ABSTRACT
Background
Rabies vaccines are lifesaving in human post animal exposure. However, the compliance to the complete course of vaccine is found to be only 60%. Hence, there is a need for safe and immunogenic, shorter course vaccine that can enhance the compliance and effectively prevent the disease.
Objectives
To establish a noninferiority of a novel three-dose recombinant rabies G protein vaccine to be administered as simulated postexposure prophylaxis when compared to five-dose WHO prequalified vaccine for better safety and immunogenicity.
Methods
A multi-centric, open label, assessor blind, center-specific block randomized, parallel design, phase III clinical study was conducted among 800 subjects. The eligible subjects were randomized in 2:1 ratio for recombinant rabies G protein vaccine and the reference vaccine. Subjects in recombinant rabies G protein vaccine arm received three doses of vaccine on days 0, 3, and 7, while subjects in reference vaccine arm received five doses of WHO prequalified vaccine on days 0, 3, 7, 14, and 28.
Results
The socio-demographic characteristics of the two arms were comparable. About 9.9% subjects in recombinant rabies G protein vaccine arm and 17.2% subjects in reference arm reported adverse events. The sero-protection on day 14 was found to be 99.24% and 97.72% in recombinant rabies G protein vaccine arm and reference vaccine arm respectively and the difference was statistically nonsignificant.
Conclusion
The novel three-dose recombinant rabies G protein vaccine administered as simulated postexposure prophylaxis was noninferior to five dose WHO prequalified vaccine in terms of safety and immunogenicity.
KEYWORDS: Recombinant rabies G protein vaccine, noninferiority, safety, immunogenicity, simulated postexposure prophylaxis
Introduction
Rabies is a vaccine-preventable disease.1 The modern rabies vaccines remain the mainstay for postexposure prophylaxis (PEP) in animal exposures and has proved to be safe and effective in preventing the disease.2 Annually, more than 15 million people worldwide receive postexposure vaccination and it is estimated to prevent thousands of rabies deaths.3
A variety of empirical schedules and vaccine doses for PEP have been recommended over time, based on immunogenicity and clinical experience in different parts of the world with enzootic canine or wildlife rabies.4 As the scientific knowledge improved, the total number of rabies vaccine doses administered for PEP has decreased.5 The PEP was initially for 90 days with six injections (1-1-1-1-1-1; Original Essen regimen); but with better understanding of the immunology, this extended regimen was reduced to 30 days using five injections (1-1-1-1-1; Essen regimen) and to later to 21 days duration using four doses of vaccine (2-1-1; Zagreb regimen).6–8 However, the studies shown that the compliance to complete course of standard Essen regimen was only 60%.9 Hence, the emphasis was on reducing the long duration PEP with a shorter course, resulting in saving of vaccine, reduced number of visits and travel costs.
In this regard, WHO recommended that in healthy and fully immune competent person, who receives wound care along with high-quality rabies immunoglobulin (RIG)/Rabies monoclonal antibody (RmAb) and WHO prequalified rabies vaccines, a PEP vaccine regimen consisting of four doses administered intramuscularly on days 0, 3, 7, and 14 can be used as an alternative to the five-dose intramuscular regimen.10
The studies for further revision and reduction of PEP doses in humans have been encouraged by WHO and a novel vaccine with improved immunological outcomes through accelerated PEP schedule was desirable.11,12 In this regard, the Cadila Pharmaceuticals Ltd., Ahmedabad, India has developed a novel recombinant nanoparticle-based rabies G protein vaccine (Thrabis®) prepared by using Virus Like Particle technology (VLP). A genetic sequence encoding the rabies G protein sequence is selected for generating Thrabis® using VLP platform. The genes are then cloned into baculovirus. The recombinant baculovirus are made to infect insect cells (sf9). The target antigens are expressed in the sf9 cells which are purified using various chromatographic techniques. The purified target antigen exists as assembly of polypeptides that is present in multiple copies in subunit antigens in a well-ordered arrays with defined orientations. This can potentially mimic the repetitiveness, geometry, size, and shape of the natural host–pathogen surface interactions. Such nanoparticles offer a collective strength of multiple binding sites (avidity) and can provide improved antigen stability and immunogenicity.13,14
The dose and schedule of recombinant rabies G protein vaccine was evaluated in phase I/II trial. In the phase I trial, 16 different regimens of intramuscular recombinant rabies G protein vaccine were administered in 170 healthy volunteers. Based on the safety, as well as immunogenicity results, four dosing regimens (10 and 50 μg per 0.5 ml dose were scheduled on days 0, 3 and 0, 3, 7) of the same vaccine was evaluated in phase II trial which included 225 healthy volunteers. Considering the safety and immunogenicity from the above studies, the present study was performed to establish the noninferiority of novel three-dose recombinant rabies G protein vaccine given on days 0, 3, and 7 as simulated PEP, when compared to WHO prequalified vaccine given as standard Essen five-dose regimen.
