Abstract
Gut-related symptoms and an increase in markers of gut dysfunction have been observed in patients with chronic obstructive pulmonary disease (COPD). It remains unclear whether exercise, in relation to inducing hypoxia, plays a role in disturbances in protein digestion and amino acid absorption and whole body protein kinetics. Sixteen clinically stable patients with moderate-to-very severe COPD and 12 matched healthy subjects completed the study. Protein digestion and amino acid absorption, whole body protein kinetics were measured in the postabsorptive state via a continuous infusion of stable tracers in combination with orally administered stable tracer sips during 20 min of walking exercise and up to 4 h post exercise. In addition, concentrations of short-chain fatty acid (SCFA) and amino acids were measured. Patients with COPD completed one study day, walking at maximal speed, whereas healthy subjects completed two, one matched to the speed of a patient with COPD and one at maximal speed. The patients with COPD tolerated 20 min of vigorous intensity walking with an elevated heart rate (P < 0.0001) and substantial desaturation (P = 0.006). During exercise, we observed lower protein digestion (P = 0.04) and higher SCFA acetate (P = 0.04) and propionate (P = 0.02) concentrations on max speed study days, lower amino acid absorption (P = 0.004) in subjects with oxygen desaturation, and lower net protein breakdown (P = 0.03) and propionate concentrations (P = 0.04) in patients with COPD. During late recovery from exercise, amino acid absorption (P = 0.02) and net protein breakdown (P = 0.02) were lower in patients with COPD. Our data suggest that 20 min of walking exercise is sufficient to cause perturbations in gut function and whole body protein metabolism during and up to 4 h post exercise in older adults and in patients with COPD with exercise-induced hypoxia.
NEW & NOTEWORTHY Gut function is disturbed in older adults with COPD. As exercise is the cornerstone of pulmonary rehabilitation in COPD, knowledge of the response of the gut to aerobic exercise is of importance.
Keywords: aerobic exercise, amino acid absorption, COPD, protein digestion
INTRODUCTION
Chronic obstructive pulmonary disease (COPD) is a chronic low-grade inflammatory disease, negatively affecting the pulmonary but also extrapulmonary compartments like the gut. Gastrointestinal diseases are prevalent in patients with COPD (1), with gastroesophageal reflux (2) and inflammatory bowel diseases being 55% higher than in the general population (3), leading to increased mortality (4). Although studies examining the underlying pathology are limited, recent animal models of COPD revealed structural changes in the intestinal mucosal barrier (5), intestinal ischemia and epithelial barrier dysfunction (6).
Research studies in human clinical populations have employed a variety of methods to measure gastrointestinal function. In patients with COPD, higher small intestine passive permeability was observed using oral inert sugars intake in response to various stressors such as exacerbations (7) and household activities, as a model of daily physical exercise (8). We recently confirmed impaired gut function in patients with COPD using novel stable tracer approaches (9).
In young adults, strenuous exercise induces intestinal mucosal damage and lower postmeal plasma essential amino acid concentrations were suggested to indicate an attenuation of the anabolic response to a bolus intake of intrinsically labeled casein protein (10). Using a novel oral stable tracer method, utilizing a combination of labeled spirulina protein and phenylalanine, we were able to quantify the reduction in protein digestion in patients with cystic fibrosis (11) and COPD (9). Recently in pigs, we extended this method by adding the inert amino acid l-allo-isoleucine to simultaneously estimate the absorption of amino acids by the intestinal enterocytes (12). This method measures amino acid absorption by the enterocytes as there is no intestinal metabolism of l-allo-isoleucine.
We have previously shown that 20 min of submaximal aerobic exercise was able to alter whole body protein metabolism in patients with COPD for up to 3 h post exercise in the fasted (13) and fed (14) state. We also observed that exercise induced an increase in whole body protein breakdown in COPD during high quality protein feeding (14) which was associated with changes in splanchnic extraction. However, combining nutrition with exercise makes determining the effect of exercise separately difficult. Therefore, in the present study, we took the approach to measure the effects of exercise in the postabsorptive state on protein digestion and amino acid absorption in COPD.
In healthy subjects, diminished perfusion of the gut and intestinal damage has been observed during strenuous physical exercise (15, 16) and at high altitude (17). In these studies and in patients with COPD (8), hypoxia was suggested to play a role behind the observed exercise-induced gut dysfunction.
Recently, changes in the gut microbiome in relation to gut health in COPD has gained interest, specifically the microbiome’s ability to alter circulating concentrations of metabolites such as short-chain fatty acids (acetate, butyrate, propionate; SCFA) (18). Additionally, microbiota-derived butyrate can act as a hypoxia signal in the gut to increase epithelial barrier function (19) whereas endurance performance, which was reduced with antibiotic treatment, can be recovered with an intravenous infusion of acetate (20). We hypothesized that exercise induces perturbations in SCFA concentrations in patients with COPD with a potential influence of hypoxia.
To better understand gut function in patients with COPD in the postabsorptive state and the effect of exercise, we examined gut function by studying simultaneously protein digestion, amino acid absorption, and whole body protein kinetics in response to 20 min of treadmill walking exercise using a panel of comprehensive tracer-based methods. Additionally, we evaluated the concentration of SCFA in plasma as a marker of gut microbiome health. We studied these endpoints in moderate-to-severe patients with COPD and compared those with a group of healthy matched subjects. We hypothesized that protein digestion and amino acid absorption would be reduced following walking exercise in patients with COPD.
MATERIALS AND METHODS
Subjects
Sixteen older adults with a clinical diagnosis of moderate-to-severe airflow obstruction (grade 2–4), according to the established Global Initiative for Chronic Obstructive Pulmonary Disease (GOLD) guidelines (21), and 12 healthy older adults completed the study protocol. All subjects were studied at the Clinical Research Unit of the Center for Translational Research on Aging and Longevity at Texas A&M University. Subjects were recruited via advertisements in the surrounding hospitals and local community. Medical history and medication use were assessed as part of the screening process. Exclusion criteria for all subjects were preexistent untreated metabolic or renal disease (including diabetes mellitus requiring daily insulin administration), malignancy, recent surgery, and ability to walk independently. Healthy subjects specifically were not diagnosed with airflow obstruction and were permitted medication use for common indications such as blood pressure and cholesterol. Patients with COPD were clinically stable and not suffering from an acute exacerbation, infection, or use of systemic corticosteroids for at least 4 wk before the study. Written informed consent was obtained from all subjects, and the study was approved by the Institutional Review Board of Texas A&M University.
Anthropometrics, Body Composition, and Lung Function
Body weight and height were measured by a digital beam scale and stadiometer, respectively, and regional values for fat mass and fat-free mass were obtained by dual-energy X-ray absorptiometry (DXA; Hologic QDR 4500/v 12.7.3.1; Bedford, MA). Anthropometric and body composition measures were standardized for height, to obtain body mass index (BMI), fat-free mass index (FFMI), fat mass index (FMI), and appendicular skeletal muscle index (ASMI). Postbronchodilator forced expiratory volume in 1 s (FEV1) was assessed by spirometry, with the highest value from ≥3 technically acceptable maneuvers.
