Abstract
Background:
Previous studies of severe acute respiratory syndrome coronavirus 2 infection in infants have incompletely characterized factors associated with severe illness or focused on infants born to mothers with coronavirus disease 2019 (COVID-19). Here we highlight demographics, clinical characteristics and laboratory values that differ between infants with and without severe acute COVID-19.
Methods:
Active surveillance was performed by the Overcoming COVID-19 network to identify children and adolescents with severe acute respiratory syndrome coronavirus 2–related illness hospitalized at 62 sites in 31 states from March 15 to December 27, 2020. We analyzed patients >7 days to <1 year old hospitalized with symptomatic acute COVID-19.
Results:
We report 232 infants >7 days to <1 year of age hospitalized with acute symptomatic COVID-19 from 37 US hospitals in our cohort from March 15 to December 27, 2020. Among 630 cases of severe COVID-19 in patients >7 days to <18 years old, 128 (20.3%) were infants. In infants with severe illness from the entire study period, the median age was 2 months, 66% were from racial and ethnic minority groups, 66% were previously healthy, 73% had respiratory complications, 13% received mechanical ventilation and <1% died.
Conclusions:
Infants accounted for over a fifth of children <18 years of age hospitalized for severe acute COVID-19, commonly manifesting with respiratory symptoms and complications. Although most infants hospitalized with COVID-19 did not suffer significant complications, longer term outcomes remain unclear. Notably, 75% of infants with severe disease were <6 months of age in this cohort study period, which predated maternal COVID-19 vaccination, underscoring the importance of maternal vaccination for COVID-19 in protecting the mother and infant.
Keywords: infant, hospitalized, COVID-19, SARS-CoV-2
Studies involving infants with the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) [the virus that causes coronavirus disease 2019 (COVID-19)] infection have generally focused on infants born to infected mothers or incompletely characterize factors associated with severe acute COVID-19.1–3 Herein we describe severe COVID-19 frequency, presentation and factors associated with severe illness among infants (>7 days to <1 year old) from Overcoming COVID-19—a US-based network registry that identifies hospitalized pediatric cases of COVID-19.4,5
METHODS
Active surveillance was performed by the Overcoming COVID-19 network to identify children and adolescents with SARS-CoV-2–related illness hospitalized at 62 sites in 31 states.5,6 This analysis was limited to patients >7 days to <1 year of age hospitalized with symptomatic acute COVID-19 from March 15 to December 27, 2020, at which point funding for this project ended. Patients were identified by positive clinical SARS-CoV-2 real-time reverse transcription–polymerase chain reaction test during admission; those who were asymptomatic (had incidental positive test) and/or had an alternative reason for admission not related to COVID-19 as adjudicated by a clinician team (C.V.H., K.W., A.P.C., M.M.P., A.G.R.), or who met criteria consistent with the case definition for multisystem inflammatory syndrome in children within the registry, were excluded (Figure, Supplemental Digital Content 3, http://links.lww.com/INF/E610).4,6,7 The study was approved by the central Institutional Review Board at Boston Children’s Hospital. Also, the study was reviewed by the Centers for Disease Control and Prevention and deemed consistent with applicable federal law and the Centers for Disease Control and Prevention policy.8 Of note, starting August 13, 2020, due to the high volume of patients enrolled and the desire to focus on more severely ill children, the registry was restricted to patients admitted to the intensive care unit (ICU) or high acuity unit for the duration of the registry. Comparisons of severe to nonsevere disease presented herein are, therefore, limited to the period of March 15 and August 13, 2021, and descriptions of severe disease encompass the period through December 27, 2020.
