adopted, with higher scores indicating more severe pain symptoms and disability.30
Harris Hip Score (HHS)
The Harris Hip Score (HHS) was originally developed as a standardized assessment following the THA. In recent years, it was also commonly used for hip osteoarthritis and ONFH patients. The HHS consists of four subscales for pain severity, function, absence of deformity, and range of motion. The total score ranges from 0 to 100, with higher scores indicating better outcomes. Generally, the HHS total score of <70 is considered as a poor result; 70–80 is considered fair; 80–90 is good; and 90–100 is excellent.31
Magnetic Resonance Imaging (MRI)
MRI examinations were performed at baseline and 12-month follow-up, using T1- and T2-weighted scans, and T2-weighted sequences with fat saturation. One radiologist and one physician who were blinded to allocation evaluated the stages of ONFH and disease progress independently according to the ARCO classification system.
Statistical Analysis
The sample size was calculated using Power Analysis and Sample Size (PASS) software (version 15.0; NCSS) based on the difference of VAS between interventions according to the previous study and our preliminary experiment. Power analysis was performed with α = 0.05 (one-sided) and β = 0.20 (power). The minimum sample size of 60 patients (N=30 for each group) was determined by considering the probable loss to follow-up (20%).
The intention-to-treat principle was applied to all analyses, and the participants’ follow-up data were analyzed according to groups they were allocated to originally. Data analysis was performed using R software version 4.0.5. Descriptive statistics were used to present the patient characteristics. The normally distributed data were presented as means±standard deviations (SDs), and non-normally distributed data as Median (Percentile 25, Percentile 75). Descriptive statistics utilized χ2 for categorical measures and t-test for continuous measures. The Mann–Whitney U-test was used to evaluate differences between the disease duration since the data were not normally distributed.
To detect the changes of primary outcomes (VAS and PPTs) and secondary outcomes (WOMAC and HHS) over time and the comparisons between groups, we constructed a linear mixed model (LMM) with repeated measures, adjusting for baseline scores of the above outcome variables, and other demographic variables. R package nlme was used to conduct the LMM analysis. Further pairwise comparisons of simple main effects based on the LMMs were performed to explore the differences between the two groups at the 12-month follow-up. Cohen’s d was calculated to assess the between-group effect size (the mean difference scores for the ESWT group were subtracted from the mean difference scores for the PRP group and divided by the standard deviation).32
We also performed post hoc exploratory analyses to detect the effects of the interventions on the primary outcomes (VAS and PPTs) at 12-month follow-up using tests for interaction, according to the baseline characteristics of the participants. A P-value less than 0.05 was considered statistically significant.
Results
Demographic Characteristics
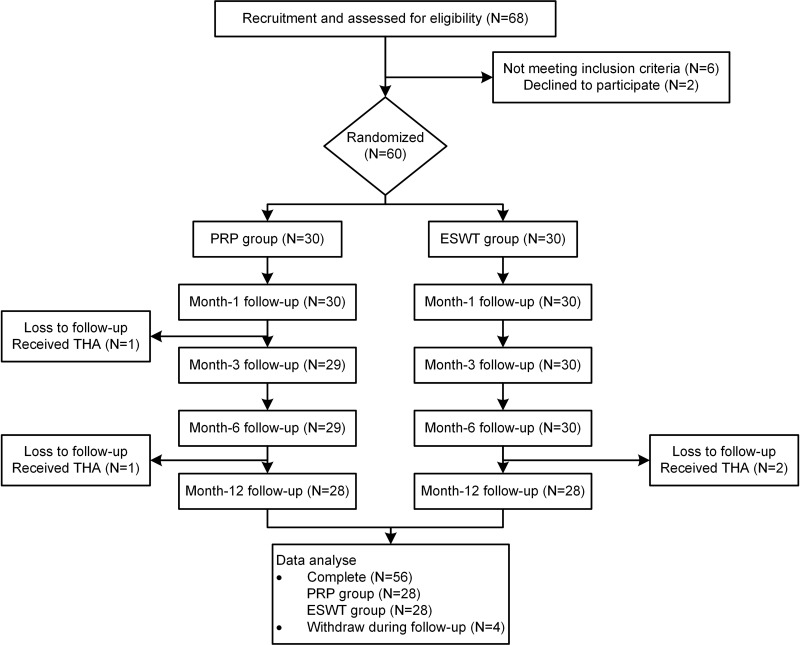
Among the 68 recruited subjects, 60 eligible participants consented to participate and were randomized to the PRP group (n=30) and ESWT group (n=30). Details of demographic characteristics are presented in Table 1. The PRP group consisted of 13 men and 17 women, while the ESWT group consisted of 9 men and 21 women. In the PRP group, 3 patients were classified as stage I, 11 as stage II, and 16 as stage III, while 3 patients as stage I, 13 as stage II, and 14 as stage III in the ESWT group. There were no significant differences in characteristics between the two groups, including age, sex distribution, disease duration, and body mass index (BMI). Before the end of the 12-month follow-up, two patients at ARCO stage III in the PRP group (2/30) and two in the ESWT group (2/30) decided to receive the THA and were lost to follow-up. The dropout rate was 6.66% for each group. Figure 2 shows the flowchart of the study. Baseline assessments of primary and secondary outcomes for both groups are displayed in Table 2.