Materials and methods
A multicentric study was conducted after obtaining approval from the regulatory authority Drugs Controller General of India (DCGI) and the trial was registered in the Clinical Trials Registry-India (CTRI/2016/08/007137). Subsequently, the Institutional Ethics Committee clearance from all study sites were also obtained.
The study was conducted in accordance with the Declaration of Helsinki following ICH-GCP guidelines at eight different hospitals across the country for period of one year. It was a simulated, open-label, assessor blind (immunogenicity), center-specific nonadaptive block randomized, parallel-arm, phase III trial. The randomization was performed by the Contract Research Organization using SAS version 9.4 (SAS Institute Inc., Cary, NC, USA).
Objectives and endpoints
The primary objective was to demonstrate noninferiority of the test vaccine on day 14 after first dose relative to the reference vaccine in terms of seroprotection rate. The seroprotection rate (RVNA titer of ≥0.5 IU/mL; rapid fluorescent focus inhibition test method) on day 14 was the primary endpoint. The secondary endpoints were the seroprotection rate on day 42 post first dose of the study vaccine and the frequency of solicited and unsolicited adverse events (AEs) reported between days 0 and 180. Participants were also monitored for compliance throughout the study.
Sample size calculation
For noninferiority comparison of seroprotection rate on day 14; considering one-sided alpha error of 0.025, 90% power, noninferiority immunogenicity margin of −0.04 (−4%) and 15% drop-out rate; the sample size was calculated as follows:
α = 0.025; power is 90% hence, β = 10%; Delta (δ0) = 4% (noninferiority immunogenicity margin); drop-out 15%; P = 98% (expected sero protection)
Sample size = 800
Study subjects
In this study 800 healthy (adjudicated by history, clinical, and laboratory investigations) human volunteers of age 18−65 years of either gender were included. Written informed consent was obtained from each volunteer before performing any study-related procedure. Routine clinical examination, laboratory investigations, radiological examinations, and electrocardiogram were performed on all participants.
The other inclusion criteria included RVNA seronegativity at screening by ELISA; Platelia® Rabies II kit, Bio-Rad, France as per the protocol; because the turnaround time for reporting and logistics by RFFIT was not feasible during screening. Additionally documented negative serological results for human immunodeficiency virus, hepatitis B surface antigen or antihepatitis C antibody. Females of reproductive age group who agreed to practice acceptable contraceptive methods were considered. The exclusion criteria included the history of potential rabies exposure or receipt of rabies vaccination, history of hypersensitivity to any investigational vaccine component, receipt of any other vaccines within past one month, body temperature of ≥38.0°C, presence of any acute infection, history of any chronic illness, receipt of any immunomodulating agents within past six months, concomitant treatment with any antimalarial drugs, history of drug or alcohol abuse, those with deficient immunoglobulins (IgG, IgM, or IgA), and pregnant or lactating female.
Test vaccine
3 intramuscular doses (each dose of 50 µg/ 0.5 ml) of recombinant rabies G protein; test vaccine (Thrabis®, Cadila Pharmaceuticals Ltd., India) administered on days 0, 3, and 7.
Reference vaccine
5 intramuscular doses (each dose of ≥2.5 IU/ml) of WHO prequalified vaccine (Rabipur®, Chiron Behring Vaccines Pvt. Ltd., marketed by Novartis Healthcare Pvt. Ltd., India) available in 1 ml, administered on days 0, 3, 7, 14, and 28 as Essen PEP regimen.
