Abstract
Objectives
A higher risk of adverse pregnancy outcomes is associated with SARS-CoV-2 infection; this could be partially explained by an altered placental function. Because histopathology is often unspecific, we aimed to assess placental weight, birthweight/placental weight (b/p) ratio, and the metabolic scaling exponent ß, an indicator of normal fetal-placental growth, to analyze placental function.
Methods
We included 153 singleton pregnancies with SARS-CoV-2–positive PCR result who delivered at three referring hospitals in Switzerland. Placental weight and b/p ratio were compared to published reference charts. Logistic regression analysis investigated the role of time of infection and other confounding factors on placental weight. The scaling exponent β was compared to the reference value of 0.75.
Results
Placental weight was inferior or equal to the tenth centile in 42.5% (65 of 153) and to the third centile in 19% (29 of 153) of the cases. The risk of low placental weight was not influenced by the trimester in which infection occurred. The b/p ratio was >50th centile in 80.4% (123 of 153) of the cases. The incidence of foetal growth restriction, preeclampsia, and gestational diabetes was 11.8% (18 of 153), 3.3% (5 of 153), and 19.6% (30 of 153). Linear regression modelling revealed a pathologic metabolic scaling exponent β of 0.871 ± 0.064 (R2 = 0.56).
Discussion
SARS-CoV-2 infection during pregnancy was associated with a higher incidence of low placental weight, an increased b/p ratio, and an abnormal scaling exponent β in our cohort. This could be particularly relevant for the still controversial issue of an increased stillbirth rate in SARS-CoV-2 infection during pregnancy. In this regard, intensified foetal surveillance should be mandatory in these pregnancies.
Keywords: Birth weight/placental weight ratio, COVID-19, Metabolic scaling exponent β, Placental weight, SARS-CoV-2
Introduction
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) was first identified in Wuhan, China, in December 2019 and since has caused a major global health crisis [1]. In its severe forms, SARS-CoV-2 infection can trigger a hyperinflammatory response, leading to a complex, immune-mediated disorder [2]. It is rational to believe that COVID-19, as a highly immunogenic viral infection, may interfere with the regular course of pregnancy and that pregnant women could be highly susceptible to a more severe course of the disease [2]. Indeed, recent systematic reviews report more severe outcomes in pregnant women with symptomatic SARS-CoV-2 infection, as well as a higher risk of being admitted to the intensive care unit and requiring invasive ventilation [[3], [4]]. The risk for adverse neonatal outcomes also seems to be elevated [5]. Moreover, the risk of stillbirth seems to be increased, as reported by a British study including more than 340 000 women [5].
To date, it is unclear to what extent placental damage could be responsible for adverse maternal and neonatal outcome in these pregnancies. Although a fair amount of attention has been focused on this topic, reports are inconclusive. Several studies report histopathological alterations, such as the presence of intervillous thrombi and placental infarcts as an expression of maternal vascular malperfusion after SARS-CoV-2 infection [[6], [7], [8], [9]]. The presence of a robust inflammatory response at the maternal-foetal interface and possible associations with long-term neurocognitive impairment in children after immune activation in the placenta have also been discussed [10]. Nevertheless, histopathological findings are still conflicting, and several studies report no differences between placentas originating from SARS-CoV-2–infected mothers and controls [11,12].
Maternal viral infection can be associated with placental alterations, such as lymphoplasmacytic villitis after cytomegalovirus infection and intervillositis after Zika or Dengue virus infection [6]. Expression of angiotensin-converting enzyme 2 in the placenta offers a potential entry mechanism for SARS-CoV-2, yet vertical transmission seems to be exceptionally rare [[7], [8], [9]]. Beyond the inconsistency of data lies one certainty: No histopathological footprint in association with SARS-CoV-2 has been found, and the described changes can be associated with other pregnancy-related pathologies such as hypertensive complications [13,14].
A healthy placenta is a prerequisite for appropriate foetal growth and development, and alterations at its level may cause hypoxia and impairment of various transport systems such as glucose and amino acids transport, which may have short- and long-term consequences for the foetus [15].
Birthweight/placental weight ratio (b/p ratio), also defined as gram foetus per gram placenta, reflects a marker of placental efficiency. A high b/p ratio seems to be associated with adverse obstetrical outcome such as foetal distress, meconium-stained amniotic fluid, or hyperbilirubinemia [16,17]. In mice, small placentas have been shown to upregulate placental transport systems to prevent foetal growth restriction [16,17]. An elevated b/p ratio could be a marker for increased nutrient transfer to the foetus, which despite its normal weight seems to be at risk by outgrowing its placenta [16,17].
