Abstract
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection often causes pneumonia and respiratory failure that may lead to postintensive care syndrome, including critical illness neuropathy (CIN) and critical illness myopathy (CIM). The data on the rehabilitation outcomes of post-novel coronavirus disease (COVID) patients with CIN and CIM following respiratory failure and mechanical ventilation are still limited. To address this, we enrolled in our prospective observational study a sample of 50 consecutive COVID-19 patients admitted to our facility between 2 November 2020 and 3 May 2021 with electrophysiologically confirmed or clinically suspected diagnosis of CIN/CIM. The functional abilities were assessed at admission and discharge with the Functional Independence Measure (FIM), The Canadian Occupational Performance Measure, 10-metre walk test, 6-min walk test and the de Morton Mobility Index. The gain in motor FIM and the length of stay were used as an index of rehabilitation efficiency. Nutritional status was also assessed using anthropometric measurements and bioelectrical Impedance analysis. Psychologic evaluation was performed at admission only. At admission, functional limitations and severe malnutrition were present in all patients with psychologic problems in about one third. At discharge (42 ± 16 days later), clinically important and statistically significant improvements were found in all outcome measures, which was also noted by the patients. The gain in motor FIM was larger with the longer length of stay up to 2 months and plateaued thereafter. We conclude that post-COVID-19 patients who develop CIN/CIM following respiratory failure can improve functional and nutritional status during inpatient rehabilitation.
Keywords: COVID-19, critical illness neuropathy and myopathy, functional independence measure, outcome, rehabilitation, respiratory failure, walk tests
Introduction
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection can cause pneumonia as well as respiratory and multi-organ failure [1] with various short- and long-term consequences. The first case reports about the extent and severity of peripheral neurologic and muscular impairments after severe novel coronavirus disease-19 (COVID-19) were published in March 2020 [2]. Soon thereafter, the European Academy for Rehabilitation Medicine called to attention the need for the rehabilitation of mechanically ventilated patients with the postintensive care syndrome, including those with critical illness neuropathy (CIN) and critical illness myopathy (CIM). A ‘call for action’ has been sent to rehabilitation specialists and hospitals in April 2020 [3]. The development of CIN and CIM was expected based on previous experiences with critically ill patients requiring mechanical ventilation [4,5].
It has been estimated that critical illness develops in about one-fifth of hospitalized COVID-19 patients [6]. However, in a prospective study conducted in the ICU, a neurophysiologic pattern of CIN/CIM was seen in 79% (11/14) of the COVID-19 cases and 70% (7/10) of non-COVID-19 ICU controls (all CIM) [7]. Several studies described functional impairments associated with the COVID-19 critical illness [8–11]. The first report about rehabilitation outcomes following COVID-19 respiratory failure included collectively 192 cases (58–70% men, average age 57–72 years) and suggested improvements in activities in daily living, walking ability, balance, endurance and respiratory and cognitive functions [12–15].
In Slovenia, the first COVID-19 case was confirmed on 4 March 2020. The first patient with CIN due to critical illness after COVID-19 and respiratory failure was admitted to our Institute, the only tertiary level rehabilitation hospital in Slovenia, on 16 May 2020. The patient suffered respiratory failure, required mechanical ventilation during the ICU stay, and was diagnosed with CIN. The rehabilitation program was implemented based on our experience with CIN and CIM of other origins [16] and with severe peripheral neurological impairments [17,18] following published recommendations [19,20]. Five more similar patients were admitted during the first pandemic wave between March 2020 and June 2020. In anticipation of the second wave and more such admissions come the fall of 2020, we decided to carry out a prospective observational study of rehabilitation outcomes. The specific aim was to describe changes in functional and nutritional status from admission to rehabilitation discharge in a cohort of post-COVID-19 patients who were referred to our institution for electrophysiologically confirmed or clinically suspected CIN and CIM.
Methods
Study design
This was a prospective observational study carried out between 2 November 2020 and 3 May 2021. The study endpoint was the enrolment of 50 consecutive participants, admitted to our institute after acute hospitalisation (at ICU, followed by acute and, if necessary, postacute ward). All patients were adults and did not have significant functional impairments prior to COVID-19 disease. The inclusion criteria were the ICU stay for COVID-19 infection complicated by respiratory failure and requiring mechanical ventilation, electrophysiologically confirmed or clinically suspected diagnosis of CIN/CIM at the time of rehabilitation referral, direct transfer from the acute hospital. The diagnosis of CIN/CIM was established according to the clinical and electrodiagnostic criteria [4] by ICU physicians and neurologists, respectively. The electromyography (EMG) was performed by neurologists, either in the acute hospital after discharge from the ICU or in our institute during the first week of admission if EMG was not available earlier.
