ABSTRACT
Pityriasis rosea (PR) is an acute papulosquamous cutaneous disorder that classically presents with a herald patch rapidly followed by a widespread rash along skin cleavage lines. Although the exact pathogenesis of PR is unknown, current evidence suggests that an inflammatory reaction due to a viral trigger may lead to the cutaneous manifestations. COVID-19 has been reported as one such viral trigger for PR. Previously, PR has been reported in temporal association with various viral inoculations. This article presents a case of PR in a 66-year-old black male 1 week after administration of the Pfizer-BioNTech COVID-19 vaccine.
KEYWORDS: Vaccination, COVID-19, pityriasis rosea
Introduction
Pityriasis rosea (PR) is an acute papulosquamous cutaneous disorder that classically presents with a herald patch, followed by rapid development of a widespread rash that follows skin cleavage lines. PR is generally self-limited, with lesions tending to heal spontaneously over a 6-to-8-week period.1 Although the exact pathogenesis of PR is unknown, current evidence suggests that an inflammatory reaction due to a viral trigger such as Human Herpes Virus (HHV)-6 or HHV-7 may lead to the cutaneous manifestations.2 A wide variety of PR presentations have been reported; however, those triggered by inoculations are exceedingly rare. Case reports of PR following influenza, yellow fever, and human papilloma virus, vaccination have been documented 5 days to 4 weeks following administration of the vaccines.3–7
Case presentation
A 66-year-old male with a history of mild hidradenitis suppurativa presented to his primary care physician due to the development of an ovoid eczematous plaque on his right flank. Over the next week, multiple similar slightly scaling patches and plaques appeared on his back, chest, and abdomen. The patient reported that approximately 1 week prior to the first lesion appearing, he had received the first dose of the Pfizer-BioNTech COVID-19 vaccine. His only other reported symptom was transient arm soreness. He denied any personal history of a similar rash or any generalized symptoms and reported no recent travel.
Physical exam revealed a 3-cm red-brown eczematous plaque on the right flank and multiple two-toned papules with fine overlying scale, following skin cleavage lines in a “Christmas tree” distribution on the upper body (Figure 1(a,b)). There was no mucous membrane involvement. Histopathology showed a superficial perivascular lymphohistiocytic infiltrate with scattered dermal erythrocytes, along with nonspecific chronic dermatitis, spongiosis, absent granular layer, and focal hyperkeratosis and parakeratosis. These findings were consistent with pityriasis rosea. The patient was initially treated with triamcinolone 0.1% ointment as needed for itch, then transitioned to a bland emollient. Four weeks after initial presentation, the patient reported complete resolution of the rash.
Figure 1.
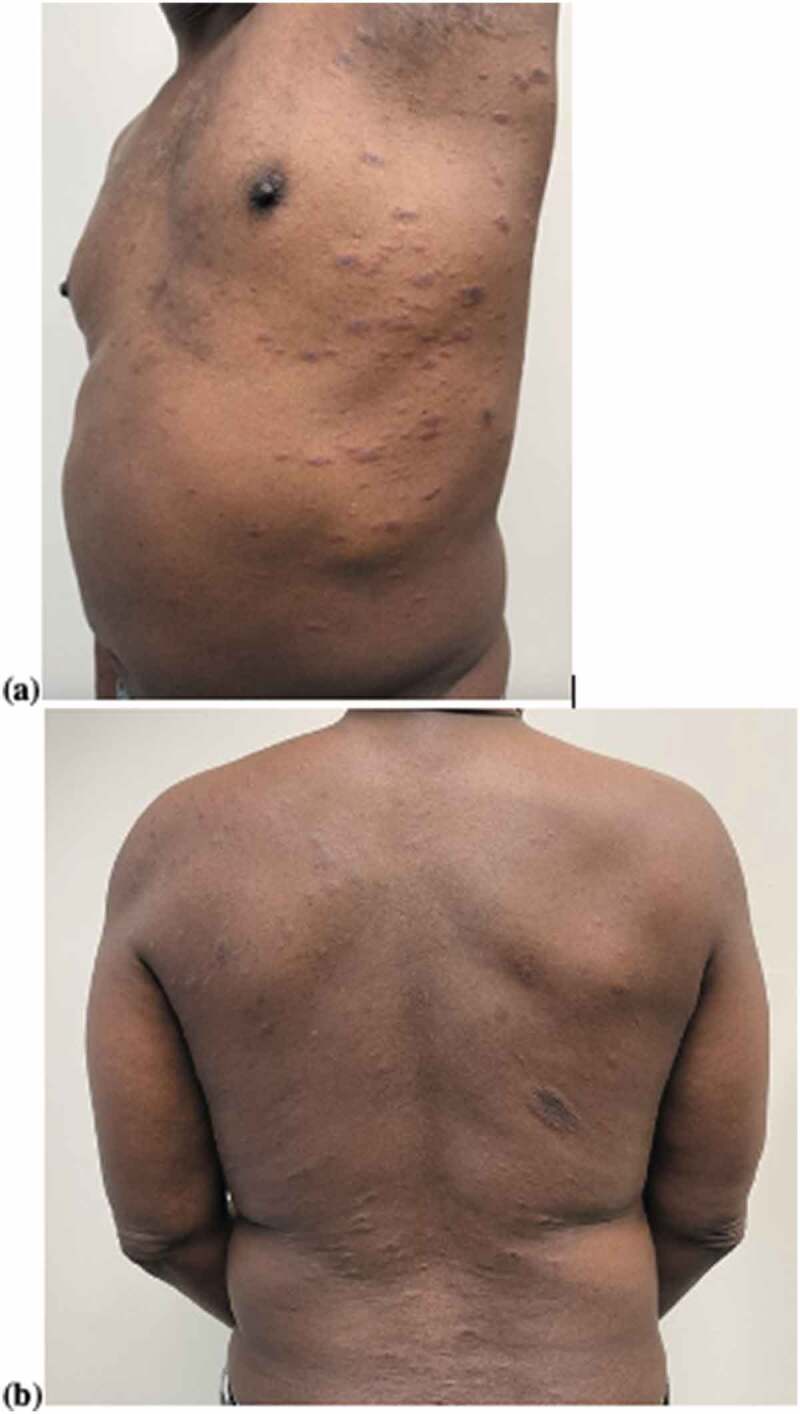
