ABSTRACT
Introduction
Brazil is one of the epicenters of COVID-19 pandemic and faces several hindrances to make his COVID-19 vaccination plan efficient.
Methods
The Brazilian COVID-19 vaccination plan was evaluated and the hindrances to make the COVID-19 vaccination plan efficient were described and discussed.
Results
High territorial extension might contribute to a delay on the COVID-19 vaccination, due to difficulty in delivering vaccines to furthest Brazilian states and to all the interior cities. The choice among the vaccines should be done based on the type of storage and must consider the transport conditions necessary to maintain its effectiveness. The indigenous individuals were included with health-care workers as the first group to be vaccinated, inflaming the number of vaccines doses distributed in states where the indigenous population have higher prevalence. The antivaccine movement and the politicization of the vaccine are also hindrances to be overcome in Brazil. The COVID-19 incidence or mortality rate and the distribution of intensive care units (ICUs) are not a criterion to distribute the vaccines, as we did not identify a correlation between these markers and the number of vaccines. However, a strong or very strong correlation occurred between the number of COVID-19 vaccines and the number of COVID-19 cases, deaths by COVID-19, gross domestic product, as well as populational density. A total of 83,280,475 doses of COVID-19 vaccines were distributed in Brazil. In the first dose, the Coronavac (Sinovac™), AZD1222 (AstraZeneca/Oxford™), and BNT162b (Pfizer/BioNTech™) vaccines were responsible to vaccinate, respectively, 9.61%, 6.69%, and 0.35% of the Brazilian population. In the second dose, the Coronavac, AZD1222, and BNT162b vaccines were responsible to vaccinate, respectively, 7.52%, 0.53%, and <0.01% of the Brazilian population.
Conclusions
The Federal Government must evaluate the hindrances and propose solutions to maximize the immunization against COVID-19 on Brazil.
KEYWORDS: COVID-19, immunization, public health, SARS-CoV-2, vaccine, viral infection
Introduction
On 11 March 2020, the World Health Organization determined the COVID-19 could be characterized as a pandemic,1 and since then, it became one of the deadliest pandemics of the century, with millions of deaths in the world.2 Several efforts have been taken in order to restrain, at least in part, the COVID-19 pandemic, such as the implementation of lockdown, home quarantine in regions with high number of cases, school vacation, a proper hand washing and the disinfection of surfaces,1,3,4 however, except clean water, vaccination is the main means to decrease the burden of infectious disease, such as SARS-CoV-2 infection.5,6 Normally, the development of a vaccine takes 10 to 15 years,7 but fortunately, by the end of December 2020 and early March 2021, that is, in less than one year, 14 vaccines have been approved for use or emergency use in several countries and territories and several others are being tested in clinical trials.8,9 Several countries have started the COVID-19 vaccination still in December 2020 and have vaccinated more than half of their population, such as Israel and United States of America (USA); on the other hand, countries like Brazil and India started the vaccination on 2021, and have a low percentage of individuals vaccinated.10
In general, vaccines can be divided into 7 classes, according to its method of production and origin, being the immunological mechanism of vaccines classified as live attenuated organism, killed whole organism, toxoid/protein, polysaccharide, glycoconjugate, recombinant and blood cell infusion, and more recently, the synthetic vaccines, which are based on genetic material, such as DNA or RNA.11 Several COVID-19 vaccines have been or are being developed worldwide, being at least 6 of them with limited use, 8 full approved for use, 27 in phase III clinical trials, 37 in phase II clinical trials, 49 in phase I clinical trials and, finally, 4 vaccines were abandoned after clinical trials.9 Two of them, the BNT162b2 (Pfizer/BioNtechTM) and mRNA-1273 (ModernaTM), are mRNA vaccines while the others approved have different immune mechanisms, such as non-viral replicating viral vector or inactivated virus.8,9
Most of the countries have their own regulatory agency, which determines the approval of a vaccine for use, for instance, in the USA, the Food and Drug Administration (FDA) was responsible for the emergency use authorization of BNT162b2 (Pfizer/BioNtechTM), mRNA-1273 (Moderna’sTM) and Ad26.COV2.S (Johnson/JanssenTM) COVID-19 vaccines,12 meanwhile, the Brazilian Health Regulatory Agency (ANVISA; acronym in Portuguese for “Agência Nacional de Vigilância Sanitária”) has approved the AZD1222 (AstraZeneca/Oxford’sTM) and BNT162b2 (Pfizer/BioNtechTM) and approved for emergency use the Ad26.COV2.S (Johnson/JanssenTM) and CoronaVac´s (SinovacTM) COVID-19 vaccines.13 The characteristics of the approved vaccines are synthetized in Table 1.9,14–22 Unfortunately, only a few vaccines have a phase III clinical trial already published, such as BNT162b2 (Pfizer/BioNtechTM), followed by mRNA-1273 (Moderna’sTM), AZD1222 (AstraZeneca/Oxford’sTM), and Sputnik V (Gamaleya Research Institute), which showed 95%, 94.1%, 70.40% and 91.6% of efficacy, respectively.14–17 Curiously, the mRNA vaccines presented an excellent efficacy, mainly, to protect against the severe infection by SARS-CoV-2.14,15
Table 1.
| Vaccine | Phase III trial | Developer | Doses needed | Time from the first dose to the second one | Efficacy (%) | Immune mechanism | Main adverse effects | Storage (oC) | Approved in | Emergency use | Cost of the dose (USD $) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| BNT162b2 | Yes | Pfizer/BioNTechTM | 2 | 3 weeks | 91 | mRNA | Pain, swelling and redness in the injection site, fatigue, headache, fever, and chills |
–25 to – 15°C | Brazil, Bahrain, New Zealand, Saudi Arabia, and Switzerland |
Argentina, Australia, Botswana, Brunei, Canada, Chile, Colombia, Costa Rica, Ecuador, European Union, Greenland, Guatemala, Hong Kong, Iceland, Iraq, Israel, Japan, Jordan, Kuwait, Lebanon, Liechtenstein, Malaysia, Maldives, Mexico, Moldova, Monaco, Mongolia, Norway, North Macedonia, Oman, Panama, Peru, Philippines, Qatar, Serbia, Singapore, South Africa, South Korea, Tunisia, Turkey, Ukraine, United Arab Emirates, United Kingdom, United States, and Uruguay | 14.7 to 19.5 |
| mRNA-1273 | Yes | ModernaTM | 2 | 4 weeks | 94.1 | mRNA | Pain, erythema, induration, tenderness, headache, fatigue, myalgia, and chills | –20°C | Switzerland | Canada, European Union, Greenland, Guatemala, Honduras, Iceland, Israel, Kuwait, Mongolia, Norway, Qatar, Singapore, United Kingdom, United States, and Vietnam | 25 to 37 |
| Sputnik V | Yes | Gamaleya Research Institute | 2 | 3 weeks | 91.6 | Viral Vector | Flu-like illness, injection site reactions, headache, and asthenia | 2 to 8°C | None | Russia, Albania, Algeria, Angola, Antigua and Barbuda, Argentina, Armenia, Azerbaijan, Bahrain, Bangladesh, Belarus, Bolivia, Bosnian Serb Republic, Cameroon, Congo Republic, Djibouti, Egypt, Honduras, Gabon, Ghana, Guatemala, Guinea, Guyana, Hungary, India, Iran, Iraq, Jordan, Kazakhstan, Kenya, Kyrgyzstan, Laos, Lebanon, Mali, Mauritius, Mexico, Moldova, Mongolia, Montenegro, Morocco, Myanmar, Namibia, Nepal, Nicaragua, North Macedonia, Pakistan, Palestinian Authority, Panama, Paraguay, Philippines, San Marino, Slovakia, Sri Lanka, St. Vincent and the Grenadines, Serbia, Seychelles, Syria, Tunisia, Turkey, Turkmenistan, United Arab Emirates, Uzbekistan, Venezuela, Vietnam, and Zimbabwe | <10 |
| AZD1222 | Yes | AstraZeneca/OxfordTM | 2 | 4 months (recommended) | 70.4 | Viral Vector | Fatigue, headache, reactions at the injection site, fever, nausea, vomiting, and dizziness | 2to 8°C | Brazil | Algeria, Argentina, Australia, Bahamas, Bahrain, Bangladesh, Barbados, Bhutan, Botswana, Brunei, Canada, Chile, Colombia, Costa Rica, Dominican Republic, Ecuador, Egypt, El Salvador, Ethiopia, European Union, Fiji, Georgia, Ghana, Greenland, Guatemala, Honduras, Hungary, Iceland, India, Indonesia, Iran, Iraq, Jamaica, Kenya, Kuwait, Liechtenstein, Malaysia, Maldives, Mexico, Moldova, Mongolia, Morocco, Namibia, Nepal, Nigeria, North Macedonia, Norway, Pakistan, Papua Guinea, Peru, Philippines, Saudi Arabia, Seychelles, Sri Lanka, South Africa, South Korea, Sudan, Taiwan, Thailand, Ukraine, United Kingdom, Vietnam, and Zambia. Emergency use validation from the World Health Organization. Endorsed by the Africa Regulatory Taskforce. Recommended for emergency use by the Caribbean Regulatory System | 2 to 10 |
| Ad5-nCov | No | CanSinoBioTM | 1 | NA | 65.4 | Viral Vector | Pain at injection site, fever, fatigue, nausea, and vomiting | 2 to 8°C | China | Chile, Hungary, Mexico, and Pakistan | <4 |
| Ad26.COV2.S | Yes | Johnson/JanssenTM | 1 | NA | 69 | Viral Vector | Pain in the injection site, headache, fatigue, myalgia, and fever | 2 to 8°C | Denmark | Bahrain, Brazil, Canada, Colombia, European Union, Greenland, Iceland, Liechtenstein, Norway, Philippines, South Africa, South Korea, Switzerland, Thailand, United States, and Zambia. Emergency use validation from the World Health Organization. Endorsed by the Africa Regulatory Taskforce | 2.80 to 9 |
| EpiVacCorona | No | BektopTM | 2 | 3 weeks | NA | Protein Based | NA | 2 to 8°C | Turkmenistan | Russia | NA |
| ZF2001 | No | ZFSW/Institute of Medical Biology | 3 | 4 weeks | NA | Protein Based | Pain at injection site, redness, itch, cough, fever, and headache | 2 to 8°C | None | China | NA |
| Unnamed | No | SinopharmTM | NA | NA | 72.5 | Inactive or attenuated virus | Not informed | 2 to 8°C | China | United Arab Emirates | 30 to 72.5 |
| BBIBP-CorV | No | SinopharmTM | 2 | 3 weeks | 78.1 | Inactive or attenuated virus | Pain at injection site, fever, fatigue, nausea, and vomiting | 2 to 8°C | Bahrain, China, and United Arab Emirates. | Argentina, Bangladesh NEW, Brunei, Cambodia, Egypt, Gabon, Guyana, Hungary, Indonesia, Iran, Iraq, Jordan, Lebanon, Maldives, Morocco, Namibia, Nepal, North Macedonia, Pakistan, Peru, Venezuela, and Zimbabwe. Emergency use validation from the World Health Organization | 30 to 72.5 |
| CoronaVac | No | SinovacTM | 2 | 2 weeks | 50.7 (Brazil) | Inactive or attenuated virus | Pain, swelling, redness, fatigue, diarrhea, fever, and headache | 2 to 8°C | China | Azerbaijan, Brazil, Cambodia, Chile, Colombia, Ecuador, Egypt, Hong Kong, Indonesia, Laos, Malaysia, Mexico, Pakistan, Panama, Philippines, Thailand, Tunisia, Turkey, Ukraine, Uruguay, and Zimbabwe | 30 |
| Covaxin | No | Bharat BiotechTM | 2 | 4 weeks | 78 | Inactive or attenuated virus | Headache, fatigue, nausea, and vomiting | 2 to 8°C | None | Botswana, Guatemala, Guyana, India, Iran, Mauritius, Mexico, Nepal, Nicaragua, Paraguay, Philippines, and Zimbabwe | 2 to 3 |
| QazCovid-in | No | RIBSPTM | NA | NA | NA | Inactive or attenuated virus | NA | 2 to 8°C | None | Kazakhstan | NA |
| CoviVac | No | Chumakov Center | 2 or 3 | 2 weeks | NA | Inactive or attenuated virus | NA | 2 to 8°C | None | Russia | NA |
NA; Not available or it was not possible to identify the data.
