Abstract
Background
Prehabilitation is a promising method to enhance postoperative recovery, especially in patients suffering from cancer. Particularly during times of social distancing, providing home-based programmes may have become a suitable solution to increase compliance and effectiveness.
Methods
In line with the PRISMA guidelines, a systematic review was conducted including trials that investigated the effect of home-based prehabilitation (HBP) in patients undergoing surgery for cancer. The primary outcome was postoperative functional capacity (6 min walk test, 6MWT). Secondary outcomes were postoperative complications and compliance.
Results
Five randomized controlled trials were included with 351 patients undergoing surgery for colorectal cancer, oesophagogastric cancer, bladder cancer and non-small cell lung cancer. Three studies presented results of significant progress after eight weeks. The meta-analysis showed a significant improvement of the 6MWT in the prehabilitation group compared to the control group preoperatively (MD 35.06; 95% CI 11.58 to 58.54; p = .003) and eight weeks postoperatively (MD 44.91; 95% CI 6.04 to 83.79; p = .02) compared to baseline. Compliance rate varied from 63% to 83% with no significant difference between prehabilitation and control groups. These data must be interpreted with caution because of a high amount of heterogeneity and small sample sizes.
Discussion
In conclusion, HBP may enhance overall functional capacity of patients receiving oncological surgery compared to standard of care. This could be a promising alternative to hospital-based prehabilitation regarding the current pandemic and further digitalization in the future. In order to increase accessibility and effectiveness of prehabilitation, home-based solutions should be further investigated.
Keywords: Cancer, Surgery, Prehabilitation, Exercise therapy and clinical trials
1. Background
According to the World Health Organization (WHO), an average of 18.1 million people are diagnosed with cancer annually worldwide [1,2]. A surgical intervention remains the primary curative treatment for most types of cancer. However, surgery can lead to postoperative complications, functional disability and an overall decrease in physical condition. This can result in delayed recovery, extent of hospital stay (LOS) and risk of readmission [3,4]. As such, abdominal surgery causes a 20–40% reduction in overall functional capacity. Patients not suffering complications may still encounter physical disability for up to six months following surgery [3].
Successful perioperative outcomes rely on a multi-faceted team approach through optimisation of patients before, during and after surgery [[5], [6], [7]]. Enhanced recovery programmes have shown great merit in improving outcomes through intra- and postoperative interventions. An upcoming field of interest is the enhancement of patients' clinical condition through interventions delivered in the time-window available before surgery; prehabilitation [8,9]. This is a multimodal way of enhancing the patients' functional capacity prior to surgery with the primary aim to help withstand the physiological and psychological stress of surgery, and to reduce the recovery period [[10], [11], [12], [13], [14]]. By enhancing the body's perioperative resilience, prehabilitation interventions may reduce adverse outcomes [[15], [16], [17]]. Furthermore, by becoming actively involved, prehabilitation offers patients to play a key role in their own treatment and recovery.
Initially, prehabilitation programmes were trialed in hospital settings, which demonstrated beneficial effects for patients awaiting surgery. Patients showed improvements in functional capacity, physical and mental health, a faster recovery and an overall better quality of life after surgery [[18], [19], [20], [21], [22], [23]]. However, hospital-based initiatives are not suitable for up to 50% of patients, mainly due to other commitments, travel difficulties, hospital distance and costs, multi-morbidity and discomfort in group settings [24]. The COVID-19 pandemic has shifted the focus to providing health care at home for a variety of reasons, including the need for shielding in many patients, and enforced closure of leisure facilities [25,26]. Especially for patients with cancer who may be immunocompromised and at increased risk for infections, it might be more convenient and safer to prepare for an operation at home. This review aimed to assess the effectiveness of home-based prehabilitation (HBP) compared to standard of care on functional capacity and treatment outcomes, including compliance and postoperative complications, in patients undergoing cancer surgery.
2. Methods
2.1. Literature search
This systematic review and meta-analysis was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) statement [27]. A comprehensive search was performed in the bibliographic databases Pubmed, Embase and Cinahl from inception up to October 13th, 2021 in collaboration with a medical information specialist (LS). The following search terms were used (including synonyms and closely related words) as index terms or free-text words: ‘cancer’ and ‘surgery’ and ‘prehabilitation’ and ‘exercise therapy’ and ‘clinical trials’ (Supplementary Information A). The search was performed without date or language restriction. Duplicate articles were excluded (LS) using EndNote X20.01 (Clarivatetm), following the Amsterdam Efficient Deduplication (AED)-method and the Bramer-method [28,29].
2.2. Study selection
All titles and abstracts were reviewed for relevance. Full-text articles were read when eligible or when the eligibility was unclear. Two independent reviewers (TG and JP) screened all articles to assess them for eligibility (Supplementary Information B). A third reviewer (EB) was available for discussion in case of a disagreement.
Inclusion criteria were: all randomized controlled trials of patients of 18 years and older undergoing elective surgery for all types of cancer. Studies had to include multi-modal interventions (including physical training (aerobic and resistance training) and nutritional support) for a median of two weeks. The term ‘home-based’ prehabilitation encompasses intervention delivery of the majority of the program a) at home residence and b) at local health/leisure facilities such as with a physiotherapist or in a community gym. All studies had to assess exercise capacity using the 6-min walk test (6MWT) at several timepoint pre- and postoperative. The effects had to be reported as mean change from baseline to each timepoint at which the measurement was assessed. Also, the effect on treatment outcome had to be reported (i.e. postoperative complication, length of stay, re-admission rate).
Exclusion criteria were: metastatic disease, multi-organ resection, palliative surgery and specific cancer related prehabilitation (e.g. arm exercises after breast cancer surgery or pelvic exercises after prostate cancer surgery). The control group consisted of participants receiving standard of care consisting of operative risk and anesthesia assessment, medication management, perioperative blood management and smoking cessation. Control groups using standardized perioperative care according to the Enhanced Recovery After Surgery (ERAS) programs were also included. Those programs consist of preoperative nutrition and exercise advise, perioperative minimal invasive surgery, epidural analgesia when possible, postoperative early oral nutrition, physiotherapy and early mobilization to shorten hospital stay [5,30].
