Abstract
Background
Pulmonary aspergillosis may complicate coronavirus disease 2019 (COVID-19) and contribute to excess mortality in intensive care unit (ICU) patients. The disease is poorly understood, in part due to discordant definitions across studies.
Objectives
We sought to review the prevalence, diagnosis, treatment, and outcomes of COVID-19–associated pulmonary aspergillosis (CAPA) and compare research definitions.
Data sources
PubMed, Embase, Web of Science, and MedRxiv were searched from inception to October 12, 2021.
Study eligibility criteria
ICU cohort studies and CAPA case series including ≥3 patients were included.
Participants
Adult patients in ICUs with COVID-19.
Interventions
Patients were reclassified according to four research definitions. We assessed risk of bias with an adaptation of the Joanna Briggs Institute cohort checklist tool for systematic reviews.
Methods
We calculated CAPA prevalence using the Freeman-Tukey random effects method. Correlations between definitions were assessed with Spearman's rank test. Associations between antifungals and outcome were assessed with random effects meta-analysis.
Results
Fifty-one studies were included. Among 3297 COVID-19 patients in ICU cohort studies, 313 were diagnosed with CAPA (prevalence 10%; 95% CI 8%–13%). Two hundred seventy-seven patients had patient-level data allowing reclassification. Definitions had limited correlation with one another (ρ = 0.268–0.447; p < 0.001), with the exception of Koehler and Verweij (ρ = 0.893; p < 0.001); 33.9% of patients reported to have CAPA did not fulfill any research definitions. Patients were diagnosed after a median of 8 days (interquartile range 5–14) in ICUs. Tracheobronchitis occurred in 3% of patients examined with bronchoscopy. The mortality rate was high (59.2%). Applying CAPA research definitions did not strengthen the association between mould-active antifungals and survival.
Conclusions
The reported prevalence of CAPA is significant but may be exaggerated by nonstandard definitions.
Keywords: Aspergillus, CAPA, Fungal infection, ICU, Mycosis, Secondary infection, SARS-CoV-2
Introduction
Given the high morbidity and mortality associated with severe coronavirus disease 2019 (COVID-19), it is imperative to rule out coinfections that may contribute to poor outcomes. In patients with severe COVID-19 requiring admission to the intensive care unit (ICU), pulmonary aspergillosis has been reported to be a relatively common and important complication contributing to increased mortality [1].
An association between viral respiratory tract infection and aspergillosis has been established in influenza: The incidence of influenza-associated pulmonary aspergillosis (IAPA) ranges from 7% to 30% within 3 days of ICU admission and is associated with ∼50% mortality [2,3]. Similarly, aspergillosis has been reported to occur in up to a third of patients with critical COVID-19 [[4], [5], [6]]. However, diagnosis and classification remain challenging.
Most reported cases of COVID-19–associated pulmonary aspergillosis (CAPA) have occurred in immunocompetent patients, and thus patients without histologic evidence of invasive fungal disease cannot meet the classic research definitions of the European Organization for Research and Treatment of Cancer–Mycoses Study Group Education and Research Consortium (EORTC-MSGERC) for probable aspergillosis, because these require the presence of immunocompromising host factors [7]. Alternative research definitions for CAPA have been proposed, first modified from IAPA and subsequently refined given the rapid pace of data generation and knowledge synthesis [5,[8], [9], [10], [11]].
A limitation in common among these definitions is the characterization of CAPA by nonspecific clinical and radiographic findings that are difficult to distinguish from COVID-19 alone. Moreover, mycological findings (e.g. detection of Aspergillus in respiratory samples by culture, antigen detection, or PCR) cannot reliably distinguish between infection and colonization.
The lack of a validated research case definition may result in missed or misidentified individual cases, thereby delaying appropriate antifungal therapy or resulting in unnecessary therapy, both of which may result in poor outcomes. We conducted a systematic review of case series and cohort studies in which patients were evaluated for CAPA while in the ICU and compared research definitions and report prevalence, diagnostics, treatments, and mortality.
Methods
Protocol and registration
The review protocol has been registered in the PROSPERO International Prospective Register of Systematic Reviews (CRD42020204123). The review and reporting were conducted according to PRISMA guidelines.
Eligibility criteria
We included cohort studies of patients with COVID-19 admitted to ICUs who were evaluated for pulmonary aspergillosis using fungal diagnostics (including direct microscopy, fungal culture, Aspergillus PCR, galactomannan testing on respiratory tract specimens, or galactomannan or 1,3-β-D-glucan (BDG) testing in blood) and case series of ICU patients with CAPA. We excluded case reports with fewer than three patients.
Information sources
We searched three electronic databases (PubMed, Embase, and Web of Science) from database inception to October 12, 2021, irrespective of language to identify relevant studies. A separate search was conducted on medRxiv for studies posted ahead of peer review. The search was restricted to studies of humans. Reference lists and cited bibliographies were hand searched. Two reviewers assessed for study eligibility, selected studies, and extracted data. Where assessments were discordant and could not be settled by discussion between the reviewers, these were arbitrated by a third reviewer.
Search
The following search strategy was employed on PubMed from inception to October 12, 2021: (("aspergillus"[All Fields]) OR ("aspergillosis"[All Fields]))) AND (((("sars cov2"[All Fields]) OR ("covid"[All Fields])) OR ("2019 ncov"[All Fields])) OR ("novel coronavirus"[All Fields]). Similar search terms were used for the other databases.
