Abstract
Background
Healthcare workers (HCWs) exposed to coronavirus 19 (COVID-19) are at high risk of developing mental health concerns across several domains. The aim of this study is to determine the updated, global frequency of these outcomes.
Methods
A multistep literature search was performed from database inception until March 1, 2021. PRISMA/MOOSE-compliant systematic review and PROSPERO protocol were used to identify studies reporting on depression, anxiety, acute stress, post-traumatic symptoms, insomnia, and burnout in HCWs exposed to COVID-19. A quantitative meta-analysis with random effects was conducted to analyze the proportion rate of the mental health disorders. Sensitivity analyses were performed to investigate the effect of the different continents and scales. Meta-regression analyses were conducted to examine the effect of gender, age, and work position.
Results
239 articles were included (n = 271,319 HCWs, mean age = 36.08 ± 8.33 (66.99% female). 33% HCWs exposed to COVID-19 reported depressive symptoms (95% confidence intervals [CI] = 28–38%), 42% anxiety features (95% CI = 35–48), 40% acute stress (95% CI = 32–47), 32% post-traumatic symptoms (95% CI = 26–37%), 42% insomnia (95% CI = 36–48), 37% burnout (95% CI = 31–42). Sensitivity analyses did not show statistically significant differences. Meta-regressions found a statistically significant lower prevalence of post-traumatic symptoms in Asia.
Conclusions
HCWs exposed to COVID-19 were found to have a significant prevalence of mental health concerns in all domains analyzed. The effects of COVID-19 on HCWs’ mental health could be underestimated and the future consequences dismissed.
Keywords: Coronavirus, COVID-19, healthcare workers, mental health
Introduction
On December 31, 2019, the WHO warned of the first cases of pneumonia caused by a new coronavirus in the city of Wuhan [1]. As of September 1, 2021, the disease caused by this virus (the COVID-19) has infected more than 215 million people worldwide and caused 4.5 million deaths, thus being considered a global pandemic [2].
Large outbreaks such as the one caused by COVID-19 place healthcare workers (HCWs) in a position of particular vulnerability [3]. HCWs are not only one of the groups most at risk of being infected by COVID-19 [4], but they are exposed to a huge workload [5], the absence of adequate protective equipment [6] and the extensive media coverage [7,8]. Routine clinical practice has been significantly changed, and many professionals have been removed from their usual workplace and redirected to higher-risk frontline works while also having to adhere to continuously changing guidelines [9].
The literature published during the SARS and MERS pandemics more than a decade ago suggests that HCWs present, due to all the above, an increased risk of suffering adverse mental health effects in pandemic situations, including anxiety, depression, and post-traumatic stress symptoms [10–14]. In line with these results, recently numerous scientific articles have been published on this subject. Most of these studies and reviews, however, focus on one or few mental health domains or offer results from very specific populations, either in terms of geographical origin (mainly from mainland China) or professional category and medical specialty [15–18].
No updated meta-analyses analyze the effects of the COVID-19 pandemic on the different domains of mental health impact in HCWs worldwide, including depression, anxiety, burnout, acute stress, post-traumatic symptoms, and insomnia. Therefore, the aim of this study is to synthesize the available scientific evidence about the state of mental health of HCWs during the COVID-19 pandemic.
Methods
This study protocol was registered on PROSPERO (registration number: CRD42021247610). The study was conducted in accordance with “Preferred Reporting Items for Systematic Reviews and Meta-Analyses” (PRISMA) [19], (Supplementary Table S1) and “Meta-analyses of Observational Studies in Epidemiology” (MOOSE) checklist [20] (Supplementary Table S2), following “EQUATOR Reporting Guidelines” [21].
Search strategy and selection criteria
A systematic literature search was carried out by two independent researchers (C.A. and B.P.). Web of Science database (Clarivate Analytics) was searched, incorporating the Web of Science Core Collection, the BIOSIS Citation Index, the KCI-Korean Journal Database, MEDLINE®, the Russian Science Citation Index, and the SciELO Citation Index as well as Cochrane Central Register of Reviews, and Ovid/PsycINFO databases, from inception until March 1, 2021.
The following keywords were used: “CoV-19” OR “SARS-CoV-2” OR “2019 nCoV” OR “2019nCoV” OR “2019 novel coronavirus” OR “new coronavirus” OR “novel coronavirus” OR “SARS CoV-2” OR “Wuhan coronavirus” OR “COVID 19” OR “2019-nCoV” AND “professionals” OR “worker*” OR “doctor*” OR “nurse*” OR “occupation*” OR “employee*” OR “healthcare provider*” OR “healthcare worker*” OR “healthcare employee*” OR “personnel” OR “emergency worker” OR “paramedic*”.
Articles identified were first screened as abstracts, and after the exclusion of those which did not meet the inclusion criteria, the full texts of the remaining articles were assessed for eligibility and inclusion.
Inclusion criteria for the systematic review and meta-analysis were (a) individual studies with original data, (b) focusing on HCWs exposed to COVID-19 (defined as HCWs who have been working during COVID-19 pandemic tending to patients potentially infected with SARS-COV-2, but not necessarily limited toHCWs working in frontline units), (c) reporting meta-analyzable proportions about mental health outcomes included in at least one of the following categories: anxiety, depression, acute stress/distress, post-traumatic symptoms, burnout, and sleep disturbances, (d) using validated, structured, evaluation scales, (e) nonoverlapping samples (overlap was determined by looking at the inclusion dates, type of population and country in which the study was carried out, and the study with the largest sample was then selected), (f) sample size ≥50 participants, and (g) written in English. Exclusion criteria were (a) reviews, clinical cases, study protocols or qualitative studies, conferential proceedings, letters, and commentaries, (b) reporting outcomes on populations other than HCWs, including the general population, medical and nursing students, dentists, and podologists.