All data elements were collected from hospital medical records as reported by site clinicians. Severe COVID-19 was defined as described previously,6 with severe disease indicators including new oxygen requirement, receipt of invasive mechanical ventilation, noninvasive mechanical ventilation, vasopressors, new renal dialysis, cardiopulmonary resuscitation or death (Table, Supplemental Digital Content 4, http://links.lww.com/INF/E610). Presenting signs, symptoms and complications were defined by organ system, including constitutional (fever, lethargy), upper respiratory (rhinorrhea, congestion, sore throat), lower respiratory (cough, shortness of breath, chest pain, wheezing, lower chest wall retractions), gastrointestinal (refusal to eat, vomiting, abdominal pain, diarrhea), mucocutaneous (rash, inflammation of the oral mucosa, conjunctivitis) and extremity findings (erythema or edema of the hands or feet or periungual peeling), hematologic (abnormal cell counts or clotting function) or neurologic (altered mental status/confusion) as described previously5 and as described in the Table, Supplemental Digital Content 4, http://links.lww.com/INF/E610. Patients whose data were included in other reports are listed in the Table, Supplemental Digital Content 5, http://links.lww.com/INF/E610.4,6,9
Statistical Analysis
We determined baseline demographics, clinical characteristics and laboratory values for infants with severe and nonsevere COVID-19. Continuous variables were expressed as medians and interquartile ranges (IQRs); categorical variables were expressed as counts and percentages. For univariate comparisons between infants with and without severe COVID-19, a Kruskal-Wallis test was used for continuous variables and χ2 and Fisher’s exact tests were used for categorical variables, where appropriate. The significance threshold was set at P < 0.05. We did not impute missing data. All analyses were conducted in R 4.0.2 (R Project for Statistical Computing).
RESULTS
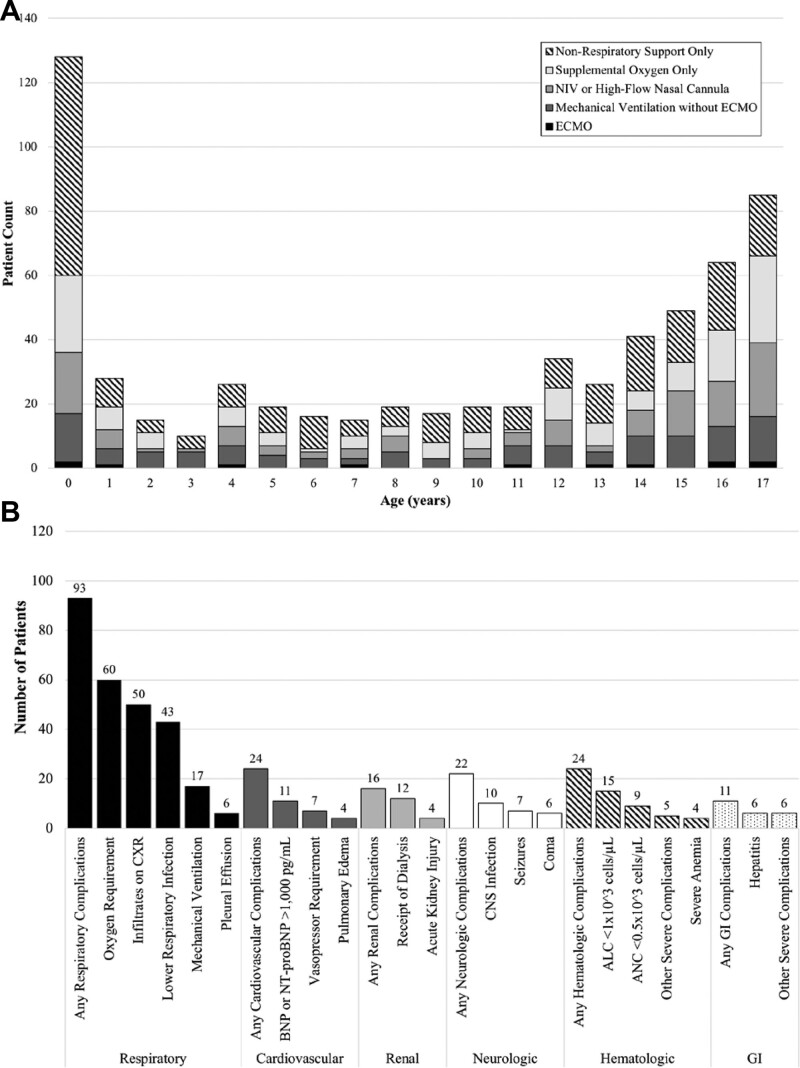
A total of 2113 children were reported through the Overcoming COVID-19 registry between March 15 and December 27, 2020, including 232 (11.0%) symptomatic infants <1 year of age reported from 37 sites in 26 states (Figure, Supplemental Digital Content 5, http://links.lww.com/INF/E610), notably preceding vaccine availability to pregnant women and adults. Among the patients reported, 630 cases of severe acute COVID-19 were identified in children >7 days and <18 years of age from 50 sites in 31 states. By year of age, infants (128/630, 20.3%) made up a disproportionate number of severe acute COVID-19 cases among children reported to the Overcoming COVID-19 registry (Fig. 1A) both before August 13, 2020 (104/468, 22.2%), when inclusion was restricted to ICU/high acuity unit patients, as well as after this date (24/162, 14.8%). Of the 128 infants with severe acute COVID-19, 68 (53.1%) did not require any respiratory support, 24 (53.1%) received supplemental oxygen only, 19 (14.8%) received oxygen support through noninvasive mechanical ventilation or high-flow nasal cannula, 15 (11.7%) received mechanical ventilation and 2 (1.6%) required extracorporeal membrane oxygenation (Fig. 1A).