2.3. Data extraction and outcomes
The data extraction of the included articles was conducted by two reviewers, where one review author extracted all data (TG) and one review author independently extracted all numeric data (HZ). A data collection form was used to perform the data extraction (Supplementary Information C).
The primary outcome was postoperative functional capacity evaluated by the change of 6MWT (baseline vs postoperative) in all trials. The 6MWT is a validated test used as a measurement for postoperative recovery in surgery where studies aim to improve the functional capacity [31,32]. The 6MWT evaluates functional exercise capacity, where a change in walking distance in metres (m) reflects a change in functional capacity and the individuals ability to perform basic activities of daily living [33,34]. In this setting the maximum distance covered in 6 min (at a comfortable pace) was used as the outcome assessed at different time point pre- and postoperatively. The change in covered metres from baseline to specific timepoints reflected the change in functional capacity. This data was expressed as mean (standard deviation).
The secondary outcomes were compliance, length of hospital stay (LOS), re-admission rate, emergency department visits, mortality and postoperative complication rate using the Clavien-Dindo classification [35].
2.4. Risk of bias assessment
To ascertain the validity or methodological heterogeneity of each included study, two reviewers (TG and EB) individually assessed seven domains on possible sources of bias according to the Cochrane tool for risk of bias [36].
2.5. Statistical analysis
To perform a consistent quantitative analysis all studies had to report the same outcome measurements to be included in the meta-analysis. Continuous data were presented using the mean difference (MD), estimating the average change of the intervention compared to the control [37]. Continuous outcomes were measured using an inverse variance method. The Mantel-Haenszel estimator was used to measure dichotomous outcomes like odds ratio's (OR) or risk ratio's (RR).
To address inconsistencies in results amongst studies that exceed differences due to chance, the statistical heterogeneity was assessed. Heterogeneity was described as I2 (deriving from Q as the chi-squared statistic and df its degree of freedom). According to the Cochrane Handbook it can be classified in low (<25%), moderate (25–75%) and considerable (>75%) heterogeneity [38,39]. The data were considered to be statistically significant at an alpha of 0.05. A random effect model was used because fewer than ten studies got included in this review. All data was displayed using a forest plot. The meta-analyses were performed using Review Manager software 5.340.
3. Results
3.1. Search results
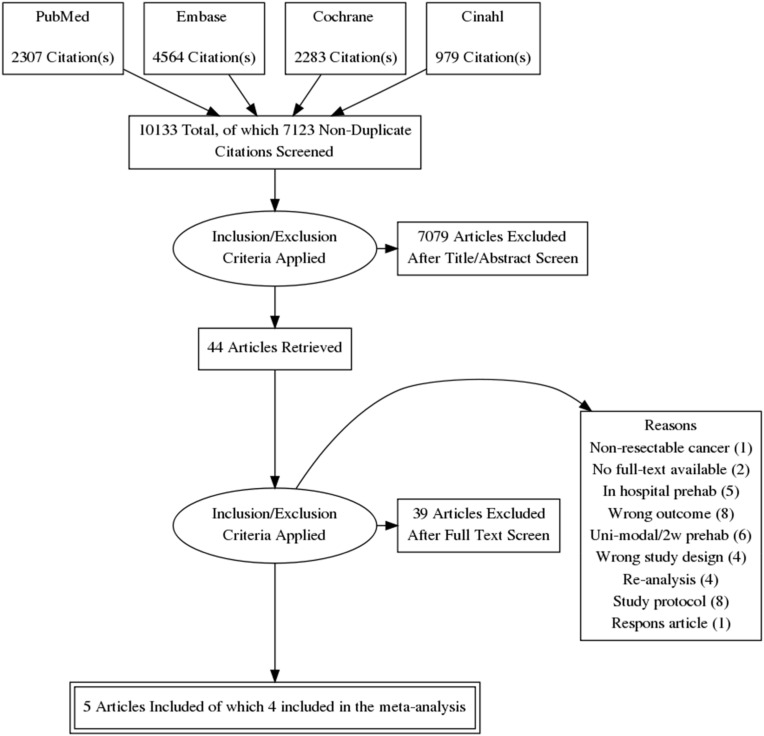
The literature search generated a total of 10.133 references. After deduplication the search identified 7123 articles of which 44 were included. Of these 44 studies again 39 were excluded for not meeting inclusion criteria which made 5 RCT's relevant for analysis (Fig. 1 ) [[41], [42], [43], [44], [45]]. There were a noticeable number of pilot studies among the articles. These were separately discussed since they did not fit the inclusion criteria but were considered relevant. For the meta-analysis one study was excluded due to unpublished data [42].
Fig. 1.
PRISMA flow diagram displaying the selection of studies and reasons for exclusion.
3.2. Risk of bias assessment
Because of the nature of prehabilitation it was not possible to blind participants and personnel to group allocation and therefore all included trials had a high risk of performance bias [[41], [42], [43], [44], [45]]. The risk of bias summary presents the judgement of each source of bias for each study (Fig. 2 and supplementary E1). All trials stated that outcome assessors were blinded to the allocation of patients. Nonetheless, they did not specify the methods of blinding and were therefore judged as unclear risk of bias [[41], [42], [43], [44], [45]]. Three trials had a low risk of bias regarding handling missing data. They either used imputations to handle missing data or had well-motivated loss to follow-up [41,44,45]. There was almost 50% reporting bias across the included trials mainly on account of a selective choice of data for an outcome and selective under-reporting of data. Liu et al. only reported data on mean difference of the change in functional capacity [42]. The study of Minnella et al. (2019) had a high risk of reporting bias due to an inadequate way of showing their results in a whisker plot (using standard error instead of standard mean) and made a conclusion which was not in line with their results [44].
Fig. 2.
Risk of bias summary.