Data extraction
Three researchers (J.L.P., M.L., and J.H.) independently extracted data from all the included studies. The three databases were then cross-checked, and discrepancies were resolved through consensus under the supervision of a senior researcher (A.C.). A summary of selected variables included: first author and year of publication, country and city, HCW category involved, sample size, age (mean ± standard deviation [SD]), sex (% female), mental health domain studied, evaluation tool used, quality assessment (see below), and key findings.
Risk of bias (quality) assessment
Risk of bias was assessed using a modified version of the Newcastle–Ottawa Scale (NOS) for assessing the quality of nonrandomized studies due to the heterogeneity expected in the included studies [22] (Supplementary Methods S1).
Strategy for data synthesis
First, we provided a systematic synthesis (Supplementary Table S4) of the findings from the included studies structured around the selected six mental health outcomes: anxiety, depression, acute stress/distress, post-traumatic symptoms, burnout, and sleep disturbances. Second, we performed meta-analyses using, as primary effect size, the proportion (% and standard error [SE], when available) of mental health outcomes in HCWs exposed to COVID-19.
Meta-regressions were performed to determine the effect of the (a) sex, (b) age, and (c) NOS score on the mental health domains. Sensitivity analyses were conducted to estimate the association between the mental health domains and (a) continent of the study, (b) type of mental health worker (doctor, nurse, or multi-professional), and (c) used scale.
Heterogeneity among studies was assessed using the Q statistic, with the proportion of the total variability in effect size estimates evaluated using the I2 index (with an I2 > 50% representing significant heterogeneity) [23]. Publication biases were assessed for the proportion of remission or recovery by inspecting funnel plots and assessing Egger’s test [24].
All analyzes were conducted using STATA version 17 [25]. The significance level was set at a p < 0.05, two-sided.
Results
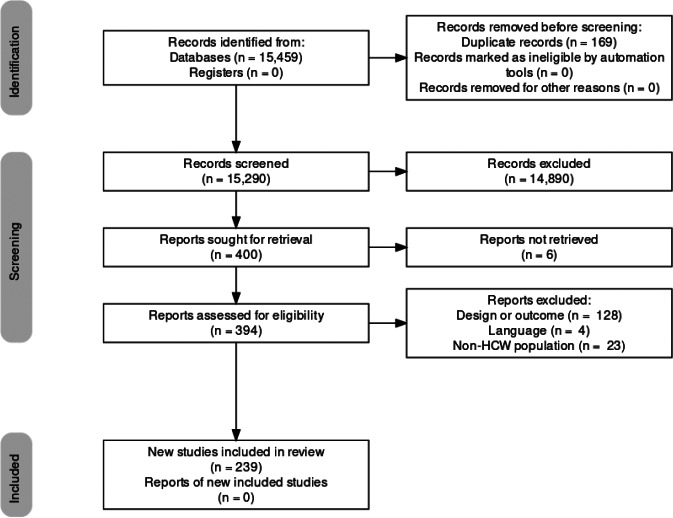
The literature search yielded 15,459 citations through electronic database, which were screened for eligibility; 394 articles were assessed in full text, and 155 were excluded (reasons for exclusion are detailed in Supplementary Table S3). The final database for the systematic review and meta-analysis included 239 studies (Figure 1).
Figure 1.
PRISMA 2009 flow diagram [26].
One hundred and sixty studies (66.95%) focused on depression, 179 (74.90%) on anxiety, 57 (23.85%) on acute stress/distress, 55 (23.01%) on sleep problems, 39 (16.32%) on post-traumatic symptoms, and 24 (10.04%) on burnout. The full sample includes 271,319 HCWs, including articles with sample sizes ranging from 54 to 21,199 HCWs. The mean age of the sample was 36.08 years, ranging from 21 to 55.13 years (SD = 8.33). 66.99% were female. Studies included HCWs from 50 countries in five continents: 150 (62.76%) from Asia, 55 (23.01%) from Europe, 20 (8.37%) from America, 11 (4.60%) from Africa, and 2 (0.84%) from Oceania; there was also one multicontinental study [27].
Depression
Depression prevalence was reported in 160 studies, including a total sample of 210,762 participants. Multiple evaluation scales were used, including Patient Health Questionnaire-9 (PHQ-9) [28], PHQ-2 [29], PHQ-4 [30], Zung Self-Rating Depression Scale (SDS) [31], Hospital Anxiety and Depression Scale (HADS) [32], Beck Depression Inventory (BDI) [33], Center for Epidemiologic Studies Depression Scale (CES-D) [34], and Depression, Anxiety, and Stress Scale-21 (DASS-21) [35]. The pooled prevalence of depression was 0.33 (95% confidence intervals [CI] 0.28–0.38). Prevalence varied widely depending on the scale used, from 0.53 with PHQ-2/4 to 0.26 with CES-D. Detailed results of depression and the other mental health domains are displayed in Table 1. Sensitivity analyses and meta-regressions revealed no statistically significant differences regarding age, gender, NOS score, or continent.
Table 1.
