Abstract
Background:
Olfactory impairment is increasingly common with older age, which may be in part explained by cumulative effects of exposure to inhaled toxins. However, population-based studies investigating the relationship between air pollution and olfactory ability are scarce.
Objectives:
We aimed to investigate associations between exposure to common air pollutants and longitudinal change in odor identification.
Methods:
Our study of 2,468 participants (; 61.1% female), of which 1,774 participants (; 61.9% female) had at least two olfactory assessments over 12 y of follow-up from the Swedish National Study on Aging and Care in Kungsholmen (SNAC-K), Stockholm, Sweden. Participants were free from cognitive impairment and neurodegenerative disease at baseline. Odor identification ability was assessed with Sniffin’ Sticks. Change in olfactory performance was estimated with linear mixed models. Exposure to two major airborne pollutants [particulate matter with aerodynamic diameter () and nitrogen oxides ()] for the 5 y preceding baseline was assessed using spatiotemporal dispersion models for outdoor levels at residential addresses.
Results:
Participants showed significant decline in odor identification ability for each year in the study { [95% confidence interval (CI): , ; ]}. After adjustment for all covariates, residents of third [ (95% CI: , ; )] and fourth [ (95% CI: , ; )] exposure quartiles of had faster rates of olfactory decline than residents from the first quartile. Similar results were observed for the third [ (95% CI: , ; )] and fourth [ (95% CI: , ; ) quartiles of ].
Discussion:
Our results suggest an association between air pollution exposure and subsequent olfactory decline. We speculate that cumulative effects of airborne pollutants on the olfactory system may be one underlying cause of olfactory impairment in aging. https://doi.org/10.1289/EHP9563
Introduction
Among sensory dysfunctions, loss in the sense of smell, olfaction, is particularly pronounced in older age (Doty et al. 1984; Doty and Kamath 2014). Olfactory deficits are associated with a number of health conditions such as depressive symptoms (Croy et al. 2014; Negoias et al. 2010) and frailty (Harita et al. 2019; Laudisio et al. 2019), as well as shorter survival (Devanand et al. 2015a; Ekström et al. 2017; Pinto et al. 2014) and diminished quality of life (Blomqvist et al. 2004). An important fact is that olfactory impairment has exceptionally high prevalence rates among patients with neurodegenerative diseases, such as Alzheimer disease (AD) and Parkinson disease (PD; as reviewed by Doty 2012 and Sun et al. 2012) and may constitute one of the first noncognitive manifestations of an impending dementia (Devanand et al. 2015b; Stanciu et al. 2014).
Given that the olfactory system is directly exposed to the outside environment, it has been speculated that part of the olfactory loss observed in older age may arise from cumulative damage of xenobiotics (Doty 2008; Pinto 2011). For example, an increased exposure to air pollution may lead to olfactory loss, especially among middle-age or older adults for whom xenobiotic exposure has accumulated over a longer time (Doty and Kamath 2014). Sourcing mainly from traffic exhaust and other fuel-burning operations, the smallest particulates [particulate matter with aerodynamic diameter ] are among the most harmful forms of air pollution for human health. Ranking as the sixth leading risk factor for premature death globally, they are associated with severe diseases, such as stroke, heart disease, chronic lung disease, lung cancer, and respiratory infections, at levels that are commonly experienced by general populations across the globe (State of Global Air 2020). Due to its small size, can penetrate the brain via the olfactory route, where it is likely to cause damage to the olfactory system (Calderón-Garcidueñas et al. 2003). Additional types of pervasive air pollutants that can enter the brain via the olfactory system are different forms of nitrogen oxides (; Shusterman 2011). These gases are usually produced during combustion of fuels in air, such as in car engines, and are therefore a significant source of air pollution in urban areas with high motor vehicle traffic (Olivier et al. 1998).
To date, we are aware of only two studies that have directly examined the impact of air pollution on olfactory impairment in the general population of older adults. Ajmani et al. (2016a) found that exposure to fine , averaged over 3–12 months, was associated with worse olfactory ability in a large sample of urban-dwelling respondents. Similarly, another U.S. population-based study found higher exposure to nitrogen dioxide () to be associated with an increased risk for olfactory impairment among older adults (Adams et al. 2016). A few other studies have examined associations between air pollution and olfactory function using indirect measures for pollutant exposure. For example, younger adults living in Mexico City, where particulate concentrations are substantially elevated, have been found to exhibit poorer olfactory ability in comparison with residents of nearby cities with lower pollution levels (Calderón-Garcidueñas et al. 2010; Hudson et al. 2006). Likewise, a study of older German women (ages 68–79 y) found olfactory impairment to be associated with proximity to the nearest busy roadway, a proxy for exposure to traffic-related pollutants (Ranft et al. 2009). In sum, these previous—mainly ecological, cross-sectional, or indirect—studies suggest that increased airborne pollutant exposure may be associated with olfactory deficits. An important fact is that cross-sectional variation in olfactory ability between individuals does not equal intraindividual olfactory loss. However, to the best of our knowledge, no previous study has investigated the association between air pollution and longitudinally measured olfactory change. To examine whether air pollution exposure may be linked to decline in olfactory ability in older age, longitudinal population-based studies with repeated olfactory measurements are needed.
We hypothesized higher exposure to common airborne pollutants to be associated with a faster rate of decline in olfactory identification ability. We tested this hypothesis using a well-characterized population-based sample with spatially detailed data of long-term exposure to air pollution ( or ) and repeated olfactory identification tests across 12 y of follow-up.
Methods
Data collection
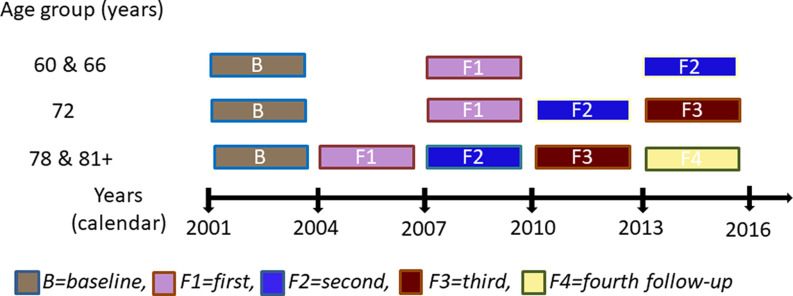
Participants were from the Swedish National Study on Aging and Care in Kungsholmen (SNAC-K), an ongoing longitudinal population-based study on aging and health that started in 2001 (Lagergren et al. 2004). The original study population consisted of 4,590 persons, randomly drawn from the population registry of Kungsholmen, a central area of Stockholm, Sweden. Out of these, 3,363 persons participated in the baseline examination (2001–2003). The participants came from 11 prespecified age cohorts: 60, 66, 72, 78, 81, 84, 87, 90, 93, 96, and 99 y and older. The examination involved a social interview and assessment of physical functioning (performed by nurses); a clinical assessment of geriatric, neurological, and psychiatric information (performed by physicians); and neuropsychological testing (performed by psychologists). All parts of the SNAC-K project have been approved by the Ethics Committee at Karolinska Institutet or by the Regional Ethical Review Board in Stockholm, Sweden. All participants provided written informed consent or, in cases where the participants were severely cognitively impaired, the consent was provided by their next of kin. Figure 1 presents an overview of the design of the SNAC-K study.
Figure 1.
The SNAC-K study design.
Most participants () completed a neuropsychological test battery (Laukka et al. 2013). We excluded participants who had a diagnosis of dementia (), schizophrenia (), Parkinson disease (), or developmental disorder () at baseline, as well as those with a Mini-Mental State Examination (MMSE) score below 24, indicative of cognitive impairment (). Of the remaining participants, 2,475 completed the olfactory testing (Ekström et al. 2020) at baseline. Seven participants had missing data on environmental concentration, resulting in a final sample of 2,468 participants; 1,774 participants had at least one additional olfactory assessment during the 12-y follow-up, of which 522 (29.4%) had participated in one, 898 (50.6%) in two, 268 (15.1%) in three, and 86 (4,8%) in four follow-up testing waves [, standard deviation ()]. Their average follow-up time was 9.23 y (). Loss of follow-up after baseline concerned 694 individuals [ (10.6); years of y (4.1); baseline odor identification (3.6); 59.1% female].