Definitions
Several research definitions for CAPA have been proposed (Table S1). We analyzed these by extracting patient-level data and reclassifying patients according to four research definitions (which we herein designate after the first author): an expert consensus definition for IAPA adapted to COVID-19 (Verweij) [9], the CAPA definitions from Wales (White) [1], expert consensus definitions of the European Confederation of Medical Mycology and the International Society for Human and Animal Mycoses for CAPA (Koehler) [10], and the EORTC-MSGERC Intensive Care Working Group definitions for invasive aspergillosis in ICU patients (Bassetti) [11].
Given that some data were not always explicitly stated, we made some assumptions. First, all patients admitted to an ICU with COVID-19 were assumed to meet the clinical criteria for the White definition (i.e. had ≥1 of the following: refractory fever despite >3 days of antibiotics; recrudescent fever of ≥48 hours despite antibiotics; dyspnoea; haemoptysis; pleural rub or chest pain; worsening respiratory function despite antibiotics and ventilatory support). Second, where radiological findings were not provided, abnormal imaging was assumed for critically ill patients with COVID-19. Third, for the purpose of White classification, radiographic abnormalities reported simply as being typical of COVID-19 were assumed to not be specific for pulmonary aspergillosis. On the other hand, because of the specific imaging requirements needed to meet the Bassetti classification, if radiographic results were not provided, these were considered unclassifiable. Immunocompromise was defined as per EORTC-MSGERC host factors for aspergillosis [7]. We defined survival as being reported to be alive and discharged from the ICU, as reported by the authors. Patients reported to be alive but still in the ICU were excluded from survival analysis.
Risk-of-bias quality assessment
A formal assessment for risk of bias was conducted using an adaptation of the Joanna Briggs Institute cohort checklist tool for systematic reviews [12]. These tools rated the quality of the study by assessing for appropriate patient population, fungal diagnostics, definitions, and outcomes and/or follow-up. Two reviewers assessed the quality of all included studies, and discrepancies were arbitrated by a third reviewer.
Statistical analysis
CAPA prevalence was calculated for each study and pooled using the Freeman-Tukey random effects method. We visualized summary estimates with forest plots. Descriptive data were reported for continuous outcomes. Dichotomous data and outcomes were reported as frequencies and proportions. The associations between antifungal therapy and mortality for patients with CAPA—defined as reported by the authors and proven or probable/putative according to the four research definitions—were tested using DerSimonian-Laird random effects meta-analysis. For this, we excluded from the ‘as reported’ analysis those studies that used a nonstandard definition for CAPA that specifically excluded patients who improved without antifungal therapy. In addition, to be included in the survival meta-analysis, the study needed to have both treated and untreated patients as well as a record at least one death and one survival. Heterogeneity in all meta-analyses was measured using the I2 statistic. Agreement between diagnostic definitions was assessed with Spearman's rank correlation (ρ), with p = 0.05. A sensitivity analysis dichotomized classification as proven/probable (or putative) CAPA vs. not CAPA/possible CAPA. Analyses were done using SPSS v26.0 (Armonk, NY, USA) and Stata v17.0 (College Station, TX, USA).
Results
Literature search