Prevalence of mental health impacts across each of the domains and scales studied.
| Scale | No. Studies | Sample size | Proportion | 95% CI | z Score | p | I 2 (%) |
|---|---|---|---|---|---|---|---|
| Depression | 160 | 210,762 | 0.33 | 0.28–0.38 | 13.36 | 0.00 | 99.95 |
| PHQ9 | 74 | 115,185 | 0.33 | 0.28–0.38 | 11.98 | 0.00 | 99.85 |
| SDS | 12 | 26,207 | 0.38 | 0.10–0.65 | 2.69 | 0.01 | 99.97 |
| HADS | 23 | 12,984 | 0.34 | 0.27–0.42 | 9.40 | 0.00 | 98.85 |
| BDI | 5 | 2,325 | 0.35 | 0.22–0.47 | 5.40 | 0.00 | 96.54 |
| CES-D | 3 | 16,118 | 0.30 | 0.24–0.36 | 9.78 | 0.00 | n.a. |
| DASS-21 | 36 | 23,497 | 0.33 | 0.24–0.42 | 7.42 | 0.00 | 99.87 |
| PHQ2/PHQ4 | 4 | 4,005 | 0.25 | 0.19–0.30 | 8.78 | 0.00 | 91.56 |
| Anxiety | 179 | 206,513 | 0.42 | 0.35–0.48 | 12.35 | 0.00 | 99.94 |
| GAD-7 | 87 | 123,895 | 0.42 | 0.35–0.48 | 12.72 | 0.00 | 99.87 |
| DASS-21 | 32 | 22,698 | 0.37 | 0.29–0.44 | 9.59 | 0.00 | 99.22 |
| HADS | 22 | 14,515 | 0.44 | 0.39–0.50 | 15.41 | 0.00 | 96.25 |
| SAS | 16 | 32,000 | 0.35 | 0.08–0.62 | 2.52 | 0.00 | 99.98 |
| BAI | 4 | 1,569 | 0.34 | 0.11–0.58 | 2.94 | 0.00 | 98.49 |
| STAI-S | 10 | 5,875 | 0.68 | 0.48–0.88 | 6.68 | 0.00 | 99.77 |
| PHQ-4 | 4 | 4,005 | 0.37 | 0.23–0.51 | 5.20 | 0.00 | 98.52 |
| CAS | 4 | 2,018 | 0.47 | 0.27–0.67 | 4.66 | 0.00 | n.a. |
| Acute stress | 57 | 48,042 | 0.40 | 0.32–0.47 | 10.12 | 0.00 | 99.87 |
| SASRQ | 4 | 12,365 | 0.33 | 0.10–0.55 | 2.83 | 0.00 | 99.73 |
| DASS-21 | 32 | 22,561 | 0.26 | 0.21–0.32 | 9.37 | 0.00 | 99.42 |
| PSS | 21 | 13,116 | 0.62 | 0.50–0.73 | 10.35 | 0.00 | 99.67 |
| Post-trauma | 39 | 58,995 | 0.32 | 0.26–0.37 | 11.01 | 0.00 | 99.69 |
| IES-R | 23 | 24,225 | 0.38 | 0.29–0.47 | 8.33 | 0.00 | 99.70 |
| PCL-C | 12 | 26,057 | 0.20 | 0.12–0.28 | 4.94 | 0.00 | 99.53 |
| PC-PTSD | 4 | 8,713 | 0.32 | 0.21–0.43 | 5.77 | 0.00 | 99.07 |
| Insomnia | 55 | 37,068 | 0.42 | 0.36–0.48 | 12.92 | 0.00 | 99.59 |
| ISI | 27 | 23,366 | 0.39 | 0.30–0.47 | 8.96 | 0.00 | 99.66 |
| SQS | 3 | 920 | 0.28 | 0.02–0.54 | 2.11 | 0.04 | n.a. |
| AIS | 6 | 4,079 | 0.42 | 0.33–0.51 | 9.42 | 0.00 | 96.66 |
| PSQI | 19 | 8,703 | 0.49 | 0.37–0.61 | 8.26 | 0.00 | 99.29 |
| Burnout | 25 | 30,873 | 0.37 | 0.31–0.42 | 12.62 | 0.00 | 99.81 |
| MBI-EE | 19 | 26,776 | 0.39 | 0.30–0.48 | 8.90 | 0.00 | 99.50 |
| MBI-DP | 19 | 26,776 | 0.34 | 0.22–0.46 | 5.59 | 0.00 | 99.77 |
| MBI-RPA | 19 | 26,776 | 0.36 | 0.28–0.45 | 8.54 | 0.00 | 99.38 |
| CBI | 3 | 3,238 | 0.53 | 0.52–0.54 | 59.85 | 0.00 | n.a. |
| Mini-Z | 3 | 859 | 0.22 | 0.14–0.30 | 5.31 | 0.00 | n.a. |
Abbreviations: AIS, Athens Insomnia Scale; BAI, Beck Anxiety Inventory; BDI, Beck Depression Inventory; CAS, Coronavirus Anxiety Scale; CBI, Copenhagen Burnout Inventory; CES-D, Center for Epidemiologic Studies Depression Scale; DASS-21, Depression, Anxiety, and Stress Scale-21; GAD-7, Generalized Anxiety Disorder-7; HADS, Hospital Anxiety and Depression Scale; IES-R, Impact of Event Scale—Revised; ISI, Insomnia Severity Index; MBI-DP, Maslach Burnout Inventory—depersonalization; MBI-EE, Maslach Burnout Inventory—emotional exhaustion; MBI-RPA, Maslach Burnout Inventory—reduced personal accomplishment; Mini-Z, Mini-Z Burnout Survey; n.a., not applicable; PC-PTSD, Primary Care—Post Traumatic Stress Disorder Scale; PCL-C, Post Traumatic Stress Disorder Checklist—Civilian Version; PHQ, Patient Health Questionnaire; PSQI, Pittsburgh Sleep Quality Index; SAS, Zung Self-Rating Anxiety Scale; SASRQ, Stanford Acute Stress Reaction Questionnaire; SDS, Zung Self-Rating Depression Scale; SQS, Sleep Quality Scale; STAI-S, State-–Trait Anxiety Inventory—State Subscale.