A similar approach to assess placental efficacy is to calculate the metabolic scaling exponent ß, which reflects the fractal structure of the placental vasculature [18]. This model proposes an explanation for how the placenta translates into foetal mass, thus metabolism into organism [18].
Given the inconsistency of data regarding placental histopathology after SARS-CoV-2 infection during pregnancy, we aimed to follow the more basic approach of assessing placental function by assessing its weight, calculating the b/p ratio, and analyzing whether the scaling exponent β in our population is close to 0.75, which would be congruent with optimal placental metabolic efficiency.
Methods
We included prospective data originating from singleton pregnancies between 24 and 44 weeks of gestation affected by SARS-CoV-2 infection who delivered between May 2020 and July 2021 at three referring hospitals in Switzerland, irrespective of maternal symptoms. Infection was diagnosed with evidence of SARS-CoV-2 RNA in real-time PCR of nasopharyngeal swabs. The weight of wet, untrimmed placentas was assessed in a standard manner within minutes after delivery, after removal of blood clots.
Written consent was obtained from all women for use of COVID-19–related data. Institutional review board approval from the Cantonal Ethical Committees of Bern, Lausanne, and Lugano was obtained. The study was performed in accordance with the principles of the Declaration of Helsinki.
Statistical analysis was performed with GraphPad Prism version 8.0 for Windows, (GraphPad Software, San Diego CA). Independent-sample Student's t-test was used to compare continuous variables. Proportions were analyzed by Fisher's exact test or the χ2 test where appropriate. Spearman rank correlation and linear logistic regression were used to assess the relationship between gestational age, birthweight, and placental weight. Placental weight scale and percentiles were calculated according to Thompson et al. [19]. A case-control analysis comparing low to normal placental weight was performed, considering low placental weight as inferior or equal to the third and tenth percentile, respectively. A p value of <0.05 was considered significant.
For multivariate analysis, covariates were considered in the regression model if the univariate analysis showed a difference between groups with a p value of <0.25.
To verify the foetal-placental scaling exponent ß, the metabolic scaling equation was applied and fitted as described by Salafia et al. [18]. Because human neonatal birthweight does not scale linearly with the placental weight but follows the rules of the allometric metabolic scaling model described by Keiber's law and Ahern's adaptation for the feto-placental unit [20,21] we considered following formula:
| Placental weight = α (Birthweight)β |
which reveals the relationship between placental weight and birthweight, under the hypothesis that the placenta and the foetus interact like a fractal supply system [18]. We considered as reference the β value close to the value of 0.75, which has been previously described as normal in allometric metabolic studies in singleton pregnancies [18,[20], [21], [22]]. Briefly, Ahern's power function relationship—Placental weight = α(Birthweight)β—was transformed in a linear form by applying the natural logarithm to both sides: Ln (Placental weight) = Lnα+ß∗Ln (Birthweight). The data were then fitted by ordinary linear least-square regression using the curve-fitting tool of the statistical software.