3.3. Patient characteristics
In all studies combined, there were a total of 351 participants of whom 177 received a prehabilitation intervention before elective surgery, and 174 participants who received standard of care. Two studies evaluated prehabilitation in patients with colorectal cancer undergoing surgery [41,43]. One study evaluated prehabilitation in patients undergoing Video Assisted Thoracoscopy (VATS) lobectomy for non-small cell lung cancer (NSCLC) [42], one study evaluated patients undergoing radical cystectomy for bladder cancer [44] and one study evaluated patients undergoing upper abdominal surgery for oesophagogastric cancer [45].
Baseline characteristics between groups were similar in every study except for the age distribution in the study by Bousquet-Dion et al. [43] (Table 1 ). Participants were significantly younger in the control group compared to those in the prehabilitation group (mean age 71 years [range 54.5–74.5] versus (vs) 74 [67.5–78], respectively; P = .05); the control group had fewer participants >75 years of age although this did not reach significance (23% vs 43%, P = .098). Participants in the study by Liu et al. were generally younger than other included trials, as they excluded all patients older than 70 years [42]. Four out of five studies included patients receiving neo-adjuvant therapy, with one study excluding these patients [42]. Minnella et al. (2018) included unequally sized groups regarding patients receiving neo-adjuvant therapy (I: 77% (20) vs C: 60% (15)) [45].
Table 1.
Patient characteristics (p = prehab, c = control, ERAS = enhanced recovery program,amedian and interquartile range, Δ = mean change).
| Study | Design | Inclusion Date |
Type of cancer | Patients (n) |
Age (Mean, %) |
Male (n, %) |
Neoadjuvant therapy (n, %) |
ERAS | Duration prehab (median days) | Primary outcome | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| P | C | P | C | P | C | P | C | |||||||
| Gillis, 2014 [41] | RCT | 11-2011/03-2013 | Colorectal cancer | 38 | 39 | 65.7 (13.6) | 66.0 (9.1) | 21 (55) | 27 (69) | 10 (26) | 8 (21) | Yes | 24 | Δ 6MWT |
| Liu, 2019 [42] | RCT | 03-2017/12-2017 | NSCLC | 37 | 36 | 56.2 (10.3) | 56.2 (8.7) | 12 (32) | 11 (31) | – | – | No | 15 | Δ 6MWT |
| Bousquet-Dion, 2017 [43] | RCT | 12-2013/08-2015 | Colorectal cancer | 37 | 26 | 74 [67.5 to 78] a | 71 [54.5 to 74.5] a | 30 (81) | 16 (62) | 5 (14%) | 4 (15%) | Yes | 32 | Δ 6MWT |
| Minnella, 2018 [45] | RCT | 02-2013/02-2017 | Esophago-gastric cancer | 26 | 25 | 67.3 (7.4) | 68 (11.6) | 18 (69) | 20 (80) | 20 (77) | 15 (60) | Yes | 36 | Δ 6MWT |
| Minnella, 2019 [44] | RCT | 08-2013/10-2017 | Bladder cancer | 35 | 35 | 69.7 (10.2) | 66.0 (10.2) | 22 (62.9) | 27 (77.1) | 18 (51.4) | 17 (48.6) | No | 27 | Δ 6MWT |
3.4. Intervention characteristics
All studies were single-blinded RCTs performed in a single center of which four trials were conducted in the same center in Canada [41,[43], [44], [45]]. The duration of intervention differed from a median of 15–36 days.
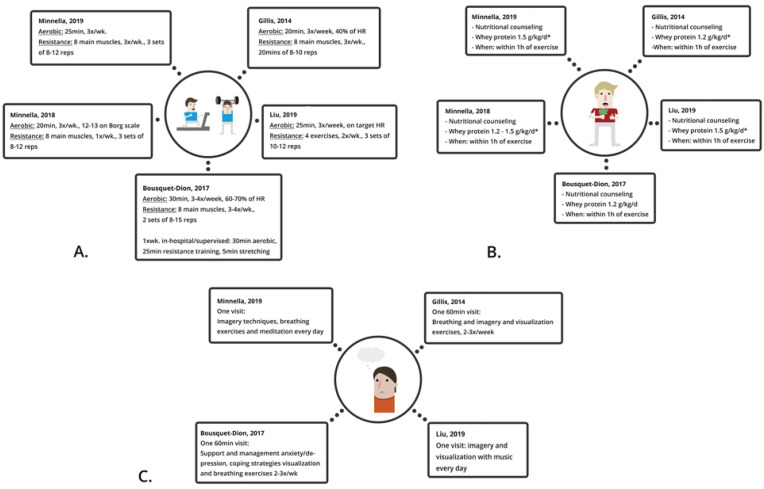
All studies included at-home prehabilitation consisting of 1) training which was divided in aerobic and resistance training. Aerobic training consisted of walking, jogging, cycling or swimming and the intensity was measured using a heart rate monitor or the Borg Scale of Perceived Exertion (RPE) (a scale from 6 to 20 where 6 rates an easy work out and 20 a hard work out). Participants had to reach an exertion rate of 12 or 13 which equates to ‘somewhat hard but able to continue’ [46]. Sessions varied from 25 to 30 min, 3–4 days a week. Resistance training consisted of four to eight exercises per training focused on the main muscle groups of the body where two studies specified the main muscle groups (quadriceps, hamstrings, chest, back, biceps, triceps, shoulders and abdominal wall) [42,45]. The frequency differed from 1 to 4 times a week with an intensity of 1–3 sets including 8–15 repetitions. The intensity could be enhanced using resistance bands [[41], [42], [43], [44]]. Bousquet-Dion et al. contained a once a week supervised, in-hospital, exercise training [43]. 2) Nutritional counseling consisted of protein intake using whey supplements. The dosage varied from 1.2 to 1.5 g/kg/d. Two trials subscribed whey supplements only if the daily requirement of protein was not met in their diet [43,44]. All participants received nutritional counseling, where Minnella et al. (2019) specified the goal to avoid underfeeding and overfeeding [40]. Four studies included 3) psychological support. This included one appointment with a psychologist who taught participants breathing exercises, imagery techniques and coping strategies to reduce distress symptoms [[41], [42], [43], [44]]. In three studies both groups received medication management and smoking cessation counseling [42,44,45]. All intervention characteristics are shown in Fig. 3 .