Table 1.
Demographic Characteristics of the Two Groups
| PRP Group (N=30) | ESWT Group (N=30) | P | |
|---|---|---|---|
| Age, mean (SD) | 63.4 (11.8) | 61.6 (11.8) | 0.542 |
| Sex | 0.422 | ||
| Male | 13 (43.3%) | 9 (30.0%) | |
| Female | 17 (56.7%) | 21 (70.0%) | |
| Disease duration (years), Median (P25, P75) | 2.75(1.00, 3.88) | 2.00(1.00, 4.75) | 0.820 |
| ARCO stages | 0.929 | ||
| I | 3 (10.0%) | 3(10.0%) | |
| II | 11 (36.7%) | 13(43.3%) | |
| III | 16 (53.3%) | 14(46.7%) | |
| BMI, mean (SD) | 24.6 (3.78) | 24.6 (3.68) | 0.969 |
Abbreviations: PRP, platelet-rich plasma; ESWT, extracorporeal shock wave therapy; P25, 25th percentiles; P75, 75th percentile; ARCO, Association Research Circulation Osseous; BMI, body mass index.
Figure 2.
Flow diagram of the study.
Abbreviations: PRP, platelet-rich plasma; ESWT, extracorporeal shock wave therapy; THA, total hip arthroplasty.
Table 2.
Between-Group Comparisons for Primary Outcomes and Secondary Outcomes for the PRP and ESWT Groups at All Follow-Ups
| Follow-Up Time | PRP Group (N=30), Mean (SD) | ESWT Group (N=30), Mean (SD) | Treatment Effects (95% CI)a | Liner Mixed Model Results, P valuea,c | ||
|---|---|---|---|---|---|---|
| Groupb | Time | Group×Time | ||||
| Primary outcomes | ||||||
| VAS (0–10) | ||||||
| Baseline | 6.13 (1.28) | 5.97 (1.19) | – | 0.820 | – | – |
| 1-month | 3.40 (1.19) | 3.10 (1.06) | 0.13(−0.42, 0.69) | – | <0.001 | 0.640 |
| 3-month | 2.97 (1.30) | 3.67 (1.27) | −0.83 (−1.39, −0.27) | – | <0.001 | 0.004 |
| 6-month | 3.00 (1.36) | 3.83 (1.39) | −0.96 (−1.52, −0.40) | – | <0.001 | 0.001 |
| 12-month | 3.11 (1.23) | 3.75 (1.11) | −0.82 (−1.39, −0.25) | – | <0.001 | 0.005 |
| PPTs (kg/cm2/second) | ||||||
| Baseline | 2.11 (0.48) | 2.07 (0.45) | – | 0.636 | – | – |
| 1-month | 2.59 (0.37) | 2.61 (0.27) | −0.07 (−0.22, 0.08) | – | <0.001 | 0.341 |
| 3-month | 2.84 (0.31) | 2.63 (0.32) | 0.15 (0.001, 0.30) | – | <0.001 | 0.049 |
| 6-month | 2.73 (0.31) | 2.51 (0.37) | 0.16 (0.01, 0.30) | – | <0.001 | 0.041 |
| 12-month | 2.68 (0.29) | 2.53 (0.27) | 0.11 (−0.04, 0.26) | – | <0.001 | 0.138 |
| Secondary outcomes | ||||||
| WOMAC (0–96) | ||||||
| Baseline | 41.87 (9.52) | 40.67 (8.88) | – | 0.727 | – | – |
| 1-month | 29.00 (7.54) | 25.93 (5.76) | 1.87 (−0.89, 4.62) | – | <0.001 | 0.185 |
| 3-month | 24.45 (6.84) | 27.30(6.45) | −4.05 (−6.82, −1.28) | – | <0.001 | 0.005 |
| 6-month | 24.79 (6.75) | 28.40 (6.25) | −4.80 (−7.57, −2.04) | – | <0.001 | 0.001 |
| 12-month | 25.71 (6.42) | 28.36 (5.44) | −4.19 (−7.00, −1.37) | – | <0.001 | 0.004 |
| HHS (0–100) | ||||||
| Baseline | 51.73 (9.15) | 55.64 (9.35) | – | 0.227 | – | – |
| 1-month | 71.25 (9.67) | 73.92 (6.16) | 1.24 (−2.03, 4.51) | – | <0.001 | 0.458 |
| 3-month | 76.07 (8.58) | 73.88 (7.79) | 5.57 (2.28, 8.86) | – | <0.001 | 0.002 |
| 6-month | 75.65 (6.80) | 73.07 (7.56) | 5.96 (2.67, 9.25) | – | <0.001 | <0.001 |
| 12-month | 74.17 (8.15) | 72.37 (5.79) | 5.28 (1.94, 8.62) | – | <0.001 | 0.002 |
Notes: aBetween-group difference for mean change from baseline (95% CI); bP of Group indicates the baseline comparisons between the two groups; cLinear mixed model based on five time points (baseline, 1-month, 3-month, 6-month and 12-month follow-up) and adjusted for baseline of the outcome variables, and other baseline demographic characteristic (age, disease duration, body mass index, sex and ARCO stage).