Results
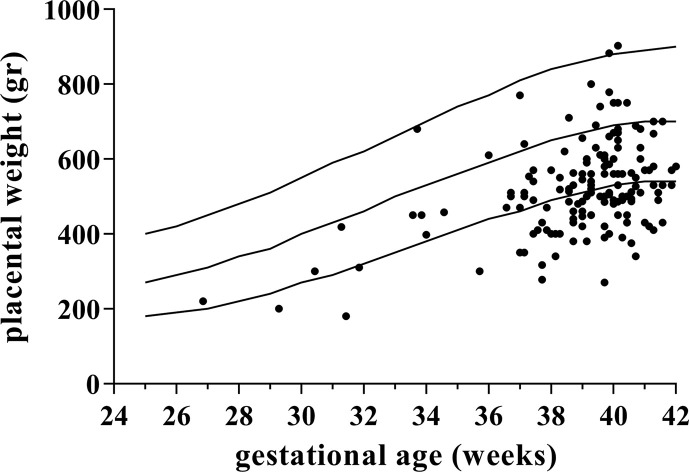
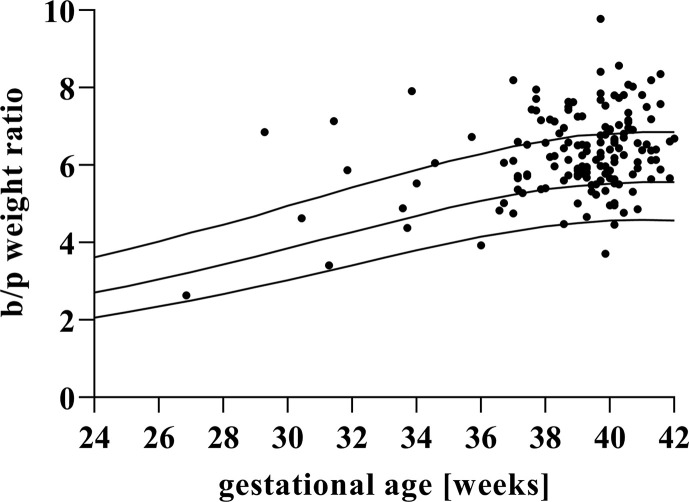
During the study period, 153 placentas from pregnancies affected by SARS-CoV-2 infection were included. Baseline characteristics are described in Table 1 . Placental weight was inferior or equal to the tenth centile in 42.5% (65 of 153) and inferior or equal to the third centile in 19% (29 of 153) of the cases (Fig. 1 ). The b/p ratio was >50th centile in 80.4% (123 of 153) of the cases and >90th centile in 31.37% (48 of 153) of the cases (Fig. 2 ). Linear regression modelling of the analyzed population revealed a metabolic scaling exponent β of 0.871 ± 0.064 (R2 = 0.56) and Ln (Placental weight) = –0.786 + 0.871∗Ln (Birthweight) (Fig. 3 ).
Table 1.
Clinical characteristics and outcomes of the study population
| Characteristics | Values |
|---|---|
| Body mass index (kg/m2) | 24.8 (22.2–29.4) |
| Parity | 2 (1–2) |
| Gestational age at delivery (wk) | 38.8 ± 2.71 |
| Preterm deliverya | 16 (10.45%) |
| Gestational diabetes | 30 (19.6%) |
| Preeclampsia | 5 (3.3%) |
| Fetal growth restrictionb | 18 (11.76%) |
| Gestational age at infection (wk) | 31 (26–37) |
| Birthweight (g) | 3206.57 ± 637.49 |
| Placental weight (g) | 520.42 ± 124.81 |
Values are shown as median (range), number (%), or mean ± standard deviation where appropriate.
<37 weeks of gestation.
Fetal growth restriction defined by abdominal circumference <5th percentile/foetal weight <10th percentile with altered hemodynamic or abnormal growth trajectory.
Fig. 1.
Placental weights (black dots) plotted on reference ranges derived from Thompson et al. [19]. The lines represent the 10th, 50th, and 90th percentile for gestational age.
Fig. 2.
Birthweight/placental weight ratio (b/p weight ratio). Placental weights (black dots) plotted on reference ranges derive from Thompson et al. [19]. The lines represent the 10th, 50th, and 90th percentile for gestational age.
Fig. 3.
Relationship between birthweight and placental mass. Fitted straight line to natural logarithms (LN) of birthweight (BW) and placental weight (PW).
Trimester 1, 2, and 3 were defined as conception up to 11 + 6 weeks, between 12 and 23 + 6 weeks, and >24 weeks of gestation, respectively. In 62.1% (95 of 153) of cases, infection occurred in the third trimester of gestation. In 29.41% (45 of 153) and 4.58% (7 of 153) of cases, infection occurred in the second and first trimester, respectively. In 3.92% (6 of 153) of cases, the time point of infection was unknown. Univariate logistic regression revealed that the risk of low placental weight was not significantly different between each trimester of infection after OR analysis for the third and tenth centile, adjusted for trimester of infection and multiparty status.
In multivariate logistic regression analysis considering adjusted OR on available covariates (body mass index >35 kg/m2, ethnicity, tobacco consumption, multiparty, gestational diabetes, pregestational diabetes, and preeclampsia), multiparty was the only significant factor negatively associated with low placental weight defined as inferior or equal to the tenth percentile (OR 0.49; 95% CI, 0.25–0.97; p = 0.034) (Table 2 ).
