Abstract
The etiology of multiple inflammatory syndrome in children (MIS-C) remains poorly understood. As clues to elucidate the pathogenic condition, several characteristic peripheral immunophenotypes have been reported in MIS-C. However, no report has demonstrated the time course of the peripheral immunophenotype along with the clinical course in the same patient. Herein, we clarified the immunological characteristics of a Japanese patient with MIS-C. There was an initial cytokine storm followed by T-cell activation, especially of CD8+ T cells, with the expansion of T-cell receptor Vβ 21.3-expressing cells, which suggests superantigen-mediated T-cell activation. In addition, we also found an increase in IgG-producing cells (plasmablasts and switched memory B cells), which were accompanied by elevated serum levels of anti-SARS-CoV-2 spike antigen-specific IgG antibodies. These time course of peripheral immunophenotypes support that immunological activation against SARS-CoV-2 spike protein plays a central role in the etiology of MIS-C.
Keywords: Antibody, IgG, MIS-C, SARS-CoV-2, Spike protein, Vβ 21.3
Abbreviations: ASA, aspirin; CDC, Centers for Disease Control and Prevention; COVID-19, coronavirus disease 2019; ECG, electrocardiogram; IFN, interferon; IL, interleukin; IVIG, intravenous immunoglobulin; MCP, monocyte chemotactic protein; MIS-C, multiple inflammatory syndrome in children; NK, natural killer; PCR, polymerase chain reaction; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; PBMCs, peripheral blood mononuclear cells; TCR, T cell receptor; Th, T-helper; TNF, tumor necrosis factor; WHO, World Health Organization
1. Introduction
Multiple inflammatory syndrome in children (MIS-C) occurs 2–5 weeks after severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection [1]. MIS-C is characterized by fever, signs of generalized inflammation including gastrointestinal symptoms, shock with acute heart failure, and multiple organ dysfunction with cytokine storm [2,3]. Although MIS-C is considered the consequence of a delayed immune response to SARS-CoV-2 infection [1], its etiology remains poorly understood.
Several characteristic peripheral immunophenotypes have been reported in MIS-C as clues to the origin of the pathogenic condition. Analysis of peripheral blood mononuclear cells (PBMCs) from patients with MIS-C revealed decreased numbers of natural killer (NK) and T cells, suggesting extravasation of these cells into peripheral tissues [4]. Substantial T-cell dysregulation, particularly that of CD8+ T cells, increases in the populations of activated T cells [5]. Moreover, expression levels of HLA-DR on γδ T cells and CCR7+ CD4+ T cells are high, and those of HLA-DR and CD86 on monocytes are low, suggesting impaired antigen presentation capacity [6]. Additionally, a profound expansion of T cells expressing the T cell receptor (TCR) β variable 11.2 (TRBV11.2) gene, which encodes Vβ 21.3, has been observed in patients with MIS-C [7,8], suggesting superantigen-mediated T-cell activation as an underlying pathophysiological event in MIS-C. A superantigen-like motif near the S1/S2 cleavage site on the SARS-CoV-2 spike protein is believed to drive this hyperinflammatory response in MIS-C [9,10], and patients with MIS-C have significantly higher concentrations of anti-SARS-CoV-2 spike antibody [11].
However, no study has shown the time course of the peripheral immunophenotype along with the clinical course in the same patient. Herein, we report the case of a 14-year-old boy with MIS-C and conduct a detailed analysis of the phenotype of the peripheral blood. This case provides insights into the pathophysiology of MIS-C, including novel kinetics of IgG-producing cells and anti-SARS-CoV-2 spike antigen-specific IgG antibodies in addition to T-cell activation characterized by the expansion of Vβ 21.3-expressing cells.
2. Patient and methods
2.1. The patient
The 14-year-old Japanese patient was admitted and followed at our center. Immunologic analyses were conducted in the context of a research project (H29–310) approved by the hospital ethics committee. Written informed consent was obtained from the patient and patient's parents. The day of onset of MIS-C when fever appeared was defined as day 0.