The search for publications identified 572 articles from PubMed (n = 414), Embase (n = 140), Web of Science (n = 13), and grey literature and citations from general and systematic review papers (n = 5). After removing duplicates, 361 relevant studies were identified for title and abstract review. One hundred fifty-two studies were eligible for full-text review, of which 51 met the inclusion criteria, including 31 with patient-level data (Fig. 1 ; Table S2). There were 45 cohort studies and 6 case series included. Individual patient details from studies meeting inclusion criteria are presented in Table S2.
Fig. 1.
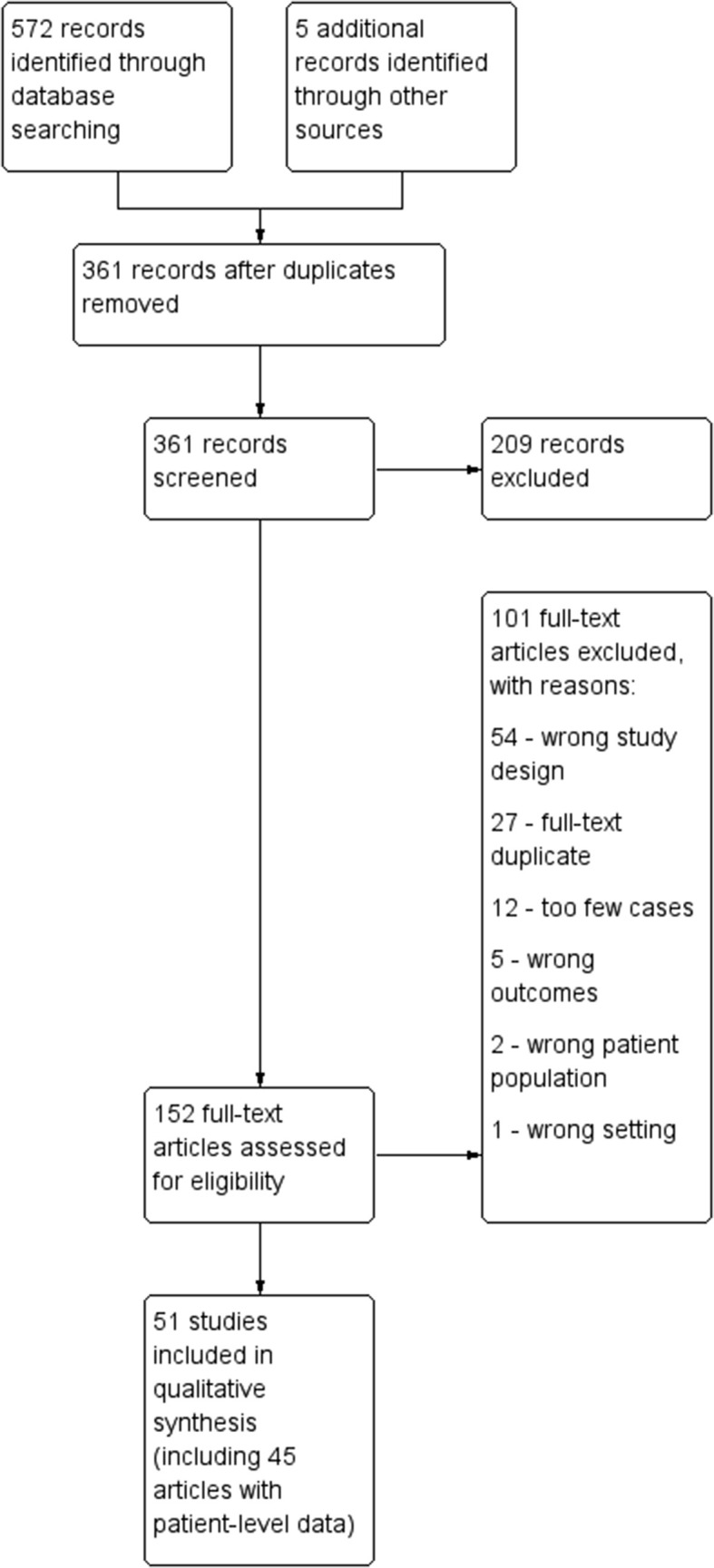
PRISMA flowchart.
Risk-of-bias assessment quality assessment
All studies met overall inclusion criteria, with differences noted in Table S3. Given the low quality of evidence, a GRADE analysis could not be performed. A quality assessment checklist adapted from the Joanna Briggs Institute checklist for cohort and case studies is shown in Table S3.
Correlations between definitions
Four proposed definitions for CAPA (Verweij [9], White [1], Koehler [10], Bassetti [11]) are summarized in Table S1. From 31 studies, we identified 277 patients reported as having CAPA for whom patient-level data were reported (Table S4). Among these, 147 (53.1%) met the criteria of the Verweij definitions (4 proven tracheobronchitis and 143 probable, without documented tracheobronchitis), 125 (45.1%) met the White definitions (4 proven tracheobronchitis, 77 putative), and 179 (64.6%) met the Koehler definitions (4 proven tracheobronchitis; 137 probable, other pulmonary forms; and 38 possible, other pulmonary forms). Two hundred thirty-seven patients could be classified according to the Bassetti definitions, and 40 were unclassifiable due to lack of radiographic details. Among the 237 classifiable patients, 42 (17.7%) met the Bassetti criteria for CAPA (4 proven, 38 probable invasive aspergillosis). Ninety-four patients (33.9%) did not meet criteria for any of the definitions for CAPA: 75 (27.1%) were classified as not meeting the criteria for CAPA for all four definitions, and another 19 (6.9%) did not meet CAPA criteria for any of the Verweij, White, and Koehler definitions and were unclassifiable by Bassetti criteria because of lack of reported radiographic details. Sixteen patients (5.8%) failed to meet criteria for CAPA according to Verweij, White, and Bassetti and were only classified as possible CAPA by Koehler (the only definition to include this classification category). Koehler and Verweij definitions (ρ = 0.893; p < 0.001) showed high levels of concordance, although agreement was modest between other definitions: The correlation coefficient, ρ, ranged between 0.263 and 0.447 (p < 0.001). Moreover, a binary analysis comparing the number of patients who did not meet any criteria comb and with those classified as possible CAPA to those patients with proven or probable/putative CAPA across each definition revealed similar correlation coefficients (ρ = 0.263–0.429; p < 0.001) with moderate correlation between the Koehler and Verweij definitions (ρ = 0.851; p < 0.001) (Tables S5 and S6 and Fig. 2 ).
Fig. 2.
Venn diagram illustrating number of patients meeting each COVID-19–associated pulmonary aspergillosis (CAPA) classification, among 277 patients with individual-level data. Ninety-four patients (33.9%) did not meet criteria for any of the definitions for CAPA: 75 (27.1%) were classified as not meeting criteria for CAPA for all four definitions, and another 19 (6.9%) did not meet CAPA criteria for any of the Verweij, White, and Koehler definitions and were unclassifiable by Bassetti criteria because of lack of reported radiographic details.