Amino Acid Tracer Administration Protocol
Each study day started in the early morning after an overnight fast, and lasted 7 h, during which participants continued their fast. Catheters were inserted into an antecubital vein in both lower arms/hands, for blood draws and continuous stable isotope tracer infusion. A visual of the isotope model can be found in Fig. 1. A blood sample was taken pre-infusion to measure natural background isotope enrichment. At time t = −180 min, we started a continuous intravenous infusion of l-[13C9]-phenylalanine (infusion rate = 11.12 μmol/h) to assess whole body protein metabolism and l-allo-[2H10-15N]-isoleucine (infusion rate = 9.67 μmol/h) to assess amino acid absorption (healthy, n = 12; COPD, n = 16). However due to limited isotope availability, the l-[13C9]-phenylalanine tracer was only used for a subset of subjects (healthy, n = 7; COPD, n = 7). At the same time, oral sips were started (every 20 min) of 15n-spirulina protein (17.96 μmol/h, spirulina protein that contains ∼2.5% l-[15N]-phenylalanine) and an oral phenylalanine: as either l-[1-13C]-phenylalanine (9.14 μmol/h) or l-[13C6]-phenylalanine (51.18 μmol/h) and l-allo-[13C6]-Isoleucine (9.16 μmol/h) to assess protein digestion and amino acid absorption, respectively (healthy, n = 12; COPD, n = 16; Cambridge Isotope Laboratories, Woburn, MA). The blood draw hand was placed in a thermostatically controlled heated box (internal temperature, 60°C), a technique to mimic direct arterial sampling (22, 936–940). We obtained arterialized-venous blood samples at t = −180, −60, −20, 0, 10, 20, 40, 80, 120, 140, 180, and 240 min for the measurement of isotope enrichment values and SCFA concentrations.
Figure 1.
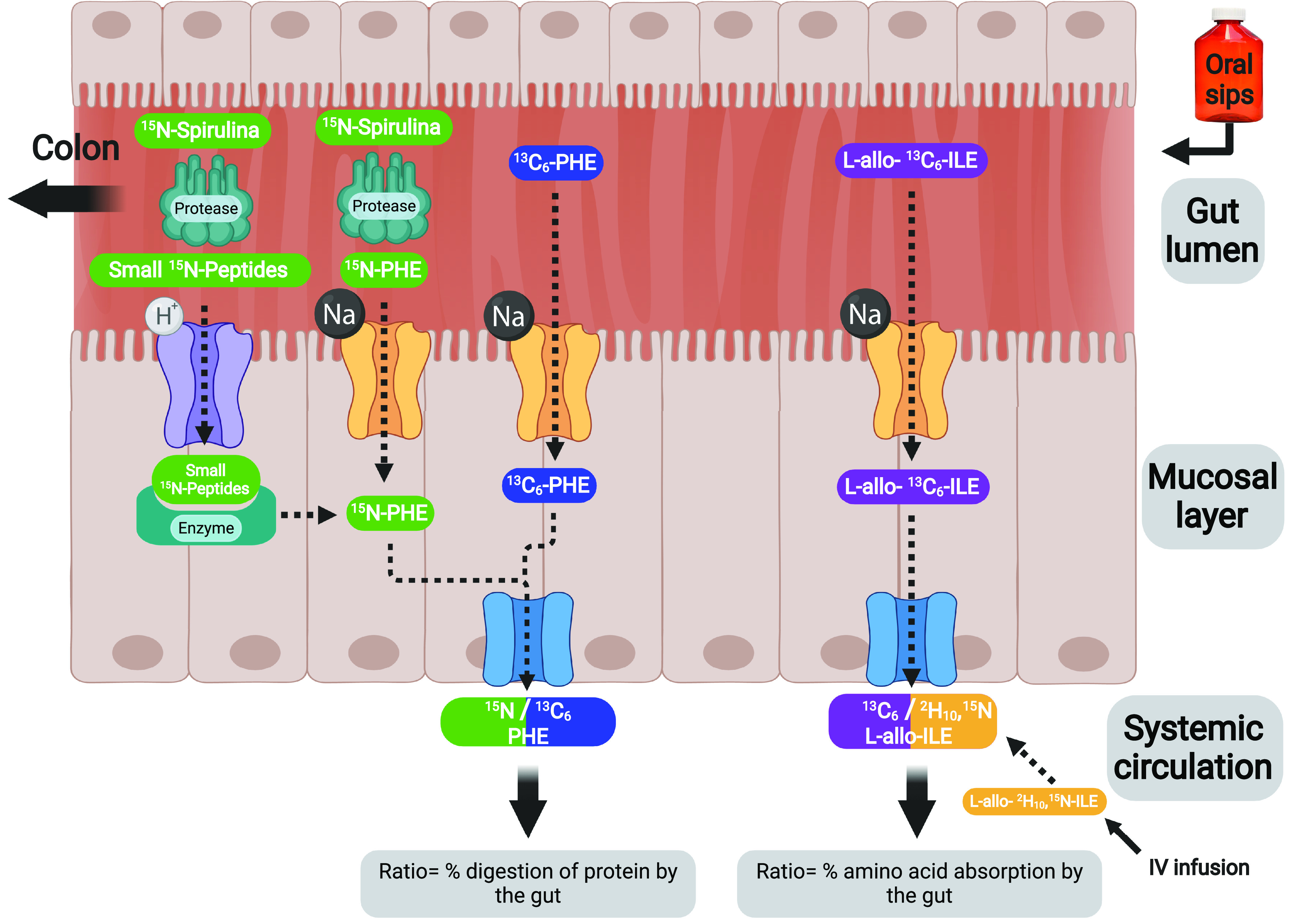
The basic physiological model of oral sips and intravenous infusion of stable isotope tracers to simultaneously measure protein digestion and amino acid absorption. The protein bound amino acids (15N-labeled spirulina), free amino acid (13C6 or 1-13C phenylalanine), and free inert amino acid (l-allo-[13C6]-Isoleucine) were given orally, whereas the free inert amino acid (l-allo-[2H10;15N]-Isoleucine) was given as iv Created with BioRender.com.
Exercise Protocol
At the screening visit, the subjects received 20 min of familiarization with the motorized treadmill (TT8, Sole Fitness, Jonesboro). Subjects were allowed to adjust the speed to become familiar with various intensities. On the study day, at 180 min into infusion of the stable tracers (t = 0 min), all subjects performed an exercise bout on the same treadmill for 20 min. Instructions were given to maintain the desired walking speed throughout the bout with standardized verbal encouragement (e.g., “keep going,” “you’re doing great,” “good job”). Patients with COPD completed one study day and were given instructions to maintain the fastest walking speed possible throughout the bout (Max Speed).
Healthy subjects completed two study days. On their first day, they walked the same speed of a patient with COPD matched for gender and BMI (Matched Speed). On their second day, they were given the same instructions as those with COPD, e.g., to maintain the fastest speed possible (Max Speed) during the 20 min of treadmill exercise. In this way, the healthy subjects walked at two different workloads: one at the same absolute workload (Max Speed) as the patients with COPD and the one at the same relative workload (Matched Speed). The same absolute work rate was done because in real-life situations, subjects perform absolute work, whereas the same relative exercise intensity was selected to induce a comparable physical stress in both groups. Oxygen saturation, heart rate, perceived exertion, and dyspnea were measured throughout the exercise protocol.