All the study subjects in both the groups were followed up for any adverse events (AEs) post vaccination. The subjects were observed for an hour after each dose to rule out any possible immediate solicited AEs i.e., local reactions such as pain, pruritus, induration and/or systemic reactions such as malaise, dizziness, headache, arthralgia, nausea and abdominal pain. They were given a follow up card indicating the date of the next dose of vaccination and blood sampling; and also to record unsolicited late AEs such as pain, induration, erythema, itching, fever, serum sickness, arthralgia and any others. The follow up cards were checked on every visit and the AEs, if any, were recorded during subsequent visits on days 3, 7, 14, 28, 180 and 365 and the appropriate treatment was provided free of cost. All the study subjects were also monitored for the compliance to complete the course of vaccination.
The sera samples were collected from both the groups for estimation of rabies virus neutralizing antibody (RVNA) titers on day 14 & 42 and were analyzed by Rapid Fluorescent Focus Inhibition Test (RFFIT) at the Department of Neurovirology, National Institute of Mental Health and Neurosciences (NIMHANS), Bangalore, India; which is a World Health Organization (WHO) collaborating center for Reference & Research on Rabies.
Procedure for RFFIT
It was carried out as per WHO recommended procedure with some modifications. The cell line used was BHK 21 (ATCC CCL 10) and 96 well tissue culture plates (Sigma) and BHK21 adapted CVS 13 strain of rabies virus. The reference serum used was an in-house serum calibrated against 2nd international reference standard having a titer of 30 IU/ml (obtained from National Institute of Biological standards, UK). Briefly, doubling dilutions of serum samples and reference serum (after heat inactivation at 56 C for 30 min in a water bath) in duplicate were made in 96 well plates using IMDM (Sigma Cat No.17633). To each 100 µl of serum dilution 100 µl of CVS (100 FFD 50) was added and the plate was incubated at 37°C for 1 hour. A confluent monolayer of BHK 21 cells were trypsinized and re-suspended in 10 ml of IMDM with 10% FCS (Sigma, cat No. F2442). Further cell control and virus controls were also included. To each well of the 96 well plate 100 µl of cell suspension was added and the plate was incubated at 37°C in a CO2 incubator (Sanyo, Japan). After 24 hours the cells were fixed in cold acetone for 30 minutes and stained by direct FAT using commercially available rabies N conjugate (Light diagnostics USA, Cat No. F199). The plates were then observed under an inverted fluorescence microscope (Nikon Eclipse). The highest dilution of serum showing 50% inhibition of fluorescence foci was taken as end point dilution. The titer was converted to IU/ ml in comparison with reference serum.15
All statistical analyses were performed using SAS version 9.4 (SAS Institute Inc., Cary, NC, USA). A P-value of <0.05 was considered statistically significant. The continuous variables were compared using Student’s t-test, while the categorical variables were compared using the chi-square test.
Results
A total of 1023 participants were screened and 800 eligible participants were randomized in 2:1 ratio (533 participants in the test vaccine arm and 267 in the reference vaccine arm). The disposition of the study participants is as shown in Figure 1.
Figure 1.
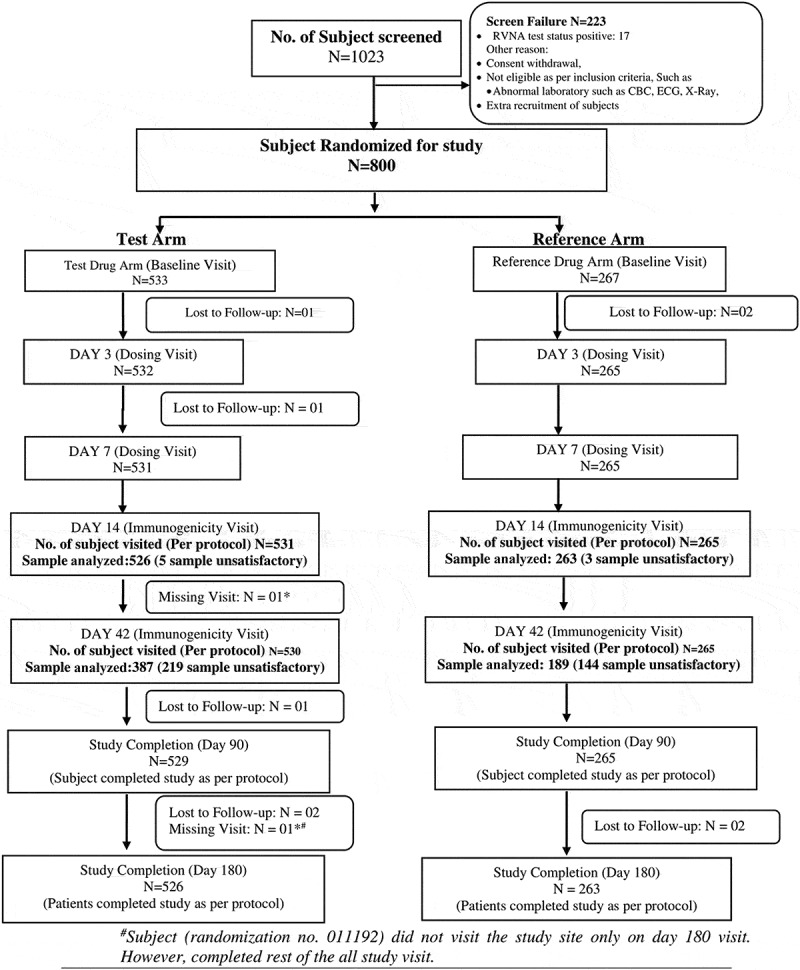
Disposition of the study participants.
