Highlights
-
•
COVID-19 myocardial injury results from immune and hypercoagulability responses.
-
•
Long-term cardiac consequences of COVID-19 include structural and functional changes.
-
•
Myocarditis after COVID-19 vaccination is uncommon (highest risk in teenage males).
-
•
Larger population-based studies are necessary to validate these early results.
Key Words: athlete, cardiovascular magnetic resonance imaging, COVID-19, inflammation, myocardial injury, myocarditis, sudden cardiac death, troponin
Abbreviations and Acronyms: CMR, cardiovascular magnetic resonance; COVID-19, coronavirus disease-2019; CT, Computerized Tomography; LGE, late gadolinium enhancement; MI, myocardial infarction; SARS-CoV-2, severe acute respiratory syndrome coronavirus-2
Summary
The mechanisms of coronavirus disease-2019 (COVID-19)–related myocardial injury comprise both direct viral invasion and indirect (hypercoagulability and immune-mediated) cellular injuries. Some patients with COVID-19 cardiac involvement have poor clinical outcomes, with preliminary data suggesting long-term structural and functional changes. These include persistent myocardial fibrosis, edema, and intraventricular thrombi with embolic events, while functionally, the left ventricle is enlarged, with a reduced ejection fraction and new-onset arrhythmias reported in a number of patients. Myocarditis post-COVID-19 vaccination is rare but more common among young male patients. Larger studies, including prospective data from biobanks, will be useful in expanding these early findings and determining their validity.
Central Illustration
Background
Biochemical Cues to Cardiac Involvement in Coronavirus Disease-2019
Approximately 15 months after the World Health Organization declared coronavirus disease-2019 (COVID-19) a pandemic, the global case load exceeds 217 million, with more than 4.5 million deaths worldwide as of September 2021.1 Although severe respiratory failure is considered the main cause of death,2 cardiovascular symptoms such as palpitations, dyspnea, fatigue, and chest pain are common both in the subacute and chronic stages of the disease.3, 4, 5, 6 Additionally, a significant proportion of patients with COVID-19 were found to have biochemical evidence of myocardial injury. Myocardial injury is common in patients with COVID-19, its frequency increases with greater severity of illness, and it carries relevant prognostic information.7 The first case series reported on clinical outcomes and cardiac involvement in COVID-19 suggested that up to 28% of hospitalized patients had elevated troponin levels.8 Subsequent studies have reported an association between biochemical evidence of myocardial injury and increased risk for arrhythmic events, extracardiac complications (acute respiratory distress syndrome, mechanical ventilation), and mortality in hospitalized patients.9,10 However, the majority of patients with COVID-19 with elevated cardiac enzymes have no underlying epicardial coronary artery occlusion (effectively excluding type 1 myocardial infarction [MI]) or clinical symptoms of a cardiac pathology.11 This prompted some to propose that COVID-19 predisposes to a nonspecific myocardial injury biomarker leak not indicative of a type 2 MI.12 Nevertheless, recent autopsy series of patients with COVID-19 have demonstrated venous thromboembolism and pulmonary embolism as frequent findings.13 The underlying hypercoagulability may contribute to hypoxia or thrombosis, which precipitate organ failure, including sudden ventricular dysfunction and cor pulmonale.14 Potential mechanisms for myocardial injury engendered by severe acute respiratory syndrome-coronavirus-2 (SARS-CoV-2) are discussed in detail under “SARS-CoV-2-Related Myocardial Injury.”
Imaging and histological evidence of SARS-CoV-2-related myocardial injury and the controversial diagnosis of myocarditis
As mentioned, elevated cardiac biomarkers in patients with COVID-19 are common, but this does not necessarily indicate clinically significant cardiac involvement. When coronary angiography fails to indicate an ischemic cause of the myocardial injury, it cannot be assumed that the elevated cardiac enzymes result from myocardial inflammation.15 Additional investigations are therefore required to ascertain the existence of myocardial injury.
Cardiovascular magnetic resonance (CMR) imaging has been used to assess cardiac involvement in recovered patients with COVID-19.16,17 In general, a positive correlation is found between abnormal CMR findings (eg, increased T1 or late gadolinium enhancement [LGE]) and increased troponin levels, suggesting that the cardiac biomarker is sensitive but not specific to myocardial injury found in recovered patients with COVID-19. Studies to date indicate that cardiac involvement, as measured by CMR, is fairly common among recovered patients with COVID-19 (range: 30%-78%), even when these patients were included nonselectively for cardiovascular presentations during their acute illness. Furthermore, the nonischemic LGE patterns found among participants suggest a non-MI cause of cardiac injury.
More controversial, however, is the diagnosis of myocarditis ascribed to patients with COVID-19 with cardiac symptoms. Many case reports of myocarditis in patients with COVID-19 rely on clinical diagnoses, and several lack robust advanced imaging and/or biopsy-derived evidence (including conventional and immunohistochemistry and polymerase chain reaction) to help establish the diagnosis. This has led some to suggest that myocarditis is a rare complication of COVID-19; nevertheless, a recent study showed that 4.5% of COVID-19-related cases examined with autopsy or endomyocardial biopsy showed histologic evidence of myocarditis.18 However, with elevated cardiac enzymes, the priority is to exclude an acute coronary syndrome, and in cases in which culprit occlusions cannot be found, other investigations are used to refine the diagnosis. The diagnoses of myocarditis and other cardiovascular involvement of COVID-19 are discussed in the next section.
SARS-CoV-2-Related Myocardial Injury
Prevalence
The prevalence of myocardial injury as a consequence of COVID-19 remains uncertain. An early case series of COVID-19-related mortalities attributed as much as 7% of all COVID-19 deaths to myocarditis.2 However, the diagnosis of myocarditis was loosely based on clinical presentation and cardiac biomarkers, both of which have low specificity to the disease. This likely contributed to an overestimation of the incidence of SARS-CoV-2-related myocarditis. A recent retrospective cohort study showed that new heart failure was seen in 0.6% of patients with COVID-19 (37 of 6,439), and among these patients, 22% (8 of 37) had no predisposing risk factors or preexisting cardiovascular disease.19 The Centers for Disease Control and Prevention revealed that the risk for myocarditis is 0.146% among inpatients and outpatients with COVID-19; this is 15.7 times the background risk in those without COVID-19, after matching for patient and hospital characteristics.20 Although not all diagnoses had histologic or radiological proof of myocarditis, a subset of these patients might have had myocarditis secondary to SARS-CoV-2. Therefore, when applying conventional diagnostic criteria for myocarditis (ie, the updated Lake Louise criteria for CMR imaging or the [outdated] Dallas criteria for endomyocardial biopsy), only a handful of case reports would be truly identified as myocarditis.18 However, in this setting of acute myocardial injury of unknown mechanism(s), the validity of these criteria is questionable, as is the attribution to myocarditis (rather than to another noncoronary pathological process) of abnormalities detected. Additionally, a prospective multicenter cohort study of cardiac involvement in young athletes with COVID-19 revealed a low prevalence of cardiac involvement.21 In the study, only 12.6% of patients who were clinically indicated for CMR were found to have findings that satisfied Lake Louise criteria for myocarditis. CMR and endomyocardial biopsy were underused in these early patients, in part because of restrictions from infection control measures, redeployment of staff, and lack of staff and equipment to justify these tests in infectious and critically ill patients. Nevertheless, it is reasonable to suggest that even within the select group of patients with COVID-19 with the clinical presentation of myocarditis, radiologically or histologically confirmed myocarditis remains an uncommon diagnosis.