2.2. Cytokine analysis
Serum was collected from a patient and stored at −80 °C. The concentrations of interleukin-1β (IL-1β), IL-2, IL-4, IL-6, IL-8, IL-10, IL-12 / IL-23p40, IL-17A, tumor necrosis factor (TNF), interferon-α (IFN-α), IFN-β, and monocyte chemotactic protein 1 (MCP-1) were measured in serum samples on day 5, 6, 7, 8, 10, 12, and 24 using a BD LSRfortessa flow cytometer (Becton Dickinson, Franklin Lakes, NJ, USA) with BD Cytometric Bead Array Flex Sets (Becton Dickinson, Franklin Lakes, NJ, USA) according to the manufacturer instructions.
2.3. Leukocytes immunophenotyping
Immune cells from peripheral blood mononuclear cells (PBMCs) were phenotyped using four separate flow cytometry panels (Supplementary Table 1), which were created based on the Human Immunology Project [12].
Whole blood samples were collected from a patient on day 5, 7, 10, 24, and 54. PBMCs were isolated from whole blood by using Lymphoprep (Axis Shield Diagnostics, Dundee, Scotland, UK) and SepMate (STEMCELL technologies, Vancouver, BC, Canada) according to the manufacturer instructions. PBMCs were resuspended in 1 mL RBC Lysis Buffer (Sony Biotechnology, Champaign, IL, USA) and incubated at room temperature for 15 min and washed twice. PBMCs (1 × 106 cells per tube) were resuspended in 100 μL staining each cocktail (Supplementary Table 1) and incubated at 4 °C in the dark for 30 min. For data acquisition, a BD LSRFortessa flow cytometer (Becton Dickinson, Franklin Lakes, NJ, USA) was used and analyzed using Flowjo (10.6.2; Treestar). The gating strategies are outlined in Supplementary Fig. 3, using a patient sample on day 7. Each lymphocyte subset counts were obtained by multiplying subset percentages times anchor marker percentages of total CD45 lymphocyte population times the absolute lymphocyte count.
2.4. T-cell receptor repertoire analysis
The phenotypic analysis of TCR Vβ repertoire on PBMCs was performed on day 12 and 54 using the IOTest Beta Mark kit (Beckman Coulter, South Kraemer Boulevard Brea, CA, USA) containing 24 monoclonal antibodies (mAbs) identifying ~70% of the T cell repertoire. PBMCs were stained with surface markers in 8 sample tubes (Supplementary Table 2). All samples were acquired on a BD LSRFortessa flow cytometer (Becton Dickinson, Franklin Lakes, NJ, USA) and analyzed using FlowJo (10.6.2; Treestar). Lymphocytes were first gated according to FSC/SSC parameter, then by selection of CD3+CD4+CD45RO+, CD3+CD8+CD45RO+, CD3+CD4+HLA-DR+, and CD3+CD8+HLA-DR+ positive cells.
2.5. Anti-SARS-CoV-2 spike IgG antibody analysis
Serum anti-SARS-CoV-2 spike IgG antibody was measured on day 5, 6, 7, 10, 12, 24, and 54 using a human SARS-CoV-2 spike IgG ELISA kit (Thermo Fisher Scientific, Waltham, MA, USA) according to the manufacturer instructions. Serum samples were dilute 1:100 with assay buffer.
3. Results
3.1. Clinical course
A 14-year-old previously healthy Japanese boy was transferred to our hospital because of suspected MIS-C. He had been in contact with patients who had coronavirus disease 2019 (COVID-19) and had a history of positive SARS-CoV-2 polymerase chain reaction (PCR) results. He was asymptomatic for 26 days after being tested. Subsequently, the patient experienced fever, pharyngeal pain, cough, dyspnea, chest pain, and diarrhea. On day 4 of fever, oliguria and physical disability occurred and he was taken to an emergency medical center. The electrocardiogram (ECG) and echocardiogram results suggested myocardial damage, and he was transferred to our hospital on day 5. On arrival, the patient had negative nasopharyngeal SARS-CoV-2 PCR results. He had a fever of 38.8 °C, blood pressure of 99/61 mmHg, and a pulse rate of 110 bpm. Physical examination revealed bilateral conjunctival congestion and pharyngeal erythema. No other abnormal findings like rashes were observed during admission. Blood test results showed thrombocytopenia, lymphopenia, and elevated C-reactive protein, fibrinogen, ferritin, and troponin T levels (Supplementary Fig. 1). Chest radiography revealed cardiac enlargement (cardiothoracic ratio, 54%). ECG revealed negative T waves in II, III, aVF, biphasic T waves in V3–4, and flat T waves in V5–6 leads (Supplementary Fig. 2), but no recognized cardiac conduction abnormalities such as those previously reported in patients with MIS-C [13]. The echocardiogram showed impaired systolic function (ejection fraction, 43%), increased myocardial echogenicity, and slight pericardial effusion. Valvular regurgitation and coronary artery dilatation were not observed. The patient was diagnosed with MIS-C based on both the Centers for Disease Control and Prevention (CDC) and the World Health Organization (WHO) criteria [14,15]. He did not meet the diagnostic criteria for Kawasaki disease [16]. Intravenous immunoglobulin (IVIG) (2 g/kg) and oral aspirin (ASA) (30 mg/kg) were administered as initial therapy on day 5. Subsequently, the patient defervesced rapidly and other symptoms and clinical parameters gradually improved. ASA was reduced to 4 mg/kg on day 7. Cardiac magnetic resonance imaging was performed on day 9 and no myocardial edema or gadolinium enhancement [17] was observed during the recovery phase. Enalapril was initiated on day 10, and the patient was discharged on day 13. The echocardiogram and ECG findings normalized on days 24 and 56, respectively.