Comparisons with two-sample -tests and chi-square tests between the 1,774 participants with follow-up assessments and the 695 individuals who were lost to follow-up (of which 38.8% had died) showed statistically significant group differences regarding age, education, and baseline olfactory identification performance (statistics presented in Supplementary Table S1). The dropout group was older (, ) than the main sample (, ), had fewer years of education (, ) than the main sample (, ) and lower odor identification scores (, ) than the main sample (, ). We found a similar sex distribution in participants who were lost to follow-up (40.92% male/59.08% female) in comparison with the main sample (38.22% male/61.78% female). Likewise, we found similar average pollution concentrations for the 5 y preceding baseline for (, ) and (, ) in comparison with the concentrations of (, ) and (, ) that were found in the main sample.
Odor Identification Assessment
Odor identification ability was assessed with the Sniffin’ Sticks, a well-established and norm-referenced olfactory test kit with high test-retest reliability (Croy et al. 2015; Hummel et al. 1997). The testing procedure is described in detail elsewhere (Ekström et al. 2020). In brief, participants were presented with 16 household odors and instructed to freely identify the presented odor by providing a verbal descriptor. If they failed to provide a correct label, they were presented with four written response alternatives from which they were instructed to choose the label that best matched the odor. In a few cases, items were skipped due to allergy to the specific odor or test-leader mistakes ( at baseline; at first follow-up, at second follow-up, at third follow-up, and at fourth follow-up). Participants received a score of 0.25, representing performance at chance level, for that item. Participants received a score of 0 if they were unable to identify an item because they could not perceive the presented odor. The odor identification score was calculated as number of correctly identified odors, with a performance range of 0 to 16. To minimize retest effects across follow-up occasions, participants were randomly assigned one of three test versions, with different presentation orders of the odors. At the next follow-up, the participant would receive a different version. Notably, the same odors were used for each version, in different presentation orders.
Air Pollution Assessment
We used two different measures of air pollution. Local contributions of consisted of combustion particles from residential wood burning and exhaust and wear particles from road traffic. Nitrogen oxides () represented exhaust emissions from road traffic. Pollutant levels at the participants’ residential addresses were estimated with Gaussian dispersion modeling based on local emission inventories described in detail elsewhere (Segersson et al. 2017). Briefly, annual average air pollution levels from local sources were calculated using emission inventories, considering both traffic and nontraffic sources, for the years 1990, 1995, 2000, 2005, and 2011. Annual average levels of and for the period 1990–2011 were obtained from linear interpolation over the years between each model simulation. Annual long-range pollutant contributions were added to the simulation of locally generated and . These contributions were based on measurements from a rural site located outside the calculation domain ( northeast of Stockholm). To allow high resolution in vicinity of roads, a quadtree receptor grid was used. The size of the domain on which the model was based was , covering the city cores with suburbs, many smaller villages, and some rural areas. The exposure and impact assessment presented here is limited to the location covering the residential addresses of the included SNAC-K participants, an area the size of 391 hectares. Reliability measures of the dispersion model derived from yearly measurements at three sites within the City of Stockholm (of which this district is part) were high ( of 0.86 for and 0.97 for ; Grande et al. 2020). Average particulate emissions from the 5 y preceding baseline olfactory assessment (2001–2003) were calculated for and . Thus, for participants who were assessed in 2001, average concentration values from the years 1996 to 2000 were used. Similarly, we used average values from the years 1997 to 2001 for participants assessed in 2002, and so forth.
Demographic and Occupational Variables
Age, sex, and years of education were collected following standard protocols. Age was measured as years since birth and education as years of formal schooling. We centered these variables on their respective means. Participants longest held professional occupation was obtained through self-reports and dichotomized into manufacturing (“blue-collar”) or nonmanufacturing (“white-collar”) work.
Cognition
The odor identification test involves matching olfactory input to word labels. Performance in this test may therefore be affected by cognitive ability in semantic memory (word knowledge). To control for the potential effects of semantic ability, we included a Swedish vocabulary test, SRB1 (Dureman 1960), as a covariate. Here, participants are presented with 30 words together with 5 additional words, and the task is to choose the correct synonym in forced-choice format, similar to the format of the odor identification test.
Health and Lifestyle Variables
Heart diseases and cerebrovascular diagnoses at baseline and during follow-up were based on the clinical assessment, self-report, medications lists, laboratory data, and information from the computerized Stockholm inpatient and outpatient register (Calderón-Larrañaga et al. 2017). Cerebrovascular disease (ICD-10 I60–I69) was dichotomized as 1 (yes) or 0 (no). Heart disease was defined as a diagnosis of either atrial fibrillation (ICD-10 I48), heart failure (ICD-10 I50), or ischemic heart disease (I20–I25) and dichotomized as 1 (yes) if having at least one of these diagnoses or 0 (no) if not. Diagnosis of diabetes type 1 or 2 was obtained from medical history, use of diabetes drugs (ATC code A10), diagnosis in the Stockholm inpatient register (ICD-10 code E11), or (; Marseglia et al. 2019). Smoking was derived from self-reports at baseline and categorized into former, current, or never smoked. Height and weight were measured with light clothes and no shoes. Body mass index (BMI) was calculated as weight (kilograms) divided by height (meters) squared.
Statistical Analysis
All analysis were performed in STATA (version 14; Stata Corp.), and the threshold for statistical significance was set at . We used linear mixed models to analyze olfactory level (baseline) and rate of change as a function of particulate concentration. We used unstructured variance-covariance matrices for all models. The random effects included random intercept and slope, allowing for individual differences at baseline and across time. In creating the estimates for the longitudinal trajectories, STATA also considers the hypothetical trajectories of the people who dropped out of the study. The basic model was adjusted for age at baseline, sex, and years of education. The covariates in the multiadjusted model were chosen a priori and based on the findings of previous studies. In addition to age, sex, and education, we included cognitive performance in vocabulary, professional background (blue vs. white collar), BMI, smoking, history of heart disease, and cerebrovascular disease or diabetes at baseline because these diseases have been shown to be related to olfactory impairment or decline in aging (Ekström et al. 2020; Fluitman et al. 2019; Lietzau et al. 2018; Schubert et al. 2015; Siegel et al. 2019). Potential effects of changes over time in the level of air pollution during the years surrounding baseline were considered by adjusting for year of baseline assessment, with 2001 as the reference category. We also included test versions of the odor identification test to certify that the results were not affected by differences in presentation order of the odors. The analytical equation of the linear mixed-effect models is:
where is the intercept in the reference group; i [range 1,…, ] represents the ith subjects at jth (1,…, ) measurement occasion; h (range 1,…, ) refers to the covariates; and is the measurement or sampling errors (modeled in the random effect part of the mixed model).
First, we treated concentration of and pollutants as continuous variables. To test departure from linearity, we further categorized average pollution emissions into quartiles with increasing emission concentrations, each comprising 25% of the total sample size. The first quartile was considered the reference category, representing participants exposed to the lowest average pollutant concentration of or .
We performed sensitivity analyses in which the average concentrations of and for 1 y (the year of baseline assessment for each participant) were used as predictors of olfactory change, instead of the average pollution concentration for the 5 y preceding baseline. This approach allowed us to explore potential effects of temporal variation in pollution concentration on our results. Sensitivity analyses further considered the possibility that our results may be influenced by individuals who already had a severe olfactory dysfunction at baseline, as well as by those with a history of cerebrovascular disease. For this purpose, we repeated main analyses in subsamples free of a) anosmia (anosmia defined as an odor identification score of ; Hummel et al. 2007; Seubert et al. 2017) () or b) history of cerebrovascular disease () at baseline.
The potentially biasing effects of attrition were investigated in follow-up multiadjusted mixed models by comparing the impact that two extreme scenarios would have on our results. In a first step, we gave all participants who had not participated in an additional olfactory assessment after baseline a score of 0 in the odor identification test, assuming that they would have developed a functionally absent sense of smell during follow-up. In a second step, all participants with missing follow-up data received the same odor identification score as at the baseline assessment, assuming that there was no olfactory decline in this group.