COVID-19-related myocardial injury: The proposed mechanisms
Various mechanisms have been proposed for SARS-CoV-2-mediated cardiac injury: 1) direct injury to cardiomyocytes; 2) direct damage to endothelial cells and endotheliitis; 3) indirect injury from hypercoagulability; 4) indirect injury from cytokine storm; and 5) a form of cell- or antibody-mediated autoimmunity triggered following the host response to the virus in susceptible individuals (Figure 1).22 These suggestions have been refined according to the evolving evidence gradually being made available from autopsy series reported from centers around the globe. One early suggestion was direct cell injury by means of SARS-CoV-2 entry into the myocardium. This proposition is based on the presence of angiotensin-converting enzyme 2, a membrane protein, on cardiomyocytes. Angiotensin-converting enzyme 2 acts as a docking site for SARS-CoV-2 to gain cell entry.23 In an in vitro study using human induced pluripotent stem cell–derived cardiomyocytes, Sharma et al24 demonstrated that SARS-CoV-2 can infect and induce apoptosis in these cardiomyocytes. Direct viral infection of human induced pluripotent stem cell–derived cardiomyocytes was also shown to cause up-regulation of certain proteins, including brain natriuretic peptide, interleukin-6, interleukin-8, and tumor necrosis factor–α, to the detriment of angiotensin-converting enzyme 2 expression.25 The crucial flaw in these studies is that cardiac tropism of SARS-CoV-2 was forced in an artificial cellular model, when in fact presence of the binding proteins does not always predict tropism,26 and so far, there is little in vivo evidence to support this model. For example, direct detection of SARS-CoV-2 in the heart is rare but reported;27 among these highly selected cases, localization of SARS-CoV-2 has been confined principally to interstitial cells or macrophages, rather than the cardiomyocytes.18,28
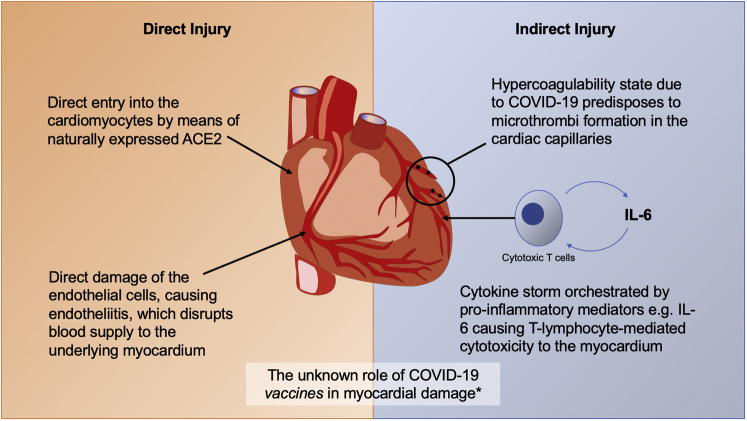
Figure 1.
Summary of the Proposed Mechanisms for Myocardial Cell Injury by SARS-CoV-2 Infection
Direct injury might be caused by cell entry via the angiotensin-converting enzyme 2 (ACE2) protein expressed naturally on cardiomyocytes or endothelial cell damage (endotheliitis) due to severe acute respiratory syndrome-coronavirus-2 (SARS-CoV-2) infection. Indirect damage might be brought on by the hypercoagulability state of coronavirus disease-2019 (COVID-19), which engenders microthrombus formation, disrupting cardiac capillary flow or by means of T lymphocyte–mediated cytotoxicity as part of the phenomenon called cytokine storm. ∗Since April 2021, there have been case reports of myocarditis and pericarditis following COVID-19 vaccination across all vaccine types, especially in young male subjects. The mechanism remains unknown. IL = interleukin.
An alternative proposal is that SARS-CoV-2 infects the endothelial cells, which in turn mediates cellular injury to the tissues supplied by the affected vasculature. Endotheliitis, an inflammatory capillary injury with fibrin deposition and activation of the terminal portion of the complement cascade, was histologically confirmed in autopsies of patients with COVID-19.29 The investigators reported direct involvement of SARS-CoV-2 in the endothelium in addition to the proposed immune-mediated inflammation. However, the study did not address the pattern of tissue injury beyond the endothelium (ie, the suggestion that endotheliitis was responsible for local hypoxia and tissue ischemia is speculative, as other plausible causes of organ failure, including cytokine storm and type I/II respiratory failure, cannot be negated).
Another hypothesis is that SARS-CoV-2 causes myocardial injury indirectly, as there is often a temporal delay between the cardiac presentations and SARS-CoV-2 infection.27,30,31 A handful of mechanisms may be relevant and may coexist in some patients, such as, venous thromboembolism, with pulmonary embolism being an important cause of adverse outcomes associated with COVID-19.32 An autopsy report from Germany reported deep venous thrombosis in 58% of patients (7 of 12) in whom venous thromboembolism was not suspected before death,13 and the incidence of alveolar capillary microthrombi was significantly higher than following H1N1 influenza.12 Microthrombi have also been identified in the cardiac vasculature of patients with COVID-19; nevertheless, the study lacks cardiac imaging and clinical correlation to corroborate the postmortem findings, which may well be incidental rather than causal of the myocardial injury.33 The reasons for greater thrombosis risk in patients with COVID-19, compared with other infectious diseases, remain unknown. Additionally, it is unclear whether the microthrombosis was due to the procoagulable state of COVID-19 or the endothelial injury brought on by SARS-CoV-2 or both. However, the high thrombosis burden among patients with COVID-19 may predispose them to poorer health outcomes, especially in patients with concurrent ST-segment elevation myocardial infarction.34 Figure 1 summarizes the possible mechanism of myocardial injury secondary to SARS-CoV-2 infection.
Nevertheless, the most likely pathophysiology for SARS-CoV-2-related myocardial injury is based on a phenomenon known as cytokine storm. Proinflammatory cytokines, released by innate and adaptive immune cells, result in hyperactive and dysregulated immune responses. These responses are pleiotropic and complex but may include polarization toward cardiotropic CD4 and CD8 T cells and suppression of T regulatory cells. Evidence supporting this hypothesis includes elevated proinflammatory cytokines, such as interleukin-6 and interferon-γ in patients with COVID-19,35 as well as documented cases of cytokine storm syndrome in severely ill inpatients.36 Elevated interleukin-6, which was found to be one of the predictors of more rapid COVID-19 deterioration, may partly explain the acute and long-term consequences of COVID-19, including myocardial inflammation, as it is known to be a mediator of the prothrombotic state, platelet function, and antibody production.37 Other interleukins may also be involved in acute heart failure and myocarditis. For instance, intense NOD-, LRR-, and pyrin domain-containing protein 3 inflammasome formation in the heart is observed in myocarditis, producing the proinflammatory cytokine interleukin-1 which exacerbates the myocardial injury.38 Figure 2 shows the proposed molecular mechanism for the indirect and indirect myocardial injury due to SARS-CoV-2.
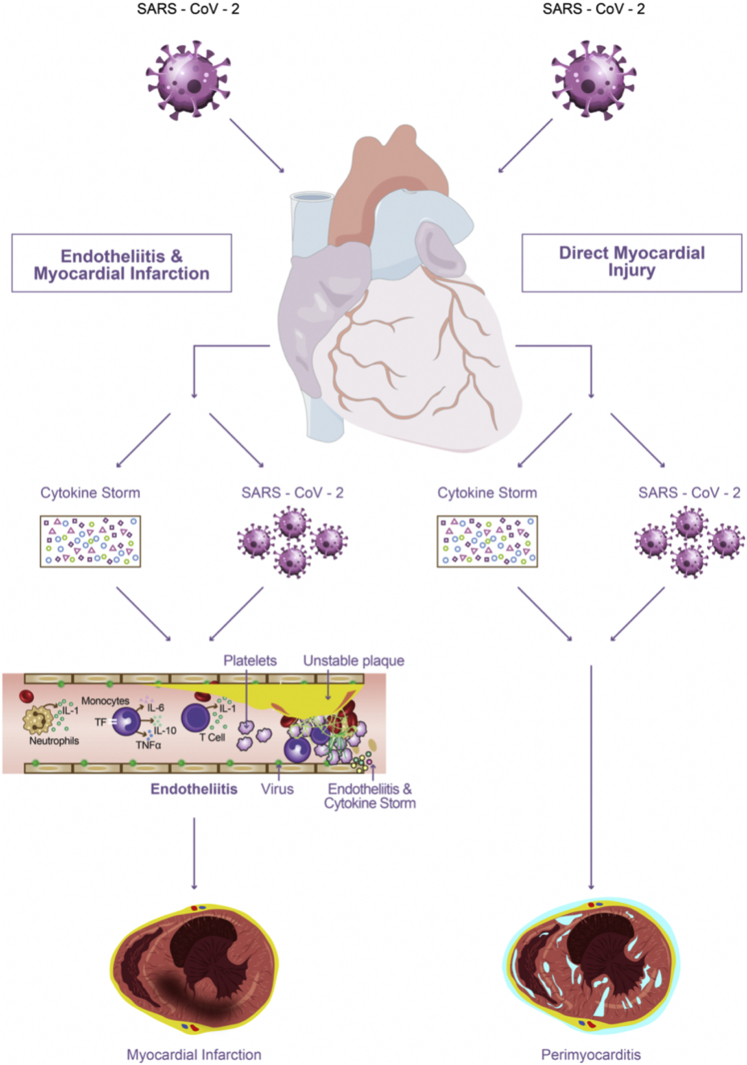
Figure 2.
Acute Cardiac Injury in Coronavirus Disease 2019
In the acute setting, both direct (viral) and indirect (immune-mediated) damage to the myocardial and other heart tissues can give rise to perimyocarditis or myocarditis. Moreover, endothelial tissue injury, by means of endotheliitis and microthrombus formation, leads to type 2 myocardial infarction. TF = tissue factor; TNF= tumor necrosis factor; other abbreviations as in Figure 1.