The study was approved by the local medical ethics committee and was carried out in accordance with the Declaration of Helsinki. All participants signed written informed consent.
Rehabilitation program
Our comprehensive individually tailored rehabilitation program included rehabilitation nursing, nutritional support, respiratory therapy, kinesiotherapy, electrotherapy, functional occupational therapy, training into activities of daily living and psychosocial support. In addition, when indicated, speech and swallowing training was performed. On average, patients received 30 min of morning bedside training into activities of daily living, followed by 60 min of functional occupational therapy (including fine motors skills training), 90 min of physiotherapy (kinesiotherapy, electrical stimulation, aerobic training, balance and walking training) and 30 min of respiratory therapy five times per week (Monday to Friday). They were also included in individual and group psychological and social support therapy according to their needs. The activities were performed throughout the day, mostly between 7 a.m. and 4 p.m. The therapy schedule and intensity were adjusted according to individual capabilities, and the availability of therapists and treatment facilities. At the time of rehabilitation admission, COVID-19 protective measures were no longer required.
Assessment
All assessments were performed at admission and discharge, except psychological evaluation (admission only). The functional assessment included the Functional Independence Measure (FIM) and two walk tests. Total and motor FIM scores were analysed. The gain in total and motor FIM from admission to discharge and the length of rehabilitation stay (days) were calculated to assess rehabilitation efficiency. Walking ability was assessed with the 10-metre walk test (10 MWT; expressed as walking speed in m/s) and the 6-minute walk test (6 MinWT; expressed in metres) [21]. The mobility was also assessed with de Morton Mobility Index (DEMMI) [22,23], which is a valid instrument for patients with a critical illness [24].
The Canadian Occupational Performance Measure (COPM) was used to assess client outcomes in the areas of self-care, productivity and leisure. Through a semi-structured interview, the COPM is a five-step process that measures client-identified problem areas in daily function. Two COPM scores were obtained: performance and satisfaction with performance [25].
After assessing the nutritional risk at admission with the nutritional risk screening 2002 [26], the patients were referred for anthropometric measurements and bioelectrical impedance analysis (BIA) carried out by QuadScan 4000 (BODYSTAT – Body Composition Technology).
Psychologic evaluation was also performed. Depressive symptoms were assessed using Beck Depression Inventory-II (BDI- II) [27]. The Anxiety/Worry (AW) symptom scale of comprehensive assessment of depressive symptomatology (CAD) was used for assessing anxiety [28].
Statistical analysis
Data collection, visualization and statistical analyses were performed using R [29], 3.6.3 version. All continuous data were expressed as mean, SD, minimum and maximum value, when normally distributed, and as the median and interquartile range (IQR) when not normally distributed. Categorical data were expressed as frequencies and percentages. The Kolmogorov–Smirnov test was carried out to test the normality of the continuous variables.
Differences in outcomes before and after rehabilitation were tested using the exact Wilcoxon signed-rank test for total and motor FIM, walk tests and COPM or paired Student’s t-test for phase angle, body fat percentage and body mass. If patients were unable to walk at admission (n = 12), 0 was assigned for 10 MWT and 6 MinWT. The change in the use of the assistive device was presented as a cross-table. Spearman correlation was used to assess the association between the length of stay and motor FIM gain (not normally distributed). The significance level was set to 0.05.
Results
Sample characteristics
The admission characteristics of 50 recruited participants are presented in Table 1. One or more comorbidities before the COVID-19 disease were reported by 42 (84%) patients: 33 had hypertension, 5 chronic atrial fibrillation, 15 other cardiovascular diseases, 15 diabetes, 27 other endocrine diseases (hypothyroidism, dyslipidemia, obesity and adrenal gland insufficiency), 10 pulmonary disease, 7 kidney disease, 7 musculoskeletal disease and 4 oncological diagnoses. The number of comorbidities ranged from 0 to 8 (median 3). There were 20 (40%) current or former smokers. Significant respiratory symptoms were still present in 36 patients (72%). None of them received the SARS-COV-2 vaccine as it was not available to the general population in Slovenia before and during the study period.
Table 1.