We investigated the potential modifying effects of increasing age and vascular diseases that developed during follow-up on the association between air pollution and olfactory decline. For this purpose, we added interaction terms between odor identification, time in study and the potential modifiers to our multiadjusted models. First, we added an interaction term between age, pollution quartile, and level and change of odor identification. Second, we investigated whether there was as an interaction between newly occurring diagnoses of cerebrovascular disease or heart disease during follow-up. Given the decrease in traffic exhaust in the Stockholm area during recent decades, we investigated the extent of the decline of air pollution concentration from and between 1996 and 2003, a timespan encompassing baseline for each participant and the 5 y preceding this assessment.
Results
Study Participants Characteristics
Participant characteristics for the total sample () are presented in Table 1. On average, participants were 72.3 y old (; range 58.4–100.9 y), had completed 12.2 y of formal education (), correctly identified 11.6 of 16 odors at baseline (), and 61.0% were female.
Table 1.
Sample characteristics at baseline assessment (2001–2003) for the total sample () and by air pollutant quartiles of and . Participants are from the SNAC-K Study on Aging and Care in Kungsholmen, Stockholm, Sweden.
| Total sample () | quartiles | quartiles | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1st |
2nd |
3rd |
4th |
1st |
2nd |
3rd |
4th |
|||
| Age () | 2,468 | |||||||||
| Female/male (%) | 2,468 | 61.0/39.0 | 55.6/44.4 | 62.9/37.1 | 61.4/38.6 | 64.2/35.8 | 56.1/43.9 | 61.8/38.3 | 62.6/37.4 | 63.7/36.3 |
| Years of education () | 2,468 | |||||||||
| Vocabulary () | 2,448 | |||||||||
| Blue-collar/white-collar profession (%) | 2,465 | 20.1/79.8 | 16.4/83.6 | 19.5/80.6 | 21.2/78.4 | 23.3/76.5 | 14.9/85.1 | 20.9/78.9 | 20.4/79.4 | 24.2/75.7 |
| BMI () | 2,419 | |||||||||
| Smoking yes/no (%) | ||||||||||
| Currently | 363 | 14.7/85.3 | 12.3/87.7 | 15.7/84.3 | 14.4/85.6 | 16.4/83.6 | 13.1/86.9 | 12.6/87.4 | 16.7/83.3 | 16.4/83.6 |
| Ever | 973 | 39.4/60.6 | 40.0/60.0 | 40.0/60.0 | 41.3/58.7 | 36.3/63.7 | 40.4/59.6 | 41.2/58.8 | 40.0/60.0 | 36.1/63.9 |
| Never | 1,118 | 45.3/54.7 | 47.5/52.5 | 43.8/56.2 | 42.9/57.1 | 46.5/53.5 | 46.4/53.6 | 45.1/54.9 | 43.1/56.9 | 46.7/53.3 |
| Diabetes yes/no (%) | 2,468 | 8.6/91.4 | 7.9/92.1 | 8.8/91.3 | 9.1/90.9 | 8.6/91.4 | 6.2/93.8 | 11.0/89.0 | 8.3/91.7 | 8.9/91.1 |
| Heart disease yes/no (%) | 2,468 | 20.8/79.2 | 19.9/80.1 | 19.8/80.2 | 20.8/79.3 | 22.7/77.3 | 16.2/83.8 | 22.2/77.8 | 22.5/77.5 | 22.2/77.8 |
| Cerebrovascular disease yes/no (%) | 2,468 | 5.8/94.3 | 4.2/95.8 | 5.0/95.0 | 6.8/93.2 | 7.0/93.0 | 4.1/95.9 | 5.4/94.6 | 6.3/93.7 | 7.3/92.7 |
| (; ) | 2,468 | |||||||||
| (; ) | 2,468 | |||||||||
| Odor identification score () | 2,468 | |||||||||
Note: Variables with missing values: Smoking ; Occupational background . BMI, body mass index; , nitrogen oxide; , particulate matter with aerodynamic diameter less than or equal to ; SD, standard deviation.
Longitudinal Analysis of Olfactory Change as a Function of Air Pollution
Participants declined on average 0.20 items [ (95% CI: , ; )] on the odor identification task for each year in the study.
Pollution concentration in our study sample averaged for the 5 y prior baseline ranged from 6.– (; ) for , and from 12.8– (; ) for . The range of was 8.1– (; ) in 1996 and 7.2– (; ) in 2003. The range of was 15.2– (; ) in 1996 and 12.0– (; ) in 2003. Analysis of temporal trends for change in pollution concentration between the years 1996 and 2003 showed a statistically significant decrease for both (, ) and (, ) during this period. On average, concentrations for our study population decreased by 10.2%. For , the decrease was 25.6%.
In a first step, we treated and air pollution concentrations as continuous variables. In the multiadjusted model, adjusting for version of the odor identification test at baseline, year of the baseline assessment, age, sex, education, vocabulary performance, blue-collar profession, BMI, and smoking, we found a statistically significant positive association between pollutant concentration and olfactory identification at baseline, such that higher pollution concentrations were associated with higher olfactory baseline scores (Table 2). In contrast to these findings, we found that higher air pollution concentration was associated with faster rate of olfactory decline. The association was, however, not statistically significant for and approached statistical significance for (Table 2).
Table 2.
Results of basic and multiadjusted linear mixed models on associations between air pollution in and (5-y mean prior to baseline assessment, continuous and quartiles) and intercept and change (score/year) in odor identification in the total sample (), derived from the SNAC-K study on aging and care in Kungsholmen, Stockholm, Sweden (baseline assessment between 2001 and 2003; last assessment between 2013 and 2015).
| Intercept | Change | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Basic modela (95% CI) |
-Value | Adjusted modelb (95% CI) |
-Value | Basic modela (95% CI) |
-Value | Adjusted modelb (95% CI) |
-Value | ||
| () | 0.194 (0.045, 0.342) | 0.01 | 0.181 (0.031, 0.330) | 0.02 | (, 0.004) | 0.10 | (, 0.002) | 0.08 | — |
| () | 0.011 (0.002, 0.02) | 0.02 | 0.010 (0.001, 0.002) | 0.02 | (, 0.001) | 0.35 | (, 0.001) | 0.30 | — |
| quartiles (range) | — | — | — | — | — | — | — | — | Trend in change (p-value) |
| 2nd (7.98–) | 0.104 (, 0.400) | 0.49 | 0.124 (, 0.418) | 0.41 | (, 0.020) | 0.26 | (, 0.021) | 0.27 | 0.53 |
| 3rd (8.27–) | 0.215 (, 0.512) | 0.16 | 0.208 (, 0.505) | 0.17 | (, ) | 0.000 | (, ) | 0.000 | — |
| 4th (8.59–) | 0.250 (, 0.546) | 0.10 | 0.266 (, 0.565) | 0.08 | (, ) | 0.003 | (, ) | 0.005 | — |
| quartiles (range) | — | — | — | — | — | — | — | — | Trend in change (p-value) |
| 2nd (26.76–) | 0.107 (, 0.404) | 0.48 | 0.112 (, 0.407) | 0.46 | (, 0.021) | 0.27 | (, 0.022) | 0.28 | 0.11 |
| 3rd (31.85–) | 0.235 (, 0.533) | 0.12 | 0.247 (, 0.545) | 0.11 | (, ) | 0.028 | (, ) | 0.029 | — |
| 4th (36.59–) | 0.230 (, 0.527) | 0.13 | 0.259 (, 0.557) | 0.09 | (, ) | 0.006 | (, ) | 0.006 | — |
Note: —, no data; BMI, body mass index; CI, confidence interval; , nitrogen oxide; , particulate matter with aerodynamic diameter less than or equal to .
Adjusted for age, sex, and education.
bAdjusted for age, sex, education, odor test version at baseline, baseline assessment year, vocabulary, longest held occupation, BMI, smoking, diabetes, heart disease, and cerebrovascular disease.
In a second step, we used quartiles of and air pollution as predictors of olfactory level and change (Oudin et al. 2016). Table 1 summarizes participant characteristics by pollution quartile. Regarding secular trends by quartile, we found that pollution concentrations decreased the most in the fourth and thus highest air pollution quartiles between 1996 and 2003. For , concentrations declined by 9.9%, 9.5%, 10.2%, and 10.6% in the first, second, third, and fourth quartiles, respectively. For , decreases were 24%, 34.4%, 39.2%, and 55.5%, respectively.