Clinical characteristics of the reported cases of SARS-CoV-2-related myocarditis
The clinical presentation of SARS-CoV-2-related myocarditis is, in general, highly variable and nonspecific, partly because of a lack of definitive investigations discussed under “Background.” The reported presenting symptoms range from relatively mild (eg, fatigue and dyspnea)39, 40, 41, 42 to more severe (eg, chest pain on exertion and hemodynamic instability).43, 44, 45 However, even in cases in which myocarditis is eventually confirmed, presenting symptoms may not be cardiac specific.46 In general, the cardiac disease was suspected when abnormal electrocardiographic changes and elevated troponins were identified, even when the patients had minimal cardiac symptoms at presentation.44,47 Conversely, some patients without overt cardiac symptoms at presentation have gone on to develop acute heart failure and cardiogenic shock,48 demonstrating how variable COVID-19 myocarditis can be over the duration of illness.
In the most severe presentation, patients have developed fulminant myocarditis, defined as acute ventricular dysfunction and heart failure within 2 to 3 weeks of contracting the virus.49, 50, 51, 52, 53 Although most of these patients are young and recovered fully after pharmacologic management, some patients progressed to require durable mechanical circulatory support in response to persistent severe ventricular dysfunction.54 Because of the nonspecificity of COVID-19-related myocarditis reported to date, other diagnoses should also be considered and a clear diagnostic algorithm used.22 The clinical characteristics of reported cases of COVID-19-related myocarditis (as of September 2021) are summarized in Supplemental Table 1.
Management
Management of the cardiac complications of COVID-19 is still contentious. The general principle is to establish hemodynamic stability in a stepwise manner, escalating to match the patient’s clinical needs. Detailed suggested management algorithms can be found in other papers;22,55 however, we aim to provide an update to this with our current understanding of the disease and additional evidence since reported regarding the long-term consequences.
In short, patients presenting acutely with cardiogenic shock or severe hypotension may require inotropes or vasopressors to improve perfusion. If these modalities are insufficient to overcome shock or normalize cardiac filling pressures, temporary mechanical circulatory support may be required with an intra-aortic balloon pump, a percutaneous left ventricular assist device, or extracorporeal membrane oxygenation.56 In patients with severe COVID-19 pneumonia, often with signs of systemic infection, immunomodulatory treatments may be given to alleviate the respiratory symptoms. These include corticosteroids (eg, dexamethasone) and anti-interleukin-6 receptor monoclonal antibodies (eg, tocilizumab, sarilumab). Tocilizumab was shown in the RECOVERY (Randomised Evaluation of COVID-19 Therapy) trial to be effective at improving survival and clinical outcomes in patients with COVID-19 with hypoxia and systemic inflammation.57 Both the US and UK guidelines recommend tocilizumab for patients with COVID-19 with hypoxia requiring ventilatory support or high-flow nasal oxygen.58,59 Moreover, tocilizumab, at the dose recommended (8 mg/kg intravenously, up to 800 mg), was successfully used as a part of management for a patient with COVID-19 with acute heart failure.60 There have been some anecdotal reports in favor of interleukin-1 antagonists (eg, anakinra) in myocarditis, recurrent pericarditis, and heart failure, although not in the context of COVID-19.38,61 Corticosteroid treatment is a mainstay in the management of severe COVID-19; however, its use may complicate viral myocardial injury in the early phases when active inflammation is required to clear the pathogen. Acute management modalities so far reported for COVID-19-related myocarditis are summarized in Supplemental Table 2.
Long-term management is more complex, though few patients thus far have had such severe persistent disease as to require durable left ventricular assist support or heart transplantation. Nevertheless, patients with persistent severe left ventricular systolic dysfunction should be optimized on goal-directed medical therapy. This includes beta-blockers, angiotensin receptor-neprilysin inhibitors, mineralocorticoid receptor antagonists, and even sodium-glucose cotransporter 2 inhibitors, though the direct benefit of any of these therapies after COVID-19 myocarditis is only inferred from other populations with heart failure.
Long-Term Consequences of COVID-19-Related Injury and Myocarditis
Clinical outcomes of COVID-19-related myocarditis
Most surviving patients with myocarditis have achieved full or significant recovery of their systolic function. However, a small minority experienced catastrophic consequences, which ultimately led to death. A search of published studies (MEDLINE, PubMed Central, and NCBI Bookshelf) identified 77 case reports of SARS-CoV-2-related myocarditis published by the end of September 2021. In 1 case report, ventricular fibrillation was the cause of death, representing 1 of 10 deaths overall in the 77 cases reviewed.44 In 3 patients who made a full recovery, new-onset arrhythmias developed (2 complete atrioventricular block and 1 atrial fibrillation).50,62,81 In 1 case, the patient developed septic shock and disseminated intravascular coagulation.64 One patient had a stroke several days after being discharged in stable condition following a myocarditis episode.65 Other milder complications include reduced exercise tolerance and persistent reduction in systolic ejection fraction.66,67 A full summary of the 77 case reports can be found in Supplemental Table 1.
Post-COVID-19 syndrome
There is a distinct lack of data on the long-term sequelae and prognosis following SARS-CoV-2-related myocardial injury and myocarditis, although a fuller picture is beginning to emerge via observational case reports. Understandably, the main focus of the medical response to the pandemic has been on early recognition and treatment to reduce systemic inflammatory response and prevent multiorgan failure and multiorgan dysfunction syndrome. A principle-based approach on managing post-COVID-19 syndrome68 was based on the experiences of critical care patients who survived sepsis, the postsepsis syndrome. Postsepsis syndrome is well reported and seen in up to 50% of sepsis cases managed on critical care,69 but little is known about the frequency of post-COVID-19 syndrome. Proposed mechanisms first require introducing a compensatory anti-inflammatory response syndrome.70 It is postulated that a systemic inflammatory response with cytokine storm is balanced by the compensatory anti-inflammatory response syndrome to prevent widespread multiorgan dysfunction. Patients who may have a milder earlier phase of illness can enter persistent inflammation, immunosuppression, and catabolism syndrome, which is a hypothesized cause of persistent post-COVID-19 syndrome. There have also been reports of latent virus reactivation of SARS-COV-2 in recovered patients with COVID-19. These patients can then develop various post-COVID-19 manifestations, including pulmonary fibrosis, with transforming growth factor–beta implicated as a key factor. Transforming growth factor–beta is a known inducer of fibrosis and immunosuppression71 (Figure 3).
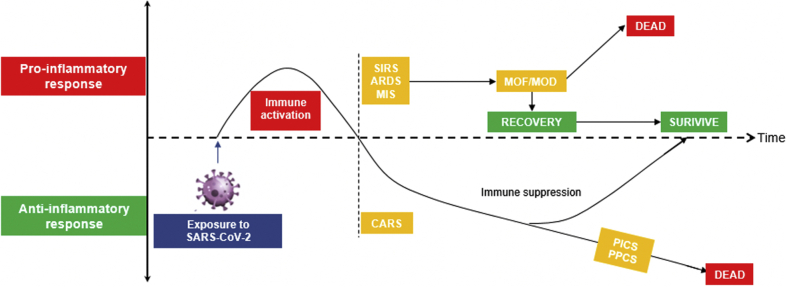
Figure 3.
Timeline of the Immune Response to COVID-19
The diagram shows the proposed immunologic reaction to SARS-CoV-2 infection, in which the proinflammatory response predominates in the acute phase, which could culminate in acute respiratory response syndrome (ARDS), multisystem inflammatory syndrome (MIS), or systemic inflammatory response syndrome (SIRS). Should there be no counterbalancing anti-inflammatory response, these syndromes could lead to multiorgan failure (MOF) or multiorgan dysfunction (MOD) and/or death. With adequate counteracting anti-inflammatory cytokines produced, the body is in the immune-suppressed phase, a process called compensatory anti-inflammatory response syndrome (CARS). With prolonged immune suppression, persistent infection can occur (ie, in the form of persistent inflammation, immunosuppression, and catabolism syndrome [PICS] or persistent post-COVID-19 syndrome [PPCS]). Abbreviations as in Figure 1.