Anxiety
Anxiety prevalence was reported in 179 studies, including a total sample of 206,513 participants. Multiple evaluation scales were used, including Generalized Anxiety Disorder-7 (GAD-7) [36], DASS-21 [35], HADS [32], Zung Self-Rating Anxiety Scale (SAS) [37], Beck Anxiety Inventory (BAI) [38], State–Trait Anxiety Inventory—State Subscale (STAI-S) [39], PHQ-4 [30], and Coronavirus Anxiety Scale (CAS) [40]. The pooled prevalence of anxiety was 0.42 (95% CI 0.35–0.48). Again, prevalence varied substantially depending on the scale used, from 0.34 with BAI to 0.68 with STAI-S. Sensitivity analyses and meta-regressions did not show statistically significant differences regarding age, gender, NOS score, or continent.
Acute stress
Acute stress prevalence was reported in 57 studies, including a total sample of 48,042 participants. Multiple evaluation scales were used, including Stanford Acute Stress Reaction Questionnaire (SASRQ) [41], DASS-21 [35], and Perceived Stress Scale (PSS) [42]. The pooled prevalence of acute stress was 0.40 (95% CI 0.32–0.47). Prevalence varied from 0.26 as measured with DASS-21 to 0.62 with PSS. Again, sensitivity analyses and meta-regressions revealed no statistically significant differences regarding age, gender, NOS score, continent, or professional category.
Insomnia
Insomnia prevalence was reported in 55 studies, including a total sample of 37,068 participants. Multiple evaluation scales were used, including Insomnia Severity Index (ISI) [43], Sleep Quality Scale (SQS) [44], Athens Insomnia Scale (AIS) [45], and Pittsburgh Sleep Quality Index (PSQI) [46]. The pooled prevalence of insomnia was 0.42 (95% CI 0.36–0.48). Sensitivity analyses and meta-regressions revealed no statistically significant differences regarding age, gender, NOS score, or continent.
Post-traumatic symptoms
Relevant post-traumatic symptoms prevalence was reported in 39 studies, including a total sample of 58,995 participants. Multiple evaluation scales were used, including Impact of Event Scale—Revised (IES-R) [47], Post Traumatic Stress Disorder Checklist—Civilian Version (PCL-C) [48], and Primary Care—Post Traumatic Stress Disorder Scale (PC-PTSD) [49]. The pooled prevalence of post-traumatic symptoms was 0.32 (95% CI 0.26–0.37). Prevalence varied from 0.20 with PCL-C to 0.38 with IES-R. No statistical statistically significant differences regarding age, gender, or NOS score were found in meta-regressions. Sensitivity analyses found a statistically significant lower prevalence of post-traumatic symptoms in Asia (0.29; 95% CI 0.18–0.34) compared to North America (0.41; 95% CI 0.34–0.48).
Burnout
Burnout prevalence was reported in 25 studies, including a total sample of 30,873 participants. Three scales were used to evaluate it: Mini-Z Burnout Survey (Mini-Z) [50], Copenhagen Burnout Inventory (CBI) [51], and Maslach Burnout Inventory (MBI) [52]. MBI has three measurable domains: emotional exhaustion (MBI-EE), depersonalization (MBI-DP), and reduced personal accomplishment (MBI-RPA). Scoring positively to any of these areas implies a relevant level of professional burnout. The pooled prevalence of burnout symptoms was 0.37 (95% CI 0.31–0.42). Prevalence varied from 0.22 with Mini-Z to 0.53 with CBI. In MBI, emotional exhaustion was the most deteriorated area among the sample. Sensitivity analyses and meta-regressions revealed no statistically significant differences regarding age, gender, NOS score, or continent.
Quality assessment and meta-regressions
According to NOS Scale, the mean quality of the included studies was 5.12 ± 0.80 and ranged from three to seven. Scores for each individual article are available in Supplementary Table S4.
Discussion
This meta-analysis has identified, for the first time on a large scale and at a global level, the prevalence of mental health symptoms in several domains in the HCWs group. HCW exposed to COVID-19 were found to have a significant prevalence rate of anxiety, depression, acute stress, insomnia, post-traumatic symptoms and burnout.
Thirty three percentage of the HCWs exposed to COVID-19 presented depressive symptomatology. This prevalence is higher than that reported in the general population during the pandemic, between 20.9% [53] and 27.8% [54]. The prevalence of anxiety in HCWs reported by this meta-analysis, 42%, is also much higher than that detected in the general population, 27.3% [55]. These results are consistent with previous findings in the literature; both Dutta et al. [56] and Saragih et al. [57] reported very similar data to those found in this study. These authors reported a total prevalence of depression of 0.32 and 0.37 and anxiety of 0.33 and 0.40, respectively. Another recent meta-analysis [58] reported a lower prevalence, 0.24 for depression and 0.26 for anxiety. However, this meta-analysis [58] included only 29 articles published in the initial months of the pandemic. All of this suggests that a progressive worsening in the mental health of HCWs may have occurred as the COVID-19 pandemic dragged on.
Insomnia was found to have a prevalence of 42%, higher than the 18–31% prevalence identified in other meta-analyses studying the general population for the same period [59–61]. This difference between the samples may be caused at least in part by the long and strenuous work shifts that characterize the duties of HCWs, which worsen insomnia and sleep quality [62,63].
As for acute stress, a prevalence of 40% was found in the sample included in our meta-analysis. While these results are similar to those previously reported in another recent meta-analysis [64], the prevalence of relevant post-traumatic stress symptoms in our sample (32%) was unexpectedly high, more than doubling the 15% prevalence previously reported [65,66]. This may be due to several reasons. Firstly, post-traumatic stress symptoms, as per definition, take time to appear, so it is reasonable to expect an increase in its prevalence as months go by. Furthermore, the previous meta-analysis included a lower number of studies, including mostly samples from Asia, limiting the generalization of its results to a global sample. General population samples also report a significantly lower prevalence of post-traumatic symptoms during the same time frame [67].