In multiadjusted mixed models, we found no significant association between air pollution and olfactory level at baseline, neither for quartiles of nor (Table 2).
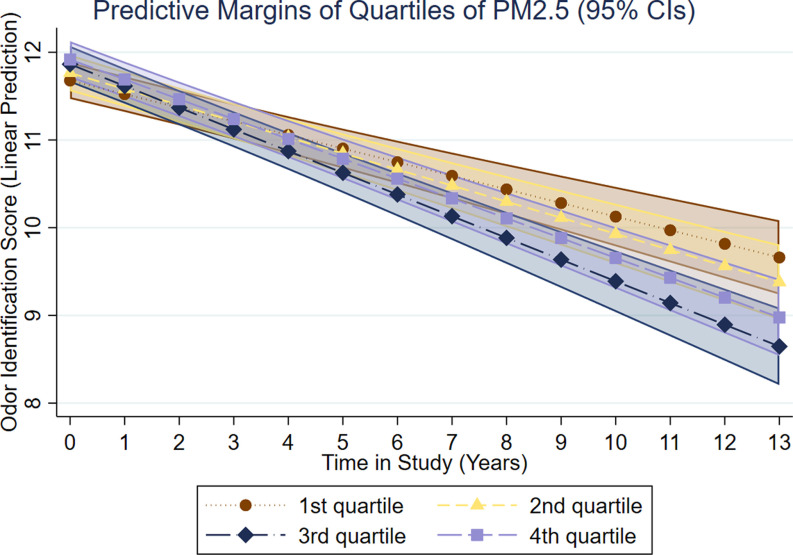
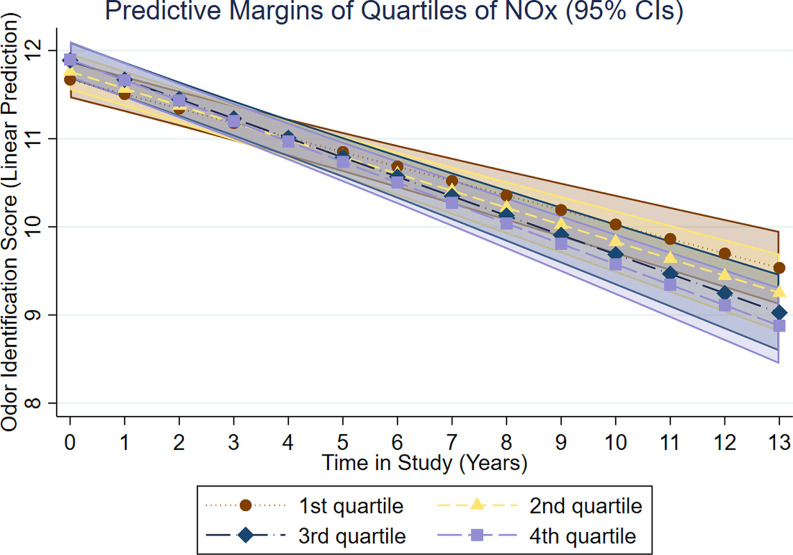
Regarding change, we found that belonging to the two highest pollutant quartiles was associated with a significantly faster rate of olfactory decline (Table 2). For , belonging to the third and fourth concentration quartiles was associated with a faster decline in odor identification change by (95% CI: , ) and (95% CI: , ) points/y, respectively (Figure 2). For , belonging to the third and fourth concentration quartiles was associated with a faster decline in odor identification change by (95% CI: , ) and (95% CI: , ) points/y, respectively (Figure 3). Multiadjusted associations between pollution quartiles of or and olfactory decline remained largely unchanged in analyses of participants without anosmia or a history of cerebrovascular disease at baseline (Table 3).
Figure 2.
Results of multiadjusted linear mixed models (adjusted for age, sex, education, odor test version at baseline, baseline assessment year, vocabulary, longest held occupation, BMI, smoking, diabetes, heart disease, and cerebrovascular disease) on associations between quartiles of air pollution in (5-y mean prior baseline assessment) and intercept and change (score/year) in odor identification in the total sample (), derived from the SNAC-K study on Aging and Care in Kungsholmen, Stockholm, Sweden (baseline assessment between 2001 and 2003; last assessment between 2013 and 2015).
Figure 3.
Results of multiadjusted linear mixed models (adjusted for age, sex, education, odor test version at baseline, baseline assessment year, vocabulary, longest held occupation, BMI, smoking, diabetes, heart disease, and cerebrovascular disease) on associations between quartiles of air pollution in (5-y mean prior baseline assessment) and intercept and change (score/year) in odor identification in the total sample (), derived from the SNAC-K Study on Aging and Care in Kungsholmen, Stockholm, Sweden (baseline assessment between 2001 and 2003; last assessment between 2013 and 2015).
Table 3.
Results of multiadjusted linear mixed models on associations between air pollution in and (5-y mean prior baseline assessment, quartiles) and change (score/year) in odor identification in SNAC-K participants without anosmia at baseline () and in those without a history cerebrovascular disease at baseline ().
| Participants without anosmia () | Participants without cerebrovascular disease () | |||
|---|---|---|---|---|
| Change (95% CI) | -Value | Change (95% CI) | -Value | |
| quartiles | ||||
| 2nd | (, 0.020) | 0.25 | (, 0.027) | 0.39 |
| 3rd | (, ) | 0.000 | (, ) | 0.000 |
| 4th | (, ) | 0.008 | (, ) | 0.016 |
| quartiles | ||||
| 2nd | (, 0.020) | 0.24 | ( ,0.013) | 0.16 |
| 3rd | (, ) | 0.031 | (, ) | 0.039 |
| 4th | (, ) | 0.005 | (, ) | 0.011 |
Note: Adjusted for age, sex, education, odor test version at baseline, baseline assessment year, vocabulary, longest held occupation, BMI, smoking, diabetes, heart disease, and cerebrovascular disease. BMI, body mass index; CI, confidence interval; , nitrogen oxide; , particulate matter with aerodynamic diameter less than or equal to .
We found a significant interaction between age and with regard to olfactory change. Increasing age modified olfactory decline in the fourth pollution concentration quartile of quartile, with each additional year accelerating the average olfactory decline rate for this quartile by (Table 4). By contrast, no statistically significant interactions with age were found between pollution quartiles of and olfactory decline (Table 4). We further investigated the modifying effects of incident cerebrovascular or heart disease among participants without a history of these diseases at baseline. During follow-up, 11.8% of 2,362 participants developed a cerebrovascular disease, and 22.9% of 1,955 participants developed a heart disease. We found no interaction between the development of cerebrovascular or heart disease during follow-up and associations of pollution quartiles and olfactory change, suggesting no modifying effect of these variables (Table 4).
Table 4.
Results of multiadjusted linear mixed models on associations between air pollution in and (5-y mean prior baseline assessment, quartiles) and change (score/year) in odor identification in interaction with age at baseline (continuous), development of a cerebrovascular disease during follow-up (yes/no) and development of heart disease during follow-up (yes/no).
| a (95% CI) | -Value | of cerebrovascular diseaseb (95% CI) | -Value | of heart diseaseb (95% CI) | -Value | |
|---|---|---|---|---|---|---|
| quartiles | ||||||
| 2nd | 0.002 (, 0.007) | 0.57 | 0.026 (, 0.165) | 0.72 | 0.051 (, 0.165) | 0.38 |
| 3rd | (, ) | 0.82 | 0.040 (, 0.178) | 0.57 | 0.058 (, 0.170) | 0.31 |
| 4th | (, 0.001) | 0.11 | 0.079 (, 0.223) | 0.28 | 0.102 (, 0.218) | 0.08 |
| quartiles | ||||||
| 2nd | (, 0.004) | 0.56 | 0.055 (, 0.197) | 0.45 | (, 0.096) | 0.74 |
| 3rd | (, 0.000) | 0.05 | (, 0.112) | 0.79 | (, 0.120) | 0.86 |
| 4th | (, ) | 0.03 | 0.019 (, 0.149) | 0.88 | 0.063 (, 0.177) | 0.28 |
Note: BMI, body mass index; CI, confidence interval; , nitrogen oxide; , particulate matter with aerodynamic diameter less than or equal to .