3.2. Time course of serum cytokines
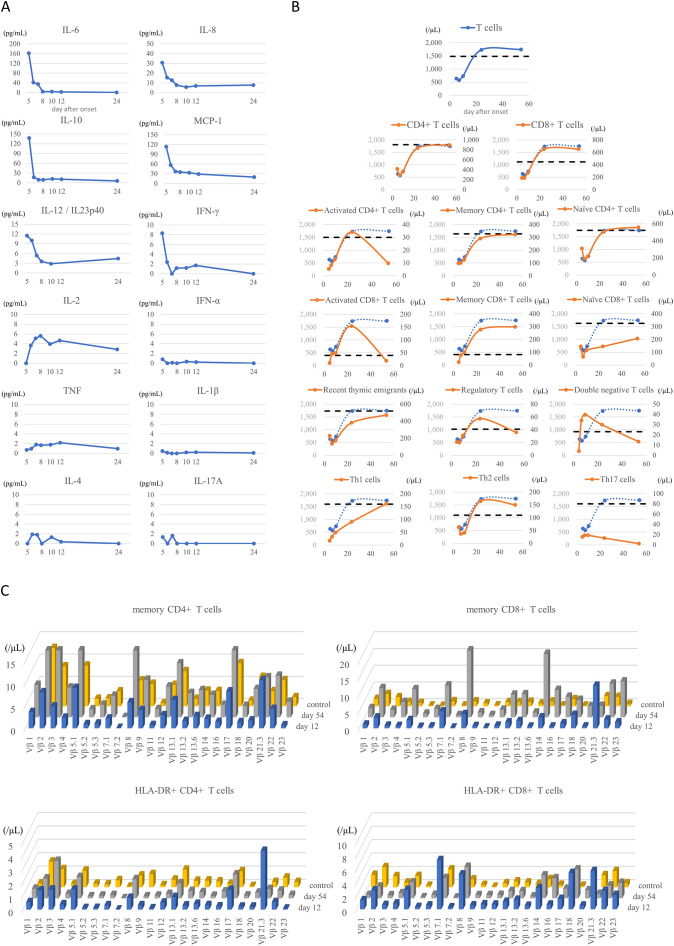
The acute phase was characterized by increased serum levels of a broad spectrum of cytokines, including IL-6, IL-8, IL-10, IL-12/IL-23p40, MCP-1, and IFN-γ. The elevated cytokine levels normalized rapidly after treatment (Fig. 1A), along with convalescence. There was no elevation in the serum IFN-α levels. These changes are consistent with those previously reported [[4], [5], [6],[18], [19], [20]]. However, in our case, there was no increase in TNF, IL-1β, IL-4, or IL-17A levels.
Fig. 1.
(A) Time course of serum cytokines. The values of day 5 was measured before treatment. Note that the elevated cytokine levels were normalized rapidly after treatment, along with the convalescence from the illness. (B) Time course of T-cell subsets. Some T-cell subsets had characteristic kinetics. The dashed lines indicate the median value of healthy children around the same age as the patient according to reference [[28], [29], [30], [31]]. The total T-cell kinetics were included in each graph as comparison. (C) Time course of T-cell receptor repertories. The expansion of Vβ 21.3 in both memory CD4+ and CD8+ T cells was seen on day 12. A similar trend was seen in HLA-DR+ CD4+ and CD8+ T cells. The peripheral blood from a healthy 11-year-old boy was used as control.