The demographic characteristics of the study participants in both the study arms were comparable (Table 1).
Table 1.
Demographic characteristics of the study subjects
| Parameters | Test vaccine arm (N = 533) |
Reference vaccine arm (N = 267) |
||
|---|---|---|---|---|
| Male; N (%) | Female; N (%) | Male; N (%) | Female; N (%) | |
| Number of subjects | 440 (82.55%) | 93 (17.45%) | 225 (84.27%) | 42 (15.73%) |
| Age (years) (Mean ± SD) |
33.116 ± 8.310 | 37.419 ± 12.108 | 35.347 ± 10.176 | 39.119 ± 14.049 |
| Height (cm) (Mean ± SD) |
164.609 ± 7.018 | 155.845 ± 12.818 | 164.715 ± 5.817 | 154.995 ± 6.030 |
| Weight (kg) (Mean ± SD) |
63.413 ± 9.249 | 56.772 ± 7.080 | 64.043 ± 8.374 | 55.643 ± 7.197 |
Safety
A total of 170 AEs were reported by 99 (12.38%) study subjects; out of which 53 (09.94%) were in test vaccine arm and 46 (17.23%) in the reference vaccine arm, showing that though the AEs were equal in numbers but a significantly higher number of participants in the reference arm had AEs (P=0.0032). All the AEs were mild to moderate in nature, which resolved without any complications. The most frequently observed local AEs were pain, redness and swelling at the injection site. The systemic AEs were fever, headache, ear pain, urticaria, joint pain and nausea (Table 2).
Table 2.
Adverse drug events among the study subjects
| Adverse drug events | Test vaccine (n = 533) |
Reference vaccine (n = 267) |
P value |
|---|---|---|---|
| Number of subjects with ADEs | 53 (9.94%) | 46 (17.23%) | 0.0032 |
| Local ADEs* | |||
| Pain | 59 (7.69%) | 59 (13.56%) | 0.0075 |
| Redness | 16 (2.81%) | 12 (3.37%) | 0.065 |
| Swelling | 4 (0.75%) | 6 (1.87%) | 0.1559 |
| Systemic ADEs* | |||
| Fever | 2 (0.38%) | 4 (1.5%) | 0.1931 |
| Headache | 2 (0.38%) | 2 (0.75%) | 0.8607 |
| Ear pain | 1 (0.19%) | 0 (0%) | 0.0826 |
| Urticaria | 1 (0.19%) | 0 (0%) | 0.0826 |
| Joint pain | 0 (0%) | 1 (0.19%) | 0.0826 |
| Nausea | 0 (0%) | 1 (0.19%) | 0.0826 |
*Multiple response.
None of the study subjects in either groups discontinued the vaccination course due to AEs and no death, serious AE or anaphylactic reaction was observed during the entire study. The compliance to complete course of vaccination was 99.5% among the 800 participants. All the study subjects were healthy up to day 365 in both the arms.