Prevalence of CAPA in the ICU
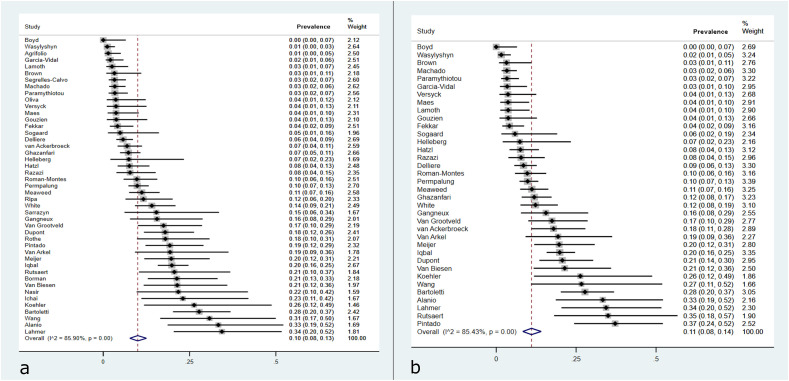
The as-reported prevalence of CAPA in the ICU was calculated based on 5091 patients across 45 cohort studies from 19 countries (Table S3). This ranged from 0% to 34.3% (Fig. 3 a and b). Together, these studies reported 480 cases of CAPA, for an (as-reported) prevalence of 10% (95% CI 8%–13%; I2 = 86%) in ICU patients. Among 3779 patients who were recorded as receiving invasive mechanical ventilation, there were 413 cases of CAPA reported, for a prevalence of 11% (95% CI 9%–15%; I2 = 85%).
Fig. 3.
Forest plot of reported prevalence of COVID-19–associated pulmonary aspergillosis in (a) intensive care unit (ICU) cohort studies and (b) among only those ICU patients receiving invasive mechanical ventilation.
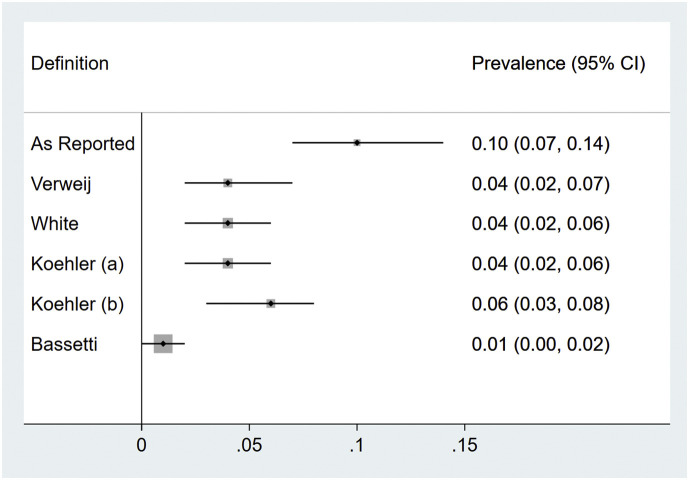
Among only ICU cohort studies with patient-level details, the pooled prevalence of CAPA—as reported—was 10% (95% CI 7%–14%); upon reclassification to Verweij, White, Koehler, and Bassetti definitions, prevalence was 4% (95% CI 2%–7%), 4% (95% CI 2%–6%), 4% (95% CI 2%–6%), and 1% (95% CI 0%–2%), respectively (Fig. 4 ; Table S7; Fig. S1a–e). Heterogeneity was quite high in all meta-analyses (64% for Bassetti; >80% for all other outcomes), indicating highly variable prevalence rates across the studies.
Fig. 4.
Summary forest plots for prevalence of COVID-19–associated pulmonary aspergillosis in cohort studies with individual patient-level data permitting reclassification, as reported and upon reclassification per four published research definitions.
Patient characteristics and diagnosis
Thirty-one articles reporting 277 individuals with CAPA were included in the patient-level analysis (Table S4). Demographic and clinical features of these cases are summarized in Table 1 . Patients came from Europe (n = 181), North America (n = 48), Asia (n = 43), and South America (n = 5). The median age was 65 years (IQR 55–74), and 67.8% were male. The presence of comorbidities was reported for 252 patients (91.0%). Immunocompromised status was present in 17 patients (6.7%). One hundred fifty-four patients (of 220 with such data; 70%) received immunomodulatory therapy alone or in combination: 137 patients (62.3%) received corticosteroids (in addition to 4 patients already on steroids at admission), 35 (15.9%) received tocilizumab, and 2 (0.9%) received anakinra.
Table 1.
Demographic and clinical features reported for 277 patients reported to have CAPA for whom patient-level details are available
| Characteristic | |
|---|---|
| Age (y), median (IQR) | 65 (55–74)a |
| Male sex, n/N (%) | 125/172 (67.8%) |
| Immune compromised, n (%)b | 17 (6.7%) |
| Any immunomodulation, n/N (%) | 154/220 (70%) |
| Systemic steroids, n/N (%) | 136/220 (64.8%)c |
| Tocilizumab, n/N (%) | 35/220 (15.9%) |
| Anakinra, n/N (%) | 2/220 (0.9%) |
| Invasive mechanical ventilation, n (%) | 233 (90.7%) |
| ECMO, n/N (%) | 11/262 (4.2%) |
| Radiographic findings of nodules, n/N (%) | 23/208 (11.1%)d |
| Radiographic findings of cavitations, n (%) | 22 (10.6%) |
| Radiographic findings of nodules or cavitations, or reported as suspicious for fungal infection, n/N (%) | 41/208 (19.7%) |
| Bronchoscopy, n/N (%) | 127/155 (45.8%) |
| Tracheobronchial abnormalities, n/N (%) | 4/127 (3.1%) |
| Mould-active antifungals, n/N (%) | 177/262 (67.6%) |
| Deaths, n/N (%) | 147/248 (59.3%) |
| Timing of CAPA diagnosis after ICU admission (d), median (IQR)b | 8 (5–14)e |
| Length of mechanical ventilation before CAPA diagnosis (d), median (IQR) | 6 (3–10)f |
CAPA, COVID-19–associated pulmonary aspergillosis; ECMO, extracorporeal membrane oxygenation; ICU, intensive care unit; IQR, interquartile range.