Table 2.
Clinical characteristics of the study population dichotomized between placental weight ≤10th and >10th centile for gestational age
| Characteristics | Placental weight |
p | |
|---|---|---|---|
| ≤10th centile (n = 65; 42.5%) |
>10th centile (n = 88; 57.5%) |
||
| Body mass index (kg/m2) | 24.0 (22–28.1) | 25.6 (22.2–31.2) | ns |
| Tobacco consumption (%) | 2 (3.1%) | 4 (4.5%) | ns |
| Parity | 2 (1–2) | 2 (1–3) | ns |
| Gestational diabetes (%) | 12 (18.5%) | 18 (20.5%) | ns |
| Preeclampsia (%) | 2 (3.1%) | 3 (3.4%) | ns |
| Gestational age at infection (wk) | 31 (26–37) | 30 (22–37) | ns |
| Gestational age at delivery (wk) | 39 (38–40) | 39 (37–40) | ns |
Values are shown as median (range) or number (%) where appropriate. ns, not significant.
The incidence of foetal growth restriction (FGR), defined as a foetal weight <10th centile for gestational age, was 11.8% (18 of 153), whereas 3.3% (5 of 153) of cases were complicated by preeclampsia. Gestational diabetes was present in 19.6% (30 of 153) of the cases (Table 1).
After infection in the first trimester, no pregnancy was complicated by FGR (0 of 7). Of pregnancies where infection occurred in the second trimester, 13.33% (6 of 45) resulted in FGR, vs 9.5% (9 of 95) after infection in the third trimester. In three cases of FGR (2%), the time point of infection was unknown.
Discussion
The main finding of our study was the increased incidence of low placental weight after SARS-CoV-2 infection during pregnancy. This finding contrasts with the predominantly normal birth weight of the corresponding neonates and leads to an elevated b/p ratio in our cohort. Furthermore, we found a distinctively higher value of the metabolic scaling exponent β than expected in normal singleton pregnancies.
Understanding the possible implications of a pathologically altered b/p ratio is important for further interpretation of these findings. A low b/p ratio is commonly found in FGR foetuses, where both placental and foetal weight are low [17]. In contrast, the combination of small placenta and normal-sized foetus seems to be a sign of upregulated nutrient transfer capacity in an apparently normal pregnancy, where the resulting normal foetal weight possibly masks an altered placenta/foetus dyad.
One of the largest studies investigating the meaning of the b/p ratio relies on data from over 500 000 singleton deliveries in Norway [23]. The authors analyzed the relative risk of foetal death in the lowest and highest b/p ratio quartiles for both preterm and term deliveries in their population. In the preterm group, in both the lowest and highest b/p ratio quartiles, the odds ratio for foetal death was increased, whereas at term, an elevated risk for foetal demise was found in the highest quartile. This finding is highly relevant and suggests that a small placenta as related to foetal size could be a risk factor for foetal death at term.
By relating these findings to our cohort, this could indicate that presumably low-risk foetuses are actually at high risk and that SARS-CoV-2 could act as a promoter for the destabilization of the placental/foetal dyad in these pregnancies.
Data on placental weight after maternal SARS-CoV-2 infection are limited, as most publications focus on histopathological and immunohistochemical examinations. One report documents a rate of 66.7% placentas <10th centile (n = 20), which is in line with our findings but nevertheless associated with an accordingly high prevalence of FGR [12].
As previously mentioned, there are preliminary data linking foetal death to SARS-CoV-2 infection during pregnancy. The mechanism behind these findings is not yet elucidated, and so far, placental inflammation and/or generic consequences of maternal illness in pregnancy were invoked as a potential cause [5]. Our data also cannot fully explain the underlying mechanism, but they raise further concerns regarding these insights. Given the scenario of increased risk for foetal demise in relation with an elevated b/p ratio, we believe it is legitimate to be concerned about the stability of placental function in COVID-19 during pregnancy.