Abbreviations: PRP, platelet-rich plasma; ESWT, extracorporeal shock wave therapy; VAS, visual analogue scale; PPTs, pressure pain thresholds; WOMAC, Western Ontario and McMaster Universities Osteoarthritis Index; HHS, Harris Hip Score.
Primary Outcomes
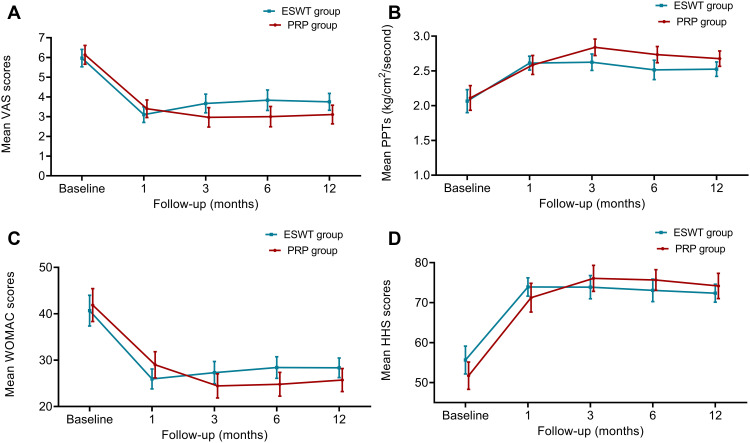
Results from LMM analysis with the between-group simple main effects are displayed in Table 2. Trends for the primary outcomes (VAS and PPTs) over time are presented in Figure 3A and B. Our results indicated significant Group × Time interactions between the two groups at 3-month, 6-month, and 12-month follow-up. The VAS scores of the PRP group, when compared to the ESWT group, exhibited significant improvements since 3-month (mean difference = −0.83 points, 95% CI [−1.39, −0.27], P = 0.004) and maintained up to 12-month (mean difference = −0.82 points, 95% CI [−1.39, −0.25], P=0.005). At 12-month follow-up, the VAS scores of the PRP group (from 6.13 to 3.11) decreased significantly compared to the ESWT group (5.97 to 3.75), with a standard effect size (Cohen d) of 0.73.
Figure 3.
Changes in Visual Analogue Scale (VAS), pressure pain thresholds (PPTs), Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC), and Harris Hip Score (HHS) in the 12-month follow-up period. (A) Visual Analogue Scale (VAS); (B) pressure pain thresholds; (C) Western Ontario and McMaster Universities Osteoarthritis Index; (D) Harris Hip Score.
Notes: Data are presented as mean (±95% confidence interval).
Abbreviations: PRP, platelet-rich plasma; ESWT, extracorporeal shock wave therapy.
Similarly, the LMM showed that the participants in the PRP group had significantly increased PPTs in comparison with the ESWT group, and the between-group differences in the PPTs were statistically significant for six-month post-treatment (3-month: 0.15, 95% CI [0.001 to 0.30], P=0.049, Cohen d=0.51; and 6-month: 0.16, 95% CI [0.01 to 0.30; P=0.041; Cohen d=0.53). However, simple main effects of between-group PPTs comparisons at the 12-month follow-up was not statistically significant (0.11, 95% CI [−0.04, 0.26]; P=0.138; Cohen d=0.38) (Tables 2 and 3).
Table 3.
Between Group Effect Size Assessed Using Cohen’s d
| Outcomes | Effect Sizes | 95% CI |
|---|---|---|
| Primary outcomes | ||
| VAS | ||
| 1-month | 0.12 | −0.39 to 0.63 |
| 3-month | 0.75 | 0.23 to 1.28 |
| 6-month | 0.87 | 0.34 to 1.40 |
| 12-month | 0.73 | 0.21 to 1.25 |
| PPTs | ||
| 1-month | 0.25 | −0.26 to 0.75 |
| 3-month | 0.51 | 0.001 to 1.03 |
| 6-month | 0.53 | 0.02 to 1.05 |
| 12-month | 0.38 | −0.13 to 0.90 |
| Secondary outcomes | ||
| WOMAC | ||
| 1-month | 0.34 | −0.17 to 0.85 |
| 3-month | 0.74 | 0.22 to 1.26 |
| 6-month | 0.88 | 0.35 to 1.41 |
| 12-month | 0.75 | 0.23 to 1.28 |
| HHS | ||
| 1-month | 0.19 | −0.31 to 0.70 |
| 3-month | 0.86 | 0.33 to 1.39 |
| 6-month | 0.92 | 0.39 to 1.45 |
| 12-month | 0.80 | 0.27 to 1.33 |
Notes: The effect size calculated by the Cohen’s d (delta value/SD before and after treatment sessions), interpreted as 0.20–0.40 (small), 0.50–0.70 (moderate), and large (0.80 or higher).