Characteristics of the sample at admission
| Characteristics | Values (n = 50) |
|---|---|
| Demographics | |
| Female | 14 (28%) |
| Age, years | 62 (10) (37–81) |
| Smoking | |
| Nonsmoker | 30 (60%) |
| Former smoker | 17 (34%) |
| Current smoker | 3 (6%) |
| Acute hospitalisation | |
| Length of stay, days | 67 (28) (25–123) |
| Length of mechanical ventilation, days | 27 (16) (6–79) |
| Corticosteroid treatment | 16 (32%) |
| Remdesivir treatment | 25 (50%) |
| Weight loss, % | 15.8 (6.0) (0–31.0) |
| Rehabilitation | |
| Length of stay, days | 42 (16) (11–80) |
| Vit D, nmol/l | 64.9 (23.5) (28.0–142) |
Values are mean (SD) (minimum-maximum) for normally distributed continuous data and n (%) for categorical data.
According to BDI II, six patients (12 %) had mild to moderate depression. The CAD showed a mild clinical risk for anxiety in nine patients (18 %).
EMG findings
The EMG was performed before rehabilitation admission in 18 patients and during rehabilitation in 32 patients. The EMG confirmation of CIN or CIM was obtained in 48 (96%) patients (CIN in 42, CIM in 2, both CIN and CIM in 4). In two patients examined in our institution, there was no EMG evidence of CIN or CIM. Additionally, focal neurological impairments were found in 20 patients affecting the ulnar nerve in 9 (18%), the peroneal nerve in 5 (10%), both ulnar and peroneal nerve in 4 (8%), the median nerve in 1 (2%) and brachial plexus in 1 (2%) patient.
Rehabilitation outcomes
The main results are summarized in Table 2. Both total and motor FIM increased significantly from admission to discharge (P < 0.001). Significant improvements were also found for both COPM performance and satisfaction with performance. In terms of general mobility, DEMMI increased significantly (P < 0.001).
Table 2.
Comparison of functional status on admission and discharge
| Outcome measures | Admission | Discharge | Improvement (P valuea) |
|---|---|---|---|
| Total FIM (points) | 81 (63–69) | 117 (115–120) | <0.001 |
| Motor FIM (points) | 48 (31–65) | 83 (80–85) | <0.001 |
| DEMMI (points) | 40 (25–48) | 74 (62–85) | <0.001 |
| 10MWT (m/s) | 0.36 (0–0.61) | 0.87 (0.72–1.07) | <0.001 |
| 6MinWT (m) | 53 (5–120) | 273 (210–351) | <0.001 |
| COPM- performance (points) | 2.3 (1.4–7.2) | 8.4 (7.3–9.0) | <0.001 |
| COPM- satisfaction (points) | 2.3 (1.2–3.4) | 8.8 (7.8–9.5) | <0.001 |
| PA | 3.0 (0.6) (2.0–5.2) | 3.7 (0.5) (2.7–5.2) | <0.001 |
| Fat (%) | 33.5 (8.5) (21.0–56.2) | 32.7 (8.9) (19.5–55.5) | 0.017 |
| Total body mass (kg) | 83.4 (15.7) (54.1–126.0) | 86.5 (15.4) (61.5–128.2) | <0.001 |
Values are mean (SD) (minimum-maximum) for normally distributed continuous data and median (interquartile range IQR) for nonnormally distributed continuous data.
aWilcoxon signed-rank test or paired t test
6 MinWT, 6 minute walk test; 10 MWT, 10-metre walk test; COPM, The Canadian Occupational Performance Measure; DEMMI, de Morton Mobility Index; FIM, functional independence measure; PA, phase angle.
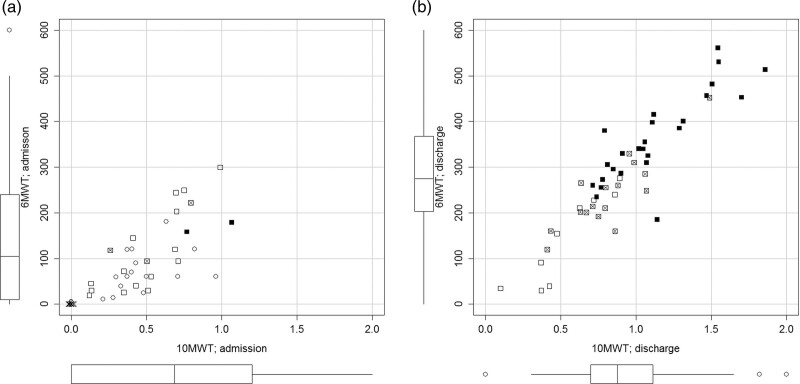
At admission, 12 (24%) patients were unable to walk and 36 (72%) could walk with a walking aid. At discharge, all 50 patients were able to walk either with no walking aid or with a less supportive walking aid (Table 3). On average, the walking speed increased more than two-fold and the walking distance more than five-fold (Table 2, Fig. 1a and b).