Immunogenicity
As per WHO recommendation, RVNA titer of ≥ 0.5 IU/ml is considered as seroprotective against rabies. While assessing the immunogenicity, there were two dropouts, one each, in arm for day 14 due to migration. Likewise, five sera samples in test vaccine arm and two sera samples in reference vaccine arm were unsatisfactory for testing. Consequently, 789 samples were analyzed for day 14; out of which 779 samples (98.73%) were seropositive, i.e. 522/526 (99.24%) in the test vaccine arm and 257/263 (97.72%) in the reference vaccine arm; the difference between the study groups was statistically nonsignificant (P = 0.718) (Table 3). The results were consistent across different age groups and genders. The observed difference in the seroprotection rate on day 14 between the test and the reference vaccine arm was 1.52% (95% CI: −0.18% to 4.17%). Thus, the study met the primary efficacy criteria as the test vaccine arm was noninferior to the reference vaccine within the prespecified margin.
Table 3.
Sero-response of the study subjects
| Day | Test vaccine (n = 533) |
Reference vaccine (n = 267) |
P value |
|---|---|---|---|
| Sero-response % | |||
| Day 0 | 0 | 0 | |
| Day 14 | 99.24 | 97.72 | P = .718 |
| Day 42 | 98.69 | 100 | |
Likewise, on day 42, 98.69% of the subjects in the test vaccine arm and 100.00% in the reference vaccine were seropositive, the difference was statistically nonsignificant (Table 3).
Discussion
Animal exposure to human is a public health problem posing potential threat to over 3.3 billion humans worldwide.16 These exposures have been documented for over 4000 years and occurs mainly in the underserved population of both rural and urban areas.17 Most of these exposures occur in Africa and Asia, where a close habitation of large human and dog population is existent.18
Post-exposure prophylaxis to these exposures is an effective way of rabies prevention.19 It should include wound washing with soap/detergent and water, followed by application of virucidal agents to reduce the viral inoculum at wound site; wound infiltration of rabies immunoglobulin (RIG)/ rabies monoclonal antibodies (RmAb) in all Category III exposures to neutralize the virus at the wound site and complete the course of post exposure vaccination to induce antibodies this prevents the risk of virus entering peripheral nerves. Early and complete PEP will prevent rabies, even after high-risk exposure to potentially rabid animals.20
Successful post-exposure vaccination against rabies relies by following complete vaccination protocol, which often involves a lengthy dosing regimen. Due to the long duration of post-exposure vaccination, many animal bite victims do not complete the course of vaccination. This problem is more prominent in the developing countries pertaining to the high cost of vaccination and loss of income due to frequent visits to health centers.21 Therefore, a global quest is to find an effective vaccine against rabies that would require less-frequent dosing but would confer similar immunogenicity and safety as those of the approved vaccines around.22
Various sites of administration and shortened vaccine regimens have been tried for PEP against rabies and country-wise recommendations also vary widely in this regard.23 Among these regimens, the one-week, four-site intradermal schedule;24–26 one-month simplified four-site intradermal schedule27,28 and one-week, two-site intradermal schedule29,30 were found to be safe and immunogenic. A recent systematic review has evaluated various vaccine regimens and endorsed the reduction in the dose or duration of rabies postexposure prophylaxis schedules.31 Likewise, a modeling study comparing the cost-effectiveness of different postexposure prophylaxis schedules has also advocated the use of an abridged rabies vaccination schedule.32
Many effective vaccines have been developed that used live-attenuated strains of pathogens or inactivated killed pathogens. Live-attenuated vaccine strains are typically highly immunogenic but have inherent safety concerns; whereas, the inactivated or killed vaccine stimulates a weaker immune reaction and may require administration of multiple dosages, an important practical limitation which may increase noncompliance rate. An effective way to address these limitations has gradually emerged through studies of self-assembling proteins, which can be used as nanoparticles mediating multicopy antigen display.33
The recombinant rabies G protein vaccine (Thrabis®) prepared by using VLP technology. The purified target antigen exists as assembly of polypeptides that is present in multiple copies in subunit antigens in a well-ordered arrays with defined orientations. This can potentially mimic the repetitiveness, geometry, size, and shape of the natural host–pathogen surface interactions. Such nanoparticles offer a collective strength of multiple binding sites (avidity) and can provide improved antigen stability and immunogenicity.13,14
The safety profile of the novel vaccine (Thrabis®) was comparable with the WHO prequalified vaccine. The ADEs reported in test vaccine arm was 9.94% and reference vaccine arm was 17.23%. These ADEs reported were similar to other vaccines studied using standard intramuscular Essen regimen. A study done by Sudarshan et al. with human diploid cell rabies vaccine (HDCV) using Essen regimen showed 8.4% ADEs with local ADEs of 7.2% and systemic ADEs of 1.2%. All the ADEs subsided without any complications.34
The requisite for vaccination is to stimulate the immune system to produce antibody titers of at least 0.5 IU/ mL by day 14 as recommended by WHO for seropositivity. In the present study 99.24% in the test vaccine arm and 97.72% in the reference vaccine arm were seropositive; the difference was statistically nonsignificant. The immunogenicity in the present study was found to be comparable with other studies using intramuscular Essen regimen for postexposure prophylaxis. A study done by Ashwath Narayana et al. showed that all the 127 study subjects who received Vaxirab N as PEP had 100% seroprotection on day 14.35
The present study also shows that, the immunogenicity elicited by the investigational recombinant rabies G protein vaccine (Thrabis®) on day 14 and 42 in healthy human volunteers was comparable to that of the reference vaccine. Further studies will be initiated to assess the immunogenicity in category III exposures, and to know whether RIGs will interfere with the antibody production and long term immunogenicity levels up to 6 months.