3.3. Time course of peripheral leukocyte subsets
We observed a decrease in number of T (Fig. 1B) and NK cells, a decrease in HLA-DR expression on monocytes, and an increase in HLA-DR expression on γδ T cells in the acute phase (data not shown), as previously reported [[4], [5], [6], [7]]. Additionally, we found characteristic kinetics in some of the T-cell subsets (Fig. 1B). Although no obvious changes were observed in the overall CD4+ / CD8+ ratio in T cells, an increase in activated T cells (HLA-DR+ CD38+) and memory T cells was observed immediately after treatment. Notably, the changes in the activation of CD8+ T cells were remarkable, and the activation lasted more than 1 month. Moreover, recovery of naïve CD8+ T cells was poor compared to that of naïve CD4+ T cells or recent thymic emigrants. We also observed an increase in double-negative T cells (TCRαβ+ CD4− CD8−) in the acute phase and an increase in regulatory T cells (CD4+ CD25+ CD127low) in the recovery phase. In T-helper (Th) subpopulations, Th2 cells (CD4+ CD45RO+ CXCR3− CCR6−) were more abundant than Th1 cells (CD4+ CD45RO+ CXCR3+ CCR6−) before treatment, but these two subpopulations became balanced immediately after treatment in the acute phase. Th17 cells (CD4+ CD45RO+ CXCR3− CCR6+) were depleted over time. No remarkable changes were observed in follicular T cells (data not shown).
3.4. Time course of T-cell receptor repertories
The expansion of Vβ 21.3 in both memory CD4+ T cells and memory CD8+ T cells was observed on day 12, as previously reported (Fig. 1C) [7,8,10]. A similar trend was observed in HLA-DR+ CD4+ or CD8+ T cells.
3.5. Time course of anti-SARS-CoV-2 spike-specific IgG antibody
The anti-SARS-CoV-2 spike-specific IgG antibody titer was already high at admission and further increased during the acute phase. This trend fitted perfectly with the trend for IgG-producing cells (plasmablasts and switched memory B cells) (Fig. 2 ). Moreover, the timing of the rise and fall of IgG titers and IgG-producing cells coincided with that of activated T cells (Figs. 1B, 2). We confirmed that the immunoglobulin preparation used for treatment did not contain SARS-CoV-2 IgG antibodies.
Fig. 2.
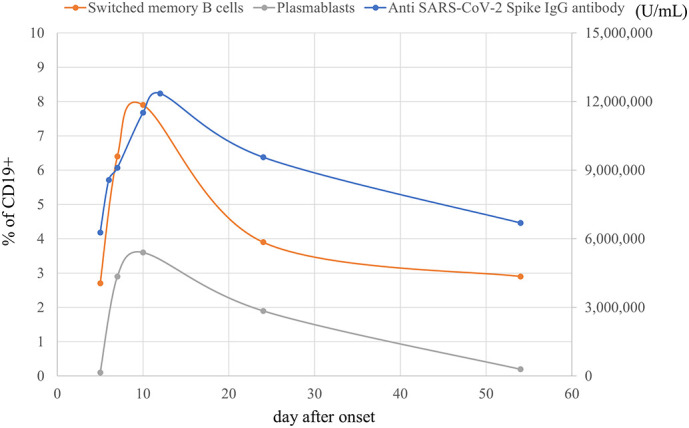
Time course of serum anti-SARS-CoV-2 spike antigen-specific IgG antibody levels and the relative number of IgG-producing cells. The antibody titer increased in the acute phase and this trend fitted well with the changes of IgG-producing cell number.
