Summary
Background
Convalescent plasma has been proposed as an early treatment to interrupt the progression of early COVID-19 to severe disease, but there is little definitive evidence. We aimed to assess whether early treatment with convalescent plasma reduces the risk of hospitalisation and reduces SARS-CoV-2 viral load among outpatients with COVID-19.
Methods
We did a multicentre, double-blind, randomised, placebo-controlled trial in four health-care centres in Catalonia, Spain. Adult outpatients aged 50 years or older with the onset of mild COVID-19 symptoms 7 days or less before randomisation were eligible for enrolment. Participants were randomly assigned (1:1) to receive one intravenous infusion of either 250–300 mL of ABO-compatible high anti-SARS-CoV-2 IgG titres (EUROIMMUN ratio ≥6) methylene blue-treated convalescent plasma (experimental group) or 250 mL of sterile 0·9% saline solution (control). Randomisation was done with the use of a central web-based system with concealment of the trial group assignment and no stratification. To preserve masking, we used opaque tubular bags that covered the investigational product and the infusion catheter. The coprimary endpoints were the incidence of hospitalisation within 28 days from baseline and the mean change in viral load (in log10 copies per mL) in nasopharyngeal swabs from baseline to day 7. The trial was stopped early following a data safety monitoring board recommendation because more than 85% of the target population had received a COVID-19 vaccine. Primary efficacy analyses were done in the intention-to-treat population, safety was assessed in all patients who received the investigational product. This study is registered with ClinicalTrials.gov, NCT04621123.
Findings
Between Nov 10, 2020, and July 28, 2021, we assessed 909 patients with confirmed COVID-19 for inclusion in the trial, 376 of whom were eligible and were randomly assigned to treatment (convalescent plasma n=188 [serum antibody-negative n=160]; placebo n=188 [serum antibody-negative n=166]). Median age was 56 years (IQR 52–62) and the mean symptom duration was 4·4 days (SD 1·4) before random assignment. In the intention-to-treat population, hospitalisation within 28 days from baseline occurred in 22 (12%) participants who received convalescent plasma versus 21 (11%) who received placebo (relative risk 1·05 [95% CI 0·78 to 1·41]). The mean change in viral load from baseline to day 7 was −2·41 log10 copies per mL (SD 1·32) with convalescent plasma and −2·32 log10 copies per mL (1·43) with placebo (crude difference −0·10 log10 copies per mL [95% CI −0·35 to 0·15]). One participant with mild COVID-19 developed a thromboembolic event 7 days after convalescent plasma infusion, which was reported as a serious adverse event possibly related to COVID-19 or to the experimental intervention.
Interpretation
Methylene blue-treated convalescent plasma did not prevent progression from mild to severe illness and did not reduce viral load in outpatients with COVID-19. Therefore, formal recommendations to support the use of convalescent plasma in outpatients with COVID-19 cannot be concluded.
Funding
Grifols, Crowdfunding campaign YoMeCorono.
Introduction
Immunotherapies that administer antibodies directly to the patient are classified as passive immunotherapies, as opposed to active immunotherapy that aims to stimulate the host's immune response. Passive immunotherapies, including the use of convalescent plasma (obtained from donors who have recovered from infection) and monoclonal antibodies targeting specific epitopes, have emerged as candidates for preventing severe illness when administered early after COVID-19 onset.1, 2 To date, various anti-SARS-CoV-2 monoclonal antibodies have shown efficacy in reducing the combined rates of hospitalisation and death in outpatients with early, mild disease, and a small benefit in reducing death rates among seronegative patients who are admitted to hospital.2, 3, 4, 5, 6 The US Food and Drug Administration has issued the Emergency Use Authorization5 for monoclonal antibodies in patients with mild to moderate COVID-19 who are at high risk of progression to severe COVID-19. However, the high cost and complexity of monoclonal antibody production is a challenge to the widespread global use of this strategy, and concern has arisen regarding how these antibodies will respond to emerging SARS-CoV-2 variants.7 For instance, the new omicron variant (B.1.1.529) of concern is resistant against almost all licensed monoclonals.8, 9
Research in context.
Evidence before this study
We searched PubMed and medRxiv databases from March 1, 2020, to Aug 20, 2021, with no language restrictions, for randomised trials or meta-analyses of trials evaluating the effect of convalescent plasma in patients with COVID-19. We used the terms (“COVID-19”, “COVID”, “SARS-CoV-2”, or “Coronavirus”) AND (“convalescent plasma”, “passive immunization”, “passive immunotherapy”, “plasma therapy”), and 13 trials and one meta-analysis were identified. 11 trials, including one with more than 10 000 participants enrolled, included hospitalised patients with severe or critical COVID-19. In hospitalised patients with COVID-19, convalescent plasma was not associated with a reduction in mortality or with benefits in other clinical outcomes. Only two trials included patients with COVID-19 who had not been admitted to hospital. Both trials were placebo-controlled and enrolled a total of 671 randomly assigned patients. The first trial was published in February, 2021, and included 160 older adults (aged ≥75 years) within 72 h after the onset of mild COVID-19 symptoms. In this Argentinian trial, early administration of convalescent plasma reduced the proportion of patients progressing to severe respiratory disease from 25 (31%) of 80 patients in the placebo group to 13 (16%) of 80 in the convalescent plasma group. The second trial (SIREN-C3PO), published in August, 2021, included 511 participants with non-severe COVID-19 recruited at an emergency room. The trial showed no benefit of treatment with convalescent plasma in preventing hospitalisation (81 [32%] of 254 had disease progression or hospitalisation in the placebo group vs 77 [30%] of 257 in the convalescent plasma group). Convalescent plasma was administered in the first week after symptom onset, with a median time of 4 days (IQR 2–5), and the patients were either aged 50 years and older or had one or more risk factors. Criticism was raised regarding the fact that 16% of patients were admitted in the index visit.
Added value of this study
We found that compared with placebo, high-titre convalescent plasma did not reduce hospitalisation up to day 28 after random assignment and did not reduce viral load at day 7 when administered to outpatients aged 50 years and older with COVID-19 with less than 7 days from symptom onset. Our results are consistent with evidence reported from the SIREN-C3PO trial of convalescent plasma in outpatients with COVID-19. Our trial is important not only for replication, but also because it addresses some of the limitations of the SIREN-C3PO trial. Unlike SIREN-C3PO, our participants were not recruited in emergency room departments and, therefore, probably presented with milder earlier symptoms. We assessed the antibody serum status in patients at enrolment and we confirmed the absence of efficacy of the early treatment with convalescent plasma in serum antibody-negative patients, who represented most of our cohort. Moreover, we confirmed the neutralising activity of plasma units against the common circulating SARS-CoV-2 variants during recruitment, and plasma units were near-sourced, reducing the risk of efficacy being affected by antigenic shifts in viral strains from regional differences. In addition, plasma was characterised and the median titre of SARS-CoV-2 neutralising antibodies administered was very high (median 50% inhibitory dilution for original virus strain Wuhan-Hu-1 lineage B was 1:1379 [IQR 1:602–1:2801] and for alpha [B.1.1.7] variant was 1:943 [1:428–1:2236]).
Implications of all the available evidence
The results on the efficacy of convalescent plasma generated to date do not allow a formal recommendation to support its use in outpatients with COVID-19. Our results suggest that methylene blue-treated convalescent plasma does not prevent progression from mild to severe illness and does not reduce viral load in outpatients with COVID-19. The findings of our study need to be taken with caution due to a possible reduced activity of plasma collected during former COVID-19 waves against the alpha variant and the potential effect of methylene blue inactivation on the observed efficacy, as well as in the context of early termination of the study.