Data missing for 25 patients.
Per European Organization for Research and Treatment of Cancer–Mycoses Study Group Education and Research Consortium consensus definitions for host factors.
Plus four already on steroids.
One additional patient had nodules attributed to known pulmonary metastases.
Data missing for 168 patients.
Data missing for 176 patients.
Two hundred thirty-three patients (90.7% for whom this was known) received invasive mechanical ventilation, and 11 received extracorporeal membrane oxygenation. The duration of invasive mechanical ventilation at CAPA diagnosis, known for 57 patients (20.6%), was a median of 6 days (IQR 3–10); duration of ICU admission at time of CAPA diagnosis, known for 109 patients (39.4%), was a median of 8 days (IQR 5–14).
Radiographic findings were described for 208 patients (75.1%). In total, 41 patients (19.7%) had specific findings of nodules, cavitations, or results not specified but reported as suspicious for IPA. Forty-seven described consolidations (without cavitation). The remaining 119 patients had radiographic findings described as nonspecific, diffuse bilateral infiltrates, ground glass opacities, or typical for COVID-19 (without nodules, consolidation, or cavitation), and 1 other patient had nodules attributed to another process.
Among patients reported to have CAPA, bronchoscopy was performed on 127 (45.8%), among whom just 4 (3.1%) were reported to have tracheobronchial abnormalities; in these patients, tracheobronchial biopsies had histopathological evidence of invasive aspergillosis.
Microbiological investigations
Bronchial sampling was done on 180 CAPA patients, including bronchoscopic (n = 127, 71%) and nonbronchoscopic lavage (NBL) (n = 53, 29%). One hundred eighty-six patients had Aspergillus spp. cultured from 192 respiratory specimens. These included A. fumigatus in 130 (70%); A. flavus in 20 (10%); A. niger in 7 (4%); A. terreus in 3 (2%); A. japonicus in 3 (2%); A. citronotterreus, A. lentulus, A. nidulans, A. versicolor, Aspergillus section fumigati, and A. calidoustus in 1 (0.5%) each; and 10 (5%) unspeciated Aspergillus spp. Mixed infections were encountered in seven (4%) additional patients: three with A. fumigatus and A. flavus, two with A. fumigatus and A. niger, one with A. fumigatus and A. versicolor, and one with A. fumigatus, A. awamori, and A. terreus . These were cultured from BAL (n = 79, 42%), bronchial aspirates (n = 19, 10%), NBL (n = 18, 9%), endotracheal aspirates (n = 74, 39%), and sputum (n = 2, 1%). PCR for Aspergillus spp. was done in 105 patients (37.9%) and was positive in 68 of these (64.7%). These included BAL/BA in 21 patients (31%), NBL in 10 patients (15%), NBL and plasma in 2 patients (3%), NBL and serum in 2 patients (3%), bronchial aspirate in 11 patients (16%), tracheal aspirate (TA) in 5 patients (7%), and TA and BAL/BA in 7 patients (10%). Eight had PCR detection of Aspergillus spp. in serum (12%), one from plasma (1.5%), and one from sputum (1.5%).
Galactomannan was measured in bronchial fluid for 125 (45.21%) patients. Ninety-eight had GM measured on BAL, of which 65 were positive (optical density index [ODI] ≥1), whereas another 29 patients had GM measured on NBL, 27 of which were ≥1.0 ODI (Table S4). Serum galactomannan testing was conducted for 168 patients, with 69 (41.1%) being positive (ODI ≥0.5); only 32 (19.0%) had values ≥ 1.0 ODI. Serum BDG was conducted on 109 (39.4%) patients, of whom 50 (45.9%) had levels ≥80 pg/mL.
Treatment and outcomes
Treatment data were available for 262 patients (94.6%). One hundred seventy-seven patients (67.6%) were treated with mould-active antifungals, including (alone or in combination) a mould-active azole (voriconazole, itraconazole, isavuconazole, or posaconazole; n = 139), amphotericin B (n = 41), an echinocandin (n = 28; used with a mould-active azole or amphotericin B in 25 cases, and alone in 3 cases); in 18 patients, the mould-active antifungal used was unclear. Eighty-five (32.4%) patients did not receive mould-active antifungals.