Adjusted for sex, education, odor test version at baseline, baseline assessment year, vocabulary, longest held occupation, BMI, smoking, diabetes, heart disease, and cerebrovascular disease.
bAdjusted for age, sex, education, odor test version at baseline, baseline assessment year, vocabulary, longest held occupation, BMI, smoking, diabetes, heart disease, and cerebrovascular disease.
Sensitivity analysis in which we used average pollution concentrations from the year of baseline for each participant, instead of the average of the 5 y preceding baseline, showed a similar pattern of results, but with somewhat attenuated associations (Table 5). To investigate the potential effects of attrition on our results, we performed additional multiadjusted mixed models in which all individuals with missing olfactory values at follow-up (i.e., participants with a completed baseline assessment but no additional olfactory assessment) were first given a score of zero for the odor identification test at follow-up to assume the development of a completely absent sense of smell. This approach resulted in a pattern of results similar to that in the initial analysis, but with somewhat larger associations between the third and fourth quartiles of pollution concentration and olfactory decline (Table 5). Imputing missing olfactory values at follow-up with the same scores as those the participants received at baseline, assuming no olfactory decline in this group, resulted in largely the same results as in the main analysis (Table 5).
Table 5.
Results of multiadjusted linear mixed models on associations between air pollution in and (1-y mean prior baseline assessment, quartiles) and change (score/year) in odor identification in the total sample (). Results of multiadjusted linear mixed models on associations between and (5-y mean prior to baseline assessment, quartiles) and change (score/year) in odor identification in the total sample with manually imputed missing values for participants lost at follow-up () with a) a score of zero and b) the baseline score in odor identification for these participants.
| 1-y pollution exposure | Manual imputation of values for participants lost at follow-up () | |||||
|---|---|---|---|---|---|---|
| Odor identification score imputed by zero | Odor identification score imputed by baseline value | |||||
| Change (95% CI) |
-Value | Change (95% CI) |
-Value | Change (95% CI) |
-Value | |
| quartiles | ||||||
| 2nd | 0.003 (, 0.050) | 0.91 | (, 0.025) | 0.34 | (, 0.021) | 0.26 |
| 3rd | (, ) | 0.046 | (, ) | 0.000 | (, ) | 0.000 |
| 4th | (, 0.002) | 0.06 | (, ) | 0.012 | (, ) | 0.004 |
| quartiles | ||||||
| 2nd | (, 0.014) | 0.16 | (, 0.016) | 0.18 | (, 0.021) | 0.27 |
| 3rd | (, 0.003) | 0.068 | (, ) | 0.040 | (, ) | 0.034 |
| 4th | (, ) | 0.004 | (, ) | 0.006 | (, ) | 0.006 |
Note: Adjusted for age, sex, education, odor test version at baseline, baseline assessment year, vocabulary, longest held occupation, BMI, smoking, diabetes, heart disease, and cerebrovascular disease. BMI, body mass index; CI, confidence interval; , nitrogen oxide; , particulate matter with aerodynamic diameter less than or equal to .
Discussion
In this population-based sample of older adults, we found exposure to higher quartiles of outdoor air pollution to be associated with faster decline in olfactory identification ability across follow-up, in comparison with exposure to the lowest quartile. Individuals in the two highest quartiles were estimated to follow a trajectory leading to a lower score corresponding to 1 less identified odor over the 12-y follow-up, in comparison with the lowest quartile. The corresponding trajectories for showed a similar pattern but with smaller group differences. Notably, we observed associations between air pollution and olfactory decline at commonly occurring and concentrations, the former being on average well below the current World Health Organization (WHO) guideline of (WHO 2005). These longitudinal relationships persisted after adjusting for important potential confounders, including age, sex, education, occupational background, semantic memory performance, smoking, BMI, and history of cerebrovascular disease, heart disease, or diabetes.
Pathophysiological studies indicate that the olfactory system may be especially susceptible to injury from inspired air, and these findings have been corroborated by epidemiological studies based on proxies for pollution exposure estimations (as reviewed by Ajmani et al. 2016b). However, population-based studies with access to estimates of individual-level pollution levels at residential addresses are scarce. To our knowledge, no longitudinal study has so far examined associations between pollutant exposure and olfactory function over time. A previous cross-sectional population-based study found that residential exposure to in home-dwelling U.S. adults age 57–85 y was significantly associated with olfactory dysfunction (Ajmani et al. 2016a). Likewise, Adams et al. (2016) found similar associations between olfactory impairment and exposure to the nitrogen oxide . In contrast to these studies, we did not observe a negative association between or exposure and baseline olfactory ability in our sample. However, in longitudinal analyses, we found an association between the two highest quartiles of and and faster olfactory decline trajectories. Importantly, excluding participants with anosmia at baseline did not influence these findings, suggesting that the association between air pollution and olfactory decline was not driven by participants who already exhibited an olfactory dysfunction at the start of the study.
In addition to , we found exposure to another air pollutant, , to be linked to olfactory ability. Although research on the effects of and related ambient pollutants with the human olfactory system is scarce, previous studies suggest an association of with brain health. For example, residing in the two highest quartiles of exposure has been linked to an increased risk of dementia in two Swedish population-based studies involving pollutant concentrations comparable to those observed in our study (Andersson et al. 2018; Oudin et al. 2016). Similarly, a previous study on SNAC-K participants showed associations between and and a higher risk of dementia (Grande et al. 2020). It should be noted that in our study area is modeled with road traffic as the primary source, which is why the effects associated with should be seen as effects of the complex mixture of traffic-related pollutants, and most important, the exhaust-related pollutants.
We did not find an association between air pollution and rate of olfactory change when treating pollutant levels as continuous predictors. Similarly, our analyses based on quartiles of pollution exposure did not suggest a statistically significant linear exposure–response relationship between air pollution and change in olfactory ability. This finding is in line with the results from a previous cross-sectional study (Ajmani et al. 2016b) suggesting a nonlinear relationship between pollution exposure and olfactory function. It is conceivable that potential harmful effects of air pollution on the olfactory system may emerge only after reaching a certain threshold of exposure levels. Similar nonlinear associations have previously been obtained regarding pollution exposure from road traffic and future dementia (Oudin et al. 2016). At this point, we cannot offer a satisfactory explanation as to why effect sizes were somewhat stronger for the third quartile in comparison with the fourth quartile of . It is conceivable that our results were affected by variations in indoor air quality, which is influenced by more factors than outside concentrations of air pollution. The amount of air pollution transported inside a dwelling depends significantly on the ventilation and infiltration rate of the outdoor air as well as on human behavior. In turn, these factors are influenced by outdoor air pollution, and compensatory mechanisms are common in areas subjected to high levels of exhaust (as reviewed by Leung 2015). For example, individuals living near highly trafficked roads are less likely to open their windows for a longer amount of time due to disturbance from traffic noise (Leung 2015).
It is noteworthy that we found positive baseline associations between pollutant exposure and olfactory performance when treating air pollution as continuous predictors. The difference at baseline could either result from chance or possibly be reflective of a variety of uncontrolled aspects related to for example overall health and/or socioeconomic status of the participants in residential areas with higher pollution exposure. Although we tried to control for the latter by including years of education and occupational background as covariates, we had no access to a more direct measure of economic status, which may be related to residential address within the area of Kungsholmen. Whereas different confounding factors may influence olfactory performance at the starting point, the longitudinal associations constitute a stronger indication of a possible causal link between pollution exposure and olfactory function. One may also speculate that in populations with comparatively low pollution exposure concentrations in combination with comparatively good health, long-term associations between pollutant exposure and olfaction, reflecting gradual and cumulative damage, are more likely to be detected than cross-sectional associations. We found that older age was related to accelerated olfactory decline trajectories for higher concentration quartiles of , but not for . Conceivably, the olfactory system may be more vulnerable to toxic insults in older age (Doty and Kamath 2014), whereas older adults have also been subjected to longer time periods of accumulation during their lifespans. The discrepancy between and may arise from the fact that emissions, in contrast to , have decreased substantially during the past 30 y in the examined area (Segersson et al. 2017). Consequently, older adults in our sample were exposed to significantly higher exposure concentrations of for longer time periods than were relatively younger participants.