Historically, Brazil is an example of how to manage a vaccination campaign, for example, in the 2009 H1N1 flu (swine flu) pandemic, the Brazilian Health System (SUS, acronym in Portuguese for “Sistema Único de Saúde”) was responsible for the vaccination of 93 million individuals (43% of the population) in only 3 months, in fact, Brazil was the country that most vaccinated against H1N1, even ahead of USA and México, which vaccinated 26% and 24% of their population, respectively.23 However, the Brazilian Federal government struggles to vaccinated the majority of the Brazilian population against SARS-CoV-2 infection, which can be demonstrated by the low index of vaccinated individuals (only 16% of the population), even after 4 months since the first vaccine was approved for emergency use in Brazil.10,24 Thus, the aim of this study is to evaluate the major hindrances, such as political and territorials, in the COVID-19 vaccination in Brazil.
Materials and methods
Our findings were discussed in different topics, such as: (a) Sociodemographic features and its influence to perform the vaccination plan on Brazil, in which we try to evaluate how the sociodemographic characteristics of the Brazilian population and the size of the country (territorial extension) might influence on the vaccination campaign, also, we evaluated several neglected population, as indigenous ones, and how their vaccination is being done; (b) The anti-vaccine movement and the politicization of the vaccine, in which we discuss how the anti-vaccine movement and the politicization of the vaccine could decrease the vaccination rate, mainly in places with low rate of vaccination, such as Brazil; (c) Vaccine quantities, transportation and government vaccine regulatory agency, in which we discuss how the amount of vaccines is distributed across the country considering the first and second doses and the immunological mechanism of vaccines used in Brazil and how the ANVISA and the transportation might be related to a vaccination barrier. Also, we demonstrated the decision making from the Brazilian regulatory agency and the Federal management regarding the COVID-19 vaccines, in which we discuss the necessary documentation needed by the ANVISA in order to approve a vaccine and how the Federal government acted in the purchase of the vaccines in Brazil.
The Brazilian COVID-19 vaccination plan was evaluated25 and the hindrances to make the COVID-19 vaccination plan efficient in our country were described, such as the political issues associated with the COVID-19 vaccination, the choice of priority groups to be vaccinated, the epidemiologic data regarding the Brazilian population, the territorial extension of Brazil, and the anti-vaccine movement. The Brazilian COVID-19 vaccination plan was described for number of vaccines distributed by states and Federal District according to the priority groups such were individuals aged ≥60 years old or institutionalized; institutionalized disabled people; indigenous living at indigenous lands and health workers.25
The vaccination schedule, availability of vaccines and the kinds of vaccines were retrieved from the Ministry of Health in Brazil. The hindrances to perform the vaccination plan in Brazil were based on expertise opinions from Brazil according to media declarations and expertise from the authors, according to the previous studies with COVID-19 and previous vaccinations campaigns regarding other diseases. Also, a data search was done in the PubMed about the clinical trials availability for each vaccine and the description of vaccines in use in the world as described in Table 1.
The epidemiologic data from Brazil for number of COVID-19 cases, number of deaths by COVID-19, COVID-19 incidence by 100,000 inhabitants, COVID-19 mortality by 100,000 inhabitants, gross domestic product (GDP), number of inhabitants, % of the population from Brazil, area (km2), population density (persons per km2), number of intensive care units (ICUs) beds by 10,000 habitants, number of COVID-19 vaccines doses, number of COVID-19 vaccines by 1 M inhabitants, number of vaccines adjusted by COVID-19 cases and deaths were also presented in the manuscript according the states and Federal District from Brazil.25–28
In our data, it was also included the number of vaccines according to the first and second doses distributed of the different COVID-19 vaccines [Coronavac (Sinovac™), AZD1222 (AstraZeneca/Oxford™), and BNT162b (Pfizer/BioNTech™)] approved in Brazil. The calculation of number of vaccines per 1 M inhabitants was done based on the following formula: (number of vaccines by inhabitants) * 1 M. The number of vaccines adjusted by number of COVID-19 cases was calculated using the following formula: (number of COVID-19 cases by state or Federal District by all COVID-19 cases) * total number of vaccines doses. Also, the number of vaccines adjusted by number of deaths by COVID-19 was calculated using the following formula: (number of deaths by state or Federal District by all deaths by COVID-19) * total number of vaccines doses. In addition, it was calculated an estimative regarding the number of vaccines in each state and Federal District versus the number of vaccines after the adjustment for COVID-19 cases and deaths. Finally, the percentage of vaccinated population was calculated using the following formula: (number of vaccine doses by number of individuals in each state and Federal District) * 100. The percentage of vaccinated people was also calculated for all the Brazilian population.
A Spearman correlation between COVID-19 vaccine doses [Coronavac (Sinovac™), AZD1222 (AstraZeneca/Oxford™), and BNT162b (Pfizer/BioNTech™)], number of COVID-19 vaccines per 1 M inhabitants, number of vaccines after the adjustment for COVID-19 cases and deaths, and the number and percentage of population vaccinated with the first and second doses with the number of COVID-19 cases, demographics data from Brazilian territory, GDP, ICUs availability in Brazil by states and Federal District was performed. It was considered the following categorization for the Spearman correlation: (very high positive/negative correlation) 0.9 to 1.0; (high positive/negative correlation) 0.7 to 0.9; (moderate positive/negative correlation) 0.5 to 0.7; (low positive/negative correlation) 0.30 to 0.50; (negligible correlation) 0.00 to 0.30. The statistical analysis was done using the Statistical Package for the Social Sciences software (IBM SPSS Statistics for Macintosh, Version 27.0) and the GraphPad Prism version 8.00 for Apple Mac, GraphPad Software, San Diego California USA, www.graphpad.com. An alpha of 0.05 was used in all statistical analysis.
Results
In Table 1, it was shown the characterization of the approved COVID-19 vaccines worldwide. A total of 14 vaccines were approved for emergency use in several countries, being 8 of them approved in at least one country. Only 5 vaccines had a phase III clinical trial published. Also, 2 vaccines had immunological mechanism the mRNA, 5 viral vectors, 2 proteins based, and, finally, 6 inactive or attenuated virus. This last type is the mechanism of the Coronavac (Sinovac™), which is the most used vaccine used in Brazil. Besides having a great efficacy, both mRNA vaccines should be preserved at a low temperature, which is difficult for the transportation and conditioning, mainly in places of Brazil with limited access to potent refrigerators. The efficacy and the cost were different among all vaccines. The lowest cost was described for Covaxin (2–3 USD per dose; Bharat BiotechTM); and the three vaccines available in Brazil, Coronavac (Sinovac™), AZD1222 (AstraZeneca/Oxford™), and BNT162b (Pfizer/BioNTech™), presented respectively the costs of 30 USD, 2–10 USD and 14.7–19.5 USD per dose.8,9,14–22 Also, Table 1 describes the side-effects related to each vaccine and the countries or territories where the vaccines were authorized in the world.8,9,14–22
Table 2 presents the descriptive analysis of the COVID-19 pandemic in Brazil, such as COVID-19 cases, deaths by COVID-19, COVID-19 prevalence by 100,000 inhabitants and COVID-19 mortality by 100,000 inhabitants. Also, it was demonstrated the demographics data from Brazilian territory, GDP, and ICUs availability.25–30
Table 2.
Descriptive analysis of the COVID-19, demographics data from Brazilian territory, gross domestic product (GDP) and intensive care units (ICUs) availability.25–30.
| States and the Federal District | COVID-19 cases | Deaths by COVID-19 | Prevalence by 100,000 inhabitants | Mortality by 100,000 inhabitants | GDP (R$) x 103 | Number of inhabitants | % Of the population from Brazil | Area (km2) | Population density (persons per km2) | ICUs bed by 10,000 habitants |
|---|---|---|---|---|---|---|---|---|---|---|
| Acre | 80,177 | 1,608 | 9,091 | 182 | 13,751 | 881,935 | 0.4 | 164,123.74 | 5.37 | 1.60 |
| Alagoas | 182,969 | 4,462 | 5,482 | 133 | 49,456 | 3,337,357 | 1.6 | 27,843.30 | 119.86 | 1.60 |
| Amapá | 108,719 | 1,610 | 12,855 | 190 | 14,339 | 845,731 | 0.4 | 142,470.76 | 5.94 | 0.99 |
| Amazonas | 378,138 | 12,808 | 19,124 | 309 | 89,017 | 4,144,597 | 2.0 | 1,559,168.12 | 2.66 | 1.29 |
| Bahia | 948,753 | 19,739 | 6,379 | 133 | 258,649 | 14,873,064 | 7.1 | 564,722.61 | 26.34 | 1.85 |
| Ceará | 734,766 | 18,995 | 8,046 | 208 | 138,379 | 9,132,078 | 4.3 | 148,894.76 | 61.33 | 1.74 |
| Federal district | 390,805 | 8,275 | 12,961 | 274 | 235,497 | 3,015,268 | 1.4 | 5,760.78 | 523.41 | 4.33 |
| Espírito Santo | 456,999 | 19,151 | 11,372 | 253 | 109,227 | 4,018,650 | 1.9 | 46,074.44 | 87.22 | 3.63 |
| Goiás | 577,347 | 16,027 | 8,226 | 228 | 181,692 | 7,018,354 | 3.3 | 340,125.72 | 20.63 | 2.49 |
| Maranhão | 278,137 | 7,674 | 3,931 | 108 | 85,286 | 7,075,181 | 3.4 | 329,642.17 | 21.46 | 1.37 |
| Mato Grosso | 378,708 | 10,189 | 10,868 | 292 | 123,834 | 3,484,466 | 1.7 | 903,207.00 | 3.86 | 2.95 |
| Mato Grosso do Sul | 262,551 | 6,163 | 9,448 | 222 | 91,866 | 2,778,986 | 1.3 | 357,145.54 | 7.78 | 3.16 |
| Minas Gerais | 1,451,836 | 37,005 | 6,858 | 175 | 544,634 | 21,168,791 | 10.1 | 586,521.12 | 36.09 | 2.55 |
| Paraná | 1,012,015 | 24,330 | 8,851 | 213 | 401,662 | 11,433,957 | 4.1 | 199,305.24 | 57.37 | 2.59 |
| Paraíba | 307,542 | 7,178 | 7,654 | 179 | 59,089 | 4,018,127 | 1.9 | 56,467.24 | 71.16 | 1.86 |
| Pará | 494,328 | 13,828 | 5,746 | 161 | 138,068 | 8,602,865 | 5.4 | 1,245,759.31 | 6.91 | 1.41 |
| Pernambuco | 437,783 | 14,841 | 4,581 | 155 | 167,290 | 9,557,071 | 4.5 | 98,068.02 | 97.45 | 2.56 |
| Piauí | 255,372 | 5,469 | 7,802 | 167 | 41,406 | 3,273,227 | 1.6 | 251,616.82 | 13.01 | 1.76 |
| Rio Grande do Norte | 247,512 | 5,785 | 7,058 | 165 | 59,661 | 3,506,853 | 1.7 | 52,809.60 | 66.41 | 2.30 |
| Rio Grande do Sul | 1,026,998 | 26,550 | 9,027 | 233 | 408,645 | 11,377,239 | 5.4 | 281,707.15 | 40.39 | 2.32 |
| Rio de Janeiro | 809,971 | 47,699 | 4,691 | 276 | 640,186 | 17,264,943 | 8.2 | 43,750.42 | 394.62 | 3.73 |
| Rondônia | 220,719 | 5,457 | 12,419 | 307 | 39,451 | 1,777,225 | 0.8 | 237,765.23 | 7.47 | 2.56 |
| Roraima | 99,459 | 1,568 | 16,419 | 259 | 11,011 | 605,761 | 0.3 | 224,273.83 | 2.70 | 1.29 |
| Santa Catarina | 924,602 | 14,351 | 12,905 | 200 | 256,661 | 7,164,788 | 3.4 | 95,730.92 | 74.84 | 2.53 |
| Sergipe | 215,831 | 4,669 | 9,389 | 203 | 38,867 | 2,298,696 | 1.1 | 21,926.91 | 104.83 | 1.80 |
| São Paulo | 3,069,804 | 103,493 | 6,685 | 225 | 2,038,005 | 45,919,049 | 21.9 | 248,219.48 | 184.99 | 2.43 |
| Tocantins | 167,684 | 2,704 | 10,661 | 172 | 31,576 | 1,572,866 | 0.7 | 277,720.40 | 5.66 | 1.80 |
The Brazilian GDP for 2017 was used. The number of Brazilian inhabitants, % of the population in each state or the Federal District and population density were estimated in 2019.