Biochemical Analysis and Calculations
Arterialized-venous blood was put in Li-heparinized tubes (Becton Dickinson Vacutainer system, Franklin Lakes, NJ), immediately put on ice to minimize enzymatic reactions, and centrifuged to obtain plasma. A part of the plasma was aliquoted into tubes with trichloroacetic acid for denaturation of proteins. Samples were immediately frozen and stored at −80°C until further analysis. Tracer enrichments [tracer:tracee ratio (TTR)] and plasma SCFA concentrations were analyzed batch-wise by LC-MS/MS or GC-MS, respectively.
For metabolic calculations we used previously described steady state tracer dilution equations to calculate whole body protein breakdown (23) and whole body net protein breakdown (24), protein digestion (9, 11), and amino acid absorption (12):
1) Whole body protein breakdown = (1/lean body mass) × (I/Art-PHE-cTTR)
2) Whole body net protein breakdown = (whole body protein breakdown × 0.73) × (l-[13C9]-tyrosine-cTTR/Art-PHE-cTTR)
3) Protein digestion = plasma (BoundOral-PHE-cTTR/Oral-PHE-cTTR)/given (BoundOral-PHE/Oral-PHE)
4) Amino acid absorption = plasma (Art-allo-ILE-cTTR/Oral-allo-ILE-cTTR)/given (Art-allo-ILE/Oral-allo-ILE)
Where lean body mass is total lean body mass in kilograms, I = rate of continuous infusion of l-[13C9]-phenylalanine, and Art-PHE-cTTR is the arterialized plasma TTR of l-[13C9]-phenylalanine corrected for natural background abundance. Whole body protein breakdown is corrected by 0.73 to represent the ratio of the fluxes of tyrosine and phenylalanine arising from protein catabolism (24) and l-[13C9]-tyrosine-cTTR is the arterialized plasma TTR of l-[13C9]-tyrosine, coming from l-[13C9]-phenylalanine, corrected for natural background abundance. BoundOral-PHE-cTTR is the arterialized plasma TTR of l-[15N]-phenylalanine, from 15n-spirulina, whereas Oral-PHE-cTTR is the arterialized plasma TTR of either l-[1-13C]-phenylalanine or l-[13C6]-phenylalanine, all corrected for natural background abundance. This ratio is corrected for the amount of l-[15N]-phenylalanine and either l-[1-13C]-phenylalanine or l-[13C6]-phenylalanine in the oral sips as BoundOral-PHE or Oral-PHE, respectively. Art-allo-ILE-cTTR is the arterialized plasma TTR of l-allo-[2H10;15N]-isoleucine, whereas Oral-allo-ILE-cTTR is the arterialized plasma TTR of l-allo-[13C6]-Isoleucine, both corrected for natural background abundance. This ratio is corrected for the amount of l-allo-[2H10-15N]-Isoleucine in the infusion bag and l-allo-[13C6]-Isoleucine in the oral sips, Art-allo-ILE and Oral-allo-ILE, respectively.
To measure acetate, propionate, and butyrate concentrations, plasma was derivatized with pentafluorobenzyl bromide (PFB-Br) in acetone before extraction with hordenine (9). PFB-hordenine was extracted with sulfuric acid in water, whereas SCFAs were extracted into an organic phase with hexane. Samples were analyzed with a 15 m poly stationary phase column (Supelco, Inc. Bellefonte, PA) on a SCION 436-GC gas chromatograph with programmable temperature vaporization injection, CP-8400 autosampler, and SCION TQ Triple Quadrupole Mass Spectrometer (Bruker, Billerica).
Statistical Analysis
Results are expressed as means ± SE. We compared the clinical characteristics of the study populations with the unpaired Student’s t test. Variables obtained in response to exercise like heart rate, oxygen saturation, exertion, and dyspnea were compared with two-way repeated-measures analysis of variance (RM-ANOVA) with “group” (COPD Max Speed, Healthy Matched Speed, Healthy Max Speed) and “time” (0, 5, 10, 15, and 20 min into exercise) as main factors and post hoc correction for multiple comparisons via the two-stage step-up method of Benjamini et al. (25).
Three distinct periods were defined for analyzing metabolic data (e.g., protein digestion, amino acid absorption, whole body protein kinetics, and SCFA concentrations). Area under the curve calculation was performed on a per subject basis for protein digestion, amino acid absorption, and whole body protein kinetics to enable calculation of the average value for each period. Pre-exercise period values were calculated between t = −60 and t = −20 min (= 120–160 min after the start of the isotope protocol). During-exercise period values were calculated between t = 0 and t = 40 min (= 0–40 min after the start of the treadmill walking exercise). We included the 20-min period after the treadmill walk ended because subjects had to walk from the exercise testing area to the Clinical Research Unit, at their own pace, to get settled to continue the study day. Late recovery from exercise period values was calculated between t = 80 and t = 240 min (= 60–220 min after the end of the treadmill walking exercise).
To examine group differences across these periods, the analysis of covariance (ANCOVA) procedure was used. Metabolic data obtained during and late recovery from exercise were used as the dependent variables (e.g., protein digestion, amino acid absorption, protein breakdown, and net protein breakdown). As dummy variables, we used the presence of COPD (0 = no, 1 = yes) and speed (0 = matched, 1 = max), and as covariates, we used pre-exercise values, gender, BMI, age, and level of exercise-induced oxygen desaturation. For analyzing SCFA concentrations, we used the individual time points t = −180, 20, 120, and 240. The level of significance was set at P < 0.05. The statistical package within Graphpad Prism (v 8.2.0, GraphPad Software Inc, San Diego) was used for data analysis.
RESULTS
General Characteristics
Patients with COPD were characterized by moderate-to-severe airflow obstruction and increased self-reported shortness of breath (Table 1). The average age of all participants was around 70 yr and body composition and gender distribution were comparable.
Table 1.
Subject characteristics and distance walked
| Healthy (n = 12 subjects) | COPD (n = 16 patients) | |
|---|---|---|
| General characteristics | ||
| Age, y | 68.1 ± 1.7 | 70.7 ± 1.8 |
| Gender, n; female/male | 7/5 | 9/7 |
| Body mass index, kg/m2 | 27.5 ± 1.2 | 29.9 ± 1.7 |
| Fat free mass index, kg/m2 | 17.7 ± 0.7 | 19.1 ± 1.0 |
| Fat mass index, kg/m2 | 9.0 ± 0.9 | 10.9 ± 1.1 |
| Charlson Comorbidity Index, score | 0.42 ± 0.23 | 1.63 ± 0.26** |
| Pulmonary function and COPD-related measures | ||
| FEV1, % of predicted | 91.3 ± 3.9 | 42.9 ± 4.7*** |
| FVC, % of predicted | 86.3 ± 2.7 | 54.7 ± 4.0*** |
| FEV1/FVC, ratio | 80.0 ± 2.3 | 56.7 ± 3.2*** |
| Oxygen saturation, % | 96.8 ± 0.4 | 95.7 ± 0.8 |
| mMRC dyspnea scale | 0 ± 0.0 | 2.2 ± 0.3*** |
| GOLD stage, n; I, II, III, IV | 0 | 1,2,7,5 |
| Distance walked | ||
| Distance walked, m | Matched Speed: 832 ± 91***; Max Speed: 1,827 ± 82 | Matched Speed: 778 ± 113*** |
Data are means ± SE. Statistics are by unpaired Student’s t test or one-way ANOVA. **P < 0.01 denotes difference from healthy. ***P < 0.001 denotes difference from healthy. COPD, chronic obstructive pulmonary disease; FEV1, forced expiratory volume in 1 s; FVC, forced vital capacity; mMRC, modified Medical Research Council.