Abbreviations: VAS, visual analogue scale; PPTs, pressure pain thresholds; WOMAC, Western Ontario and McMaster Universities Osteoarthritis Index; HHS, Harris Hip Score.
Secondary Outcomes
For the WOMAC and HHS assessment, there was a trend that the PRP group patients achieved better functional outcomes compared to the ESWT group during follow-up (see Figure 3C and D). Considering the between-group differences, PRP groups showed significantly improved WOMAC and HHS scores compared to the ESWT group since month-3 (WOMAC: P = 0.005, HHS: P = 0.002) and maintained up to the end of follow-up (WOMAC: P = 0.004, HHS: P =0.002). At 12 months, compared to the ESWT group, the estimated mean improvement of PRP group in WOMAC scores was −4.19 (95% CI [−7.00, −1.37], P = 0.004; Cohen d=0.75) and in HHS was 5.28 (95% CI [1.94, 8.62], P = 0.002; Cohen d=0.80), respectively (Tables 2 and 3).
No discrepant MRI evaluations were reported between the radiologist and the physician. At the end of the study, the improvements in radiographic findings of ARCO I–II individuals were observed in two groups (one patient in the PRP group: from ARCO stage II to I, and one in the ESWT group: from ARCO stage I to 0), respectively. And two patients at ARCO stage III (one in the PRP group and one in the ESWT group) progressed to ARCO stage IV. Other participants in both groups remained unchanged on radiographic assessments.
Exploratory Subgroup Analysis
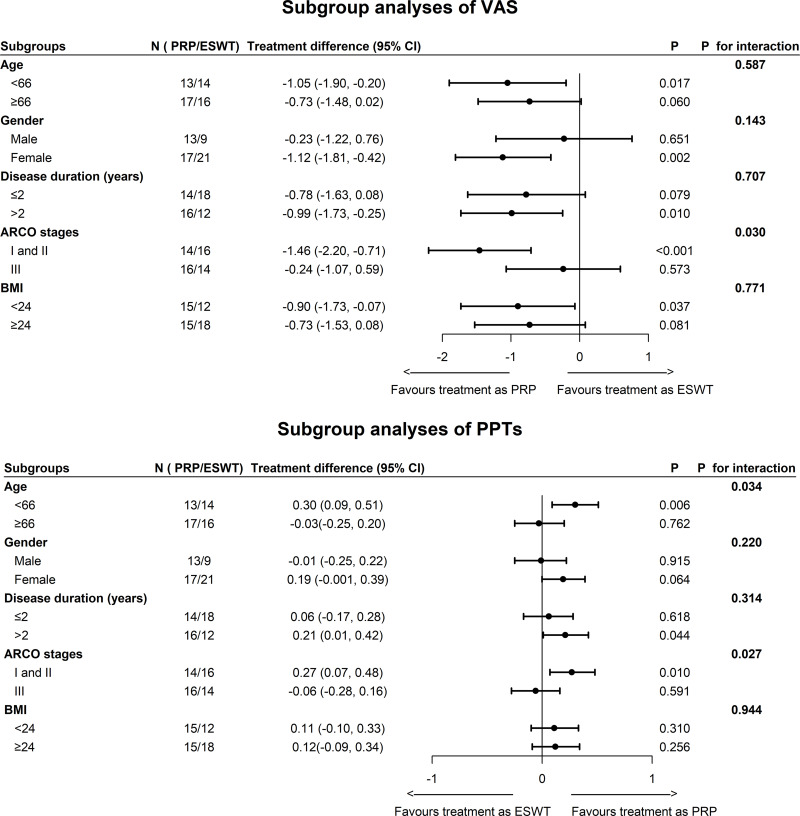
Except for ARCO stages, no significant interactions were found in the post hoc exploratory analyses of VAS, including age, gender distribution, disease duration, and BMI. Patients at ARCO stages I and II had statistically greater improvements of VAS than those at ARCO stage III. For the PPTs, significant interactions were found in the age and ARCO stage, which indicated that younger participants with early-stage ONFH were more likely to benefit from PRP injection treatment (see Figure 4).
Figure 4.
Changes in primary outcomes in two groups by participant characteristics. Subgroup analyses of visual analogue scale and pressure pain thresholds.
Abbreviations: PRP, platelet-rich plasma; ESWT, extracorporeal shock wave therapy; VAS, visual analogue scale; PPTs, pressure pain thresholds; ARCO, Association Research Circulation Osseous; BMI, body mass index.
Discussion
ONFH is a refractory devastating orthopedic disease characterized by pain, stiffness, and joint dysfunction. Up to date, there are still no standardized treatments for these patients. Previous pilot studies proved that both the PRP injection and ESWT might contribute to pain relief and slowing down the progression of ONFH.16,33 Our current results confirm our hypothesis that there were significant improvements in the PRP group for pain relief and functional recovery compared to the conventional ESWT.