Convalescent plasma, the traditional approach to passive immunotherapy, has yielded promising results in other viral respiratory infections.10 Compared with monoclonal antibodies, convalescent plasma has the drawback of lacking standardisation in dose, affinity, and specificity of antibodies, which might lead to varying neutralising activity in different plasma units. The overall dose of specific antibodies is generally lower in convalescent plasma, although convalescent plasma has the potential advantage of a broader antiviral activity than monoclonal antibody therapy. However, randomised controlled trials involving patients admitted to hospital (severe disease) with COVID-19 have found no survival benefit with convalescent plasma treatment.11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22 The results of one recent randomised controlled trial (SIREN-C3P0) of convalescent plasma in 511 high-risk outpatients with COVID-19 showed no benefit in preventing disease progression from mild to severe disease when given at a median of 4 days (IQR 2–5) after symptom onset.23 However, in this trial, patients were recruited at emergency rooms and were, therefore, likely to present with moderate-to-severe symptoms. Moreover, 25 (16%) of 158 patients who met the primary outcome of disease progression within 15 days after randomisation were ultimately admitted to hospital during the index visit (at baseline). Additionally, serological tests were not done at enrolment, and benefit of convalescent plasma is most likely in seronegative individuals. In addition, plasma units were sourced more than 150 miles (>240 km) from plasma recipients, which might affect efficacy if they are derived from donors infected with different strains of SARS-CoV-2.24 A smaller randomised trial done in Argentina in 160 outpatients, aged 75 years and older and treated within 72 h of symptom onset (mild disease), found that high-titre convalescent plasma was associated with a lower likelihood of progression to severe disease (relative risk [RR] 0·52 [95% CI 0·29–0·94]).25
More conclusive information on convalescent plasma efficacy in outpatients is required. In this randomised controlled trial, we investigated whether near-sourced, high-titre convalescent plasma, administered within 7 days after symptom onset, would prevent hospital admission or reduce SARS-CoV-2 viral load in outpatients with mild-to-moderate COVID-19.
Methods
Study design
The CONV-ERT study was a multicentre, double-blind, randomised, placebo-controlled trial to assess the efficacy of convalescent plasma in preventing severe COVID-19 in patients infected with SARS-CoV-2 with mild and moderate illness. The trial was done at four health-care centres providing universal health care to a catchment population of 3·9 million people in Catalonia, Spain (appendix p 3).
The study was done according to the Helsinki Declaration of the World Medical Association. The study protocol was approved by the Ethics Committee at Hospital Germans Trias i Pujol (number PI 20-313) and the institutional review boards of participating centres. The study was supervised by an independent data and safety monitoring board.
Participants
To be eligible for participation, patients had to be aged 50 years or older and non-hospitalised (not admitted to hospital) with mild-to-moderate COVID-19. All patients had to have a confirmed SARS-CoV-2 infection, with a positive PCR or validated antigen rapid test result received no more than 5 days before randomisation, and symptom onset no more than 7 days before randomisation. Mild and moderate COVID-19 were defined according to international guidelines:26 patients with fever, cough, sore throat, malaise, headache, and muscle pain were considered to have mild COVID-19, whereas evidence of lower respiratory disease by clinical assessment or imaging and a saturation of oxygen 94% or more on room air was considered moderate COVID-19. Patients were excluded if they had severe COVID-19 or required hospitalisation for any cause, a history of a previous SARS-CoV-2 infection, received one or two doses of a COVID-19 vaccine, contraindications to the investigational product, increased thrombotic risk, history of clinically significantly abnormal liver function (eg, Child-Pugh C), or chronic kidney disease stage 4 or worse. We excluded patients who were pregnant, breastfeeding, or planning a pregnancy during the study period. Further details on the eligibility criteria are listed in the trial protocol (appendix p 21).
We identified study participants from two sources: (1) active screening of laboratory-confirmed new infections at study sites and (2) individuals who voluntarily registered on an institutional website launched by the sponsor and the Catalan Institute of Health. Investigators contacted participants by telephone or in person to inform them about the study, invite participation, and check their eligibility. We scheduled eligible participants for a baseline visit, done either at hospital or at home by the hospital domiciliary homecare unit, during which written informed consent was obtained, and eligibility confirmed.
Randomisation and masking
We used a central web-based randomisation system with allocation concealment and no stratification to randomly assign participants (1:1) to receive convalescent plasma or placebo. Study researchers confirmed eligibility of participants and contacted an independent technician based at the central blood bank (Banc de Sang i Teixits de Catalunya, Barcelona, Spain), with no information about the participant, who used the web-based system to assign participants to the trial groups. Blood bank staff masked the investigational products with opaque tubular bags that covered the entire unit of plasma or saline solution and the infusion catheter. Finally, an unmasked study nurse, who was not involved in patient follow-up, administered the investigational product. All participants and other investigators (including all personnel involved in patient follow-up, laboratory staff, and statisticians) were masked to treatment allocation. Random assignment and infusion were always done on the same day.
Unmasking was permitted only if a clinical emergency occurred during or immediately after the infusion or an unexpected severe adverse event occurred during follow-up. Only the principal investigator was allowed to unmask individual study participants using a specific command in the electronic case report form.
Procedures
Participants received one intravenous infusion of either 250–300 mL of ABO-compatible high-titre methylene blue-treated convalescent plasma (experimental group) or 250 mL of sterile 0·9% saline solution (control group). For participants with a bodyweight of less than 45 kg, dosing of intervention (ABO-compatible high-titre methylene blue-treated convalescent plasma or 0·9% saline solution) was bodyweight adjusted to 5 mL/kg. All patients also received standard medical treatment. The study convalescent plasma units were sourced from the central blood bank located 12 km or less from the two largest study sites, and 90 km or less from all study sites (appendix pp 3, 12). Plasma was selected after screening for high anti-SARS-CoV-2 IgG titres with ELISA (EUROIMMUN ratio ≥6), according to international guidelines.27 After transfusion, we further characterised plasma post hoc with a pseudovirus-based neutralising antibody assay that used a spike from the virus lineage Wuhan-Hu-1.28 The plasmid SARS-CoV-2.SctΔ19 was generated (GeneArt) from the full protein sequence of the original Wuhan-Hu-1 lineage B SARS-CoV-2 spike (Genbank MN908947.3) with a deletion of the last 19 amino acids in C-terminal. The sequence was human-codon optimised and inserted into pcDNA3.1(+). To assess the neutralising activity against the alpha variant (B.1.1.7), post hoc we repeated the neutralising antibody testing using an alpha-variant pseudotyped virus.28 Also, to assess the effect of methylene blue treatment on neutralising antibodies, we compared the neutralising activity of stored biospecimens from the donor (ie, before methylene blue treatment) and that of the plasma unit (ie, after methylene blue treatment) in a subset of participants. To establish calibrating factors for conversion of ID50 geometric mean titres into IU/mL, we used a panel of plasma samples developed and distributed by the National Institute for Biological Standards and Control (UK, number 20/136). For the purpose of data analysis, neutralising results were used to define high-titre convalescent plasma with a threshold of 50% inhibitory dilution (ID50) of more than 1:250 (equivalent to more than 60 IU/mL; details are provided in the appendix p 5).