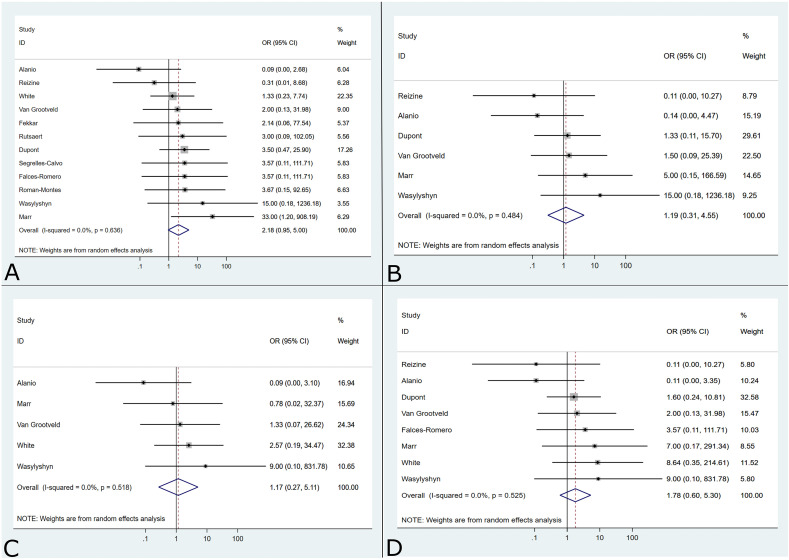
After excluding studies that used survival without antifungals as an exclusion for CAPA diagnosis, survival data were available for 240 patients; 4 additional patients were reported as still alive in ICU and are excluded from further analysis. In total, 98 patients survived (40.8%) and 142 patients died (59.2%). Among patients treated with mould-active antifungals, the survival rate was 46.8% (73/156), vs. 29.8% (25/84) for patients not treated with mould-active antifungals (p = 0.01). A meta-analysis of the association between mould-active antifungal treatment and survival varied across the studies as reported by authors, but the association did not reach statistical significance (OR = 2.18; 95% CI 0.95–5.00) (Fig. 5 a). This finding remained consistent even when only patients who met research definitions were analyzed, including White (OR = 1.17; 95% CI 0.27–5.11), Koehler (OR = 1.78; 95% CI 0.60–5.30), and Verweij (OR = 1.19; 95% CI 0.31–4.55) definitions (Fig. 5b–d). There were insufficient studies that included patients who met Bassetti criteria for this meta-analysis. There was no indication of heterogeneity (I2 = 0%) in any of the analyses.
Fig. 5.
Summary forest plots for antifungal treatment and survival amongst patients with COVID-19–associated pulmonary aspergillosis as reported (a) and when reclassified according to Verweij (b), White (c), and Koehler (d). Analysis of patients who met classification by Bassetti could not be performed because an insufficient number of studies met the minimum criteria for meta-analysis.
Discussion
In this systematic review, we found that the total prevalence of CAPA, as reported, was 10% and varied widely among studies, which may relate in part to surveillance practices and definitions used. These findings are consistent with those reported by other groups [[13], [14], [15]]. However, when reported cases were reclassified to four proposed research definitions for CAPA, the prevalence was 40%–90% lower. These results suggest that a lack of—or inconsistent use of—standard definitions overinflated the reported prevalence of CAPA early in the pandemic.
Most patients with CAPA received invasive mechanical ventilation, although this association may be due to easier access to the lower respiratory tree for sampling and diagnosis. Most studies did not report bronchoscopy rates across the ICU cohort, so it is difficult to know if CAPA rates reflected different rates of this procedure in different centres. A small proportion of patients (3.1%) who had bronchoscopies were reported to have tracheobronchial abnormalities. Although the presence of tracheobronchial disease in a COVID-19 patient should increase suspicion for CAPA, radiographic findings are insufficient to distinguish patients with CAPA from those with COVID-19 alone. In fact, few CAPA patients were reported to have pulmonary nodules or cavitations, findings classically associated with IPA.
Aspergillus spp. were most commonly detected through evaluation of bronchial fluid with fungal culture and, to a lesser extent, galactomannan or PCR. Many patients had bronchial fluid sampling by NBL. The precise role for NBL in diagnosing CAPA is unknown: Although it has sometimes been favoured in the pandemic because of lower concern for aerosolization, it cannot visualize signs of tracheobronchitis or obtain biopsies. In general, more proximal samples such as TA and sputum are less specific and may overestimate CAPA, although one prospective study found reasonable concordance between fungal culture of TA and BAL [16]. Some centres have incorporated screening protocols for CAPA with routine measurement of serum galactomannan and/or BDG [5,17,18]. Although galactomannan detection in serum is likely more specific for invasive disease than that in bronchial fluid (depending on cut-off values), the test lacks sensitivity. Among 168 CAPA patients with serum galactomannan, 69 were positive using the recommended cut-off of 0.5 ODI. Although the rate of positivity of serum BDG was slightly higher than galactomannan, this test lacks specificity.
Various research definitions for CAPA have been used in the emerging literature. Research definitions for invasive aspergillosis in other settings (e.g. the EORTC-MSGERC definitions [7]) are poorly suited because biopsies—required for antemortem diagnosis of proven invasive aspergillosis—are rarely pursued in ICU patients due to risk of complications, and most patients with CAPA lack underlying host factors required for classification as probable invasive aspergillosis. Consequently, most early studies used various (inconsistent) modifications of the AspICU score, adopted from studies of IAPA [3,8]. Later, in a consensus definition for IAPA, Verweij et al. proposed a parallel definition for CAPA [9]. White et al. proposed another definition [1]. In December 2020, an expert panel convened by the European Confederation of Medical Mycology and International Society for Human and Animal Mycoses proposed yet another research definition for the disease [10]. Most recently, the EORTC-MSGERC Intensive Care Working Group proposed definitions for invasive aspergillosis in ICU patients, including those with influenza or COVID-19 [11].
Our analysis of these definitions identified several findings. First, the prevalence of CAPA may be overestimated in the literature because over a third of cases did not fulfil any of these four standardized definitions (or were unclassifiable). For example, among ICU cohort studies with patient-level data, the prevalence of CAPA as reported by authors was 10%, and this rate dropped considerably—between 1% and 6%—upon reclassification of cases according to the four proposed definitions. Second, although there was overlap in the definitions, only the Verweij and Koehler definitions had high correlation with one another; correlation between any other combination of definitions was modest. Consequently, studies using discordant definitions may not be discussing the same patient populations. Third, all definitions are hampered by lack of specificity because they all rely on clinical, radiographic, and mycological findings that may be difficult to distinguish from critically severe COVID-19.