For ONFH patients, the pain symptoms and radiologic findings do not necessarily parallel each other. Among all of its symptoms, pain is one of the most disabling symptoms of ONFH and the unsolved persistent pain is also the main reason for patients to seek THA. In this study, we chose the VAS and PPTs as the primary outcomes, and we confirmed that the pain improvements between the two groups were maintained over time in favor of intra-articular injection (except for PPTs at 12-month). In particular, even though we did not detect the between-group simple main effect of PPTs at 12-month follow-up, we observed the significant Group × Time interactions at 3-month and 6-month for PPTs. In previous studies of musculoskeletal disease, VAS has been widely used for pain assessment, but objective pain assessment tools are still urgently needed. PPTs, as one of the widely used quantitative sensory tests, usually work as an objective supplementary tool. Unfortunately, the measurement protocol of PPTs has not been unified and few prior studies have reported the reference range or the MCID for the PPTs in hip disorders.34 Our results provided the available evidence-based PPTs in ONFH patients for future reference. Based on our post hoc subgroup analysis of VAS and PPTs, we found that younger early-stage patients (ARCO I and II) achieved better results than those at late stages (ARCO III), similar to the findings in other published studies.35 Early interventions before the femoral head collapse usually lead to better and long-lasting pain relief.
For hip function assessments, we observed similar patterns of WOMAC and HHS improvements in both groups during the 12-month follow-up. For the radiographic assessments at the end-point of follow-up, the staging progression did not occur in ARCO stages I and II patients in both groups. It is commonly accepted that the natural history of ONFH is of progression, thus our results might be encouraging. Interestingly, the MRI scannings at 12-month showed radiological improvements based on the ARCO stages in two early-stage patients (one patient in the PRP group: from ARCO stage II to I, and one in the ESWT group: from ARCO stage I to 0), accompanied by improved clinical outcomes. It is important to acknowledge that the effectiveness of PRP and ESWT is highly related to the disease stages of ONFH. Generally, THA is inevitable in most cases with femoral head collapse (ARCO stage III, IV) in the long term. We also found that two ARCO stage III patients (one in each group) progressed to ARCO stage IV. It is still unclear how PRP injection and ESWT affect the disease course, and future clinical trials with longer follow-up periods are warranted.
Related evidence about the beneficial effects of ESWT is accumulating in both basic and clinical research. It is commonly recognized that the ESWT promotes many cellular activities, which are critical to neovascularization and bone tissue regeneration.15 Ludwig et al once reported positive results of ESWT in treating ARCO I–III stage patients.36 In 2012, Vulpiani et al reported that after four sessions of ESWT treatment at 48–72 h intervals (2400 impulses, 0.50 mJ/mm2), the patients were assessed with VAS scores, HHS, and Roles and Maudsley score at 3, 6, 12, and 24 months. They found patients from ARCO stage I–II groups were more likely to benefit from ESWT than those from the ARCO stage III group.16 The results of our study are basically in line with those observations obtained in the published studies mentioned above. Results from LMM analysis indicated that both PRP injection and ESWT significantly promoted pain relief and functional recovery in ONFH patients over the 12-month follow-up. Notably, the doses of EFD and pulses varied considerably in the clinical trial protocols, which might explain the potential discrepancies among different studies. Generally, most researchers adopted a total of 2400–6000 impulses (single or multiple points) at 0.16–0.62 mJ/mm in one session. In this study, the ESWT doses were determined as 4000 impulses at 0.50 mJ/mm2 for four sessions according to the published evidence and our pilot study experience. Future researches might focus on the intensity and impulses adjustment according to patients’ tolerance.
Promising advances have been made in the surgical treatments for ONFH in the past decades, especially in combination with biological treatments. PRP was originally used in conjunction with the classical core decompression procedure since PRP could augment the therapeutic effects of joint-preserving surgeries for the ONFH. The PRP in association with core decompression has a major regenerative role on necrosis.17 In intra-articular infiltrations, it could have a pain-reducing role by acting on the synovial membrane, where synovitis is the basis of the joint degenerative process. In a 4.5- to 6-year prospective comparative study, the authors reported that the PRP use after core decompression provided obvious pain relief and functional improvements, which enhanced the survivorship free from THA and retarded ONFH progression.37 Relatively, the intra-articular injections are less invasive and more convenient than the surgical procedures, thus the injection therapy might be preferred by the young early-stage patients, especially when the pain symptoms persist. For decades, many studies have explored the possible mechanisms by which PRP promotes repair and tissue regeneration. The PRP treatment has gained widespread attention because of its potential to release a large pool of growth factors, including concentrated levels of vascular endothelial growth factor (VEGF), transforming growth factor-β 1 (TGF-β1), and insulin-like growth factor (IGF).38 However, few studies have addressed the analgesic effects of PRP. Recently, more and more PRP-related clinical studies focused on its possibility in relieving symptoms and delaying the more invasive surgical treatments in musculoskeletal disease management, especially for osteoarthritis and ONFH. First, some researchers revealed the direct analgesic effects of PRP, which might be achieved by the augmentation of cannabinoid receptors (CB1 and CB2) in pain regulation.39 Second, recent studies found that a large amount of pain-modulating serotonin (5-hydroxytryptamine, 5-HT) could be discharged from activated PRP, thus the partial analgesic effects could be attributed to the increased 5-HT level. More efforts should be made to further understand the modulatory roles of PRP-derived 5-HT in peripheral nociceptive transmission.40 Another important possible reason might be the long-lasting effects of PRP in maintaining joint homeostasis.41 In our study, the intra-articular activation of PRP is finished more physiologically and various biologically active components released from platelets helped promote sequential restoration.