Table 3 presents the COVID-19 vaccination plan in Brazil and the number of vaccines applied according to the vaccine approved in Brazil and the first or second dose. The vaccines distribution is being done in phases and the proportion of people vaccinated with different kinds of vaccines is disproportionate in Brazil. The vaccines were applied first on people aged ≥60 or institutionalized; institutionalized disabled people; indigenous population living on indigenous lands and for 34% of health workers who act, mainly, in the first line to treat, the severe cases of individuals infected by the SARS-CoV-2. Also, a total of 83,280,475 doses of COVID-19 vaccines were distributed in Brazil until 15 May 2021; being the major part Coronavac (Sinovac™; 20,204,265 doses as first dose and 15,813,505 doses as second dose), followed by AZD1222 (AstraZeneca/Oxford™,14,054,334 doses as first dose and 1,105,835 as second dose) and in third place, the BNT162b (Pfizer/BioNTech™, 726,726 doses as first dose and 1,783 as second dose) vaccines. The data presented in Tables 2 and 3 were used to perform the Spearman correlation with the vaccine markers and to calculate the adjustments presented in Table 4. Also, the first dose of the Coronavac (Sinovac™), AZD1222 (AstraZeneca/Oxford™), and BNT162b (Pfizer/BioNTech™) vaccines was responsible to vaccinate, respectively, 9.61%, 6.69%, and 0.35% of the Brazilian population while the second dose was responsible to vaccinate 7.52%, 0.53% and <0.01% of the Brazilian population, respectively (Table 5).13,29 To date, on 15 May 2021, only 16.64% and 8.04% of the Brazilian population was vaccinated, respectively, for the first and second doses (Table 5) putting the Brazil ranked as the 56° country worldwide.13,29
Table 3.
COVID-19 vaccination plan in Brazil and the number of COVID-19 vaccines applied according to vaccine type and first or second dose.13,25,29.
| States and the Federal District | Individuals aged ≥60 years old or institutionalized | Institutionalized disabled people | Indigenous living at indigenous lands | Health workers (34%) | Phase 1 – target population | Vaccines available | COVID-19 Vaccines applied |
|||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Coronavac (Sinovac™) dose |
AZD1222 (AstraZeneca/Oxford™) dose |
BNT162b (Pfizer/BioNTech™) dose |
||||||||||
| First | Second | First | Second | First | Second | |||||||
| Acre | 244 | 0 | 12,815 | 6,343 | 19,402 | 298,320 | 57,296 | 41,872 | 49,981 | 2,652 | 1,187 | 2 |
| Alagoas | 1,246 | 10 | 7,946 | 32,594 | 41,796 | 1,185,350 | 280,668 | 224,271 | 209,422 | 15,187 | 1,417 | 4 |
| Amapá | 76 | 0 | 7,616 | 7,057 | 14,749 | 229,880 | 63,421 | 41,863 | 37,827 | 3,800 | 370 | 1 |
| Amazonas | 400 | 60 | 101,156 | 32,813 | 134,429 | 2,022,100 | 330,120 | 240,741 | 253,102 | 70,348 | 525 | 3 |
| Bahia | 9,788 | 285 | 27,201 | 142,087 | 179,361 | 5,683,440 | 1,362,603 | 1,019,904 | 1,108,944 | 80,291 | 35,470 | 59 |
| Ceará | 2,398 | 132 | 20,250 | 86,380 | 109,160 | 3,212,360 | 771,285 | 558,360 | 382,059 | 44,462 | 11,405 | 59 |
| Federal district | 648 | 178 | 95 | 49,629 | 50,550 | 1,064,240 | 255,140 | 224,702 | 207,797 | 28,179 | 4,737 | 9 |
| Espírito Santo | 2,970 | 210 | 2,793 | 42,273 | 48,246 | 1,566,100 | 473,905 | 259,443 | 288,776 | 27,054 | 8,864 | 19 |
| Goiás | 8,828 | 475 | 320 | 77,549 | 87,172 | 2,435,120 | 592,519 | 515,776 | 495,249 | 51,252 | 18,063 | 17 |
| Maranhão | 264 | 110 | 19,626 | 58,223 | 78,223 | 2,394,810 | 503,303 | 418,496 | 450,915 | 34,877 | 14,631 | 17 |
| Mato Grosso | 2,382 | 190 | 28,758 | 28,744 | 60,074 | 1,109,830 | 288,891 | 214,595 | 192,994 | 19,507 | 76,002 | 254 |
| Minas Gerais | 38,578 | 1,160 | 7,878 | 227,472 | 275,088 | 8,909,924 | 2,120,270 | 1,649,241 | 1,366,387 | 95,684 | 39,843 | 52 |
| Mato Grosso do Sul | 2,966 | 95 | 46,180 | 26,356 | 75,597 | 1,057,320 | 333,495 | 209,217 | 244,727 | 22,337 | 5,760 | 5 |
| Paraná | 12,224 | 482 | 10,816 | 102,959 | 126,481 | 4,491,120 | 1,117,981 | 971,689 | 880,613 | 67,493 | 33,036 | 35 |
| Paraíba | 1,212 | 120 | 10,432 | 42,925 | 54,689 | 1,548,360 | 467,137 | 674,024 | 281,482 | 25,769 | 2,694 | 2 |
| Pará | 962 | 10 | 23,184 | 58,334 | 82,490 | 2,687,220 | 518,706 | 411,405 | 393,164 | 45,098 | 19,412 | 7 |
| Pernambuco | 2,462 | 130 | 26,506 | 99,924 | 129,022 | 2,451,830 | 898,861 | 674,024 | 489,088 | 47,358 | 438 | 17 |
| Piauí | 460 | 10 | 21 | 28,651 | 29,142 | 1,159,860 | 277,124 | 217,406 | 208,211 | 13,399 | 5,419 | 20 |
| Rio Grande do Norte | 1,400 | 10 | 0 | 37,848 | 39,258 | 1,291,880 | 359,706 | 249,417 | 215,336 | 25,377 | 993 | 2 |
| Rio Grande do Sul | 9,510 | 380 | 14,348 | 138,523 | 162,761 | 5,481,310 | 1,578,788 | 1,043,126 | 1,086,688 | 78,743 | 9,883 | 39 |
| Rio de Janeiro | 10,892 | 783 | 381 | 220,495 | 232,551 | 8,133,939 | 1,810,293 | 1,256,903 | 1,216,248 | 87,456 | 54,944 | 118 |
| Rondônia | 140 | 0 | 7,784 | 15,595 | 23,519 | 527,758 | 138,831 | 88,951 | 63,560 | 6,829 | 2,383 | 7 |
| Roraima | 100 | 0 | 36,834 | 4,833 | 41,767 | 221,620 | 61,171 | 51,043 | 10,952 | 374 | 763 | 1 |
| Santa Catarina | 3,460 | 263 | 8,317 | 56,540 | 68,580 | 2,752,720 | 645,753 | 564,707 | 461,968 | 27,076 | 57 | 3 |
| Sergipe | 240 | 22 | 250 | 22,760 | 23,272 | 792,790 | 177,610 | 133,787 | 129,250 | 4,576 | 3,535 | 10 |
| São Paulo | 42,604 | 1,357 | 3,727 | 598,518 | 646,206 | 20,067,074 | 4,585,390 | 3,746,120 | 3,259,160 | 253,009 | 135,622 | 416 |
| Tocantins | 424 | 0 | 6,749 | 13,803 | 20,976 | 504,200 | 133,998 | 112,422 | 70,434 | 6,391 | 239,273 | 605 |
| Total | 156,878 | 6,472 | 431,983 | 2,259,227 | 2,854,560 | 83,280,475 | 20,204,265 | 15,813,505 | 14,054,334 | 1,105,835 | 726,726 | 1,783 |
COVID-19 vaccination plan in Brazil was published by the Health Ministry from Brazil.
Table 4.