The strength of this study includes a robust protocol, the inclusion of a fairly large number of participants from multiple sites, good compliance rate, inclusion of primary endpoints as approved by WHO and the robust analytical methods followed. There are a few limitations; as the open-label design could lead to few reporting bias as subjective outcomes and the study has not included special population (pediatric/elderly population and pregnant/lactating women, etc.); which will be considered for the future studies.
In conclusion, the novel three-dose recombinant rabies G protein vaccine (Thrabis®) was found to be safe and immunogenic and was comparable to five doses of WHO prequalified vaccine in simulated postexposure prophylaxis. The reduced number of vaccine doses leads to reduction in number of visits and travel cost as well as increases the compliance, which is important to prevent rabies and ultimately help in eliminating dog-mediated human rabies by 2030.36
Funding Statement
This study was funded by Cadila Pharmaceuticals Ltd., Ahmedabad, India.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Trial registration
CTRI/2016/08/007137.
References
- 1.WHO Expert Consultation on Rabies prevention . Third report. Technical report series no. 1012. Geneva, Switzerland: World Health Organization; 2018. [Google Scholar]
- 2.World Health Organization . Rabies Vaccines and Immunoglobulins: WHO position, WHO/CDS/NTD/NZD/2018.04. Geneva, Switzerland: World Health Organization; 2018. [Google Scholar]
- 3.Minghui R, Stone M, Semedo MH, Nel L. New global strategic plan to eliminate dog-mediated rabies by 2030. Lancet Glob Health. 2018 Aug;6(8):e828–e829. doi: 10.1016/S2214-109X(18)30302-4. Epub 2018 Jun 18. PMID: 29929890. [DOI] [PubMed] [Google Scholar]
- 4.World Health Organization. Rabies vaccines: WHO position paper – April 2018. Weekly Epidemiological Record, No. 16. 2018;93:201–20 . [Google Scholar]
- 5.Vaccine Investment Strategy . Global alliance for vaccines and immunization. [accessed 2021 Mar 30] https://www.gavi.org/about/strategy/vaccine-investment-strategy.
- 6.World Health Organization . WHO Expert Consultation on Rabies. Second report. Technical report series no. 931. Geneva (Switzerland): World Health Organization; 2005. [PubMed] [Google Scholar]
- 7.Rupprecht CE, Nagarajan T, Ertl H.. Current status and development of vaccines and other biologics for human rabies prevention. Expert Rev Vaccin. 2016;15(6):731–49. doi: 10.1586/14760584.2016.1140040. [DOI] [PubMed] [Google Scholar]
- 8.Chutivongse S, Wilde H, Fishbein DB, Baer GM, Hemachudha T.. One year study of the 2-1-1 intramuscular post exposure rabies vaccine regimen in 100 severely exposed Thai patients using rabies immune globulin and Vero cell rabies vaccine. Vaccine. 1991;9(8):573–76. doi: 10.1016/0264-410X(91)90244-Z. [DOI] [PubMed] [Google Scholar]
- 9.Ravish HS, Rachana AR, Veena V, Ashwath Narayana DH. Compliance to anti-rabies vaccination in post-exposure prophylaxis. Indian J Public Health. 2015;59(1):58–60. doi: 10.4103/0019-557X.152867. [DOI] [PubMed] [Google Scholar]
- 10.Rupprecht CE, Briggs D, Brown CM, Franka R, Katz SL, Kerr HD, Lett S, Levis R, Meltzer MI, Schaffner W, et al. Evidence for a 4-dose vaccine schedule for human rabies post-exposure prophylaxis in previously non-vaccinated individuals. Vaccine. 2009 Nov 27;27(51):7141–8. [DOI] [PubMed] [Google Scholar]
- 11.Haradanahalli RS, Banerjee R, Kalappa MS, Narayana A, Annadani RR, Bilagumba G. Safety and immunogenicity of rabies vaccine as 4 - dose Essen Intramuscular regimen for post exposure prophylaxis: A non - randomized, comparative controlled study. Hum Vaccin Immunother. 2021 Feb 23:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.World Health Organization . Rabies working group report, SAGE meeting October 2017. [accessed 2021 Mar 30] http://www.who.int/immunization/sage/meetings.