Fig. 3.
Intervention characteristics
a) Physical intervention b) Nutritional intervention c) Psychological intervention
∗if patients didn't meet daily requirement in diet
∗∗enhanced recovery after surgery [5].
Three studies used the ERAS protocol as standard of care [41,43,45]. To assess compliance all studies gave participants a daily diary or logbook to record all activities and they monitored participants through weekly telephone calls.
All studies reported multiple assessments time-points (Table 2 ). The first assessment was at baseline which indicated the time before prehabilitation commenced. The second assessment was preoperatively, just before surgery when the prehabilitation intervention had been completed (only Liu et al. specified the timing; one day before surgery) [42]. Three studies performed a third and fourth assessment at four and eight weeks postoperatively [41,43,44]. Minnella et al. (2018) performed a third assessment between four and eight weeks postoperatively without further specification [45]. Liu et al. performed a third assessment thirty days postoperatively [42].
Table 2.
Summary of reported data on different time points. (postop = postoperative).
| Mean change in 6MWT (compared to baseline) | Preoperatively | Four weeks postop | Four to eight weeks postop | Eight weeks postop | 30 days postop | Conclusion of mean change in 6MWT (compared to baseline) |
|---|---|---|---|---|---|---|
| Gillis, 2014 [41] | X | X | + Significant difference in the prehab group at both time points | |||
| Liu, 2019 [42] | X | X |
+ Overall significant increase of 60.9 m in prehab group (mixed effects model) − No significant difference at both time points |
|||
| Bousquet-Dion, 2017 [43] | X | X | X |
+ Significant increase in inactive participants (subgroup analysis) − No significant difference at any time point |
||
| Minnella, 2018 [45] | X | X | + Significant difference in prehab group at both time points | |||
| Minnella, 2019 [44] | X | X | X |
+ Significant difference at 4 weeks of less decline in prehab group − No significant difference preoperatively and at 8 weeks |
The primary outcome in all studies was the change in functional capacity in relation to baseline [[41], [42], [43], [44], [45]].
3.5. Primary outcome
Functional capacity was the primary outcome in all included studies using the 6MWT (Supplementary D1).
Gillis et al. measured an overall significant increase in functional capacity between groups, in favour of the prehabilitation group (P = .032). They measured a significant increase in walking distance preoperatively (intervention (I) +25.2 m vs control (C) −16.4 m, P < .001) and after eight weeks postoperatively (I: +23.4 m vs C: 21.8 m, P = .020) both compared to baseline [41].
Liu et al. reported an overall significant increase of 60.9 m in 6MWT in the prehabilitation group compared to the control group derived from a mixed effects model (95%CI (32.4–89.5), P < .0001). They measured an increase of functional capacity compared to baseline preoperatively (I: +45.1 m vs C: +3.8 m) and at 30-days postoperatively (I: +21.5 m vs C: 36.1 m) although not reported if this is significant [42].
Bousquet et al. did not find a significant difference between the prehabilitation and control group. They performed a subgroup analyses where they categorized participants as inactive and active. They reported that participants in the prehabilitation group who initially categorized as inactive had a greater change in their functional capacity (OR 7.07 [1.10–45.51]) [43].
Minnella et al. (2018) reported an overall significant increase of functional capacity. They measured an increase preoperatively (I: +36.9 m vs C: 22.8 m, P < .001) and 4–8wk postoperatively (I: +15.4 m vs C: 81.8 m, P < .001), both compared to baseline [45].
Minnella et al. (2019) showed no significant difference in functional capacity between groups preoperatively and at 8 weeks postoperatively compared to baseline. However, at 4 weeks postoperatively compared to baseline they reported less decline in functional capacity in the prehabilitation group versus the control group (I: 15.4 m vs C: 97.9 m, P = .014) [44].
A meta-analysis was performed on the change in 6MWT from baseline to different time points pre- and postoperatively [41,[43], [44], [45]]. Liu et al. was not included in the meta-analysis due to selective under-reporting of the data of several time measurements [42].
A meta-analysis of the preoperative change in 6MWT distance compared to baseline showed a significantly higher walking distance in the prehabilitation group (MD 35.06; 95% CI 11.58 to 58.54; p = .003, I2 = 67%) (Supplementary E2).
Only 2 studies reported the change in 6MWT distance measured 4 weeks postoperatively from baseline due to incomplete data, therefore no meta-analysis was performed on this timepoint.
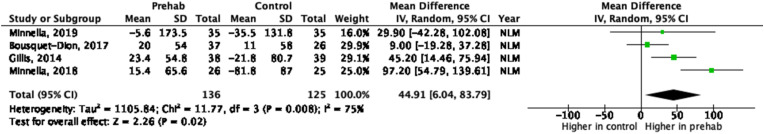
The meta-analysis of the 8 weeks changes postoperatively from baseline in 6MWT showed a higher walking distance in the prehabilitation group compared to the control group (MD 44.91; 95% CI 6.04 to 83.79; p = .02, I2 = 75%) (Fig. 4 ). Strong evidence of a considerable heterogeneity was observed (75%, p = .02).
Fig. 4.
Forest plot and meta-analysis of change in 6MWT distance between baseline and 8 weeks post-operative.
3.6. Secondary outcomes
Compliance, postoperative complication rate, length of hospital stay, readmission and emergency visit and compliance rate were secondary outcomes of this review (Supplementary D1).
Adherence rate varied from 63% to 83% between studies (Supplementary D2) [[41], [42], [43], [44], [45]]. Liu et al. considered a compliance level of 70% as the minimal requirement to perform analysis where no participants were excluded due to low compliance [42]. Both studies from Minnella et al. showed only an overall postoperative compliance result [44,45]. Compliance was stimulated by peer-to-peer motivation through weekly telephone calls through which assessors collected standardized questionnaire. Three studies specified the information obtained in those calls (information on frequency, intensity and duration of exercise, additional physical exercise, quantity and frequency of whey supplements) [41,43,44]. All participants received a daily diary or logbook where they could track their compliance. Compliance was calculated based on the information in logbooks and the information obtained in the phone calls.