COVID-19 vaccine [Coronavac (Sinovac™), AZD1222 (AstraZeneca/Oxford™), and BNT162b (Pfizer/BioNTech™)] doses status in Brazil by states and Federal District.13,29.
| States and Federal District | COVID-19 vaccines available (A) | Vaccines by 1 M inhabitants | Vaccines adjusted by number of cases (B) | Difference between (B) and (A) | Vaccines adjusted by number of deaths (C) | Difference between (C) and (A) |
|---|---|---|---|---|---|---|
| Acre | 298,320 | 338,256 | 425,570 | 127,250 | 299,936 | 1,616 |
| Alagoas | 280,668 | 84,099 | 971,178 | 690,510 | 832,286 | 551,618 |
| Amapá | 229,880 | 271,812 | 577,068 | 347,188 | 300,309 | 70,429 |
| Amazonas | 2,022,100 | 487,888 | 2,007,112 | −14,988 | 2,389,045 | 366,945 |
| Bahia | 5,683,440 | 382,130 | 5,035,868 | −647,572 | 3,681,867 | −2,001,573 |
| Ceará | 3,212,360 | 351,767 | 3,900,051 | 687,691 | 3,543,091 | 330,731 |
| Federal district | 1,064,240 | 352,950 | 2,074,346 | 1,010,106 | 1,543,516 | 479,276 |
| Espírito Santo | 1,566,100 | 389,708 | 2,425,696 | 859,596 | 3,572,189 | 2,006,089 |
| Goiás | 2,435,120 | 346,965 | 3,064,489 | 629,369 | 2,989,477 | 554,357 |
| Maranhão | 2,394,810 | 338,480 | 1,476,318 | −918,492 | 1,431,412 | −963,398 |
| Mato Grosso | 1,109,830 | 318,508 | 2,010,137 | 900,307 | 1,900,529 | 790,699 |
| Mato Grosso do Sul | 1,057,320 | 380,470 | 1,393,589 | 336,269 | 1,149,569 | 92,249 |
| Minas Gerais | 8,909,924 | 420,899 | 7,706,173 | −1,203,751 | 6,902,452 | −2,007,472 |
| Paraná | 4,491,120 | 392,788 | 5,371,655 | 880,535 | 4,538,216 | 47,096 |
| Paraíba | 1,548,360 | 385,344 | 1,632,396 | 84,036 | 1,338,895 | −209,465 |
| Pará | 2,687,220 | 312,363 | 2,623,834 | −63,386 | 2,579,303 | −107,917 |
| Pernambuco | 2,451,830 | 256,546 | 2,323,700 | −128,130 | 2,768,256 | 316,426 |
| Piauí | 1,159,860 | 354,348 | 1,355,484 | 195,624 | 1,020,119 | −139,741 |
| Rio Grande do Norte | 1,291,880 | 368,387 | 1,313,764 | 21,884 | 1,079,062 | −212,818 |
| Rio Grande do Sul | 5,481,310 | 481,779 | 5,451,183 | −30,127 | 4,952,307 | −529,003 |
| Rio de Janeiro | 8,133,939 | 471,125 | 4,299,230 | −3,834,709 | 8,897,178 | 763,239 |
| Rondônia | 527,758 | 296,956 | 1,171,550 | 643,792 | 1,017,881 | 490,123 |
| Roraima | 221,620 | 365,854 | 527,917 | 306,297 | 292,475 | 70,855 |
| Santa Catarina | 2,752,720 | 384,201 | 4,907,677 | 2,154,957 | 2,676,857 | −75,863 |
| Sergipe | 792,790 | 344,887 | 1,145,605 | 352,815 | 870,897 | 78,107 |
| São Paulo | 20,067,074 | 437,010 | 16,294,155 | −3,772,919 | 19,304,297 | −762,777 |
| Tocantins | 504,200 | 320,561 | 890,047 | 385,847 | 504,371 | 171 |
1 M, one million. The number of number of Brazilian inhabitants, % of the population in each state or the Federal District and population density were estimated in 2019. (A) Vaccines available in each state and federal district; Vaccines by 1 M inhabitants were calculated using the following formula: (number of vaccines by inhabitants) * 1 M; (B) Vaccines adjusted by the number of COVID-19 cases were calculated using the following formula: (number of COVID-19 cases by state or Federal District by all COVID-19 cases) * total number of vaccine dosages; (C) Vaccines adjusted by number of deaths by COVID-19 was calculated using the following formula: (number of deaths by state or Federal District by all deaths by COVID-19) * total number of vaccine dosages.
Table 5.
Number of vaccines and percentage of the population that received each COVID-19 vaccine in Brazil.13,29.
| Vaccines | Number of COVID-19 vaccines |
Percentage of the population that received each COVID-19 vaccine |
||
|---|---|---|---|---|
| First dose | Second Dose | First dose | Second Dose | |
| Coronavac (Sinovac™) | 20,204,265 | 15,813,505 | 9.61 | 7.52 |
| AZD1222 (AstraZeneca/Oxford™) | 14,054,334 | 1,105,835 | 6.69 | 0.53 |
| BNT162b (Pfizer/BioNTech™) | 726,726 | 1,783 | 0.35 | <0.01 |
| Total | 34,985,325 | 16,921,123 | 16.64 | 8.04 |
Table 4 presents the COVID-19 vaccine [Coronavac (Sinovac™), AZD1222 (AstraZeneca/Oxford™), and BNT162b (Pfizer/BioNTech™)] doses status in Brazil by states and Federal District regarding the number of doses and the adjustment by number of COVID-19 cases and deaths, as well as the ratio per 1 M of inhabitants. Moreover, the percentage of population vaccinated according the first and second doses by state and Federal District is shown in Figure 1. It was observed that the state with highest first dose applied was Tocantins (28.21%), followed by Rio Grande do Sul (23.52%) and Mato Grosso do Sul (18.70%) states, whereas the highest rate for the second dose was observed in Paraíba (17.42%) state, followed by Rio Grande do Sul (9.86%) and Paraná (9.10%) states. Curiously, in the Paraíba state, nearly all individuals who received the first dose of the vaccine received also the second dose, which is different from mostly of the states, in which only a small percentage of individuals who received the first dose received also the second one.
Figure 1.
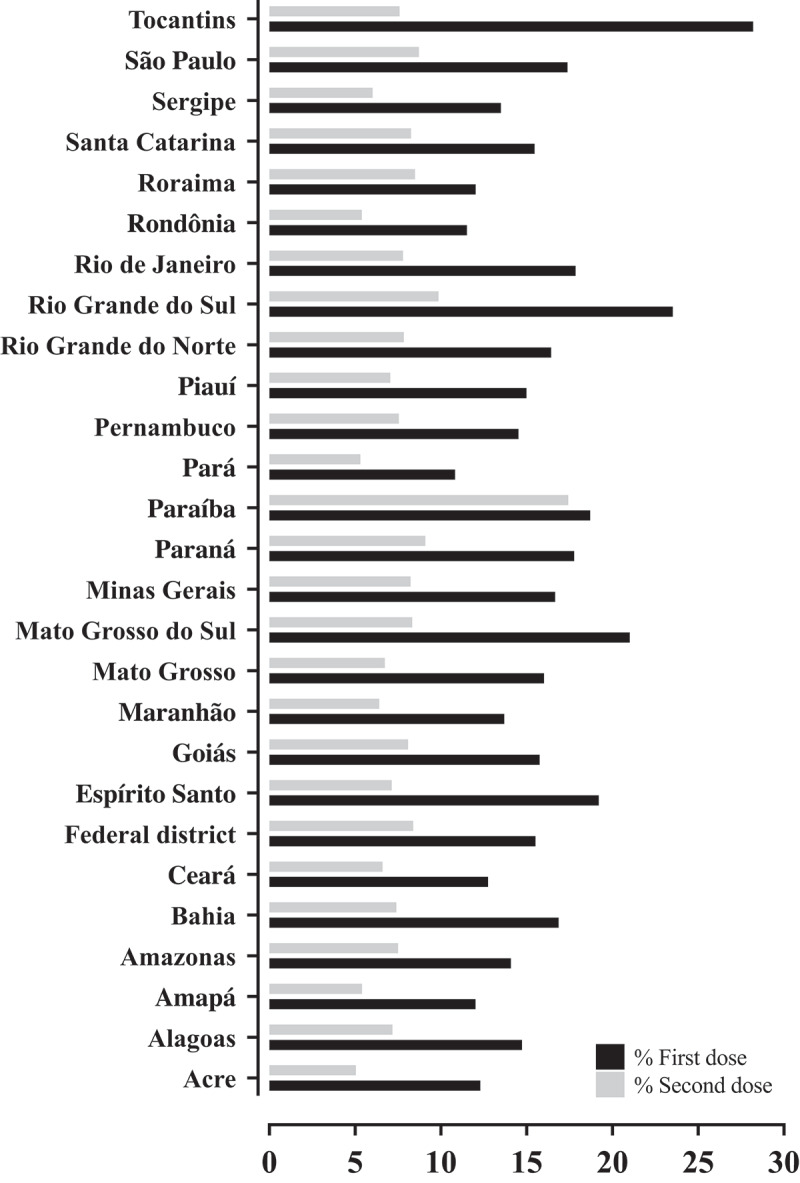
Percentage of population vaccinated according the first and second doses by states and Federal District.
Also in our data, it was described a different number of vaccines according to the ratio per 1 M of inhabitants varying between 84,099 in Alagoas state and 487,888 in Amazonas state. In addition, both adjustments done using the number of COVID-19 cases and the number of deaths by COVID-19 showed a great difference for the number of vaccines really used to vaccinate the population. Maybe, the number of deaths by COVID-19, as well as the number of confirmed cases of COVID-19, should also be used as a criterion to distribute the COVID-19 vaccines in Brazil. Using our formula, some states prone to receive more COVID-19 vaccines doses than others. We observed that the number of COVID-19 vaccines available minus the vaccines adjusted by number of cases in the Federal district was extremely high (1,010,106), whereas in others states, such as the Rio de Janeiro, this coefficient is extremely low (−3,834,709). Regarding the total of COVID-19 vaccines minus the vaccines adjusted by the number of deaths, it was observed the highest coefficient in the Espírito Santo state (2,006,089), whereas the lowest was observed in the Minas Gerais state (−2,007,472) (Table 4). Furthermore, the number for vaccines per 1 M inhabitants is high in states where the number of indigenous population or elderly individuals are high, such as Amazonas and Minas Gerais states. The number of COVID-19 vaccines doses were distributed without consideration of the number of cases, deaths, incidence, mortality and/or the presence of healthcare support, such as, ICUs to treat the patients with severe SARS-CoV-2 infection.
Finally, we included a Spearman correlation between the number of COVID-19 vaccines by inhabitants, number of vaccines per 1 M of inhabitants, number of vaccines adjusted by COVID-19 cases and deaths by COVID-19, number and percentage of population vaccinated with the first and second doses with number of individuals with COVID-19, number of deaths by COVID-19, incidence by 100,000 inhabitants, mortality by 100,000 inhabitants, GDP, the number of inhabitants by states and Federal District, populational density and the number of ICUs beds to prove our hypothesis previously demonstrated in the last paragraph (Figure 2). In our statistical analysis, the number of deaths did not show any significant P-value. Also, the incidence by 100,000 inhabitants, mortality by 100,000 inhabitants, populational density and the number of ICUs beds did not present any correlation with the vaccine’s markers above 0.7 as correlation coefficient, indicating a non-strong correlation. The same was observed for vaccines per 1 M inhabitants, and percentage of population vaccinated with the first and second doses with the other markers evaluated.
Figure 2.