Long-term effects on the heart
In the setting of COVID-19, myocarditis and cardiac myocyte damage can lead to residual morphologic and functional impact on the myocardium (Figure 4), particularly in those with preexisting cardiac disease. Optimal assessment of long-term outcomes of COVID-19-related cardiac injury, whether myocarditis or other injury, requires regular follow-up with clinical investigation, cardiac arrhythmia monitoring, and multimodality imaging. Thus far, however, there are very few studies assessing the long-term effects and complications after recovery from COVID-19-mediated myocarditis.22,72 In a study from Germany, patients recently recovered from COVID-19 had lower left ventricular ejection fraction, higher left ventricular volumes, and higher native T1 (suggestive of myocardial interstitial fibrosis73) and T2 (suggestive of myocardial edema74) values compared with healthy control subjects and risk factor–matched control subjects.17 Abnormal CMR findings were present in 78% of patients and ongoing myocardial inflammation in 60%. Native T1 and T2 mapping provided the best discriminatory ability to detect COVID-19-associated myocardial disease. These findings correlated with higher levels of high-sensitivity troponin T and active lymphocytic inflammation on endomyocardial biopsy specimens.17 In summary, this study also highlights that myocardial inflammation can be observed radiologically (on CMR) in patients recovering from COVID-19, in both asymptomatic and symptomatic patients.75
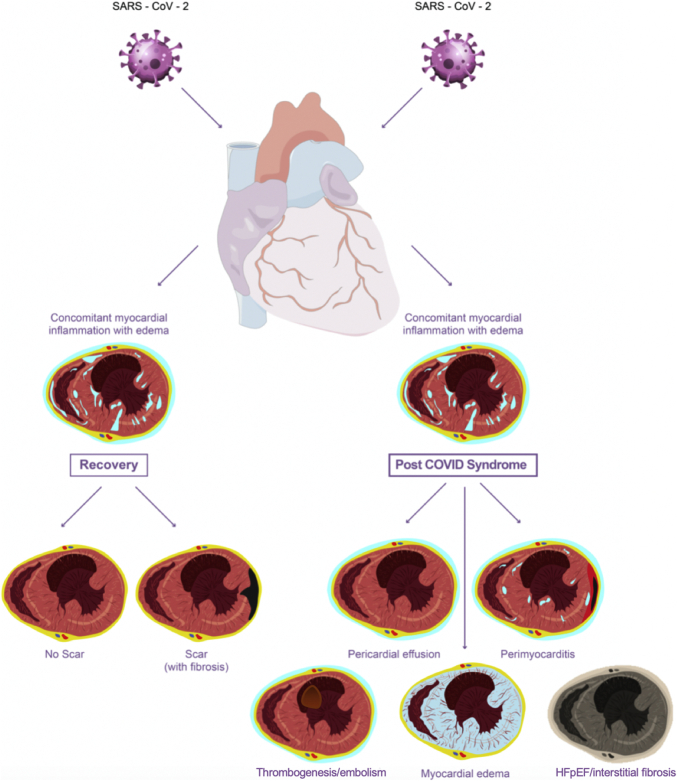
Figure 4.
Chronic Consequences of COVID-19 Infection on the Heart
Most patients with cardiac involvement do fully recover, either with or without scarring (as evident in some follow-up studies). Some patients with persistent infection can develop post-COVID-19 syndrome. The cardiac presentation of this includes pericardial effusion, perimyocarditis (early) or myocardial edema, embolism, and interstitial fibrosis with heart failure with preserved ejection fraction (HFpEF) (late). Abbreviations as in Figure 1.
The long-term cardiac consequences of COVID-19 may also be dependent on the severity of initial COVID-19 itself. A recent study of health workers seropositive for SARS-CoV-2 and either mild or no symptoms detected no differences in the CMR findings or cardiac biomarkers (ie, N-terminal pro–B-type natriuretic peptide and troponin) between 74 positive and 75 matched seronegative participants.76
One multicenter study involved 148 COVID-19 survivors with elevated troponin who underwent CMR after a median of 68 days (range: 30-103 days) following hospital discharge.11 The investigators reported a myocarditis-like LGE pattern in 26% (39 of 148), evidence of occlusive coronary disease (MI-pattern LGE and/or abnormal stress perfusion) in 22% (32 of 148), and dual pathology in 6% (9 of 148). Myocardial injury was limited to ≤3 of the 17 American Heart Association segments, and there was no measurable impact on left ventricular ejection fraction. Neither peak nor admission serum troponin was predictive of a diagnosis of myocarditis; of the 39 patients with myocarditis-like LGE patterns, 8 (20%) also had elevated native T1 and T2 values indicative of myocardial edema. In the absence of pre-COVID-19 imaging, determining antecedence and prior myocardial scar or structural abnormalities is difficult, despite the use of matched non-COVID-19 control subjects and healthy control subjects.77
In a US study of 26 competitive athletes recovering from COVID-19, 4 male athletes (15%) had CMR findings suggestive of myocarditis on the basis of the Lake Louise criteria.75 Twelve athletes (46%) had myocardial LGE (mean of 2 of the 17 American Heart Association segments), of whom 8 (30.8%) had LGE without concomitant T2 elevation. However, this was a small study, and it is unclear whether these CMR changes predated these patients’ COVID-19 illness. A recent multicenter cohort study of nearly 789 professional athletes referred for CMR according to the American College of Cardiology’s return-to-play guideline found that only 5 athletes (0.6%) ultimately had CMR findings suggestive of inflammatory heart disease (myocarditis in 3 and pericarditis in 2) that resulted in restriction from play.78 Another prospective multicenter observational cohort study with data from 42 colleges and universities from the United States identified SARS-COV-2 cardiac involvement in 21 of 3,018 athletes (0.7%).21 During a median clinical surveillance period of 113 days, no COVID-19-related adverse cardiac events occurred, confirming a low prevalence of cardiac involvement and a low risk for cardiac events in short-term follow-up among young competitive athletes. Applying the current evidence, athletes with COVID-19-related cardiac disease should be screened for cardiovascular abnormality as per each sport’s regulatory guideline. CMR is recommended only when initial screenings reveal significant abnormalities, and those who clear the screening tests and/or CMR imaging should be able to return to play safely.
Electrocardiographic and cardiac conduction abnormalities are not uncommon among recovered patients with COVID-19 who had moderate to severe disease during their acute illness. Liu et al79 reported that among 486 Chinese patients with COVID-19 followed at 3, 6, and 12 months postdischarge, arrhythmias were present in 12.4%, 7.6%, and 16.3%, respectively. However, it is unclear how many of these abnormal electrocardiographic findings predated these patients’ COVID-19 illness and, more important, which of these resulted from COVID-19. Among the cases of COVID-19-related myocarditis reviewed, however, the majority of new-onset arrhythmias resolved spontaneously before discharge50,80 and rarely required a treatment.63,81 The true long-term arrhythmic burdens arising from COVID-19 are likely clarified in large population-based prospective studies focusing on cardiovascular monitoring82 (Central Illustration).
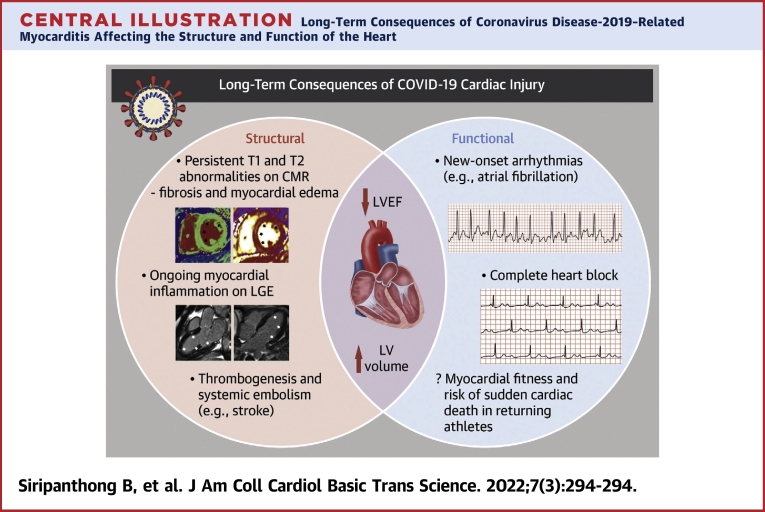
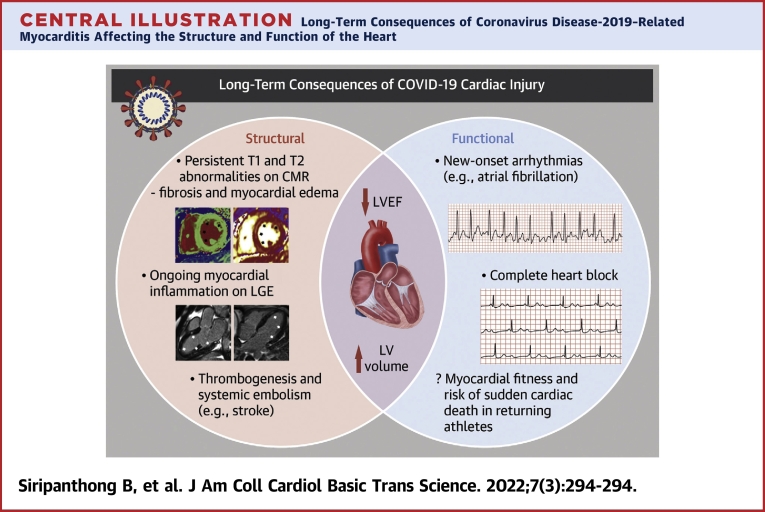
Central Illustration.
Long-Term Consequences of Coronavirus Disease-2019–Related Myocarditis Affecting the Structure and Function of the Heart
Some persistent structural abnormalities, including on T1 and T2 sequences on cardiovascular magnetic resonance, were noted in asymptomatic patients, indicating fibrosis and myocardial edema. Ongoing myocardial inflammation as shown by late gadolinium enhancement was also observed in recovered patients. A number of patients with healed myocarditis also showed reduced left ventricular (LV) ejection fraction and increased LV volume long after the episode. Functionally, in a small number of patients, new-onset arrhythmias, including atrial fibrillation and complete heart block, occurred. Concerns regarding myocardial fitness remain to be addressed, especially in young adult athletes considering return to play.