All studies provided data on the postoperative complications using the Clavien-Dindo classification [[41], [42], [43], [44], [45]]. The mean complication rate across 4 of the 5 studies was 43% in the prehabilitation group versus 50% in the control group (Liu et al. failed to publish overall complication scores) [41,[43], [44], [45]]. None of the studies showed a significant difference in any complication rate between groups. A meta-analysis was performed on the 30-day postoperative complication rate and this showed no significant difference between prehabilitation and control groups across all studies (OR 0.78; 95% CI 0.47 to 1.32; p = .36, I2 = 0%) (Supplementary E3).
All studies reported LOS. None of them reported a significant difference between the prehabilitation and control group in terms of LOS [[41], [42], [43], [44], [45]].
Four studies reported readmission rate and emergency visits. None of the studies found a significant difference between groups in either readmission rate nor emergency visits (Supplementary E4/E5) [41,[43], [44], [45]].
4. Discussion
This review suggests that pre- and postoperative functional capacity can be enhanced by HBP prior to oncological surgery. However, the results are not sufficient to illustrate a reduction of postoperative complications, hospital LOS and readmission rates.
The research field regarding prehabilitation is promising but relatively young (all included studies were published in 2014 or later). Due to its multifactorial character, it continues to introduce a number of research questions regarding the type of patients, the method of prehabilitation, its duration and its location. Although frail patients might benefit from prehabilitation, they may also experience difficulties attending the hospital on a frequent basis [47,48]. The aim of this review was to assess the possibility of at-home prehabilitation.
There is an ongoing debate as to whether the clinical effectiveness and intervention fidelity of HBP can be enhanced with more remote supervision [49,50]. The general thought used to be that adherence was larger in supervised prehabilitation, however just as seen in Bousquet-Dion et al. a supervised session does not necessarily improve adherence rate [43]. Adherence rates differed among all included studies yet none were significant [[41], [42], [43], [44], [45]]. A possible explanation may be the lack of a validated score measuring adherence rate which make outcomes more reliable [51]. Recent studies have shown that if prehabilitation is tailored to the environment of the patient motivation and adherence will increase [49,52,53]. There is a short supply of data on the effect of HBP programmes, the present data showed a significant improvement in pre- and postoperative functional capacity in the prehabilitation groups in a home-based setting. This can support previous data on prehabilitation trialed in the hospital setting and can hereby broaden the impactability of prehabilitation programmes [[18], [19], [20], [21], [22], [23]].
The effectiveness of HBP on treatment outcomes cannot be confirmed by this review. Due to the multifactorial nature of prehabilitation and its number needed to treat for prehabilitation to work these included studies might underestimate the effect of prehabilitation. For studies to confirm a reliable effect the number of participants should be higher. Furthermore, at the level of methodology, prehabilitation as a research field is challenging. There is an overall lack of pioneering research groups investigating worldwide resulting in four studies originating from the same research group in a Canadian hospital, posing a risk for location bias [41,[43], [44], [45]]. The overall validity of this study is weakened by the small amount of included studies. Sample sizes are small most likely due to the loss to follow up which was substantial among four studies [41,[43], [44], [45]]. Liu et al. used an intention to treat analysis although they excluded the patients lost to follow-up [42]. Minnella et al. (2018) had the most lost to follow up due to hospital distance [45].
Included studies showed a variation of study designs regarding the composition and duration of prehabilitation programmes, different cancer types and variation in operations. Furthermore, outcome measures used to quantify the effect of prehabilitation differ among studies. The lack of standardized prehabilitation programs and key standards avoids study comparability. One study included home based prehabilitation combined with a supervised, in-hospital training once a week which makes it hard to weigh up to the completely HBP of the other studies [43]. Unfortunately, the combination of exercises included in the prehabilitation differed, as did the patient-specific execution of the exercises.
The required duration of prehabiliation in order to obtain a significant effect is still a matter of debate. Recent literature emphasizes the fact that delayed surgery in colorectal cancer is not associated with adverse survival rates [54,55]. For most types of cancer this could potentially mean that there is a wider window to optimize patients before and after surgery with prehabilitation, with regard to the mental burden of patients that have been diagnosed with cancer and their desire to be operated as quickly as possible [54]. Although one study excluded patients receiving neoadjuvant therapy [42], it is becoming more usual in standard of care in multiple types of cancer, which will broaden the window of opportunity allowing patients to prehabilitate during and before (neo)adjuvant therapy as well as preoperatively [56,57].
The coming years will show an increasing shift of healthcare being provided at home, especially for vulnerable groups. Especially in times of social distancing, this is specifically relevant given the patient population who are generally older, more frail and may experience logistical difficulties (e.g. financial, physical, travel) and are more at risk for infections when attending the hospital [24]. This specific patient population is at risk for a critical infection with bad health outcomes when infected with COVID-19 [58]. This pandemic revealed health care obstacles including closure of leisure facilities and inequality of health care accessibility. HBP may offer a more patient tailored program that will; increase accessibility to the program closer to home, avoid unnecessary public activity, enhance compliance and associated effectiveness, make it more affordable for both patients as healthcare, and promote a long lasting lifestyle change through supported self-management [25,26].
In conclusion, the present data showed improvement of pre- and postoperative functional capacity after HBP. Regarding the high amount of heterogeneity, small sample sizes, underpowered studies and considerable risk of bias there is a need for high-quality data. This includes large sample size, studies specified on operation or cancer type and vulnerable population to enhance generalizability. Prehabilitation programs and outcome measures require standardization on a large scale. Regarding the current pandemic and further digitalization in the future, at home prehabilitation will be an indispensable alternative to hospital-based prehabilitation and should be further investigated and developed.