Patients were asked to complete a symptom inventory every day for 14 days after random assignment by means of an electronic form. In-person follow-up visits were scheduled on days 7 and 28 at participants' residence, or at the hospital if the participant was hospitalised. Additionally, we contacted study participants by telephone on days 3, 14, and 60 to assess their clinical status. WHO Clinical Progression Scale score (range 0–10) was determined at each study visit (appendix p 7). During follow-up visits, we obtained blood samples (at baseline and day 7) to assess anti-SARS-CoV-2 serum antibodies and inflammatory biomarkers, and nasopharyngeal swabs (at baseline and days 7 and 28) for quantification of SARS-CoV-2 viral load. We used a structured electronic case report form to record data.
Serum antibody status of all enrolled participants was prospectively characterised from baseline samples by chemiluminescence immunoassay in a fully automated platform (LIAISON XL, DiaSorin, Vercelli, Italy). Patients were designated serum antibody-negative if they were negative for both of the following antibodies: IgG anti-SARS-CoV-2 trimeric spike glycoprotein (DiaSorin, Vercelli, Italy) and IgM anti-SARS-CoV-2 S1-RBD (DiaSorin, Vercelli, Italy; appendix p 6). Viral load was determined by real-time quantitative RT-PCR in a single step with the Allplex 2019-nCoV assay (Werfen, Hospitalet de Llobregat, Spain) on the CFX96 instrument (BIO-RAD, Hercules, CA, USA). For absolute quantification, a standard curve was built using 2-fold serial dilutions of a SARS-CoV2 plasmid RNA of known concentration (Amplirun Coronavirus RNA Control, catalogue reference MBC090, Vircell Microbiologists, Granada, Spain). Study samples were run in parallel to the set of prequantified samples covering all thermal cycles used in the analysis. The viral load was extrapolated from the standard curve using the corresponding cycle threshold values in the RdRP gene results (appendix p 6). We tested biomarkers with most evidence as predictors for severe COVID-19 at baseline and on day 7, including D-dimer, ferritin, interleukin (IL)-6, lymphocytes, C-reactive protein, and prealbumin.29
Outcomes
We defined two coprimary outcomes regarding treatment efficacy. First, the clinical outcome was the incidence of hospitalisation within 28 days from baseline. Second, the virological outcome was the mean change in viral load (in log10 copies per mL) in nasopharyngeal swabs from baseline to day 7.
Prespecified secondary outcomes were time to complete symptom resolution, change in the 10-point WHO Clinical Progression Scale score30 within the 60 days following infusion, change from baseline in inflammatory biomarkers on day 7 of follow-up, mean change in viral load in nasopharyngeal swabs at day 28, death rate, titres of neutralising antibodies against SARS-CoV-2 in plasma of a subgroup of participants at day 7, and rate of adverse events. Details of all secondary outcomes are included in the study protocol (appendix p 21), including three prespecified outcomes that will be reported elsewhere as they were related to ancillary substudies.
Safety was assessed as the proportion of patients with adverse events that occurred or worsened during the follow-up period. Adverse events were assessed for severity and causality. The safety population included all patients who received the investigational product.
Statistical analysis
We estimated that a sample size of 474 participants (237 per group) would provide 80% power to detect a 50% reduction in hospitalisation incidence by day 28,31 assuming an expected rate of hospitalisation of 15%, at a significance level of α=0·05, and allowing a 5% loss to follow-up. Approximately 150 participants per group were required to have 80% power to detect a difference of 0·5 log10 copies per mL in the mean reduction of SARS-CoV-2 viral load at a two-sided significance level of α=0·05, assuming an expected overall SD of 1·5. A 0·5 log10 copies per mL difference in reduction was chosen to represent the minimal threshold for a biologically relevant change for our analyses. On May 28, 2021, despite the sample size not being reached, the data and safety monitoring board recommended halting recruitment to the trial because more than 85% of the target population had received SARS-CoV-2 vaccination.
Primary efficacy analyses were done in the intention-to-treat population. Hospitalisation rate was compared between groups using the RR obtained by fitting a generalised estimating equation log-binomial model that accounted for clustering (centre of recruitment). To determine whether the estimator was significantly different from zero, we used the Wald test on the robust SE from the fitter treatment effect coefficient. Virological efficacy was determined by comparing the mean reduction of the viral load from baseline to days 7 and 28. The mean reduction of viral load (in log10 scale) was compared by fitting linear mixed-effect models using the centre of recruitment and the individual as nested random effects (cluster or individual) in the intercept to adjust for intra-individual and intra-cluster correlation. According to available evidence on factors influencing the successful treatment of COVID-19, prespecified analyses of the primary outcomes were done in subgroups (as an interaction term with the treatment) defined by participant's baseline antibody serum status (IgG or IgM anti-SARS-CoV-2 positive and negative), duration of illness (≤3 days and >3 days), and according to the neutralisation activity of the plasma received (ID50 >1:250 and ID50 ≤1:250). Prespecified sensitivity analysis of the primary outcomes were done in the per-protocol population.
The days to complete resolution of symptoms were analysed using Kaplan-Meier survival functions and hazard ratios (HRs) obtained by fitting Cox proportional hazards regression models based on the assumptions of proportional risks. The Kaplan-Meier curves were compared using the log-rank test. The mean reduction of the 10-point WHO Clinical Progression Scale score was compared by fitting linear mixed-effect models. The median values of laboratory parameters at day 7 were compared between treatment groups by means of the non-parametric Wilcoxon-Mann-Whitney test. Death rate and adverse events rate were compared between groups using the RR obtained according to Deeks and Higgins.32 Comparison of median of neutralising antibody titres against SARS-CoV-2 in plasma of a subgroup of participants at day 7 was done by Wilcoxon matched-pairs signed rank test.
Post-hoc comparison of median titres of neutralising antibody of alpha variant versus original virus was done by Wilcoxon matched-pairs signed rank test.
All analyses were done with the R statistical package (version 4.1 or higher) with a significance level of 0·05. We did not adjust the type I error for multiplicity because we considered that both coprimary endpoints individually must show statistically significant treatment benefit.
This study is registered with ClinicalTrials.gov, NCT04621123.
Role of the funding source
The funder of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report.
Results
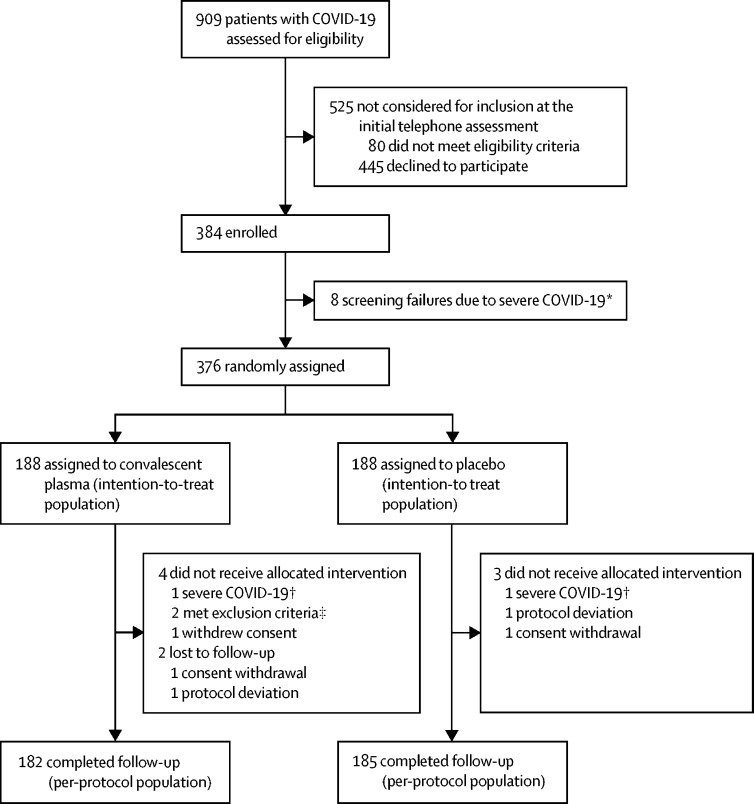
Between Nov 10, 2020, and July 28, 2021, we assessed 909 patients with confirmed COVID-19 for eligibility. The recruitment and follow-up of study participants are shown in figure 1. 525 (58%) of 909 screened patients did not meet the selection criteria or declined to participate and were therefore not enrolled. Additionally, eight (2%) of 384 consented participants were not randomly assigned to intervention and were excluded from the intention-to-treat analysis because of screening failure. Therefore, 376 participants were randomly assigned (convalescent plasma n=188; placebo n=188). All 376 participants were included in the intention-to-treat analysis.