Myocarditis and Pericarditis After COVID-19 Vaccination
Recently, several cases of myocarditis-like illness in COVID-19 vaccine recipients have been reported. As of July 3, 2021, with more than 177 million people who received at least 1 dose of COVID-19 vaccine in the United States,83 the Vaccine Adverse Event Reporting System received 780 reports of myocarditis or pericarditis among people aged ≤30 years, with most cases being reported after messenger ribonucleic acid COVID-19 vaccination, particularly in male adolescents and young adults. As of the end of May 2021, in the European Economic Area, the exposure was about 221 million doses of COVID-19 vaccine, and cases of myocarditis and pericarditis reported from the EudraVigilance database were 176 and 192, respectively.84 These events have been reported with all 3 of the US Food and Drug Administration–approved vaccines: messenger ribonucleic acid (Moderna and Pfizer/BioNTech) and adenovirus (Janssen, Johnson & Johnson). In a series of 7 adult patients presenting with myocarditis-like illness temporarily associated with COVID-19 vaccination, CMR between 3 and 37 days showed multifocal subepicardial LGE in all 7 patients and additional midmyocardial LGE in 4 of 7 patients. Corresponding myocardial edema was detected in 3 of 7 patients. Treatment varied and included beta-blockers and anti-inflammatory medications. Mean length of hospital stay was 3 ± 1 days, and symptoms had resolved in all patients by hospital discharge. Despite the temporal association with COVID-19 vaccination, causality of myocarditis in these patients cannot be assumed.
Data from the US military health system85 showed that the incidence of myocarditis among those who were fully vaccinated by the organization did not exceed the expected incidence of myocarditis (∼20 cases per 1,065,000). However, those affected are all male, with a median age of 25 years, and all received messenger ribonucleic acid–based vaccines (Pfizer/BioNTech or Moderna). Among the fully vaccinated male military members, the incidence of myocarditis is significantly greater than the expected background level (19 vs 8 per 436,000). Another large study among 2,000,287 individuals86 who received at least a single dose of COVID-19 vaccine (52.6% Pfizer/BioNTech, 44.1% Moderna, and 3.1% Johnson & Johnson) revealed 20 cases of myocarditis (11 after Moderna, 9 after Pfizer/BioNTech) a median of 3.5 days after vaccination. The majority of these patients were men (15 of 20), with a median age of 36 years. Nevertheless, a higher incidence of myocarditis after COVID-19 vaccination was reported in Israel,87 especially after the second dose. Compared with unvaccinated individuals, the rate ratio of myocarditis in the following 30 days after the second dose of the Pfizer/BioNTech vaccine was 2.35. Strikingly, among the highest risk demographic (male, 16-19 years of age), the rate ratio is 8.96 compared with the unvaccinated, which translates into 1 case in 6,637 second vaccinations (0.015%). This still compares favorably with the incidence of myocarditis in patients with COVID-19 across all age groups (0.146%).20 Immunophenotyping studies investigating potential mechanisms of vaccine-associated myocardial injury are necessary to determine whether populations at higher risk for this potential outcome exist. Systematic evaluation of patients who develop chest pain and/or fever should be conducted with timing of doses and symptoms noted to demonstrate temporal antecedence.
On the basis of the limited evidence to date, the rate of COVID-19 vaccine–associated myocarditis-like illness appears low overall in comparison with doses administered, and its clinical course favorable, with resolution of symptoms in all patients reported thus far. We are not aware of studies suggesting that vaccine-related myocarditis is associated with increased an risk for death or heart failure. Moreover, most studies are based on imaging findings, with no clear clinical correlation or concrete endpoints.85,86
Future Directions
Despite the wealth of data regarding COVID-19 cardiac involvement hitherto reported, more information must be curated over a much longer period and in a systematic manner to allow us to fully appreciate the full spectrum and prevalence of long-term cardiac consequences of COVID-19. A number of clinical trials have been initiated to collect the cardiovascular parameters in post-COVID-19 patients using a variety of inclusion criteria. Several recently launched studies aim to use CMR and/or computed tomography, including cardiac functional evaluation and myocardial tissue characterization, to assess the long-term outcome of COVID-19-related cardiac disease in adult and pediatric patients.88 The CoViDEx (Effect of COVID-19 (Coronavirusdisease-19) and Exercise on Myocardial Fibrosis and Ventricular Arrhythmias; NCT04726150) is investigating the association of myocardial fibrosis and ventricular arrhythmias using an implantable loop recorder and stress echocardiography in adult athletes, who are at least 1 month after diagnosis of COVID-19. This study is particularly important in the context of the available knowledge, suggesting that monomorphic ventricular arrhythmias are frequent after recovery from the acute phase of myocarditis caused by other viral antigens.89,90
Another study (NCT04794062) aims to perform repeat CMR with contrast enhancement at 6 months of dynamic follow-up in participants with established COVID-19-related myocardial injury at inclusion to assess the percentage of participants with ongoing myocardial injury. The CO-Qo-ICU (One-Year Outcomes in Survivors of the Severe COVID-19 Pneumonia; NCT04401111), whose aim is to study 1-year outcomes in survivors of severe COVID-19 pneumonia, will study echocardiographic parameters of right and left ventricular function during the first year after intensive care unit discharge. The MOIST (Multi-Organ Imaging With Serial Testing in COVID-19 Infected Patients; NCT04525404) will enroll participants with newly or recently diagnosed COVID-19 infection (inpatients and outpatients), who will undergo multiorgan magnetic resonance imaging (heart, brain, lungs, liver), blood work, and functional testing at 1 or more time points. These studies will hopefully elucidate the puzzling issue of COVID-19-related myocardial injury and inflammation and aid in understanding its potential long-term consequences.91
With the increased diagnosis of new COVID-19 subtypes, our appreciation of cardiac involvement in COVID-19 and its long-term consequences may change over time. Currently, it is unknown if the myocardial damage and inflammation caused by new COVID-19 subtypes are any different from the accumulated knowledge. Whether a combination of viral subtypes can cause a more severe disease presentation, and whether such cases will have significantly more myocardial inflammation or damage, remains unknown and should be investigated in future studies.
The logistical difficulties associated with acute evaluation and follow-up using multimodality imaging and/or endomyocardial biopsy present considerable obstacles to prospective studies. Furthermore, the inclusion of only hospitalized patients in studies adds a potentially profound source of bias, particularly as it remains far from certain that the myocardial consequences of SARS-CoV-2-infection are associated with the severity of the acute illness. Some sources of inclusion bias may be addressed by the use of biobank-based studies. Biobanks are a type of biorepository in which biological samples from participating volunteers are stored for research use. The value of biobanks may overcome the knowledge gaps in COVID-19-related cardiac disease because of their scale and wider participation from both inpatients and outpatients. The UK Biobank, for example, has rapidly tackled the global pandemic by undertaking several major initiatives, including the COVID-19 repeat imaging study, which aims to perform repeat CMR in 3,000 subjects.92
Large-scale investigations planned by Public Health England through the Post-Hospitalisation COVID-19 national consortium (with its 2 magnetic resonance imaging substudies, C-MORE and COVERSCAN)93 and several other biobanks around the world have also started to collate data and to make available health information regarding COVID-19 from participating patients.92
The first holistic data of post–hospital discharge patients with COVID-19 showed medium-term effects of SARS-CoV-2 infection on multiple vital organs, exercise tolerance, and mental, cognitive, and physical health in a significant proportion of patients with long-haul symptoms. Notably, serum biomarkers of inflammation and severity of acute illness correlated with magnetic resonance imaging evidence of multiorgan abnormalities (including native T1 values of the myocardium) and reduced exercise tolerance.94 Future initiatives, including the use of follow-up questionnaires assessing the physical and mental health of recovered patients with COVID-19, will go a long way toward helping discover other long-term consequences of the disease, which may be elusive to small single- and multicenter studies. It is from these datasets that clinicians can obtain a holistic, integrated, multidisciplinary picture of COVID-19 sequelae, including from its associated myocarditis.
Nevertheless, to study myocardial disease, it is crucial to use standardized cardiac imaging, such as CMR protocols with consistent-strength scanners, including gadolinium contrast–based enhancement and multiparametric myocardial mapping (eg, native T1 and T2). However, the reliance on CMR-based diagnostics for the stratification of myocardial involvement is also a limitation; for instance, distinguishing endotheliitis, microvascular obstruction, type 1 or 2 MI, inflammatory-pattern injury, and new or old myocardial injury can be challenging, and the specificity of these techniques has not been validated for SARS-CoV-2-related myocardial injury, let alone in other groups of patients. The sensitivity of CMR-based detection of myocardial injury also remains a concern; abnormal LGE was detected in only half of troponin-positive patients with SARS-CoV-2 infection.11 Furthermore, the classic cellular distinctions of myocardial inflammation have thus far not been reproduced in the few autopsy and biopsy reports. Studies have largely focused on left ventricular function, but right ventricular function, ventricular thrombus and thromboembolic risk, arrhythmia,95, 96, 97 and autonomic dysfunction,98 are clinically apparent within this patient group and should also be systematically evaluated.