Author's contributions
EB designed the project. TG and LS designed the search strategy. LS performed the search and TG and JP screened the articles. TG and HZ did the data analysis. EB and SR drafted the manuscript and LG, HZ, FD and GD critically revised the manuscript. All authors approved the final version of the manuscript.
Declaration of competing interest
None.
Footnotes
This systematic review is not preregistered with an analysis plan.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ejso.2022.02.010.
(review authors' judgements about each risk of bias item for each included study. (+: low risk of bias, -: high risk of bias, ?: unclear of bias)).
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.SEER. Cancer Stat facts: cancer of any site.
- 2.World Health Organization . GLOBOCAN; 2018. Estimated age-standardized incidence rates (World) in 2018, all cancers, both sexes, all ages. [Google Scholar]
- 3.Lawrence V.A., Hazuda H.P., Cornell J.E., et al. Functional independence after major abdominal surgery in the elderly. J Am Coll Surg. 2004;199(5):762-72 doi: 10.1016/j.jamcollsurg.2004.05.280. [DOI] [PubMed] [Google Scholar]
- 4.Brittney M., Kohlnhofer B.S., Sarah E., et al. Multiple complications and short length of stay are associated with postoperative readmissions. Am J Surg. 2014;207(4), 449–456 doi: 10.1016/j.amjsurg.2013.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Robinson Carli F. 2015. The SAGES/ERAS society manual of enhanced recovery programs for gastrointestinal surgery. [Google Scholar]
- 6.van Rooijen S.J., Huisman D., Stuijvenberg M., et al. Intraoperative modifiable risk factors of colorectal anastomotic leakage: why surgeons and anesthesiologists should act together. Int J Surg. 2016;36:183–200. doi: 10.1016/j.ijsu.2016.09.098. [DOI] [PubMed] [Google Scholar]
- 7.Huisman D.E., Reudink M., van Rooijen S.J., et al. LekCheck: a prospective study to identify perioperative modifiable risk factors for anastomic leakage in colorectal surgery. Ann Surg. 2020;(Xx):1–9. doi: 10.1097/SLA.0000000000003853. Publish Ah. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.van Rooijen S., Carli F., Dalton S.O., Johansen C., Dieleman J., Roumen R., et al. Preoperative modifiable risk factors in colorectal surgery: an observational cohort study identifying the possible value of prehabilitation. Acta Oncol (Madr) 2017;56(2):329–334. doi: 10.1080/0284186X.2016.1267872. [DOI] [PubMed] [Google Scholar]
- 9.Santa Mina D., Clarke H., Ritvo P., Leung Y.W., Matthew A.G., Katz J., et al. Effect of total-body prehabilitation on postoperative outcomes: a systematic review and meta-analysis. Physiotherapy. 2014;100(3):196–207. doi: 10.1016/j.physio.2013.08.008. [DOI] [PubMed] [Google Scholar]
- 10.Van Rooijen S., Carli F., Dalton S., Thomas G., Bojesen R., Le Guen M., et al. Multimodal prehabilitation in colorectal cancer patients to improve functional capacity and reduce postoperative complications: the first international randomized controlled trial for multimodal prehabilitation. BMC Cancer. 2019;19(1):1–11. doi: 10.1186/s12885-018-5232-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Barberan-Garcia A., Ubré M., Roca J., Lacy A.M., Burgos F., Risco R., et al. Personalised prehabilitation in high-risk patients undergoing elective major abdominal surgery : a randomized blinded controlled trial. Ann Surg. 2018;267(1):50–56. doi: 10.1097/SLA.0000000000002293. [DOI] [PubMed] [Google Scholar]
- 12.Mayo N.E., Feldman L., Scott S., Zavorsky G., Kim D.J., Charlebois P., et al. Impact of preoperative change in physical function on postoperative recovery: argument supporting prehabilitation for colorectal surgery. Surgery. 2011 Sep;150(3):505–514. doi: 10.1016/j.surg.2011.07.045. [DOI] [PubMed] [Google Scholar]
- 13.Carli F., Scheede-Bergdahl C. Prehabilitation to enhance perioperative care. Anesthesiol Clin. 2015;33(1):17-33 doi: 10.1016/j.anclin.2014.11.002. [DOI] [PubMed] [Google Scholar]
- 14.van Rooijen S.J., Molenaar C.J.L., Schep G., van Lieshout Rhma, Beijer S., Dubbers R., et al. Making patients fit for surgery: introducing a four pillar multimodal prehabilitation program in colorectal cancer. Am J Phys Med Rehabil. 2019;98(10):888–896. doi: 10.1097/PHM.0000000000001221. [DOI] [PubMed] [Google Scholar]
- 15.Egholm J.W.M., Pedersen B., Møller A.M., Adami J., Juhl C.B., Tønnesen H. Perioperative alcohol cessation intervention for postoperative complications. Cochrane Database Syst Rev. 2018;2018(11) doi: 10.1002/14651858.CD008343.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Myels S.P., Lacono A.G., Hunt O.J., Fletcher H., Morris J., Mcllroy D., et al. Risk of respiratory complications and wound infection in. Anesthesiology. 2002;97(4):842–847. doi: 10.1097/00000542-200210000-00015. [DOI] [PubMed] [Google Scholar]
- 17.Dronkers J.J., Chorus A.M.J., Van Meeteren N.L.U., Hopman-Rock M. The association of pre-operative physical fitness and physical activity with outcome after scheduled major abdominal surgery. Anaesthesia. 2013;68(1):67–73. doi: 10.1111/anae.12066. [DOI] [PubMed] [Google Scholar]
- 18.Gillis C., Fenton T.R., Sajobi T.T., Minnella E.M., Awasthi R., Carli F. Trimodal prehabilitation for colorectal surgery attenuates post-surgical losses in lean body mass: a pooled analysis of randomized controlled trials. Clin Nutr. 2019;38(3):1053–1060. doi: 10.1016/j.clnu.2018.06.982. [DOI] [PubMed] [Google Scholar]