Figure 1.
Trial profile
*Worsening of clinical status before random assignment. †Worsening of clinical status before infusion. ‡1 history of chronic kidney disease stage 4 or worse and 1 pre-existing condition with increased risk of thrombosis.
Baseline demographics and clinical characteristics were similar in the convalescent plasma group and the placebo group (table 1). The median age of the participants was 56 years (IQR 52–62), 173 (46%) were women, 203 (54%) were men, and 278 (74%) had at least one risk factor for progression to severe COVID-19 related to coexisting conditions. The mean time from symptom onset to random assignment was 4·4 days (SD 1·4). Overall, 366 (97%) of 376 patients had mild COVID-19. Baseline serum antibody status was negative in 326 (88%) of 369 patients for whom results were available. The mean viral load from the nasopharyngeal swab at baseline was 6·7 log10 copies per mL (SD 1·6) for the convalescent plasma group and 6·8 log10 copies per mL (1·4) for the placebo group.
Table 1.
Baseline characteristics
| Convalescent plasma group (n=188) | Placebo group (n=188) | |
|---|---|---|
| Demographics | ||
| Age, years | 56 (52–62) | 56 (53–63) |
| Women | 83 (44%) | 90 (48%) |
| Men | 105 (56%) | 98 (52%) |
| Body-mass index, kg/m2 | 27·9 (4·5) | 27·6 (4·5) |
| Primary coexisting risk factors for severe COVID-19 | ||
| At least one risk factor | 137 (73%) | 141 (75%) |
| Smoker | 94 (50%) | 97 (52%) |
| Obesity | 51 (27%) | 45 (24%) |
| Cardiovascular disease | 14 (7%) | 9 (5%) |
| Lung disease (chronic obstructive pulmonary disease, asthma, or both) | 17 (9%) | 16 (9%) |
| Diabetes | 20 (11%) | 19 (10%) |
| Chronic renal failure | 3 (2%) | 3 (2%) |
| Immune-compromised | 6 (3%) | 3 (2%) |
| COVID-19 duration | ||
| Days from symptoms onset to random assignment* | 4·4 (1·4; 185) | 4·4 (1·4; 187) |
| Days from positive test† to random assignment | 2·8 (1·0; 185) | 2·7 (1·1; 187) |
| COVID-19 severity | ||
| Mild COVID-19 | 183 (97%) | 183 (97%) |
| Moderate COVID-19 | 5 (3%) | 5 (3%) |
| Serum IgM and IgG antibody status‡ | ||
| Negative | 160/183 (87%) | 166/186 (89%) |
| Positive | 23/183 (13%) | 20/186 (11%) |
| Laboratory parameters§ | ||
| D-dimer, ng/mL | 325 (250–516; 181) | 355 (250–513; 180) |
| Ferritin, ng/mL | 222·0 (106·8–410·0; 184) | 223·5 (107·8–368·3; 184) |
| IL-6, pg/mL | 5·1 (3·1–12·9; 186) | 5·1 (2·8–10·9; 185) |
| Lymphocytes, × 109 cells per L | 1·2 (1·0–1·6; 188) | 1·2 (0·9–1·6; 188) |
| C-reactive protein, mg/L | 5·5 (2·3–14·1; 187) | 5·4 (2·5–12·5; 186) |
| Prealbumin, mg/dL | 27·0 (20·9–38·8; 182) | 27·5 (22·0–47·2; 178) |
Data are median (IQR), n (%), mean (SD), mean (SD; N), n/N (%), or median (IQR; N). IL=interleukin.
Random assignment and infusion were always done on the same day.
Positive PCR or validated antigen rapid test result for SARS-CoV-2 infection.
Patients were designated serum antibody-negative if they were negative for both of the following antibodies: IgG anti-SARS-CoV-2 trimeric spike glycoprotein, and IgM anti-SARS-CoV-2 S1-RBD.
Laboratory reference ranges: D-dimer 0–500 ng/mL; ferritin 30·0–400·0 ng/mL; IL-6 0·0–6·4 pg/mL; lymphocytes 1·2–3·5 × 109 cells per L; C-reactive protein 0·0–5·0 mg/L; prealbumin 20·0–40·0 mg/dL.
Of the 148 units of methylene blue-treated convalescent plasma with available neutralising antibody titres, 132 (89%) had a SARS-CoV-2 neutralising ID50 of more than 1:250. The median ID50 was 1:1379 (IQR 1:602–1:2801 [equivalent to median 1:342 IU/mL, IQR 1:147–1:705]) for the original virus (Wuhan-Hu-1 [Genbank MN908947.3]; appendix p 8). Distribution of neutralising antibody titres against Wuhan-Hu-1 and the alpha variant pseudovirus in a subset of 40 samples showed a decrease of 1·33-fold (median ID50 1:1256 [IQR 1:709–1:2712] against Wuhan-Hu-1 and median ID50 1:943 [1:428–1:2236] against the alpha variant; p=0·0032; appendix p 9). Neutralising activity titres to Wuhan-Hu-1 remained unchanged after methylene blue treatment (median ID50 1:1256 [IQR 1:709–1:2712] before treatment vs 1:1287 [1:349–1:3333] after treatment; p=0·32; appendix p 9). Convalescent plasma donations were collected at a time when the B1, B1.1, and B1.177 variants of the SARS-CoV-2 virus were predominant in Catalonia (April, 2020, to January, 2021), and all trial participants were recruited during the second wave (largely B1.177, October, 2020, to January, 2021) and third wave (largely the alpha variant, February–May, 2021; appendix p 10) of the COVID-19 pandemic. The plasma units were sourced 12 km or less from the two largest study sites that recruited 174 (93%) of the participants in the convalescent plasma group, and 90 km or less from all study sites (appendix p 12).
For the clinical primary outcome, there was no significant difference in hospitalisation up to day 28 between the two groups (table 2). Hospitalisations occurred in 22 (12%) of 188 participants in the convalescent plasma group and 21 (11%) of 188 participants in the placebo group (RR 1·05 [95% CI 0·78–1·41]). According to the log-binomial regression model, age, body-mass index, lymphocytes, and ferritin were independently associated with the hospitalisation event (appendix p 14). In prespecified subgroup analyses according to the patients' baseline serum antibody status, duration of illness, and neutralisation activity of the convalescent plasma, hospitalisation rates were not significantly different between groups (table 2).
Table 2.