To our knowledge, ultrasound-guided PRP injection for ONFH treatment has rarely been discussed in clinical studies to date, especially in randomized controlled trials. Our current data proved that the intra-articular PRP injection exerted better effects in terms of pain relief and function improvement on ONFH patients when compared to conventional ESWT. A strength of the study was that we conducted the repeated measures analysis using LMMs, and the LMMs are more flexible at handling the dropout and missing data. Another strength of this study was that we reported the relative effect sizes, which indicated the practical significance of the current results for future reference.
The current study still has some limitations. First, the subjects were only followed for up to 12 months, and the follow-up period was relatively short. In this study, we did not perform the MRI at each time point to assess dynamic evolution. Second, we chose the symptomatic ONFH patients as objects of the study. Whether the PRP injection and ESWT influence the disease progression of asymptomatic patients still needs further discussion. Considering the potential PRP preparation and injection regimen variations that existed among different studies, future studies are still required to standardize the PRP treatment protocol.
Funding Statement
This study was funded by Sun Yat-sen Clinical Research Cultivating Program (SYS-C-202002).
Abbreviations
PRP, platelet-rich plasma; ESWT, extracorporeal shock wave therapy; THA, total hip arthroplasty; ARCO, Association Research Circulation Osseous; BMI, body mass index; VAS, visual analogue scale; PPTs, pressure pain thresholds; WOMAC, Western Ontario and McMaster Universities Osteoarthritis Index; HHS, Harris Hip Score; LMM, linear mixed model; VEGF, vascular endothelial growth factor; TGF-β1, transforming growth factor-β 1; IGF, insulin-like growth factor.
Data Sharing Statement
The data of this study are available from the first author, Shuo Luan, on request contact luanshuo@mail2.sysu.edu.cn. De-identified data will be made available to qualified researchers by reasonable request.
Ethics Approval and Informed Consent
This study was approved by the Medical Ethics Committee of Sun Yat-sen Memorial Hospital (2019-KY-086). All participants provided written informed consent. The study was conducted according to the guidelines of the Declaration of Helsinki.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Mont MA, Cherian JJ, Sierra RJ, Jones LC, Lieberman JR. Nontraumatic osteonecrosis of the femoral head: where do we stand today? A ten-year update. J Bone Joint Surg Am. 2015;97(19):1604–1627. doi: 10.2106/JBJS.O.00071 [DOI] [PubMed] [Google Scholar]
- 2.Zhao D, Zhang F, Wang B, et al. Guidelines for clinical diagnosis and treatment of osteonecrosis of the femoral head in adults (2019 version). J Orthop Translat. 2020;21:100–110. doi: 10.1016/j.jot.2019.12.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cui Q, Jo WL, Koo KH, et al. ARCO consensus on the pathogenesis of non-traumatic osteonecrosis of the femoral head. J Korean Med Sci. 2021;36(10):e65. doi: 10.3346/jkms.2021.36.e65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Di Benedetto P, Niccoli G, Beltrame A, Gisonni R, Cainero V, Causero A. Histopathological aspects and staging systems in non-traumatic femoral head osteonecrosis: an overview of the literature. Acta Biomed. 2016;87(Suppl 1):15–24. [PubMed] [Google Scholar]
- 5.Yoon BH, Mont MA, Koo KH, et al. The 2019 revised version of association research circulation osseous staging system of osteonecrosis of the femoral head. J Arthroplasty. 2020;35(4):933–940. doi: 10.1016/j.arth.2019.11.029 [DOI] [PubMed] [Google Scholar]
- 6.Bennardo F, Buffone C, Giudice A. New therapeutic opportunities for COVID-19 patients with Tocilizumab: possible correlation of interleukin-6 receptor inhibitors with osteonecrosis of the jaws. Oral Oncol. 2020;106:104659. doi: 10.1016/j.oraloncology.2020.104659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Choi HR, Steinberg ME, Cheng EY. Osteonecrosis of the femoral head: diagnosis and classification systems. Curr Rev Musculoskelet Med. 2015;8(3):210–220. doi: 10.1007/s12178-015-9278-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cao H, Guan H, Lai Y, Qin L, Wang X. Review of various treatment options and potential therapies for osteonecrosis of the femoral head. J Orthop Translat. 2016;4:57–70. doi: 10.1016/j.jot.2015.09.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Osmani F, Thakkar S, Vigdorchik J. The utility of conservative treatment modalities in the management of osteonecrosis. Bull NYU Hosp Jt Dis. 2017;75(3):186–192. [PubMed] [Google Scholar]