Physiological Response to Walking Exercise
Patients with COPD covered 832 ± 91 m (range, 97–1,416 m) during their Max Speed walking exercise. Healthy subjects covered 1,827 ± 82 m (range, 1,400–2,237 m) on their Max Speed study day, on average more than twice as far as patients with COPD in the same amount of time (Table 1). On Max Speed study days, patients with COPD and healthy subjects showed continuously increased heart rate throughout exercise (P < 0.0001), whereas healthy subjects on their Matched Speed study day showed increased heart rate after 5 min (P = 0.002) but then plateaued (Fig. 2A). This translated to a 40% and 44% increase in heart rate in COPD and healthy Max Speed study days, respectively, but only a 26% increase in Healthy Matched Speed. Transcutaneous O2 saturation dropped within 5 min after the start of exercise in COPD Max Speed study days (P = 0.006) and remained lower throughout (Fig. 2B). A similar trend was seen for Healthy Max Speed study days (P = 0.04) but at higher absolute saturation (96% vs. 94%). No change in saturation was seen on Healthy Matched Speed study days. Dyspnea and exertion during exercise were continuously elevated to the same extent on Max Speed study days for patients with COPD (P = 0.0007 and P = 0.002, respectively) and healthy subjects (P = 0.0004 and P < 0.0001, respectively) but unchanged for Healthy Matched Speed (Fig. 2C and D).
Figure 2.

Change in heart rate, oxygen saturation, and physical symptoms in patients with COPD and healthy subjects during 20 min of walking exercise. A: heart rate. B: oxygen saturation. C: perceived exertion. D: dyspnea. Healthy matched speed (n = 12 subjects), COPD max speed (n = 16 patients), healthy max speed (n = 11 subjects). Means ± SE, statistics were obtained by two-way repeated-measures analysis of variance, with “group” and “time” as main factors with post hoc correction for multiple comparisons via the two-stage step-up method of Benjamini et al. A: there was a significant time effect during exercise across study days (†††P < 0.0001). There was a significant group effect between study days (##P = 0.002). B: there was a significant time effect during exercise across study days (††P = 0.001). There was a significant group effect between study days (P = 0.003, ##). C: there was a significant time effect during exercise across study days (†††P < 0.0001). There was a significant group effect between study days (###P < 0.0001). D: there was a significant time effect during exercise across study days (†††P < 0.0001). There was a significant group effect between study days (###P < 0.0001). COPD, chronic obstructive pulmonary disease.
Protein Digestion and Amino Acid Absorption in Response to Walking Exercise
Protein digestibility pre-exercise was >85%, with no significant difference between study days (Fig. 3A). During exercise, protein digestion was 11% lower on Max Speed study days (P = 0.04) and in all subjects with less exercise-induced oxygen desaturation (P = 0.04). However, there were no significant effects during the late recovery period.
Figure 3.
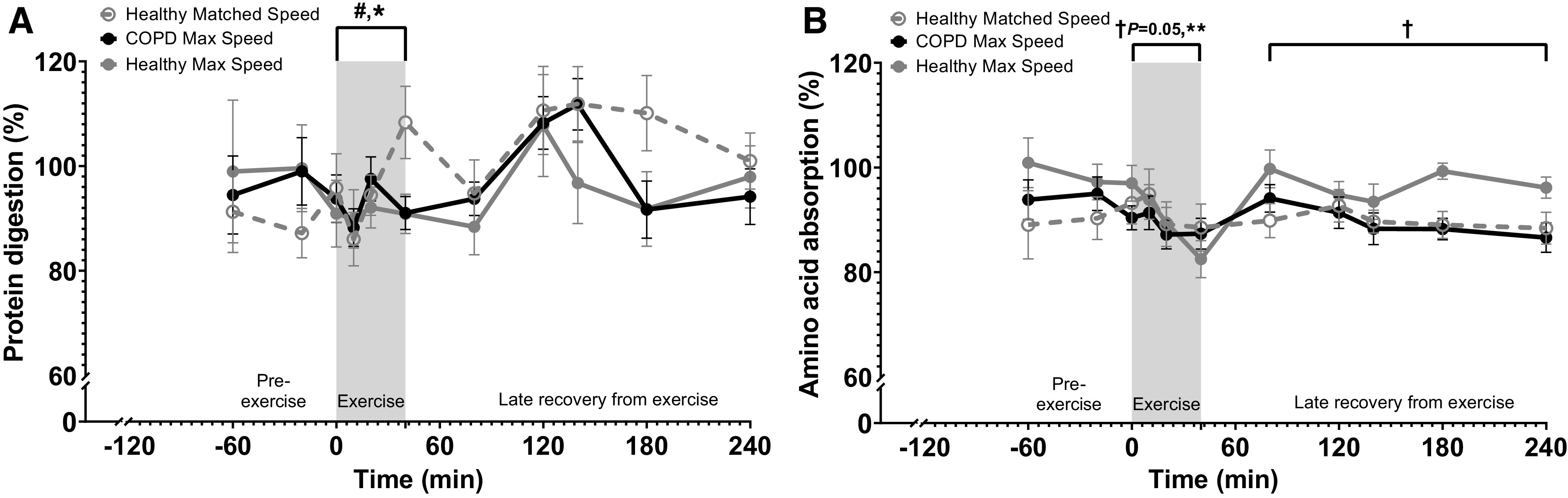
Change in gut function in patients with COPD and healthy subjects before, during, and after 20 min of walking exercise. A: protein digestion. B: amino acid absorption. Healthy Matched Speed (n = 12 subjects), COPD Max Speed (n = 16 patients), and Healthy Max Speed (n = 11 subjects). Means ± SE, statistics were obtained by analysis of covariance with COPD, speed, pre-exercise values, gender, BMI, age, and level of exercise-induced oxygen desaturation. A: pre-exercise digestion was not different among study days. There were significant speed (#P < 0.05) and oxygen desaturation (*P < 0.05) effects during exercise (A). Pre-exercise absorption was not different among study days (B). There was a significant oxygen desaturation (**P < 0.01) effect and trend for COPD (†P = 0.05) effect during exercise. There was a significant COPD (†P < 0.05) effect during late recovery from exercise. BMI, body mass index; COPD, chronic obstructive pulmonary disease.
Amino acid absorption pre-exercise was also >85%, with no significant difference between study days (Fig. 3B). During exercise, amino acid absorption was lower in all subjects with exercise-induced oxygen desaturation (P = 0.004) and tended to be lower in patients with COPD (P = 0.05). During late recovery from exercise, absorption was lower in patients with COPD (P = 0.02).