Finally, our study also analyzed the prevalence of burnout in HCWs exposed to the COVID-19 pandemic, a mental health domain little studied in previous meta-analyses. The sample included in our meta-analysis presented a 37% burnout prevalence. This is consistent with data reported by a previous study [3]. Burnout was already a relevant problem in HCWs before the COVID-19 pandemic, heavily related to a decrease in occupational well-being [68], so an increase in burnout prevalence is a growingly concerning phenomenon. During the pandemic, burnout has been especially high among young professionals due to increased workload, the loss of formational activities, and the perceived lack of proper supervision [69].
These results may have several clinical implications. First, our study confirms that HCWs are an especially vulnerable population during the COVID-19 pandemic, being more prone to mental health impairment than the general population. These findings suggest that the deterioration in the mental health of HCWs is not due to measures of general confinement, social distance and concern about the pandemic, but to the particularities of the health professions and their conditions during the pandemic. These challenges include the lack of protective equipment [70], increased workload and strenuous work shifts [71], but also ethical challenges and moral distress [72,73]. In addition to this, residents and fellows have seen their training deprioritized while also increasing their responsibility and workload [74]. Institutions should provide their professionals with proper formation, coping tools and strategies to alleviate the effects of the pandemic on their mental health. Preventive approaches should also be improved for HCWs facing these challenges, including the implementation of screening instruments to identify professionals with mental health symptomatology [3].
The meta-regression results reveal fewer symptoms in the post-traumatic domain in Asia than in other continents. Asia—and especially China, where most of the articles from this continent have been published—was initially the continent most affected by the pandemic [75], so these results may seem paradoxical. However, some studies have detected that proximity to the pandemic’s epicenter is inversely correlated with levels of distress, in a phenomenon known as the “Psychological Typhoon Eye” effect [76]. Previous research shows that deep emotional feelings, such as those related to health emergencies, decrease more quickly than less deep feelings since they activate internal psychological mechanisms designed to mitigate them [77]. Coping efficacy has been identified as a mediating factor between both events [78], which could, in turn, be stimulated by cultural factors related to collectivist cultures [79].
This study has several strengths. To the best of the authors’ knowledge, this is the largest meta-analysis published to date evaluating mental health outcomes in HCWs exposed to COVID-19. In addition, it evaluates domains of mental health less studied in previous meta-analyses, such as burnout or post-traumatic symptoms. Studies from more than 50 countries on 5 continents have been included, so its results are highly generalizable.
On the other hand, this study also has several limitations, mainly the considerable heterogeneity in the outcomes evaluated. Some authors have used different scales and cut-off points in the different domains of mental health. The exposure levels and the length of exposition duration of the HCWs included in the studied samples have not been analyzed due to the lack of data. Meta-regressions have been carried out to assess the impact of gender, professional category, and geographic origin on heterogeneity. COVID-19 pandemic has stimulated the publication of many studies in a short time, some of them of limited quality [80]. It is necessary to improve the design of the studies and standardize the methods and populations evaluated. Also, further studies should be conducted to determine in-depth the factors associated with mental health problems in HCWs during the pandemic.
In conclusion, HCWs worldwide exposed to COVID-19 were found to have a significant prevalence of concerning symptoms in a wide range of mental health domains. The effects of COVID-19 on HCWs’ mental health should not be underestimated. Further studies should be carried out to follow its evolution during the pandemic, and effective measures should be implemented to prevent and alleviate mental health deterioration in HCWs.
Supplementary material
For supplementary material accompanying this paper visit https://doi.org/10.1192/j.eurpsy.2022.1.
click here to view supplementary material
Data Availability Statement
The data that support the findings of this study are available from the corresponding author/first author, A.C./C.A., upon request.
Author Contributions
Conceptualization: C.A., B.P., L.A., G.S.P., and A.C.; Data curation: C.A., B.P., J.L.P., J.H., J.B., and G.M.; Formal analysis: C.A. and M.L.; Investigation: B.P., J.L.P., M.L., J.H., J.B., G.M., L.A., O.E., and M.F.; Methodology: B.P., J.L.P., M.L., J.H., J.B., G.M., L.A., O.E., G.S.P., and M.F.; Project administration: B.P.; Supervision: A.C. and M.A.G.-T.; Writing—original draft: C.A. and A.C.; Writing—review and editing: C.A., M.F., A.C., and M.A.G.-T.
Financial Support
This research received funding from the OSI Bilbao Basurto Research Commission.
Conflict of Interest
The authors declare no potential conflicts of interest.
References
- [1]. World Health Organization. WHO statement regarding cluster of pneumonia cases in Wuhan, China [accessed 31 December 2019].
- [2]. World Health Organization. Coronavirus disease (COVID-19), https://www.who.int/emergencies/diseases/novel-coronavirus-2019?gclid=Cj0KCQjw7MGJBhD-ARIsAMZ0eesjMiAoiau_iEk-616CPVWtErNDSN1hu6TMGslv2w7FlHfb07j7yYEaAg-8EALw_wcB; 2021. [accessed 2 September 2021].