- 10.Rajpura A, Wright AC, Board TN. Medical management of osteonecrosis of the hip: a review. Hip Int. 2011;21(4):385–392. doi: 10.5301/HIP.2011.8538 [DOI] [PubMed] [Google Scholar]
- 11.Hua KC, Yang XG, Feng JT, et al. The efficacy and safety of core decompression for the treatment of femoral head necrosis: a systematic review and meta-analysis. J Orthop Surg Res. 2019;14(1):306. doi: 10.1186/s13018-019-1359-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Saito S, Saito M, Nishina T, Ohzono K, Ono K. Long-term results of total hip arthroplasty for osteonecrosis of the femoral head. A comparison with osteoarthritis. Clin Orthop Relat Res. 1989;244:198–207. [PubMed] [Google Scholar]
- 13.Schreurs BW, Hannink G. Total joint arthroplasty in younger patients: heading for trouble? Lancet. 2017;389(10077):1374–1375. doi: 10.1016/S0140-6736(17)30190-3 [DOI] [PubMed] [Google Scholar]
- 14.Wang CJ, Cheng JH, Huang CC, Yip HK, Russo S. Extracorporeal shockwave therapy for avascular necrosis of femoral head. Int J Surg. 2015;24:184–187. doi: 10.1016/j.ijsu.2015.06.080 [DOI] [PubMed] [Google Scholar]
- 15.Wang CJ, Wang FS, Ko JY, et al. Extracorporeal shockwave therapy shows regeneration in hip necrosis. Rheumatology. 2008;47(4):542–546. doi: 10.1093/rheumatology/ken020 [DOI] [PubMed] [Google Scholar]
- 16.Vulpiani MC, Vetrano M, Trischitta D, et al. Extracorporeal shock wave therapy in early osteonecrosis of the femoral head: prospective clinical study with long-term follow-up. Arch Orthop Trauma Surg. 2012;132(4):499–508. doi: 10.1007/s00402-011-1444-9 [DOI] [PubMed] [Google Scholar]
- 17.Grassi M, Salari P, Massetti D, Papalia GF, Gigante A. Treatment of avascular osteonecrosis of femoral head by core decompression and platelet-rich plasma: a prospective not controlled study. Int Orthop. 2020;44(7):1287–1294. doi: 10.1007/s00264-020-04628-4 [DOI] [PubMed] [Google Scholar]
- 18.Bennell KL, Hunter DJ, Paterson KL. Platelet-rich plasma for the management of hip and knee osteoarthritis. Curr Rheumatol Rep. 2017;19(5):24. doi: 10.1007/s11926-017-0652-x [DOI] [PubMed] [Google Scholar]
- 19.Lin MT, Wei KC, Wu CH. Effectiveness of platelet-rich plasma injection in rotator cuff tendinopathy: a systematic review and meta-analysis of randomized controlled trials. Diagnostics. 2020;10(4). doi: 10.3390/diagnostics10040189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fortunato L, Bennardo F, Buffone C, Giudice A. Is the application of platelet concentrates effective in the prevention and treatment of medication-related osteonecrosis of the jaw? A systematic review. J Craniomaxillofac Surg. 2020;48(3):268–285. doi: 10.1016/j.jcms.2020.01.014 [DOI] [PubMed] [Google Scholar]
- 21.Bennardo F, Bennardo L, Del DE, et al. Autologous platelet-rich fibrin injections in the management of facial cutaneous sinus tracts secondary to medication-related osteonecrosis of the jaw. Dermatol Ther. 2020;33(3):e13334. doi: 10.1111/dth.13334 [DOI] [PubMed] [Google Scholar]
- 22.Everts P, Onishi K, Jayaram P, Lana JF, Mautner K. Platelet-rich plasma: new performance understandings and therapeutic considerations in 2020. Int J Mol Sci. 2020;21(20):7794. doi: 10.3390/ijms21207794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Han J, Gao F, Li Y, et al. The use of platelet-rich plasma for the treatment of osteonecrosis of the femoral head: a systematic review. Biomed Res Int. 2020;2020:2642439. doi: 10.1155/2020/2642439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tao SC, Yuan T, Rui BY, Zhu ZZ, Guo SC, Zhang CQ. Exosomes derived from human platelet-rich plasma prevent apoptosis induced by glucocorticoid-associated endoplasmic reticulum stress in rat osteonecrosis of the femoral head via the Akt/Bad/Bcl-2 signal pathway. Theranostics. 2017;7(3):733–750. doi: 10.7150/thno.17450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dallari D, Stagni C, Rani N, et al. Ultrasound-guided injection of platelet-rich plasma and hyaluronic acid, separately and in combination, for hip osteoarthritis: a randomized controlled study. Am J Sports Med. 2016;44(3):664–671. doi: 10.1177/0363546515620383 [DOI] [PubMed] [Google Scholar]