Coprimary endpoints and virological secondary endpoint in the intention-to-treat population
| Convalescent plasma group | Placebo group | RR or crude difference (95% CI) | p value | |||
|---|---|---|---|---|---|---|
| Clinical primary end point: hospitalisation within 28 days of random assignment | ||||||
| Overall population | 22/188 (12%) | 21/188 (11%) | RR 1·05 (0·78 to 1·41) | 0·76 | ||
| Subgroups according to serostatus at baseline* | ||||||
| Negative baseline serum antibody status | 20/160 (13%) | 19/166 (11%) | RR 1·09 (0·83 to 1·44) | 0·54 | ||
| Positive baseline serum antibody status | 2/23 (9%) | 2/20 (10%) | RR 0·87 (0·20 to 3·88) | 0·86 | ||
| Subgroups according to duration of illness† | ||||||
| ≤3 days | 4/49 (8%) | 6/52 (12%) | RR 0·83 (0·56 to 1·25) | 0·37 | ||
| >3 days | 18/136 (13%) | 15/135 (11%) | RR 1·19 (0·89 to 1·60) | 0·24 | ||
| Subgroups according to plasma neutralisation activity‡ | ||||||
| ID50 >1:250§ | 13/132 (10%) | 21/188 (11%) | RR 0·88 (0·70 to 1·12) | 0·30 | ||
| ID50 ≤1:250 | 2/16 (13%) | 21/188 (11%) | RR 1·12 (0·77 to 1·63) | 0·56 | ||
| Virological primary and secondary endpoints: change in viral load from baseline¶‖ | ||||||
| Overall population | ||||||
| Day 7 | −2·41 (1·32; 174) | −2·32 (1·43; 174) | −0·10 (−0·35 to 0·15) | 0·42 | ||
| Day 28 | −3·86 (1·56; 180) | −4·00 (1·45; 172) | 0·12 (−0·17 to 0·40) | 0·33 | ||
| Subgroups according to serostatus at baseline* | ||||||
| Negative baseline serum antibody status | ||||||
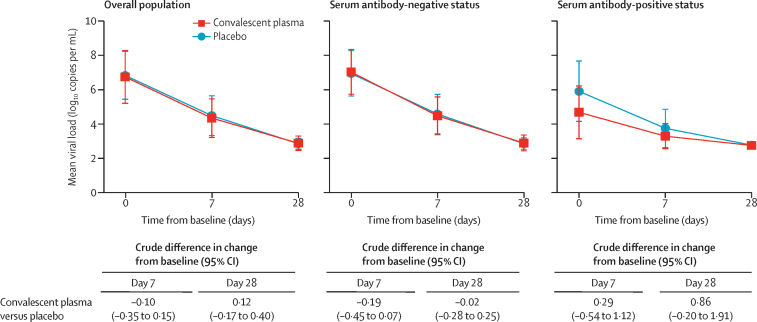
| Day 7 | −2·54 (1·31; 149) | −2·35 (1·43; 155) | −0·19 (−0·45 to 0·07) | 0·16 | ||
| Day 28 | −4·12 (1·35; 154) | −4·10 (1·37; 154) | −0·02 (−0·28 to 0·25) | 0·89 | ||
| Positive baseline serum antibody status | ||||||
| Day 7 | −1·45 (1·19; 21) | −1·85 (1·42; 17) | 0·29 (−0·54 to 1·12) | 0·49 | ||
| Day 28 | −1·91 (1·60; 22) | −2·97 (1·87; 16) | 0·86 (−0·20 to 1·91) | 0·11 | ||
Data are n/N (%) or mean (SD; N) except where otherwise stated. ID50=50% inhibitory dilution. RR=relative risk.
Seven of 376 participants did not have baseline serological test.
Four of 376 participants did not have records on duration of illness.
40 of 188 participants in the convalescent plasma group did not have a plasma neutralisation activity test.
ID50 value of 1:250 is equivalent to 60 IU/mL (appendix p 5).
28 of 376 participants did not have nasal swab collected on day 7.
24 of 376 participants did not have nasal swab collected on day 28.
For the coprimary virological outcome, the mean difference in viral load from baseline to day 7 was −2·41 log10 copies per mL (SD 1·32) in the convalescent plasma group and −2·32 log10 copies per mL (1·43) in the placebo group (crude difference −0·10 log10 copies per mL [95% CI −0·35 to 0·15]; table 2, figure 2). The analysis of the reduction of the viral load followed a similar trend at day 28: −3·86 log10 copies per mL (SD 1·56) in the convalescent plasma group versus −4·00 log10 copies per mL (1·45) in the placebo group (crude difference 0·12 log10 copies per mL [95% CI −0·17 to 0·40]). Results for the virological outcomes from the subgroup analyses according to the patients' baseline serum antibody status were not significantly different between groups (table 2). Primary outcomes in the per protocol population are shown in the appendix (p 15).
Figure 2.
Viral load change from baseline to day 7 and day 28
Comparison of the mean reduction of the viral load between treatment groups was done using a linear mixed-effect model. Error bars are 95% CIs.
In the analysis of the secondary outcome of median time from random assignment to the resolution of COVID-19 symptoms, there was no significant difference between the convalescent plasma group (12·0 days [IQR 6·0–21·3]) and the placebo group (12·0 days [6·0–22·0]; HR 1·05 [95% CI 0·85–1·30]; appendix p 16). The proportional hazard assumption of the Cox regression of the risk over time was satisfied (Schoenfeld test p=0·81; appendix p 17). There were no differences between the groups in the secondary endpoint of change in the 10-point WHO Clinical Progression Scale score within the 60 days following infusion (appendix p 18). Two (1%) of 188 convalescent plasma recipients and four (2%) of 188 placebo recipients required mechanical ventilation (reached ordinal score ≥7). No participants in the convalescent plasma group died, whereas two (1%) participants in the placebo group died (RR 0·20 [95% CI 0·01–4·14]).
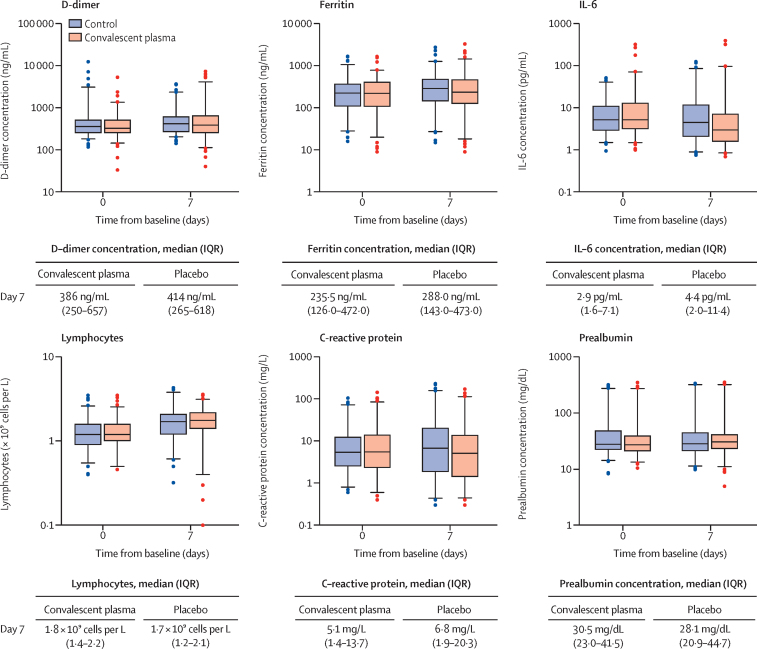
There were no significant differences in inflammatory parameters between the groups at day 7 of follow-up, except a minor difference in IL-6 with no clinical significance (figure 3).
Figure 3.