- 13.López-Sagaseta J, Malito E, Rappuoli R, Bottomley MJ. Self-assembling protein nanoparticles in the design of vaccines. Comput Struct Biotechnol J. 2016;14:58–68. doi: 10.1016/j.csbj.2015.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hicks DJ, Fooks AR, Johnson N. Developments in rabies vaccines. Clin Exp Immunol. 2012;169(3):199–204. doi: 10.1111/j.1365-2249.2012.04592.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zalan E, Wilson C, Pukitis D. A microtest for the quantitation of rabies virus neutralizing antibodies. J Biol Stand. 1979;7:213–20. doi: 10.1016/S0092-1157(79)80024-4. [DOI] [PubMed] [Google Scholar]
- 16.World Health Organization. Rabies vaccines: WHO position paper. Weekly Epidemiological Record. No 32, 2010;85:309–29. [Google Scholar]
- 17.World Health Organization. Strategic framework for elimination of human rabies transmitted by dogs in the South-East Asia Region. WHO Library Cataloguing-in-Publication data 2012 who.int/docs/default-source/searo/india/health-topic-pdf/zoonoses-sfehrtd-sear.pdf. [Google Scholar]
- 18.Tarantola A. Four thousand years of concepts relating to rabies in animals and humans, its prevention and its cure. Trop Med Infect Dis. 2017;2:e5. doi: 10.3390/tropicalmed2020005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sudarshan MK, Haradanhalli RS. Facilities and services of postexposure prophylaxis in anti-rabies clinics: a National assessment in India. Indian J Public Health. 2019;63:S26–30. [DOI] [PubMed] [Google Scholar]
- 20.Haradanhalli SR, Krishna C, Kumar DP, Siddareddy I, Annadani RR. Safety, immunogenicity and clinical efficacy of post exposure prophylaxis in confirmed rabies exposures. Glob Vaccines Immunol. 2016;1:56–59. doi: 10.15761/GVI.1000116. [DOI] [Google Scholar]
- 21.Tran CH, Kligerman M, Andrecy LL, Etheart MD, Adrien P, Blanton JD, Millien M, Wallace RM. Rabies vaccine initiation and adherence among animal-bite patients in Haiti, 2015. PLoS Negl Trop Dis. 2018;12(11):e0006955. doi: 10.1371/journal.pntd.0006955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lilare RR, Rathod N, Narlawar UW. Compliance of post exposure rabies vaccination among patients attending anti-rabies OPD in the government medical college, Nagpur. Int J Community Med Public Health. 2018;5:3045–48. doi: 10.18203/2394-6040.ijcmph20182646. [DOI] [Google Scholar]
- 23.Buchy P, Preiss S, Singh V, Mukherjee P. Heterogeneity of rabies vaccination recommendations across Asia. Trop Med Infect Dis Internet. 2017;1;2(3). doi: 10.3390/tropicalmed2030023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sudarshan MK, Narayana DHA, Madhusudana SN, Holla R, Ashwin BY, Gangaboraiah B, Ravish HS. Evaluation of a one week intradermal regimen for rabies post-exposure prophylaxis: results of a randomized, open label, active-controlled trial in healthy adult volunteers in India. Hum Vaccines Immunother. 2012;8(8):1077–81. doi: 10.4161/hv.20471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Narayana A, Manoharan A, Narayan MS, Kalappa SM, Biligumba G, Haradanahalli R, Anand AM. Comparison of safety and immunogenicity of 2 WHO prequalified rabies vaccines administered by one week, 4 site intra dermal regimen (4-4-4-0-0) in animal bite cases. Hum Vaccines Immunother. 2015;11(7):1748–53. doi: 10.1080/21645515.2015.1048938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shantavasinkul P, Tantawichien T, Wilde H, Sawangvaree A, Kumchat A, Ruksaket N, Lohsoonthorn V, Khawplod P, Tantawichien T. Postexposure rabies prophylaxis completed in 1 week: preliminary study. Clin Infect Dis. 2010;50(1):56–6038. doi: 10.1086/649211. [DOI] [PubMed] [Google Scholar]