We used as reference placental weight charts for singleton pregnancies published by Thomson et al. in 2007, which are based on data originating from 231 806 deliveries from Norway [19]. To our knowledge, these are the largest published placental weight reference charts originating from a population similar to ours in terms of ethnic distribution, gestational age, and type of pregnancy (multiple pregnancies have been excluded from our analysis, to best fit the reference curves). In the reference population, 85.7% of the women were born in Norway; thus, the ethnicity can be extrapolated to our Swiss, predominantly Caucasian population, as opposed to other placental weight curves available, where an important percent of the population was of African (>80%) or Asian (>95%) ethnicity [24,25]. Furthermore, the reference values were generated by using outcomes from pregnancies between 24 and 44 weeks of gestation, identical to our cohort. Although Swiss reference curves for placental weight are also available, these are based on a population starting at 37 weeks of gestation and derive from a significantly lower cohort, thus our decision in favour of the Scandinavian curves [19,26]. Given the size and characteristics of the available references, we consciously decided against a case-control approach. We are aware that some may regard this as a weak point of our analysis; thus, we intended to counterbalance it with the calculation of the scaling exponent β in our population.
On this note, we found a distinctively higher value of the scaling exponent β than expected in normal singleton pregnancies. A higher value of β correlates with a newborn weight lower than that predicted by Kleiber's metabolic scaling law [18]. It is assumed that a deviation from a β ˜ 0.75 may reflect decreased metabolic efficiency of the placenta, as β reflects the fractal structure of the placental vasculature [18]. This correlates with the elevated b/p ratio.
Looking beyond the presumed risk for foetal death associated with an elevated b/p ratio, there is evidence linking placental growth and metabolism to the development of chronic diseases in later life. Placental weight seems to play a critical role in foetal programming, without necessarily influencing size at birth [27,28]. This may occur due to developmental plasticity, where adaptation to a low transplacental supply of nutrients can influence the long-term development of the offspring on an epigenetic basis [28].
Our study shows no significant difference in the risk of low placenta weight in pregnant women infected by SARS-CoV-2 across trimester of infection. This finding of an apparent lack of association between early infection during pregnancy and the incidence of placental insufficiency is somewhat anticyclical, as the expression of angiotensin-converting enzyme 2 and transmembrane protease serine-2 receptors in the placenta are highest in the first trimester of pregnancy [29]. Given the low absolute number of cases with FGR in our cohort, we believe it is not possible to draw any solid conclusion relying on these data.
The incidence of preterm delivery and FGR in our cohort were both in line with the Swiss incidence of these adverse pregnancy outcomes (Table 1). In only four cases (2.61%), both FGR and premature delivery were present, so an association between the two cannot be clearly stated.
Our study is the only one to date describing a relevant association between low placental weight and an altered metabolic scaling value in pregnancies complicated by SARS-CoV-2 infection, without cofounding factors such as FGR. The main strengths of the study are its prospective and multicentric nature. For the moment, it is still speculative to assert that SARS-CoV-2 alone may be responsible for these findings, the size of the cohort being the main limitation of our study. A further limitation is not being able to assess severity of disease in all patients, owing to data inconsistency; thus, it was not considered in multivariate logistic regression.
It seems urgent to continue research on placental defensive and potentially altered adaptive mechanisms during infection, in particular after SARS-CoV-2 viral infection. Analyzing placental weight, b/p ratio, and the scaling exponent ß may offer additional clues to understand the processes at the maternal-placental interface.
A healthy, normal-sized, and adequately functioning placenta is not only important for the direct outcome of the pregnancy, but is likely to provide lifetime benefits for the offspring [27,28]. Our study reveals that the maternal-foetal unit could be at risk for placental-related impairment after SARS-CoV-2 infection during pregnancy, and we believe that intensified foetal surveillance should be mandatory in these cases [30].
Transparency declaration
The authors report no conflict of interest and no funding.
Author contributions
A-PR: conception and design of the study, acquisition of data, analysis and interpretation of data, drafting the article; DB: acquisition of data, analysis and interpretation of data, revising the article critically for important intellectual content; GF: acquisition of data, analysis and interpretation of data; AP: acquisition of data, revising the article critically for important intellectual content; DS: acquisition of data, revising the article critically for important intellectual content; MB: analysis and interpretation of data; LR: conception and design of the study, analysis and interpretation of data, revising the article critically for important intellectual content.