- [3]. Salazar de Pablo G, Vaquerizo-Serrano J, Catalan A, Arango C, Moreno C, Ferre F, et al. Impact of coronavirus syndromes on physical and mental health of health care workers: systematic review and meta-analysis. J Affect Disord. 2020;275:48–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4]. Galanis P, Vraka I, Fragkou D, Bilali A, Kaitelidou D. Seroprevalence of SARS-CoV-2 antibodies and associated factors in healthcare workers: a systematic review and meta-analysis. J Hosp Infect. 2021;108:120–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5]. Almaghrabi RH, Alfaraidi HA, Al Hebshi WA, Albaadani MM. Healthcare workers experience in dealing with coronavirus (COVID-19) pandemic. Saudi Med J. 2020;41(6):657–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6]. Rosenbaum L. Facing covid-19 in Italy: ethics, logistics, and therapeutics on the epidemic’s front line. Recenti Prog Med. 2020;111(4):192–7. [DOI] [PubMed] [Google Scholar]
- [7]. Hart PS, Chinn S, Soroka, S. Politicization and polarization in COVID-19 news coverage. Sci Commun. 2020;42:679–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8]. Lynch J, Evans N, Ice E, Costa DK. Ignoring nurses: media coverage during the COVID-19 pandemic. Ann Am Thorac Soc. 2021;18(8):1278–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9]. Houghton C, Meskell P, Delaney H, Smalle M, Glenton C, Booth A, et al. Barriers and facilitators to healthcare workers’ adherence with infection prevention and control guidelines for respiratory infectious diseases: a rapid qualitative evidence synthesis. Emergencias. 2021;33(1):62–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10]. Lee SM, Kang WS, Cho AR, Kim T, Park JK. Psychological impact of the 2015 MERS outbreak on hospital workers and quarantined hemodialysis patients. Compr Psychiatry. 2018;87:123–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11]. Liu X, Kakade M, Fuller CJ, Fan B, Fang Y, Kong J, et al. Depression after exposure to stressful events: lessons learned from the severe acute respiratory syndrome epidemic. Compr Psychiatry. 2012;53(1):15–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12]. Chong MY, Wang WC, Hsieh WC, Lee CY, Chiu NM, Yeh WC, et al. Psychological impact of severe acute respiratory syndrome on health workers in a tertiary hospital. Br J Psychiatry. 2004;185:127–33. [DOI] [PubMed] [Google Scholar]
- [13]. McAlonan GM, Lee AM, Cheung V, Cheung C, Tsang KW, Sham PC, et al. Immediate and sustained psychological impact of an emerging infectious disease outbreak on health care workers. Can J Psychiatr. 2007;52(4):241–7. [DOI] [PubMed] [Google Scholar]
- [14]. Su TP, Lien TC, Yang CY, Su YL, Wang JH, Tsai SL, et al. Prevalence of psychiatric morbidity and psychological adaptation of the nurses in a structured SARS caring unit during outbreak: a prospective and periodic assessment study in Taiwan. J Psychiatr Res. 2007;41(1,2):119–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15]. Luo Y, Chua CR, Xiong Z, Ho RC, Ho CSH. A systematic review of the impact of viral respiratory epidemics on mental health: an implication on the coronavirus disease 2019 pandemic. Front Psychiatry. 2020;11:565098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16]. Pappa S, Ntella V, Giannakas T, Giannakoulis VG, Papoutsi E, Katsaounou P. Prevalence of depression, anxiety, and insomnia among healthcare workers during the COVID-19 pandemic: a systematic review and meta-analysis. Brain Behav Immun. 2020;88:901–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17]. Muller AE, Hafstad EV, Himmels JPW, Smedslund G, Flottorp S, Stensland SO, et al. The mental health impact of the covid-19 pandemic on healthcare workers, and interventions to help them: a rapid systematic review. Psychiatry Res. 2020;293:113441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18]. Danet Danet A. Psychological impact of COVID-19 pandemic in western frontline healthcare professionals. a systematic review. Med Clin (Barc). 2021;156(9):449–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19]. Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. 2009;151(4):264–9. [DOI] [PubMed] [Google Scholar]
- [20]. Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. meta-analysis of observational studies in epidemiology (MOOSE) group. J Am Med Assoc. 2000;283(15):2008–12. [DOI] [PubMed] [Google Scholar]
- [21]. Altman DG, Simera I, Hoey J, Moher D, Schulz K. EQUATOR: Reporting guidelines for health research. Lancet. 2008;371(9619):1149–50. [DOI] [PubMed] [Google Scholar]
- [22]. Wells GA, Shea B, O’Connell D, Peterson J, Welch V, Losos M, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of non-randomized studies in meta-analyses. 2012; Available at: http://wwwohrica/programs/clinical_epidemiology/oxfordasp.
- [23]. Lipsey MW, Wilson DB. Practical meta-analysis. 1st ed. Thousand Oaks: Sage; 2001. [Google Scholar]
- [24]. Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. Br Med J. 1997;315:629–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25]. StataCorp. Stata Statistical Software: Release 17. 2021.
- [26]. PRISMA. PRISMA Flow Diagram, http://prisma-statement.org/prismastatement/flowdiagram.aspx; 2021. [accessed September 7th, 2021].