FIGURE 1.
Age distribution of patients with severe acute coronavirus disease 2019 (COVID-19) and types of complications experienced by infants with severe acute COVID-19. A: Patients >7 days to <18 years old (n = 630) with severe acute COVID-19 by age from 50 sites in 31 states, March 15 to December 27, 2020. Patients identified from Overcoming COVID-19 sentinel surveillance with positive SARS-CoV-2 polymerase chain reaction test, >7 days to <18 years of age with severe respiratory, cardiovascular, renal, neurologic, gastrointestinal, or hematologic acute COVID-19 as defined by previously outlined criteria6 (also see Table, Supplemental Digital Content 4, http://links.lww.com/INF/E610, and B, below) are shown herein. Children with multisystem inflammatory syndrome in children (MIS-C) as defined by the Centers for Disease Control and Prevention7 and as reported previously4 were excluded from this report. Total number of patients fulfilling criteria for severe disease are shown by patient count on the y axis, with age in years shown on the x axis, with nonrespiratory involvement only, any respiratory (support), or mechanical ventilation color coded (see shading legend). By year of age, infants (128/630, 20.3%) made up a disproportionate number of severe acute COVID-19 cases among children reported to the Overcoming COVID-19 registry (A) both before August 13, 2020 (104/468, 22.2%), when inclusion was restricted to ICU/high acuity unit patients, as well as after this date (24/162, 14.8%). Of the 128 infants with severe acute COVID-19, 68 (53.1%) did not require any respiratory support, 24 (53.1%) received supplemental oxygen only, 19 (14.8%) received oxygen support through noninvasive mechanical ventilation or high-flow nasal cannula, 15 (11.7%) received mechanical ventilation and 2 (1.6%) required extracorporeal membrane oxygenation (ECMO). Note that on August 13, 2020, the registry was restricted to patients admitted to the intensive care unit or high acuity step-down unit for patients without MIS-C, but data presented in this figure are only severe cases and, therefore, encapsulate the entire study period. B: Complications experienced by infants >7 days to <1 year of age with severe acute COVID-19 illness (n = 128 patients) from 37 sites in 26 states, March 15 to December 27, 2020. Severe COVID-19 was defined as meeting at least one of the criteria listed on the x axis. Of the total children enrolled, 242 were infants, 232 remained after adjudication to confirm that the reason for admission was due to SARS-CoV-2–related illness, with 128 of those 232 infants having fulfilled criteria for severe COVID-19. Criteria for complications were previously defined6 (also see Table, Supplemental Digital Content 4, http://links.lww.com/INF/E610), and complications by organ system detail are provided (x axis). Acute kidney injury was defined as a creatinine level equal to or above 0.62 mg/dL. Severe anemia was defined as hemoglobin level less than 7 g/dL. If fewer than 3 patients fulfilled a condition, they are not depicted herein. Also, for each patient, any instance of specific organ system involvement is represented for each category shown, with many patients meeting more than one criterion. ALC indicates absolute lymphocyte count; ANC, absolute neutrophil count; BNP, B-type natriuretic peptide; CNS, central nervous system; CXR, chest radiograph; GI, gastrointestinal; NIV, non-invasive ventilation; NT-proBNP, N-terminal B-type natriuretic peptide; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
Prior to August 13, 2020, infants from racial and ethnic minority groups (Black non-Hispanic, Hispanic and other non-Hispanic) accounted for the majority of severe COVID-19 cases (69.2%; Table 1). The most frequent age group of infants hospitalized with COVID-19 was <3 months of age, but infants with severe acute COVID-19 were older than infants with nonsevere disease [median age, 1 month (IQR, 1–4) vs. 1 month (IQR, 0–2), respectively, P = 0.001] and more likely to have at least one underlying condition (31.7% of severe compared with 6.9% of nonsevere, P < 0.001; Table 1). In infants with severe acute COVID-19, 8.7% and 12.5% had underlying respiratory or cardiovascular conditions, respectively (Table 1).