- 26.Luan S, Liu C, Lin C, Ma C, Wu S. Platelet-rich plasma for the treatment of adolescent late-stage femoral head necrosis: a case report. Regen Med. 2020;15(9):2067–2073. doi: 10.2217/rme-2020-0057 [DOI] [PubMed] [Google Scholar]
- 27.Ehrich EW, Davies GM, Watson DJ, Bolognese JA, Seidenberg BC, Bellamy N. Minimal perceptible clinical improvement with the Western Ontario and McMaster Universities osteoarthritis index questionnaire and global assessments in patients with osteoarthritis. J Rheumatol. 2000;27(11):2635–2641. [PubMed] [Google Scholar]
- 28.Fischer AA. Pressure algometry over normal muscles. Standard values, validity and reproducibility of pressure threshold. Pain. 1987;30(1):115–126. doi: 10.1016/0304-3959(87)90089-3 [DOI] [PubMed] [Google Scholar]
- 29.Rienstra W, Blikman T, Dijkstra B, et al. Validity of the Dutch modified painDETECT questionnaire for patients with hip or knee osteoarthritis. Disabil Rehabil. 2019;41(8):941–947. doi: 10.1080/09638288.2017.1413429 [DOI] [PubMed] [Google Scholar]
- 30.Bellamy N, Buchanan WW, Goldsmith CH, Campbell J, Stitt LW. Validation study of WOMAC: a health status instrument for measuring clinically important patient relevant outcomes to antirheumatic drug therapy in patients with osteoarthritis of the hip or knee. J Rheumatol. 1988;15(12):1833–1840. [PubMed] [Google Scholar]
- 31.Harris WH. Traumatic arthritis of the hip after dislocation and acetabular fractures: treatment by mold arthroplasty. An end-result study using a new method of result evaluation. J Bone Joint Surg Am. 1969;51(4):737–755. doi: 10.2106/00004623-196951040-00012 [DOI] [PubMed] [Google Scholar]
- 32.Cohen J. A power primer. Psychol Bull. 1992;112(1):155–159. doi: 10.1037//0033-2909.112.1.155 [DOI] [PubMed] [Google Scholar]
- 33.Xu HH, Li SM, Fang L, et al. Platelet-rich plasma promotes bone formation, restrains adipogenesis and accelerates vascularization to relieve steroids-induced osteonecrosis of the femoral head. Platelets. 2020:1–10. doi: 10.1080/09537104.2020.1810221 [DOI] [PubMed] [Google Scholar]
- 34.Izumi M, Petersen KK, Arendt-Nielsen L, Graven-Nielsen T. Pain referral and regional deep tissue hyperalgesia in experimental human hip pain models. Pain. 2014;155(4):792–800. doi: 10.1016/j.pain.2014.01.008 [DOI] [PubMed] [Google Scholar]
- 35.Mont MA, Jones LC, Hungerford DS. Nontraumatic osteonecrosis of the femoral head: ten years later. J Bone Joint Surg Am. 2006;88(5):1117–1132. doi: 10.2106/JBJS.E.01041 [DOI] [PubMed] [Google Scholar]
- 36.Ludwig J, Lauber S, Lauber HJ, Dreisilker U, Raedel R, Hotzinger H. High-energy shock wave treatment of femoral head necrosis in adults. Clin Orthop Relat Res. 2001;387:119–126. doi: 10.1097/00003086-200106000-00016 [DOI] [PubMed] [Google Scholar]
- 37.Aggarwal AK, Poornalingam K, Jain A, Prakash M. Combining platelet-rich plasma instillation with core decompression improves functional outcome and delays progression in early-stage avascular necrosis of femoral head: a 4.5- to 6-year prospective randomized comparative study. J Arthroplasty. 2021;36(1):54–61. doi: 10.1016/j.arth.2020.07.010 [DOI] [PubMed] [Google Scholar]
- 38.Giusti I, D’Ascenzo S, Macchiarelli G, Dolo V. In vitro evidence supporting applications of platelet derivatives in regenerative medicine. Blood Transfus. 2020;18(2):117–129. doi: 10.2450/2019.0164-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lee HR, Park KM, Joung YK, Park KD, Do SH. Platelet-rich plasma loaded hydrogel scaffold enhances chondrogenic differentiation and maturation with up-regulation of CB1 and CB2. J Control Release. 2012;159(3):332–337. doi: 10.1016/j.jconrel.2012.02.008 [DOI] [PubMed] [Google Scholar]
- 40.Mammadova-Bach E, Mauler M, Braun A, Duerschmied D. Autocrine and paracrine regulatory functions of platelet serotonin. Platelets. 2018;29(6):541–548. doi: 10.1080/09537104.2018.1478072 [DOI] [PubMed] [Google Scholar]
- 41.Filardo G, Kon E, Roffi A, Di Matteo B, Merli ML, Marcacci M. Platelet-rich plasma: why intra-articular? A systematic review of preclinical studies and clinical evidence on PRP for joint degeneration. Knee Surg Sports Traumatol Arthrosc. 2015;23(9):2459–2474. doi: 10.1007/s00167-013-2743-1 [DOI] [PMC free article] [PubMed] [Google Scholar]