Conclusions
Myocardial involvement in SARS-CoV-2-mediated disease is a consequence of multiple pathophysiological mechanisms, including severe hypoxia-mediated injury, thromboembolic disease, systemic inflammatory response, and direct myocardial inflammation. Studies of human engineered heart tissues confirm that SARS-CoV-2 can directly infect cardiac myocytes, causing cytokine production and cardiomyocyte death. Rigorous analyses of cardiac tissue obtained from affected patients are limited, and most knowledge of myocardial inflammation in COVID-19 is based on autopsy findings that have limited value for prognostication in patients with mild or moderate COVID-19. Although full recovery with good short-term outcomes have been reported in small case series of fulminant COVID-19 myocarditis, persistent inflammation with resulting myocardial damage with or without residual symptoms is also possible in patients with supposed “recovery” from acute COVID-19. Patients with COVID-19-associated new-onset myocardial dysfunction and inflammation or scarring on CMR might be at increased risk for heart failure or re-entrant arrhythmias. Myocarditis following COVID-19 vaccination remains rare, and to date, no conclusive proof of causality is available. Regular follow-up with clinical examination, arrhythmia monitoring, and serial imaging studies is therefore warranted in patients with significant acute myocardial damage related to COVID-19.
Funding Support and Author Disclosures
Dr Nazarian has received grants from the National Institutes of Health, ImriCor, Biosense Webster, and ADAS; and is a consultant to CardioSolv and ImroCor. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Footnotes
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
Appendix
For supplemental tables and references, please see the online version of this paper.
Appendix
References
- 1.Worldometer COVID-19 pandemic live update. https://www.worldometers.info/coronavirus/ Accessed July 3, 2021.
- 2.Ruan Q., Yang K., Wang W., Jiang L., Song J. Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Med. 2020;46:846–848. doi: 10.1007/s00134-020-05991-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Carvalho-Schneider C., Laurent E., Lemaignen A., et al. Follow-up of adults with noncritical COVID-19 two months after symptom onset. Clin Microbiol Infect. 2021;27:258–263. doi: 10.1016/j.cmi.2020.09.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Carfi A., Bernabei R., Landi F., for the Gemelli Against COVID-19 Post–Acute Care Study Group Persistent symptoms in patients after acute COVID-19. JAMA. 2020;324:603–605. doi: 10.1001/jama.2020.12603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Arnold D.T., Hamilton F.W., Milne A., et al. Patient outcomes after hospitalisation with COVID-19 and implications for follow-up: results from a prospective UK cohort. Thorax. 2021;76:399–401. doi: 10.1136/thoraxjnl-2020-216086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chopra V., Flanders S.A., O’Malley M., Malani A.N., Prescott H.C. Sixty-day outcomes among patients hospitalized with COVID-19. Ann Intern Med. 2021;174:576–578. doi: 10.7326/M20-5661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sandoval Y., Januzzi J.L., Jr., Jaffe A.S. Cardiac troponin for assessment of myocardial injury in COVID-19: JACC review topic of the week. J Am Coll Cardiol. 2020;76:1244–1258. doi: 10.1016/j.jacc.2020.06.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Guo T., Fan Y., Chen M., et al. Cardiovascular implications of fatal outcomes of patients with coronavirus disease 2019 (COVID-19) JAMA Cardiol. 2020;5:811–818. doi: 10.1001/jamacardio.2020.1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shi S., Qin M., Shen B., et al. Association of cardiac injury with mortality in hospitalized patients with COVID-19 in Wuhan, China. JAMA Cardiol. 2020;5:802–810. doi: 10.1001/jamacardio.2020.0950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Papageorgiou N., Sohrabi C., Prieto Merino D., et al. High sensitivity troponin and COVID-19 outcomes. Acta Cardiol. 2021:1–8. doi: 10.1080/00015385.2021.1887586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kotecha T., Knight D.S., Razvi Y., et al. Patterns of myocardial injury in recovered troponin-positive COVID-19 patients assessed by cardiovascular magnetic resonance. Eur Heart J. 2021;42:1866–1878. doi: 10.1093/eurheartj/ehab075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Siripanthong B., Hanff T.C., Levin M.G., et al. Coronavirus disease 2019 is delaying the diagnosis and management of chest pain, acute coronary syndromes, myocarditis and heart failure. Future Cardiol. 2021;17:3–6. doi: 10.2217/fca-2020-0088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wichmann D., Sperhake J.P., Lutgehetmann M., et al. Autopsy findings and venous thromboembolism in patients with COVID-19: a prospective cohort study. Ann Intern Med. 2020;173:268–277. doi: 10.7326/M20-2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hanff T.C., Mohareb A.M., Giri J., Cohen J.B., Chirinos J.A. Thrombosis in COVID-19. Am J Hematol. 2020;95:1578–1589. doi: 10.1002/ajh.25982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guzik T.J., Mohiddin S.A., Dimarco A., et al. COVID-19 and the cardiovascular system: implications for risk assessment, diagnosis, and treatment options. Cardiovasc Res. 2020;116:1666–1687. doi: 10.1093/cvr/cvaa106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang H., Li R., Zhou Z., et al. Cardiac involvement in COVID-19 patients: mid-term follow up by cardiovascular magnetic resonance. J Cardiovasc Magn Reson. 2021;23:14. doi: 10.1186/s12968-021-00710-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Puntmann V.O., Carerj M.L., Wieters I., et al. Outcomes of cardiovascular magnetic resonance imaging in patients recently recovered from coronavirus disease 2019 (COVID-19) JAMA Cardiol. 2020;5:1265–1273. doi: 10.1001/jamacardio.2020.3557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kawakami R., Sakamoto A., Kawai K., et al. Pathological evidence for SARS-CoV-2 as a cause of myocarditis: JACC review topic of the week. J Am Coll Cardiol. 2021;77:314–325. doi: 10.1016/j.jacc.2020.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Alvarez-Garcia J., Jaladanki S., Rivas-Lasarte M., et al. New heart failure diagnoses among patients hospitalized for COVID-19. J Am Coll Cardiol. 2021;77:2260–2262. doi: 10.1016/j.jacc.2021.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Boehmer T.K. Association between COVID-19 and myocarditis using hospital-based administrative data—United States, March 2020–January 2021. MMWR Morb Mortal Wkly Rep. 2021;70(35):1228–1232. doi: 10.15585/mmwr.mm7035e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Moulson N., Petek B.J., Drezner J.A., et al. SARS-CoV-2 cardiac involvement in young competitive athletes. Circulation. 2021;144(4):256–266. doi: 10.1161/CIRCULATIONAHA.121.054824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Siripanthong B., Nazarian S., Muser D., et al. Recognizing COVID-19-related myocarditis: the possible pathophysiology and proposed guideline for diagnosis and management. Heart Rhythm. 2020;17:1463–1471. doi: 10.1016/j.hrthm.2020.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hoffmann M., Kleine-Weber H., Schroeder S., et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181:271–280.e8. doi: 10.1016/j.cell.2020.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sharma A., Garcia G., Jr., Wang Y., et al. Human iPSC-derived cardiomyocytes are susceptible to SARS-CoV-2 infection. Cell Rep Med. 2020;1:100052. doi: 10.1016/j.xcrm.2020.100052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wong C.-K., Luk H.K.-H., Lai W.-H., et al. Human-induced pluripotent stem cell-derived cardiomyocytes platform to study SARS-CoV-2 related myocardial injury. Circ J. 2020;84(11):2027–2031. doi: 10.1253/circj.CJ-20-0881. [DOI] [PubMed] [Google Scholar]