- 27.Warrell MJ, Riddell A, Yu LM, Phipps J, Diggle L, Bourhy H, Deeks JJ, Fooks AR, Audry L, Brookes SM, et al. A simplified 4-site economical intradermal post-exposure rabies vaccine regimen: a randomised controlled comparison with standard methods. PLoS Negl Trop Dis. 2008;2(4):e224. 35. doi: 10.1371/journal.pntd.0000224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ambrozaitis A, Laiškonis A, Balčiuniene L, Banzhoff A, Malerczyk C. Rabies post-exposure prophylaxis vaccination with purified chick embryo cell vaccine (PCECV) and purified Vero cell rabies vaccine (PVRV) in a four-site intradermal schedule (4-0-2-0-1-1): an immunogenic, cost– effective and practical regimen. Vaccine. 2006;24(19):4116–21. doi: 10.1016/j.vaccine.2006.02.036. [DOI] [PubMed] [Google Scholar]
- 29.Cantaert T, Borand L, Kergoat L, Leng C, Ung S, In S, Peng Y, Phoeun C, Hing C, Taing CN, et al. A 1-week intradermal dose-sparing regimen for rabies post-exposure prophylaxis (RESIST-2): an observational cohort study. Lancet Infect Dis. 2019;19(12):1355–62. doi: 10.1016/S1473-3099(19)30311-1. [DOI] [PubMed] [Google Scholar]
- 30.Tarantola A, Ly S, Chan M, In S, Peng Y, Hing C, Taing CN, Phoen C, Ly S, Cauchemez S, et al. Intradermal rabies post-exposure prophylaxis can be abridged with no measurable impact on clinical outcome in Cambodia, 2003-2014. Vaccine. 2019;37(Suppl 1):A118–27. doi: 10.1016/j.vaccine.2018.10.054. [DOI] [PubMed] [Google Scholar]
- 31.Kessels J, Tarantola A, Salahuddin N, Blumberg L, Knopf L. Rabies post-exposure prophylaxis: a systematic review on abridged vaccination schedules and the effect of changing administration routes during a single course. Vaccine. 2019;37:A107–17. doi: 10.1016/j.vaccine.2019.01.041. [DOI] [PubMed] [Google Scholar]
- 32.Hampson K, Abela-Ridder B, Bharti O, Knopf L, Léchenne M, Mindekem R, Tarantola A, Zinsstag J, Trotter C. Modelling to inform prophylaxis regimens to prevent human rabies. Vaccine. 2019;37(Suppl 1):A166–73. doi: 10.1016/j.vaccine.2018.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Plotkin S. History of vaccination. Proc Natl Acad Sci U S A. 2014;111(34):12283–87. doi: 10.1073/pnas.1400472111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sudarshan MK, Bhardwaj S, Mahendra BJ, Sharma H, Sanjay TV, Ashwathnarayana DH. An immunogenicity, safety and postmarketing surveillance of a novel adsorbed human diploid cell rabies vaccine (Rabivax®) in Indian subjects. Hum Vaccin. 2008;4(4):275–79. doi: 10.4161/hv.4.4.5588. [DOI] [PubMed] [Google Scholar]
- 35.Ashwathnarayana DH, Madhusudhana SN, Sampath G, Tripathy RM, Sudarshan MK. Gangaboraiah. Safety and Immunogenicity Study of a New Purified Chick Embryo Cell Rabies Vaccine Vaxirab-N (Pitman Moore Strain) Manufactured in India. Human Vaccines & Immuntherapeutics. 2014;10:120–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.World Health Organization. Global elimination of dog-mediated human rabies: report of the rabies global conference, 10-11 December 2015, Geneva, Switzerland.