Editor: L. Leibovici
References
- 1.Sohrabi C., Alsafi Z., O'Neill N., Khan M., Kerwan A., Al-Jabir A., et al. World Health Organization declares global emergency. A review of the 2019 novel coronavirus (COVID-19) Int J Surg. 2020;76:71–76. doi: 10.1016/j.ijsu.2020.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mor G., Aldo P., Alvero A. The unique immunological and microbial aspects of pregnancy. Nat Rev Immunol. 2017;17:469–482. doi: 10.1038/nri.2017.64. [DOI] [PubMed] [Google Scholar]
- 3.Elsaddig M., Khalil A. Effects of the COVID pandemic on pregnancy outcomes. Best Pract Res Clin Obstet Gynaecol. 2021;73:125–136. doi: 10.1016/j.bpobgyn.2021.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Allotey J., Stallings E., Bonet M., Yap M., Chatterjee S., Kew T., et al. Clinical manifestations, risk factors, and maternal and perinatal outcomes of coronavirus disease 2019 in pregnancy: living systematic review and meta-analysis. BMJ. 2020;370:m3320. doi: 10.1136/bmj.m3320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gurol-Urganci I., Jardine J.E., Carroll F., Draycott T., Dunn F., Fremeaux A., et al. Maternal and perinatal outcomes of pregnant women with SARS-CoV-2 infection at the time of birth in England: national cohort study. Am J Obstet Gynecol. 2021;225:e1–e11. doi: 10.1016/j.ajog.2021.05.016. 522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shanes E.D., Mithal L.B., Otero S., Azad H.A., Miller E.S., Goldstein J.A. Placental pathology in COVID-19. Am J Clin Pathol. 2020;154:23–32. doi: 10.1093/ajcp/aqaa089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gengler C., Dubruc E., Favre G., Greub G., de Leval L., Baud D. SARS-CoV-2 ACE-receptor detection in the placenta throughout pregnancy. Clin Microbiol Infect. 2021;27:489–490. doi: 10.1016/j.cmi.2020.09.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jaiswal N., Puri M., Agarwal K., Singh S., Yadav R., Tiwary N., et al. COVID-19 as an independent risk factor for subclinical placental dysfunction. Eur J Obstet Gynecol Reprod Biol. 2021;259:7–11. doi: 10.1016/j.ejogrb.2021.01.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hecht J.L., Quade B., Deshpande V., Mino-Kenudson M., Ting D.T., Desai N., et al. SARS-CoV-2 can infect the placenta and is not associated with specific placental histopathology: a series of 19 placentas from COVID-19-positive mothers. Mod Pathol. 2020;33:2092–2103. doi: 10.1038/s41379-020-0639-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lu-Culligan A., Chavan A.R., Vijayakumar P., Irshaid L., Courchaine E.M., Milano K.M., et al. SARS-CoV-2 infection in pregnancy is associated with robust inflammatory response at the maternal-fetal interface. medRxiv. 2021 doi: 10.1016/j.medj.2021.04.016. [Preprint] 2021.01.25.21250452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gao L., Ren J., Xu L., Ke X., Xiong L., Tian X., et al. Placental pathology of the third trimester pregnant women from COVID-19. Diagn Pathol. 2021;16:8. doi: 10.1186/s13000-021-01067-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.He M., Skaria P., Kreutz K., Chen L., Hagemann I.S., Carter E.B., et al. Histopathology of third trimester placenta from SARS-CoV-2-positive women. Fetal Pediatr Pathol. 2020:1–10. doi: 10.1080/15513815.2020.1828517. [DOI] [PubMed] [Google Scholar]
- 13.Bustamante Helfrich B., Chilukuri N., He H., Cerda S.R., Hong X., Wang G., et al. Maternal vascular malperfusion of the placental bed associated with hypertensive disorders in the Boston Birth Cohort. Placenta. 2017;52:106–113. doi: 10.1016/j.placenta.2017.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Weiner E., Feldstein O., Tamayev L., Grinstein E., Barber E., Bar J., et al. Placental histopathological lesions in correlation with neonatal outcome in preeclampsia with and without severe features. Pregnancy Hypertens. 2018;12:6–10. doi: 10.1016/j.preghy.2018.02.001. [DOI] [PubMed] [Google Scholar]