- 19.Bruns E., et al. Oral nutrition as a form of pre-operative enhancement in patients undergoing surgery for colorectal cancer: a systematic review. Surg Infect. 2018;19(1):1-10 doi: 10.1089/sur.2017.143. [DOI] [PubMed] [Google Scholar]
- 20.Dunne D., Jones R., Lythgoe D., Malik H. Prehabilitation before liver surgery. Eur J Surg Oncol. 2014;40(11):S52. [Google Scholar]
- 21.Lindbäck Y., Tropp H., Enthoven P., Abbott A., Öberg B. PREPARE: presurgery physiotherapy for patients with degenerative lumbar spine disorder: a randomized controlled trial. Spine J. 2018;18(8):1347–1355. doi: 10.1016/j.spinee.2017.12.009. [DOI] [PubMed] [Google Scholar]
- 22.Santa Mina D., Hilton W.J., Matthew A.G., Awasthi R., Bousquet-Dion G., Alibhai S.M.H., et al. Prehabilitation for radical prostatectomy: a multicentre randomized controlled trial. Surg Oncol. 2018;27(2):289–298. doi: 10.1016/j.suronc.2018.05.010. [DOI] [PubMed] [Google Scholar]
- 23.Minnella E.M., Bousquet-Dion G., Awasthi R., Scheede-Bergdahl C., Carli F. Multimodal prehabilitation improves functional capacity before and after colorectal surgery for cancer: a five-year research experience. Acta Oncol (Madr) 2017;56(2):295–300. doi: 10.1080/0284186X.2016.1268268. [DOI] [PubMed] [Google Scholar]
- 24.Denson A.C., Mahipal A. Participation of the elderly population in clinical trials: barriers and solutions. Cancer Control. 2014;21(3):209–214. doi: 10.1177/107327481402100305. [DOI] [PubMed] [Google Scholar]
- 25.Ferreira V., Agnihotram R.V., Bergdahl A., van Rooijen S.J., Awasthi R., Carli F., et al. Maximizing patient adherence to prehabilitation: what do the patients say? Support Care Cancer. 2018;26(8):2717–2723. doi: 10.1007/s00520-018-4109-1. [DOI] [PubMed] [Google Scholar]
- 26.Danjoux G. Preparing for surgery the PREP-WELL. Proj Rep. 2019;(November):1–21. [Google Scholar]
- 27.Moher D., Liberati A., Tetzlaff J., Altman D.G., Altman D., Antes G., et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7) doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Otten R., de Vries Ls R. 2019. Amsterdam efficient deduplication (AED) method. [Google Scholar]
- 29.Bramer W.M., Giustini D., De Jonge G.B., Holland L., Bekhuis T. De-duplication of database search results for systematic reviews in EndNote. J Med Libr Assoc. 2016;104(3):240–243. doi: 10.3163/1536-5050.104.3.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chao Li M., Ferri L.E., et al. An enhanced recovery pathway decreases duration of stay after esophagectomy. Cent Surg Assoc. 2012;152(4):606-14 doi: 10.1016/j.surg.2012.07.021. [DOI] [PubMed] [Google Scholar]
- 31.Pecorelli N., Fiore J.F., Gillis C., Awasthi R., Mappin-Kasirer B., Niculiseanu P., et al. The six-minute walk test as a measure of postoperative recovery after colorectal resection: further examination of its measurement properties. Surg Endosc. 2016;30(6):2199–2206. doi: 10.1007/s00464-015-4478-1. [DOI] [PubMed] [Google Scholar]
- 32.Antonescu I., Scott S., Tran T.T., Mayo N.E., Feldman L.S. Measuring postoperative recovery: what are clinically meaningful differences? Surgery. 2014;156(2):319–327. doi: 10.1016/j.surg.2014.03.005. [DOI] [PubMed] [Google Scholar]
- 33.Finch E., Brooks D., Stratford P.W., Mayo N. second ed. Canadian Physiotherapy Association; 2002. Physical rehabilitation outcome measures, A guide to enhanced clinical decision making; pp. 248–253. 2nd editio. [Google Scholar]
- 34.Gibbons W., Fruchter N., Sloan S., Levy R. Reference values for a multiple repetition 6-minute walk test in healthy adults older than 20 years. J Cardiopulm Rehabil. 2001;21(2):87-93 doi: 10.1097/00008483-200103000-00005. [DOI] [PubMed] [Google Scholar]
- 35.Dindo D., Demartines N., Clavien P.A. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240(2):205–213. doi: 10.1097/01.sla.0000133083.54934.ae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Higgins J.P.T., Altman D.G., Gøtzsche P.C., Jüni P., Moher D., Oxman A.D., et al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ. 2011;343(7829):1–9. doi: 10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Higgins JPT G s. Cochrane Handbook for systematic reviews of interventions. Version 5. The Cochrane Collaboration. 2011;2011 [Google Scholar]
- 38.Melsen W.G., Bootsma M.C.J., Rovers M.M., Bonten M.J.M. The effects of clinical and statistical heterogeneity on the predictive values of results from meta-analyses. Clin Microbiol Infect. 2014;20(2):123–129. doi: 10.1111/1469-0691.12494. [DOI] [PubMed] [Google Scholar]
- 39.Higgins J.P.T., Thompson S.G., Deeks J.J., Altman D.G. Measuring inconsistency in meta-analyses. Br Med J. 2003;327(7414):557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Collaboration T.C. 2014. Review manager (RevMan) [Google Scholar]
- 41.Gillis C., Li C., Lee L., Awasthi R., Augustin B., Gamsa A., et al. Prehabilitation versus rehabilitation: a randomized control trial in patients undergoing colorectal resection for cancer. Anesthesiology. 2014 Nov;121(5):937–947. doi: 10.1097/ALN.0000000000000393. [DOI] [PubMed] [Google Scholar]