TABLE 1.
Baseline Characteristics and Clinical Course of Infants Hospitalized Between March 15 and August 13, 2021, From 37 Sites in 26 States With Severe Compared With Nonsevere Acute COVID-19 and Initial Laboratory Values Within 48 Hours of Admission*
| All Infants (n=206) | Severe COVID-19 (n = 104) | Nonsevere COVID-19 (n = 102) | P | |
|---|---|---|---|---|
| Age (mo) | 1 (0–3) | 1 (1–4) | 1 (0–2) | 0.001 |
| 7–28 d of life | 58 (28.2%) | 20 (19.2%) | 38 (37.3%) | 0.008 |
| 1 to <3 mo | 89 (43.2%) | 45 (43.3%) | 44 (43.1%) | |
| 3 to <6 mo | 31 (15.0%) | 20 (19.2%) | 11 (10.8%) | |
| 6 to <12 mo | 28 (13.6%) | 19 (18.3%) | 9 (8.8%) | |
| Sex, race and ethnicity† | ||||
| Male sex | 112 (54.4%) | 58 (55.8%) | 54 (52.9%) | 0.78 |
| White, non-Hispanic | 36 (17.5%) | 22 (21.2%) | 14 (13.7%) | 0.20 |
| Black, non-Hispanic | 40 (19.4%) | 22 (21.2%) | 18 (17.6%) | |
| Hispanic or Latino | 91 (44.2%) | 46 (44.2%) | 45 (44.1%) | |
| Other, non-Hispanic | 15 (7.3%) | 4 (3.8%) | 11 (10.8%) | |
| Unknown | 24 (11.7%) | 10 (9.6%) | 14 (13.7%) | |
| At least one underlying condition‡ | 40 (19.4%) | 33 (31.7%) | 7 (6.9%) | <0.001 |
| Respiratory | 9 (4.4%) | 9 (8.7%) | 0 (0%) | 0.003 |
| Cardiovascular | 14 (6.8%) | 13 (12.5%) | 1 (1.0%) | 0.001 |
| Neurologic | 10 (4.9%) | 7 (6.7%) | 3 (2.9%) | 0.33 |
| Other underlying condition | 32 (15.5%) | 27 (26.0%) | 5 (4.9%) | <0.001 |
| Prematurity | ||||
| Premature | 25 (12.1%) | 16 (15.4%) | 9 (8.8%) | 0.20 |
| <28 weeks | 2/25 (8.0%) | 2/16 (12.5%) | 0/9 (0%) | 0.52 |
| 29–33 weeks | 2/25 (8.0%) | 2/16 (12.5%) | 0/9 (0%) | 0.52 |
| 34–36 weeks | 21/25 (84.0%) | 12/16 (75.0%) | 9/9 (100.0%) | 0.26 |
| Presentation conditions | ||||
| Duration of symptoms pre-hospitalization (d) | 1 (1–2.5) | 1 (1–3) | 1 (1–2) | 0.37 |
| Organ systems involved§ | 2 (1–2) | 2 (1–3) | 1 (0–1) | <0.001 |
| Signs and symptoms on presentation¶ | ||||
| Constitutional | 167 (81.1%) | 74 (71.2%) | 93 (91.2%) | <0.001 |
| Gastrointestinal | 101 (49.0%) | 48 (46.2%) | 53 (52.0%) | 0.49 |
| Mucocutaneous | 23 (11.2%) | 14 (13.5%) | 9 (8.8%) | 0.38 |
| Lower respiratory | 97 (47.1%) | 64 (61.5%) | 33 (32.4%) | <0.001 |
| Upper respiratory | 73 (35.4%) | 38 (36.5%) | 35 (34.3%) | 0.77 |
| Neurologic | 25 (12.1%) | 20 (19.2%) | 5 (4.9%) | 0.002 |
| Initial laboratory values | ||||
| Absolute lymphocyte count (103 cells/μL) | 3.68 (2.5–5.3) | 3.72 (1.89–5.25) | 3.64 (2.68–5.35) | 0.75 |
| Absolute neutrophil count (103 cells/μL) | 2.65 (1.48–4.0) | 3.17 (1.8–5.18) | 2.03 (1.35–3.09) | 0.002 |
| Neutrophil:lymphocyte ratio | 0.77 (0.40–1.19) | 0.91 (0.5–1.31) | 0.6 (0.33–1.06) | 0.007 |
| Platelet count (103 cells/μL) | 325 (250–397) | 355 (268–416) | 310 (242–362) | 0.054 |
| Hemoglobin (g/dL) | 11.7 (10.7–13.2) | 11.3 (10.5–12.6) | 12 (10.9–13.9) | 0.02 |