- 26.Weiss S.R. Forty years with coronaviruses. J Exp Med. 2020;217(5) doi: 10.1084/jem.20200537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ishikura H., Maruyama J., Hoshino K., et al. Coronavirus disease (COVID-19) associated delayed-onset fulminant myocarditis in patient with a history of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection. J Infect Chemother. 2021;27(12):1760–1764. doi: 10.1016/j.jiac.2021.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lindner D., Fitzek A., Brauninger H., et al. Association of cardiac infection with SARS-CoV-2 in confirmed COVID-19 autopsy cases. JAMA Cardiol. 2020;5:1281–1285. doi: 10.1001/jamacardio.2020.3551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Varga Z., Flammer A.J., Steiger P., et al. Endothelial cell infection and endotheliitis in COVID-19. Lancet. 2020;395:1417–1418. doi: 10.1016/S0140-6736(20)30937-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Meyer M., Vogel T., Meyer A., et al. Presence of active myocarditis at the 6 month follow-up appointment for a severe form of COVID-19: a case report. ESC Heart Fail. 2021;8(5):4307–4312. doi: 10.1002/ehf2.13461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nedeljkovic I.P., Giga V., Ostojic M., et al. Focal myocarditis after mild COVID-19 infection in athletes. Diagnostics (Basel) 2021;11(8):1519. doi: 10.3390/diagnostics11081519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sakr Y., Giovini M., Leone M., et al. Pulmonary embolism in patients with coronavirus disease-2019 (COVID-19) pneumonia: a narrative review. Ann Intensive Care. 2020;10:124. doi: 10.1186/s13613-020-00741-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bois M.C., Boire N.A., Layman A.J., et al. COVID-19-associated nonocclusive fibrin microthrombi in the heart. Circulation. 2021;143:230–243. doi: 10.1161/CIRCULATIONAHA.120.050754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Choudry F.A., Hamshere S.M., Rathod K.S., et al. High thrombus burden in patients with COVID-19 presenting with ST-segment elevation myocardial infarction. J Am Coll Cardiol. 2020;76:1168–1176. doi: 10.1016/j.jacc.2020.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Han H., Ma Q., Li C., et al. Profiling serum cytokines in COVID-19 patients reveals IL-6 and IL-10 are disease severity predictors. Emerg Microbes Infect. 2020;9:1123–1130. doi: 10.1080/22221751.2020.1770129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tang Y., Liu J., Zhang D., Xu Z., Ji J., Wen C. Cytokine storm in COVID-19: the current evidence and treatment strategies. Front Immunol. 2020;11:1708. doi: 10.3389/fimmu.2020.01708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kerr R., Stirling D., Ludlam C.A. Interleukin 6 and haemostasis. Br J Haematol. 2001;115:3–12. doi: 10.1046/j.1365-2141.2001.03061.x. [DOI] [PubMed] [Google Scholar]
- 38.Abbate A., Toldo S., Marchetti C., Kron J., Van Tassell B.W., Dinarello C.A. Interleukin-1 and the inflammasome as therapeutic targets in cardiovascular disease. Circ Res. 2020;126:1260–1280. doi: 10.1161/CIRCRESAHA.120.315937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ciuca C., Fabi M., Di Luca D., et al. Myocarditis and coronary aneurysms in a child with acute respiratory syndrome coronavirus 2. ESC Heart Fail. 2021;8:761–765. doi: 10.1002/ehf2.13048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Volis I., Livneh I., Hussein K., Raz-Pasteur A. COVID-19-associated suspected myocarditis as the etiology for recurrent and protracted fever in an otherwise healthy adult. Am J Med Sci. 2021;361:522–525. doi: 10.1016/j.amjms.2020.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rehman M., Gondal A., Rehman N.U. Atypical manifestation of COVID-19-induced myocarditis. Cureus. 2020;12 doi: 10.7759/cureus.8685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ng M.Y., Ferreira V.M., Leung S.T., et al. Patients recovered from COVID-19 show ongoing subclinical myocarditis as revealed by cardiac magnetic resonance imaging. J Am Coll Cardiol Img. 2020;13:2476–2478. doi: 10.1016/j.jcmg.2020.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Craver R., Huber S., Sandomirsky M., McKenna D., Schieffelin J., Finger L. Fatal eosinophilic myocarditis in a healthy 17-year-old male with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2c) Fetal Pediatr Pathol. 2020;39:263–268. doi: 10.1080/15513815.2020.1761491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Auer J., Neuhierl F., Hetzmann Z. COVID-19-related fatal myocarditis in a 42-year-old female patient. Cardiol J. 2020;27:642–643. doi: 10.5603/CJ.2020.0155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Beaudry J.T., Dietrick B., Lammert D.B., et al. Fatal SARS-CoV-2 inflammatory syndrome and myocarditis in an adolescent: a case report. Pediatr Infect Dis J. 2021;40:e72–e76. doi: 10.1097/INF.0000000000002978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sala S., Peretto G., Gramegna M., et al. Acute myocarditis presenting as a reverse tako-tsubo syndrome in a patient with SARS-CoV-2 respiratory infection. Eur Heart J. 2020;41:1861–1862. doi: 10.1093/eurheartj/ehaa286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bonnet M., Craighero F., Harbaoui B. Acute myocarditis with ventricular noncompaction in a COVID-19 patient. J Am Coll Cardiol HF. 2020;8:599–600. doi: 10.1016/j.jchf.2020.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Coyle J., Igbinomwanhia E., Sanchez-Nadales A., Danciu S., Chu C., Shah N. A recovered case of COVID-19 myocarditis and ARDS treated with corticosteroids, tocilizumab, and experimental AT-001. J Am Coll Cardiol Case Rep. 2020;2:1331–1336. doi: 10.1016/j.jaccas.2020.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kesici S., Aykan H.H., Orhan D., Bayrakci B. Fulminant COVID-19-related myocarditis in an infant. Eur Heart J. 2020;41:3021. doi: 10.1093/eurheartj/ehaa515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lara D., Young T., Del Toro K., et al. Acute fulminant myocarditis in a pediatric patient with COVID-19 infection. Pediatrics. 2020;146(2) doi: 10.1542/peds.2020-1509. [DOI] [PubMed] [Google Scholar]
- 51.Irabien-Ortiz A., Carreras-Mora J., Sionis A., Pamies J., Montiel J., Tauron M. Fulminant myocarditis due to COVID-19. Rev Esp Cardiol (Engl Ed) 2020;73:503–504. doi: 10.1016/j.rec.2020.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bernal-Torres W., Herrera-Escandon A., Hurtado-Rivera M., Plata-Mosquera C.A. COVID-19 fulminant myocarditis: a case report. Eur Heart J Case Rep. 2020;4:1–6. doi: 10.1093/ehjcr/ytaa212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Garot J., Amour J., Pezel T., et al. SARS-CoV-2 fulminant myocarditis. J Am Coll Cardiol Case Rep. 2020;2:1342–1346. doi: 10.1016/j.jaccas.2020.05.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Papageorgiou J.M., Almroth H., Tornudd M., van der Wal H., Varelogianni G., Lawesson S.S. Fulminant myocarditis in a COVID-19 positive patient treated with mechanical circulatory support—a case report. Eur Heart J Case Rep. 2021;5(2):ytaa523. doi: 10.1093/ehjcr/ytaa523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ranard L.S., Fried J.A., Abdalla M., et al. Approach to acute cardiovascular complications in COVID-19 infection. Circ Heart Fail. 2020;13 doi: 10.1161/CIRCHEARTFAILURE.120.007220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kociol R.D., Cooper L.T., Fang J.C., et al. Recognition and initial management of fulminant myocarditis: a scientific statement from the American Heart Association. Circulation. 2020;141:e69–e92. doi: 10.1161/CIR.0000000000000745. [DOI] [PubMed] [Google Scholar]
- 57.Abani O., Abbas A., Abbas F., et al. Tocilizumab in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial. Lancet. 2021;397(10285):1637–1645. doi: 10.1016/S0140-6736(21)00676-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.National Institutes of Health Interleukin-6 inhibitors. https://www.covid19treatmentguidelines.nih.gov/therapies/immunomodulators/interleukin-6-inhibitors/ Accessed October 28, 2021.