Table 3.
Summary of the changes in the use of walking aids from admission to discharge
| Admission | Discharge | Total | ||||
|---|---|---|---|---|---|---|
| Unable to walk | Forearm walker | Frame or rollator | Crutches | No aids | ||
| Unable to walk | 0 | 0 | 5 | 3 | 4 | 12 |
| Forearm walker | 0 | 0 | 4 | 6 | 8 | 18 |
| Frame or rollator | 0 | 0 | 0 | 6 | 9 | 15 |
| Crutches | 0 | 0 | 0 | 1 | 2 | 3 |
| No aids | 0 | 0 | 0 | 0 | 2 | 2 |
| Total | 0 | 0 | 9 | 16 | 25 | 50 |
Fig. 1.
Scatter plot of 6-Minute Walk test (6MinWT) and Timed 10-Metre Walk Test (10MWT) at admission (a, left) and discharge (b, right). Boxplots on the margins indicate the group results for 10 MWT (horizontal axis) and 6 MinWT (vertical axis). Different symbols denote the ambulatory status and the use of walking aids (crosses, does not walk, circles, forearm walker, empty square, walking frame or rollator, crossed square, crutches, black square, no walking aid).
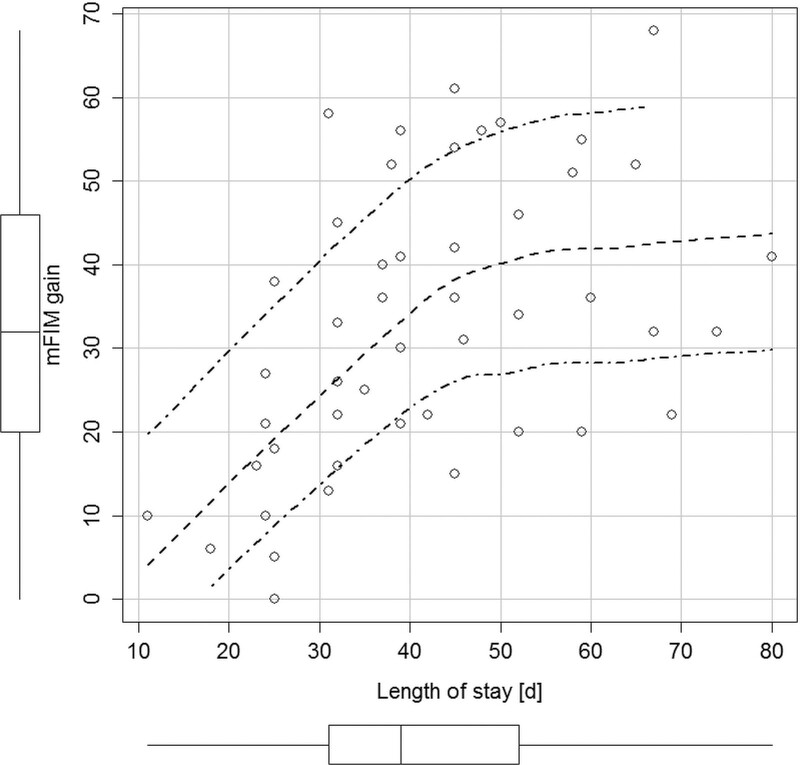
The correlation between the length of stay and motor FIM gain was moderate (ρ = 0.55; P < 0.001). The average change per day in motor FIM was 0.8 points (SD 0.4; range 0–1.9) and in total FIM 0.8 points (SD 0.4; range 0–2.0). Patients reached a functional plateau after 50–60 days of rehabilitation when little or no further improvement seems to be expected (Fig. 2). At discharge, one patient did not improve in motor FIM and one patient scored a maximum of 91 points. On average they achieved 75 % (SD 18%) of the possible max gain.
Fig. 2.
Scatter plot of gain in motor Functional Independence Measure (mFIM) and length of stay. Boxplots on the margins show univariate distributions. Inner dashed line shows LOESS fit (local regression smoother; two outer dash-dot lines indicate 95% confidence interval).