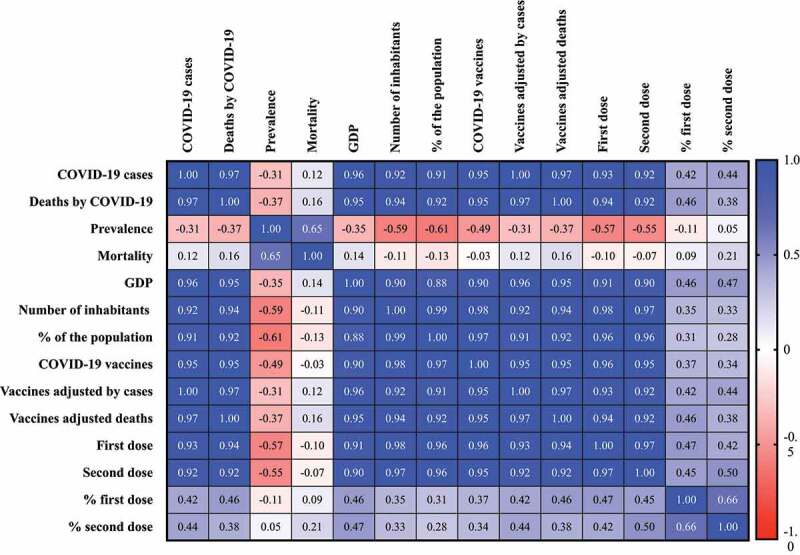
Spearman correlation matrix between the number of COVID-19 vaccines by Brazilian inhabitants, number of COVID-19 vaccines per 1 M of inhabitants, number of COVID-19 vaccines adjusted by COVID-19 cases and deaths by COVID-19, number and percentage of Brazilian population vaccinated with the first and second doses with number of individuals with COVID-19, number of deaths by COVID-19, incidence by 100,000 inhabitants, mortality by 100,000 inhabitants, gross domestic product (GDP), the number of Brazilian inhabitants by states and Federal District, % of the population and populational density and the number of intensive care units (ICUs) beds in Brazil. The calculation of number of vaccines per 1 M inhabitants was done based on the following formula: (number of vaccines by inhabitants) * 1 M. The number of vaccines adjusted by number of COVID-19 cases was calculated using the following formula: (number of COVID-19 cases by state or Federal District by all COVID-19 cases) * total number of vaccine doses. The number of vaccines adjusted by number of deaths by COVID-19 was calculated using the following formula: (number of deaths by state or Federal District by all deaths by COVID-19) * total number of vaccine doses. The percentage of vaccinated population was calculated using the following formula: (number of vaccine doses by number of individuals in each state and Federal District) * 100. In the figure it was not shown the results of correlations for population density, ICUs beds and vaccines per 1 M of inhabitants because the correlation coefficient was low. It was considered the following categorization: (very high positive/negative correlation) 0.9 to 1.0; (high positive/negative correlation) 0.7 to 0.9; (moderate positive/negative correlation) 0.5 to 0.7; (low positive/negative correlation) 0.30 to 0.50; (negligible correlation) 0.00 to 0.30.
Curiously, a strong or very strong correlation (P-value<0.001) between the number of COVID-19 vaccines, number of COVID-19 vaccines adjusted by COVID-19 cases and deaths, number of the Brazilian population vaccinated with the first and second doses with other markers, such as number of COVID-19 cases, number of deaths by COVID-19, GDP, number of Brazilian inhabitants and % of the population was observed (Figure 2). However, it was evident that these correlations are spurious ones, which indicated that the higher number of vaccines are dispensed to places with a higher number of individuals and, respectively, higher absolute number of cases and deaths related with the COVID-19. This correlation also did not correspond to the COVID-19 severity, due to a negative correlation that occurs between number of vaccines and other markers. Also, the no significant correlation between number of vaccines and, (i) incidence per 100,000 habitants, (ii) mortality per 100,000 habitants and (iii) ICUs bed per 10,000 habitants can demonstrate that the impact of the vaccination cannot cause the reduction in the mortality where the mortality rate is high and a reduction in the impact of health system in places where the ICUs bed availability is low.
Discussion
Hindrances toward the COVID-19 vaccination in Brazil
(A) Sociodemographic features an its influence to perform the vaccination plan in Brazil
Brazil is one of the most territorially extensive countries in the world, with 8,510,295.914 km2, only behind Russia, Canada, USA, and China.26,31 In order to facilitate the management, Brazil was divided into 26 states and 1 Federal District, where the headquarters of the federal government is located; however, these states and Federal District are neither similar in extension nor sociodemographic characteristics.27 For instance, the territorial extension among the states and Federal District can range between 1,559,167.889 km2, for Amazonas, to 5,760.783 km2, for the Federal District,27 in addition, the GDP also has a very wide variation, ranging from R$ 2,210,562 × 106 in São Paulo state to R$ 13,370 × 106 in Roraima state.28 The same scenario occurs for the number of inhabitants, demographic density, the number of ICUs beds and health care workers that have a huge variability among the states and Federal District (Table 2).26,30
The high territorial extension might contribute to a delay on the COVID-19 vaccination, due to difficulty in delivering vaccines to furthest states and to all the interior cities, since the bigger the state the farther the cities are from each other. Several others complications should be attributed to the high territorial extension, such as the difficulty to reach some cities, mainly, from the North region, especially in Amazonas state, which can only be reachable by boat and the transportation to cities provide a vaccine wastage. Finally, not all states have a proper storage system for the vaccine, which can also lead to vaccine wastage.
The Brazilian population is mostly descendant from the Portuguese, since its discovery in the 1500´s, however, several individuals from others countries, such as France, Germany, Netherlands and countries from Africa, also settled in different regions of Brazil, leading to an extremely mixed population,32 even nowadays, there are quite cultural and sociodemographic differences between all the 5 Brazilian regions. This fact can corroborate with a social hindrance to deal with the pandemic, because unfortunately, the access to the SUS is different in each region and it is also different for several populations. For instance, it was observed a higher concentration of high or medium complexity equipment’s in only a few cities, which makes the displacement of patients who need high complex treatment necessary,33,34 also, several populations have low access to the health system, such as the black and indigenous population35,36 which demonstrates how heterogeneous the country is in access to health and might impose a difficulty to the COVID-19 vaccination campaign. Furthermore, according to the Ministry of Education, in 2014, Brazil accounted for a total of 8.7% of illiterate, however, this illiteracy is not uniform, the Northeast accounts for 16.6%, whereas the South region accounts for 4.4%,37 which can lead to a different perception, in each region, regarding the necessity of vaccination, since higher years of study were associated with higher vaccination rate.38
Also, the Brazilian people, as discussed before, is mixed. For example, among all citizens, Brazil has more than 572,000 indigenous that are considered as risk population for pandemics. Indigenous comprises a rich culture with several languages and behaviors that are passed down from generation to generation. Part of the customs are transmitted mainly by the older individuals, and these are part of the main population affected by COVID-19.35,39 In addition, historically, indigenous have low access healthcare system35 regardless of whether they live at the city or forests, recognized as indigenous lands where the local community is namely as “tribe.” Obviously, the restrictions for the healthcare are greater for indigenous that live under social isolation, into the “tribes.” Besides, into the “tribes,” the indigenous live-in community, sharing environments and household utensils, including those used for food.40–42
The forests devastation to create pasture, to get wood, to raise livestock and to mine ores raise an approximation between the indigenous and the other ethnic groups from Brazil that increase the disease dispersion such as the COVID-19.40 Simultaneously, during the COVID-19 pandemic the action from people who devastated the forests increased with no Federal policy to restrain its activities.43 Then, the COVID-19 spread among the indigenous and the SARS-CoV-2 infected more than 48,000 indigenous and caused the death of at least 672 individuals, mainly elderly ones.44
The indigenous peoples were included with healthcare workers as the first group to be vaccinated in Brazil, which probably occurred due to a poor management of environmental protection, which increases contact with the non-indigenous individuals, increasing the COVID-19 cases among indigenous peoples.40,45 There are no studies about the COVID-19 vaccine efficacy and its side effects in this population. It is important to determine both, the types of side-effects and the efficacy in this group, considering that the indigenous have a distinct genetic background46 that could influence the response to the vaccine. Moreover, historically, the contact with pathogens in indigenous population were restricted for some microorganisms and, maybe, due to high genetic homozygosity, the indigenous immune system is not as efficient as non-indigenous,47 which could alter the efficacy of the vaccine.
The vaccines should be distributed equally to all individuals, regardless ethnicity and wealthier, however, the indigenous peoples might be neglected, due to low access to the SUS, which can result in a low immunization rate, thus the government has a duty to provide equal access to the vaccination. This fact inflames the number of vaccines doses to be distributed in states where the Indigenous population has a great prevalence.
(B) The antivaccine movement and the politicization of the vaccine
The vaccine opposition is not new, reports exist since the 18th with Reverend Edmund Massey in England, who judged the vaccine as “diabolical operations,”48,49 however since the publication by Andrew Wakefield on The Lancet associating the measles, mumps and rubella vaccine to autism in children50 the movement became stronger, even after the retraction of the paper and the publication of several others studies which did not find any association between the vaccine and autism.51–53
The antivaccine movement is present in Brazil and affects all the immunization programs. For instance, the dropout rate in any free national immunization plan in 2018 was 15.34%, and in 2020 it increased to 18.88%, being more intense in states from the North and Northeast region,54 in part, due to the antivaccine movement. Even President Jair Bolsonaro appears to be a part of this movement, due to several declarations against the vaccine, such as these in which he admitted lack of interest in COVID-19 vaccination, or even worse, when the first report of the Coronavac (Sinovac™) was available, he celebrated a death which occurred in the study, stating the vaccine does not work, even though the overall efficacy was prove to be 50.38%.55 The leader of the executive should be the first one to encourage the vaccination, and not invent adverse effects such as a transformation into crocodile,56 since a great number of individuals see him as an example to be followed.57
Another important aspect which can affect the vaccination is its politicization. Since the beginning of the pandemic, the governor of São Paulo State, Mr. João Doria, invested in the Coronavac (Sinovac™) vaccine, claiming it was the São Paulo vaccine, using it to promote himself for the 2022 presidential election, in contrast, President Jair Bolsonaro demoralized this vaccine in every way possible, and even did not finance it with Federal budget. Nevertheless, after the approval of ANVISA, the President Jair Bolsonaro and his sons, who are also politicians, claimed it was a Brazilian vaccine developed with Federal budget, in order to benefit himself in the 2022 election. This politicization makes individuals who votes for Bolsonaro to be less willing to take the vaccines.
Unfortunately, the Brazilian congress approved the law project 948/21 in which private companies can purchase vaccines, donating half of them to the Brazilian public health system, and to use the other half on the company employees, however, the law project allows the companies to purchase vaccines that are not approved by the ANVISA. In that scenario, the Brazilian public health system is not allowed to use these vaccines, since it was not approved by the ANVISA. Another aspect of this law project causes SUS and private companies to compete for vaccines, which can cause prices to rise.56,58
Even though several studies showed limited results regarding children vaccination using communication strategies, such as pamphlets or brochures,59,60 the Federal government should invest in propaganda, especially on social media, to explain how important the vaccination is and to clarify all the possible doubts the population has, demystifying the untruths of the anti-vaccine movement. Allied to that, the physicians should listen61 to their patients doubts about the COVID-19 vaccine and try to help them in the best possible way, in order to increase the vaccine adhesion. The vaccine politicization only polarizes even more the population, so that individuals with a certain political propensity will not vaccinate, in order to maximize the immunization, the vaccination must be seen as a scientific and nonpolitical issue.