| ALT (U/L) | 29 (23–38) | 30.5 (23–50) | 27 (23.25–33) | 0.12 |
| CRP (mg/dL) | 0.5 (0.27–1.65) | 0.5 (0.33–2.0) | 0.5 (0.25–0.88) | 0.54 |
| Clinical course and outcomes | ||||
| ICU/HAU admission | 41 (19.9%) | 32 (30.8%) | 9 (8.8%) | <0.001 |
| Hospital length of stay (d) | 2 (1–2) | 2 (1–4) | 1 (1–2) | <0.001 |
| Any respiratory support | 40 (19.4%) | 40 (38.5%) | 0 (0%) | <0.001 |
| Mechanical ventilation | 10 (4.9%) | 10 (9.6%) | 0 (0%) | 0.002 |
| Vasopressor requirement | 5 (2.4%) | 5 (4.8%) | 0 (0%) | 0.06 |
| ECMO | 1 (0.5%) | 1 (1.0%) | 0 (0%) | 1.00 |
| Death | 1 (0.5%) | 1 (1.0%) | 0 (0%) | 1.00 |
Continuous variables are expressed as median and IQR, while discrete variables are expressed as counts and percentages.
*Severe acute COVID-19 was defined as severe complications involving 1 organ system or more and evidence of infection with severe acute respiratory syndrome coronavirus 2 based on having a positive RT-PCR test result as previously as defined by previously outlined criteria4 (Table, Supplemental Digital Content 4, http://links.lww.com/INF/E610; Fig. 1B).
†Race and ethnicity were recorded from hospital medical records as reported by the site clinicians who cared for the patients.
‡Underlying conditions excluded obesity. Other category includes oncologic, immunosuppressive, rheumatologic, autoimmune, hematologic, renal, urologic, gastrointestinal, hepatic, endocrine and metabolic conditions. Respiratory conditions include “asthma,” chronic restrictive lung disease, tracheomalacia/bronchomalacia, bronchopulmonary dysplasia, cystic fibrosis, obstructive sleep apnea, recurrent aspiration into lungs or pulmonary hypertension, as well as other underlying conditions judged by the study team to involve the respiratory system. Cardiovascular includes congenital heart disease (nonspecific), acquired heart disease (nonspecific), history of cardiac repair, as well as other underlying conditions judged by the study team to involve the cardiovascular system.
§Organ systems involved includes cardiovascular, respiratory, renal, neurologic, gastrointestinal, hematologic, mucocutaneous and musculoskeletal.
¶Presenting signs and symptoms were recorded from hospital medical records and included constitutional symptoms (fever, fatigue, muscle aches/joint pain), gastrointestinal symptoms (nausea/refusal to eat, vomiting, abdominal pain, diarrhea), upper respiratory (rhinorrhea, congestion, sore throat), lower respiratory (cough, shortness of breath, chest pain, wheezing, lower chest wall indrawing), mucocutaneous findings (rash, inflammation of the oral mucosa, conjunctivitis and extremity findings, including erythema or edema of the hands or feet, or periungual peeling), hematologic signs (abnormal cell counts or clotting function) and neurologic symptoms (headache, altered mental status/confusion).