The mortality rate of patients with CAPA was approximately 60%, and survival was higher in patients who received mould-active antifungals. Although data were sparse, a meta-analysis showed no significant difference between survival rate and use of antifungals as reported by authors and after the use of various definitions, highlighting significant challenges in treatment recommendations for this population. Nonetheless, some patients classified as having CAPA survived without antifungal therapy, which brings into question the specificity of the diagnosis. Some lines of evidence suggest that not all cases of CAPA represent invasive disease. A systematic review found that proven invasive mould disease is an uncommon autopsy finding in patients dying with COVID-19 (<2%) [19], although rates have been higher in some single-centre studies [20,21]. Moreover, in some cases of probable CAPA, post-mortem examination failed to confirm the diagnosis [22]. Currently, distinguishing which patients with CAPA require treatment is challenging and not clearly addressed by our study.
We observed a number of similarities and some contrasts with IAPA. Like with IAPA—and unlike invasive aspergillosis outside of ICU settings—most CAPA patients lacked pre-existing immunocompromising conditions. In contrast with IAPA, the timing of diagnosis of CAPA was late (occurring after a median 8 days after ICU admission), compared to after a median 2 days reported in the literature in IAPA [3]. In addition, tracheobronchitis was rare, noted in just 3.1% of CAPA patients in whom bronchoscopy was done, in contrast to IAPA patients, in whom it is reported to occur in one-third to one-half of affected patients [3,9].
Limitations
This study has some important limitations. Studies employed different surveillance methods and diagnostic tests to screen for and diagnose CAPA, and thus pooled analyses should be interpreted with caution. For example, studies that employed more aggressive surveillance (e.g. by routine bronchoscopy with fungal culture and galactomannan) would likely report higher rates of CAPA than studies in which fungal investigations were obtained only based on clinical suspicion; conversely, they may also overrepresent those with Aspergillus spp. colonization. There was also inconsistent reporting related to clinical characteristics and diagnostics, both in patients reported to have CAPA and the rest of the ICU cohorts, limiting comparisons. For example, use of corticosteroids and other immunomodulators was infrequently reported in non-CAPA patients, precluding meaningful comparison with those who developed CAPA. Study durations varied or were inconsistently reported, precluding a precise calculation of the incidence rate over a specified time. Similarly, duration of patient follow-up was not uniformly reported, which may have biased the estimation of case fatality. Lastly, the dearth of studies from low- and middle-income countries may limit the generalizability of this systematic review.
Conclusions
The reported prevalence of CAPA in ICU patients with COVID-19 varies greatly by study and may be related to surveillance protocols and inconsistent definitions, which may inflate the prevalence of this complication. Further research should refine CAPA definitions and identify patients most likely to benefit from pre-emptive antifungal therapy.
Transparency declaration
This study was not funded. ISS received personal fees from AVIR Pharma, outside the submitted work. TCD has received research grant funding from AVIR Pharma and clinical trial support from BioMérieux, outside the submitted work. All other authors declare no conflicts.
Author contributions
RMK designed the literature search, created the figures, and wrote the first draft of the manuscript. RMK, TCD, BEK, and ISS screened and reviewed articles and extracted and analyzed data. BV, RMK, WIS, and ISS performed statistical analysis. ISS designed the study and was responsible for overall supervision. All authors critically reviewed and revised the manuscript and approved the final draft.
Editor: E. Roilides
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.cmi.2022.01.027.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
Supplementary Figure 1.
Prevalence of COVID-19-associated pulmonary aspergillosis in cohort studies with patient-level data (a) as reported and when reclassified according to Verweij (b), White (c) Koehler - proven/probable classifications only (d) Koehler - any classifications and (e) Bassetti.