The COVID-19 vaccination started at 18 January 2021 in Brazil as described in Table 3. However, after three days, in some cases described in social media people did not follow the recommendation and used their status to get ahead in the priority line for vaccination. For example, mayors, medical doctors, and other health care workers who were not on the front line to treat, mainly, the severe cases of SARS-CoV-2 infection were vaccinated even though they are not the group to be included in phase I. The Amazonas state is lacking in oxygen and with 100% capacity of ICUs, with patients under treatment and, at this moment, the vaccination is impareid62 due to bad indole of people who did not follow the immunization rules. Finally, the patients from Amazonas state were transferred to other states to be treat; but it is worrisome because in this state a new variant from the SARS-CoV-2 were identified, such as the P.1 and P.2 lineage.63–65 In both these variants, the E484K and N501K mutations were observed, and in vitro reports show that the E484K mutation is associated with low neutralization by polyclonal antibodies.64–66
(C) Vaccine quantities, transportation, and government vaccine regulatory agency
To date, Brazil started its vaccine plan for COVID-19 vaccination using the Coronavac (Sinovac™), AZD1222 (AstraZeneca/Oxford™), and BNT162b (Pfizer/BioNTech™) (Table 3); and the distribution of these vaccines will be done in phases. The vaccines were applied first in people aged ≥60 or institutionalized; institutionalized disabled people; indigenous population living on indigenous lands and for 34% of health workers who act, mainly, in the first line to treat the COVID-19 patients, that is, those considered to be a part of the first phase of the Federal plan of vaccination against COVID-19 (Table 3).25
Brazil, on 15 May 2021, accounts for a total of 84,355,357 vaccines available, however, only 51,906,448 (61.5%) were applied, being 34,985,325 corresponding to the first dose and 16,921,123 to the second dose (Table 3).13,29 Unfortunately, nearly one third of the vaccinated individuals did not receive the second dose of the vaccine, which shows that most people who have already been vaccinated are not fully immunized, as a dose is still missing. Furthermore, several media outlets stated a great number of individuals received the wrong vaccine as a second dose, even though it has been demonstrated that mixing vaccines could enhance the immune response and boost the immunization campaign, it has not yet been demonstrated for COVID-19 vaccines, since the trial have not yet been published.67–69
Several others studies also observed that the first phase of the vaccination should be directed to health-care workers who are acting in the COVID-19 frontline and to institutionalized patients,70,71 it is unquestionable that the vaccination of these groups is essential, however, we observed a mismatch in the relation of the total confirmed cases of COVID-19, total deaths by COVID-19 and the availability of vaccines. Our data suggest that several regions with a relatively lower index of confirmed COVID-19 cases, such as the Federal District, and lower index of deaths by COVID-19, such as Espírito Santo, received proportionally more vaccines than states with high index of confirmed COVID-19 cases, such as Minas Gerais state, and the index of deaths by COVID-19, such as Rio de Janeiro. The number of cases and deaths related to COVID-19, as well as the incidence, mortality, and ICUs availability (Table 2) should also be considered in the distribution of the COVID-19 vaccine, since one of the pillars of the SUS is equity,72 that is, there should an inequality between the doses distributed to the states, in order to achieve the equality. Also, in our data, curiously, the number for vaccines per 1 M inhabitants was high in states where the number of indigenous population or elderly individuals are also high (Tables 2 and 4).
The Brazilian National Immunization plan is 48 years old, and collects several achievements in the field of vaccination in Brazil.73,74 Since its creation, the prevalence of several contagious infectious diseases that can be prevented, such as measles, rubella, meningococcal disease decreased.73,75 However, perhaps one of the most impressive vaccination campaign in Brazil was accomplished in the 2009 H1N1 pandemic, in which Brazil vaccinated 93 million individuals (43% of the population) in nearly 3 months, whereas countries like USA and Mexico vaccinated, respectively, only 26% and 24% of their population.23 It is clear that Brazil is extremely efficient in vaccination campaigns, however, this is not being demonstrated in vaccination against COVID-19. It is imperative to Brazil to make the most of your ability and experience in vaccination, in order to try to increase the rate of individuals vaccinated against COVID-19.
Even though the acceptable wastage for vaccines is 1%,76 the minimum waste implicates in less individuals vaccinated, which in a pandemic era, can bring countless consequences. As observed in our data for the Brazilian territorial extension and the immunological mechanism of COVID-19 vaccine, the choice among the vaccines should be done based in the type of storage and must consider the transport conditions necessary to maintain the effectiveness of the vaccine, as well as the cost to purchase them. Then, the vaccines based on non-viral replicating viral vector and inactivated virus can be the best choice to be used on Brazil. Another aspect which contributes to a delay in a homogenously vaccination is the high variation of GDP in the states and Federal District, which could lead to a concentration of vaccines in the wealthiest states (Table 2).
For the vaccination campaign against a COVID-19 to be more effective in Brazil and overcome these adversities, Brazil’s Federal government ought to invest more in states with the lowest GDP and also invest in means of transport that can deliver the vaccines to most part of the Brazilian cities. Investment in education is more than necessary to vaccination adhesion, however, it is a long-term solution. In the short term, the Federal government should focus on mass media report about COVID-19 including the vaccination, mainly on social media and television channel, and how it is beneficial to get vaccinated, since it elevates the vaccination rates for Influenza virus.77
The ANVISA is the Brazilian regulatory agency, responsible to evaluate the vaccines and to approve or disapprove its use in national territory. In order for the ANVISA to evaluate the emergency use of a vaccine, the company that developed the vaccine ought to submit several documents, such as the vaccine description, the interaction history, the international status approval, justification for the emergency use considering the Brazilian public health context, evaluation of risks with a high benefit/risk ratio, reports of safety and immunogenicity pre clinic studies, immunogenicity report containing the results of the immunological parameters evaluated in the clinical studies, reports of the phase III clinical trials, validation reports of bioanalytical assays used to assess study outcomes phase III clinician, safety data of phase III trial with a median follow up of 2 months after de last immunization, data of efficacy and safety of the subgroup analysis, a report with all the locations where the vaccine are being or will be fabricated, information of expiration date and how to storage the vaccine, information on forecasting the quantity of finished product available for import and/or availability, bull text, plan of risk management and, finally, the informed consent form to be signed by the patient.78,79
The first two vaccines approved by the ANVISA to be used in emergency character were the Coronavac (Sinovac™) and AZD1222 (AstraZeneca/Oxford™), on January 2021, followed by the Ad26.COV2-S (Johnson/JanssenTM), on March 2021,24 however, even though this last vaccine was approved, there is no record in the ministry of health that it was distributed to the states.29 The ANVISA has only approved and registered the first vaccine on February 2021, being the BNT162b (Pfizer/BioNTech™) the first one, followed by the AZD1222 (AstraZeneca/Oxford™), registered on March 2021.80 Unfortunately, Brazil was extremely slow in approving the COVID-19 if compared to others countries, such as, the USA, which approved the first two vaccines [BNT162b (Pfizer/BioNTech™) and mRNA-1273 (ModernaTM) already on December 2020, and the United Kingdom, which also approved the vaccines on December 2020.12,81 The delay to approved the vaccines might have contributed, at least in part, for the low number of individuals vaccinated. Two more vaccines, Sputnik-V (Gamaleya Research Institute) and Covaxin (Bharat BiotechTM), are being analyzed for emergency use by the ANVISA, however they were not approved yet.13
Another important aspect that may have delayed the vaccination in Brazil is the refusal of the Federal government in the purchased of vaccines in 2020.82 In fact, the Brazilian government is being investigated for misconduct in the pandemic by a parliamentary inquiry committee (PIC), which demonstrated the Government refused to purchase nearly 70 million doses of the Pfizer/BioNtechTM vaccine in 2020,82 in fact, according to the PIC, the company, which tried to contact the Brazilian government six times, did not receive an answer regarding the purchase of the vaccines for at least 2 months.83
It is unquestionable the crucial role of the ANVISA and the Federal government in the management of the COVID-19 pandemic, however, several aspects might have contributed to a delay in the vaccination of the Brazilian population, such as the delay in the approval of the emergency use of the vaccines by the regulatory agency and the fact that only two vaccines have been approved so far, exemplifying the high rigor, perhaps unnecessary in this case, of the ANVISA. Others countries, such as USA and United Kingdom, have at least 3 vaccines totally approved, which can enhance the vaccination.12,84 Several decisions of the Federal government might have contributed to a more intense COVID-19 pandemic in Brazil85 however the lack of investment and interest in the acquisition of the 70 million doses of the vaccine, perhaps is one of the most important factor that contributed to the Brazilian low vaccination index since these vaccines could have been used to immunize a part of the Brazilian population.
Final considerations
As discussed, several factors might be associated with the poor COVID-19 vaccination campaign in Brazil, however, quite can be done to attenuate the impact of these hindrances. The investment of proper storage and proper transportation is crucial, in order to minimize the waste, to enable purchased of vaccines that need more rigorous storage, such as the Pfizer/BioNtechTM and ModernaTM and perhaps the most important to deliver the vaccines in cities be difficult to do. For the indigenous people, the Ministry of Health should consider the low access of this people to the SUS, which could decrease the immunization campaign for the indigenous, measures like vaccinations in the tribes and the active search for individuals that need vaccination and are a priority might mitigate the impact of low access to the health care system faced the indigenous. Regarding the anti-vaccine and the polarization of the vaccine, the Federal government should invest in publicity and how benefits of the vaccination can be, in order to disprove the antivaccine movement, also, the Federal government should not treat the vaccine as a social demand based on scientific evidence, since several lives depends on the vaccination. Another important aspect is the availability of the vaccines, more vaccines should be purchased and distributed, with equity based on the COVID-19 incidence and mortality, as well as ICUs availability, to the states, in order to attenuate the particularly pandemic effects of each state and Federal District. Finally, the ANVISA should be more dynamic and responsive in the analysis of a vaccine approval, since we are facing a pandemic, and the safer vaccines we have, the more lives we can protect and save.
Limitations
Our study has several limitations, for instance, (i) we were unable to summarize all the potential hindrances related to the COVID-19 vaccination in Brazil, we only discussed the main ones; (ii) the number of COVID-19 cases and deaths are underestimated in Brazil. The difficulty is the analysis of the real impact of the pandemic; (iii) the information about the loss of vaccines regarding distribution is scarce; (iv) the access of information about the analysis of new vaccines for the ANVISA is limited; (v) daily, the number of vaccines and other COVID-19 features change, which is a difficulty for the analysis of how to overcome the obstacles associates with the COVID-19 vaccination in Brazil.
Conclusions
Brazil faces hindrances to make his COVID-19 vaccination plan efficient, from geographical and sociodemographic characteristics to antivaccine movement and poorly management of the vaccines. The Federal Government must evaluate all the hindrances and propose solutions to maximize the immunization against COVID-19, because whether the immunization rate does not increase, individuals will continue to die due to this disease. In brief, Brazil started the COVID-19 vaccination on January 16, 2021, however, on May 17, 2021 only 17 million individuals were vaccinated (~8.0% of the population for the second dose), what puts Brazil behind several other countries, such as Israel, United Kingdom and USA.
Funding Statement
Matheus Negri Boschiero received an grant ID 2021/05810-7 from Fundação de Amparo à Pesquisa do Estado de São Paulo [São Paulo Research Foundation; FAPESP]
Authors’ contributions
All authors conceived of the presented idea; performed the data collection and collaborate writing the manuscript. Also, all authors discussed the results and contributed to the final manuscript.
Disclosure of potential conflicts of interest
All authors have approved the manuscript and agreed with its submission to the journal. Also, all authors wrote and revised the manuscript.