- [27]. Tan YQ, Wang Z, Yap QV, Chan YH, Ho RC, Hamid ARAH, et al. Psychological health of surgeons in a time of COVID-19: a global survey. Ann Surg. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28]. Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;9:606–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29]. Kroenke K, Spitzer RL, Williams JB. The patient health questionnaire-2: validity of a two-item depression screener. Med Care. 2003;41:1284–92. [DOI] [PubMed] [Google Scholar]
- [30]. Kroenke K, Spitzer RL, Williams JB, Lowe B. An ultra-brief screening scale for anxiety and depression: the PHQ-4. Psychosomatics 2009;50(6):613–21. [DOI] [PubMed] [Google Scholar]
- [31]. Zung W. Self-rating depression scale. Arch Gen Psychiatry. 1965;12:63–70. [DOI] [PubMed] [Google Scholar]
- [32]. Snaith RP. The Hospital Anxiety and Depression Scale. Health Qual Life Outcomes. 2003;1:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33]. Beck AT, Ward CH, Mendelson M. An inventory for measuring depression. Arch Gen Psychiatry. 1961;4:561–71. [DOI] [PubMed] [Google Scholar]
- [34]. Radloff LS. The CES-D scale: a self-report depression scale for research in the general population. Appl Psychol Meas. 1977;1(3):385–401. [Google Scholar]
- [35]. Henry JD, Crawford JR. The short-form version of the Depression Anxiety Stress Scales (DASS-21): construct validity and normative data in a large non-clinical sample. Br J Clin Psychol. 2005;44(Pt 2):227–39. [DOI] [PubMed] [Google Scholar]
- [36]. Spitzer RL, Kroenke K, Williams JB, Lowe B. A brief measure for assessing generalized anxiety disorder: the GAD-7. Arch Intern Med. 2006;166(10):1092–7. [DOI] [PubMed] [Google Scholar]
- [37]. Zung W. A rating instrument for anxiety disorders. Psychosomatics. 1971;12:371–9. [DOI] [PubMed] [Google Scholar]
- [38]. Beck AT, Epstein N, Brown G, Steer RA. An inventory for measuring clinical anxiety: psychometric properties. J Consult. 1988;56:893. [DOI] [PubMed] [Google Scholar]
- [39]. Spielberger CD. State-trait anxiety inventory for adults; 1983.
- [40]. Lee SA. Coronavirus anxiety scale: a brief mental health screener for COVID-19 related anxiety. Death Stud. 2020;44(7):393–401. [DOI] [PubMed] [Google Scholar]
- [41]. Cardena E, Koopman C, Classen C, Waelde LC, Spiegel D. Psychometric properties of the Stanford Acute Stress Reaction Questionnaire (SASRQ): a valid and reliable measure of acute stress. J Trauma Stress. 2000;13(4):719–34. [DOI] [PubMed] [Google Scholar]
- [42]. Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. J Health Soc Behav. 1983;24(4):385–96. [PubMed] [Google Scholar]
- [43]. Morin CM, Belleville G, Belanger L, Ivers H. The Insomnia Severity Index: psychometric indicators to detect insomnia cases and evaluate treatment response. Sleep. 2011;34(5):601–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44]. Yi H, Shin K, Shin C. Development of the sleep quality scale. J Sleep Res. 2006;15(3):309–16. [DOI] [PubMed] [Google Scholar]
- [45]. Soldatos CR, Dikeos DG, Paparrigopoulos TJ. Athens Insomnia Scale: validation of an instrument based on ICD-10 criteria. J Psychosom Res. 2000;48(6):555–60. [DOI] [PubMed] [Google Scholar]
- [46]. Buysse DJ, Reynolds CF, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28(2):193–213. [DOI] [PubMed] [Google Scholar]
- [47]. Weiss DS, Marmar CR. The impact of event scale—Revised. In: Wilson JP, Keane TM, editors. Assessing psychological trauma and PTSD: a handbook for practitioners, New York:– Guilford Press; 1997, pp. 399–411. [Google Scholar]
- [48]. The PTSD checklist: reliability, validity, and diagnostic utility.In: Annual meeting of the international society for traumatic stress studies, San Antonio, CA; 1993. [Google Scholar]
- [49]. Prins A, Ouimette P, Kimerling R, et al. The primary care PTSD screen (PC-PTSD): development and operating characteristics. Prim Care Psychiatry. 2003;9:9–14. [Google Scholar]
- [50]. Dolan ED, Mohr D, Lempa M, Joos S, Fihn SD, Nelson KM, et al. Using a single item to measure burnout in primary care staff: a psychometric evaluation. J Gen Intern Med. 2015;30(5):582–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51]. Kristensen TS, Borritz M, Villadsen E, Christensen KB. The Copenhagen burnout inventory: a new tool for the assessment of burnout. Work & Stress. 2005;19:192–207. [Google Scholar]
- [52]. Maslach C, Jackson SE, Leiter MP. Maslach Burnout Inventory manual. In: Zalaquett P, Wood RJ, editors. Evaluating stress: a book of resources, London: The Scarecrow Press; 1997. pp. 191–218. [Google Scholar]
- [53]. Kim SW, Park IH, Kim M, Park AL, Jhon M, Kim JW, et al. Risk and protective factors of depression in the general population during the COVID-19 epidemic in Korea. BMC Psychiatry. 2021;21(1):445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54]. Ettman CK, Abdalla SM, Cohen GH, Sampson L, Vivier PM, Galea S. Prevalence of depression symptoms in US adults before and during the COVID-19 pandemic. JAMA Netw Open. 2020;3(9):e2019686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55]. Pashazadeh Kan F, Raoofi S, Rafiei S, Khani S, Hosseinifard H, Tajik F, et al. A systematic review of the prevalence of anxiety among the general population during the COVID-19 pandemic. J Affect Disord. 2021;293:391–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56]. Dutta A, Sharma A, Torres-Castro R, Pachori H, Mishra S. Mental health outcomes among health-care workers dealing with COVID-19/severe acute respiratory syndrome coronavirus 2 pandemic: a systematic review and meta-analysis. Indian J Psychiatry. 