The nutritional risk was confirmed in all patients at admission for which they received nutritional support (adjusted daily intake of macro and micronutrients according to the calculated needs). BIA was performed in 48 patients on admission (contraindicated in two due to cardiac pacemaker) and in 47 at discharge (1 discharged after 11 days only). A significant improvement from admission to discharge was observed for both BIA and anthropometric measures (Table 2). On average, phase angle increased over 20% (on average 0.7°, SD 0.4), the proportion of body fat decreased by less than 1 %, and total body mass increased 3.1 kg (SD 2.4; range −2.5 to 8.2 kg).
Discussion
The main results of this study indicate many functional limitations on admission and significant improvements at rehabilitation discharge in functional independence, general mobility, walking speed and distance, self-perception of performance and performance satisfaction and nutritional status among patients with CIN/CIM following post-COVID-19 respiratory failure and mechanical ventilation. To the best of our knowledge, this is a rare prospective study on inpatient rehabilitation outcomes in a well-defined COVID-19 CIN/CIM cohort. The results need to be interpreted in the context of challenges associated with providing inpatient rehabilitation under the extraordinary circumstances caused by the pandemic.
Due to the extent of the epidemic in Slovenia, the admission of COVID-19 patients with CIN/CIM became the priority of our department. Given limited recommendations [19,20], we based the rehabilitation program on our experience with CIN/CIM of other origins [16], which seems justified considering an overlap in clinical and EMG findings. However, the characteristics of COVID-19 patients and circumstances were unique, which required many adjustments. First, we observed a high rate of comorbidities (84%), similar to previous reports [12–15], which amplified functional limitations and restricted therapy participation. Second, although we admitted patients about 1–4 months after developing respiratory failure, 72% still had respiratory symptoms requiring daily respiratory therapy. Third, 40% showed additional focal impairments in one or more peripheral nerves, consistent with compressive neuropathies due to prolonged lying, prone positioning and the use of muscle relaxants during mechanical ventilation [30–33]. The case of a flaccid arm plegia was reportedly due to a brachial plexus stretch while handling a relaxed patient in the ICU. Lastly, the majority acquired multi-resistant hospital bacteria during the ICU stay for which we implemented protective isolation protocol, reorganized rehabilitation services, and employed additional staff.
The limitations in functional independence present at admission were mainly in the motor domain. The gains in total and motor FIM from admission to discharge can be considered clinically important. Although not directly comparable, Spielmanns et al. [13] reported an 11-point gain in total FIM (starting from 100 points) over 20 days of pulmonary rehabilitation of COVID patients with respiratory failure [13]. The pattern of moderate correlation between the improvement in motor FIM and length of stay (Fig. 2) suggests that the cohort was making progress mainly over the first 2 months. Although this group result does not apply to individual patients, the information may help plan the rehabilitation stay.
Walking and mobility improved significantly. At discharge, walking speed doubled, distance increased five times and the overall mobility (DEMMI) nearly doubled. However, the walking speed remained lower than in the healthy population, considering age and gender [34]. On average, our CIN/CIM patients walked a shorter distance (53 m) at admission compared to 176–323 m reported for COVID-19 patients admitted for rehabilitation after respiratory failure [13–15]. This lower starting distance may have contributed to the comparably larger improvements in our patients (220 m vs. 63–181 m). The increase in walking speed here cannot be compared to the latter studies because 10 MWT was not performed. In addition, all of our patients were able to walk at discharge and half of them used no walking aid or progressed to a less supported aid.
The patients’ perspectives on performance and satisfaction with performance in the areas of self-care, productivity and leisure, as assessed by COPM, were more favourable by the time of discharge. Although COPM was used before in various peripheral neurological conditions (chronic inflammatory demyelinating polyneuropathy, multifocal motor neuropathy, neuralgic amyotrophy and Guillain-Barre syndrome) [35–37], we found no reports in patients after critical illness for comparison. With heightened distress caused by the pandemic, we favour the use of patient-reported outcomes not only as complementary to clinical instruments but also for setting individual goals and resolving self-identified problems.
Perhaps somewhat surprisingly, we observed on admission a rather low rate of mild to moderate depressive symptoms (12%) and mild risk for anxiety (18%) considering the history of respiratory failure and the need for mechanical ventilation. These results are at the low end of the range reported in the previous studies in COVID-19 patients (depression 4.5–45%; anxiety 11–47%) [38–41]. The large variability may be explained by the use of different assessment instruments, including self-administered instruments that are prone to bias.