- 59.National Institute for Health and Care Excellence COVID-19 rapid guideline: managing COVID-19. NG191. https://www.nice.org.uk/guidance/ng191 Accessed October 28, 2021. [PubMed]
- 60.Chitturi K.R., Thacker S., Al-Saadi M.A., Kassi M. Successful treatment of acute heart failure in COVID-19-induced cytokine storm with tocilizumab: a case report. Eur Heart J Case Rep. 2020;4(FI1):1–6. doi: 10.1093/ehjcr/ytaa188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Cavalli G., Pappalardo F., Mangieri A., Dinarello C.A., Dagna L., Tresoldi M. Treating life-threatening myocarditis by blocking interleukin-1. Crit Care Med. 2016;44:e751–e754. doi: 10.1097/CCM.0000000000001654. [DOI] [PubMed] [Google Scholar]
- 62.Lozano Gomez H., Pascual Bielsa A., Arche Banzo M.J. Fulminant myocarditis and cardiogenic shock during SARS-CoV-2 infection. Med Clin. 2020;155:463–464. doi: 10.1016/j.medcle.2020.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ashok V., Loke W.I. Case report: high-grade atrioventricular block in suspected COVID-19 myocarditis. Eur Heart J Case Rep. 2020;4(FI1):1–6. doi: 10.1093/ehjcr/ytaa248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zeng J.H., Liu Y.X., Yuan J., et al. First case of COVID-19 complicated with fulminant myocarditis: a case report and insights. Infection. 2020;48:773–777. doi: 10.1007/s15010-020-01424-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ford J.S., Holmes J.F., Jones R.F. Cardioembolic stroke in a patient with coronavirus disease of 2019 (COVID-19) myocarditis: a case report. Clin Pract Cases Emerg Med. 2020;4:332–335. doi: 10.5811/cpcem.2020.6.47856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hussain H., Fadel A., Alwaeli H., Guardiola V. Coronavirus (COVID-19) fulminant myopericarditis and acute respiratory distress syndrome (ARDS) in a middle-aged male patient. Cureus. 2020;12 doi: 10.7759/cureus.8808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Oleszak F., Maryniak A., Botti E., et al. Myocarditis associated with COVID-19. Am J Med Case Rep. 2020;8:498–502. [Google Scholar]
- 68.National Institute for Health Research. Living with COVID19. 10.3310/themedreview_41169 [DOI]
- 69.Angus D.C. The lingering consequences of sepsis: a hidden public health disaster? JAMA. 2010;304:1833–1834. doi: 10.1001/jama.2010.1546. [DOI] [PubMed] [Google Scholar]
- 70.Bone R.C. Sir Isaac Newton, sepsis, SIRS, and CARS. Crit Care Med. 1996;24:1125–1128. doi: 10.1097/00003246-199607000-00010. [DOI] [PubMed] [Google Scholar]
- 71.Russell B., Moss C., George G., et al. Associations between immune-suppressive and stimulating drugs and novel COVID-19—a systematic review of current evidence. Ecancermedicalscience. 2020;14:1022. doi: 10.3332/ecancer.2020.1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Driggin E., Madhavan M.V., Bikdeli B., et al. Cardiovascular considerations for patients, health care workers, and health systems during the COVID-19 pandemic. J Am Coll Cardiol. 2020;75:2352–2371. doi: 10.1016/j.jacc.2020.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hinojar R., Varma N., Child N., et al. T1 mapping in discrimination of hypertrophic phenotypes: hypertensive heart disease and hypertrophic cardiomyopathy: findings from the international T1 multicenter cardiovascular magnetic resonance study. Circ Cardiovasc Imaging. 2015;8(12) doi: 10.1161/CIRCIMAGING.115.003285. [DOI] [PubMed] [Google Scholar]
- 74.Li H., Zhu H., Yang Z., Tang D., Huang L., Xia L. Tissue characterization by mapping and strain cardiac MRI to evaluate myocardial inflammation in fulminant myocarditis. J Magn Reson Imaging. 2020;52:930–938. doi: 10.1002/jmri.27094. [DOI] [PubMed] [Google Scholar]
- 75.Rajpal S., Tong M.S., Borchers J., et al. Cardiovascular magnetic resonance findings in competitive athletes recovering from COVID-19 infection. JAMA Cardiol. 2021;6:116–118. doi: 10.1001/jamacardio.2020.4916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Joy G., Artico J., Kurdi H., et al. Prospective case-control study of cardiovascular abnormalities 6 months following mild COVID-19 in healthcare workers. J Am Coll Cardiol Img. 2021;14:2155–2166. doi: 10.1016/j.jcmg.2021.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Raisi-Estabragh Z., McCracken C., Cooper J., et al. Adverse cardiovascular magnetic resonance phenotypes are associated with greater likelihood of incident coronavirus disease 2019: findings from the UK Biobank. Aging Clin Exp Res. 2021;33:1133–1144. doi: 10.1007/s40520-021-01808-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Martinez M.W., Tucker A.M., Bloom O.J., et al. Prevalence of inflammatory heart disease among professional athletes with prior COVID-19 infection who received systematic return-to-play cardiac screening. JAMA Cardiol. 2021;6(7):745–752. doi: 10.1001/jamacardio.2021.0565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Liu T., Wu D., Yan W., et al. Twelve-month systemic consequences of COVID-19 in patients discharged from hospital: a prospective cohort study in Wuhan, China. Clin Infect Dis. 2021;Aug 14 doi: 10.1093/cid/ciab703. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Gaine S., Devitt P., Coughlan J.J., Pearson I. COVID-19-associated myocarditis presenting as new-onset heart failure and atrial fibrillation. BMJ Case Rep. 2021;14(7) doi: 10.1136/bcr-2021-244027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kohli U., Meinert E., Chong G., Tesher M., Jani P. Fulminant myocarditis and atrial fibrillation in child with acute COVID-19. J Electrocardiol. 2020 doi: 10.1016/j.jelectrocard.2020.10.004. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Horn A., Krist L., Lieb W., et al. Long-term health sequelae and quality of life at least 6 months after infection with SARS-CoV-2: design and rationale of the COVIDOM-study as part of the NAPKON population-based cohort platform (POP) Infection. 2021:1–11. doi: 10.1007/s15010-021-01707-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Centers for Disease Control and Prevention Selected adverse events reported after COVID-19 vaccination. https://www.cdc.gov/coronavirus/2019-ncov/vaccines/safety/adverse-events.html Accessed July 3, 2021.
- 84.European Medicines Agency COVID-19 vaccines: update on ongoing evaluation of myocarditis and pericarditis. https://www.ema.europa.eu/en/news/covid-19-vaccines-update-ongoing-evaluation-myocarditis-pericarditis Accessed July 3, 2021.
- 85.Montgomery J., Ryan M., Engler R., et al. Myocarditis following immunization with mRNA COVID-19 vaccines in members of the US military. JAMA Cardiol. 2021;6(10):1202–1206. doi: 10.1001/jamacardio.2021.2833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Diaz G.A., Parsons G.T., Gering S.K., Meier A.R., Hutchinson I.V., Robicsek A. Myocarditis and pericarditis after vaccination for COVID-19. JAMA. 2021;326:1210–1212. doi: 10.1001/jama.2021.13443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Mevorach D., Anis E., Cedar N., et al. Myocarditis after BNT162b2 mRNA vaccine against COVID-19 in Israel. N Engl J Med. 2021 doi: 10.1056/NEJMoa2109730. 385:2140–2149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.National Health Service, Health Research Authority COVID-HEART study [COVID-19] [UPH] https://www.hra.nhs.uk/planning-and-improving-research/application-summaries/research-summaries/covid-heart-study-covid-19-uph/ Accessed May 22, 2021.
- 89.Peretto G., Sala S., Rizzo S., et al. Ventricular arrhythmias in myocarditis: characterization and relationships with myocardial inflammation. J Am Coll Cardiol. 2020;75:1046–1057. doi: 10.1016/j.jacc.2020.01.036. [DOI] [PubMed] [Google Scholar]
- 90.Pelargonio G., Pinnacchio G., Narducci M.L., et al. Long-term arrhythmic risk assessment in biopsy-proven myocarditis. J Am Coll Cardiol EP. 2020;6:574–582. doi: 10.1016/j.jacep.2019.12.010. [DOI] [PubMed] [Google Scholar]
- 91.Nalbandian A., Sehgal K., Gupta A., et al. Post-acute COVID-19 syndrome. Nat Med. 2021;27:601–615. doi: 10.1038/s41591-021-01283-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Khanji M.Y., Aung N., Chahal C.A.A., Petersen S.E. COVID-19 and the UK Biobank—opportunities and challenges for research and collaboration with other large population studies. Front Cardiovasc Med. 2020;7:156. doi: 10.3389/fcvm.2020.00156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.NIHR Leicester Biomedical Research Centre PHOSP-COVID. https://www.leicesterbrc.nihr.ac.uk/themes/respiratory/research/phosp-covid/ Accessed June 28, 2021.
- 94.Raman B., Cassar M.P., Tunnicliffe E.M., et al. Medium-term effects of SARS-CoV-2 infection on multiple vital organs, exercise capacity, cognition, quality of life and mental health, post-hospital discharge. EClinicalMedicine. 2021;31:100683. doi: 10.1016/j.eclinm.2020.100683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Abrams M.P., Coromilas E.J., Wan E.Y., Rubin G.A., Garan H., Dizon J.M. Malignant ventricular arrhythmias in patients with severe acute respiratory distress syndrome due to COVID-19 without significant structural heart disease. HeartRhythm Case Rep. 2020;6(11):858–862. doi: 10.1016/j.hrcr.2020.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Eneizat Mahdawi T., Wang H., Haddadin F.I., Al-Qaysi D., Wylie J.V. Heart block in patients with coronavirus disease 2019: a case series of 3 patients infected with SARS-CoV-2. HeartRhythm Case Rep. 2020;6:652–656. doi: 10.1016/j.hrcr.2020.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Mountantonakis S.E., Saleh M., Fishbein J., et al. Atrial fibrillation is an independent predictor for in-hospital mortality in patients admitted with SARS-CoV-2 infection. Heart Rhythm. 2021;18:501–507. doi: 10.1016/j.hrthm.2021.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Goldstein D.S. The possible association between COVID-19 and postural tachycardia syndrome. Heart Rhythm. 2021;18:508–509. doi: 10.1016/j.hrthm.2020.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.