4. Discussion
We clarified the detailed immunological characteristics of a Japanese patient with MIS-C. There was an initial cytokine storm followed by T-cell activation, especially of CD8+ T cells, with the expansion of Vβ 21.3-expressing cells. Surprisingly, we also found B cell activation, which was accompanied by elevated serum levels of anti-SARS-CoV-2 spike antigen-specific IgG antibody (Fig. 2). T-cell activation was characterized by the expansion of Vβ 21.3-expressing cells (which suggests superantigen-mediated T-cell activation) and serum anti-SARS-COV-2 spike antibody levels also increased along with IgG-producing cells. Although this is a single case study, such dynamic and characteristic immunological change in a typical MIS-C patient supports that immunological activation against SARS-CoV-2 spike protein plays a central role in the etiology of MIS-C and may be useful for MIS-C diagnosis. The possible trigger for these immune responses could be the prolonged presence of SARS-CoV-2 in the gastrointestinal tract [21] which may increase gut permeability, allowing SARS-CoV-2 antigens, including the superantigen-like motif of the spike protein, into the bloodstream [22].
We found other characteristic kinetics in some of the T-cell subsets (Fig. 1B). Poor recovery of naïve CD8+ T cells may support T-cell dysregulation [4]. Increase in regulatory T cells in the recovery phase may be associated with the induction of immune regulation against activated T cells, which continued to increase in number in this phase. We observed an increase in Th1 cytokine (IL-2, IFN-γ), as previously reported [19]. However, peripheral immunophenotyping of T cell subpopulations revealed Th2 skewing during the acute phase, which might suggest extravasation of Th1 cells into peripheral tissues. Although the changes in double-negative T cells and Th17 cells are interesting, the association with these MIS-C pathologies is currently unknown and warrants further research.
MIS-C has predominantly been reported in Hispanic or non-Hispanic Blacks but rarely in Asians [1]. This Japanese MIS-C case showed immunological findings similar to those of non-Asian patients. Therefore, host genetic factors may possibly influence the regional differences in incidence, but not the pathophysiology of MIS-C. These findings may be of great value in differentiating MIS-C from Kawasaki disease, which has a high incidence in Asians in contrast to MIS-C. In particular, the increase in anti-SARS-CoV-2 spike antigen-specific IgG antibody levels and significant activation of CD8+ T cells with expansion of Vβ 21.3, which is not seen in Kawasaki disease [8], may be useful for MIS-C diagnosis.
Our patient seemed to respond well to IVIG alone, which did not contain anti-SARS-CoV-2 antibodies. The serum cytokine levels normalized rapidly after treatment with IVIG and correlated well with clinical symptoms, as previously reported [20]. Although it remains unclear how IVIG modulates the immune system, mechanisms such as the neutralization of cytokines and the expansion of Treg cells has been proposed [23]. The kinetics of cytokines and Treg cells in our case may be the result of these mechanisms. Currently, it has been shown that IVIG led to a decrease in IL-1β-producing neutrophils and an increase in lymphocytes in patients with MIS-C [24]. This effect may have influenced the immunophenotypes seen in our case. In contrast to the favorable effect, there is a concern that IVIG containing anti-SARS-CoV-2 antibody may enhance rather than decrease inflammation [[25], [26], [27]]. Our case suggests that the serum anti-SARS-CoV-2 spike antigen-specific IgG antibody levels increased because of a series of immune responses, and that the antibody itself is unlikely to be a factor that exacerbates the pathogenic condition. Nonetheless, further research is needed on the functional role of anti-SARS-CoV-2 antibody as an enhancer of MIS-C or as an autoantibody, in addition to that of IVIG itself.
5. Conclusion
We described a patient with MIS-C who had not only T-cell activation characterized by the expansion of Vβ 21.3-expressing cells but also the increase of serum anti-SARS-CoV-2 spike antigen-specific IgG antibody levels accompanied by an increase in IgG-producing cells. The current case supports that immunological activation against SARS-CoV-2 spike protein plays a central role in the etiology of MIS-C.
Funding/support
The authors received no financial support for the research, authorship and/or publication of this article.
Authorship contributions
Atsushi Morita (Conceptualization, Data curation, Formal analysis, Investigation, Visualization, Writing original draft), Sho Hosaka (Data curation, Investigation, Writing reviews and editing), Kazuo Imagawa (Data curation, Investigation, Writing reviews and editing), Takumi Ishiodori (Resources, Investigation, Writing reviews and editing), Yoshihiro Nozaki (Resources, Investigation, Writing reviews and editing), Takashi Murakami (Resources, Investigation, Writing reviews and editing), Hidetoshi Takada (Conceptualization, Project administration, Supervision, Writing reviews and editing)
Declaration of Competing Interest
The authors have no conflicts of interest relevant to this article to disclose.