References
- 1.Archived: WHO Timeline - COVID-19 [Internet]. [accessed 2021 May 15]. https://www.who.int/news/item/27-04-2020-who-timeline—covid-19
- 2.WHO Coronavirus (COVID-19) Dashboard . WHO Coronavirus (COVID-19) Dashboard with Vaccination Data [Internet]. [accessed 2021 Apr 26]. https://covid19.who.int/
- 3.Atalan A. Is the lockdown important to prevent the COVID-19 pandemic? Effects on psychology, environment and economy-perspective. Ann Med Surg (Lond). 2020;56:38–42. doi: 10.1016/j.amsu.2020.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lotfi M, Hamblin MR, Rezaei N.. COVID-19: transmission, prevention, and potential therapeutic opportunities. Clin Chim Acta. 2020;508:254–66. doi: 10.1016/j.cca.2020.05.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Andre FE, Booy R, Bock HL, Clemens J, Datta SK, John TJ, Lee BW, Lolekha S, Peltola H, Ruff TA, et al. Vaccination greatly reduces disease, disability, death and inequity worldwide. Bull World Health Organ. 2008;86:140–46. doi: 10.2471/blt.07.040089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Plotkin S. History of vaccination. Proc Natl Acad Sci USA. 2014;111:12283–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Han S. Clinical vaccine development. Clin Exp Vaccine Res. 2015;4:46–53. doi: 10.7774/cevr.2015.4.1.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vaccines – COVID19 vaccine Tracker [Internet] [accessed 2021 May 15]. https://covid19.trackvaccines.org/vaccines/
- 9.Covid-19 vaccine tracker: latest updates - The New York Times [Internet]. [accessed 2021 May 15]. https://www.nytimes.com/interactive/2020/science/coronavirus-vaccine-tracker.html
- 10.Coronavirus (COVID-19) vaccinations - statistics and research [Internet]. Our World Data [accessed 2021 May 13]. https://ourworldindata.org/covid-vaccinations
- 11.Delany I, Rappuoli R, De Gregorio E. Vaccines for the 21st century. EMBO Mol Med. 2014;6:708–20. doi: 10.1002/emmm.201403876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Commissioner O of the. COVID-19 vaccines. FDA [Internet]; 2021. [accessed 2021 May 15]. https://www.fda.gov/emergency-preparedness-and-response/coronavirus-disease-2019-covid-19/covid-19-vaccines
- 13.Ministério da Saúde . Plano Nacional de Operacionalização da Vacinação Contra a COVID-19 [Internet]. [accessed 2021 May 15]. https://www.gov.br/saude/pt-br/vacinacao
- 14.Polack FP, Thomas SJ, Kitchin N, Absalon J, Gurtman A, Lockhart S, Perez JL, Pérez Marc G, Moreira ED, Zerbini C, et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med. 2020;383:2603–15. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Baden LR, El Sahly HM, Essink B, Kotloff K, Frey S, Novak R, Diemert D, Spector SA, Rouphael N, Creech CB, et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med. 2020;384:403–416.NEJMoa2035389. doi: 10.1056/NEJMoa2035389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Logunov DY, Dolzhikova IV, Shcheblyakov DV, Tukhvatulin AI, Zubkova OV, Dzharullaeva AS, Kovyrshina AV, Lubenets NL, Grousova DM, Erokhova AS, et al. Safety and efficacy of an rAd26 and rAd5 vector-based heterologous prime-boost COVID-19 vaccine: an interim analysis of a randomised controlled phase 3 trial in Russia. The Lancet. 2021;397(10275):671–81. doi: 10.1016/S0140-6736(21)00234-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Voysey M, Clemens SAC, Madhi SA, Weckx LY, Folegatti PM, Aley PK, Angus B, Baillie VL, Barnabas SL, Bhorat QE, et al. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. The Lancet. 2021;397:99–111. doi: 10.1016/S0140-6736(20)32661-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sadoff J, Gray G, Vandebosch A, Cárdenas V, Shukarev G, Grinsztejn B, Goepfert PA, Truyers C, Fennema H, Spiessens B, et al. Safety and efficacy of single-dose Ad26.COV2.S vaccine against COVID-19. N Engl J Med. 2021. [epub ahead of print]. doi: 10.1056/NEJMoa2101544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang Y, Zeng G, Pan H, Li C, Hu Y, Chu K, Han W, Chen Z, Tang R, Yin W, et al. Safety, tolerability, and immunogenicity of an inactivated SARS-CoV-2 vaccine in healthy adults aged 18-59 years: a randomised, double-blind, placebo-controlled, phase 1/2 clinical trial. Lancet Infect Dis. 2021;21(2):181–92. doi: 10.1016/S1473-3099(20)30843-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kaur RJ, Dutta S, Bhardwaj P, Charan J, Dhingra S, Mitra P, Singh K, Yadav D, Sharma P, Misra S. Adverse events reported from COVID-19 vaccine trials: a systematic review. Indian J Clin Biochem. 2021. [epub ahead of print]. doi: 10.1007/s12291-021-00968-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Funk CD, Laferrière C, Ardakani A. Target product profile analysis of COVID-19 vaccines in phase III clinical trials and beyond: an early 2021 perspective. Viruses. 2021;13:418. doi: 10.3390/v13030418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yang S, Li Y, Dai L, Wang J, He P, Li C, Fang X, Wang C, Zhao X, Huang E, et al. Safety and immunogenicity of a recombinant tandem-repeat dimeric RBD-based protein subunit vaccine (ZF2001) against COVID-19 in adults: two randomised, double-blind, placebo-controlled, phase 1 and 2 trials. Lancet Infect Dis. 2021. [epub ahead of print]. doi: 10.1016/S1473-3099(21)00127-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Combate à epidemia de H1N1: um histórico de sucesso . CEE Fiocruz [Internet]. [accessed 2021 May 13]. https://cee.fiocruz.br/?q=node/1314
- 24.Vacinas: uso emergencial [Internet]. Agência Nac. Vigilância Sanitária - Anvisa [accessed 2021 May 15]. https://www.gov.br/anvisa/pt-br/assuntos/paf/coronavirus/vacinas-covid/vacinas-uso-emergencial
- 25.Plano Nacional de Operacionalização da Vacinação contra Covid-19 [Internet]. Minist. Saúde [accessed 2021 May 15]. https://www.gov.br/saude/pt-br/coronavirus/vacinas/plano-nacional-de-operacionalizacao-da-vacina-contra-a-covid-19
- 26.Áreas Territoriais | instituto Brasileiro de Geografia e Estatística (IBGE) [Internet] [accessed 2021 May 15]. https://www.ibge.gov.br/geociencias/organizacao-do-territorio/estrutura-territorial/15761-areas-dos-municipios.html?=&t=o-que-e.
- 27.IBGE | portal do Instituto Brasileiro de Geografia e Estatística (IBGE) | IBGE [Internet] [accessed 2021 May 15]. https://www.ibge.gov.br/cidades-e-estados.
- 28.IBGE | portal do Instituto Brasileiro de Geografia e Estatística (IBGE) | IBGE [Internet]. [accessed 2021 May 15]. https://www.ibge.gov.br/explica/pib.php.
- 29.Localiza SUS [Internet] [accessed 2021 May 15]. https://localizasus.saude.gov.br/
- 30.Palamim CVC, Marson FAL. COVID-19 – the Availability of ICU Beds in Brazil during the Onset of Pandemic. Ann Glob Health. 2020;86:100. doi: 10.5334/aogh.3025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Largest countries in the world | statista [Internet] [accessed 2021 May 15]. https://www.statista.com/statistics/262955/largest-countries-in-the-world/
- 32.dos Santos M, Stur E, Maia LL, Agostini LP, Peterle GT, Mendes SO, Tajara EH, de Carvalho MB, Louro ID, Silva-Conforti AM. Genetic variability of inflammatory genes in the Brazilian population. Genet Test Mol Biomarkers. 2013;17:844–48. doi: 10.1089/gtmb.2013.0264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Relatórios de Pesquisa | saúde Amanhã [Internet] [accessed 2021 May 19]. https://saudeamanha.fiocruz.br/relatorios-de-pesquisa/#.YKV8QahKhik
- 34.De Albuquerque MV, d’Ávila VAL, De Lima LD, Ferreira MP, Fusaro ER, Iozzi FL. Desigualdades regionais na saúde: mudanças observadas no Brasil de 2000 a 2016. Ciênc Saúde Coletiva. 2017;22:1055–64. doi: 10.1590/1413-81232017224.26862016. [DOI] [PubMed] [Google Scholar]
- 35.Mendes MF, Pereira LR, Lima TM, Melani VF, Palamim CVC, Boschiero MN, Marson FAL. COVID-19 pandemic evolution in the Brazilian Indigenous population. J Racial Ethn Health Disparities. 2021. [online ahead of print]. doi: 10.1007/s40615-021-01031-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Da Silva NN, Favacho VBC, de Boska GA, da Andrade EC, Das Merces NP, de Oliveira MAF, Da Silva NN, Favacho VBC, de Boska GA, da Andrade EC, et al. Acesso da população negra a serviços de saúde: revisão integrativa. Rev Bras Enferm. 2020;73:e20180834. doi: 10.1590/0034-7167-2018-0834. [DOI] [PubMed] [Google Scholar]
- 37.Analfabetismo - Ministério da Educação [Internet] [accessed 2021 May 15]. http://portal.mec.gov.br/component/tags/tag/34167
- 38.Cutler DM, Lleras-Muney A. Understanding differences in health behaviors by education. J Health Econ. 2010;29:1–28. doi: 10.1016/j.jhealeco.2009.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Palamim CVC, Ortega MM, Marson FAL. COVID-19 in the indigenous population of Brazil. J Racial Ethn Health Disparities. 2020;7:1053–58. doi: 10.1007/s40615-020-00885-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cupertino GA, Do Cupertino MC, Gomes AP, Braga LM, Siqueira-Batista R. COVID-19 and Brazilian indigenous populations. Am J Trop Med Hyg. 2020;103:609–12. doi: 10.4269/ajtmh.20-0563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Davy C, Harfield S, McArthur A, Munn Z, Brown A. Access to primary health care services for Indigenous peoples: a framework synthesis. Int J Equity Health. 2016;15:163. doi: 10.1186/s12939-016-0450-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Aspin C, Brown N, Jowsey T, Yen L, Leeder S. Strategic approaches to enhanced health service delivery for Aboriginal and Torres Strait Islander people with chronic illness: a qualitative study. BMC Health Serv Res. 2012;12:143. doi: 10.1186/1472-6963-12-143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Brancalion PHS, Broadbent EN, De-miguel S, Cardil A, Rosa MR, Almeida CT, Almeida DRA, Chakravarty S, Zhou M, Gamarra JGP, et al. Emerging threats linking tropical deforestation and the COVID-19 pandemic. Perspect Ecol Conserv. 2020;18:243–46. doi: 10.1016/j.pecon.2020.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Saúde Indígena [Internet]. [accessed 2021 May 15]. http://www.saudeindigena.net.br/coronavirus/mapaEp.php
- 45.Chen S-L, Yu H, Luo H-M, Wu Q, Li C-F, Steinmetz A. Conservation and sustainable use of medicinal plants: problems, progress, and prospects. Chin Med. 2016;11:37. doi: 10.1186/s13020-016-0108-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lins TC, Vieira RG, Abreu BS, Grattapaglia D, Pereira RW. Genetic composition of Brazilian population samples based on a set of twenty-eight ancestry informative SNPs. Am J Hum Biol Off J Hum Biol Counc. 2010;22:187–92. doi: 10.1002/ajhb.20976. [DOI] [PubMed] [Google Scholar]
- 47.Walker RS, Sattenspiel L, Hill KR. Mortality from contact-related epidemics among indigenous populations in Greater Amazonia. Sci Rep. 2015;5:14032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Massey E A sermon against the dangerous and sinful practice of inoculation. Preach’d at St. Andrew’s Holborn, on Sunday, July the 8th, 1722./By Edmund Massey, M.A. Lecturer of St. Alban Woodstreet. [Internet]; 2008. http://name.umdl.umich.edu/N02782.0001.001
- 49.Hussain A, Ali S, Ahmed M, Hussain S. The anti-vaccination movement: a regression in modern medicine. Cureus. 2018;10:e2919. doi: 10.7759/cureus.2919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wakefield AJ, Murch SH, Anthony A, Linnell J, Casson DM, Malik M, Berelowitz M, Dhillon AP, Thomson MA, Harvey P, et al. Ileal-lymphoid-nodular hyperplasia, non-specific colitis, and pervasive developmental disorder in children. The Lancet. 1998;351:637–41. doi: 10.1016/s0140-6736(97)11096-0. [DOI] [PubMed] [Google Scholar]
- 51.Taylor B, Miller E, Farrington CP, Petropoulos MC, Favot-Mayaud I, Li J, Waight PA. Autism and measles, mumps, and rubella vaccine: no epidemiological evidence for a causal association. The Lancet. 1999;353:2026–29. doi: 10.1016/s0140-6736(99)01239-8. [DOI] [PubMed] [Google Scholar]
- 52.Fombonne E, Chakrabarti S. No evidence for a new variant of measles-mumps-rubella-induced autism. Pediatrics. 2001;108:E58. doi: 10.1542/peds.108.4.e58. [DOI] [PubMed] [Google Scholar]
- 53.Farrington CP, Miller E, Taylor B. MMR and autism: further evidence against a causal association. Vaccine. 2001;19:3632–365. doi: 10.1016/s0264-410x(01)00097-4. [DOI] [PubMed] [Google Scholar]
- 54.Imunizações - Taxa de Abandono - Brasil [Internet]. [accessed 2021 May 15]. http://tabnet.datasus.gov.br/cgi/dhdat.exe?bd_pni/tpnibr.def
- 55.Andamento da análise das vacinas na Anvisa [Internet]. Agência Nac. Vigilância Sanitária - Anvisa [accessed 2021 May 15]. https://www.gov.br/anvisa/pt-br/assuntos/noticias-anvisa/2020/andamento-da-analise-das-vacinas-na-anvisa.