References
- 1.White P.L., Dhillon R., Cordey A., Hughes H., Faggian F., Soni S., et al. A national strategy to diagnose coronavirus disease 2019–associated invasive fungal disease in the intensive care unit. Clin Infect Dis. 2020:ciaa1298. doi: 10.1093/cid/ciaa1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wauters J., Baar I., Meersseman P., Meersseman W., Dams K., De Paep R., et al. Invasive pulmonary aspergillosis is a frequent complication of critically ill H1N1 patients: a retrospective study. Intensive Care Med. 2012;38:1761–1768. doi: 10.1007/s00134-012-2673-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schauwvlieghe A.F.A.D., Rijnders B.J.A., Philips N., Verwijs R., Vanderbeke L., Van Tienen C., et al. Invasive aspergillosis in patients admitted to the intensive care unit with severe influenza: a retrospective cohort study. Lancet Respir Med. 2018;6:782–792. doi: 10.1016/S2213-2600(18)30274-1. [DOI] [PubMed] [Google Scholar]
- 4.Alanio A., Dellière S., Fodil S., Bretagne S., Mégarbane B. Prevalence of putative invasive pulmonary aspergillosis in critically ill patients with COVID-19. Lancet Respir Med. 2020;8:e48–e49. doi: 10.1016/S2213-2600(20)30237-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brown L.-A.K., Ellis J., Gorton R., De S., Stone N. Surveillance for COVID-19-associated pulmonary aspergillosis. Lancet Microbe. 2020;1:e152. doi: 10.1016/S2666-5247(20)30091-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mahase E. Covid-19: WHO declares pandemic because of “alarming levels” of spread, severity, and inaction. BMJ. 2020:m1036. doi: 10.1136/bmj.m1036. [DOI] [PubMed] [Google Scholar]
- 7.Donnelly J.P., Chen S.C., Kauffman C.A., Steinbach W.J., Baddley J.W., Verweij P.E., et al. Revision and update of the consensus definitions of invasive fungal disease from the European organization for research and treatment of cancer and the Mycoses study group education and research Consortium. Clin Infect Dis. 2020;71:1367–1376. doi: 10.1093/cid/ciz1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Blot S.I., Taccone F.S., Van den Abeele A.-M., Bulpa P., Meersseman W., Brusselaers N., et al. A clinical algorithm to diagnose invasive pulmonary aspergillosis in critically ill patients. Am J Respir Crit Care Med. 2012;186:56–64. doi: 10.1164/rccm.201111-1978OC. [DOI] [PubMed] [Google Scholar]
- 9.Verweij P.E., Gangneux J.-P., Bassetti M., Brüggemann R.J.M., Cornely O.A., Koehler P., et al. Diagnosing COVID-19-associated pulmonary aspergillosis. Lancet Microbe. 2020;1:e53–e55. doi: 10.1016/S2666-5247(20)30027-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Koehler P., Bassetti M., Chakrabarti A., Chen S.C.A., Colombo A.L., Hoenigl M., et al. Defining and managing COVID-19-associated pulmonary aspergillosis: the 2020 ECMM/ISHAM consensus criteria for research and clinical guidance. Lancet Infect Dis. 2020 doi: 10.1016/S1473-3099(20)30847-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bassetti M., Azoulay E., Kullberg B.-J., Ruhnke M., Shoham S., Vazquez J., et al. EORTC/MSGERC definitions of invasive fungal diseases: summary of activities of the intensive care unit working group. Clin Infect Dis. 2021;72:S121–S127. doi: 10.1093/cid/ciaa1751. [DOI] [PubMed] [Google Scholar]
- 12.Moola S., Munn Z., Tufanaru C., Aromataris E., Sears K., Sfetc R., et al. In: JBI Manual for Evidence Synthesis. Aromataris E, Munn Z, editors. JBI; 2020. Chapter 7: Systematic reviews of etiology and risk.https://synthesismanual.jbi.global [DOI] [Google Scholar]
- 13.Salmanton-García J., Sprute R., Stemler J., Bartoletti M., Dupont D., Valerio M., et al. COVID-19–associated pulmonary aspergillosis, March–August 2020. Emerg Infect Dis. 2021;27:1077–1086. doi: 10.3201/eid2704.204895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chong W.H., Neu K.P. Incidence, diagnosis and outcomes of COVID-19-associated pulmonary aspergillosis (CAPA): a systematic review. J Hosp Infect. 2021;113:115–129. doi: 10.1016/j.jhin.2021.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mitaka H., Perlman D.C., Javaid W., Salomon N. Putative invasive pulmonary aspergillosis in critically ill patients with COVID-19: an observational study from New York City. Mycoses. 2020;63:1368–1372. doi: 10.1111/myc.13185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Garcia-Vidal C., Sanjuan G., Moreno-García E., Puerta-Alcalde P., Garcia-Pouton N., Chumbita M., et al. Incidence of co-infections and superinfections in hospitalized patients with COVID-19: a retrospective cohort study. Clin Microbiol Infect. 2021;27:83–88. doi: 10.1016/j.cmi.2020.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Helleberg M., Steensen M., Arendrup M.C. Invasive aspergillosis in patients with severe COVID-19 pneumonia. Clin Microbiol Infect. 2021;27:147–148. doi: 10.1016/j.cmi.2020.07.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.van Arkel A.L.E., Rijpstra T.A., Belderbos H.N.A., van Wijngaarden P., Verweij P.E., Bentvelsen R.G. COVID-19-associated pulmonary aspergillosis. Am J Respir Crit Care Med. 2020;202:132–135. doi: 10.1164/rccm.202004-1038LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kula B.E., Clancy C.J., Hong Nguyen M., Schwartz I.S. Invasive mould disease in fatal COVID-19: a systematic review of autopsies. Lancet Microbe. 2021;2:e405–e414. doi: 10.1016/S2666-5247(21)00091-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Evert K., Dienemann T., Brochhausen C., Lunz D., Lubnow M., Ritzka M., et al. Autopsy findings after long-term treatment of COVID-19 patients with microbiological correlation. Virchows Arch. 2021;479:97–108. doi: 10.1007/s00428-020-03014-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fortarezza F., Boscolo A., Pezzuto F., Lunardi F., Acosta M.J., Giraudo C., et al. Proven COVID-19—associated pulmonary aspergillosis in patients with severe respiratory failure. Mycoses. 2021;64:1223–1229. doi: 10.1111/myc.13342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Grootveld R., Paassen J., Boer M.G.J., Claas E.C.J., Kuijper E.J., Beek M.T., et al. Systematic screening for COVID-19 associated invasive aspergillosis in ICU patients by culture and PCR on tracheal aspirate. Mycoses. 2021;64:641–650. doi: 10.1111/myc.13259. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.