Inflammatory parameters on day 7
Box plots indicate median (middle line) and IQR (box), 2·5th and 97·5th percentile (whiskers), and outliers (single points). Difference (Wilcox test p value) between median value of the convalescent plasma group compared with the median value of the placebo group: D-dimer p=0·23; ferritin p=0·26; IL-6 p=0·0042; lymphocyte count p=0·084; C-reactive protein p=0·052; prealbumin p=0·41. Laboratory reference ranges: D-dimer 0–500 ng/mL; ferritin 30·0–400·0 ng/mL; IL-6 0·0–6·4 pg/mL; lymphocytes 1·2–3·5 × 109 cells per L; C-reactive protein 0·0–5·0 mg/L; prealbumin 20·0–40·0 mg/dL. IL=interleukin.
Levels of neutralising antibodies at day 7 after infusion, measured in a subcohort of 125 (33%) of 376 participants, did not differ between the convalescent plasma group (n=67; median ID50 1:1017 [IQR 1:296–1:2501]) and the placebo group (n=58; median ID50 1:989 [1:424–1:2321]; appendix p 13).
32 treatment-related adverse events were reported, in 24 (13%) of 188 patients in the convalescent plasma group and eight (4%) of 188 patients in the placebo group (RR 3·00 [95% CI 1·38–6·51]). The most common treatment-related adverse events reported were mild allergic reactions, fever, and local reactions (appendix p 19). One participant with mild COVID-19 signs and symptoms developed a thromboembolic event 7 days after convalescent plasma infusion, which was reported as a serious adverse event possibly related to COVID-19 or to the experimental intervention.
Discussion
In this randomised double-blind trial of high-titre methylene blue-treated convalescent plasma for adult patients aged 50 years and older who had mild to moderate COVID-19 for a week or less, we found that patients receiving convalescent plasma had no better clinical or virological outcomes than those who received a placebo infusion. There was also no evidence of benefit in the convalescent plasma group for any of our secondary endpoints nor in any of our prespecified subgroup analyses.
Our data indicate no significant difference in the proportion of participants who had to be hospitalised within 28 days of entering the trial (RR 1·05 [95% CI 0·78–1·41]). This absence of effect was also observed in the subgroup of serum-antibody-negative patients, who were the majority of our cohort and among whom benefit of other passive immunotherapy such as monoclonal antibodies is predicted to be the highest.2 Moreover, convalescent plasma did not enhance reduction of viral load in the nasopharynx 7 and 28 days after the intervention.
Previous randomised trials have reported either partial benefits21, 22 or failure11, 12, 13, 14, 15, 16, 17, 18, 19, 20 of convalescent plasma to improve any relevant outcome in patients admitted to hospital with COVID-19 or patients recruited at emergency rooms.23 The only evidence of a potential benefit of convalescent plasma in the outpatient setting comes from a smaller randomised trial done in Argentina with a study population more similar than the other randomised trials to ours.25 The main differences between that trial and ours include an earlier convalescent plasma administration timing (mean time since onset of symptoms 39·6 h [SD 13·9] vs 4·4 days [1·4]) and the selection of older patients (mean age 77 years [SD 8·5] vs 58 years [8]) in the Argentinian trial.
Several limitations of our clinical trial should be mentioned. A major limitation is that the data safety and monitoring board recommended to terminate the trial early because more than 85% of the population aged 50 years or older were fully vaccinated in Spain (and those who were not were unlikely to participate in a clinical trial), and because monoclonal antibodies became available for outpatients who were at high risk of progression to severe COVID-19. The trial was therefore underpowered. Vaccination was one of the exclusion criteria of our trial but it does not necessarily preclude the use of convalescent plasma in real life, especially considering the immunity conferred by vaccines wanes over time.
Moreover, we need to consider a number of factors that might reduce the efficacy of convalescent plasma, including the clinical time course when therapy is administered, the dose, the affinity of antibodies, and the effect of plasma pathogen inactivation procedures on immunoglobulin function.
First, we enrolled participants up to 7 days from symptom onset and we cannot rule out the potential efficacy if treatment was started earlier. Nonetheless, the fact that 326 (88%) of 369 patients were SARS-CoV-2 IgM and IgG negative at the time of inclusion confirms that they were recruited before the endogenous immune response was initiated.
Second, patients in our trial received a single high-titre plasma unit. Although this approach was similar to other outpatient trials,23, 25 higher plasma volumes are typically administered in patients who have been admitted to hospital for COVID-19. We acknowledge that higher doses might be needed in early stages, when pathology is driven by infection as opposed to inflammation. Our data do not directly address whether higher doses of convalescent plasma or titres of neutralising antibodies would be efficacious. To better understand the kinetics of antibodies in the recipients, we measured neutralisation antibodies 7 days after infusion in the peripheral blood of participants, and we found no differences between the convalescent plasma and placebo groups. It is likely that by 7 days after enrolment, endogenous antibody response will have reached high levels.33 An earlier comparison of neutralising antibody concentrations between the placebo and intervention groups on days 2–3 after infusion might have provided a better insight into the pharmacokinetics of antibodies delivered.
Third, antigenic shifts, due to discrepancy between donor and recipient infecting variants, might have affected efficacy. Convalescent plasma units for this trial were collected during a wave sustained by SARS-CoV-2 variants (B.1, B.1.1, and B.1.177), which also dominated during the first half of the recruitment period but were different to the one (alpha variant) dominating in the second half. To assess plasma neutralisation activity, we first used a pseudoviral neutralisation assay that used a spike from an original virus lineage (Wuhan-Hu-1), and then repeated testing with an alpha pseudotyped virus. We observed a 1·33-fold decrease in neutralising activity against the alpha variant compared with Wuhan-Hu-1. This finding is in line with previous reports of a 1·5-fold to 3·0-fold decrease in neutralising activity (appendix p 11). The negative results of our study could be partly influenced by a reduction of efficacy of antibodies due to differences in viral variants of donors and recipients. Of note, most previous laboratory studies did not show a statistically significant reduction in neutralising activity against the alpha variant of concern, whereas the reduction was larger and statistically significant for the beta (B.1.351) and delta (B.1.617.2) variants of concern (appendix p 11). To a lesser extent, antigen shifts in viral strains is expected to be region dependent.24 In our study, plasma units were sourced 12 km or less from the two largest study sites that recruited more than 90% of study participants.
Finally, studies focusing on the effect of methylene blue on SARS-CoV-2 neutralisation have produced mixed results. Methylene blue is a method of pathogen inactivation for plasma that is widely used in some countries in Europe. A study from Russia showed that some units of plasma lost neutralising activity with methylene blue inactivation,34 whereas other studies found no difference.35, 36 We analysed the neutralising activity of stored donor samples (ie, before methylene blue treatment) compared with the plasma unit (ie, after methylene blue treatment) in a subgroup of plasma units and we found no differences in neutralising antibody titres (median ID50 1:1256 [IQR 1:709–1:2712] vs 1:1287 [1:349–1:3333]; p=0·32). Although we observed preserved neutralising activity after methylene blue treatment, we could not evaluate the potential risk of damage to the Fc-region of the immunoglobulins. Fc-dependent functions have important antimicrobial effects, including phagocytosis, complement activation, and antibody-dependent cellular toxicity.37 Previous studies suggest that the main driver of clinical benefit from convalescent plasma units relies on their neutralising antibody content,38 and that the cell receptor binding capacity of the Fc-region is preserved after methylene blue treatment.36 Still, a concern remains that the dye might react with the glycosylation domain and affect Fc-region functionality and thus the overall response.39
The relatively low cost and straightforward production of convalescent plasma have resulted in its widespread use for patients with COVID-19. Our analysis builds on previous data23 suggesting that COVID-19 convalescent plasma does not prevent progression from mild to severe illness in non-hospitalised participants and that convalescent plasma does not reduce viral load. Taking together all the results on the efficacy of convalescent plasma generated to date, formal recommendations to support its use in outpatients with COVID-19 cannot be concluded. The findings of this study need to be taken with caution due to limitations related to a possible reduced activity of plasma collected during former waves against the alpha variant and the potential effect on efficacy of methylene blue inactivation, as well as in the context of the early termination of the trial.