Whole Body Protein Turnover in Response to Walking Exercise
Whole body protein breakdown over time (Fig. 4A) did not change during exercise or in the late recovery period from exercise. During exercise, whole body net protein breakdown was lower in patients with COPD (P = 0.03) (Fig. 4B), which remained during the late recovery period (P = 0.02).
Figure 4.
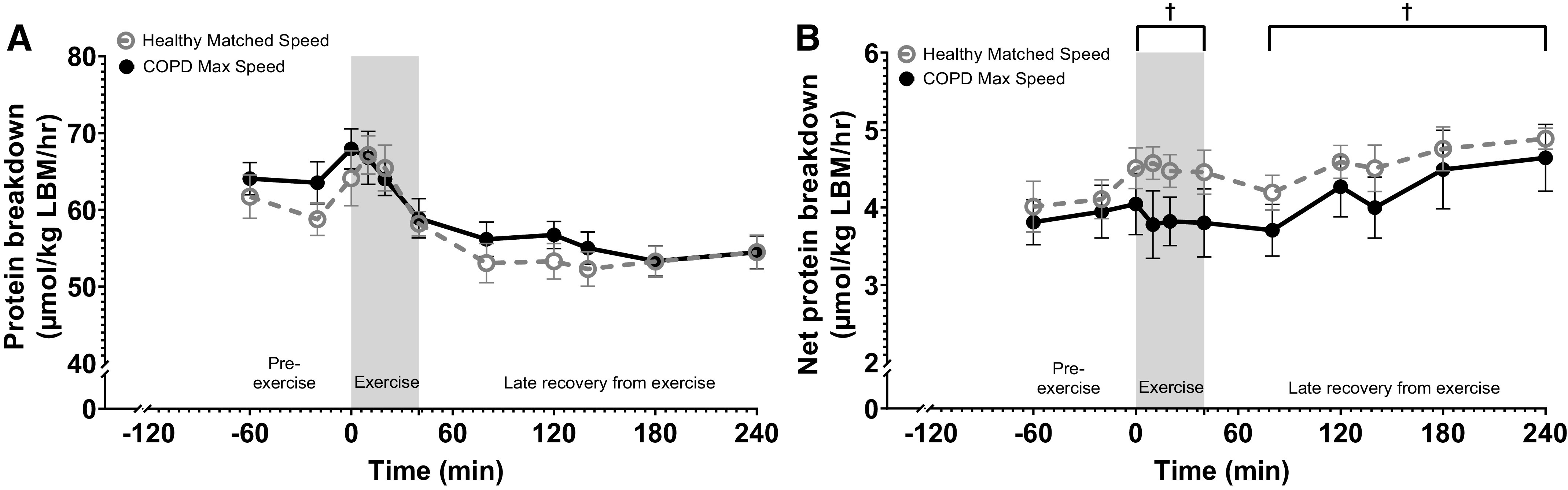
Change in whole body protein kinetics in patients with COPD and healthy subjects before and after 20 min of walking exercise. A: whole body protein breakdown. B: whole body net protein breakdown. Healthy Matched Speed (n = 7 subjects) and COPD Max Speed (n = 7 patients). Means ± SE, statistics were obtained by analysis of covariance with COPD, speed, pre-exercise values, gender, BMI, age, and level of exercise-induced oxygen desaturation. Protein breakdown was not different throughout the study day (A). There was a significant COPD (†P < 0.05) effect during exercise that remained during the late recovery period (†P < 0.05) (B). BMI, body mass index; COPD, chronic obstructive pulmonary disease.
Plasma Short-Chain Fatty Acid Concentrations
Pre-exercise plasma SCFA concentrations were not different across study days (Fig. 5A, B, and C). During exercise, acetate (P = 0.04) and propionate (P = 0.02) concentrations increased on Max Speed study days, whereas butyrate remained unchanged. Additionally, patients with COPD had lower propionate concentrations (P = 0.04) and tended to be lower in all subjects with exercise-induced oxygen desaturation (P = 0.05). There were no significant differences during the late recovery period.
Figure 5.
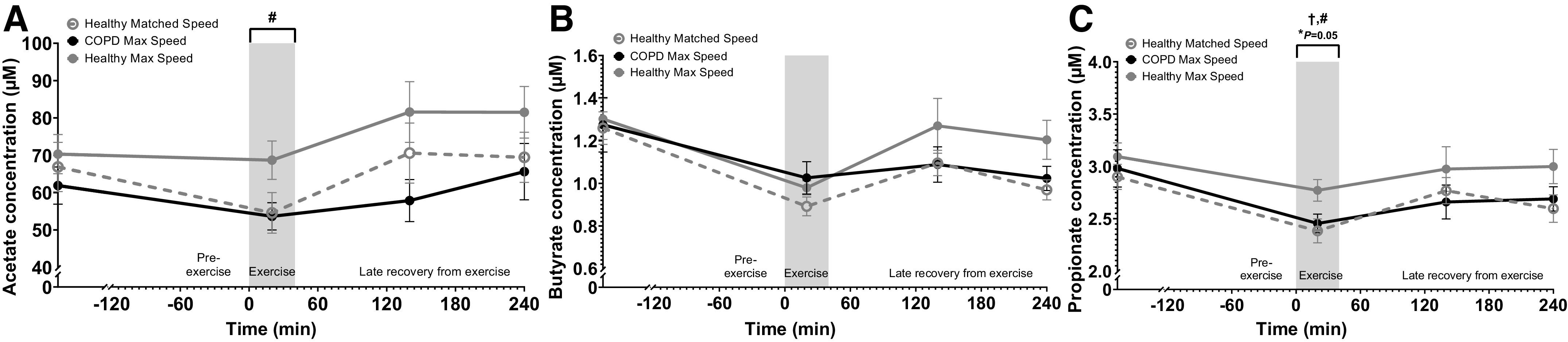
Change in plasma short-chain fatty acid concentrations in patients with COPD and healthy subjects before and after 20 min walking exercise. A: acetate. B: butyrate. C: propionate. Healthy Matched Speed (n = 12 subjects), COPD Max Speed (n = 16 patients), and Healthy Max Speed (n = 11 subjects). Means ± SE, statistics were obtained by analysis of covariance with COPD, speed, pre-exercise values, gender, BMI, age, and level of exercise-induced oxygen desaturation. Pre-exercise concentrations were not different among study days (A). There was a significant speed (#P < 0.05) effect during exercise. Butyrate was not different throughout the study day (B). There were significant COPD (†P < 0.05) and speed ( #P < 0.05) effects and trend for oxygen desaturation (*P = 0.05) effect during exercise (C). BMI, body mass index; COPD, chronic obstructive pulmonary disease.