ALT indicates alanine aminotransferase; CRP, C-reactive protein; ECMO, extracorporeal membrane oxygenation; HAU, high acuity unit; RT-PCR, reverse transcription–polymerase chain reaction.
The most common presenting signs among infants with severe disease were constitutional, upper and lower respiratory, although gastrointestinal and neurologic signs were also identified in this group (Table 1). The most frequently identified complications in infants with severe disease were respiratory (93/128, 73%; Fig. 1B). The most frequently identified respiratory complications included respiratory failure requiring mechanical ventilation (13%), oxygen requirement (47%), infiltrates on chest radiograph (39%) and lower respiratory infection (34%; Fig. 1B). Infants with severe COVID-19 had significantly higher neutrophil-to-lymphocyte ratio than infants without severe COVID-19 (P = 0.007; Table 1). Hematologic complications (24%) and cardiac complications (24%) were the second most commonly identified behind respiratory but overall similar to the reported frequency of neurologic (22%) and renal (16%) complications (Fig. 1B). Admission to the ICU (30.8% vs. 8.8%, P < 0.001) and longer length of hospital stay [median, 2 days (IQR, 1–4) vs. 1 day (IQR, 1–2), respectively, P < 0.001] were more common among infants with severe COVID-19 compared with infants with nonsevere COVID-19; 2 (1.6%) infants with severe COVID-19 received extracorporeal membrane oxygenation, and 1 infant (0.8%) died, as was reported previously.5
Separately, when evaluating the contribution of prematurity to severe disease, 68.8% of premature infants with severe disease had underlying conditions, with the most frequently identified underlying conditions falling into respiratory (43.8%) and cardiovascular (31.3%) categories. Premature infants with severe disease also frequently required respiratory support (62.5%) when compared with premature infants with nonsevere disease (0%; see Table, Supplemental Digital Content 6, http://links.lww.com/INF/E610). Laboratory parameters indicated a trend to a higher neutrophil-to-lymphocyte ratio in these premature infants with severe disease [1.09 (0.87–1.95) for severe compared with 0.61 (0.29–0.65) for nonsevere].
When evaluating infants who did not require respiratory support, but who had organ system involvement that led to them being categorized as severe, most were in the 1- to <3-month range (47.1% compared with 25% who required respiratory support), were less frequently noted to have at least one underlying condition (22.1% compared with 46.7% who required respiratory support) and were less likely to be premature (10.3% compared with 26.7% who required respiratory support). The group requiring respiratory support did more frequently require ICU/high acuity unit admission (68.3%; Table, Supplemental Digital Content 7, http://links.lww.com/INF/E610). Characteristics of all 128 infants with severe acute COVID-19 from the entire study period can be found in Table, Supplemental Digital Content 7, http://links.lww.com/INF/E610.
DISCUSSION
Infants in the Overcoming COVID-19 US public health surveillance registry comprised the highest number of cases adjusted per year of life within the cohort. Of these, the majority had severe involvement of one or more organ systems, mostly respiratory, that led to hospitalization. Overall, short-term outcomes in infants hospitalized with COVID-19 were reassuring, with few intubated patients, mostly brief hospital stays and over 99% survival to hospital discharge. Of note, infants from racial and ethnic minority groups (Black non-Hispanic, Hispanic and other non-Hispanic) accounted for the majority of these cases.
Earlier studies in children had conflicting results regarding frequency and severity of SARS-CoV-2 infection in infants. In the first and largest pediatric COVID-19 study reported from China, a nationwide survey of over 2000 pediatric patients, infants composed the largest percentage of those with severe disease.2 An early Italian study similarly showed that infants <6 months of age had increased risk of disease severity, and those requiring ICU admission most frequently needed respiratory support.10 However, a large French study showed that of infants positive for SARS-CoV-2, few had severe disease.11 Differences among those studies and our findings may be accounted for in the selection of patients, differing definitions of disease severity and comparison across age groups instead of per year of life.