The initial nutritional assessment indicated that the patients lost on average 16% of total body weight during the acute stay and presented with diffuse muscle atrophies at rehabilitation admission. A comparably lower mean phase angle at admission reported here than in previous studies [42] may be due to a greater decline in cell mass. The loss of cell mass can be ascribed not only to prolong acute hospitalisation and hyper catabolism during the COVID-19 cytokine storm but also the subsequent development of CIN/CIM [42,43]. At discharge, the largest average improvements were found for the phase angle (20%) and total body mass (3 kg) with only a minor decrease in the proportion of body fat. This suggests equal gain in muscle mass and fat that can be ascribed to both physical training and nutritional interventions.
We noted some similarities between this cohort and our previous non-COVID-19 CIN/CIM cohort [16]. The average age (62 vs. 59 years, respectively) and the length of stay in acute hospitals (67 days in both) and our rehabilitation institute (42 vs. 38 days) were similar. Whereas we previously admitted slightly more women than men (16 vs. 11), in this study there were far more men (36 vs. 14). This is consistent with the meta-analysis of more than three million global cases of COVID-19 showing that men have almost three times higher odds of requiring treatment in the ICU [44]. The FIM scores, FIM gains, rehabilitation efficiency, walking speed and walking distance are also comparable to our previous CIN/CIM non-COVID-19 study. Overall, we consider it a remarkable outcome given the challenges associated with providing rehabilitation in the middle of the COVID-19 epidemic.
Our study has several limitations. First, no control group was included. Second, we could not isolate the effects of individual therapeutic procedures. Third, due to technical reasons, obtained respiratory parameters were not reliable enough to be reported. Fourth, only outcomes of inpatients rehabilitation are reported, lacking the information about long-term functioning.
Conclusion
In summary, our prospective study provided evidence that the patients who develop CIN/CIM following respiratory failure due to COVID-19 infection can improve functional and nutritional status during inpatient rehabilitation. The concurrence between the clinically observed and subjectively reported findings provides confidence that the improvements are likely meaningful. The replication of our previous results in the non-COVID-19 cohort suggests that our program for the rehabilitation of CIN/CIM seems to be effective. Additional studies are required to develop a more specific rehabilitation protocol for patients after COVID-19.
Acknowledgements
The authors are grateful to prof. Gaj Vidmar, PhD for his useful suggestions.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Amatya B, Khan F. Rehabilitation response in pandemics. Am J Phys Med Rehabil 2020; 99:663–668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tankisi H, Tankisi A, Harbo T, Markvardsen LK, Andersen H, Pedersen TH. Critical illness myopathy as a consequence of Covid-19 infection. Clin Neurophysiol 2020; 131:1931–1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stam HJ, Stucki G, Bickenbach J; European Academy of Rehabilitation Medicine. Covid-19 and post intensive care syndrome: a call for action. J Rehabil Med 2020; 52:jrm00044. [DOI] [PubMed] [Google Scholar]
- 4.Latronico N, Bolton CF. Critical illness polyneuropathy and myopathy: a major cause of muscle weakness and paralysis. Lancet Neurol 2011; 10:931–941. [DOI] [PubMed] [Google Scholar]
- 5.Visser LH. Critical illness polyneuropathy and myopathy: clinical features, risk factors and prognosis. Eur J Neurol 2006; 13:1203–1212. [DOI] [PubMed] [Google Scholar]