DISCUSSION
We studied in patients with COPD and matched healthy subjects the effects of 20 min of walking exercise in the postabsorptive state on gut function using a panel of comprehensive stable tracer methods. Although the average drop in oxygen saturation during-exercise in our COPD group was only 2%, 56% of our patients desaturated >4%, indicating an individual response of exercise-induced hypoxia (26). During exercise, we observed lower protein digestion rate and higher acetate and propionate concentrations on Max Speed study days, lower amino acid absorption particularly in subjects with more oxygen desaturation, and lower net protein breakdown and propionate concentrations in patients with COPD. During late recovery from exercise, amino acid absorption and net protein breakdown were lower in patients with COPD. These data suggest that 20 min of walking exercise is sufficient to cause perturbations in gut function and whole body protein metabolism during and up to 4 h postexercise in older adults and patients with COPD, with exercise-induced hypoxia playing a role.
Physiological Responses to Walking Exercise in Patients with COPD
The American College of Sports Medicine (ACSM) guidelines consider an exercise intensity of 60%–80% peak work rate and 4–6 on a Borg CR10 scale to be vigorous for aerobic exercise in patients with COPD (27). Our patients with COPD reached 60% of their maximum predicted heart rate (28) just 5 min after the start of exercise and reached a maximum of 75% by the end. The 20 min of vigorous aerobic exercise in the COPD group was achieved on a self-selected speed after treadmill familiarization, which supports previous studies in older adults on the validity of self-selected speed to meet ventilatory threshold effort (29). Used modality (flat treadmill walking) and duration of exercise (20 min) are commonly used in pulmonary rehabilitation programs with patients with COPD (30), supporting our protocol as easily translatable to exercise in the clinic and daily life in patients with COPD.
Response of Protein Digestion to Walking Exercise
Protein digestion during exercise was 11% lower in subjects walking under Max Speed conditions, suggestive of an intensity dependent rapid disturbance of gut function during the short walking period. Hypoperfusion and signs of enterocyte damage were shown to occur in the gut of fasted subjects during and shortly after endurance exercise (16). In agreement with our data, these disturbances were normalized within 2 h post exercise, and thus, it appears that the gut is able to restore protein digestion shortly after exercise. Another study utilizing resistance exercise showed similar acute signs of enterocyte damage in addition to reduced circulating amino acids following postexercise nutrition supplementation (10). However, resistance exercise was also seen to have no effect on circulating amino acids following post exercise supplementation (31). Thus, postexercise protein digestion appears to be meal and exercise type-dependent. Future studies would be valuable to assess different meal and exercise types in diseased patients.
Response of Amino Acid Absorption to Walking Exercise
We observed significantly lower amino acid absorption during exercise in all subjects with exercise-induced oxygen desaturation along with lower absorption during late recovery in patients with COPD specifically with our innovative method that directly measures amino acid absorption by intestinal enterocytes independent of intestinal metabolism. The novel use of the inert amino acid l-allo-Isoleucine allows this calculation, with sensitivity to detect small (<12%) changes. As free amino acids are absorbed through the gut via sodium dependent-active transporters (32), hypoxia appears to be an inhibitor of this process during exercise, independent of exercise intensity. Additionally, patients with COPD suffer from reduced absorption during the late recovery period which should be considered when implementing postexercise nutritional supplements.
Mechanisms and Markers of Gut Function in Response to Walking Exercise
A reduced gastrointestinal barrier function in response to exercise is linked to a limited oxygen supply to the gastrointestinal tract of humans (16, 33). As previously mentioned, 56% of our patients desaturated >4% during exercise, a sign of whole body hypoxia. In mice exposed to hypobaric conditions, expression of hypoxia-inducible factor 1α and inducible nitric oxide synthase, known markers of a hypoxic environment, were increased concomitantly with physical degradation of the mucosal barrier (34). Additionally, in inflammatory bowel diseases (IBDs), which are more common in patients with COPD than the general population (3), intestinal epithelial cells have severe metabolic shifts toward hypoxia (15). We confirm now in vivo in older adults and patients with COPD that the gastrointestinal barrier is affected by exercise.
As SCFA are novel biomarkers of gut and microbial health in patients with COPD (18) and exercise has been shown to alter the composition and functional capacity of the gut microbiome with possible benefits to overall health (35), we included SCFA plasma measurements before and after exercise. We now show that higher intensity exercise induces a more elevated acetate and propionate concentrations on Max Speed study days. Interestingly, intravenous acetate infusion was able to normalize antibiotic-reduced endurance exercise performance in mice, supporting the notion that intestinally produced acetate is an important energy source for muscle during exercise (20). As our subjects are in a fasted state, higher exercise intensity could promote the mobilization of SCFA into circulation for energy in muscle (36). In humans when on high altitude mountaineering expeditions who endure hypoxia, an increase in abundance of proinflammatory gut bacteria and gastrointestinal permeability was found (37), also suggesting a link between the gut microbiota and the host response to exercise and hypoxia. Our data support future studies onto the potential link between SCFA and exercise in chronic diseases characterized by (exercise-induced) hypoxia such as COPD.
Response of Whole Body Protein Turnover to Walking Exercise in Patients with COPD
A net catabolic state during and after exercise in the postabsorptive state has been observed previously, lasting at least 2 h post exercise (38, 39). We observed an attenuated net catabolic response in patients with COPD during exercise which remained throughout the late recovery period. In combination with the reduction in amino acid absorption during late recovery, extended net catabolism after only 20 min of walking could favor intake of a delayed acting protein (e.g., casein) which delivers amino acids over a prolonged period for a better overall balance post exercise. This is supported by our previous findings of higher anabolism with casein supplementation compared with whey after aerobic exercise in patients with COPD (14).
Limitations
Owing to limited isotope availability of l-[13C9]-phenylalanine, whole body protein breakdown and net protein breakdown data are only available for a subgroup of Healthy Matched Speed and COPD Max Speed study days. However, our study was still able to assess the effect of absolute work rate, which is done in real-life situations, on whole body protein metabolism and displayed a significant effect of COPD during and late recovery from exercise. Second, although we anticipate that transcutaneous measured hypoxia would be related to a gut hypoxia, we did not measure gut oxygen saturation and so do not know the actual relation. It is therefore unclear whether specifically in patients with COPD, exercise-induced gut hypoxia is more severe and better explains the observed changes. Lastly, although caloric amount and volume of intake alter gastric emptying (40), we measured postabsorptive gut function by providing a small (∼1 g) amount of protein as sips throughout the protocol. Although gut function in daily life is particularly of importance in the fed state, we purposefully studied the postabsorptive state to get better insight on the direct effects of exercise on gut function in COPD.
Future Directions
Most of the available research in patients with COPD examined the effect of exercise while in the fed state (8, 13, 14). In future studies, our oral stable isotope methods can readily be combined with different nutritional loads and compositions to further elucidate whether targeted nutritional modulation can minimize exercise-induced gut dysfunction in COPD.
Conclusions
Walking exercise plays a large role in pulmonary rehabilitation and daily life in older adults and patients with COPD. Our data showed that all subjects were able to tolerate 20 min of vigorous-intensity walking despite elevated heart rate and mild-to-moderate desaturation. However, this type of exercise affects gut function and whole body protein metabolism up to 4 h postexercise. We hypothesize that oxygen desaturation plays an important role. Future studies are needed to examine how certain dietary compositions can attenuate protein digestion and amino acid absorption reductions during and throughout recovery from exercise.