Importantly, our analysis indicates that infants with severe acute COVID-19 most frequently present with respiratory symptoms and develop respiratory complications (Fig. 1). This is consistent with other studies that have shown that respiratory complications occur frequently in severely ill SARS-CoV-2–infected infants10 and children.12 In a study of neonates diagnosed at >7 days of life, constitutional signs (hyperthermia, coryzal signs and poor feeding) and respiratory distress were most common on presentation.3 In our present analysis, constitutional signs and symptoms, including fever, were frequent overall but significantly more commonly noted in the nonsevere group. This may reflect the standard practice of admitting febrile infants for sepsis evaluations. Neurologic symptoms were also frequently reported on presentation in infants including altered mental status, which could also represent lethargy.
In our study, infants with severe COVID-19 more frequently had underlying conditions, consistent with data from a large UK pediatric prospective observational study that showed prematurity and respiratory and cardiac comorbidities were significantly associated with admission to critical care (of note, almost half of children <19 years of age in this study admitted to the ICU were <1 year of age), when excluding multisystem inflammatory syndrome in children cases.13 In this study, severely ill infants were also more frequently admitted to the ICU, and of the 1% (6 deaths in 627 children reported in this study overall), 3/6 of them were in neonates with severe comorbidities.13 Of premature infants, those with severe disease more frequently had underlying conditions, with the most frequently identified underlying conditions falling into the respiratory and cardiovascular categories (Table, Supplemental Digital Content 6, http://links.lww.com/INF/E610). Indeed, premature infants with severe disease more commonly required respiratory support. When looking at all infants, those with severe disease not requiring respiratory support were younger, less frequently noted to have at least one underlying condition and less likely to be premature (Table, Supplemental Digital Content 7, http://links.lww.com/INF/E610).
The majority of severe COVID-19 cases occurred in children of racial and ethnic minority groups (Black non-Hispanic, Hispanic and other non-Hispanic), and many other reports have found that minority groups of all ages are disproportionately affected by COVID-19,14 including independent reports from sites included within the Overcoming COVID-19 network.15
Limitations of our study include that SARS-CoV-2 local epidemiology and testing practices may have varied over the study period. The impact of maternal infection and breastfeeding were not assessed. Newborns (<7 days old) were not systematically reported to Overcoming COVID-19 and were excluded from this analysis. Importantly, this analysis is limited to infants hospitalized with COVID-19, and population-based disease burden data are needed to describe COVID-19 among infants. In addition, this study was limited to sentinel surveillance from Overcoming COVID-19 with limiting to patients with ICU level admissions after August 13, 2020, due to funding limitations. As such, our patient population and conclusion may not be fully extrapolated to the entire population.
In conclusion, in our cohort, infants accounted for over a fifth of children <18 years old hospitalized for severe acute COVID-19, commonly manifesting with respiratory symptoms and complications, with 13% of infants with severe COVID-19 requiring mechanical ventilation. Although most infants hospitalized with COVID-19 do not suffer significant complications, longer term outcomes remain unclear. We also highlight that 75% of infants with severe disease were less than 6 months of age in this cohort which predated maternal COVID-19 vaccine availability. Recent data have indicated that women who receive BNT162b2 messenger RNA COVID-19 vaccine have detectable IgG and IgA antibodies in their breast milk, which likely confers some degree of protection to their infant.16,17 Taken together, all these data demonstrate the importance of encouraging maternal vaccination, including vaccination of pregnant women for which the recent Centers for Disease Control and Prevention recommendations highlight safety and efficacy of COVID-19 vaccination against COVID-19,18 to protect especially younger infants from infection and severe disease. Further studies to characterize predictors of severe COVID-19 and sequelae in infants are needed.
Supplementary Material
Footnotes
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
This study was supported by the Centers for Disease Control and Prevention.
M.M.P. and A.G.R. contributed equally to this study.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s website (www.pidj.com).