So far, the potential mechanisms behind associations of air pollution and olfactory function are poorly understood, but both direct and indirect pathways are possible. Olfactory neurons are situated in the roof of the nasal cavity and directly exposed to the outside environment (Doty 2003, 2009). As a result, ultrafine airborne pollutants can bypass the blood–brain barrier via the olfactory nerves (Block and Calderón-Garcidueñas 2009; Lucchini et al. 2012; Oberdörster et al. 2004). Animal data and postmortem studies indicate that inhalation of these toxins can lead to cytotoxic and DNA damage of the cells of the olfactory system already at its peripheral stages (i.e., the olfactory epithelium) and that particles deposited in the olfactory mucosa may translocate to the olfactory bulb, where they can induce cytotoxic, inflammatory, and cell stress responses (as reviewed by Ajmani et al. 2016b).
Alternatively, air pollution could affect the olfactory system indirectly. Air pollution is an established risk factor for cardiovascular conditions such as myocardial infarction (Peters et al. 2001), heart failure (Shah et al. 2013), and atherosclerosis (Adar et al. 2013). At the same time, these conditions are overrepresented among individuals with olfactory dysfunction (Palmquist et al. 2020; Schubert et al. 2011) and predictive of a faster rate of olfactory decline (Ekström et al. 2020; Schubert et al. 2015. A previous study based on SNAC-K participants suggested that cerebrovascular and cardiovascular diseases may modify the association between and cognitive decline, such that these diseases may accelerate detrimental effects of pollution on cognition (Grande et al. 2021). In contrast, in follow-up analyses, we did not find that incident cerebrovascular or heart disease accelerated the rate of olfactory decline as a function of pollution exposure. More research is needed before conclusions on potential mechanisms based on vascular pathways can be drawn.
A main strength of our study concerns the longitudinal nature of our data, allowing for inferences about associations between ambient pollution and long-term changes in olfactory function. Previous studies on associations between air pollution and olfaction have used cross-sectional measurements of olfactory ability. However, olfactory function measured over multiple times in the same individuals is likely a more valid measure of olfactory loss than comparisons between individuals. Attrition is an inevitable concern in longitudinal research, and we found that participants with a comparatively better sense of smell at baseline were more likely to remain in the study to undergo subsequent olfactory testing. However, follow-up analyses suggested that this selective dropout was unlikely to affect our results. First, we did not find any evidence for attrition being associated with pollutant quartiles. Second, the rate of olfactory decline and its associations with and exposures remained largely unchanged by modeling olfactory values that represented either the development of an absent sense of smell or no olfactory change during follow-up in the group with only one olfactory assessment.
Another main strength concerns our use of dispersion models of and , enabling participant-specific estimations of exposure levels at residential address. Although we did not have access to dense exposure measurements within our district, reliability of the dispersion model for obtaining pollution levels at individual addresses in the city of Stockholm has previously been shown to be high (Segersson et al. 2017).
Although the dispersion model from which pollution concentrations for residential addresses were derived was based on a large area, covering central and rural regions within the Stockholm county, our study population was from a small residential district within Stockholm city, with comparatively good air quality. Owing to increasingly strict control of ambient air pollution in recent decades, even the higher pollution levels observed in our study are below current WHO guidelines. Although exposure gradients in small-area studies may be smaller than in studies that compare participants over a larger area, they are not necessarily less valid and are certainly quite relevant for evaluating health effects from very local air pollution, e.g., from traffic. In addition, small-area studies may also have some advantages over studies performed over larger regions. For example, variation between participants regarding potentially confounding variables is typically small, or at least smaller than in studies that compare participants over a large geographical area. However, it is likely that our results were affected by the comparatively small range of and at the residential addresses of our participants, such as that the magnitude of the association between air pollution and olfactory loss may be underestimated in our study. Importantly, the fact that we found an association between air pollution and olfactory decline at these low concentration levels of exhaust stresses the importance of further assessments of longitudinal olfactory change in population-based participants subjected to higher contrasts of exposure.
As a result of targeted actions to reduce exhaust in particularly affected areas during recent decades (Segersson et al. 2017), we observed that pollution concentrations significantly declined in our sample for the 5 y before the olfactory baseline assessment. An important finding was that this decrease was most pronounced in the two highest pollution quartiles of and , for which significant associations with olfactory decline were obtained. Follow-up analyses showed attenuated associations between pollution concentrations and olfactory decline when we considered pollution exposure from the baseline year only (when pollution rates were lower), instead of exposure concentrations averaged over the 5 y preceding baseline. Thus, the obtained associations between air pollutants and olfactory decline were likely a result of not only spatial but also temporal variation in air pollution concentrations.
The slope of olfactory decline observed in our study was of a relatively small magnitude, which may in part explain why also its association with air pollution quartiles was relatively weak in effect size. Indeed, average olfactory decline trajectories in normal aging are likely to be subtle, with small changes occurring over the course of several years (Doty and Kamath 2014). It is possible that the assessment of olfactory sensitivity may have added information in terms of these subtle changes in smell function. However, olfactory identification has several advantages over sensitivity in the current research setting. Odor identification is the most commonly selected method in population-based studies and has also been used in most research on the relationship between air pollution and olfaction (as reviewed by Ajmani et al. 2016a), which increases comparability of our results to other studies. Odor identification is also easy to assess, reducing selective dropout from the olfactory test in longitudinal studies. In comparison with identification, olfactory sensitivity is typically operationalized based on perception thresholds for one odorant only. However, even among persons with a normal sense of smell, sensitivity to monomolecular odorants varies greatly, and an individual can be insensitive to one odorant while having a normal perception of another (Bremner et al. 2003; Keller et al. 2012). Given that the odor identification task includes 16 common household odors, it enables assessment of olfactory decline that may have adverse effects for the daily life of the individual.
Longitudinal studies on cognitive decline suggest that associations with potential predictors of age-related change are generally small and difficult to detect (Ritchie et al. 2016). Although longitudinal studies on predictors of olfactory decline are sparse, similar small effect sizes have been observed regarding olfactory loss (Ekström et al. 2020; Schubert et al. 2015). Previous research suggests that olfactory decline in the adult population is mainly driven by either aging or neurodegenerative processes (as reviewed in Olofsson et al. 2021). Apart from these two major factors, interindividual characteristics related to demographics, lifestyle, health, and genetic factors likely play a role. Independently, each of these factors may marginally contribute to the damage of the olfactory system. It is therefore not surprising that our results suggest a contribution of environmental air pollution, with effect sizes that are weak but similar in magnitude to those previously observed. In addition, the relatively weak effect sizes obtained in our study may be explained by the comparatively low and declining pollution concentrations in the Stockholm region. Whereas the low exposure concentrations in this study decrease generalizability of our findings, our results also suggest that airborne pollution is associated with long-term damage to the olfactory system at comparatively low pollution levels.
Conclusion
We found exposure to airborne and particulates to be associated with a faster rate of decline in olfactory identification ability in older adults. Our results suggest that cumulative effects of airborne pollutants on the olfactory system may contribute to olfactory loss in aging, even at comparatively low levels of pollution exposure.
Supplementary Material
Acknowledgments
The authors thank the participants and the staff involved in the data collection and management of the SNAC-K study and M. Josefsson for valuable statistical consulting.
SNAC-K is financially supported by the Swedish Ministry of Health and Social Affairs, the participating County Councils and Municipalities, and the Swedish Research Council. This work was further funded by a research grant from the Swedish Research Council awarded to EL (2017-01759). All authors contributed to the conception and design of the study. I.E. conducted the statistical analyses with the help of D.R. All authors contributed to interpretation of the results. I.E. drafted the first version of the manuscript. All authors critically revised the manuscript for important intellectual content. All authors made a significant contribution to the research and the development of the manuscript and approved the final version for publication.