Data sharing
Individual participant data that underlie the results reported in this article, after de-identification (text, tables, figures, and appendices) are available from the corresponding author on reasonable request.
Declaration of interests
We declare no competing interests.
Acknowledgments
Acknowledgments
The trial was sponsored by the Fight AIDS and Infectious Diseases Foundation (Badalona, Spain) with funding from the pharmaceutical company, Grifols Worldwide Operations (Dublin, Ireland), and the Crowdfunding campaign, YoMeCorono. The study received support from the Hospital Universitari Germans Trias i Pujol, and Banc de Sang i Teixits de Catalunya. We thank Gerard Carot-Sans for providing medical writing support with manuscript preparation and Roser Escrig for her support in the study design and medical writing assistance with the study documentation. We also thank Laia Bertran, Mireia Clua, Jordi Mitjà, Claudia Laporte, Sergi Gavilan, Joan Mercado, and Enric Nieto for the operational and financial management of the project. We thank the personnel from the Fight Aids and Infectious Diseases Foundation for their support in administration, human resources, and supply chain management. We thank the independent data safety monitoring board for their time and dedication: Cinta Hierro (Catalan Institute of Oncology, Badalona, Spain), Natalia Tovar (Hospital Clinic, Barcelona, Spain), Binh Ngo (University of Southern California, Los Angeles, CA, USA), David Boulware (University of Minnesota, Minneapolis, MN, USA), and Robin Mogg (Bill and Melinda Gates Research Institute, Seattle, WA, USA). We thank the reviewers for substantial comments that helped improve and clarify the strengths and drawbacks of the experimental intervention and study methodology. ISGlobal receives support from the Spanish Ministry of Science and Innovation through the Centro de Excelencia Severo Ochoa 2019–2023 Program (CEX2018-000806-S), and support from the Generalitat de Catalunya through the Centres de Recerca de Catalunya Program. Centro de Investigación en Salud de Manhiça is supported by the Government of Mozambique and the Spanish Agency for International Development. BB was supported by a Beatriu de Pinós postdoctoral fellow granted by the Government of Catalonia's Secretariat for Universities and Research, and by Marie Sklodowska-Curie Actions COFUND Programme (BP3, 801370). EP was supported by a doctoral grant from the National Agency for Research and Development of Chile (72180406). OM was supported by the European Research Council under grant agreement number 850450 (European Union's Horizon 2020 Research and Innovation Program, ERC-2019-STG funding scheme).
Contributors
OM, AA, PM-M, MC-M, BB, SV, AM, J-RG, and JB conceived and designed the study. All authors acquired, analysed, and interpreted the data. DO, FPF, and IG-F did the statistical analysis. OM, AA, and PM-M drafted the manuscript. All authors critically revised the manuscript for important intellectual content. All authors were responsible for the final decision to submit for publication. All authors have seen and approved the manuscript. AM, PM-M, DO, IG-F, FPF, and OM had full access to all of the data.
Contributor Information
Andrea Alemany, Email: aalemany@flsida.org.
CONV-ERT Group:
Susana Ferrer, Mireia Gallardo, Maria Ubals, Camila González-Beiras, Martí Vall-Mayans, Clara Suñer, Clàudia Laporte-Villar, Aroa Nieto, Xavier Comas-Leon, Zahida Jiménez, Ferran Ramírez-Viaplana, Maria Delgado-Capel, Beatriz Díez Sánchez, Maria Pons Barber, Cristian Gonzalez Ruiz, Laura Navarrete Gonzalez, David González García, Ainhoa Vivero Larraza, Victor Carceles Peiró, Clàudia Roquer López, Neus Robert, Carles Palet, Carlota Gudiol, Pablo Casares Gonzalez, Gemma Arcos Vila, Begoña Flores Aguilera, Graciela Rodríguez-Sevilla, Macarena Dastis Arias, Judit Roca Font, Katherine M. Carrasco Matos, Glòria Saüch Valmaña, Carla Vidal Obradors, Silvia Tarres García, Margarida Curriu Sabatès, Raquel Nieto Rodríguez, Rosa Línio, Míriam Fornos, Natàlia Casamitjana, Eva Alonso, Núria Martínez, Laura Analía Maglio, Laura Comellas Fernandez, Nadia Garcia, Luis Hernández, Maria Isabel González, Anna Bravo, Yolanda García, Silvia Sauleda Oliveras, Tatiana Vertiz, Sergio Benavent, Andrea Sofia Bianco, Joaquim Verdaguer, Ney Nicanor Briones Zambrano, Maria Viozquez Meya, Águeda Hernández, Cristina Casaña Lopez, Antoni E. Bordoy, Victoria González Soler, Montserrat Giménez, Alexa París, Silvia Marfil, Benjamin Trinité, and Eulàlia Grau
Supplementary Material
References
- 1.Katz LM. (A little) clarity on convalescent plasma for COVID-19. N Engl J Med. 2021;384:666–668. doi: 10.1056/NEJMe2035678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Horby PW, Mafham M, Peto L, et al. Casirivimab and imdevimab in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial. medRxiv. 2021 doi: 10.1101/2021.06.15.21258542. published online June 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Weinreich DM, Sivapalasingam S, Norton T, et al. REGN-COV2, a neutralizing antibody cocktail, in outpatients with COVID-19. N Engl J Med. 2021;384:238–251. doi: 10.1056/NEJMoa2035002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gottlieb RL, Nirula A, Chen P, et al. Effect of bamlanivimab as monotherapy or in combination with etesevimab on viral load in patients with mild to moderate COVID-19: a randomized clinical trial. JAMA. 2021;325:632–644. doi: 10.1001/jama.2021.0202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.US Food and Drug Administration Coronavirus (COVID-19) update: FDA authorizes monoclonal antibodies for treatment of COVID-19. https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-authorizes-monoclonal-antibodies-treatment-covid-19
- 6.Dougan M, Nirula A, Azizad M, et al. Bamlanivimab plus etesevimab in mild or moderate COVID-19. N Engl J Med. 2021;385:1382–1392. doi: 10.1056/NEJMoa2102685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang P, Nair MS, Liu L, et al. Antibody resistance of SARS-CoV-2 variants B.1.351 and B.1.1.7. Nature. 2021;593:130–135. doi: 10.1038/s41586-021-03398-2. [DOI] [PubMed] [Google Scholar]
- 8.Wilhelm A, Widera M, Grikscheit K, et al. Reduced neutralization of SARS-CoV-2 omicron variant by vaccine sera and monoclonal antibodies. medRxiv. 2021 doi: 10.1101/2021.12.07.21267432. published online Dec 8. (preprint, version 2). [DOI] [Google Scholar]