Acknowledgments
We would like to thank Editage (https://www.editage.com/) for English language editing.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.clim.2022.108955.
Appendix A. Supplementary data
Supplementary material
References
- 1.Belay E.D., Abrams J., Oster M.E., et al. Trends in geographic and temporal distribution of US children with multisystem inflammatory syndrome during the COVID-19 pandemic. JAMA Pediatr. 2021;175:837–845. doi: 10.1001/jamapediatrics.2021.0630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Godfred-Cato S., Bryant B., Leung J., et al. COVID-19-associated multisystem inflammatory syndrome in children - United States, march-July 2020. MMWR Morb. Mortal. Wkly Rep. 2020;69:1074–1080. doi: 10.15585/mmwr.mm6932e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bautista-Rodriguez C., Sanchez-de-Toledo J., Clark B.C., et al. Multisystem inflammatory syndrome in children: an international survey. Pediatrics. 2021;147 doi: 10.1542/peds.2020-024554. [DOI] [PubMed] [Google Scholar]
- 4.Gruber C.N., Patel R.S., Trachtman R., et al. Mapping systemic inflammation and antibody responses in multisystem inflammatory syndrome in children (MIS-C) Cell. 2020;183:982–995. doi: 10.1016/j.cell.2020.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vella L.A., Giles J.R., Baxter A.E., et al. Deep immune profiling of MIS-C demonstrates marked but transient immune activation compared to adult and pediatric COVID-19. Sci. Immunol. 2021;6:eabf7570. doi: 10.1126/sciimmunol.abf7570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carter M.J., Fish M., Jennings A., et al. Peripheral immunophenotypes in children with multisystem inflammatory syndrome associated with SARS-CoV-2 infection. Nat. Med. 2020;26:1701–1707. doi: 10.1038/s41591-020-1054-6. [DOI] [PubMed] [Google Scholar]
- 7.Ramaswamy A., Brodsky N.N., Sumida T.S., et al. Immune dysregulation and autoreactivity correlate with disease severity in SARS-CoV-2-associated multisystem inflammatory syndrome in children. Immunity. 2021;54:1083–1095. doi: 10.1016/j.immuni.2021.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Moreews M., Le Gouge K., Khaldi-Plassart S., et al. Polyclonal expansion of TCR Vbeta 21.3(+) CD4(+) and CD8(+) T cells is a hallmark of multisystem inflammatory syndrome in children. Sci. Immunol. 2021;6:eabh1516. doi: 10.1126/sciimmunol.abh1516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cheng M.H., Zhang S., Porritt R.A., et al. Superantigenic character of an insert unique to SARS-CoV-2 spike supported by skewed TCR repertoire in patients with hyperinflammation. Proc. Natl. Acad. Sci. U. S. A. 2020;117:25254–25262. doi: 10.1073/pnas.2010722117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Porritt R.A., Paschold L., Rivas M.N., et al. HLA class I-associated expansion of TRBV11-2 T cells in multisystem inflammatory syndrome in children. J. Clin. Invest. 2021;131 doi: 10.1172/JCI146614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rostad C.A., Chahroudi A., Mantus G., et al. Quantitative SARS-CoV-2 serology in children with multisystem inflammatory syndrome (MIS-C) Pediatrics. 2020;146 doi: 10.1542/peds.2020-018242. [DOI] [PubMed] [Google Scholar]
- 12.Maecker H.T., McCoy J.P., Nussenblatt R. Standardizing immunophenotyping for the human immunology project. Nat. Rev. Immunol. 2012;12:191–200. doi: 10.1038/nri3158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Choi N.H., Fremed M., Starc T., et al. MIS-C and cardiac conduction abnormalities. Pediatrics. 2020;146 doi: 10.1542/peds.2020-009738. [DOI] [PubMed] [Google Scholar]
- 14.Centers for Disease Control and Prevention Multisystem Inflammatory Syndrome in Children (MIS-C) Associated with Coronavirus Disease 2019 (COVID-19) 2020. https://emergency.cdc.gov/han/2020/han00432.asp (accessed 19 May 2020)
- 15.World Health Organization Multisystem Inflammatory Syndrome in Children and Adolescents Temporally Related to COVID-19. 2020. https://www.who.int/news-room/commentaries/detail/multisystem-inflammatory-syndrome-in-children-and-adolescents-with-covid-19 (accessed 15 May 2020)