References
- Adams DR, Ajmani GS, Pun VC, Wroblewski KE, Kern DW, Schumm LP, et al. 2016. Nitrogen dioxide pollution exposure is associated with olfactory dysfunction in older U.S. adults. Int Forum Allergy Rhinol 6(12):1245–1252. December, PMID: , 10.1002/alr.21829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adar SD, Sheppard L, Vedal S, Polak JF, Sampson PD, Diez Roux AV, et al. 2013. Fine particulate air pollution and the progression of carotid intima-medial thickness: a prospective cohort study from the multi-ethnic study of atherosclerosis and air pollution. PLoS Med 10(4):e1001430, PMID: , 10.1371/journal.pmed.1001430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ajmani GS, Suh HH, Pinto JM. 2016b. Effects of ambient air pollution exposure on olfaction: a review. Environ Health Perspect 124(11):1683–1693, PMID: , 10.1289/EHP136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ajmani GS, Suh HH, Wroblewski KE, Kern DW, Schumm LP, McClintock MK, et al. 2016a. Fine particulate matter exposure and olfactory dysfunction among urban-dwelling older US adults. Environ Res 151:797–803, PMID: , 10.1016/j.envres.2016.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson J, Oudin A, Sundström A, Forsberg B, Adolfsson R, Nordin M. 2018. Road traffic noise, air pollution, and risk of dementia - results from the betula project. Environ Res 166:334–339, PMID: , 10.1016/j.envres.2018.06.008. [DOI] [PubMed] [Google Scholar]
- Block ML, Calderón-Garcidueñas L. 2009. Air pollution: mechanisms of neuroinflammation and CNS disease. Trends Neurosci 32(9):506–516, PMID: , 10.1016/j.tins.2009.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blomqvist EH, Brämerson A, Stjärne P, Nordin S. 2004. Consequences of olfactory loss and adopted coping strategies. Rhinology 42(4):189–194, PMID: . [PubMed] [Google Scholar]
- Bremner EA, Mainland JD, Khan RM, Sobel N. 2003. The prevalence of androstenone anosmia. Chem Senses 28(5):423–432, PMID: , 10.1093/chemse/28.5.423. [DOI] [PubMed] [Google Scholar]
- Calderón-Garcidueñas L, Franco-Lira M, Henríquez-Roldán C, Osnaya N, González-Maciel A, Reynoso-Robles R, et al. 2010. Urban air pollution: influences on olfactory function and pathology in exposed children and young adults. Experimental and Toxicologic Pathology 62(1):91–102, PMID: , 10.1016/j.etp.2009.02.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calderón-Garcidueñas L, Maronpot RR, Torres-Jardon R, Henríquez-Roldán C, Schoonhoven R, Acuña-Ayala H, et al. 2003. DNA damage in nasal and brain tissues of canines exposed to air pollutants is associated with evidence of chronic brain inflammation and neurodegeneration. Toxicol Pathol 31(5):524–538, PMID: , 10.1080/01926230390226645. [DOI] [PubMed] [Google Scholar]
- Calderón-Larrañaga A, Vetrano DL, Onder G, Gimeno-Feliu LA, Coscollar-Santaliestra C, Carfí A, et al. 2017. Assessing and measuring chronic multimorbidity in the older population: a proposal for its operationalization. Journals of Gerontology Series A: Biomedical Sciences and Medical Sciences 72(10):1417–1423, PMID: , 10.1093/gerona/glw233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croy I, Symmank A, Schellong J, Hummel C, Gerber J, Joraschky P, et al. 2014. Olfaction as a marker for depression in humans. J Affect Disord 160:80–86, PMID: , 10.1016/j.jad.2013.12.026. [DOI] [PubMed] [Google Scholar]
- Croy I, Zehner C, Larsson M, Zucco GM, Hummel T. 2015. Test–retest reliability and validity of the sniffin’ TOM odor memory test. Chem Senses 40(3):173–179, PMID: , 10.1093/chemse/bju069. [DOI] [PubMed] [Google Scholar]
- Devanand DP, Lee S, Manly J, Andrews H, Schupf N, Doty RL, et al. 2015b. Olfactory deficits predict cognitive decline and Alzheimer dementia in an urban community. Neurology 84(2):182–189, PMID: , 10.1212/WNL.0000000000001132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devanand DP, Lee S, Manly J, Andrews H, Schupf N, Masurkar A, et al. 2015a. Olfactory identification deficits and increased mortality in the community. Ann Neurol 78(3):401–411, PMID: , 10.1002/ana.24447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doty RL. 2003. Morphology of the mammalian olfactory epithelium: form, fine structure, function, and pathology. In Handbook of Olfaction and Gustation Boca Raton, FL: CRC Press, 102–167. [Google Scholar]
- Doty RL. 2008. The olfactory vector hypothesis of neurodegenerative disease: is it viable? Ann Neurol 63(1):7–15, PMID: , 10.1002/ana.21327. [DOI] [PubMed] [Google Scholar]
- Doty RL. 2009. The olfactory system and its disorders. Semin Neurol 29(1):74–81, PMID: , 10.1055/s-0028-1124025. [DOI] [PubMed] [Google Scholar]
- Doty RL. 2012. Olfactory dysfunction in Parkinson disease. Nat Rev Neurol 8(6):329–339, PMID: , 10.1038/nrneurol.2012.80. [DOI] [PubMed] [Google Scholar]
- Doty RL, Kamath V. 2014. The influences of age on olfaction: a review. Front Psychol 5:20, PMID: , 10.3389/fpsyg.2014.00020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doty RL, Shaman P, Applebaum SL, Giberson R, Siksorski L, Rosenberg L. 1984. Smell identification ability: changes with age. Science 226(4681):1441–1443, PMID: , 10.1126/science.6505700. [DOI] [PubMed] [Google Scholar]
- Dureman I. 1960. SRB: 1. Stockholm: Psykologiförlaget. [Google Scholar]
- Ekström I, Larsson M, Rizzuto D, Fastbom J, Bäckman L, Laukka EJ. 2020. Predictors of olfactory decline in aging: a longitudinal population-based study. J Gerontol A Biol Sci Med Sci 75(12):2441–2449, PMID: , 10.1093/gerona/glaa221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekström I, Sjölund S, Nordin S, Nordin Adolfsson A, Adolfsson R, Nilsson L-G, et al. 2017. Smell loss predicts mortality risk regardless of dementia conversion. J Am Geriatr Soc 65(6):1238–1243, PMID: , 10.1111/jgs.14770. [DOI] [PubMed] [Google Scholar]
- Fluitman KS, Nadar HJ, Roos DS, Berendse HW, Keijser BJF, Nieuwdorp M, et al. 2019. The association of olfactory function with BMI, appetite, and prospective weight change in Dutch community-dwelling older adults. J Nutr Health Aging 23(8):746–752, PMID: , 10.1007/s12603-019-1241-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grande G, Ljungman P, Eneroth K, Bellander T, Rizzuto D. 2020. Association between cardiovascular disease and long-term exposure to air pollution with the risk of dementia. JAMA Neurol 77(7):801–809, PMID: , 10.1001/jamaneurol.2019.4914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grande G, Wu J, Ljungman PL, Stafoggia M, Bellander T, Rizzuto D. 2021. Long-term exposure to PM 2.5 and cognitive decline: a longitudinal population-based study. J Alzheimer’s Dis 80(2):1–9, PMID: , 10.3233/jad-200852. [DOI] [PubMed] [Google Scholar]
- Harita M, Miwa T, Shiga H, Yamada K, Sugiyama E, Okabe Y, et al. 2019. Association of olfactory impairment with indexes of sarcopenia and frailty in community‐dwelling older adults. Geriatr Gerontol Int 19(5):384–391, PMID: , 10.1111/ggi.13621. [DOI] [PubMed] [Google Scholar]
- Hudson R, Arriola A, Martínez-Gómez M, Distel H. 2006. Effect of air pollution on olfactory function in residents of Mexico city. Chem Senses 31(1):79–85, PMID: , 10.1093/chemse/bjj019. [DOI] [PubMed] [Google Scholar]
- Hummel T, Kobal G, Gudziol H, Mackay-Sim AJEA. 2007. Normative data for the “sniffin’ Sticks” including tests of odor identification, odor discrimination, and olfactory thresholds: an upgrade based on a group of more than 3,000 subjects. Eur Arch Otorhinolaryngol 264(3):237–243, PMID: , 10.1007/s00405-006-0173-0. [DOI] [PubMed] [Google Scholar]
- Hummel T, Sekinger B, Wolf SR, Pauli E, Kobal G. 1997. Sniffin’ sticks’: olfactory performance assessed by the combined testing of odor identification, odor discrimination and olfactory threshold. Chem Senses 22(1):39–52, PMID: , 10.1093/chemse/22.1.39. [DOI] [PubMed] [Google Scholar]