- 15.Illsley N.P., Baumann M.U. Human placental glucose transport in fetoplacental growth and metabolism. Biochim Biophys Acta Mol Basis Dis. 2020;1866:165359. doi: 10.1016/j.bbadis.2018.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Salavati N., Gordijn S.J., Sovio U., Zill-E-Huma R., Gebril A., Charnock-Jones D.S., et al. Birth weight to placenta weight ratio and its relationship to ultrasonic measurements, maternal and neonatal morbidity: a prospective cohort study of nulliparous women. Placenta. 2018;63:45–52. doi: 10.1016/j.placenta.2017.11.008. [DOI] [PubMed] [Google Scholar]
- 17.Hayward C.E., Lean S., Sibley C.P., Jones R.L., Wareing M., Greenwood S.L., et al. Placental adaptation: what can we learn from birthweight: placental weight ratio? Front Physiol. 2016;7:28. doi: 10.3389/fphys.2016.00028. PMID: 26903878; PMCID: PMC4742558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Salafia C.M., Misra D.P., Yampolsky M., Charles A.K., Miller R.K. Allometric metabolic scaling and fetal and placental weight. Placenta. 2009;30:355–360. doi: 10.1016/j.placenta.2009.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Thompson J.M., Irgens L.M., Skjaerven R., Rasmussen S. Placenta weight percentile curves for singleton deliveries. BJOG. 2007;114:715–720. doi: 10.1111/j.1471-0528.2007.01327.x. [DOI] [PubMed] [Google Scholar]
- 20.Kleiber M. Body size and metabolism. Hilgardia. 1932;6:315–353. [Google Scholar]
- 21.Gruenwald P. University Park Press; Baltimore: 1975. The placenta and its maternal supply line: effects of insufficiency on the fetus. [Google Scholar]
- 22.Baumann M.U., Marti M., Durrer L., Koumoutsakos P., Angelikopoulos P., Bolla D., et al. Placental plasticity in monochorionic twins: impact on birth weight and placental weight. Placenta. 2015;36:1018–1023. doi: 10.1016/j.placenta.2015.07.120. [DOI] [PubMed] [Google Scholar]
- 23.Haavaldsen C., Samuelsen S.O., Eskild A. Fetal death and placental weight/birthweight ratio: a population study. Acta Obstet Gynecol Scand. 2013;92:583–590. doi: 10.1111/aogs.12105. [DOI] [PubMed] [Google Scholar]
- 24.Dombrowski M.P., Berry S.M., Johnson M.P., Saleh A.A., Sokol R.J. Birth weight-length ratios, ponderal indexes, placental weights, and birth weight-placenta ratios in a large population. Arch Pediatr AdolescMed. 1994;148:508–512. doi: 10.1001/archpedi.1994.02170050066012. [DOI] [PubMed] [Google Scholar]
- 25.Ogawa M., Matsuda Y., Nakai A., Hayashi M., Sato S., Matsubara S. Standard curves of placental weight and fetal/placental weight ratio in Japanese population: difference according to the delivery mode, fetal sex, or maternal parity. Eur J Obstet Gynecol Reprod Biol. 2016;206:225–231. doi: 10.1016/j.ejogrb.2016.09.004. [DOI] [PubMed] [Google Scholar]
- 26.Burkhardt T., Schäffer L., Schneider C., Zimmermann R., Kurmanavicius J. Reference values for the weight of freshly delivered term placentas and for placental weight-birth weight ratios. Eur J Obstet Gynecol Reprod Biol. 2006;128:248–252. doi: 10.1016/j.ejogrb.2005.10.032. [DOI] [PubMed] [Google Scholar]
- 27.Barker D.J. In utero programming of chronic disease. Clin Sci (Lond) 1998;95:115–128. [PubMed] [Google Scholar]
- 28.Barker D.J., Bull A.R., Osmond C., Simmonds S.J. Fetal and placental size and risk of hypertension in adult life. BMJ. 1990;301 doi: 10.1136/bmj.301.6746.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bloise E., Zhang J., Nakpu J., Hamada H., Dunk C.E., Li S., et al. Expression of severe acute respiratory syndrome coronavirus 2 cell entry genes, angiotensin-converting enzyme 2 and transmembrane protease serine 2, in the placenta across gestation and at the maternal-fetal interface in pregnancies complicated by preterm birth or preeclampsia. Am J Obstet Gynecol. 2021;224:e1–e8. doi: 10.1016/j.ajog.2020.08.055. 298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vouga M., Favre G., Martinez-Perez O., Pomar L., Forcen Acebal L., Abascal-Saiz A., et al. Maternal outcomes and risk factors for COVID-19 severity among pregnant women. Sci Rep. 2021;11:13898. doi: 10.1038/s41598-021-92357-y. [DOI] [PMC free article] [PubMed] [Google Scholar]