(a) Pityriasis rosea (chest, flank, and axilla); (b) pityriasis rosea (back).
Discussion
To our knowledge, this is the first reported case of a COVID-19 vaccination being temporally associated with the development of PR. Our patient’s clinical presentation and histopathologic findings were consistent with PR; the temporal association with the administration of the COVID-19 vaccine suggests that our patient’s vaccination triggered development of PR. While PR most commonly presents spontaneously, with no known preceding trigger, neither this patient’s age, nor the time of year, was typical of pityriasis rosea, making the antecedent vaccine the most likely cause. It is possible that the state of altered immunity due to his recent vaccination may have led to an endogenous reactivation of HHV-6 or HHV-7, which served as the viral trigger for this cutaneous eruption. Alternatively, as COVID-19 infection has also been reported to trigger PR,8 vaccination against this viral antigen may have served as a noninfectious trigger for the classic PR skin rash.
Acknowledgments
The authors thank the patient for contribution to the case report.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
References
- 1.Drago F, Ciccarese G, Rebora A, Broccolo F, Parodi A.. Pityriasis Rosea: a comprehensive classification. Dermatology. 2016;232(4):431–37. doi: 10.1159/000445375. [DOI] [PubMed] [Google Scholar]
- 2.Watanabe T, Kawamura T, Jacob SE, Blauvelt A, Jacob SE, Orenstein JM, Black JB.. Pityriasis rosea is associated with systemic active infection with both human herpesvirus-7 and human herpesvirus-6. J Invest Dermatol. 2002;119(4):793–97. doi: 10.1046/j.1523-1747.2002.00200.x. [DOI] [PubMed] [Google Scholar]
- 3.Brzezinski P, Chiriac A. Uncommon presentation of pityriasis rosea after yellow fever inoculation. JAMA Dermatol. 2014;150(9):1020–21. doi: 10.1001/jamadermatol.2013.10505. [DOI] [PubMed] [Google Scholar]
- 4.Chen J-F, Chiang C-P, Chen Y-F, Wang W-M. Pityriasis rosea following influenza (H1N1) vaccination. J Chin Med Assoc. 2011;74(6) :280–82. doi: 10.1016/j.jcma.2011.04.010. [DOI] [PubMed] [Google Scholar]
- 5.Ang LPL, Yanqiong L, Wenfei L. Recurrent pityriasis rosea: a case report. Hum Vaccines Immunother. 2018;14(4):1024–26. doi: 10.1080/21645515.2017.1409928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Francesco Drago GC, Rebora A, Parodi A. Pityriasis rosea following human papillomavirus vaccination. Braz J Infect Dis. 2015;19(2):224–25. doi: 10.1016/j.bjid.2014.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dimitrios Papakostasa PGS, Papafragkakia D, Grigorakib E, Avgerinoua G, Antonioua C. An atypical case of pityriasis rosea gigantea after influenza vaccination. Case Rep Dermatol. 2014;6:119–23. doi: 10.1159/000362640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Veraldi S, Spigariolo CB. Pityriasis rosea and COVID-19. J Med Virol. 2021;93(7):4068. doi: 10.1002/jmv.26679. [DOI] [PMC free article] [PubMed] [Google Scholar]