- 6.Cummings MJ, Baldwin MR, Abrams D, Jacobson SD, Meyer BJ, Balough EM, et al. Epidemiology, clinical course, and outcomes of critically ill adults with COVID-19 in New York City: a prospective cohort study. Lancet 2020; 395:1763–1770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Frithiof R, Rostami E, Kumlien E, Virhammar J, Fällmar D, Hultström M, et al. Critical illness polyneuropathy, myopathy and neuronal biomarkers in COVID-19 patients: a prospective study. Clin Neurophysiol 2021; 132:1733–1740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Van Aerde N, Van den Berghe G, Wilmer A, Gosselink R, Hermans G; COVID-19 Consortium. Intensive care unit acquired muscle weakness in COVID-19 patients. Intensive Care Med 2020; 46:2083–2085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Abenza-Abildúa MJ, Ramírez-Prieto MT, Moreno-Zabaleta R, Arenas-Valls N, Salvador-Maya MA, Algarra-Lucas C, et al. Neurological complications in critical patients with COVID-19. Neurologia (Engl Ed) 2020; 35:621–627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sharifian-Dorche M, Huot P, Osherov M, Wen D, Saveriano A, Giacomini PS, et al. Neurological complications of coronavirus infection; a comparative review and lessons learned during the COVID-19 pandemic. J Neurol Sci 2020; 417:117085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Madia F, Merico B, Primiano G, Cutuli SL, De Pascale G, Servidei S. Acute myopathic quadriplegia in patients with COVID-19 in the intensive care unit. Neurology 2020; 95:492–494. [DOI] [PubMed] [Google Scholar]
- 12.Olezene CS, Hansen E, Steere HK, Giacino JT, Polich GR, Borg-Stein J, et al. Functional outcomes in the inpatient rehabilitation setting following severe COVID-19 infection. PLoS One 2021; 16:e0248824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Spielmanns M, Pekacka-Egli AM, Schoendorf S, Windisch W, Hermann M. Effects of a comprehensive pulmonary rehabilitation in severe post-COVID-19 patients. Int J Environ Res Public Health 2021; 18:2695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Curci C, Negrini F, Ferrillo M, Bergonzi R, Bonacci E, Camozzi DM, et al. Functional outcome after inpatient rehabilitation in postintensive care unit COVID-19 patients: findings and clinical implications from a real-practice retrospective study. Eur J Phys Rehabil Med 2021; 57:443–450. [DOI] [PubMed] [Google Scholar]
- 15.Puchner B, Sahanic S, Kirchmair R, Pizzini A, Sonnweber B, Wöll E, et al. Beneficial effects of multi-disciplinary rehabilitation in postacute COVID-19: an observational cohort study. Eur J Phys Rehabil Med 2021; 57:189–198. [DOI] [PubMed] [Google Scholar]
- 16.Novak P, Vidmar G, Kuret Z, Bizovičar N. Rehabilitation of critical illness polyneuropathy and myopathy patients: an observational study. Int J Rehabil Res 2011; 34:336–342. [DOI] [PubMed] [Google Scholar]
- 17.Novak P, Šmid S, Vidmar G. Rehabilitation of Guillain-Barré syndrome patients: an observational study. Int J Rehabil Res 2017; 40:158–163. [DOI] [PubMed] [Google Scholar]
- 18.Polončič P, Novak P, Puzić Ravnjak N, Majdič N. The associations between nutritional and functional status during recovery from Guillain-Barré syndrome: a retrospective study. Int J Rehabil Res 2021; 44:57–64. [DOI] [PubMed] [Google Scholar]
- 19.Sheehy LM. Considerations for postacute rehabilitation for survivors of COVID-19. JMIR Public Health Surveill 2020; 6:e19462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Barker-Davies RM, O’Sullivan O, Senaratne KPP, Baker P, Cranley M, Dharm-Datta S, et al. The Stanford Hall consensus statement for post-COVID-19 rehabilitation. Br J Sports Med 2020; 54:949–959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Crapo RO, Casaburi R, Coates AL, Enright PL, MacIntyre NR, McKay RT, et al. ATS statement: guidelines for the six-minute walk test. Am J Respir Crit Care Med 2002; 166:111–117. [DOI] [PubMed] [Google Scholar]
- 22.de Morton NA, Davidson M, Keating JL. The de Morton Mobility Index (DEMMI): an essential health index for an ageing world. Health Qual Life Outcomes 2008; 6:63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zupanc A, Vidmar G, Novak P, Puh U. Feasibility of de Morton Mobility Index for adult patients of all ages at low and basic functioning level: a study using the Slovenian translation. Int J Rehabil Res 2019; 42:352–357. [DOI] [PubMed] [Google Scholar]
- 24.Sommers J, Vredeveld T, Lindeboom R, Nollet F, Engelbert RH, van der Schaaf M. de Morton mobility index is feasible, reliable, and valid in patients with critical illness. Phys Ther 2016; 96:1658–1666. [DOI] [PubMed] [Google Scholar]
- 25.Law M, Baptiste S, McColl M, Opzoomer A, Polatajko H, Pollock N. The Canadian occupational performance measure: an outcome measure for occupational therapy. Can J Occup Ther 1990; 57:82–87. [DOI] [PubMed] [Google Scholar]
- 26.Kondrup J, Allison SP, Elia M, Vellas B, Plauth M; Educational and Clinical Practice Committee, European Society of Parenteral and Enteral Nutrition (ESPEN). ESPEN guidelines for nutrition screening 2002. Clin Nutr 2003; 22:415–421. [DOI] [PubMed] [Google Scholar]