- Keller A, Hempstead M, Gomez IA, Gilbert AN, Vosshall LB. 2012. An olfactory demography of a diverse metropolitan population. BMC Neurosci 13(1):1–17, PMID: , 10.1186/1471-2202-13-122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagergren M, Fratiglioni L, Hallberg IR, Berglund J, Elmståhl S, Hagberg B, et al. 2004. A longitudinal study integrating population, care and social services data. The Swedish National Study on Aging and Care (SNAC). Aging Clin Exp Res 16(2):158–168, PMID: , 10.1007/BF03324546. [DOI] [PubMed] [Google Scholar]
- Laudisio A, Navarini L, Margiotta DPE, Fontana DO, Chiarella I, Spitaleri D, et al. 2019. The association of olfactory dysfunction, frailty, and mortality is mediated by inflammation: results from the InCHIANTI study. J Immunol Res 2019:3128231, PMID: , 10.1155/2019/3128231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laukka EJ, Lövdén M, Herlitz A, Karlsson S, Ferencz B, Pantzar A, et al. 2013. Genetic effects on old-age cognitive functioning: a population-based study. Psychol Aging 28(1):262–274, PMID: , 10.1037/a0030829. [DOI] [PubMed] [Google Scholar]
- Leung DY. 2015. Outdoor-indoor air pollution in urban environment: challenges and opportunity. Front Environ Sci 2:69, 10.3389/fenvs.2014.00069. [DOI] [Google Scholar]
- Lietzau G, Davidsson W, Östenson C-G, Chiazza F, Nathanson D, Pintana H, et al. 2018. Type 2 diabetes impairs odour detection, olfactory memory and olfactory neuroplasticity; effects partly reversed by the DPP-4 inhibitor linagliptin. Acta Neuropathol Commun 6(1):1–15, PMID: , 10.1186/s40478-018-0517-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucchini RG, Dorman DC, Elder A, Veronesi B. 2012. Neurological impacts from inhalation of pollutants and the nose–brain connection. Neurotoxicology 33(4):838–841, PMID: , 10.1016/j.neuro.2011.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marseglia A, Fratiglioni L, Kalpouzos G, Wang R, Bäckman L, Xu W. 2019. Prediabetes and diabetes accelerate cognitive decline and predict microvascular lesions: a population-based cohort study. Alzheimer Dement 15(1):25–33, PMID: , 10.1016/j.jalz.2018.06.3060. [DOI] [PubMed] [Google Scholar]
- Negoias S, Croy I, Gerber J, Puschmann S, Petrowski K, Joraschky P, et al. 2010. Reduced olfactory bulb volume and olfactory sensitivity in patients with acute major depression. Neuroscience 169(1):415–421, PMID: , 10.1016/j.neuroscience.2010.05.012. [DOI] [PubMed] [Google Scholar]
- Oberdörster G, Sharp Z, Atudorei V, Elder A, Gelein R, Kreyling W, et al. 2004. Translocation of inhaled ultrafine particles to the brain. Inhalation Toxicology 16(6–7):437–445, PMID: , 10.1080/08958370490439597. [DOI] [PubMed] [Google Scholar]
- Olivier JGJ, Bouwman AF, Van der Hoek KW, Berdowski JJM. 1998. Global air emission inventories for anthropogenic sources of NOx, NH3 and N2O in 1990. Environ Pollut 102(1):135–148, 10.1016/S0269-7491(98)80026-2. [DOI] [Google Scholar]
- Olofsson JK, Ekström I, Larsson M, Nordin S. 2021. Olfaction and aging: a review of the current state of research and future directions. IPerception 12(3):20416695211020331, PMID: , 10.1177/20416695211020331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oudin A, Forsberg B, Adolfsson AN, Lind N, Modig L, Nordin M, et al. 2016. Traffic-related air pollution and dementia incidence in northern Sweden: a longitudinal study. Environ Health Perspect 124(3):306–312, PMID: , 10.1289/ehp.1408322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmquist E, Larsson M, Olofsson JK, Seubert J, Bäckman L, Laukka EJ. 2020. A prospective study on risk factors for olfactory dysfunction in aging. J Gerontol A Biol Sci Med Sci 75(3):603–610, PMID: , 10.1093/gerona/glz265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters A, Dockery DW, Muller JE, Mittleman MA. 2001. Increased particulate air pollution and the triggering of myocardial infarction. Circulation 103(23):2810–2815, PMID: , 10.1161/01.CIR.103.23.2810. [DOI] [PubMed] [Google Scholar]
- Pinto JM. 2011. Olfaction. Proc Am Thorac Soc 8(1):46–52, PMID: , 10.1513/pats.201005-035RN. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinto JM, Wroblewski KE, Kern DW, Schumm LP, McClintock MK. 2014. Olfactory dysfunction predicts 5-year mortality in older adults. PLoS One 9(10):e107541, PMID: , 10.1371/journal.pone.0107541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranft U, Schikowski T, Sugiri D, Krutmann J, Krämer U. 2009. Long-term exposure to traffic-related particulate matter impairs cognitive function in the elderly. Environ Res 109(8):1004–1011, PMID: , 10.1016/j.envres.2009.08.003. [DOI] [PubMed] [Google Scholar]
- Ritchie SJ, Tucker-Drob EM, Cox SR, Corley J, Dykiert D, Redmond P, et al. 2016. Predictors of ageing-related decline across multiple cognitive functions. Intelligence 59:115–126, PMID: , 10.1016/j.intell.2016.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schubert CR, Cruickshanks KJ, Fischer ME, Klein BE, Klein R, Pinto AA. 2015. Inflammatory and vascular markers and olfactory impairment in older adults. Age Ageing 44(5):878–882, PMID: , 10.1093/ageing/afv075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schubert CR, Cruickshanks KJ, Klein BE, Klein R, Nondahl DM. 2011. Olfactory impairment in older adults: five‐year incidence and risk factors. Laryngoscope 121(4):873–878, PMID: , 10.1002/lary.21416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segersson D, Eneroth K, Gidhagen L, Johansson C, Omstedt G, Nylén AE, et al. 2017. Health impact of PM10, PM2.5 and black carbon exposure due to different source sectors in Stockholm, Gothenburg and Umea, Sweden. Int J Environ Res Public Health 14(7):742, PMID: , 10.3390/ijerph14070742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seubert J, Laukka EJ, Rizzuto D, Hummel T, Fratiglioni L, Bäckman L, et al. 2017. Prevalence and correlates of olfactory dysfunction in old age: a population-based study. J Gerontol A Biol Sci Med Sci 72(8):1072–1079, PMID: , 10.1093/gerona/glx054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah AS, Langrish JP, Nair H, McAllister DA, Hunter AL, Donaldson K, et al. 2013. Global association of air pollution and heart failure: a systematic review and meta-analysis. Lancet 382(9897):1039–1048, PMID: , 10.1016/S0140-6736(13)60898-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shusterman D. 2011. The effects of air pollutants and irritants on the upper airway. Proc Am Thorac Soc 8(1):101–105, PMID: , 10.1513/pats.201003-027RN. [DOI] [PubMed] [Google Scholar]
- Siegel JK, Wroblewski KE, McClintock MK, Pinto JM. 2019. Olfactory dysfunction persists after smoking cessation and signals increased cardiovascular risk. Int Forum Allergy Rhinol 9(9):977–985. September, PMID: , 10.1002/alr.22357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanciu I, Larsson M, Nordin S, Adolfsson R, Nilsson LG, Olofsson JK. 2014. Olfactory impairment and subjective olfactory complaints independently predict conversion to dementia: a longitudinal, population-based study. J Int Neuropsychol Soc 20(2):209–217, PMID: , 10.1017/S1355617713001409. [DOI] [PubMed] [Google Scholar]
- State of Global Air. 2020. www.stateofglobalair.org/. [accessed 12 September 2021]
- Sun GH, Raji CA, MacEachern MP, Burke JF. 2012. Olfactory identification testing as a predictor of the development of Alzheimer’s dementia: a systematic review. Laryngoscope 122(7):1455–1462, PMID: , 10.1002/lary.23365. [DOI] [PubMed] [Google Scholar]
- WHO (World Health Organization). 2005. www.who.int/airpollution/publications/aqg2005/en/ [accessed 8 March 2021].
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.