- 42.Liu Z., Qiu T., Pei L., Zhang Y., Xu L., Cui Y., et al. Two-week multimodal prehabilitation program improves perioperative functional capability in patients undergoing thoracoscopic lobectomy for lung cancer: a randomized controlled trial. Anesth Analg. 2019;131(3):840-849 doi: 10.1213/ANE.0000000000004342. [DOI] [PubMed] [Google Scholar]
- 43.Bousquet-Dion G., Awasthi R., Loiselle S.-E., Minnella E.M., Agnihotram R.V., Bergdahl A., et al. Evaluation of supervised multimodal prehabilitation programme in cancer patients undergoing colorectal resection: a randomized control trial. Acta Oncol. 2018 Jun;57(6):849–859. doi: 10.1080/0284186X.2017.1423180. [DOI] [PubMed] [Google Scholar]
- 44.Minnella E.M., Awasthi R., Bousquet-Dion G., Ferreira V., Austin B., Audi C., et al. Multimodal prehabilitation to enhance functional capacity following radical cystectomy: a randomized controlled trial. Eur Urol Focus. 2019;7(1):132-138 doi: 10.1016/j.euf.2019.05.016. [DOI] [PubMed] [Google Scholar]
- 45.Minnella E.M., Awasthi R., Loiselle S.E., Agnihotram R.V., Ferri L.E., Carli F. Effect of exercise and nutrition prehabilitation on functional capacity in esophagogastric cancer surgery: a randomized clinical trial. JAMA Surg. 2018;153(12):1081–1089. doi: 10.1001/jamasurg.2018.1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Borg G.A. vol. 14. Medicine & Science in Sports & Exercise; 1982. pp. 377–381. (Borg's RPE Scale.pdf). [PubMed] [Google Scholar]
- 47.Margadant C.C., Bruns E.R.J., Sloothaak D.A.M., van Duijvendijk P., van Raamt A.F., van der Zaag H.J., et al. Lower muscle density is associated with major postoperative complications in older patients after surgery for colorectal cancer. Eur J Surg Oncol. 2016;42(11):1654–1659. doi: 10.1016/j.ejso.2016.05.040. [DOI] [PubMed] [Google Scholar]
- 48.Bruns E.R.J., van den Heuvel B., Buskens C.J., van Duijvendijk P., Festen S., Wassenaar E.B., et al. The effects of physical prehabilitation in elderly patients undergoing colorectal surgery: a systematic review. Colorectal Dis. 2016;18(8):O267–O277. doi: 10.1111/codi.13429. [DOI] [PubMed] [Google Scholar]
- 49.Oosting E., Jans M.P., Dronkers J.J., Naber R.H., Dronkers-Landman C.M., Appelman-De Vries S.M., et al. Preoperative home-based physical therapy versus usual care to improve functional health of frail older adults scheduled for elective total hip arthroplasty: a pilot randomized controlled trial. Arch Phys Med Rehabil. 2012;93(4):610–616. doi: 10.1016/j.apmr.2011.11.006. [DOI] [PubMed] [Google Scholar]
- 50.Driessen E.J., Peeters M.E., Bongers B.C., Maas H.A., Bootsma G.P., van Meeteren N.L., et al. Effects of prehabilitation and rehabilitation including a home-based component on physical fitness, adherence, treatment tolerance, and recovery in patients with non-small cell lung cancer: a systematic review. Crit Rev Oncol Hematol. 2017;114:63–76. doi: 10.1016/j.critrevonc.2017.03.031. [DOI] [PubMed] [Google Scholar]
- 51.Frost R., Levati S., McClurg D., Brady M., Williams B. What adherence measures should Be used in trials of home-based rehabilitation interventions? A systematic review of the validity, reliability, and acceptability of measures. Arch Phys Med Rehabil. 2017;98(6):1241–1256. doi: 10.1016/j.apmr.2016.08.482. e45. [DOI] [PubMed] [Google Scholar]
- 52.Anderson L., Ga S., Rj N., Dalal H., Sg D., Jolly K., et al. Home-based versus centre-based cardiac rehabilitation (Review) Cochrane Database Syst Rev. 2017;6 doi: 10.1002/14651858.CD007130.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Westmaas J.L., Newton C.C., Stevens V.L., Flanders W.D., Gapstur S.M., Jacobs E.J. Does a recent cancer diagnosis predict smoking cessation? An analysis from a large prospective US cohort. J Clin Oncol. 2015;33(15):1647–1652. doi: 10.1200/JCO.2014.58.3088. [DOI] [PubMed] [Google Scholar]
- 54.Bagaria S.P., Heckman M.G., Diehl N.N., Parker A., Wasif N. Delay to colectomy and survival for patients diagnosed with colon cancer. J Invest Surg. 2019;32(4):350–357. doi: 10.1080/08941939.2017.1421732. [DOI] [PubMed] [Google Scholar]
- 55.Hangaard Hansen C., Gögenur M., Tvilling Madsen M., Gögenur I. The effect of time from diagnosis to surgery on oncological outcomes in patients undergoing surgery for colon cancer: a systematic review. Eur J Surg Oncol. 2018 Oct;44(10):1479–1485. doi: 10.1016/j.ejso.2018.06.015. [DOI] [PubMed] [Google Scholar]
- 56.Poleszczuk J., Luddy K., Chen L., Lee J.K., Harrison L.B., Czerniecki B.J., et al. Neoadjuvant radiotherapy of early-stage breast cancer and long-term disease-free survival. Breast Cancer Res. 2017;19(1):1–7. doi: 10.1186/s13058-017-0870-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bos A.C.R.K., Van Erning F.N., Van Gestel Y.R.B.M., Creemers G.J.M., Punt C.J.A., Van Oijen M.G.H., et al. Timing of adjuvant chemotherapy and its relation to survival among patients with stage III colon cancer. Eur J Cancer. 2015;51(17):2553–2561. doi: 10.1016/j.ejca.2015.08.016. [DOI] [PubMed] [Google Scholar]
- 58.McGurnaghan S.J., Weir A., Bishop J., Kennedy S., Blackbourn L.A.K., McAllister D.A., et al. Risks of and risk factors for COVID-19 disease in people with diabetes: a cohort study of the total population of Scotland. Lancet Diabetes Endocrinol. 2021;9(2):82–93. doi: 10.1016/S2213-8587(20)30405-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.