GRANTS
This work was partially supported by a European Society for Clinical Nutrition and Metabolism (ESPEN) Research Fellowship. Results of the present study do not constitute endorsement by ESPEN.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
C.L.C., N.E.P.D., G.C.L.-M., S.Y.S., and M.P.K.J.E. conceived and designed research; C.L.C., S.Y.S., and M.P.K.J.E. performed experiments; C.L.C., N.E.P.D., G.C.L.-M., and M.P.K.J.E. analyzed data; C.L.C., N.E.P.D., G.C.L.-M, and M.P.K.J.E. interpreted results of experiments; C.L.C., N.E.P.D., G.C.L.-M., and M.P.K.J.E. prepared figures; C.L.C., N.E.P.D., G.C.L.-M., and M.P.K.J.E. drafted manuscript; C.L.C., N.E.P.D., G.C.L.-M., S.Y.S., and M.P.K.J.E. edited and revised manuscript; C.L.C., N.E.P.D., G.C.L.-M., S.Y.S., and M.P.K.J.E. approved final version of manuscript.
ACKNOWLEDGMENTS
The authors thank all participants for participating in this research study and making this work possible.
REFERENCES
- 1.Nielsen HM, Rødsgaard PA, Weinreich UM. Chronic obstructive pulmonary disease as comorbidity in patients admitted to a university hospital: a cross‐sectional study. Clin Respir J 8: 274–280, 2014. doi: 10.1111/crj.12050. [DOI] [PubMed] [Google Scholar]
- 2.Lee AL, Goldstein RS. Gastroesophageal reflux disease in COPD: links and risks. Int J Chron Obstruct Pulmon Dis 10: 1935–1949, 2015. doi: 10.2147/COPD.S77562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brassard P, Vutcovici M, Ernst P, Patenaude V, Sewitch M, Suissa S, Bitton A. Increased incidence of inflammatory bowel disease in Quebec residents with airway diseases. Eur Respir J 45: 962–968, 2015. doi: 10.1183/09031936.00079414. [DOI] [PubMed] [Google Scholar]
- 4.Vutcovici M, Bitton A, Ernst P, Kezouh A, Suissa S, Brassard P. Inflammatory bowel disease and risk of mortality in COPD. Eur Respir J 47: 1357–1364, 2016. doi: 10.1183/13993003.01945-2015. [DOI] [PubMed] [Google Scholar]
- 5.Xin X, Dai W, Wu J, Fang L, Zhao M, Zhang P, Chen M. Mechanism of intestinal mucosal barrier dysfunction in a rat model of chronic obstructive pulmonary disease: an observational study. Exp Ther Med 12: 1331–1336, 2016. doi: 10.3892/etm.2016.3493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fricker M, Goggins BJ, Mateer S, Jones B, Kim RY, Gellatly SL, Jarnicki AG, Powell N, Oliver BG, Radford-Smith G, Talley NJ, Walker MM, Keely S, Hansbro PM. Chronic cigarette smoke exposure induces systemic hypoxia that drives intestinal dysfunction. JCI Insight 3: e94040, 2018. doi: 10.1172/jci.insight.94040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sprooten RT, Lenaerts K, Braeken DC, Grimbergen I, Rutten EPA, Wouters EF, Rohde GG. Increased small intestinal permeability during severe acute exacerbations of COPD. Respiration 95: 334–342, 2018. doi: 10.1159/000485935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rutten EPA, Lenaerts K, Buurman WA, Wouters EFM. Disturbed intestinal integrity in patients with COPD: effects of activities of daily living. Chest 145: 245–252, 2014. doi: 10.1378/chest.13-0584. [DOI] [PubMed] [Google Scholar]
- 9.Kirschner SK, Deutz NEP, Jonker R, Olde Damink SW, Harrykissoon RI, Zachria AJ, Dasarathy S, Engelen MPKJ. Intestinal function is impaired in patients with chronic obstructive pulmonary disease. Clin Nutr, 2020. doi: 10.1016/j.clnu.2020.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.van Wijck K, Pennings B, van Bijnen AA, Senden JM, Buurman WA, Dejong CH, van Loon LJ, Lenaerts K. Dietary protein digestion and absorption are impaired during acute postexercise recovery in young men. Am J Physiol Regul Integr Comp Physiol 304: R356–R361, 2013. doi: 10.1152/ajpregu.00294.2012. [DOI] [PubMed] [Google Scholar]
- 11.Engelen MPKJ, Com G, Anderson P, Deutz NEP. New stable isotope method to measure protein digestibility and response to pancreatic enzyme intake in cystic fibrosis. Clin Nutr 33: 1024–1032, 2014. doi: 10.1016/j.clnu.2013.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kaur A, ten Have GAM, Hritzo B, Deutz NEP, Olsen C, Moroni M. Morphological and functional impairment in the gut in a partial body irradiation minipig model of GI-ARS. Int J Radiat Biol 96: 112–128, 2020. doi: 10.1080/09553002.2018.1552377. [DOI] [PubMed] [Google Scholar]
- 13.Engelen MPKJ, Deutz NEP, Mostert R, Wouters EF, Schols AM. Response of whole-body protein and urea turnover to exercise differs between patients with chronic obstructive pulmonary disease with and without emphysema. Am J Clin Nutr 77: 868–874, 2003. doi: 10.1093/ajcn/77.4.868. [DOI] [PubMed] [Google Scholar]
- 14.Engelen MPKJ, Rutten EPA, De Castro CLN, Wouters EFM, Schols AMWJ, Deutz NEP. Casein protein results in higher prandial and exercise induced whole body protein anabolism than whey protein in chronic obstructive pulmonary disease. Metab Clin Exp 61: 1289–1300, 2012. doi: 10.1016/j.metabol.2012.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Colgan SP, Taylor CT. Hypoxia: an alarm signal during intestinal inflammation. Nat Rev Gastroenterol Hepatol 7: 281, 2010. doi: 10.1038/nrgastro.2010.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.van Wijck K, Lenaerts K, van Loon LJC, Peters WHM, Buurman WA, Dejong CHC. Exercise-induced splanchnic hypoperfusion results in gut dysfunction in healthy men. PLoS One 6: e22366, 2011. doi: 10.1371/journal.pone.0022366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Khanna K, Mishra K, Ganju L, Kumar B, Singh SB. High-altitude-induced alterations in gut-immune axis: a review. Int Rev Immunol 37: 119–126, 2018. doi: 10.1080/08830185.2017.1407763. [DOI] [PubMed] [Google Scholar]