- 27.Beck AT, Brown GK, Steer RA. Beck depression inventory-II. Psychological Corporation; 1996. [Google Scholar]
- 28.Bracken BA, Howell K. Manual for the clinical assessment of depression. Psychological Assessment Resources; 2004. [Google Scholar]
- 29.R Core Team. A language and environment for statistical computing. R Foundation for Statistical Computing; 2013. [Google Scholar]
- 30.de Sire A, Andrenelli E, Negrini F, Patrini M, Lazzarini SG, Ceravolo MG, et al. Rehabilitation and COVID-19: a rapid living systematic review by Cochrane Rehabilitation Field updated as of December 31st, 2020 and synthesis of the scientific literature of 2020. Eur J Phys Rehabil Med. 2021; 57:181–188. [DOI] [PubMed] [Google Scholar]
- 31.Jové Ponseti E, Villarrasa Millán A, Ortiz Chinchilla D. Analysis of complications of prone position in acute respiratory distress syndrome: quality standard, incidence and related factors. Enferm Intensiva 2017; 28:125–134. [DOI] [PubMed] [Google Scholar]
- 32.DePasse JM, Palumbo MA, Haque M, Eberson CP, Daniels AH. Complications associated with prone positioning in elective spinal surgery. World J Orthop 2015; 6:351–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Demeco A, Marotta N, Barletta M, Pino I, Marinaro C, Petraroli A, et al. Rehabilitation of patients post-COVID-19 infection: a literature review. J Int Med Res 2020; 48:300060520948382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bohannon RW, Williams Andrews A. Normal walking speed: a descriptive meta-analysis. Physiotherapy 2011; 97:182–189. [DOI] [PubMed] [Google Scholar]
- 35.Erdmann PG, van Meeteren NL, Kalmijn S, Wokke JH, Helders PJ, van den Berg LH. Functional health status of patients with chronic inflammatory neuropathies. J Peripher Nerv Syst 2005; 10:181–189. [DOI] [PubMed] [Google Scholar]
- 36.Ijspeert J, Janssen RM, Murgia A, Pisters MF, Cup EH, Groothuis JT, van Alfen N. Efficacy of a combined physical and occupational therapy intervention in patients with subacute neuralgic amyotrophy: a pilot study. NeuroRehabilitation 2013; 33:657–665. [DOI] [PubMed] [Google Scholar]
- 37.Sahid MH, et al. Occupational Therapy Intervention in Functional Communication Program with Biomechanical and Rehabilitative Approach for Guillain-Barre Syndrome Patient: A Case Study. Ann Physiother Occup Ther 2021; 4:000207. [Google Scholar]
- 38.Mazza MG, De Lorenzo R, Conte C, Poletti S, Vai B, Bollettini I, et al. ; COVID-19 BioB Outpatient Clinic Study group. Anxiety and depression in COVID-19 survivors: role of inflammatory and clinical predictors. Brain Behav Immun 2020; 89:594–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gramaglia C, Gambaro E, Bellan M, Balbo PE, Baricich A, Sainaghi PP, et al. ; NO-MORE COVID Group. Mid-term psychiatric outcomes of patients recovered from COVID-19 from an Italian Cohort of Hospitalized Patients. Front Psychiatry 2021; 12:667385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Deng J, Zhou F, Hou W, Silver Z, Wong CY, Chang O, et al. The prevalence of depression, anxiety, and sleep disturbances in COVID-19 patients: a meta-analysis. Ann N Y Acad Sci 2021; 1486:90–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Venturelli S, Benatti SV, Casati M, Binda F, Zuglian G, Imeri G, et al. Surviving COVID-19 in Bergamo province: a post-acute outpatient re-evaluation. Epidemiol Infect 2021; 149:e32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Moonen HPFX, van Zanten FJL, Driessen L, de Smet V, Slingerland-Boot R, Mensink M, van Zanten ARH. Association of bioelectric impedance analysis body composition and disease severity in COVID-19 hospital ward and ICU patients: the BIAC-19 study. Clin Nutr 2021; 40:2328–2336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Moonen HPFX, van Zanten FJL, Driessen L, de Smet V, Slingerland-Boot R, Mensink M, van Zanten ARH. Association of bioelectric impedance analysis body composition and disease severity in COVID-19 hospital ward and ICU patients: the BIAC-19 study. Clin Nutr 2021; 40:2328–2336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Peckham H, de Gruijter NM, Raine C, Radziszewska A, Ciurtin C, Wedderburn LR, et al. Male sex identified by global COVID-19 meta-analysis as a risk factor for death and ITU admission. Nat Commun 2020; 11:6317. [DOI] [PMC free article] [PubMed] [Google Scholar]