- 9.Cao Y, Wang J, Jian F, et al. Omicron escapes the majority of existing SARS-CoV-2 neutralizing antibodies. bioRxiv. 2021 doi: 10.1038/s41586-021-04385-3. https://doi.rg/10.1101/2021.12.07.470392 published online Dec 22. (preprint, version 2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mair-Jenkins J, Saavedra-Campos M, Baillie JK, et al. The effectiveness of convalescent plasma and hyperimmune immunoglobulin for the treatment of severe acute respiratory infections of viral etiology: a systematic review and exploratory meta-analysis. J Infect Dis. 2015;211:80–90. doi: 10.1093/infdis/jiu396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Abani O, Abbas A, Abbas F, et al. Convalescent plasma in patients admitted to hospital with COVID-19 (RECOVERY): a randomised controlled, open-label, platform trial. Lancet. 2021;397:2049–2059. doi: 10.1016/S0140-6736(21)00897-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Janiaud P, Axfors C, Schmitt AM, et al. Association of convalescent plasma treatment with clinical outcomes in patients with COVID-19: a systematic review and meta-analysis. JAMA. 2021;325:1185–1195. doi: 10.1001/jama.2021.2747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li L, Zhang W, Hu Y, et al. Effect of convalescent plasma therapy on time to clinical improvement in patients with severe and life-threatening COVID-19: a randomized clinical trial. JAMA. 2020;324:460–470. doi: 10.1001/jama.2020.10044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Agarwal A, Mukherjee A, Kumar G, Chatterjee P, Bhatnagar T, Malhotra P. Convalescent plasma in the management of moderate COVID-19 in adults in India: open label phase II multicentre randomised controlled trial (PLACID Trial) BMJ. 2020;371 doi: 10.1136/bmj.m3939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Simonovich VA, Burgos Pratx LD, Scibona P, et al. A randomized trial of convalescent plasma in COVID-19 severe pneumonia. N Engl J Med. 2021;384:619–629. doi: 10.1056/NEJMoa2031304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gharbharan A, Jordans CCE, Geurtsvankessel C, et al. Effects of potent neutralizing antibodies from convalescent plasma in patients hospitalized for severe SARS-CoV-2 infection. Nat Commun. 2021;12 doi: 10.1038/s41467-021-23469-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bajpai M, Kumar S, Maheshwari A, et al. Efficacy of Convalescent Plasma Therapy compared to Fresh Frozen Plasma in Severely ill COVID-19 Patients: A Pilot Randomized Controlled Trial. medRxiv. 2020 doi: 10.1101/2020.10.25.20219337. published online Oct 27. [DOI] [Google Scholar]
- 18.AlQahtani M, Abdulrahman A, Almadani A, et al. Randomized controlled trial of convalescent plasma therapy against standard therapy in patients with severe COVID-19 disease. Sci Rep. 2021;11 doi: 10.1038/s41598-021-89444-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Balcells ME, Rojas L, Le Corre N, et al. Early versus deferred anti-SARS-CoV-2 convalescent plasma in patients admitted for COVID-19: a randomized phase II clinical trial. PLoS Med. 2021;18 doi: 10.1371/journal.pmed.1003415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ray Y, Paul SR, Bandopadhyay P, et al. Clinical and immunological benefits of convalescent plasma therapy in severe COVID-19: insights from a single center open label randomised control trial. medRxiv. 2020 doi: 10.1101/2020.11.25.20237883. published online Nov 29. [DOI] [Google Scholar]
- 21.Avendaño-Solá C, Ramos-Martínez A, Muñez-Rubio E, et al. A multicenter randomized open-label clinical trial for convalescent plasma in patients hospitalized with COVID-19 pneumonia. J Clin Invest. 2021;131 doi: 10.1172/JCI152740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.O'Donnell MR, Grinsztejn B, Cummings MJ, et al. A randomized, double-blind controlled trial of convalescent plasma in adults with severe COVID-19. J Clin Invest. 2021;131 doi: 10.1172/JCI150646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Korley FK, Durkalski-Mauldin V, Yeatts SD, et al. Early convalescent plasma for high-risk outpatients with COVID-19. N Engl J Med. 2021;385:1951–1960. doi: 10.1056/NEJMoa2103784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kunze KL, Johnson PW, van Helmond N, et al. Mortality in individuals treated with COVID-19 convalescent plasma varies with the geographic provenance of donors. Nat Commun. 2021;12 doi: 10.1038/s41467-021-25113-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Libster R, Pérez Marc G, Wappner D, et al. Early high-titer plasma therapy to prevent severe COVID-19 in older adults. N Engl J Med. 2021;384:610–618. doi: 10.1056/NEJMoa2033700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.National Institutes of Health Clinical Spectrum of SARS-CoV-2 Infection. COVID-19 treatment guidelines. 2021. https://www.covid19treatmentguidelines.nih.gov/overview/clinical-spectrum/ [PubMed]
- 27.Bratcher-Bowman N. Convalescent plasma EUA letter of authorization. March 9, 2021. https://www.govinfo.gov/content/pkg/FR-2020-04-01/pdf/2020-06905.pdf
- 28.Trinité B, Pradenas E, Marfil S, et al. Previous SARS-CoV-2 infection increases B.1.1.7 cross-neutralization by vaccinated individuals. Viruses. 2021;13 doi: 10.3390/v13061135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kermali M, Khalsa RK, Pillai K, Ismail Z, Harky A. The role of biomarkers in diagnosis of COVID-19—a systematic review. Life Sci. 2020;254 doi: 10.1016/j.lfs.2020.117788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Marshall JC, Murthy S, Diaz J, et al. A minimal common outcome measure set for COVID-19 clinical research. Lancet Infect Dis. 2020;20:e192–e197. doi: 10.1016/S1473-3099(20)30483-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Regeneron REGN-COV2 antibody cocktail program update. Sept 29, 2020. https://investor.regeneron.com/static-files/a596a85e-e72d-4529-8eb5-d52d87a99070
- 32.Deeks JJ, Higgins JPT. Statistical algorithms in Review Manager 5. 2010. https://training.cochrane.org/handbook/statistical-methods-revman5
- 33.Trinité B, Tarrés-Freixas F, Rodon J, et al. SARS-CoV-2 infection elicits a rapid neutralizing antibody response that correlates with disease severity. Sci Rep. 2021;11 doi: 10.1038/s41598-021-81862-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kostin AI, Lundgren MN, Bulanov AY, et al. Impact of pathogen reduction methods on immunological properties of the COVID-19 convalescent plasma. Vox Sang. 2021;116:665–672. doi: 10.1111/vox.13056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Larrea L, Castro E, Navarro L, et al. Preservation of anti-SARS-CoV-2 neutralising antibodies in convalescent plasma after pathogen reduction with methylene blue and visible light. Blood Transfus. 2021 doi: 10.2450/2021.0136-21. published online Aug 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Raster J, Zimmermann K, Wesche J, Aurich K, Greinacher A, Selleng K. Effect of methylene blue pathogen inactivation on the integrity of immunoglobulin M and G. Transfus Med Hemother. 2021;48:148–153. doi: 10.1159/000514485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bégin P, Callum J, Jamula E, et al. Convalescent plasma for hospitalized patients with COVID-19: an open-label, randomized controlled trial. Nat Med. 2021;27:2012–2024. doi: 10.1038/s41591-021-01488-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Focosi D, Franchini M, Pirofski LA, et al. COVID-19 convalescent plasma is more than neutralizing antibodies: a narrative review of potential beneficial and detrimental co-factors. Viruses. 2021;13 doi: 10.3390/v13081594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ross V. Photodynamic action of methylene blue on antipneumococcal serum. J Immunol. 1938;35:351–369. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Individual participant data that underlie the results reported in this article, after de-identification (text, tables, figures, and appendices) are available from the corresponding author on reasonable request.