- 18.Vaughan A, Frazer ZA, Hansbro PM, Yang IA. COPD and the gut-lung axis: the therapeutic potential of fibre. J Thorac Dis 11: S2173–S2180, 2019.doi: 10.21037/jtd.2019.10.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kelly CJ, Zheng L, Campbell EL, Saeedi B, Scholz CC, Bayless AJ, Wilson KE, Glover LE, Kominsky DJ, Magnuson A, Weir TL, Ehrentraut SF, Pickel C, Kuhn KA, Lanis JM, Nguyen V, Taylor CT, Colgan SP. Crosstalk between microbiota-derived short-chain fatty acids and intestinal epithelial HIF augments tissue barrier function. Cell Host Microbe 17: 662–671, 2015. doi: 10.1016/j.chom.2015.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Okamoto T, Morino K, Ugi S, Nakagawa F, Lemecha M, Ida S, Ohashi N, Sato D, Fujita Y, Maegawa H. Microbiome potentiates endurance exercise through intestinal acetate production. Am J Physiol Endocrinol Metab 316: E956–E966, 2019. doi: 10.1152/ajpendo.00510.2018. [DOI] [PubMed] [Google Scholar]
- 21.Vogelmeier CF, Criner GJ, Martinez FJ, Anzueto A, Barnes PJ, Bourbeau J, Celli BR, Chen R, Decramer M, Fabbri LM, Frith P, Halpin DMG, López Varela MV, Nishimura M, Roche N, Rodriguez-Roisin R, Sin DD, Singh D, Stockley R, Vestbo J, Wedzicha JA, Agustí A. Global strategy for the diagnosis, management, and prevention of chronic obstructive lung disease 2017 report. GOLD executive summary. Am J Respir Crit Care Med 195: 557–582, 2017. doi: 10.1164/rccm.201701-0218PP. [DOI] [PubMed] [Google Scholar]
- 22.Abumrad NN, Rabin D, Diamond MP, Lacy WW. Use of a heated superficial hand vein as an alternative site for the measurement of amino-acid-concentrations and for the study of glucose and alanine kinetics in man. Metab Clin Exp 30: 936–940, 1981. doi: 10.1016/0026-0495(81)90074-3. [DOI] [PubMed] [Google Scholar]
- 23.Jonker R, Deutz NEP, Harrykissoon R, Zachria AJ, Veley EA, Engelen MPKJ. A critical evaluation of the anabolic response after bolus or continuous feeding in COPD and healthy older adults. Clin Sci (Lond) 132: 17–31, 2018. doi: 10.1042/CS20171068. [DOI] [PubMed] [Google Scholar]
- 24.Thompson G, Pacy P, Merritt H, Ford G, Read M, Cheng K, Halliday D. Rapid measurement of whole body and forearm protein turnover using a [2H5] phenylalanine model. Am J Physiol Endocrinol Metab 256: E631–E639, 1989. doi: 10.1152/ajpendo.1989.256.5.E631. [DOI] [PubMed] [Google Scholar]
- 25.Benjamini Y, Krieger AM, Yekutieli D. Adaptive linear step-up procedures that control the false discovery rate. Biometrika 93: 491–507, 2006. doi: 10.1093/biomet/93.3.491. [DOI] [Google Scholar]
- 26.Temirbekov D, Güneş S, Yazıcı ZM, Sayın İ. The ignored parameter in the diagnosis of obstructive sleep apnea syndrome: the oxygen desaturation index. Turk Arch Otorhinolaryngol 56: 1–6, 2018. doi: 10.5152/tao.2018.3025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Garvey C, Bayles MP, Hamm LF, Hill K, Holland A, Limberg TM, Spruit MA. Pulmonary rehabilitation exercise prescription in chronic obstructive pulmonary disease: review of selected guidelines. J Cardiopulm Rehabil Prev 36: 75–83, 2016. doi: 10.1097/HCR.0000000000000171. [DOI] [PubMed] [Google Scholar]
- 28.Tanaka H, Monahan KD, Seals DR. Age-predicted maximal heart rate revisited. J Am Coll Cardiol 37: 153–156, 2001. doi: 10.1016/S0735-1097(00)01054-8. [DOI] [PubMed] [Google Scholar]
- 29.Smith AE, Eston R, Tempest GD, Norton B, Parfitt G. Patterning of physiological and affective responses in older active adults during a maximal graded exercise test and self-selected exercise. Eur J Appl Physiol 115: 1855–1866, 2015. doi: 10.1007/s00421-015-3167-z. [DOI] [PubMed] [Google Scholar]
- 30.Armstrong M, Vogiatzis I. Personalized exercise training in chronic lung diseases. Respirology 24: 854–862, 2019. doi: 10.1111/resp.13639. [DOI] [PubMed] [Google Scholar]
- 31.Pennings B, Koopman R, Beelen M, Senden JMG, Saris WHM, van Loon LJC. Exercising before protein intake allows for greater use of dietary protein-derived amino acids for de novo muscle protein synthesis in both young and elderly men. Am J Clin Nutr 93: 322–331, 2011. doi: 10.3945/ajcn.2010.29649. [DOI] [PubMed] [Google Scholar]
- 32.van der Wielen N, Moughan PJ, Mensink M. Amino acid absorption in the large intestine of humans and porcine models. J Nutr 147: 1493–1498, 2017. doi: 10.3945/jn.117.248187. [DOI] [PubMed] [Google Scholar]
- 33.de Oliveira EP, Burini RC, Jeukendrup A. Gastrointestinal complaints during exercise: prevalence, etiology, and nutritional recommendations. Sports Med 44: 79–85, 2014. doi: 10.1007/s40279-014-0153-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang F, Wu W, Deng Z, Zheng X, Zhang J, Deng S, Chen J, Ma Q, Wang Y, Yu X. High altitude increases the expression of hypoxia-inducible factor 1α and inducible nitric oxide synthase with intest-inal mucosal barrier failure in rats. Int J Clin Exp Pathol 8: 5189, 2015. [PMC free article] [PubMed] [Google Scholar]
- 35.Mailing LJ, Allen JM, Buford TW, Fields CJ, Woods JA. Exercise and the gut microbiome: a review of the evidence, potential mechanisms, and implications for human health. Exerc Sport Sci Rev 47: 75–85, 2019. doi: 10.1249/JES.0000000000000183. [DOI] [PubMed] [Google Scholar]
- 36.Hall G, Sacchetti M, Rådegran G. Whole body and leg acetate kinetics at rest, during exercise and recovery in humans. J Physiol 542: 263–272, 2002. doi: 10.1113/jphysiol.2001.014340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Adak A, Maity C, Ghosh K, Pati BR, Mondal KC. Dynamics of predominant microbiota in the human gastrointestinal tract and change in luminal enzymes and immunoglobulin profile during high-altitude adaptation. Folia Microbiol 58: 523–528, 2013. doi: 10.1007/s12223-013-0241-y. [DOI] [PubMed] [Google Scholar]
- 38.Bowtell J, Leese G, Smith K, Watt P, Nevill A, Rooyackers O, Wagenmakers A, Rennie M. Modulation of whole body protein metabolism, during and after exercise, by variation of dietary protein. J Appl Physiol (1985) 85: 1744–1752, 1998. doi: 10.1152/jappl.1998.85.5.1744. [DOI] [PubMed] [Google Scholar]
- 39.Rennie M, Edwards R, Krywawych S, Davies C, Halliday D, Waterlow J, Millward D. Effect of exercise on protein turnover in man. Clin Sci 61: 627–639, 1981. doi: 10.1042/cs0610627. [DOI] [PubMed] [Google Scholar]
- 40.Okabe T, Terashima H, Sakamoto A. A comparison of gastric emptying of soluble solid meals and clear fluids matched for volume and energy content: a pilot crossover study. Anaesthesia 72: 1344–1350, 2017. doi: 10.1111/anae.14026. [DOI] [PubMed] [Google Scholar]