REFERENCES
- 1.Bhuiyan MU, Stiboy E, Hassan MZ, et al. Epidemiology of COVID-19 infection in young children under five years: a systematic review and meta-analysis. Vaccine. 2021;39:667–677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dong Y, Mo X, Hu Y, et al. Epidemiology of COVID-19 among children in China. Pediatrics. 2020;145:e20200702. [DOI] [PubMed] [Google Scholar]
- 3.Gale C, Quigley MA, Placzek A, et al. Characteristics and outcomes of neonatal SARS-CoV-2 infection in the UK: a prospective national cohort study using active surveillance. Lancet Child Adolesc Health. 2021;5:113–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Feldstein LR, Rose EB, Horwitz SM, et al. ; Overcoming COVID-19 Investigators; CDC COVID-19 Response Team. Multisystem inflammatory syndrome in U.S. children and adolescents. N Engl J Med. 2020;383:334–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.LaRovere KL, Riggs BJ, Poussaint TY, et al. ; Overcoming COVID-19 Investigators. Neurologic involvement in children and adolescents hospitalized in the United States for COVID-19 or multisystem inflammatory syndrome. JAMA Neurol. 2021;78:536–547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Feldstein LR, Tenforde MW, Friedman KG, et al. ; Overcoming COVID-19 Investigators. Characteristics and outcomes of US children and adolescents with Multisystem Inflammatory Syndrome in Children (MIS-C) compared with severe acute COVID-19. JAMA. 2021;325:1074–1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.CDC. Multisystem Inflammatory Syndrome in Children (MIS-C) Associated with Coronavirus Disease 2019 (COVID-19) [CDC Website]. May 14, 2020. Available at: https://emergency.cdc.gov/han/2020/han00432.asp. Accessed November 1, 2021.
- 8.Office of the Federal Register; Goverment Publishing Office. Electronic Code of Federal Regulations Part 46 – Protection of Human Subjects. January 19, 2017. Available at: https://www.ecfr.gov/current/title-45/subtitle-A/subchapter-A/part-46#46.102. Accessed November 1, 2021.
- 9.Geva A, Patel MM, Newhams MM, et al. ; Overcoming COVID-19 Investigators. Data-driven clustering identifies features distinguishing multisystem inflammatory syndrome from acute COVID-19 in children and adolescents. EClinicalMedicine. 2021;40:101112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Parri N, Magistà AM, Marchetti F, et al. ; CONFIDENCE and COVID-19 Italian Pediatric Study Networks. Characteristic of COVID-19 infection in pediatric patients: early findings from two Italian Pediatric Research Networks. Eur J Pediatr. 2020;179:1315–1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ouldali N, Yang DD, Madhi F, et al. ; Investigator Group of the PANDOR Study. Factors associated with severe SARS-CoV-2 infection. Pediatrics. 2021;147:e2020023432. [DOI] [PubMed] [Google Scholar]
- 12.García-Salido A, de Carlos Vicente JC, Belda Hofheinz S, et al. ; Spanish Pediatric Intensive Care Society Working Group on SARS-CoV-2 Infection. Severe manifestations of SARS-CoV-2 in children and adolescents: from COVID-19 pneumonia to multisystem inflammatory syndrome: a multicentre study in pediatric intensive care units in Spain. Crit Care. 2020;24:666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Swann OV, Holden KA, Turtle L, et al. ; ISARIC4C Investigators. Clinical characteristics of children and young people admitted to hospital with COVID-19 in United Kingdom: prospective multicentre observational cohort study. BMJ. 2020;370:m3249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Van Dyke ME, Mendoza MCB, Li W, et al. Racial and ethnic disparities in COVID-19 incidence by age, sex, and period among persons aged <25 years - 16 U.S. Jurisdictions, January 1-December 31, 2020. MMWR Morb Mortal Wkly Rep. 2021;70:382–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Inagaki K, Garg P, Hobbs CV. SARS-CoV-2 positivity rates among children of racial and ethnic minority groups in Mississippi. Pediatrics. 2021;147:e2020024349. [DOI] [PubMed] [Google Scholar]
- 16.Romero Ramírez DS, Lara Pérez MM, Carretero Pérez M, et al. SARS-CoV-2 antibodies in breast milk after vaccination. Pediatrics. 2021;148:e2021052286. [DOI] [PubMed] [Google Scholar]
- 17.Perl SH, Uzan-Yulzari A, Klainer H, et al. SARS-CoV-2-specific antibodies in breast milk after COVID-19 vaccination of breastfeeding women. JAMA. 2021;325:2013–2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.CDC. New CDC Data: COVID-19 Vaccination Safe for Pregant People [CDC Website]. August 11, 2021. Available at: https://www.cdc.gov/media/releases/2021/s0811-vaccine-safe-pregnant.html. Accessed November 1, 2021.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.