2021;63(4):335–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57]. Saragih ID, Tonapa SI, Saragih IS, Advani S, Batubara SO, Suarilah I, et al. Global prevalence of mental health problems among healthcare workers during the Covid-19 pandemic: a systematic review and meta-analysis. Int J Nurs Stud. 2021;121:104002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58]. Salari N, Khazaie H, Hosseinian-Far A, Khaledi-Paveh B, Kazeminia M, Mohammadi M, et al. The prevalence of stress, anxiety and depression within front-line healthcare workers caring for COVID-19 patients: a systematic review and meta-regression. Hum Resour Health. 2020;18(1):100–1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59]. Liu X, Zhu M, Zhang R, Zhang J, Zhang C, Liu P, et al. Public mental health problems during COVID-19 pandemic: a large-scale meta-analysis of the evidence. Transl Psychiatry. 2021;11(1):384–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60]. Alimoradi Z, Gozal D, Tsang HWH, Lin CY, Brostrom A, Ohayon MM, et al. Gender-specific estimates of sleep problems during the COVID-19 pandemic: systematic review and meta-analysis. J Sleep Res. 2021; 31(1):e13432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61]. Huang Y, Zhao N. Mental health burden for the public affected by the COVID-19 outbreak in China: who will be the high-risk group? Psychol Health Med. 2021;26(1):23–34. [DOI] [PubMed] [Google Scholar]
- [62]. Galasso L, Mule A, Castelli L, Ce E, Condemi V, Banfi G, et al. Effects of shift work in a sample of Italian nurses: analysis of rest-activity circadian rhythm. Int J Environ Res Public Health. 2021;18(16):8378. doi: 10.3390/ijerph18168378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63]. Shehata RSA, Mohamed Nour ZA, Abdelrahim Badr AM, Khalifa EM. Serotonin variations and sleep disorders among shift workers. a cross-sectional study. Toxicol Ind Health. 2021;37:603–9. [DOI] [PubMed] [Google Scholar]
- [64]. Al Maqbali M, Al Sinani M, Al-Lenjawi B. Prevalence of stress, depression, anxiety and sleep disturbance among nurses during the COVID-19 pandemic: a systematic review and meta-analysis. J Psychosom Res. 2021;141:110343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65]. Norhayati MN, Che Yusof R, Azman MY. Prevalence of psychological impacts on healthcare providers during COVID-19 pandemic in Asia. Int J Environ Res Public Health. 2021;18(17):9157. doi: 10.3390/ijerph18179157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66]. Krishnamoorthy Y, Nagarajan R, Saya GK, Menon V. Prevalence of psychological morbidities among general population, healthcare workers and COVID-19 patients amidst the COVID-19 pandemic: a systematic review and meta-analysis. Psychiatry Res. 2020;293:113382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67]. Tromans S. Editorial on psychological distress in the Greek general population during the first COVID-19 lockdown. BJPsych Open. 2021;7(5):e170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68]. Adriaenssens J, De Gucht V, Maes S. Determinants and prevalence of burnout in emergency nurses: a systematic review of 25 years of research. Int J Nurs Stud. 2015;52(2):649–61. [DOI] [PubMed] [Google Scholar]
- [69]. Jimenez-Labaig P, Pacheco-Barcia V, Cebria A, Galvez F, Obispo B, Paez D, et al. Identifying and preventing burnout in young oncologists, an overwhelming challenge in the COVID-19 era: a study of the Spanish Society of Medical Oncology (SEOM). ESMO Open. 2021;6(4):100215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70]. Lacobucci G. Covid-19: doctors still at “considerable risk” from lack of PPE, BMA warns. British Med J. 2020;368:m1316. [DOI] [PubMed] [Google Scholar]
- [71]. Lucchini A, Lozzo P, Bambi S. Nursing workload in the COVID-19 era. Intensive Crit Care Nurs. 2020;61:102929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72]. Hossain F, Clatty A. Self-care strategies in response to nurses’ moral injury during COVID-19 pandemic. Nurs Ethics. 2021;28(1):23–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73]. Drewett GP, Gibney G, Ko D. Practical ethical challenges and moral distress among staff in a hospital COVID-19 screening service. Intern Med J. 2021;51(9):1513–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74]. Dedeilia A, Sotiropoulos MG, Hanrahan JG, Janga D, Dedeilias P, Sideris M. Medical and surgical education challenges and innovations in the COVID-19 era: a systematic review. In Vivo. 2020;34(3 Suppl):1603–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75]. Kang L, Li Y, Hu S, Chen M, Yang C, Yang BX, et al. The mental health of medical workers in Wuhan, China dealing with the 2019 novel coronavirus. Lancet Psychiatry. 2020;7(3):e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76]. Xie XF, Stone E, Zheng R, Zhang RG. The ‘typhoon eye effect’: determinants of distress during the SARS epidemic. J Risk Res. 2011;14(9):1091–107. [Google Scholar]
- [77]. Gilbert DT, Lieberman MD, Morewedge CK, Wilson TD. The peculiar longevity of things not so bad. Psychol Sci. 2004;15(1):14–19. [DOI] [PubMed] [Google Scholar]
- [78]. Zhang L, Ma M, Li D, Xin Z. The psychological typhoon eye effect during the COVID-19 outbreak in China: the role of coping efficacy and perceived threat. Glob Health. 2020;16(1):105–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79]. Hook CJ, Rose Markus H. Health in the United States: are appeals to choice and personal responsibility making Americans sick? Perspect Psychol Sci. 2020. 15(3):643–64. [DOI] [PubMed] [Google Scholar]
- [80]. Jung RG, Di Santo P, Clifford C, Prosperi-Porta G, Skanes S, Hung A, et al. Methodological quality of COVID-19 clinical research. Nat Commun. 2021;12(1):943–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
For supplementary material accompanying this paper visit https://doi.org/10.1192/j.eurpsy.2022.1.
click here to view supplementary material
Data Availability Statement
The data that support the findings of this study are available from the corresponding author/first author, A.C./C.A., upon request.