- 56.Boschiero MN, Palamim CVC, Marson FAL. COVID-19 vaccination on Brazil and the crocodile side-effect. Ethics Med Public Health. 2021;17:100654. doi: 10.1016/j.jemep.2021.100654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Portal da Câmara dos Deputados [Internet] [accessed 2021 May 17]. https://www.camara.leg.br/proposicoesWeb/fichadetramitacao?idProposicao=2274204
- 58.Compra de vacina por empresas: projeto de lei na Câmara enfraquece Anvisa e SUS, dizem especialistas [Internet]. G1 [accessed 2021 May 17]. https://g1.globo.com/bemestar/vacina/noticia/2021/04/07/nova-lei-de-compra-de-vacina-pela-iniciativa-privada-enfraquece-anvisa-e-e-inutil-a-vacinacao-nacional-dizem-especialistas.ghtml
- 59.Dubé E, Gagnon D, MacDonald NE; SAGE Working Group on Vaccine Hesitancy . Strategies intended to address vaccine hesitancy: review of published reviews. Vaccine. 2015;33:4191–203. doi: 10.1016/j.vaccine.2015.04.041. [DOI] [PubMed] [Google Scholar]
- 60.Sadaf A, Richards JL, Glanz J, Salmon DA, Omer SB. A systematic review of interventions for reducing parental vaccine refusal and vaccine hesitancy. Vaccine. 2013;31:4293–304. doi: 10.1016/j.vaccine.2013.07.013. [DOI] [PubMed] [Google Scholar]
- 61.Weigmann K. An injection of confidence: scientists explore new and old methods to counter anti-vaccine propaganda and overcome vaccine hesitancy so as to increase vaccination rates. EMBO Rep. 2017;18:21–24. doi: 10.15252/embr.201643589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Malta M, Strathdee SA, Garcia PJ. The Brazilian tragedy: where patients living at the ‘Earth’s lungs’ die of asphyxia, and the fallacy of herd immunity is killing people. EClinicalMedicine. 2021;32:100757. doi: 10.1016/j.eclinm.2021.100757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Phylogenetic relationship of SARS-CoV-2 sequences from Amazonas with emerging Brazilian variants harboring mutations E484K and N501Y in the Spike protein - SARS-CoV-2 coronavirus/nCoV-2019 Genomic Epidemiology [Internet]. Virological; 2021. [accessed 2021 May 18]. https://virological.org/t/phylogenetic-relationship-of-sars-cov-2-sequences-from-amazonas-with-emerging-brazilian-variants-harboring-mutations-e484k-and-n501y-in-the-spike-protein/585
- 64.Genomic characterisation of an emergent SARS-CoV-2 lineage in Manaus: preliminary findings - SARS-CoV-2 coronavirus/nCoV-2019 Genomic Epidemiology [Internet]. Virological; 2021. [accessed 2021 May 18]. https://virological.org/t/genomic-characterisation-of-an-emergent-sars-cov-2-lineage-in-manaus-preliminary-findings/586
- 65.Sabino EC, Buss LF, Carvalho MPS, Prete CA Jr, Crispim MAE, Fraiji NA, Pereira RHM, Parag KV, da Silva Peixoto P, Kraemer MUG, et al. Resurgence of COVID-19 in Manaus, Brazil, despite high seroprevalence. The Lancet. 2021;397:452–55. doi: 10.1016/S0140-6736(21)00183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Greaney AJ, Loes AN, Crawford KHD, Starr TN, Malone KD, Chu HY, Bloom JD. Comprehensive mapping of mutations in the SARS-CoV-2 receptor-binding domain that affect recognition by polyclonal human plasma antibodies. Cell Host Microbe. 2021;29:463–476.e6. doi: 10.1016/j.chom.2021.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mais de 16 mil brasileiros tomaram doses trocadas da vacina contra covid: quais são os riscos? [Internet]. BBC News Bras. [accessed 2021 May 15]. https://www.bbc.com/portuguese/brasil-57033107
- 68.About Com-COV2 [Internet]. [accessed 2021 May 15]. about-com-cov2
- 69.Ledford H. Could mixing COVID vaccines boost immune response? Nature. 2021;590:375–76. doi: 10.1038/d41586-021-00315-5. [DOI] [PubMed] [Google Scholar]
- 70.Yang J, Zheng W, Shi H, Yan X, Dong K, You Q, Zhong G, Gong H, Chen Z, Jit M, et al. Who should be prioritized for COVID-19 vaccination in China? A descriptive study. BMC Med. 2021;19:45. doi: 10.1186/s12916-021-01923-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Jung J. Preparing for the Coronavirus disease (COVID-19) Vaccination: evidence, Plans, and Implications. J Korean Med Sci. 2021;36:e59. doi: 10.3346/jkms.2021.36.e59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.PROADI-SUS - Conheça os princípios do SUS, que garantem democratização da saúde brasileira [Internet]. PROADI-SUS - Conheça Os Princípios SUS Que Garantem Democr. Saúde Bras [accessed 2021 May 18]. https://hospitais.proadi-sus.org.br/noticias/130/conheca-os-principios-do-sus-que-garantem-democratizacao-da-saude-brasileira
- 73.Domingues CMAS, Maranhão AGK, Teixeira AM, Fantinato FFS, Domingues RAS, Domingues CMAS, Maranhão AGK, Teixeira AM, Fantinato FFS, Domingues RAS. The Brazilian national immunization program: 46 years of achievements and challenges. Cad Saúde Pública. 2020;36:e00222919. doi: 10.1590/0102-311X00222919. [DOI] [PubMed] [Google Scholar]
- 74.Domingues CMAS, da Teixeira AMS. Coberturas vacinais e doenças imunopreveníveis no Brasil no período 1982-2012: avanços e desafios do Programa Nacional de Imunizações. Epidemiol Serv Saúde. 2013;22:9–27. doi: 10.5123/S1679-49742013000100002. [DOI] [Google Scholar]
- 75.SINANWEB - Dados Epidemiológicos Sinan [Internet]. [accessed 2021 May 19]. http://portalsinan.saude.gov.br/dados-epidemiologicos-sinan
- 76.WHO, World Health Organization . Monitoring vaccine wastage at country level : guidelines for programme managers; 2005. [accessed 2021 May 15]. https://apps.who.int/iris/handle/10665/68463
- 77.Yoo BK, Holland ML, Bhattacharya J, Phelps CE, Szilagyi PG. Effects of Mass media coverage on timing and annual receipt of Influenza vaccination among medicare elderly. Health Serv Res. 2010;45(5Pt1):1287–309. doi: 10.1111/j.1475-6773.x2010.01127.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Nacional I RESOLUÇÃO RDC No 475, DE 10 DE Março DE 2021 - DOU - Imprensa Nacional [Internet]. [accessed 2021 May 17]. https://www.in.gov.br/web/dou
- 79.Agência Nacional de Vigilância Sanitária - Anvisa [Internet]. [accessed 2021 May 17]. https://webcache.googleusercontent.com/search?q=cache:rfy9Cg_s_0kJ:https://www.gov.br/anvisa/pt-br/centraisdeconteudo/publicacoes/medicamentos/publicacoes-sobre-medicamentos/guia-sobre-os-requisitos-minimos-para-submissao-de-solicitacao-de-autorizacao-temporaria-de-uso-emergencial-em-carater-experimental-de-vacinas-covid-19+&cd=1&hl=pt-BR&ct=clnk&gl=br
- 80.Registros [Internet]. Agência Nac. Vigilância Sanitária - Anvisa [accessed 2021 May 15]. https://www.gov.br/anvisa/pt-br/assuntos/paf/coronavirus/vacinas-covid/registros
- 81.UK medicines regulator gives approval for first UK COVID-19 vaccine [Internet] . GOV.UK [accessed 2021 May 15]. https://www.gov.uk/government/news/uk-medicines-regulator-gives-approval-for-first-uk-covid-19-vaccine
- 82.CPI da COVID: governo Bolsonaro recusou 11 vezes ofertas para compras de vacina [Internet]. G1 [accessed 2021 May 16]. https://g1.globo.com/politica/blog/octavio-guedes/post/2021/04/27/cpi-da-covid-governo-bolsonaro-recusou-11-vezes-ofertas-para-compras-de-vacina.ghtml
- 83.Não acompanhou a CPI da Covid? Veja o que rolou até aqui [Internet]. G1 [accessed 2021 May 16]. https://g1.globo.com/politica/noticia/2021/05/15/cpi-da-covid-principais-pontos.ghtml
- 84.Coronavirus (COVID-19) vaccine [Internet]. nhs.uk2020 [accessed 2021 May 17]. https://www.nhs.uk/conditions/coronavirus-covid-19/coronavirus-vaccination/coronavirus-vaccine/
- 85.Boschiero MN, Palamim CVC, Ortega MM, Mauch RM, Marson FAL. One year of Coronavirus disease 2019 (COVID-19) in Brazil: a political and social overview. Ann Glob Health. 2021;87:44. doi: 10.5334/aogh.3182. [DOI] [PMC free article] [PubMed] [Google Scholar]