- 16.Kobayashi T., Ayusawa M., Suzuki H., et al. Revision of diagnostic guidelines for Kawasaki disease (6th revised edition) Pediatr. Int. 2020;62:1135–1138. doi: 10.1111/ped.14326. [DOI] [PubMed] [Google Scholar]
- 17.Blondiaux E., Parisot P., Redheuil A., et al. Cardiac MRI in children with multisystem inflammatory syndrome associated with COVID-19. Radiology. 2020;297:E283–E288. doi: 10.1148/radiol.2020202288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Diorio C., Henrickson S.E., Vella L.A., et al. Multisystem inflammatory syndrome in children and COVID-19 are distinct presentations of SARS-CoV-2. J. Clin. Invest. 2020;130:5967–5975. doi: 10.1172/JCI140970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Caldarale F., Giacomelli M., Garrafa E., et al. Plasmacytoid dendritic cells depletion and elevation of IFN-γ dependent chemokines CXCL9 and CXCL10 in children with multisystem inflammatory syndrome. Front. Immunol. 2021;12 doi: 10.3389/fimmu.2021.654587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Takasago S., Sakai A., Sugiyama M., et al. Case report: changes in cytokine kinetics during the course of disease in a Japanese patient with multisystem inflammatory syndrome in children. Front. Pediatr. 2021;9 doi: 10.3389/fped.2021.702318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gaebler C., Wang Z., Lorenzi J.C.C., et al. Evolution of antibody immunity to SARS-CoV-2. Nature. 2021;591:639–644. doi: 10.1038/s41586-021-03207-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yonker L.M., Gilboa T., Ogata A.F., et al. Multisystem inflammatory syndrome in children is driven by zonulin-dependent loss of gut mucosal barrier. J. Clin. Invest. 2021;131 doi: 10.1172/JCI149633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schwab I., Nimmerjahn F. Intravenous immunoglobulin therapy: how does IgG modulate the immune system? Nat. Rev. Immunol. 2013;13:176–189. doi: 10.1038/nri3401. [DOI] [PubMed] [Google Scholar]
- 24.Zhu P.Y., Shamie I., Lee C.J., et al. Immune response to intravenous immunoglobulin in patients with Kawasaki disease and MIS-C. J. Clin. Invest. 2021;131 doi: 10.1172/JCI147076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ricke D.O. Two different antibody-dependent enhancement (ADE) risks for SARS-CoV-2 antibodies. Front. Immunol. 2021;12 doi: 10.3389/fimmu.2021.640093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu Y., Soh W.T., Kishikawa J.I., et al. An infectivity-enhancing site on the SARS-CoV-2 spike protein targeted by antibodies. Cell. 2021;184:3452–3466. doi: 10.1016/j.cell.2021.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McArdle A.J., Cunnington A.J., Levin M. Therapy for multisystem inflammatory syndrome in children. N. Engl. J. Med. 2021;385 doi: 10.1056/NEJMc2111096. [DOI] [PubMed] [Google Scholar]
- 28.Shearer W.T., Rosenblatt H.M., Gelman R.S., et al. Lymphocyte subsets in healthy children from birth through 18 years of age: the pediatric AIDS Clinical Trials Group P1009 study. J. Allergy Clin. Immunol. 2003;112:973–980. doi: 10.1016/j.jaci.2003.07.003. [DOI] [PubMed] [Google Scholar]
- 29.van Gent R., van Tilburg C.M., Nibbelke E.E., et al. Refined characterization and reference values of the pediatric T- and B-cell compartments. Clin. Immunol. 2009;133:95–107. doi: 10.1016/j.clim.2009.05.020. [DOI] [PubMed] [Google Scholar]
- 30.Garcia-Prat M., Álvarez-Sierra D., Aguiló-Cucurull A., et al. Extended immunophenotyping reference values in a healthy pediatric population. Cytometry B Clin. Cytom. 2019;96:223–233. doi: 10.1002/cyto.b.21728. [DOI] [PubMed] [Google Scholar]
- 31.Ding Y., Zhou L., Xia Y., et al. Reference values for peripheral blood lymphocyte subsets of healthy children in China. J. Allergy Clin. Immunol. 2018;142:970–973. doi: 10.1016/j.jaci.2018.04.022. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material